Effects of Foliar Application of Paclobutrazol on Grain Yield, Aroma, and Canopy Radiation Use Efficiency of Aromatic Rice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Experimental Design
2.2. Measurements
2.2.1. Above-Ground Dry Matter Accumulation
2.2.2. Yield Evaluation
2.2.3. Photosynthetic Related Parameters
2.2.4. Grain 2-Acetyl-1-Pyrroline Content
2.2.5. Grains Δ1-Pyrroline-5-Carboxylic Acid, Proline, and Δ1-Pyrroline Contents
2.2.6. Intercepted Radiation and Radiation Use Efficiency
2.3. Data Processing and Analysis
3. Results
3.1. Above-Ground Biomass
3.2. Yield and Yield Composition
3.3. Photosynthetic Characteristics
3.4. Canopy Light Energy Resource Utilization
3.5. Grain 2-Acetyl-1-Pyrroline, Proline, P5C, and Δ1-Pyrroline Contents
3.6. Correlation Coefficient Analysis
3.7. Interaction Analysis of 2-AP Content with IPAR and RUE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutta, C.; Nath, D.J.; Phyllei, D. Aromatic rice and factors affecting aroma in rice. Int. J. Environ. Clim. Change 2022, 12, 1773–1779. [Google Scholar] [CrossRef]
- Bairagi, S.; Demont, M.; Custodio, M.C.; Ynion, J. What drives consumer demand for rice fragrance? Evidence from south and southeast Asia. Br. Food J. 2020, 122, 3473–3498. [Google Scholar] [CrossRef]
- Minten, B.; Murshid, K.; Reardon, T. Food quality changes and implications: Evidence from the rice value chain of Bangladesh. World Dev. 2013, 42, 100–113. [Google Scholar] [CrossRef]
- Khan, M.A.I.; Bhuiyan, M.R.; Hossain, M.S.; Sen, P.P.; Ara, A.; Siddique, M.A.; Ali, M.A. Neck blast disease influences grain yield and quality traits of aromatic rice. Comptes Rendus. Biol. 2014, 337, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ashraf, U.; Tian, H.; Mo, Z.; Pan, S.; Anjum, S.A.; Duan, M.; Tang, X. Manganese-induced regulations in growth, yield formation, quality characters, rice aroma and enzyme involved in 2-acetyl-1-pyrroline biosynthesis in fragrant rice. Plant Physiol. Biochem. 2016, 103, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Ling, L.C.; Mon, T.R. Quantitative analysis of 2-acetyl-1-pyrroline in rice. J. Agric. Food Chem. 1986, 34, 112–114. [Google Scholar] [CrossRef]
- Ren, Y.; Ashraf, U.; He, L.X.; Mo, Z.W.; Wang, F.; Wan, X.C.; Kong, H.; Ran, X.L.; Tang, X.R. Irrigation and nitrogen management practices affect grain yield and 2-acetyl-1-pyrroline content in aromatic rice. Appl. Ecol. Environ. Res. 2017, 15, 1447–1460. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Fu, X.; Gao, Z.; Liu, H.; Tan, J.; Potcho, M.P.; Pan, S.; Tian, H.; Duan, M. Light and water treatment during the early grain filling stage regulates yield and aroma formation in aromatic rice. Sci. Rep. 2020, 10, 14830. [Google Scholar] [CrossRef]
- Bao, G.; Ashraf, U.; Li, L.; Qiao, J.; Wang, C.; Zheng, Y. Transcription factor osbzip60-like regulating osp5cs1 gene and 2-acetyl-1-pyrroline (2-ap) biosynthesis in aromatic rice. Plants 2023, 13, 49. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.; Li, Y.; Ma, L.; Ashraf, U.; Tang, X.; Pan, S.; Tian, H.; Mo, Z. Application of γ-aminobutyric acid under low light conditions: Effects on yield, aroma, element status, and physiological attributes of fragrant rice. Ecotoxicol. Environ. Saf. 2021, 213, 111941. [Google Scholar] [CrossRef]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 9. [Google Scholar] [CrossRef]
- Xing, P.; Duan, M.; Liu, Y.; Mo, Z.; Lai, R.; Tang, X. Enhancement of yield, grain quality characters, 2-acetyl-1-pyrroline content, and photosynthesis of fragrant rice cultivars by foliar application of paclobutrazol. J. Plant Growth Regul. 2023, 42, 748–758. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; Zhao, L.; Lu, K.; Song, X.; Wang, C. Research progress in biosynthesis and influencing factors of 2-acetyl-1-pyrroline in fragrant rice. Chin. J. Rice Sci. 2022, 36, 131. [Google Scholar]
- Vineeth, T.V.; Kumar, P.; Yadav, S.; Pal, M. Optimization of bio-regulators dose based on photosynthetic and yield performance of chickpea (Cicer arietinum L.) Genotypes. Indian J. Plant Physiol. 2015, 20, 177–181. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Panneerselvam, R. Responses of antioxidant defense system of Catharanthus roseus (L.) G. Don. To paclobutrazol treatment under salinity. Acta Physiol. Plant 2007, 29, 205–209. [Google Scholar] [CrossRef]
- Kim, J.; Wilson, R.L.; Case, J.B.; Binder, B.M. A comparative study of ethylene growth response kinetics in eudicots and monocots reveals a role for gibberellin in growth inhibition and recovery. Plant Physiol. 2012, 160, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Syahputra, B.S.A.; Sinniah, U.R.; Ismail, M.R.; Swamy, M.K. Optimization of paclobutrazol concentration and application time for increased lodging resistance and yield in field-grown rice. Philipp. Agric. Sci. 2016, 99, 221–228. [Google Scholar]
- Zhang, Y.; He, Z.; Xing, P.; Luo, H.; Yan, Z.; Tang, X. Effects of paclobutrazol seed priming on seedling quality, photosynthesis, and physiological characteristics of fragrant rice. BMC Plant Biol. 2024, 24, 53. [Google Scholar] [CrossRef] [PubMed]
- Tesfahun, W. A review on: Response of crops to paclobutrazol application. Cogent Food Agric. 2018, 4, 1525169. [Google Scholar] [CrossRef]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kishor, P.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef]
- Renuka, N.; Barvkar, V.T.; Ansari, Z.; Zhao, C.; Wang, C.; Zhang, Y.; Nadaf, A.B. Co-functioning of 2ap precursor amino acids enhances 2-acetyl-1-pyrroline under salt stress in aromatic rice (Oryza sativa L.) Cultivars. Sci. Rep. 2022, 12, 3911. [Google Scholar] [CrossRef]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Qi, Z.; Li, S.; Du, S.; Guan, S.; Xu, D.; Zhang, G.; Ma, J.; Yan, X. Effects of nitrogen utilization differences among rice varieties on yield. Trans. Chin. Soc. Agric. Mach. 2025, 56, 684–692. [Google Scholar]
- Bao, G.; Ashraf, U.; Wang, C.; He, L.; Wei, X.; Zheng, A.; Mo, Z.; Tang, X. Molecular basis for increased 2-acetyl-1-pyrroline contents under alternate wetting and drying (awd) conditions in fragrant rice. Plant Physiol. Biochem. 2018, 133, 149–157. [Google Scholar] [CrossRef]
- Lu, J.; Liu, K.; Deng, J.; Feng, X.; Xiong, X.; Huang, L.; Tian, X.; Zhang, Y. Evaluating the effect of population density and the contribution of early canopy closure to grain yield of hybrid rice. J. Plant Growth Regul. 2022, 41, 830–839. [Google Scholar] [CrossRef]
- Yan, K.L.; Han, Y.; Tan, T.G.; Tang, L.D.; Wu, J.H. Effects of 15% paclobutrazol wp on growth, yield and quality in rice. Chin. J. Trop. Agric. 2016, 36, 73–76. [Google Scholar]
- Cheng, C.; Lei, K.; Cheng, H.; Wang, S.; Zhu, B.; Lu, Z.; Gao, B.; Wang, B.; Shi, Q.; Zeng, Y. Effects of different concentrations of paclobutrazol in seedling stage on seedling quality, tillering dynamics and grain yield of japonica rice during late cropping season in southern China. Chin. J. Rice Sci. 2020, 34, 150. [Google Scholar]
- Kamran, M.; Wennan, S.; Ahmad, I.; Xiangping, M.; Wenwen, C.; Xudong, Z.; Siwei, M.; Khan, A.; Qingfang, H.; Tiening, L. Application of paclobutrazol affect maize grain yield by regulating root morphological and physiological characteristics under a semi-arid region. Sci. Rep. 2018, 8, 4818. [Google Scholar] [CrossRef] [PubMed]
- Nivedithadevi, D.; Somasundaram, R.; Pannerselvam, R. Effect of abscisic acid, paclobutrazol and salicylic acid on the growth and pigment variation in Solanum trilobatum (L.). Int. J. Drug Dev. Res. 2012, 4, 236–246. [Google Scholar]
- Nouriyani, H.; Majidi, E.; Seyyednejad, S.M.; Siadat, S.A.; Naderi, A. Evaluation of interaction of paclobutrazol and nitrogen on correlation between yield and photosynthetic pigments contents in two wheat cultivars (Triticum aestivum L.). Res. Crops 2012, 13, 446–452. [Google Scholar]
- Kumar, A.; Bhuj, B.; Dhar, S.; Rajkumar; Rizwan, M.; Thapa, R.; Kumar, H.; Kumar, V.; Singh, A.; Kumar, V.; et al. Effect of paclobutrazole (pbz) on fruit production: A review. Int. Res. J. Plant Sci. 2023, 14, 1–20. [Google Scholar]
- Kamran, M.; Ahmad, S.; Ahmad, I.; Hussain, I.; Meng, X.; Zhang, X.; Javed, T.; Ullah, M.; Ding, R.; Xu, P. Paclobutrazol application favors yield improvement of maize under semiarid regions by delaying leaf senescence and regulating photosynthetic capacity and antioxidant system during grain-filling stage. Agronomy 2020, 10, 187. [Google Scholar] [CrossRef]
- Zhai, H.; Cao, S.; Wan, J.; Zhang, R.; Lu, W.; Li, L.; Kuang, T.; Min, S.; Zhu, D.; Cheng, S. Relationship between leaf photosynthetic function at grain filling stage and yield in super high-yielding hybrid rice (Oryza sativa L). Sci. China Ser. C Life Sci. 2002, 45, 637–646. [Google Scholar] [CrossRef] [PubMed]
- George-Jaeggli, B.; Jordan, D.R.; van Oosterom, E.J.; Broad, I.J.; Hammer, G.L. Sorghum dwarfing genes can affect radiation capture and radiation use efficiency. Field Crops Res. 2013, 149, 283–290. [Google Scholar] [CrossRef]
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Zhou, L.; Meng, X.; Zhang, Z.; Wu, Q. Association analysis of growth characteristics, wue, and rue of rice in cold region under different irrigation patterns. J. Inst. Eng. Ser. A 2020, 101, 421–431. [Google Scholar] [CrossRef]
- Shang, C.; Guo, Z.; Chong, H.; Xiong, X.; Deng, J.; Harrison, M.T.; Liu, K.; Huang, L.; Tian, X.; Zhang, Y. Higher radiation use efficiency and photosynthetic characteristics after flowering could alleviate the yield loss of indica-japonica hybrid rice under shading stress. Int. J. Plant Prod. 2022, 16, 105–117. [Google Scholar] [CrossRef]
- Potcho, P.M.; Imran, M.; Korohou, T.; Kamara, N.; Tang, X. Fertilizer deep placement significantly affected yield, rice quality, 2-ap biosynthesis and physiological characteristics of the fragrant rice cultivars. Agronomy 2022, 12, 162. [Google Scholar] [CrossRef]
- Okpala, N.E.; Mo, Z.; Duan, M.; Tang, X. The genetics and biosynthesis of 2-acetyl-1-pyrroline in fragrant rice. Plant Physiol. Biochem. 2019, 135, 272–276. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 2, 227–239. [Google Scholar] [CrossRef]
- Gopi, R.; Jaleel, C.A.; Sairam, R.; Lakshmanan, G.; Gomathinayagam, M.; Panneerselvam, R. Differential effects of hexaconazole and paclobutrazol on biomass, electrolyte leakage, lipid peroxidation and antioxidant potential of Daucus carota L. Colloids Surf. B Biointerfaces 2007, 60, 180–186. [Google Scholar]
- Jiang, Z.; Yao, X.; Du, B.; Wang, X.; Tang, X.; Pan, S.; Mo, Z. The biosynthesis of 2-acetyl-1-pyrroline is physiologically driven by carbon-nitrogen metabolism in fragrant rice. Eur. J. Agron. 2025, 164, 127476. [Google Scholar] [CrossRef]
- Chen, J.; Xie, W.; Huang, Z.; Ashraf, U.; Pan, S.; Tian, H.; Duan, M.; Wang, S.; Tang, X.; Mo, Z. Light quality during booting stage modulates fragrance, grain yield and quality in fragrant rice. J. Plant Interact. 2021, 16, 42–52. [Google Scholar] [CrossRef]
- Yoshihashi, T.; Huong, N.T.T.; Inatomi, H. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J. Agric. Food Chem. 2002, 50, 2001–2004. [Google Scholar] [CrossRef]
- Poonlaphdecha, J.; Gantet, P.; Maraval, I.; Sauvage, F.; Menut, C.; Morère, A.; Boulanger, R.; Wüst, M.; Gunata, Z. Biosynthesis of 2-acetyl-1-pyrroline in rice calli cultures: Demonstration of 1-pyrroline as a limiting substrate. Food Chem. 2016, 197, 965–971. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Jia, Y.; Han, D.; Yu, C.; Xu, M.; Fu, J. Study on difference of photosynthetic matter production and light energy utilization efficiency in japonica rice. J. Northeast Agric. Univ. 2021, 52, 1165. [Google Scholar]
- Jiang, S.; Zhang, L.; Hu, F.; Tang, X.; Du, B. Zinc supplementation and light intensity affect 2-acetyl-1-pyrroline (2ap) formation in fragrant rice. BMC Plant Biol. 2023, 23, 194. [Google Scholar] [CrossRef]
- Li, Q.; Hu, H.; Wang, X.; Liu, F.; Sun, M.; Ren, Y.; Qiu, X. Effects of exogenous azelaic acid (aza) application on 2-acetyl-1-pyrroline (2ap) content, the antioxidant defense system, and yield in fragrant rice. J. Plant Growth Regul. 2025, 44, 5909–5925. [Google Scholar] [CrossRef]
- Chakraborty, R.; Roy, T.S.; Sakagami, J. Influence of γ-aminobutyric acid (gaba) application on aromatic rice under shading and drought stress conditions: Effects on crop yield, grain quality, and 2-acetyl-1-pyrroline biosynthesis. J. Plant Growth Regul. 2025, 44, 1043–1051. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Wu, J.F.; Yin, Q.; Yang, X.F. Residual Risk Assessment and Application of 10% Paclobutrazol·Mepiquat Chloride Wettable Powder in Rice. Agrochemicals 2021, 4, 285–289. [Google Scholar]
- Yuan, F.; Yang, R.B.; Peng, J.Y.; She, J.R. Degradation of 15% Paclobutrazol Wettable Powder in Rice. J. Agri. Environ. Sci. 2007, 26, 4. [Google Scholar]

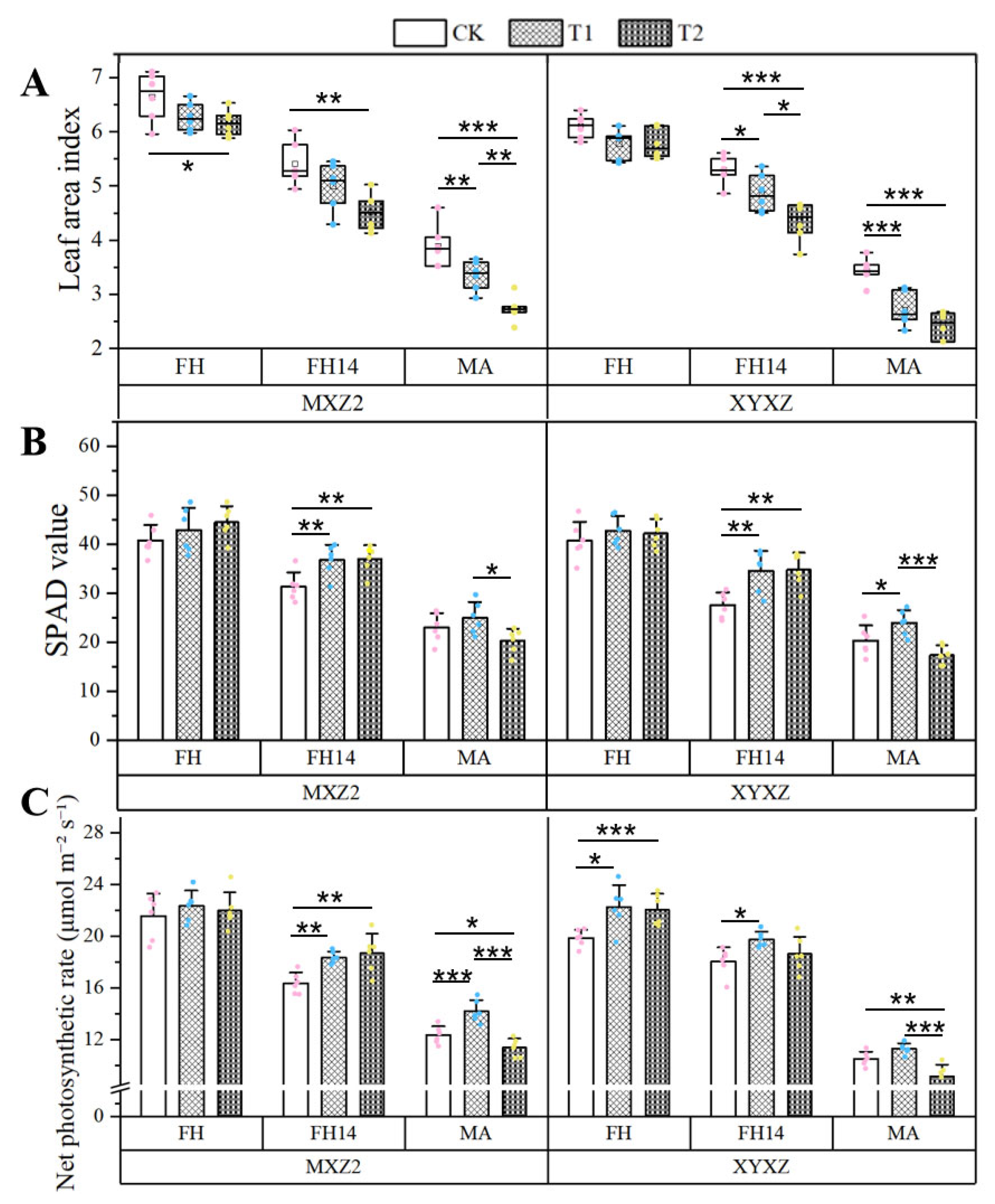

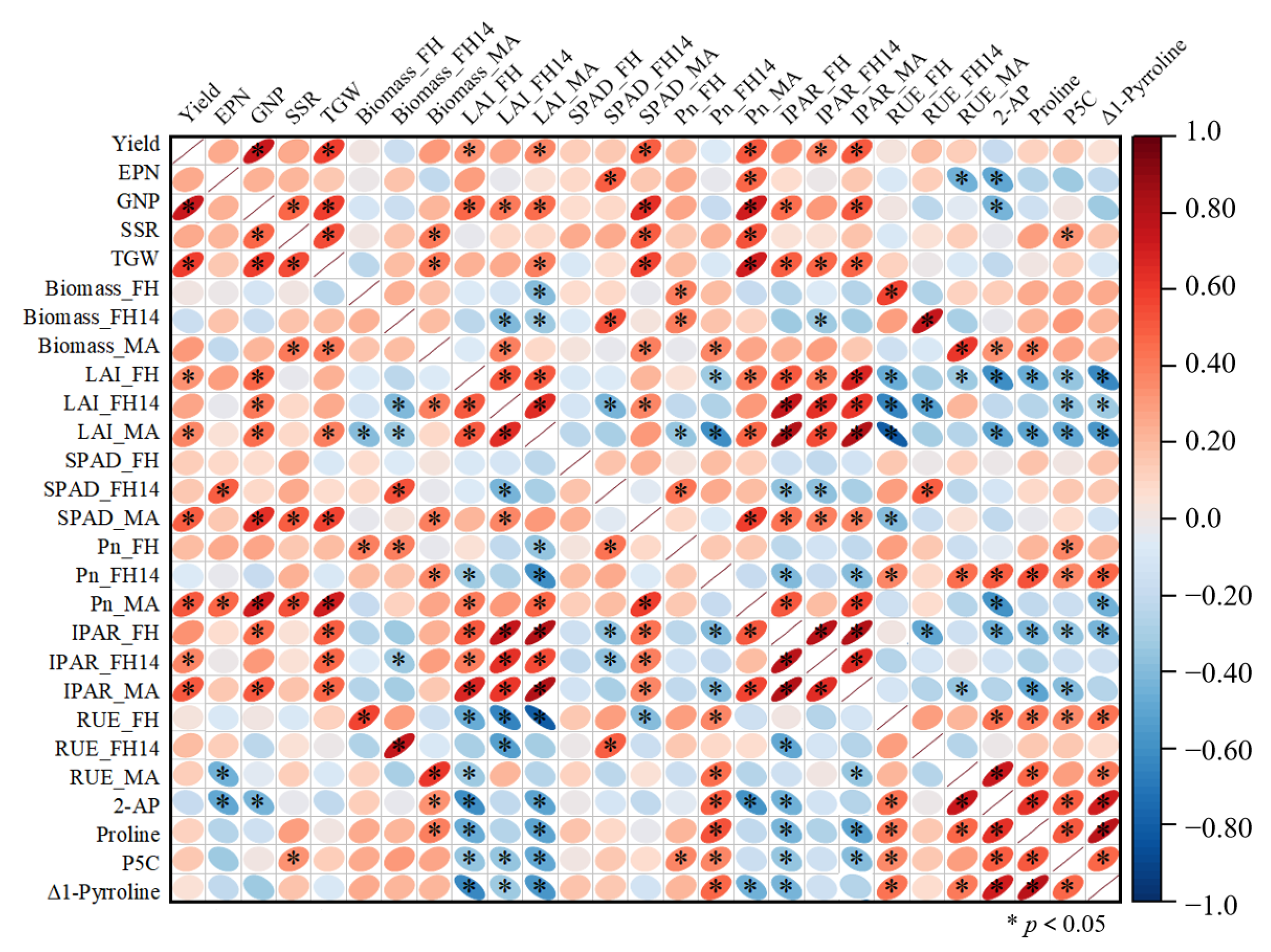
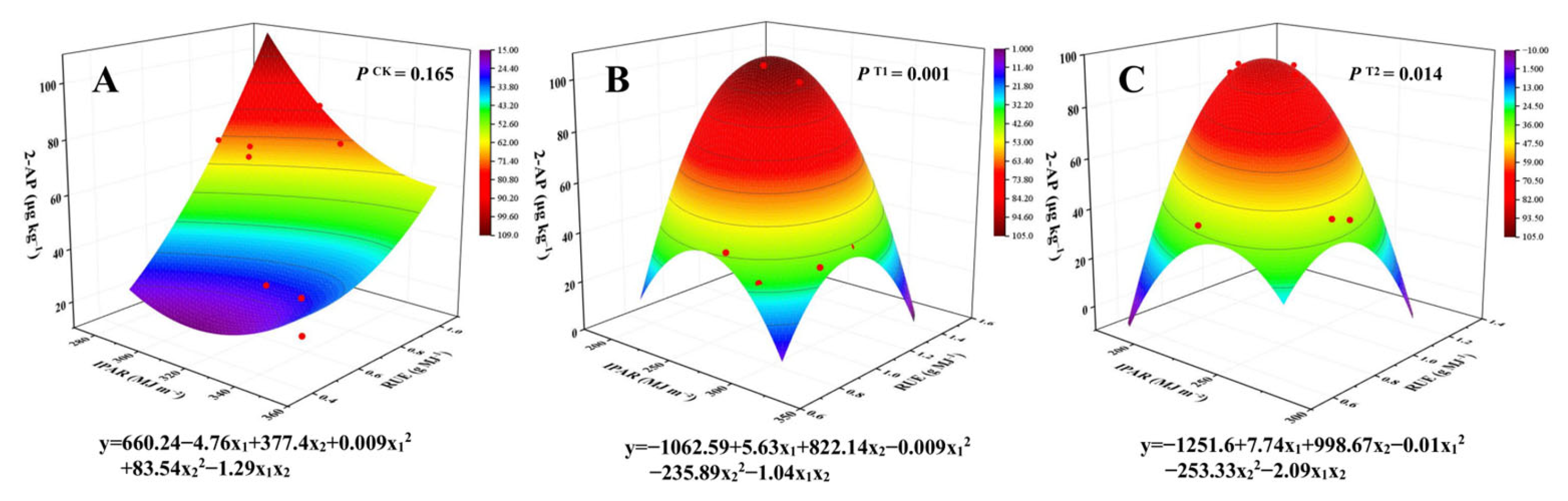
| Year | Cultivar | Treatment | FH | FH14 | MA |
|---|---|---|---|---|---|
| 2022 | MXZ2 | CK | 677.93 ± 47.13 a | 925.42 ± 38.17 b | 1196.81 ± 17.06 a |
| T1 | 684.07 ± 29.52 a | 1045.21 ± 41.33 a | 1268.61 ± 55.97 a | ||
| T2 | 698.69 ± 38.39 a | 1005.92 ± 38.86 a | 1167.87 ± 70.35 a | ||
| XYXZ | CK | 693.03 ± 20.32 a | 909.67 ± 41.52 b | 1290.58 ± 29.44 ab | |
| T1 | 715.85 ± 37.20 a | 987.71 ± 31.71 a | 1359.71 ± 46.22 a | ||
| T2 | 700.04 ± 35.76 a | 940.46 ± 34.94 ab | 1234.43 ± 45.43 b | ||
| 2023 | MXZ2 | CK | 709.66 ± 43.30 a | 877.71 ± 46.88 a | 1252.51 ± 20.70 b |
| T1 | 703.60 ± 16.87 a | 932.46 ± 39.14 a | 1316.78 ± 13.40 a | ||
| T2 | 742.60 ± 29.63 a | 916.92 ± 24.80 a | 1200.43 ± 42.40 b | ||
| XYXZ | CK | 695.42 ± 37.93 a | 879.79 ± 21.56 b | 1237.15 ± 54.27 ab | |
| T1 | 718.94 ± 50.58 a | 928.75 ± 16.24 ab | 1306.65 ± 37.62 a | ||
| T2 | 735.93 ± 22.90 a | 988.21 ± 45.81 a | 1190.02 ± 64.62 b | ||
| Analysis of variance (ANOVA) | |||||
| Year | n.s. | * | n.s. | ||
| Cultivar | n.s. | n.s. | n.s. | ||
| Treatment | n.s. | ** | *** | ||
| Year × Cultivar | n.s. | ** | ** | ||
| Year × Treatment | n.s. | n.s. | n.s. | ||
| Cultivar × Treatment | n.s. | n.s. | n.s. | ||
| Year × Cultivar × Treatment | n.s. | n.s. | n.s. | ||
| Year | Cultivar | Treatment | EPN (m2) | GNP | SSR (%) | TGW (g) |
|---|---|---|---|---|---|---|
| 2022 | MXZ2 | CK | 273.49 ± 8.64 b | 137.72 ± 4.69 ab | 76.21 ± 1.56 b | 19.29 ± 0.51 ab |
| T1 | 284.69 ± 15.44 b | 143.72 ± 7.67 a | 84.51 ± 1.16 a | 20.87 ± 1.20 a | ||
| T2 | 307.70 ± 13.90 a | 126.90 ± 9.82 b | 75.09 ± 2.49 b | 17.91 ± 0.47 b | ||
| XYXZ | CK | 254.93 ± 8.52 a | 121.37 ± 5.75 b | 73.27 ± 2.67 b | 18.15 ± 0.26 b | |
| T1 | 267.60 ± 11.27 a | 133.11 ± 6.03 a | 81.55 ± 3.59 a | 19.59 ± 0.49 a | ||
| T2 | 255.41 ± 12.44 a | 122.21 ± 2.91 b | 75.37 ± 3.13 ab | 16.93 ± 0.61 c | ||
| 2023 | MXZ2 | CK | 284.99 ± 21.85 a | 145.37 ± 7.80 ab | 75.00 ± 1.27 b | 19.01 ± 0.57 a |
| T1 | 304.99 ± 18.62 a | 153.85 ± 6.36 a | 83.32 ± 2.65 a | 19.98 ± 0.55 a | ||
| T2 | 286.14 ± 25.34 a | 133.33 ± 10.14 b | 80.19 ± 2.83 a | 17.59 ± 0.51 b | ||
| XYXZ | CK | 246.96 ± 13.82 a | 127.83 ± 6.88 b | 77.45 ± 2.52 b | 18.64 ± 0.44 a | |
| T1 | 276.18 ± 19.86 a | 142.07 ± 3.39 a | 84.03 ± 1.91 a | 19.50 ± 0.44 a | ||
| T2 | 267.72 ± 15.27 a | 114.60 ± 6.10 c | 71.53 ± 2.19 c | 17.58 ± 0.52 b | ||
| Analysis of variance (ANOVA) | ||||||
| Year | n.s. | n.s. | n.s. | n.s. | ||
| Cultivar | *** | *** | n.s. | n.s. | ||
| Treatment | n.s. | ** | *** | *** | ||
| Year × Cultivar | n.s. | n.s. | n.s. | * | ||
| Year × Treatment | n.s. | n.s. | n.s. | n.s. | ||
| Cultivar × Treatment | n.s. | n.s. | n.s. | * | ||
| Year × Cultivar × Treatment | * | n.s. | ** | n.s. | ||
| Year | Cultivar | Treatment | IPAR (MJ m−2) | RUE (g MJ−1) | ||||
|---|---|---|---|---|---|---|---|---|
| FH | FH14 | MA | FH | FH14 | MA | |||
| 2022 | MXZ2 | CK | 522.47 ± 11.56 a | 568.10 ± 8.51 a | 341.44 ± 15.8 a | 1.07 ± 0.10 b | 0.44 ± 0.08 b | 0.80 ± 0.10 a |
| T1 | 473.18 ± 8.59 b | 451.84 ± 10.72 b | 294.66 ± 10.25 b | 1.20 ± 0.04 ab | 0.80 ± 0.07 a | 0.76 ± 0.06 a | ||
| T2 | 433.99 ± 10.50 c | 408.04 ± 3.88 c | 239.60 ± 22.25 c | 1.33 ± 0.11 a | 0.75 ± 0.10 a | 0.67 ± 0.10 a | ||
| XYXZ | CK | 514.55 ± 13.24 a | 561.09 ± 6.07 a | 298.17 ± 6.24 a | 1.13 ± 0.05 b | 0.39 ± 0.07 b | 1.28 ± 0.10 b | |
| T1 | 480.87 ± 13.06 b | 528.29 ± 2.54 b | 218.99 ± 7.87 b | 1.25 ± 0.13 b | 0.52 ± 0.07 b | 1.70 ± 0.03 a | ||
| T2 | 353.63 ± 7.77 c | 329.63 ± 11.17 c | 189.62 ± 9.81 c | 1.35 ± 0.08 a | 0.73 ± 0.08 a | 1.55 ± 0.09 a | ||
| 2023 | MXZ2 | CK | 547.49 ± 13.91 a | 548.03 ± 13.19 a | 318.37 ± 15.73 a | 1.08 ± 0.10 b | 0.31 ± 0.01 c | 1.18 ± 0.07 a |
| T1 | 509.05 ± 10.74 b | 520.57 ± 40.48 ab | 320.58 ± 5.97 a | 1.15 ± 0.02 b | 0.44 ± 0.03 a | 1.20 ± 0.11 a | ||
| T2 | 419.66 ± 10.22 c | 467.82 ± 19.31 b | 271.50 ± 4.20 b | 1.30 ± 0.08 a | 0.37 ± 0.02 b | 1.04 ± 0.08 a | ||
| XYXZ | CK | 513.91 ± 10.53 a | 510.95 ± 21.80 a | 299.83 ± 23.72 a | 1.13 ± 0.03 b | 0.36 ± 0.02 b | 1.19 ± 0.02 b | |
| T1 | 415.83 ± 10.33 b | 504.58 ± 24.26 a | 254.97 ± 14.49 b | 1.44 ± 0.13 a | 0.41 ± 0.06 ab | 1.48 ± 0.06 a | ||
| T2 | 404.10 ± 7.87 b | 469.99 ± 17.78 a | 206.48 ± 24.94 c | 1.55 ± 0.04 a | 0.54 ± 0.12 a | 0.98 ± 0.08 c | ||
| Analysis of variance (ANOVA) | ||||||||
| Year | n.s. | n.s. | n.s. | n.s. | *** | n.s. | ||
| Cultivar | n.s. | n.s. | *** | * | n.s. | *** | ||
| Treatment | *** | *** | *** | *** | ** | n.s. | ||
| Year × Cultivar | ** | n.s. | n.s. | n.s. | *** | *** | ||
| Year × Treatment | ** | *** | ** | n.s. | ** | ** | ||
| Cultivar × Treatment | * | *** | * | n.s. | ** | *** | ||
| Year × Cultivar × Treatment | *** | *** | n.s. | ** | n.s. | ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, F.; Lu, J.; Zhai, L.; Qiu, X.; Du, B.; Xu, J. Effects of Foliar Application of Paclobutrazol on Grain Yield, Aroma, and Canopy Radiation Use Efficiency of Aromatic Rice. Biology 2025, 14, 1562. https://doi.org/10.3390/biology14111562
Hu F, Lu J, Zhai L, Qiu X, Du B, Xu J. Effects of Foliar Application of Paclobutrazol on Grain Yield, Aroma, and Canopy Radiation Use Efficiency of Aromatic Rice. Biology. 2025; 14(11):1562. https://doi.org/10.3390/biology14111562
Chicago/Turabian StyleHu, Fengqin, Jian Lu, Laiyuan Zhai, Xianjin Qiu, Bin Du, and Jianlong Xu. 2025. "Effects of Foliar Application of Paclobutrazol on Grain Yield, Aroma, and Canopy Radiation Use Efficiency of Aromatic Rice" Biology 14, no. 11: 1562. https://doi.org/10.3390/biology14111562
APA StyleHu, F., Lu, J., Zhai, L., Qiu, X., Du, B., & Xu, J. (2025). Effects of Foliar Application of Paclobutrazol on Grain Yield, Aroma, and Canopy Radiation Use Efficiency of Aromatic Rice. Biology, 14(11), 1562. https://doi.org/10.3390/biology14111562







