Phylogeographic Insights into Pipistrellus Species from Türkiye: Diversity, Divergence, and Regional Lineage Structure
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Sampling
2.3. DNA Extraction and Polymerase Chain Reaction (PCR)
2.4. Phylogenetic Reconstruction and Population Analyses
2.5. Divergence Time Estimations
3. Results
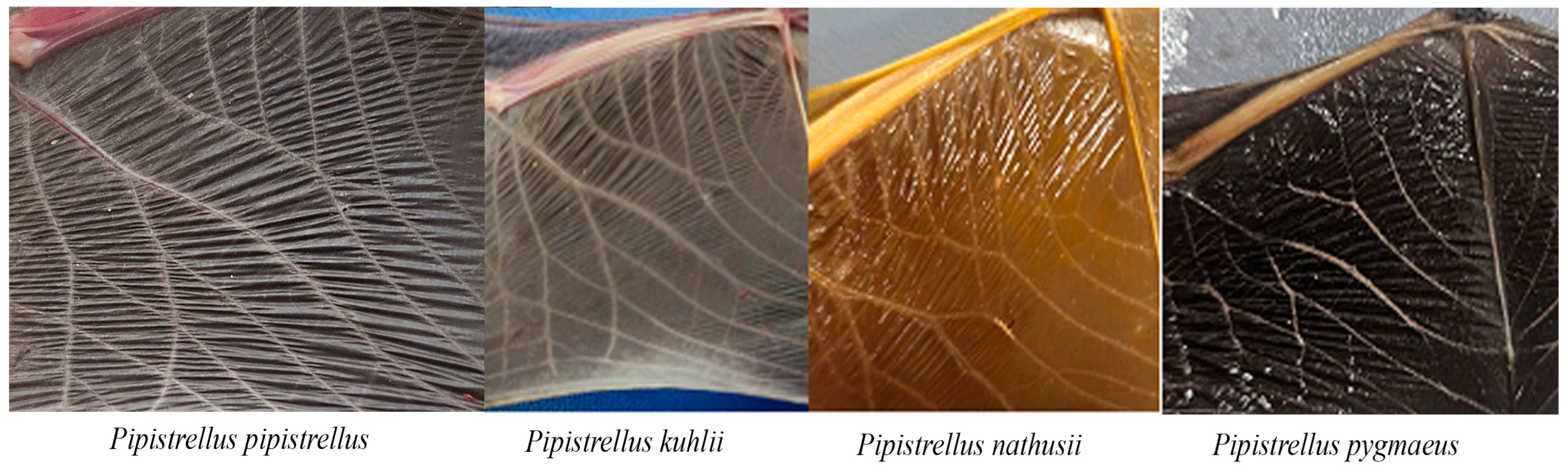
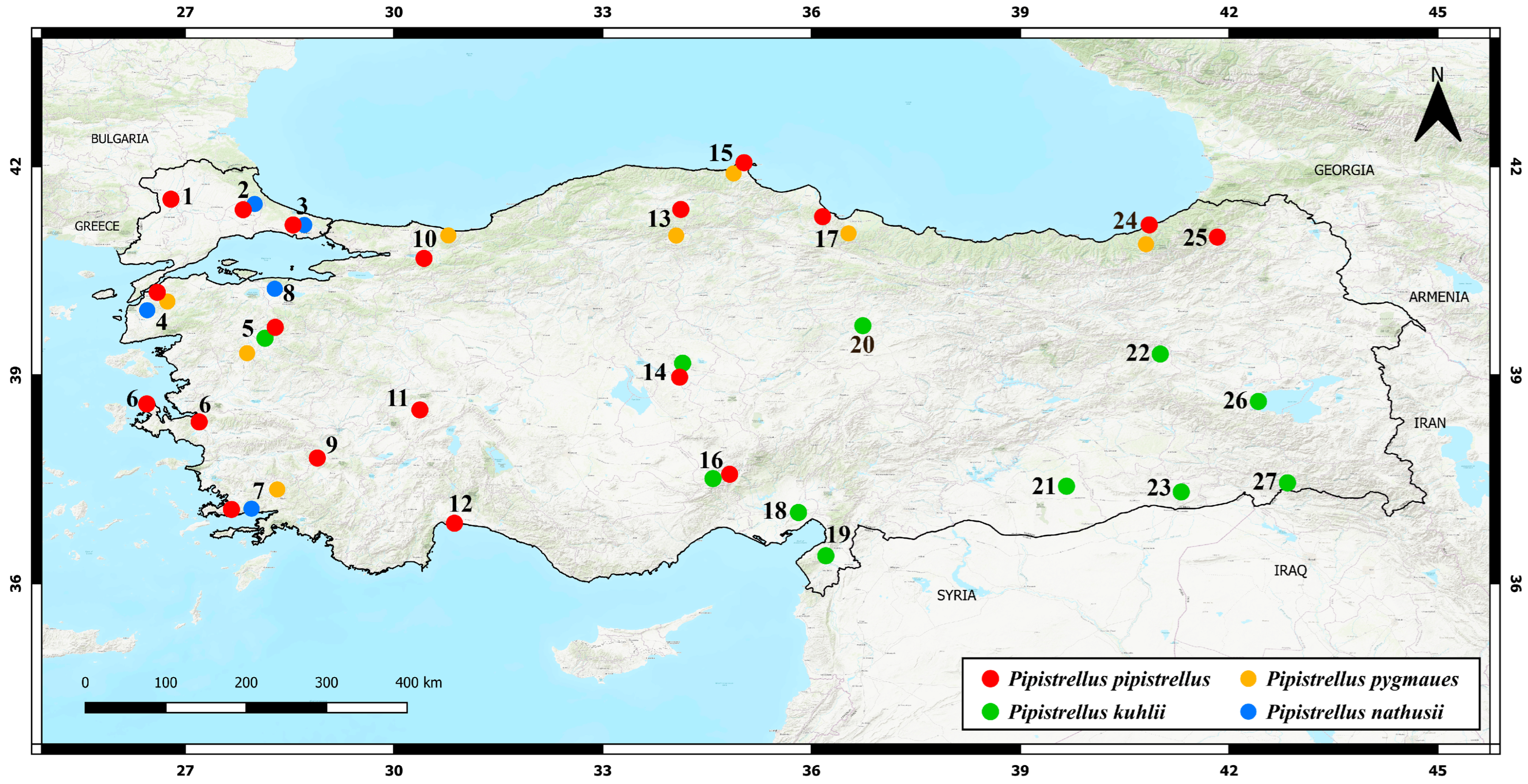
3.1. Sampling Localities
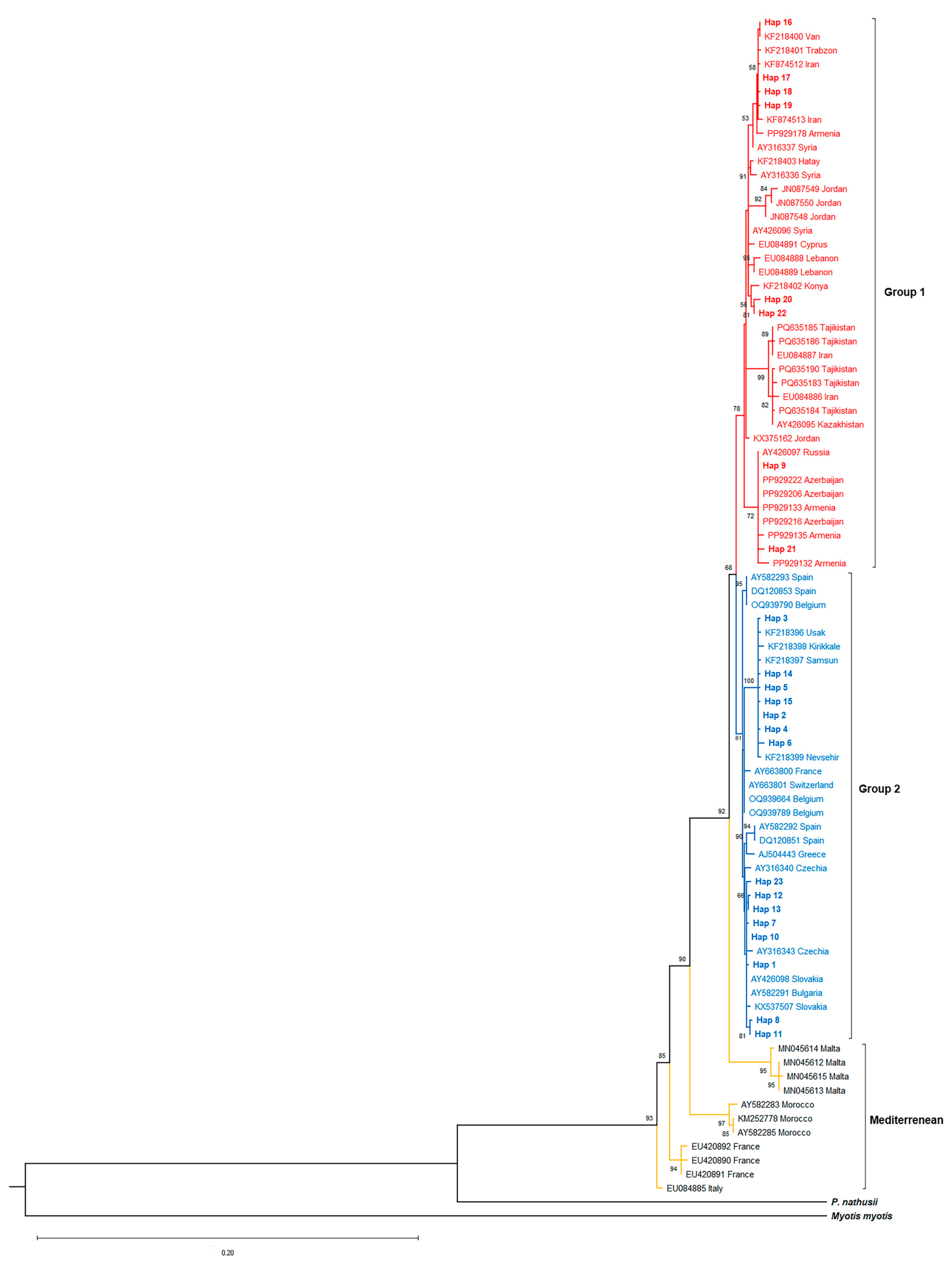
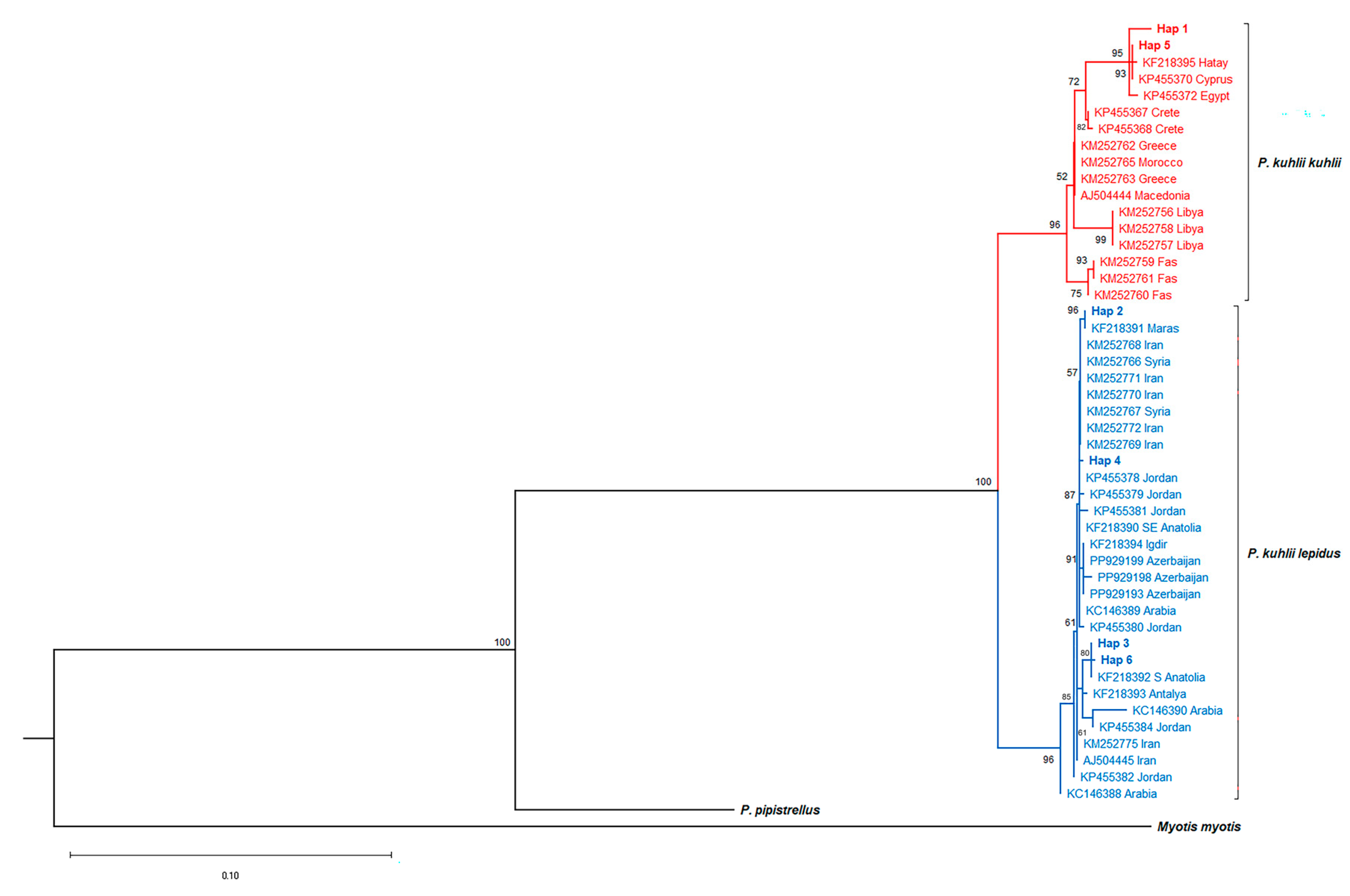
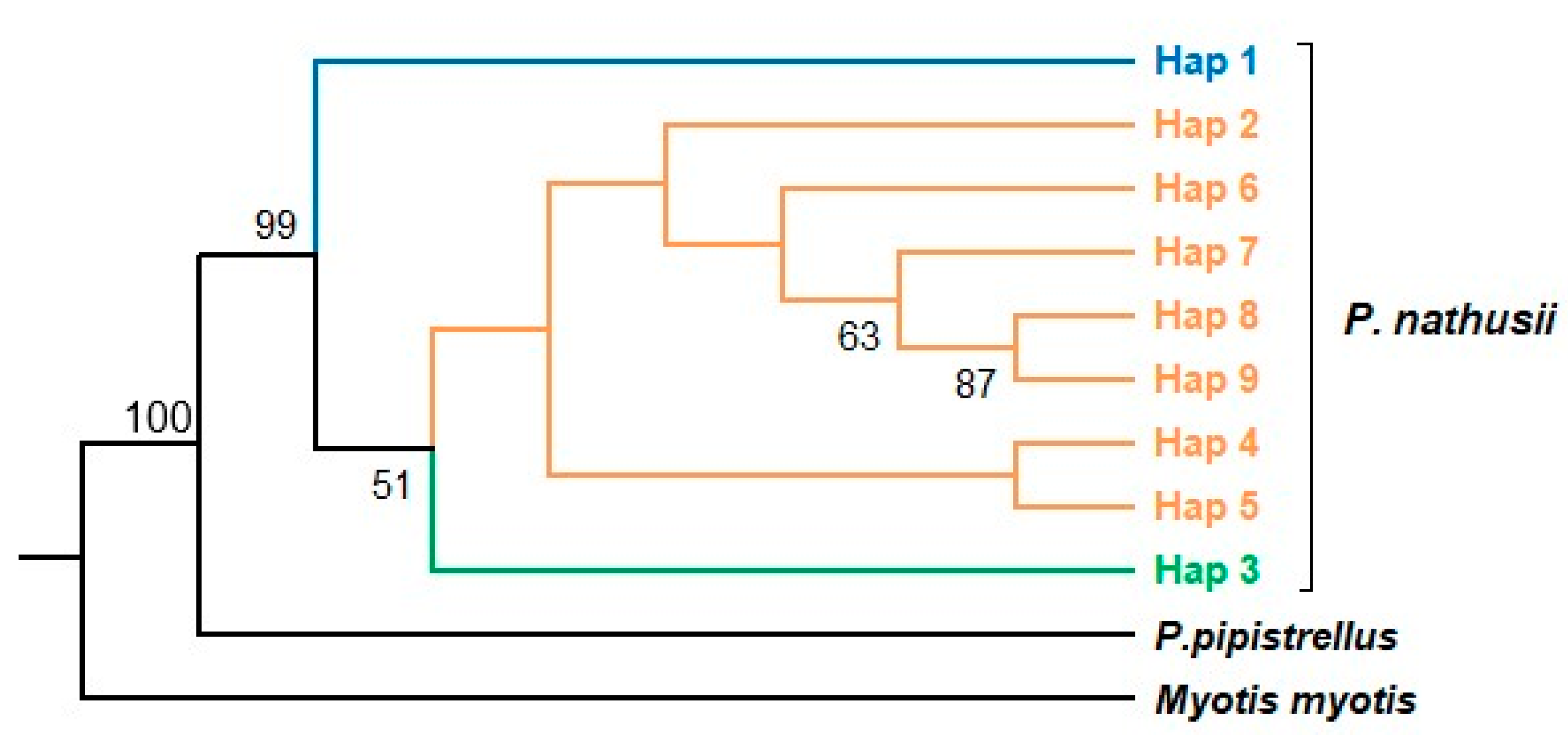
3.2. Phylogenetic Relationships, Genetic Distances, and Gene Flow
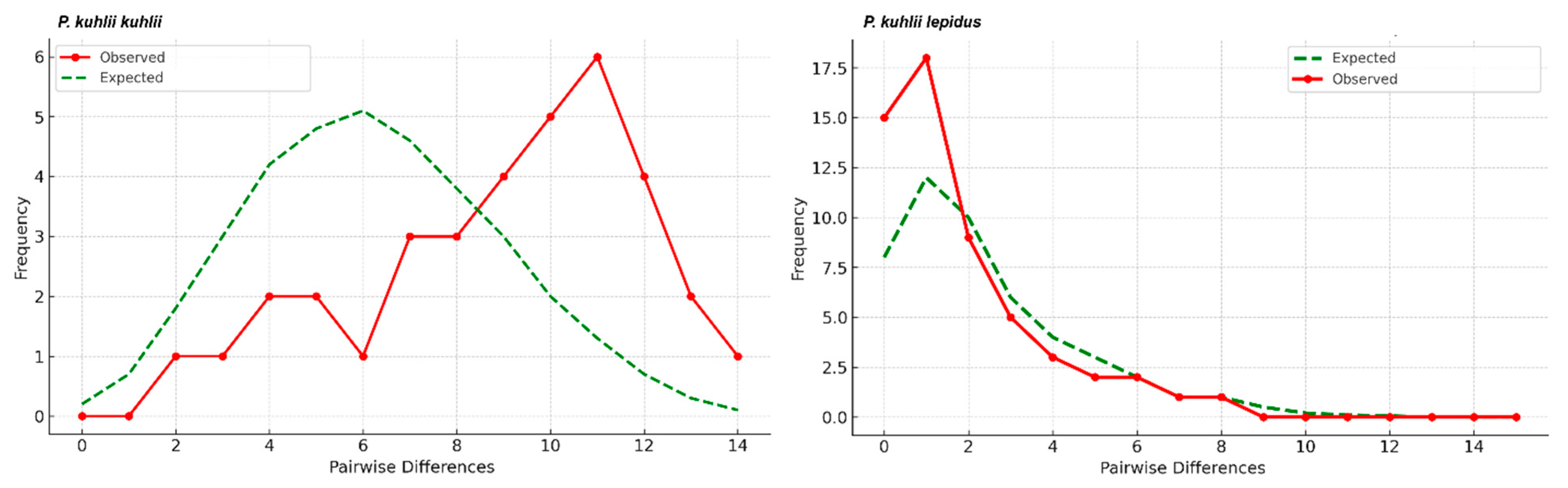
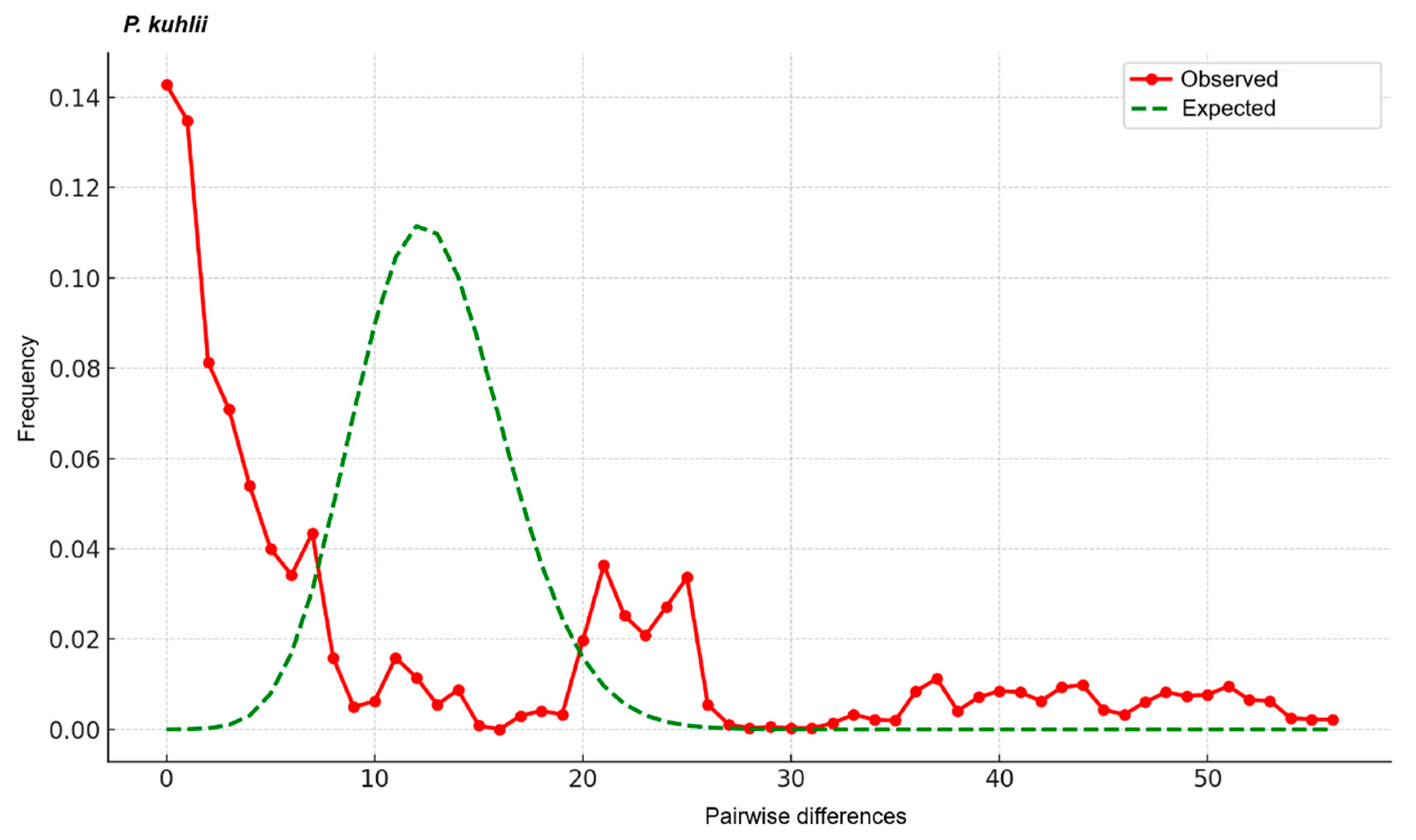
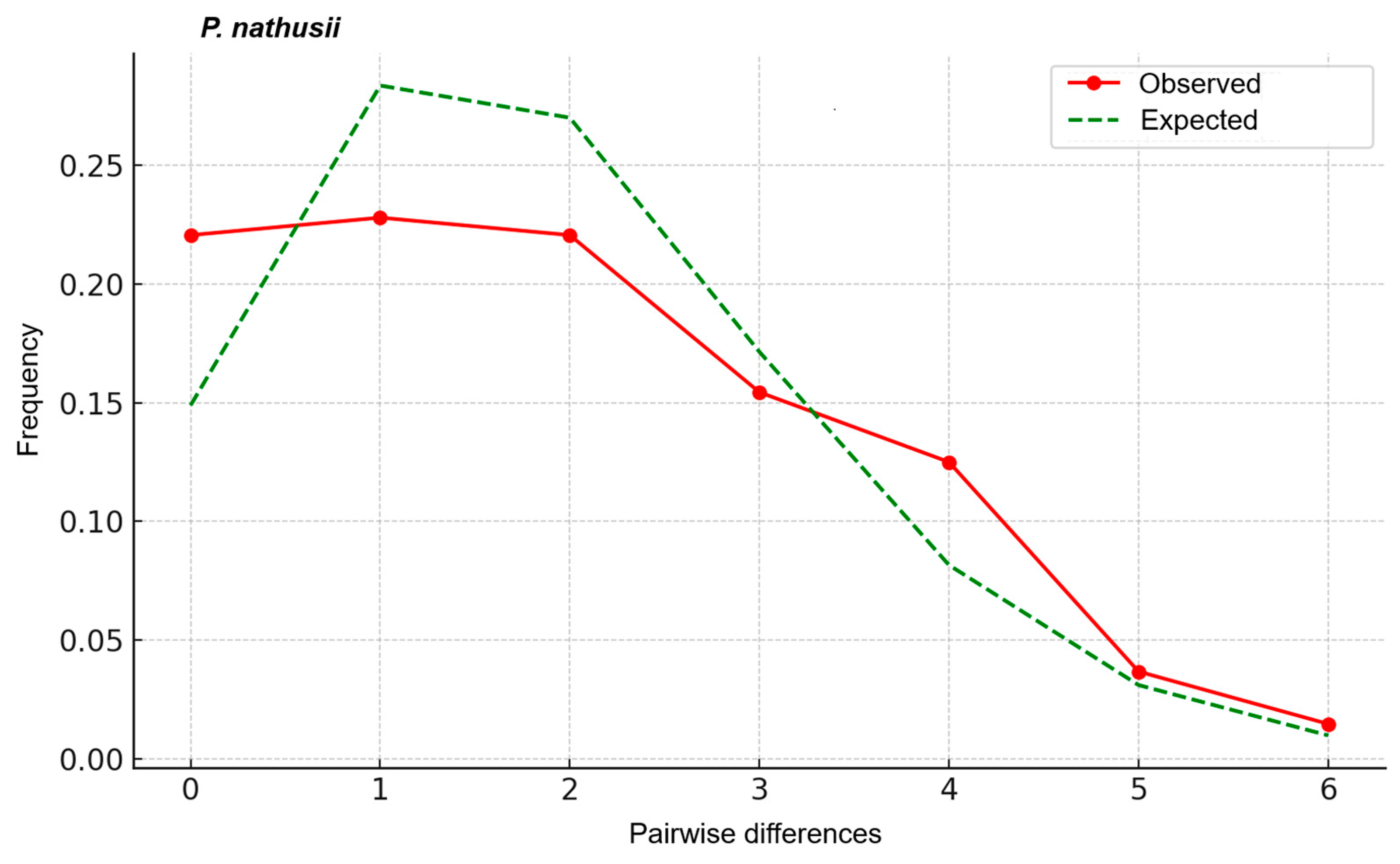
3.3. Mismatch Distribution and Neutrality Tests
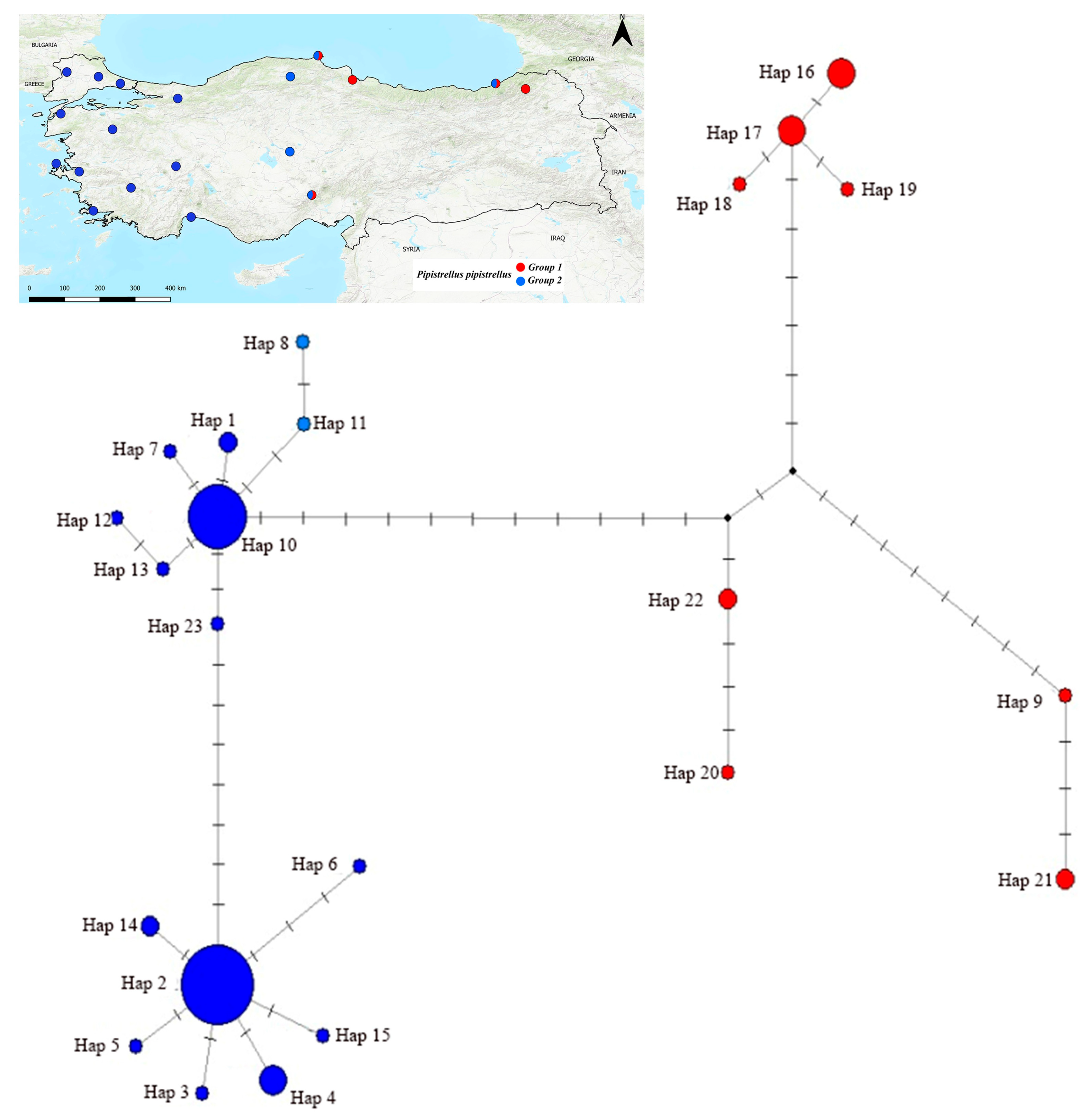
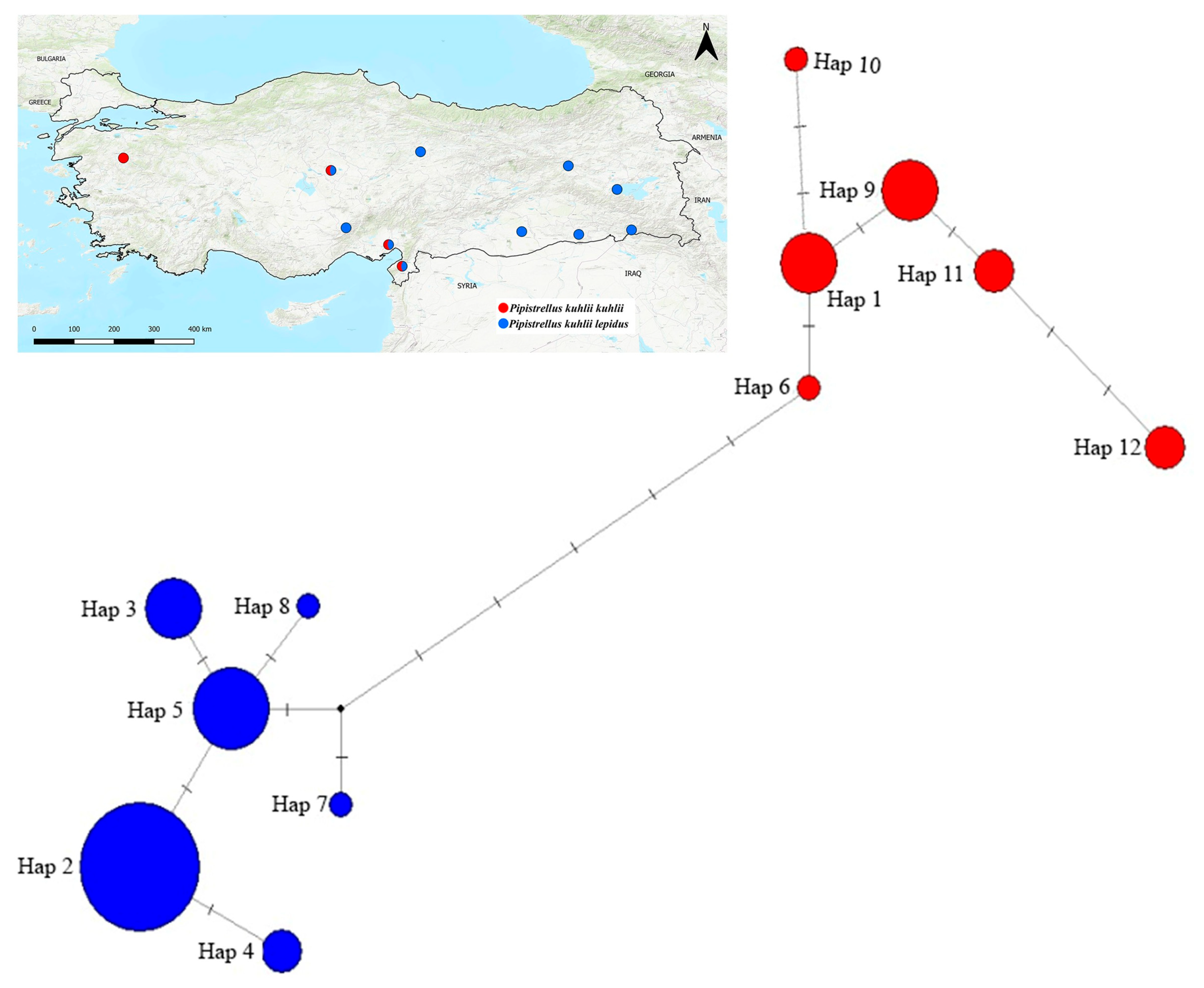
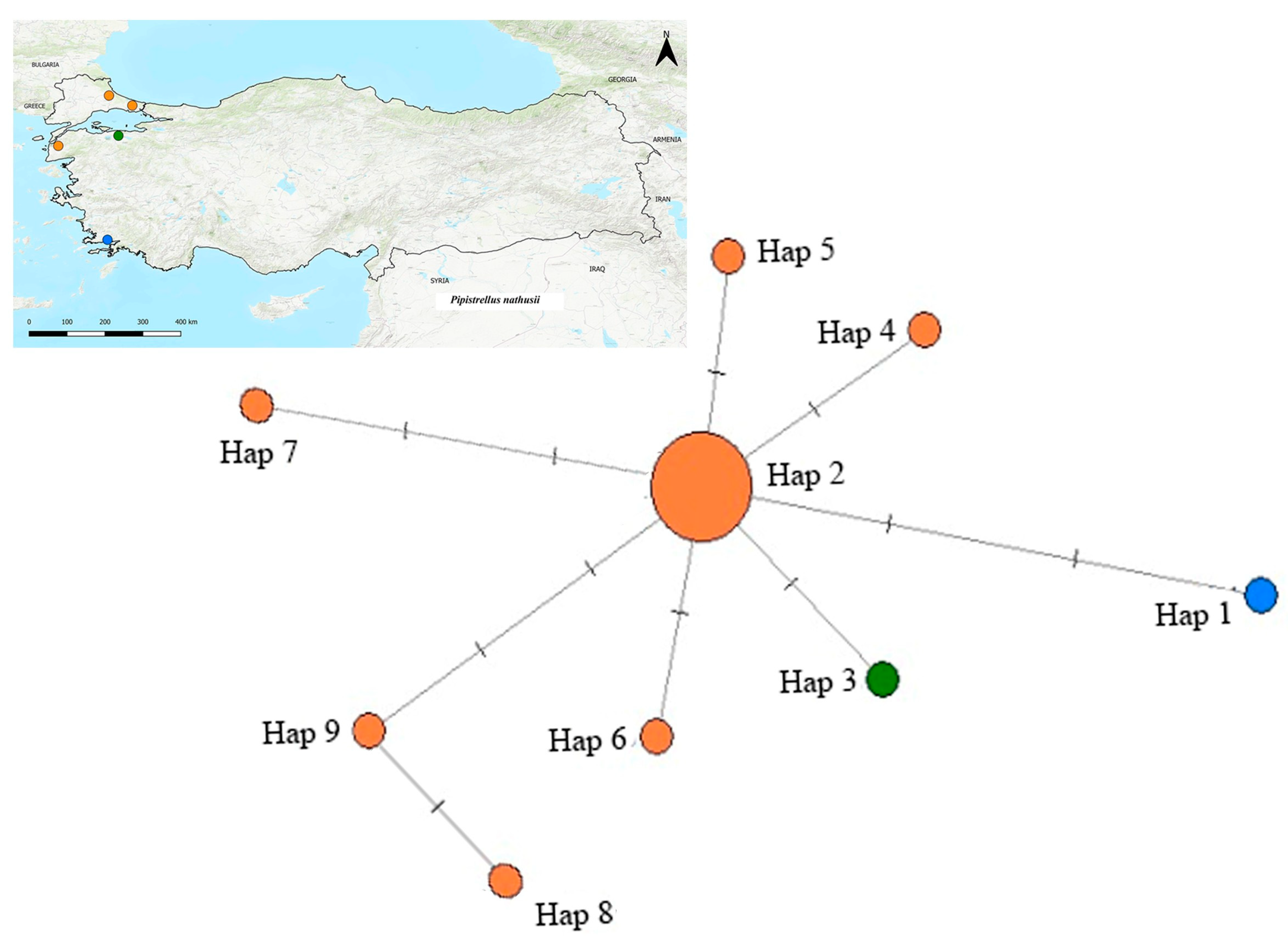
3.4. Median-Joining Network Analysis
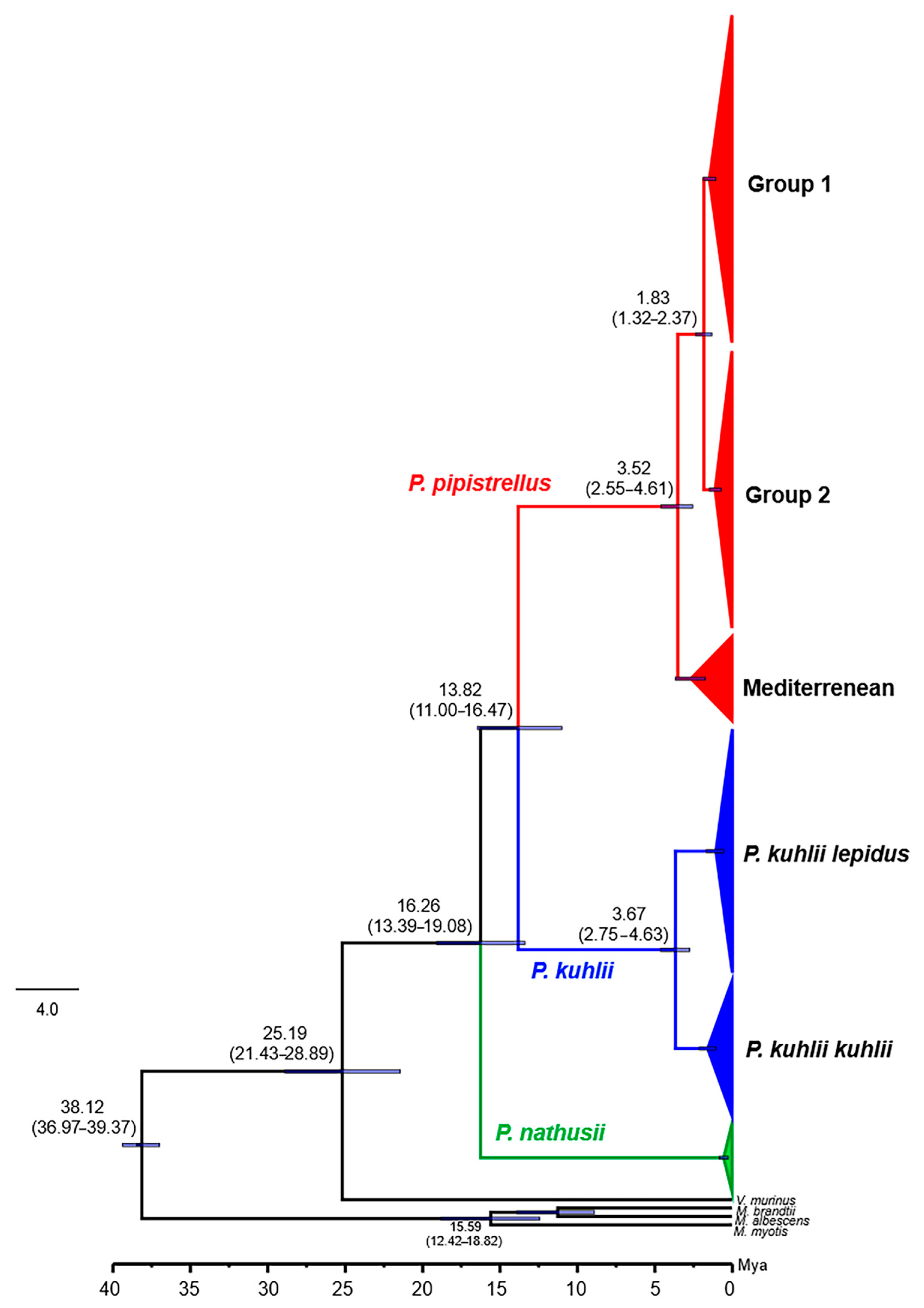
3.5. Molecular Dating and Divergence Times
4. Discussion
4.1. Resolving Cryptic Species Boundaries
4.2. The Biogeographic Role of Anatolia in P. pipistrellus Diversification and Demographic History
4.3. Lineage Diversification and Secondary Contact in P. kuhlii
4.4. Phylogeography of the Migratory P. nathusii
4.5. Temporal Framework for Diversification: Miocene-Pleistocene Influence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benda, P.; Horáček, I. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean. Part 1. Review of distribution and taxonomy of bats in Turkey. Acta Soc. Zool. Bohemicae 1998, 62, 255–313. [Google Scholar]
- Çoraman, E.; Furman, A.; Karataş, A.; Bilgin, R. Phylogeographic analysis of Anatolian bats highlights the importance of the region for preserving the chiropteran mitochondrial genetic diversity in the Western Palaearctic. Conserv. Genet. 2013, 14, 1205–1216. [Google Scholar] [CrossRef]
- Jones, K.E.; Purvis, A.; Maclarnon, A.N.; Bininda-Emonds, O.R.; Simmons, N.B. A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biol. Rev. 2002, 77, 223–259. [Google Scholar] [CrossRef] [PubMed]
- Teeling, E.C.; Springer, M.S.; Madsen, O.; Bates, P.; O’brien, S.J.; ve Murphy, W.J. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 2005, 307, 580–584. [Google Scholar] [CrossRef]
- Mammal Diversity Database. Mammal Diversity Database (Version 1.11). Zenodo. 2023. Available online: https://zenodo.org/records/8428367 (accessed on 1 November 2025).
- Wilson, D.E.; Reeder, D.M. (Eds.) Mammal Species of the World: A Taxonomic and Geographic Reference; JHU Press: Baltimore, MD, USA, 2005; Volume 1. [Google Scholar]
- Vaughan, T.A.; Ryan, J.M.; Czaplewski, N.J. Mammalogy, 6th ed.; Jones & Bartlett: Burlington, MA, USA, 2015. [Google Scholar]
- Karataş, A.; Filiz, H.; Erciyas-Yavuz, K.; Özeren, S.C.; Tok, C.V. The vertebrate biodiversity of Turkey. In Biodiversity, Conservation and Sustainability in Asia: Volume 1: Prospects and Challenges in West Asia and Caucasus; Springer: Cham, Switzerland, 2021; pp. 175–274. [Google Scholar] [CrossRef]
- Mayer, F.; von Helversen, O. Sympatric distribution of two cryptic bat species across Europe. Biol. J. Linn. Soc. 2001, 74, 365–374. [Google Scholar] [CrossRef]
- Pavlinić, I.; Tvrtković, N.; Holcer, D. Morphological identification of the soprano pipistrelle (Pipistrellus pygmaeus Leach, 1825) in Croatia. Hystrix Ital. J. Mammal. 2008, 19, 47–53. [Google Scholar] [CrossRef]
- Benda, P.; Hulva, P.; Andreas, M.; Uhrin, M. Notes on the distribution of Pipistrellus pipistrellus complex in the Eastern Mediterranean: First records of P. pipistrellus for Syria and of P. pygmaeus for Turkey. Vespertilio 2003, 7, 87–95. [Google Scholar]
- Dietz, C.; Schunger, I.; Keşapli Didrickson, Ö.; Karataş, A.; Mayer, F. First record of Pipistrellus pygmaeus (Chiroptera: Vespertilionidae) in Anatolia. Zool. Middle East 2005, 34, 5–10. [Google Scholar] [CrossRef]
- Albayrak, İ. A new record of Pipistrellus pipistrellus aladidn for Turkey. Commun. Fac. Sci. Univ. Ank. Ser. C Biol. 1987, 5, 31–37. [Google Scholar] [CrossRef]
- Karataş, A. Türkiye yarasaları (Mammalia: Chiroptera) (Tayin anahtarı). Niğde Üniversitesi Fen Bilim. Derg. 1996, 1, 107–117. [Google Scholar]
- Karataş, A.; Yiğit, N.; Kankılıç, T.; Çolak, E. Contribution to the distribution and karyology of some vespertilionid bats (Mammalia: Chiroptera) from Turkey. Zool. Middle East 2004, 31, 5–12. [Google Scholar] [CrossRef]
- Albayrak, İ. The bats of the eastern Black Sea region in Turkey (Mammalia: Chiroptera). Turk. J. Zool. 2003, 27, 269–273. [Google Scholar]
- Arslan, A.; Albayrak, İ. Taxonomic status of Kuhl’s pipistrelle Pipistrellus kuhlii (Kuhl, 1819) in Turkey (Mammalia: Chiroptera). Pak. J. Biol. Sci. 2005, 8, 1699–1702. [Google Scholar]
- Şekercioğlu, Ç.H.; Anderson, S.; Akçay, E.; Bilgin, R.; Can, Ö.E.; Semiz, G.; Dalfes, H.N. Turkey’s globally important biodiversity in crisis. Biol. Conserv. 2011, 144, 2752–2769. [Google Scholar] [CrossRef]
- Yorulmaz, T.; Ürker, O.; Özmen, R. Yarasa ve orman ilişkisi üzerine bir değerlendirme. Orman. Araştırma Derg. 2018, 5, 31–43. [Google Scholar] [CrossRef]
- Yorulmaz, T.; Arslan, N. Current status of the bats in Turkey with their ecogeographic distributions and recommendations for national conservation status (Mammalia: Chiroptera). Fresenius Environ. Bull. 2020, 29, 6691–6706. [Google Scholar]
- Tzortzakaki, O.; Papadatou, E.; Kati, V.; Giokas, S. Winners and losers in an urban bat community: A case study from southeastern Europe. Hystrix Ital. J. Mammal. 2019, 30, 134–140. [Google Scholar] [CrossRef]
- Tian, L.; Liang, B.; Maeda, K.; Metzner, W.; Zhang, Z. Molecular studies on the classification of Miniopterus schreibersii (Chiroptera: Vespertilionidae) inferred from mitochondrial cytochrome b sequences. Folia Zool. 2004, 53, 303–311. [Google Scholar]
- Hulva, P.; Benda, P.; Hanák, V.; Evin, A.; Horáček, I. New mitochondrial lineages within the Pipistrellus pipistrellus complex from Mediterranean Europe. Folia Zool. 2007, 56, 378–388. [Google Scholar]
- Stadelmann, B.; Lin, L.K.; Kunz, T.H.; Ruedi, M. Molecular phylogeny of New World Myotis (Vespertilionidae) inferred from mitochondrial and nuclear DNA genes. Mol. Phylogenetics Evol. 2007, 43, 32–48. [Google Scholar] [CrossRef]
- Uvizl, M.; Petr, B. Intraspecific variation of Myotis emarginatus (Chiroptera: Vespertilionidae) inferred from mitochondrial and nuclear genetic markers. Acta Chiropterologica 2021, 23, 285–300. [Google Scholar] [CrossRef]
- Abduriyim, S.; Kasimu, T.; Lan, J.K.; Pu, Z.L.; Bai, J.L.; Wang, Y.C. Morphological and molecular confirmation of the common pipistrelle bat, Pipistrellus pipistrellus Schreber, 1774 (Vespertilionidae: Chiroptera) in Xinjiang, China. Mammalia 2022, 86, 298–302. [Google Scholar] [CrossRef]
- Perera, T.; Schwarz, F.; Muzeniek, T.; Siriwardana, S.; Becker-Ziaja, B.; Perera, I.C.; Handunnetti, S.; Weerasena, J.; Premawansa, G.; Premawansa, S. First complete cytochrome B sequences and molecular taxonomy of bat species from Sri Lanka. Animals 2022, 12, 1674. [Google Scholar] [CrossRef]
- Hulva, P.; Fornůsková, A.; Chudárková, A.; Evin, A.; Allegrini, B.; Benda, P.; Bryja, J. Mechanisms of radiation in a bat group from the genus Pipistrellus inferred by phylogeography, demography and population genetics. Mol. Ecol. 2010, 19, 5417–5431. [Google Scholar] [CrossRef] [PubMed]
- Alivizatos, H.; Karagianni, P.; Kalpakis, S.; Karris, G.; Ioannidis, I.; Poirazidis, K. First record of Pipistrellus lepidus Blyth, 1845 in Greece. North-West. J. Zool. 2023, 19, 217–218. [Google Scholar]
- Kurvits, T.; Nellemann, C.; Alfthan, B.; Kühl, A.; Prokosch, P.; Virtue, M.; Skaalvik, J.F. (Eds.) Living Planet: Connected Planet—Preventing the End of the World’s Wildlife Migrations Through Ecological Networks. A Rapid Response Assessment; United Nations Environment Programme, GRID-Arendal: Arendal, Norway, 2011; ISBN 978-82-7701-098-4. [Google Scholar]
- Vasenkov, D.; Desmet, J.F.; Popov, I.; Sidorchuk, N. Bats can migrate farther than it was previously known: A new longest migration record by Nathusius’ pipistrelle Pipistrellus nathusii (Chiroptera: Vespertilionidae). Mammalia 2022, 86, 524–526. [Google Scholar] [CrossRef]
- Furmankiewicz, J.; Kucharska, M. Migration of bats along a large river valley in southwestern Poland. J. Mammal. 2009, 90, 1310–1317. [Google Scholar] [CrossRef]
- Hulva, P.; Horáček, I.; Strelkov, P.P.; Benda, P. Molecular architecture of Pipistrellus pipistrellus/Pipistrellus pygmaeus complex (Chiroptera: Vespertilionidae): Further cryptic species and Mediterranean origin of the divergence. Mol. Phylogenet. Evol. 2004, 32, 1023–1035. [Google Scholar] [CrossRef]
- Dietz, C.; von Helversen, O. Illustrated Identification Key to the Bats of Europe; Electronic Publication: Tuebingen, Germany, 2004; Volume 1, 72p. [Google Scholar]
- Irwin, D.M.; Kocher, T.D.; Wilson, A.C. Evolution of the cytochrome b gene of mammals. J. Mol. Evol. 1991, 32, 128–144. [Google Scholar] [CrossRef]
- Colak, R.; Olgun Karacan, G.; Kandemir, I.; Çolak, E.; Kankiliç, T.; Yigit, N.; Michaux, J. Genetic variations of Turkish bank vole, Myodes glareolus (Mammalia: Rodentia) inferred from mtDNA. Mitochondrial DNA Part A 2016, 27, 4372–4379. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Borges, M.S.; Salles, M.M.A.; Oliveira, E.F.; Magalhães, F.M.; Silva, D.C.; Oliveira, H.F.M.; Faria, K.C.; Laine, V.N.; Lilley, T.M.; Silva, J.M.; et al. Gene-Specific Substitution Rates for the Vespertilionidae (Chiroptera: Mammalia) Mitochondrial Genome, with the Description of Three new Mitogenomes. J. Mol. Evol. 2025, 93, 610–619. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Nei, M. Evolution of human races at the gene level. In Human Genetics, Part A: The Unfolding Genome; Bonne-Tamir, B., Cohen, T., Goodman, R.M., Eds.; Alan R. Liss: New York, NY, USA, 1982; pp. 167–181. [Google Scholar]
- Barratt, E.M.; Deaville, R.; Burland, T.M.; Bruford, M.W.; Jones, G.; Racey, P.A.; Wayne, R.K. DNA answers the call of pipistrelle bat species. Nature 1997, 387, 138–139. [Google Scholar] [CrossRef]
- Sztencel-Jabłonka, A.; Bogdanowicz, W. Population genetics study of common (Pipistrellus pipistrellus) and soprano (Pipistrellus pygmaeus) pipistrelle bats from central Europe suggests interspecific hybridization. Can. J. Zool. 2012, 90, 1251–1260. [Google Scholar] [CrossRef]
- Gür, H. The Anatolian diagonal revisited: Testing the ecological basis of a biogeographic boundary. Turk. J. Zool. 2016, 40, 121–134. [Google Scholar] [CrossRef]
- Mutun, S.; Dinç, S. The Anatolian Diagonal and paleoclimatic changes shaped the phylogeography of Cynips quercus (Hymenoptera, Cynipidae). Ann. Zool. Fenn. 2019, 56, 65–83. [Google Scholar] [CrossRef]
- Dietz, C.; von Helversen, O.; Nill, D.; Lina, P.H.; Hutson, A.M. Bats of Britain, Europe and Northwest Africa; A & C Black Publishers Ltd.: London, UK, 2009. [Google Scholar]
- Andriollo, T.; Naciri, Y.; Ruedi, M. Two mitochondrial barcodes for one biological species: The case of European Kuhl’s pipistrelles (Chiroptera). PLoS ONE 2015, 10, e0134881. [Google Scholar] [CrossRef]
- Sachanowicz, K.; Pıskorski, M.; Tereba, A. Systematics and taxonomy of Pipistrellus kuhlii (Kuhl, 1817) in Central Europe and the Balkans. Zootaxa 2017, 4306, 53–66. [Google Scholar] [CrossRef]
- Baker, R.J.; Bradley, R.D. Speciation in mammals and the genetic species concept. J. Mammal. 2006, 87, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Joly, S.; Stevens, M.I.; van Vuuren, B.J. Haplotype Networks Can Be Misleading in the Presence of Missing Data. Syst. Biol. 2007, 56, 857–862. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Yi, X.; Latch, E.K.; Lim, B.K.; Koroiva, R.; Da Rocha, P.A. UCE-derived mitochondrial phylogeny reveals pervasive mito-nuclear discordances in serotine bats (genus Eptesicus) and complex evolutionary history in Eptesicus (Histiotus). Mamm. Biol. 2024, 104, 417–430. [Google Scholar] [CrossRef]
- Benda, P.; Andriollo, T.; Ruedi, M. Systematic position and taxonomy of Pipistrellus deserti (Chiroptera: Vespertilionidae). Mammalia 2015, 79, 419–438. [Google Scholar] [CrossRef]
- Hutterer, R.; Ivanova, T.; Meyer-Cords, C.; Rodrigues, L. Bat Migrations in Europe: A Review of Banding Data and Literature; BfN-Schriftenvertrieb im Landwirtschaftsverlag: Münster, Germany, 2005. [Google Scholar]
- Hoffmann, F.G.; Hoofer, S.R.; Baker, R.J. Molecular dating of the diversification of Phyllostominae bats based on nuclear and mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2008, 49, 653–658. [Google Scholar] [CrossRef]
- Amador, L.I.; Moyers Arévalo, R.L.; Almeida, F.C.; Catalano, S.A.; Giannini, N.P. Bat systematics in the light of unconstrained analyses of a comprehensive molecular supermatrix. J. Mamm. Evol. 2018, 25, 37–70. [Google Scholar] [CrossRef]
- Zheng, Y.; Peng, R.; Kuro-o, M.; Zeng, X. Exploring patterns and extent of bias in estimating divergence time from mitochondrial DNA sequence data in a particular lineage: A case study of salamanders (Order Caudata). Mol. Biol. Evol. 2011, 28, 2521–2535. [Google Scholar] [CrossRef] [PubMed]
- Lukoschek, V.; Keogh, J.S.; Avise, J.C. Evaluating fossil calibrations for dating phylogenies in light of rates of molecular evolution: A comparison of three approaches. Syst. Biol. 2012, 61, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Pagani, M.; Zinniker, D.; DeConto, R.; Huber, M.; Brinkhuis, H.; Shah, S.R.; Leckie, R.M.; Pearson, A. Global cooling during the Eocene-Oligocene climate transition. Science 2009, 323, 1187–1190. [Google Scholar] [CrossRef]
- Agustí, J.; Cabrera, L.; Garcés, M. The Vallesian Mammal Turnover: A Late Miocene record of decoupled land-ocean evolution. Geobios 2013, 46, 151–157. [Google Scholar] [CrossRef]
- Zhukova, S.S.; Yuzefovich, A.P.; Lebedev, V.S.; Kruskop, S.V. Reassessment of the Taxonomic Borders Within Pipistrellus (Chiroptera, Vespertilionidae, Pipistrellini). Diversity 2025, 17, 317. [Google Scholar] [CrossRef]
- Horáček, I. On the early history of vespertilionid bats in Europe: The Lower Miocene record from the Bohemian Massif. Lynx 2001, 32, 123–154. [Google Scholar]
- Foley, N.M.; Mason, V.C.; Harris, A.J.; Bredemeyer, K.R.; Damas, J.; Lewin, H.A.; Eizirik, E.; Gatesy, J.; Karlsson, E.K.; Lindblad-Toh, K.; et al. A genomic timescale for placental mammal evolution. Science 2023, 380, eabl8189. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Böhme, M. The Miocene climatic optimum: Evidence from ectothermic vertebrates of Central Europe. Palaeogeography, Palaeoclimatology. Palaeoecology 2003, 195, 389–401. [Google Scholar] [CrossRef]
- Stadelmann, B.; Herrera, L.G.; Arroyo-Cabrales, J.; Flores-Martínez, J.J.; May, B.P.; Ruedi, M. Molecular systematics of the fishing bat Myotis (Pizonyx) vivesi. J. Mammal. 2004, 85, 133–139. [Google Scholar] [CrossRef]
- Benda, P.; Kiefer, A.; Hanák, V.; Veith, M. Systematic Status of African Populations of Long-Eared Bats, Genus Plecotus (Mammalia: Chiroptera); Institute of Vertebrate Biology: Brno, Czech Republic, 2004. [Google Scholar]
- Ibáñez, C.; García-Mudarra, J.L.; Ruedi, M.; Stadelmann, B.; Juste, J. The Iberian contribution to cryptic diversity in European bats. Acta Chiropterologica 2006, 8, 277–297. [Google Scholar] [CrossRef]
- Benda, P.; Faizolâhi, K.; Andreas, M.; Obuch, J.; Reiter, A.; Ševčík, M.; Uhrin, M.; Vallo, P.; Ashrafi, S. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 10. Bat fauna of Iran. Acta Soc. Zool. Bohemicae 2012, 76, 163–582. [Google Scholar]
- Benda, P.; Lučan, R.K.; Obuch, J.; Reiter, A.; Andreas, M.; Bačkor, P.; Bohnenstengel, T.; Eid, E.K.; Ševčík, M.; Vallo, P.; et al. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 8. Bats of Jordan: Fauna, ecology, echolocation, ectoparasites. Acta Soc. Zool. Bohemicae 2010, 74, 185–353. [Google Scholar]
- Benda, P.; Reiter, A.; Uhrin, M.; Varadínová, Z. A new species of pipistrelle bat (Chiroptera: Vespertilionidae) from southern Arabia. Acta Chiropterol. 2016, 18, 301–323. [Google Scholar] [CrossRef]
- Mifsud, C.M.; Vella, A. Mitochondrial genetic diversity of bat species from the Maltese Islands and applications for their conservation. Nat. Eng. Sci. 2019, 4, 276–292. [Google Scholar] [CrossRef]
- Benda, P.; Hulva, P.; Gaisler, J. Systematic status of African populations of Pipistrellus pipistrellus complex (Chiroptera: Vespertilionidae), with a description of a new species from Cyrenaica, Libya. Acta Chiropterol. 2004, 6, 193–217. [Google Scholar] [CrossRef]
- Bray, T.C.; Mohammed, O.B.; Butynski, T.M.; Wronski, T.; Alagaili, A.N.; Hill, J.E. Phylogenetic and demographic insights into Kuhl’s Pipistrelle, Pipistrellus kuhlii, in the Middle East. PLoS ONE 2013, 8, e57306. [Google Scholar] [CrossRef]
- Horemans, M.; Van Bets, J.; Joly Maes, T.; Maes, P.; Vanmechelen, B. Discovery and genome characterization of six new orthoparamyxoviruses in small Belgian mammals. Virus Evol. 2023, 9, vead065. [Google Scholar] [CrossRef] [PubMed]
- Medinas, D.; Marques, J.T.; Mira, A. Assessing road effects on bats: The role of landscape, road features, and bat activity on road-kills. Ecol. Res. 2013, 28, 227–237. [Google Scholar] [CrossRef]
- Benda, P.; Ševčík, M.; Horáček, I.; Uvizl, M.; Reiter, A.; Uhrin, M. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 17. New records of bats and their ectoparasites from Tajikistan with a review of these faunas of the country including a description of a new species of horseshoe bat. Acta Soc. Zool. Bohemicae 2024, 88, 1–213. [Google Scholar]
- Phelps, K.; Al Abdulasalam, Z.; Al-Hmoud, N.; Ali, S.; Alrwashdeh, M.; Bilgin, R.; Olival, K.J. Distribution of bat species in Western Asia: Occurrence records from the Western Asia Bat Research Network (WAB-Net) project. Biodivers. Data J. 2024, 12, e132199. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyfi, E.; Bulut, Ş.; Olgun Karacan, G. Phylogeographic Insights into Pipistrellus Species from Türkiye: Diversity, Divergence, and Regional Lineage Structure. Biology 2025, 14, 1549. https://doi.org/10.3390/biology14111549
Seyfi E, Bulut Ş, Olgun Karacan G. Phylogeographic Insights into Pipistrellus Species from Türkiye: Diversity, Divergence, and Regional Lineage Structure. Biology. 2025; 14(11):1549. https://doi.org/10.3390/biology14111549
Chicago/Turabian StyleSeyfi, Emin, Şafak Bulut, and Gül Olgun Karacan. 2025. "Phylogeographic Insights into Pipistrellus Species from Türkiye: Diversity, Divergence, and Regional Lineage Structure" Biology 14, no. 11: 1549. https://doi.org/10.3390/biology14111549
APA StyleSeyfi, E., Bulut, Ş., & Olgun Karacan, G. (2025). Phylogeographic Insights into Pipistrellus Species from Türkiye: Diversity, Divergence, and Regional Lineage Structure. Biology, 14(11), 1549. https://doi.org/10.3390/biology14111549




