PLIN5 Promotes Lipid Reconstitution in Goat Intramuscular Fat via the PPARγ Signaling Pathway
Simple Summary
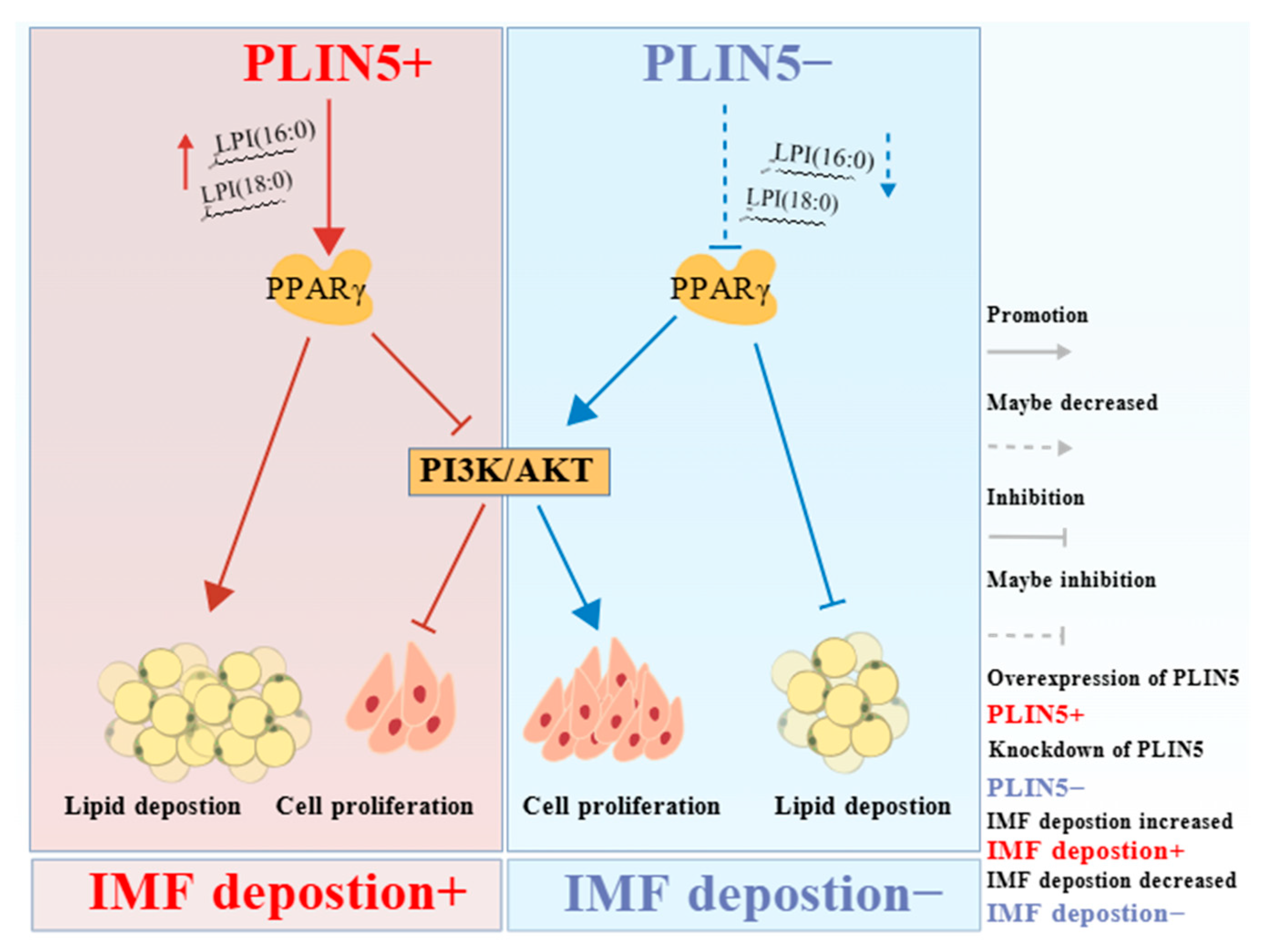
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Isolation and Culture
2.3. PLIN5 Gene Cloning and Biological Analysis
2.4. Construction of pc DNA3.1-PLIN5 Overexpression Vector and siRNA Synthesis
2.5. Cell Transfection
2.6. Oil Red O, Bodipy Staining, and Triglyceride Content Determination
2.7. CCK-8 Assay and EDU Staining
2.8. Analysis by Flow Cytometry
2.9. Total RNA Isolation, cDNA Synthesis, and q-PCR Analysis
2.10. Western Blot
2.11. Untargeted Lipidomics Sequencing
2.12. In Silico Docking Analysis
2.13. Statistical Analysis
3. Results
3.1. PLIN5 Is Associated with Intramuscular Fat Deposition
3.2. Overexpression of PLIN5 Promotes Adipogenesis and Inhibits Proliferation of Goat Intramuscular Preadipocytes
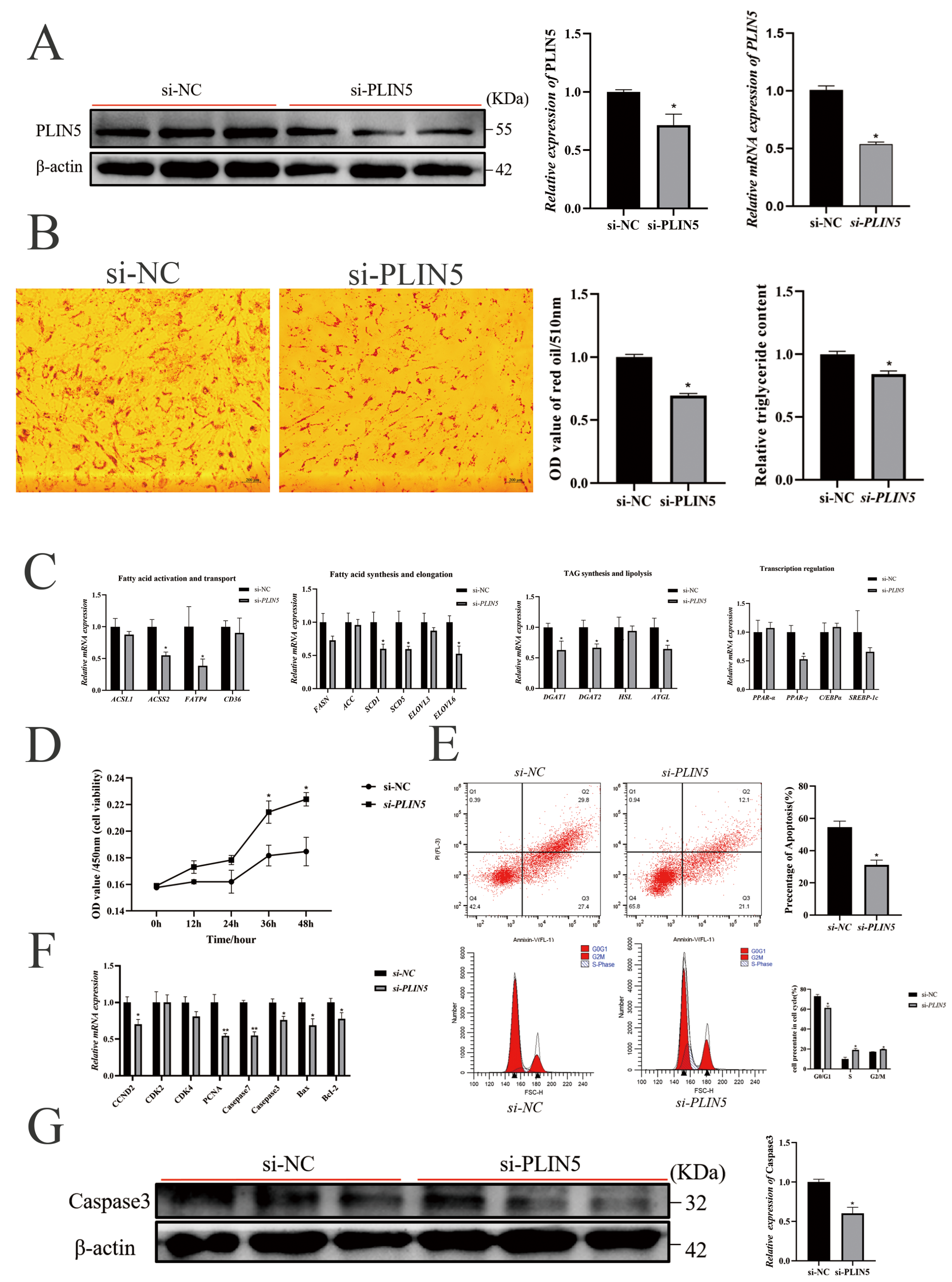
3.3. Knockdown of PLIN5 Inhibits Adipogenesis and Promotes Proliferation of Goat Intramuscular Preadipocytes
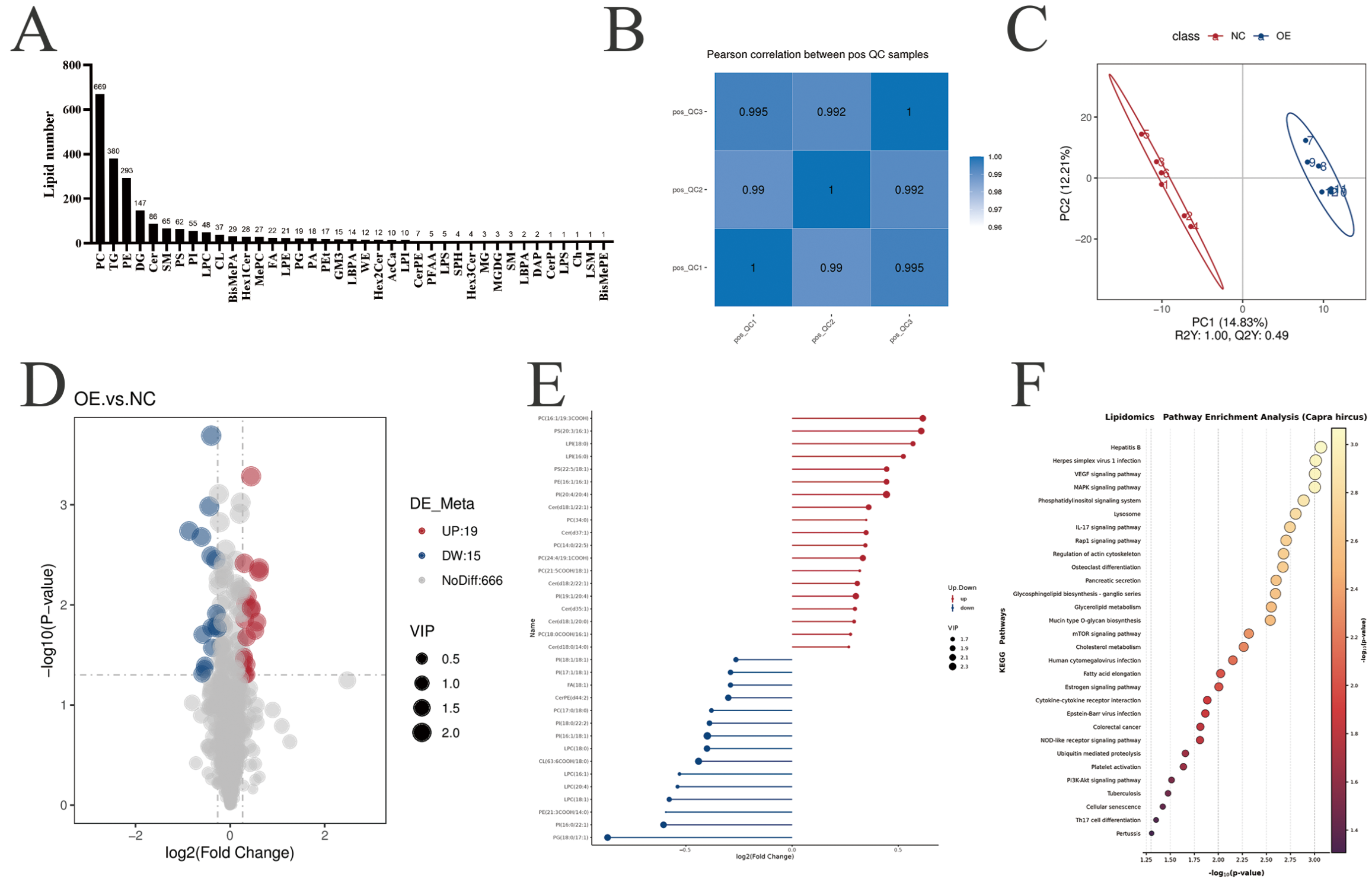
3.4. Identification and Analysis of Differential Lipids After PLIN5 Overexpression
3.5. PLIN5 Inhibits Cell Proliferation by Suppressing the PI3K-AKT Signaling Pathway
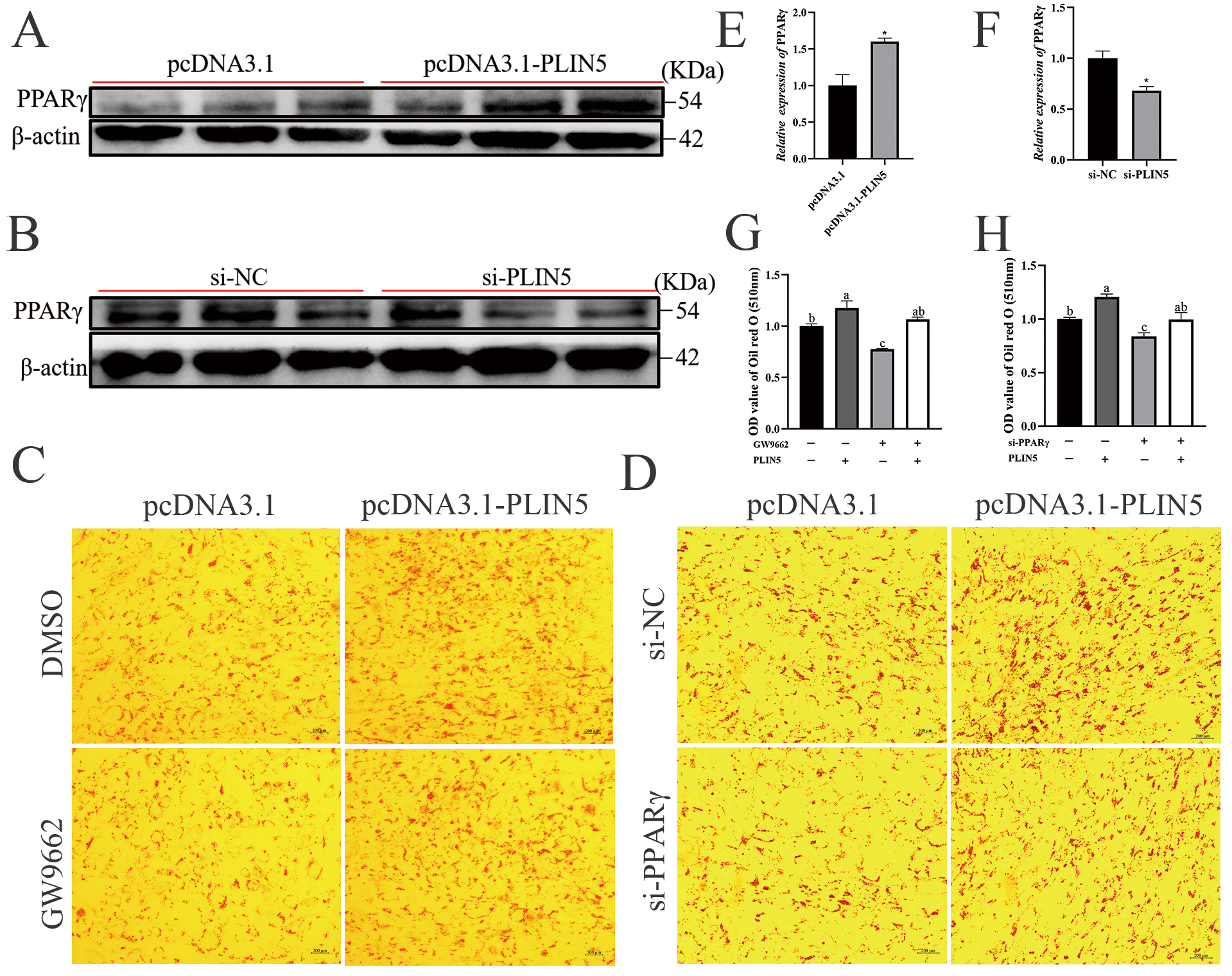
3.6. PLIN5 Promotes Cellular Lipid Deposition by Regulating PPARγ
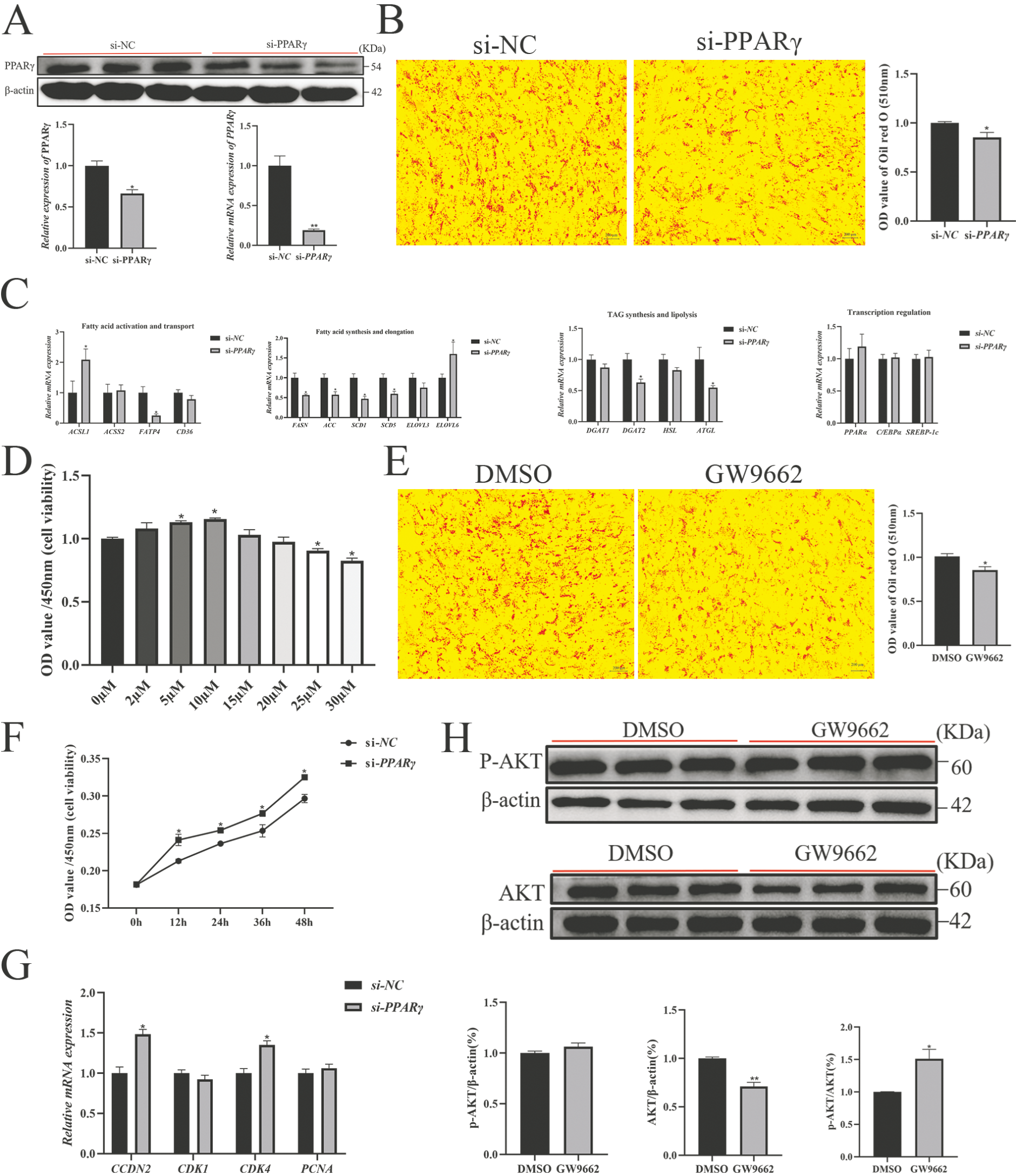
3.7. PPARγ Activation Is Required for PLIN5-Mediated Lipid Deposition and Proliferation Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef]
- Berry, R.; Jeffery, E.; Rodeheffer, M.S. Weighing in on adipocyte precursors. Cell Metab. 2014, 19, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Poulos, S.P.; Dodson, M.V.; Hausman, G.J. Cell line models for differentiation: Preadipocytes and adipocytes. Exp. Biol. Med. 2010, 235, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, B.A.; Chen, X.; Li, K.; Chen, J.; Yi, Z.; Zhang, X.; Li, Z.; Nie, Q. Control of preadipocyte proliferation, apoptosis and early adipogenesis by the forkhead transcription factor FoxO6. Life Sci. 2021, 265, 118858. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Xu, Q.; Li, A.; Yue, Y.; Ma, Y.; Lin, Y. LKB1 Regulates Goat Intramuscular Adipogenesis Through Focal Adhesion Pathway. Front. Physiol. 2021, 12, 755598. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Q.; Yang, C.; Zhang, C.; Huang, L.; Lin, Y.; Wang, Y.; Xiang, H.; Zhu, J. CIDEB promotes lipid deposition in goat intramuscular adipocytes. Anim. Biosci. 2025, 38, 884–897. [Google Scholar] [CrossRef]
- Xu, F.; Wang, H.; Qin, C.; Yue, B.; Yang, Y.; Wang, J.; Zhong, J.; Wang, H. Combined Multi-Omics Analysis Reveals the Potential Role of ACADS in Yak Intramuscular Fat Deposition. Int. J. Mol. Sci. 2024, 25, 9131. [Google Scholar] [CrossRef]
- Dalen, K.T.; Dahl, T.; Holter, E.; Arntsen, B.; Londos, C.; Sztalryd, C.; Nebb, H.I. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim. Biophys. Acta 2007, 1771, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Wolins, N.E.; Quaynor, B.K.; Skinner, J.R.; Tzekov, A.; Croce, M.A.; Gropler, M.C.; Varma, V.; Yao-Borengasser, A.; Rasouli, N.; Kern, P.A.; et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes 2006, 55, 3418–3428. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Matsushita, S.; Motojima, K.; Hirose, F.; Osumi, T. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 2006, 281, 14232–14240. [Google Scholar] [CrossRef]
- Wei, M.; Wang, Y.; Zhang, Y.; Qiao, Y. Plin5: A potential therapeutic target for type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2025, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Miner, G.E.; So, C.M.; Edwards, W.; Ragusa, J.V.; Wine, J.T.; Wong Gutierrez, D.; Airola, M.V.; Herring, L.E.; Coleman, R.A.; Klett, E.L.; et al. PLIN5 interacts with FATP4 at membrane contact sites to promote lipid droplet-to-mitochondria fatty acid transport. Dev. Cell 2023, 58, 1250–1265.e6. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Chen, Q.; Ke, S.; Ding, L.; Yang, X.; Rong, P.; Feng, W.; Cao, Y.; Wang, Q.; Li, M.; et al. Rab8a as a mitochondrial receptor for lipid droplets in skeletal muscle. Dev. Cell 2023, 58, 289–305.e6. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.R.; Mokhtar, R.; Matzaris, M.; Selathurai, A.; Kowalski, G.M.; Mokbel, N.; Meikle, P.J.; Bruce, C.R.; Watt, M.J. PLIN5 deletion remodels intracellular lipid composition and causes insulin resistance in muscle. Mol. Metab. 2014, 3, 652–663. [Google Scholar] [CrossRef]
- Li, Y.; Yang, M.; Lou, A.; Yun, J.; Ren, C.; Li, X.; Xia, G.; Nam, K.; Yoon, D.; Jin, H.; et al. Integrated analysis of expression profiles with meat quality traits in cattle. Sci. Rep. 2022, 12, 5926. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.; Mauck, J.; Loor, J.J.; Fan, W.; Tian, Y.; Yang, T.; Chang, Y.; Xie, M.; Aernouts, B.; et al. Diacylglycerol O-acyltransferase isoforms play a role in peridroplet mitochondrial fatty acid metabolism in bovine liver. J. Dairy Sci. 2024, 107, 9897–9914. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, D.; Wan, Y.; Xi, Q. Methylation of PLIN5 is a crucial biomarker and is involved in ovarian cancer development. Transl. Cancer Res. 2020, 9, 2919–2930. [Google Scholar] [CrossRef]
- Huo, K.; Ma, K.-G.; Guo, Q.-Y.; Duan, P.; Xu, J. Perilipin 5 protects against oxygen-glucose deprivation/reoxygenation-elicited neuronal damage by inhibiting oxidative stress and inflammatory injury via the Akt-GSK-3β-Nrf2 pathway. Int. Immunopharmacol. 2022, 108, 108718. [Google Scholar] [CrossRef]
- Zhang, W.; Raza, S.H.A.; Li, B.; Yang, W.; Khan, R.; Aloufi, B.H.; Zhang, G.; Zuo, F.; Zan, L. LncBNIP3 Inhibits Bovine Intramuscular Preadipocyte Differentiation via the PI3K-Akt and PPAR Signaling Pathways. J. Agric. Food Chem. 2024, 72, 24260–24271. [Google Scholar] [CrossRef]
- Dong, X.; Tang, S.; Zhang, W.; Gao, W.; Chen, Y. GPR39 activates proliferation and differentiation of porcine intramuscular preadipocytes through targeting the PI3K/AKT cell signaling pathway. J. Recept. Signal Transduct. Res. 2016, 36, 130–138. [Google Scholar] [CrossRef]
- Walczak, R.; Tontonoz, P. PPARadigms and PPARadoxes: Expanding roles for PPARgamma in the control of lipid metabolism. J. Lipid Res. 2002, 43, 177–186. [Google Scholar] [CrossRef]
- Wafer, R.; Tandon, P.; Minchin, J.E.N. The Role of Peroxisome Proliferator-Activated Receptor Gamma (PPARG) in Adipogenesis: Applying Knowledge from the Fish Aquaculture Industry to Biomedical Research. Front. Endocrinol. 2017, 8, 102. [Google Scholar] [CrossRef]
- Bensinger, S.J.; Tontonoz, P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 2008, 454, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Kadegowda, A.K.G.; Bionaz, M.; Piperova, L.S.; Erdman, R.A.; Loor, J.J. Peroxisome proliferator-activated receptor-gamma activation and long-chain fatty acids alter lipogenic gene networks in bovine mammary epithelial cells to various extents. J. Dairy Sci. 2009, 92, 4276–4289. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Zhu, J.; Zhao, Y.; Lin, Y. Molecular characterization of GTP binding protein overexpressed in skeletal muscle (GEM) and its role in promoting adipogenesis in goat intramuscular preadipocytes. Anim. Biotechnol. 2020, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Wang, Y.; Li, Y.; Wang, Y.; Zhu, J.; Lin, Y. Chi-Circ_0006511 Positively Regulates the Differentiation of Goat Intramuscular Adipocytes via Novel-miR-87/CD36 Axis. Int. J. Mol. Sci. 2022, 23, 12295. [Google Scholar] [CrossRef]
- Li, Z.; Hu, T.; Li, R.; Li, J.; Wang, Y.; Li, Y.; Lin, Y.; Wang, Y.; Jiani, X. Effect of DHCR7 on adipocyte differentiation in goats. Anim. Biotechnol. 2024, 35, 2298399. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Catalán, V.; Whyte, L.; Díaz-Arteaga, A.; Vázquez-Martínez, R.; Rotellar, F.; Guzmán, R.; Gómez-Ambrosi, J.; Pulido, M.R.; Russell, W.R.; et al. The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes 2012, 61, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, H.; Dai, Y.; Li, S.; Gu, J.; Wu, R.; Jia, J.; Shen, J.; Zhang, Y.; Li, H.; et al. CircITGB5 regulates the proliferation and adipogenic differentiation of chicken intramuscular preadipocytes through the miR-181b-5p/CPT1A axis. Int. J. Biol. Macromol. 2024, 283, 137608. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, X.; Wu, W.; Wang, W.; Pang, W.; Yang, G. MAT2B promotes adipogenesis by modulating SAMe levels and activating AKT/ERK pathway during porcine intramuscular preadipocyte differentiation. Exp. Cell Res. 2016, 344, 11–21. [Google Scholar] [CrossRef]
- Gao, X.; Jian, L.; Zhang, L.; Xu, Y.; Zhao, Y.; Yang, Y.; Yuan, Y.; Wang, Y.; Xu, S.; Ren, B.; et al. Perilipin 5 protects the mitochondrial oxidative functions and improves the alcoholic liver injury in mice. Liver Int. 2024, 44, 357–369. [Google Scholar] [CrossRef]
- Mass-Sanchez, P.B.; Krizanac, M.; Štancl, P.; Leopold, M.; Engel, K.M.; Buhl, E.M.; van Helden, J.; Gassler, N.; Schiller, J.; Karlić, R.; et al. Perilipin 5 deletion protects against nonalcoholic fatty liver disease and hepatocellular carcinoma by modulating lipid metabolism and inflammatory responses. Cell Death Discov. 2024, 10, 94. [Google Scholar] [CrossRef]
- Li, X.; Kang, K.; Shen, L.; Shen, L.; Zhou, Y. Integrative Analysis of the Predictive Value of Perilipin Family on Clinical Significance, Prognosis and Immunotherapy of Glioma. Biomedicines 2023, 11, 1009. [Google Scholar] [CrossRef]
- Zappaterra, M.; Mazzoni, M.; Zambonelli, P.; Davoli, R. Investigation of the Perilipin 5 gene expression and association study of its sequence polymorphism with meat and carcass quality traits in different pig breeds. Animal 2018, 12, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Albrecht, E.; Li, Z.; Schregel, J.; Sciascia, Q.L.; Metges, C.C.; Maak, S. Distinct Roles of Perilipins in the Intramuscular Deposition of Lipids in Glutamine-Supplemented, Low-, and Normal-Birth-Weight Piglets. Front. Vet. Sci. 2021, 8, 633898. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Liu, G.; Loor, J.J.; Bucktrout, R.; Sun, X.; Li, G.; Shu, X.; Dong, J.; Wang, Y.; et al. Perilipin 5 promotes hepatic steatosis in dairy cows through increasing lipid synthesis and decreasing very low density lipoprotein assembly. J. Dairy Sci. 2019, 102, 833–845. [Google Scholar] [CrossRef]
- Li, Y.; Torp, M.K.; Norheim, F.; Khanal, P.; Kimmel, A.R.; Stensløkken, K.O.; Vaage, J.; Dalen, K.T. Isolated Plin5-deficient cardiomyocytes store less lipid droplets than normal, but without increased sensitivity to hypoxia. Biochimica et biophysica acta. Mol. Cell Biol. Lipids 2021, 1866, 158873. [Google Scholar] [CrossRef]
- Tan, Y.; Huang, Z.; Jin, Y.; Wang, J.; Fan, H.; Liu, Y.; Zhang, L.; Wu, Y.; Liu, P.; Li, T.; et al. Lipid droplets sequester palmitic acid to disrupt endothelial ciliation and exacerbate atherosclerosis in male mice. Nat. Commun. 2024, 15, 8273. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Xiao, W.; Qin, X.; Cai, G.; Chen, H.; Hua, Z.; Cheng, C.; Li, X.; Hua, W.; Xiao, H.; et al. Myostatin regulates fatty acid desaturation and fat deposition through MEF2C/miR222/SCD5 cascade in pigs. Commun. Biol. 2020, 3, 612. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Luo, J.; He, Q.; Shi, H.; Li, J.; Wang, H.; Xu, H.; Chen, Z.; Yi, Y.; Loor, J.J. SCD1 Alters Long-Chain Fatty Acid (LCFA) Composition and Its Expression Is Directly Regulated by SREBP-1 and PPARγ 1 in Dairy Goat Mammary Cells. J. Cell. Physiol. 2017, 232, 635–649. [Google Scholar] [CrossRef]
- Khan, M.Z.; Ma, Y.; Ma, J.; Xiao, J.; Liu, Y.; Liu, S.; Khan, A.; Khan, I.M.; Cao, Z. Association of DGAT1 With Cattle, Buffalo, Goat, and Sheep Milk and Meat Production Traits. Front. Vet. Sci. 2021, 8, 712470. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Li, Z.; Li, X.; Du, Q.; Zhang, Y.; Zhong, Z.; Wang, H.; Zhang, S.; Li, P.; Li, H.; et al. Inhibition of triglyceride metabolism-associated enhancers alters lipid deposition during adipocyte differentiation. FASEB J. 2025, 39, e70347. [Google Scholar] [CrossRef]
- Chitraju, C.; Walther, T.C.; Farese, R.V., Jr. The triglyceride synthesis enzymes DGAT1 and DGAT2 have distinct and overlapping functions in adipocytes. J. Lipid Res. 2019, 60, 1112–1120. [Google Scholar] [CrossRef]
- Janani, C.; Ranjitha Kumari, B.D. PPAR gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef]
- Karpińska, O.; Baranowska-Kuczko, M.; Malinowska, B.; Kloza, M.; Kusaczuk, M.; Gęgotek, A.; Golec, P.; Kasacka, I.; Kozłowska, H. Mechanisms of l-alpha-lysophosphatidylinositol-induced relaxation in human pulmonary arteries. Life Sci. 2018, 192, 38–45. [Google Scholar] [CrossRef]
- Yamashita, A.; Oka, S.; Tanikawa, T.; Hayashi, Y.; Nemoto-Sasaki, Y.; Sugiura, T. The actions and metabolism of lysophosphatidylinositol, an endogenous agonist for GPR55. Prostaglandins Other Lipid Mediat. 2013, 107, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Arifin, S.A.; Falasca, M. Lysophosphatidylinositol Signalling and Metabolic Diseases. Metabolites 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, A.Y.; Park, S.Y.; Liu, K.H.; Im, D.S. O-1602 Promotes Hepatic Steatosis through GPR55 and PI3 Kinase/Akt/SREBP-1c Signaling in Mice. Int. J. Mol. Sci. 2021, 22, 3091. [Google Scholar] [CrossRef]
- Yu, K.; Shi, H.; Luo, J.; Li, J.; Zhao, W.; Tian, H.; Shi, H. PPARG modulated lipid accumulation in dairy GMEC via regulation of ADRP gene. J. Cell. Biochem. 2015, 116, 192–201. [Google Scholar] [CrossRef]
- Li, H.; Yao, W.; Yang, C.; Zhang, W.; Wang, Y.; Lin, Y.; Du, Z.; Zhang, C.; Huang, L.; Zhang, M.; et al. SIRT5 Regulates Lipid Deposition in Goat Preadipocytes via PI3K-Akt and MAPK Signaling Pathways. Animals 2025, 15, 1072. [Google Scholar] [CrossRef]
- Yin, X.; Dong, L.; Wang, X.; Qin, Z.; Ma, Y.; Ke, X.; Li, Y.; Wang, Q.; Mi, Y.; Lyu, Q.; et al. Perilipin 5 regulates hepatic stellate cell activation and high-fat diet-induced non-alcoholic fatty liver disease. Anim. Models Exp. Med. 2024, 7, 166–178. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, J.; Chen, Y.; Li, Y.; Xuan, B.; Gu, M.; Feng, F.; Yang, Y.; Yang, D.; Sun, X. Plin5 inhibits proliferation and migration of vascular smooth muscle cell through interacting with PGC-1α following vascular injury. Bioengineered 2022, 13, 10665–10678. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Ren, Y.; Ma, Y.; Liu, J.; Jiang, H.; Liu, C. Artesunate improves glucose and lipid metabolism in db/db mice by regulating the metabolic profile and the MAPK/PI3K/Akt signalling pathway. Phytomedicine 2024, 126, 155382. [Google Scholar] [CrossRef]
- Wang, L.; He, H.; Zhai, R.; Gao, R.; Su, M.; Duan, R.; Tu, Z.; Huang, R. Investigation of the mechanism by which FOXJ2 inhibits proliferation, metastasis and cell cycle progression of ovarian cancer cells through the PI3K/AKT signaling pathway. Eur. J. Med. Res. 2025, 30, 152. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, M.; Fu, R. Knockdown of FANCI suppresses hepatocellular carcinoma development via the PI3K/Akt/GSK-3β pathway. Heliyon 2025, 11, e42731. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pu, L.; Chen, W.; Zhao, Q.; Wu, G.; Li, D.; Zhu, H. LY294002 attenuates inflammatory response in endotoxin-induced uveitis by downregulating JAK3 and inactivating the PI3K/Akt signaling. Immunopharmacol. Immunotoxicol. 2022, 44, 510–518. [Google Scholar] [CrossRef]
- Jia, J.J.; Lai, H.J.; Sun, B.W.; Lu, J.; Zeng, Y.Y. miR-21 regulates autophagy and apoptosis of ectopic endometrial stromal cells of adenomyosis via PI3K/AKT/mTOR pathway. Sci. Rep. 2025, 15, 7639. [Google Scholar] [CrossRef] [PubMed]
- Kozar, K.; Ciemerych, M.A.; Rebel, V.I.; Shigematsu, H.; Zagozdzon, A.; Sicinska, E.; Geng, Y.; Yu, Q.; Bhattacharya, S.; Bronson, R.T.; et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell 2004, 118, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Jo, U.; Cai, W.; Wang, J.; Kwon, Y.; D’Andrea, A.D.; Kim, H. PCNA-Dependent Cleavage and Degradation of SDE2 Regulates Response to Replication Stress. PLoS Genet. 2016, 12, e1006465. [Google Scholar] [CrossRef]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar] [CrossRef]
- Ishimi, Y. Regulation of MCM2-7 function. Genes Genet. Syst. 2018, 93, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.; Aguilar-González, A.; Martín, F.; Mesas, C.; Moreno, J.; Rama, A.R. Exploring miR-21 Knock-Out Using CRISPR/Cas as a Treatment for Lung Cancer. Genes 2025, 16, 133. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Shi, L.; Sun, D.; Wang, L.; Feng, D.; Ding, C. Effect of isorhamnetin on carbonic anhydrase IX expression and tumorigenesis of bladder cancer by activating PPARγ/PTEN/AKT pathway. Tissue Cell 2023, 82, 102048. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yang, E.K.; Kim, S.G. Peroxisome proliferator-activated receptor-gamma and retinoic acid X receptor alpha represses the TGFbeta1 gene via PTEN-mediated p70 ribosomal S6 kinase-1 inhibition: Role for Zf9 dephosphorylation. Mol. Pharmacol. 2006, 70, 415–425. [Google Scholar] [CrossRef]
- Dong, Y.W.; Wang, X.P.; Wu, K. Suppression of pancreatic carcinoma growth by activating peroxisome proliferator-activated receptor gamma involves angiogenesis inhibition. World J. Gastroenterol. 2009, 15, 441–448. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Yu, C.; Zhang, P.; Gu, S.; Wang, G.; Xiao, H.; Li, S. Bergenin inhibits bladder cancer progression via activating the PPARγ/PTEN/Akt signal pathway. Drug Dev. Res. 2021, 82, 278–286. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Yang, Y.; Li, H.; Wang, Y.; Wang, Y.; Lin, Y.; Huang, L.; Du, Z.; Xiang, H.; Zhang, C.; et al. PLIN5 Promotes Lipid Reconstitution in Goat Intramuscular Fat via the PPARγ Signaling Pathway. Biology 2025, 14, 1547. https://doi.org/10.3390/biology14111547
Dai Y, Yang Y, Li H, Wang Y, Wang Y, Lin Y, Huang L, Du Z, Xiang H, Zhang C, et al. PLIN5 Promotes Lipid Reconstitution in Goat Intramuscular Fat via the PPARγ Signaling Pathway. Biology. 2025; 14(11):1547. https://doi.org/10.3390/biology14111547
Chicago/Turabian StyleDai, Yuhan, Yuling Yang, Haiyang Li, Yinggui Wang, Yong Wang, Yaqiu Lin, Lian Huang, Zhanyu Du, Hua Xiang, Changhui Zhang, and et al. 2025. "PLIN5 Promotes Lipid Reconstitution in Goat Intramuscular Fat via the PPARγ Signaling Pathway" Biology 14, no. 11: 1547. https://doi.org/10.3390/biology14111547
APA StyleDai, Y., Yang, Y., Li, H., Wang, Y., Wang, Y., Lin, Y., Huang, L., Du, Z., Xiang, H., Zhang, C., & Zhu, J. (2025). PLIN5 Promotes Lipid Reconstitution in Goat Intramuscular Fat via the PPARγ Signaling Pathway. Biology, 14(11), 1547. https://doi.org/10.3390/biology14111547





