Metabolome and Metagenome Signatures Underlying the Differential Resistance of Percocypris pingi, Crucian Carp, and Yellow Catfish to Ichthyophthirius multifiliis Infection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.3. Data Preprocessing and Metabolite Identification
2.4. Statistical Analysis of Metabolomics Data
2.5. DNA Extraction and PCR Amplification
2.6. Library Construction, Sequencing, and Data Quality Control
2.7. ASV Denoising and Taxonomic Annotation
2.8. Sample Complexity Analysis and Functional Prediction
3. Results
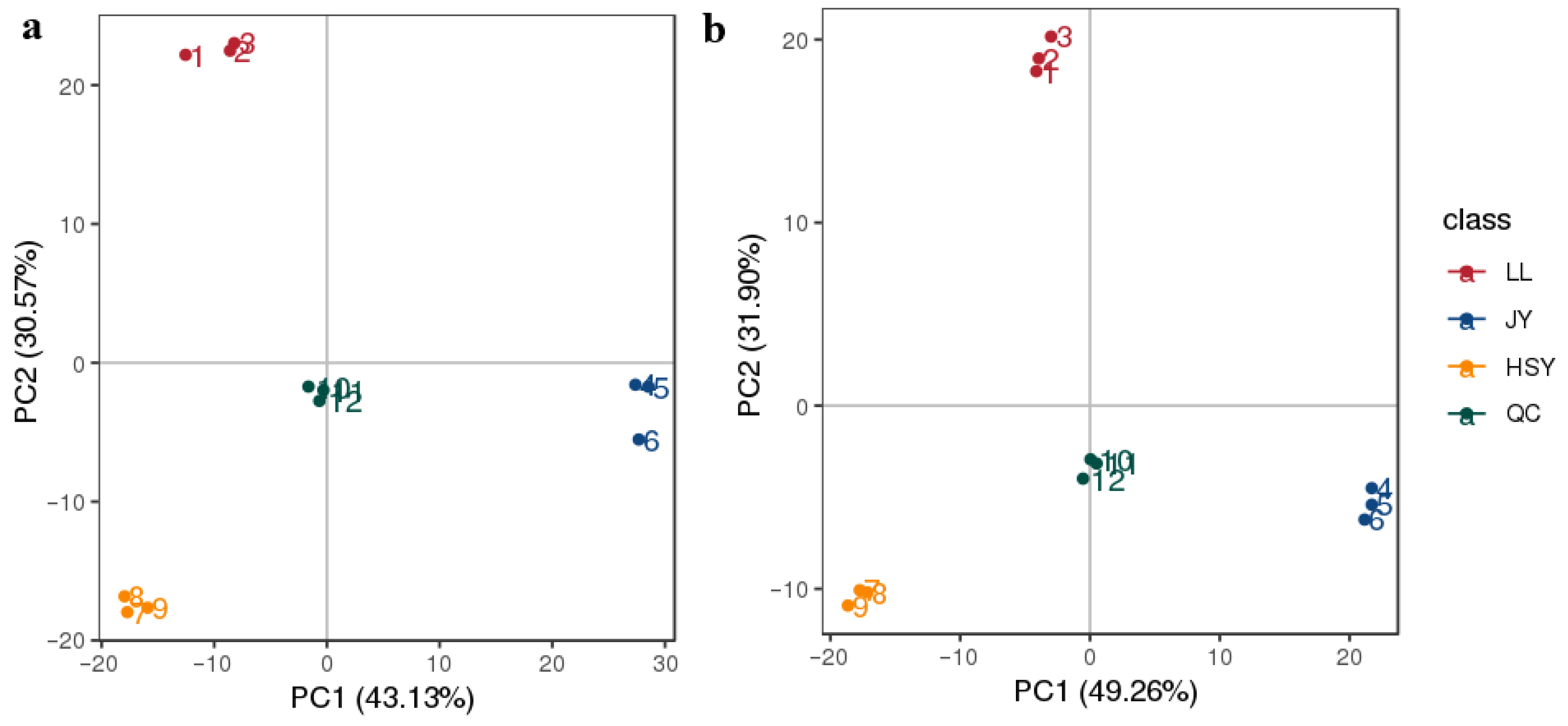
3.1. Metabolite Classification and Grouping Status
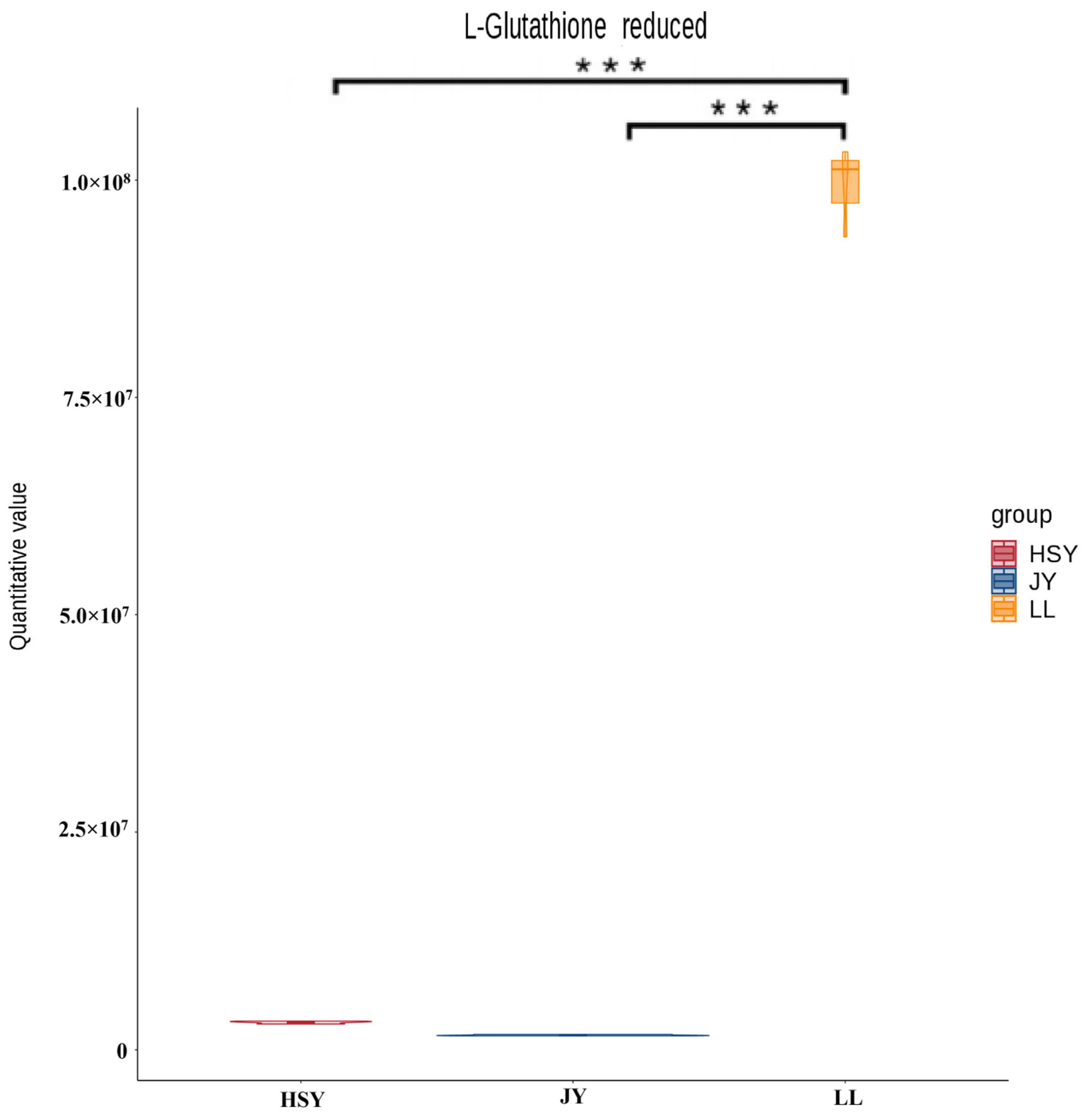
3.2. Screening of Differential Metabolites
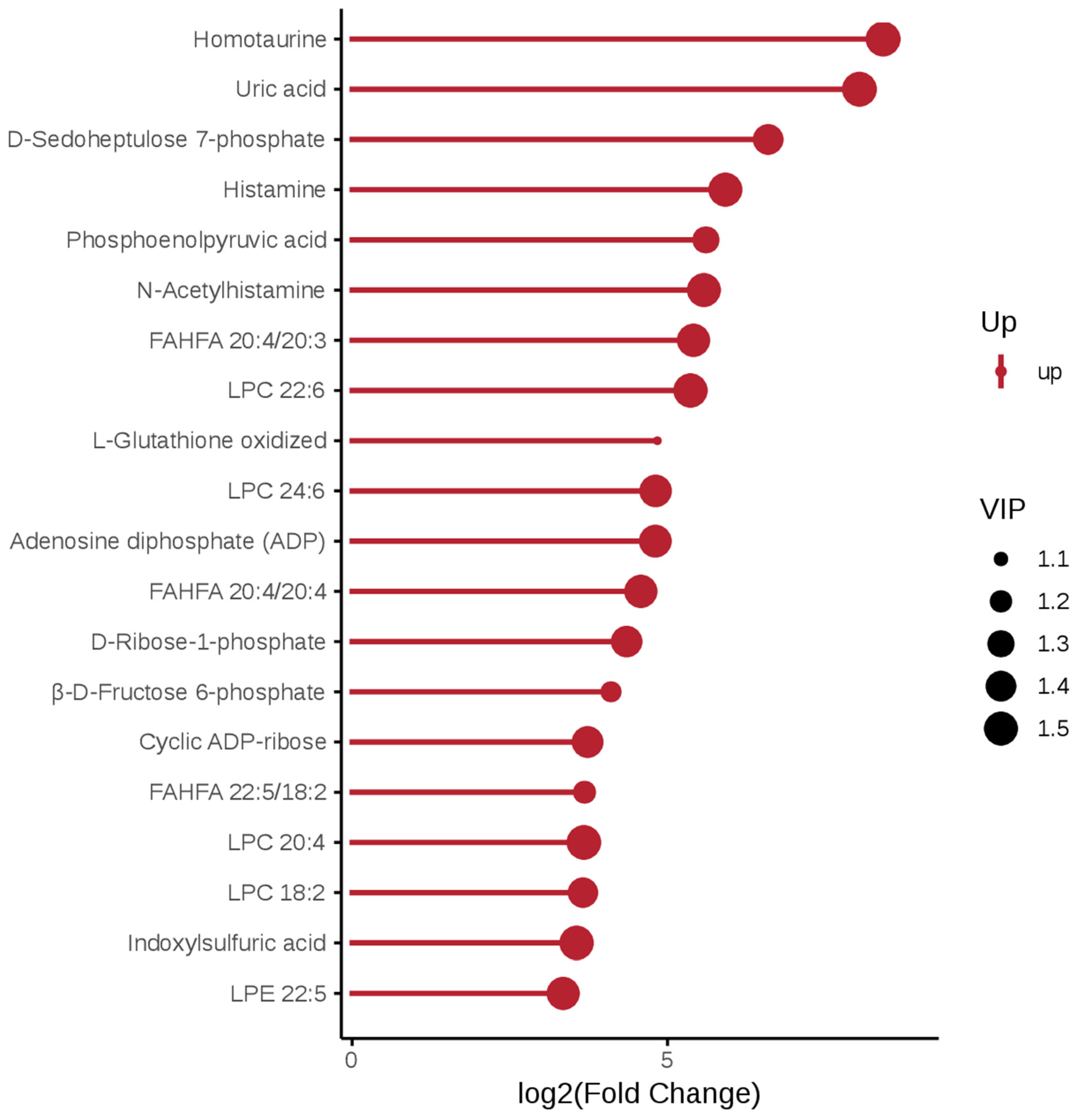
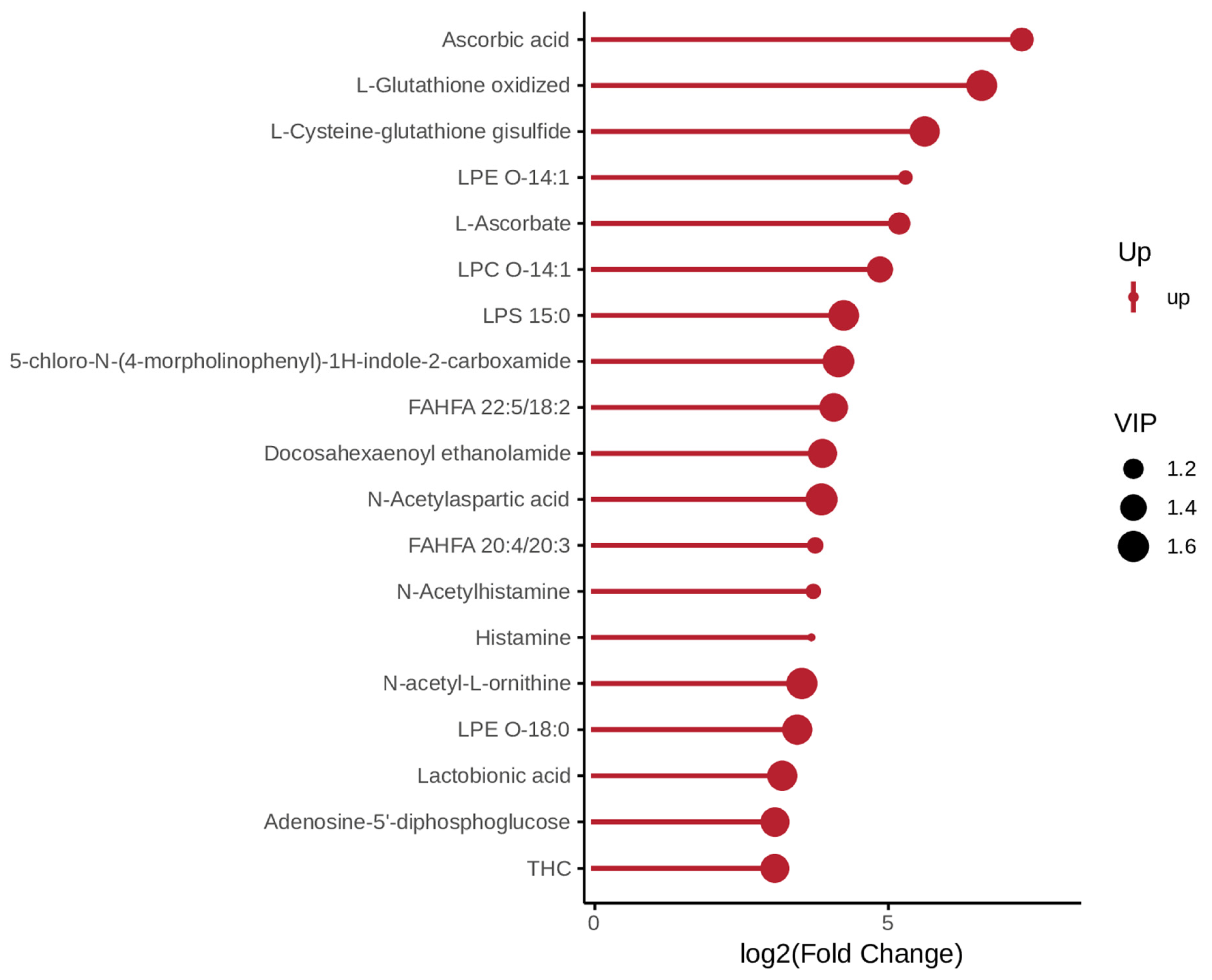
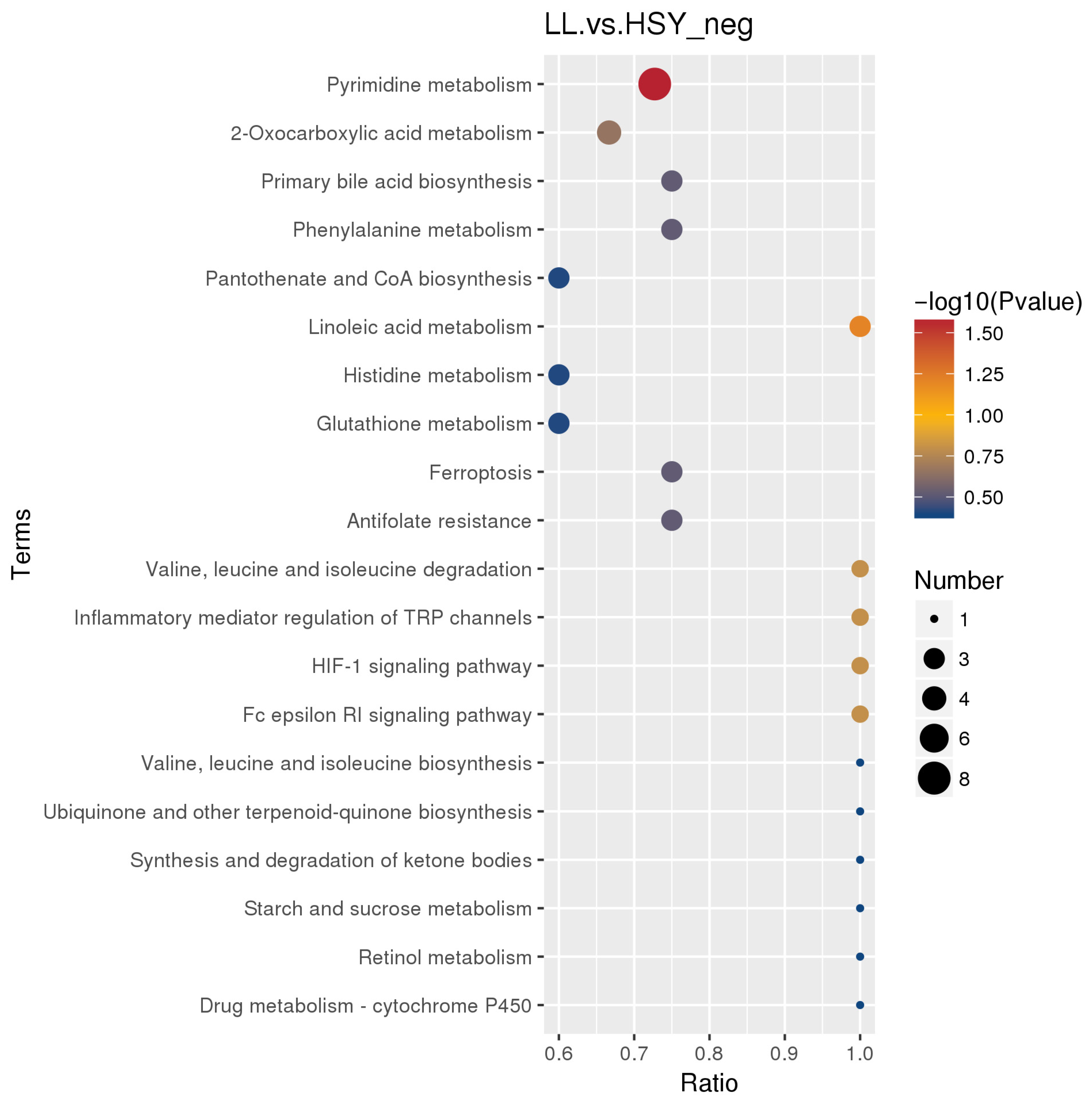
3.3. Functional Enrichment Analysis of Differential Metabolites
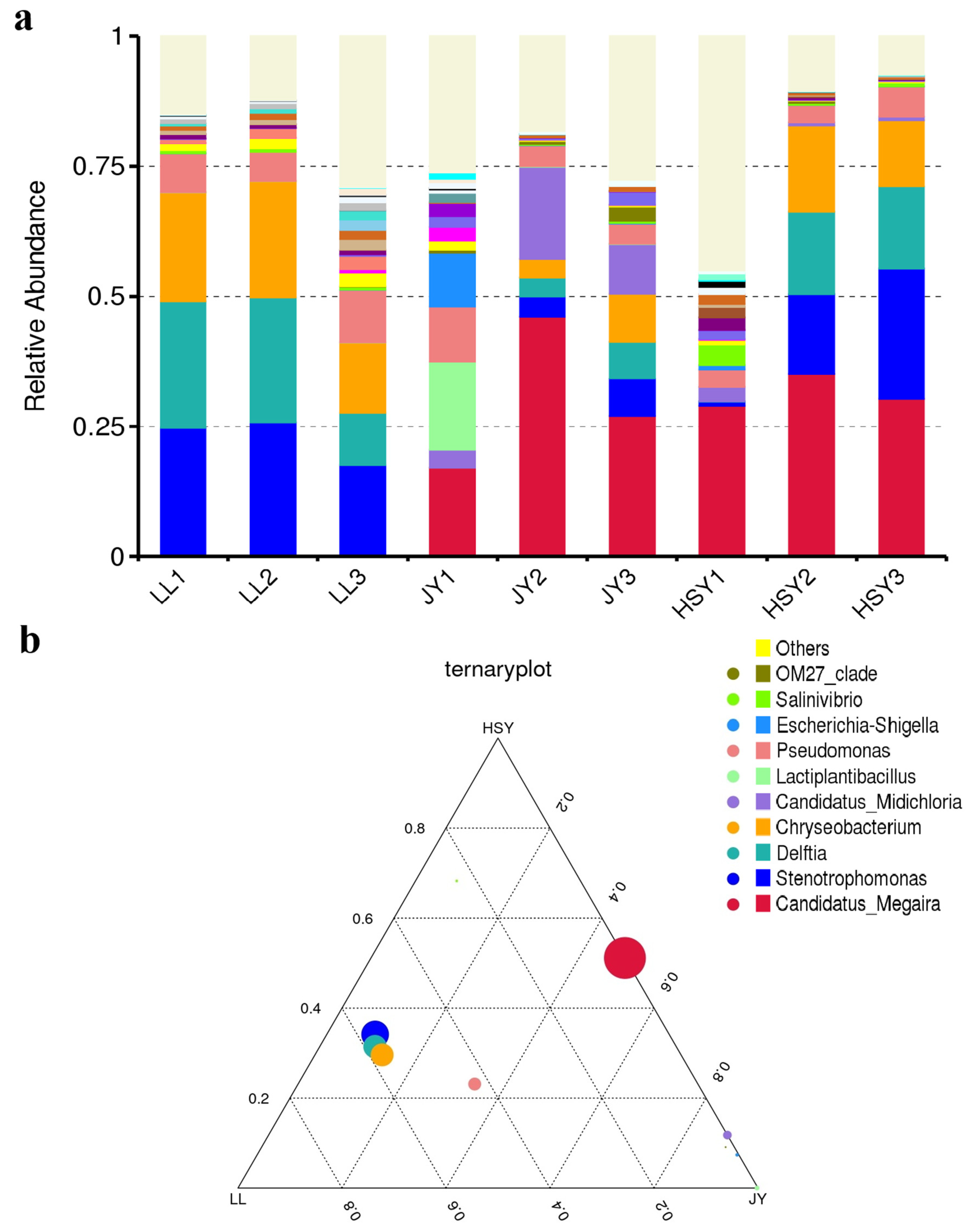
3.4. Changes in the Composition of the Skin Microbiota
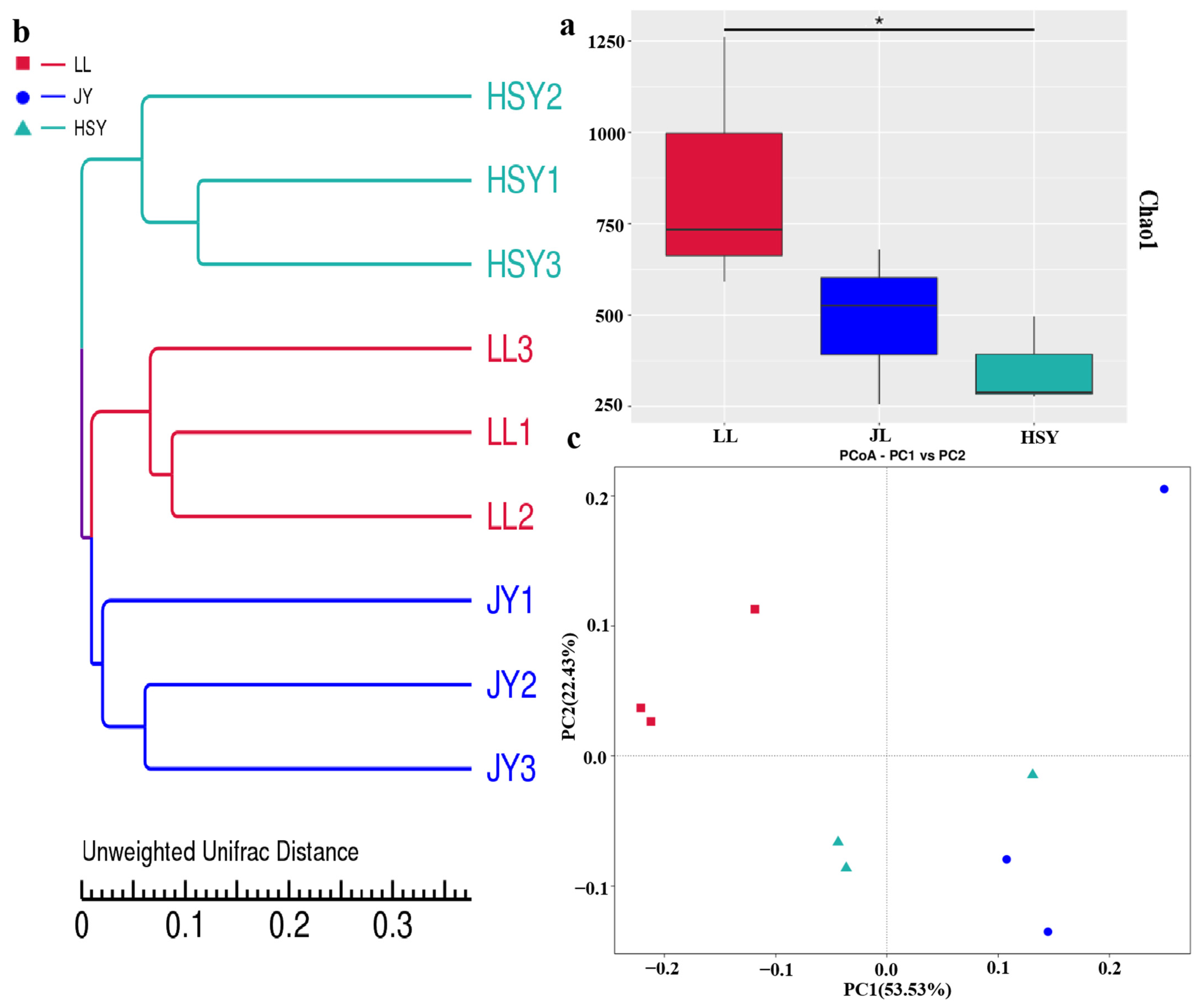
3.5. Changes in α- and β-Diversity of Skin Microbiota
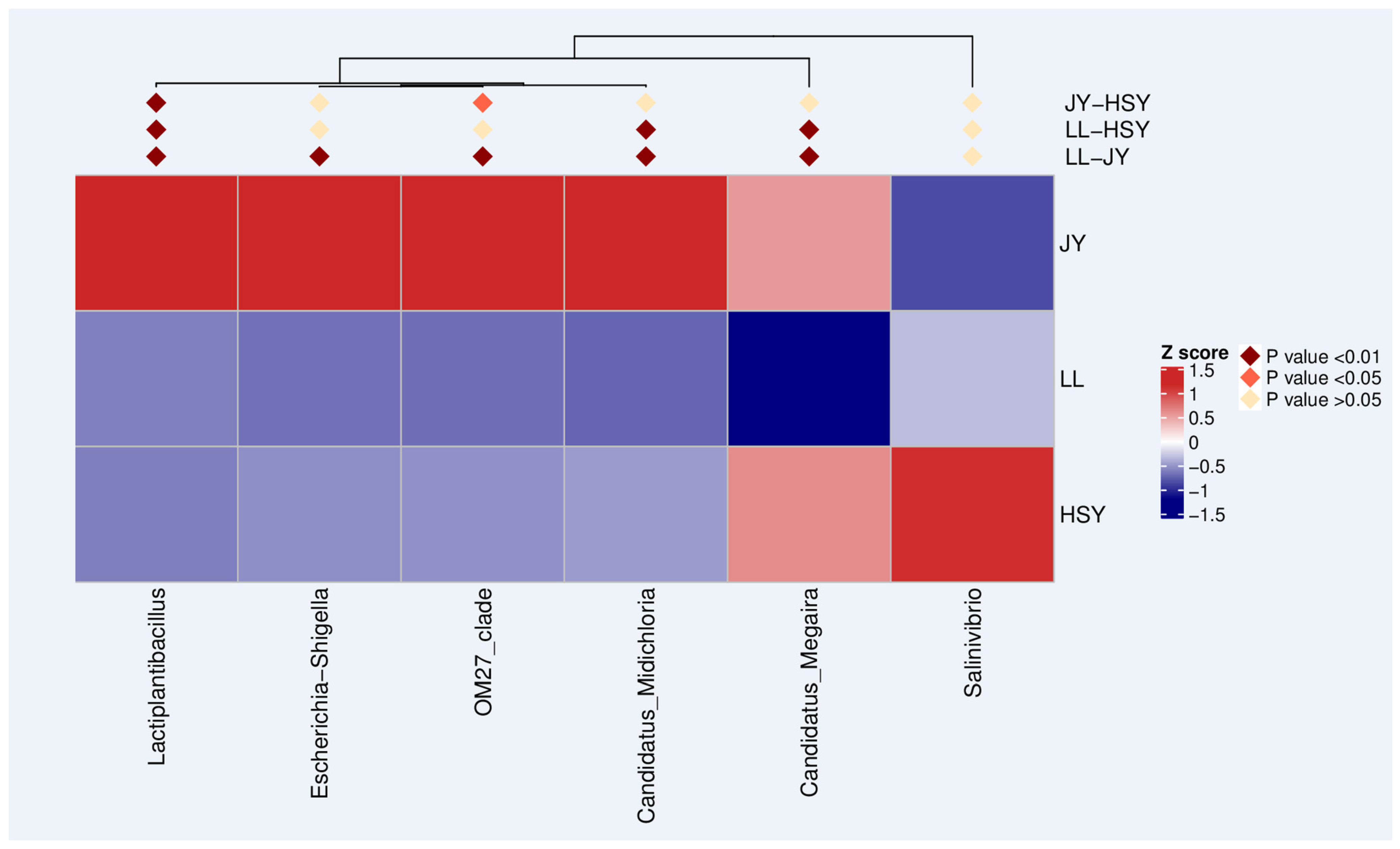
3.6. Genus with Significant Differences Between Groups
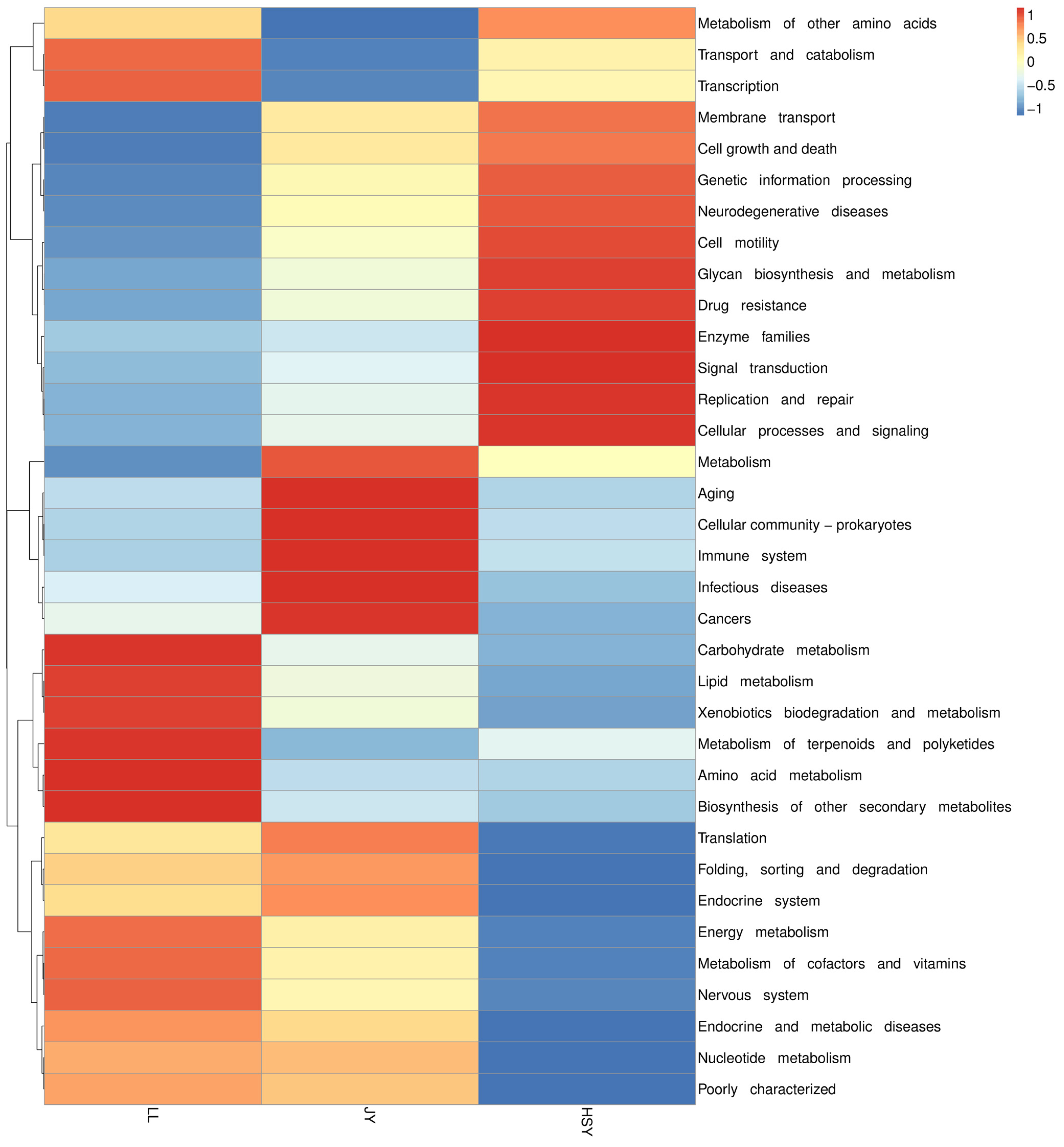
3.7. Functional Enrichment Analysis
4. Discussion
4.1. Integrated Antioxidant Defense as a Hallmark of Disease Resistance
4.2. The Protective Skin Microbiome as an Ecological Barrier
4.3. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickerson, H.W. Ichthyophthirius multifiliis and Cryptocaryon irritans (phylum Ciliophora). In Fish Diseases and Disorders, Volume 1: Protozoan and Metazoan Infections; CABI International: Wallingford, UK, 2006; pp. 116–153. [Google Scholar]
- Li, X.; Jaafar, R.; He, Y.; Wu, B.; Kania, P.; Buchmann, K. Effects of a Pseudomonas H6 surfactant on rainbow trout and Ichthyophthirius multifiliis: In vivo exposure. Aquaculture 2022, 547, 737479. [Google Scholar] [CrossRef]
- Qu, S.; Liu, J.; Wu, Z.; Li, J.; Li, P.; Wang, G.; Ling, F. Nanocomplexation is a promising strategy to enhance the solubility and anti-Ichthyophthirius multifiliis activity of magnolol. Aquaculture 2023, 565, 739105. [Google Scholar] [CrossRef]
- Dickerson, H.W.; Findly, R.C. Immunity to Ichthyophthirius infections in fish: A synopsis. Dev. Comp. Immunol. 2014, 43, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Clayton, G.M.; Price, D.J. Interspecific and intraspecific variation in resistance to ichthyophthiriasis among poeciliid and goodeid fishes. J. Fish Biol. 1992, 40, 445–453. [Google Scholar] [CrossRef]
- Clayton, G.M.; Price, D.J. Heterosis in resistance to Ichthyophthirius multifiliis infections in poeciliid fish. J. Fish Biol. 1994, 44, 59–66. [Google Scholar] [CrossRef]
- Shen, M.H. Transcriptome Analysis and Complement Component C3 Expression Analysis of Ctenopharyngodon idella and Spinibarbus hollandi Infected by Ichthyophthirius multifiliis. Master’s Thesis, Zhejiang Normal University, Jinhua, China, 2021. [Google Scholar]
- Xiang, C.Q.; Zeng, R.K.; Deng, L.J.; Dong, J.P.; Huang, J.W.; Shen, L.L. Breeding and fry cultivation techniques of Percocypris pingi. Jiangxi Aquat. Sci. Technol. 2017, 4, 22+24. [Google Scholar]
- Li, L.; Li, M.; Zeng, R.; Deng, L.; Yang, K.; Song, Z. High resistance to Ichthyophthirius multifiliis in Percocypris pingi, an endemic fish in upper Yangtze River. Aquaculture 2023, 562, 738767. [Google Scholar] [CrossRef]
- Gu, H.; Wang, H.; Deng, S.; Dai, X.; He, X.; Wang, Z. Hybridization with Percocypris pingi male can improve the cultivation characteristics of snow trout (Schizothorax wangchiachii), especially the resistance to white spot disease (Ichthyophthirius multifiliis). Aquaculture 2023, 562, 738805. [Google Scholar] [CrossRef]
- Yevtushenko, A.V. Features of the parasitic system formation in common carp in the aquaculture of the north-eastern and eastern regions of Ukraine. J. Vet. Med. Biotechnol. Biosaf. 2022, 6, 9–15. [Google Scholar] [CrossRef]
- Yang, H.; Feng, X.; Tu, X.; Liu, F.; Hu, R.; Gu, Z. Ciliated parasites as the primary agents for early epizootic ulcerative syndrome (EUS) in yellow catfish, Tachysurus fulvifraco. Aquaculture 2023, 573, 739534. [Google Scholar] [CrossRef]
- Yang, H.; Wei, F.; Jie, C.; Bi, L.; Chuan, Q.; Jun, W. Histopathological Observation of Pelteobagrus vachelli Infected with Ichthyophthirius multifiliis. J. Yunnan Agric. Univ. (Nat. Sci.) 2020, 35, 1016–1022. [Google Scholar]
- Yu, H.; Wang, Z.B.; Lu, Y.J.; Tan, S.W.; Yan, Q.G.; Li, H. Histopathological study of grass carp (Ctenopharyngodon idella) experimentally infected with Ichthyophthirius multifiliis. Afr. J. Microbiol. Res. 2012, 6, 3539–3544. [Google Scholar] [CrossRef]
- Low, C.F.; Rozaini, M.Z.H.; Musa, N.; Syarul Nataqain, B. Current knowledge of metabolomic approach in infectious fish disease studies. J. Fish Dis. 2017, 40, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; Li, X.; Han, Z.; Zhang, R.; Li, X.; Chen, Q. Metabolic responses of shrimp Palaemonetes sinensis to isopod Tachaea chinensis parasitization. Dis. Aquat. Organ. 2020, 138, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.J.; Gaulke, C.A.; Garcia-Jaramillo, M.; Leong, C.; Morre, J.; Sieler, M.J., Jr.; Stevens, J.F.; Jiang, Y.; Maier, C.S.; Kent, M.L.; et al. Gut microbiota metabolically mediate intestinal helminth infection in zebrafish. mSystems 2024, 9, e0054524. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, D.; Xie, J.; Chang, O.; Wang, Q.; Shi, C.; Zhao, F.; Gong, H.; Ren, Y.; Musa, N.; et al. Do ectoparasites on fish gills “talk” with gut microbiota far away? Aquaculture 2023, 562, 738880. [Google Scholar] [CrossRef]
- Toxqui-Rodríguez, S.; Estensoro, I.; Domingo-Bretón, R.; Del Pozo, R.; Pérez-Sánchez, J.; Sipkema, D.; Sitjà-Bobadilla, A.; Piazzon, M.C. Interactions between gilthead seabream intestinal transcriptome and microbiota upon Enteromyxum leei infection: A multi-omic approach. Anim. Microbiome 2025, 7, 22. [Google Scholar] [CrossRef]
- Becker, J.A.; Tweedie, A.; Gilligan, D.; Asmus, M.; Whittington, R.J. Experimental infection of Australian freshwater fish with epizootic haematopoietic necrosis virus (EHNV). J. Aquat. Anim. Health 2013, 25, 66–76. [Google Scholar] [CrossRef]
- Martins, M.L.; Cardoso, L.; Marchiori, N.; Benites de Pádua, S. Protozoan infections in farmed fish from Brazil: Diagnosis and pathogenesis. Rev. Bras. Parasitol. Vet. 2015, 24, 1–20. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Wang, J.B.; Pu, S.B.; Sun, Y.; Li, Z.F.; Niu, M.; Yan, X.Z.; Zhao, Y.L.; Wang, L.F.; Qin, X.M.; Ma, Z.J.; et al. Metabolomic Profiling of Autoimmune Hepatitis: The Diagnostic Utility of Nuclear Magnetic Resonance Spectroscopy. Proteome Res. 2014, 13, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, D.A.; Piccolo, B.D.; Vaziri, N.D.; Liu, S.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Moore, M.E.; Marco, M.L.; Martin, R.J.; et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. American journal of physiology. Am. J. Physiol. Ren. Physiol. 2016, 310, F857–F871. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Sui, J.; Zhang, J. Metabolomics reveals significant variations in metabolites and correlations regarding the maturation of walnuts (Juglans regia L.). Biol. Open 2016, 5, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Heischmann, S.; Quinn, K.; Cruickshank-Quinn, C.; Liang, L.P.; Reisdorph, R.; Reisdorph, N.; Patel, M. Exploratory Metabolomics Profiling in the Kainic Acid Rat Model Reveals Depletion of 25-Hydroxyvitamin D3 during Epileptogenesis. Sci. Rep. 2016, 6, 31424. [Google Scholar] [CrossRef]
- Haspel, J.A.; Chettimada, S.; Shaik, R.S.; Chu, J.H.; Raby, B.A.; Cernadas, M.; Carey, V.; Process, V.; Hunninghake, G.M.; Ifedigbo, E.; et al. Circadian rhythm reprogramming during lung inflammation. Nat. Commun. 2014, 5, 4753. [Google Scholar] [CrossRef]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Kechin, A.; Boyarskikh, U.; Kel, A.; Filipenko, M. cutPrimers: A New Tool for Accurate Cutting of Primers from Reads of Targeted Next Generation Sequencing. J. Comput. Biol. 2017, 24, 1138–1143. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, H.; Gao, X.; Wang, J. The Intratumor Microbiota Signatures Associate with Subtype, Tumor Stage, and Survival Status of Esophageal Carcinoma. Front. Oncol. 2021, 11, 754788. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Davison, H.R.; Hurst, G.D.D.; Siozios, S. ‘Candidatus Megaira’ are diverse symbionts of algae and ciliates with the potential for defensive symbiosis. Microb. Genom. 2023, 9, mgen000950. [Google Scholar] [CrossRef]
- Zaila, K.E.; Doak, T.G.; Ellerbrock, H.; Tung, C.H.; Martins, M.L.; Kolbin, D.; Yao, M.C.; Cassidy-Hanley, D.M.; Clark, T.G.; Chang, W.J. Diversity and Universality of Endosymbiotic Rickettsia in the Fish Parasite Ichthyophthirius multifiliis. Front. Microbiol. 2017, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- El Gamal, S.A.; Adawy, R.S.; Zaki, V.H.; Zahran, E. Host-pathogen interaction unveiled by immune, oxidative stress, and cytokine expression analysis to experimental Saprolegnia parasitica infection in Nile tilapia. Sci. Rep. 2023, 13, 9888. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.C.; Xie, M.Q.; Zhu, X.Q.; Li, A.X. Protective immunity in grouper (Epinephelus coioides) following exposure to or injection with Cryptocaryon irritans. Fish Shellfish Immunol. 2007, 22, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.H.; Xie, M.Q.; Li, A.X. A novel protein isolated from the serum of rabbitfish (Siganus oramin) is lethal to Cryptocaryon irritans. Fish Shellfish Immunol. 2010, 29, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, J.; Luo, H.L.; Lu, G.L.; Li, Y.W.; Li, A.X. L-amino acid oxidase expression profile and biochemical responses of rabbitfish (Siganus oramin) after exposure to a high dose of Cryptocaryon irritans. Fish Shellfish Immunol. 2017, 69, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.M.; Liu, Y.; Li, Y.C.; Tian, G.; Guo, X.Y.; Lin, J.Q.; Bai, Y.L.; Xu, P.; Zhou, T. Effects of Cryptocaryon irritans infection on physiological and biochemical indexes and immune indexes in large yellow croaker (Larimichthys crocea). J. Fish. 2024, 48, 85–93. [Google Scholar]
- Cohen-Sánchez, A.; Box, A.; Valencia, J.M.; Pinya, S.; Tejada, S.; Sureda, A. Exploring the impact of high salinity and parasite infection on antioxidant and immune systems in Coris julis in the Pityusic Islands (Spain). Sci. Total Environ. 2024, 951, 175848. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.O.; Becker, A.G.; Bertuzzi, T.; Cunha, M.A.; Kochhann, D.; Finamor, I.A.; Riffel, A.P.; Llesuy, S.; Pavanato, M.A.; Baldisserotto, B. Oxidative stress parameters in silver catfish (Rhamdia quelen) juveniles infected with Ichthyophthirius multifiliis and maintained at different levels of water pH. Vet. Parasitol. 2011, 178, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Legrand, T.P.R.A.; Catalano, S.R.; Wos-Oxley, M.L.; Stephens, F.; Landos, M.; Bansemer, M.S.; Stone, D.A.J.; Qin, J.G.; Oxley, A.P.A. The Inner Workings of the Outer Surface: Skin and Gill Microbiota as Indicators of Changing Gut Health in Yellowtail Kingfish. Front. Microbiol. 2018, 8, 2664. [Google Scholar] [CrossRef]
- Schrallhammer, M.; Ferrantini, F.; Vannini, C.; Galati, S.; Schweikert, M.; Görtz, H.D.; Verni, F.; Petroni, G. ‘Candidatus Megaira polyxenophila’ gen. nov., sp. nov.: Considerations on evolutionary history, host range and shift of early divergent rickettsiae. PLoS ONE 2013, 8, e72581. [Google Scholar] [CrossRef]
- Lobo-da-Cunha, A.; Azevedo, C. Association between xenosomes and glycogen in the cytoplasm of the ciliate Ichthyophthirius multifiliis. Int. J. Endocytobiosis Cell Res. 1988, 5, 225–231. [Google Scholar]
- Aury, J.M.; Jaillon, O.; Duret, L.; Noel, B.; Jubin, C.; Porcel, B.M.; Ségurens, B.; Daubin, V.; Anthouard, V.; Aiach, N.; et al. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature 2006, 444, 171–178. [Google Scholar] [CrossRef]
- Fokin, S.I. Bacterial endocytobionts of ciliophora and their interactions with the host cell. Int. Rev. Cytol. 2002, 236, 181–249. [Google Scholar]
- Vannini, C.; Petroni, G.; Schena, A.; Verni, F.; Rosati, G. Well-established mutualistic associations between ciliates and prokaryotes might be more widespread and diversified than so far supposed. Eur. J. Protistol. 2003, 39, 481–485. [Google Scholar] [CrossRef]
- Quackenbush, R.L.; Burbach, J.A. Cloning and expression of DNA sequences associated with the killer trait of Paramecium tetraurelia stock 47. Proc. Natl. Acad. Sci. USA 1983, 80, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Fenchel, T.; Finlay, B.J. The biology of free-living anaerobic ciliates. Eur. J. Protistol. 1991, 26, 201–215. [Google Scholar] [CrossRef]
- Kari, Z.A. Abiotic and Biotic Factors Affecting the Immune System of Aquatic Species: A review. Comp. Immunol. Rep. 2025, 9, 200230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xie, J.; He, Y.; Shi, Q.; Gong, Q.; Zhao, W.; Qin, C.; Zhou, C. Metabolome and Metagenome Signatures Underlying the Differential Resistance of Percocypris pingi, Crucian Carp, and Yellow Catfish to Ichthyophthirius multifiliis Infection. Biology 2025, 14, 1546. https://doi.org/10.3390/biology14111546
Liu Y, Xie J, He Y, Shi Q, Gong Q, Zhao W, Qin C, Zhou C. Metabolome and Metagenome Signatures Underlying the Differential Resistance of Percocypris pingi, Crucian Carp, and Yellow Catfish to Ichthyophthirius multifiliis Infection. Biology. 2025; 14(11):1546. https://doi.org/10.3390/biology14111546
Chicago/Turabian StyleLiu, Yi, Jiang Xie, Yang He, Qingchao Shi, Quan Gong, Weihong Zhao, Chuanjie Qin, and Chuang Zhou. 2025. "Metabolome and Metagenome Signatures Underlying the Differential Resistance of Percocypris pingi, Crucian Carp, and Yellow Catfish to Ichthyophthirius multifiliis Infection" Biology 14, no. 11: 1546. https://doi.org/10.3390/biology14111546
APA StyleLiu, Y., Xie, J., He, Y., Shi, Q., Gong, Q., Zhao, W., Qin, C., & Zhou, C. (2025). Metabolome and Metagenome Signatures Underlying the Differential Resistance of Percocypris pingi, Crucian Carp, and Yellow Catfish to Ichthyophthirius multifiliis Infection. Biology, 14(11), 1546. https://doi.org/10.3390/biology14111546





