Virus-Specific Defense Responses in Sweetpotato: Transcriptomic Insights into Resistance and Susceptibility to SPFMV, SPCSV, and SPVD
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Infection Experiments
2.2. Viral Transcripts and vsiRNAs in Infected Plants
2.3. RNA-Seq Data Processing and Transcriptome Sequencing
2.4. Statistical Analysis
2.5. Functional Annotation and Enrichment Analysis
2.6. Identification of Potential Molecular Markers Underlying Viral Resistance and Susceptibility
3. Results
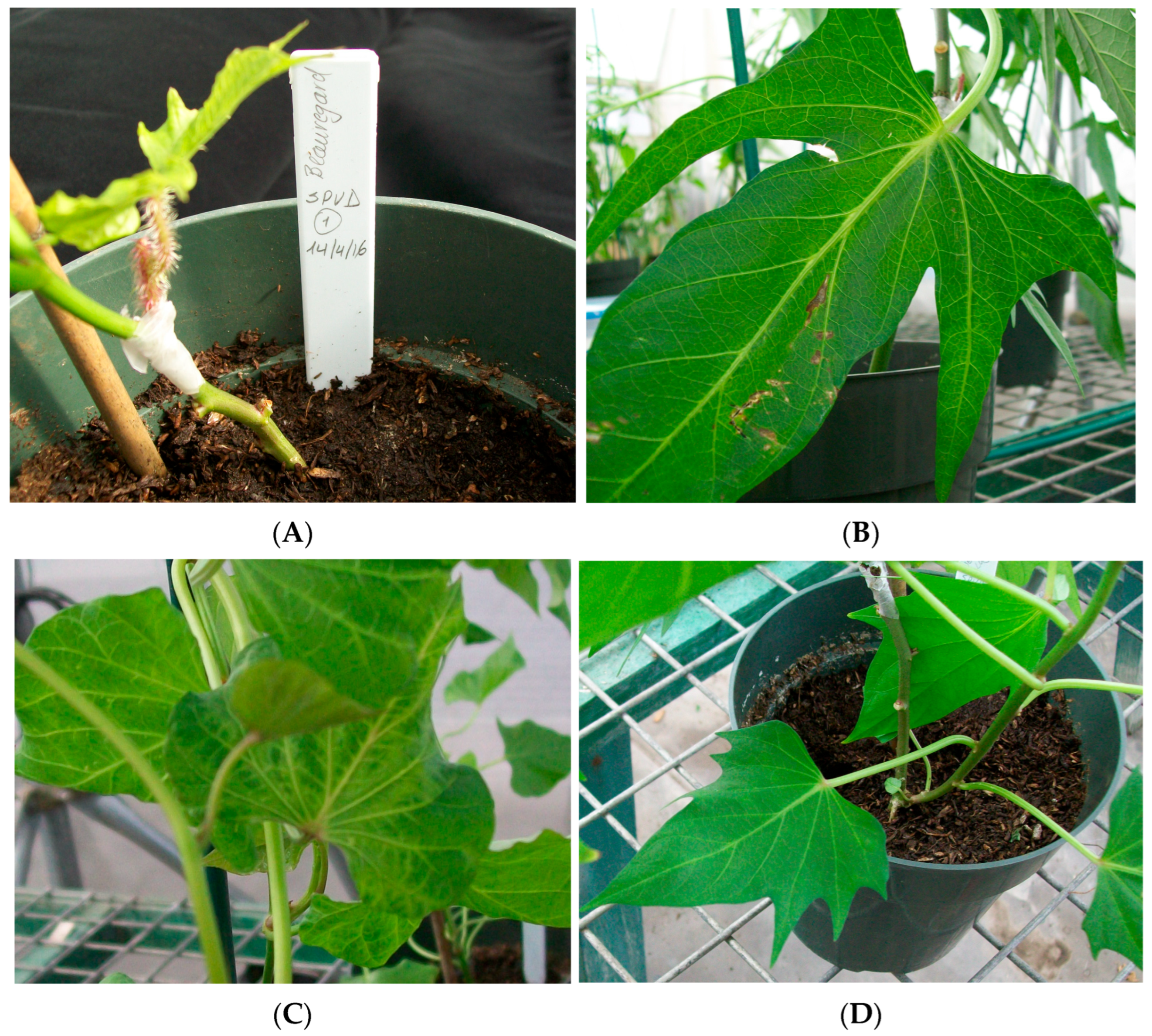
3.1. Symptoms of Sweetpotato Plants Infected
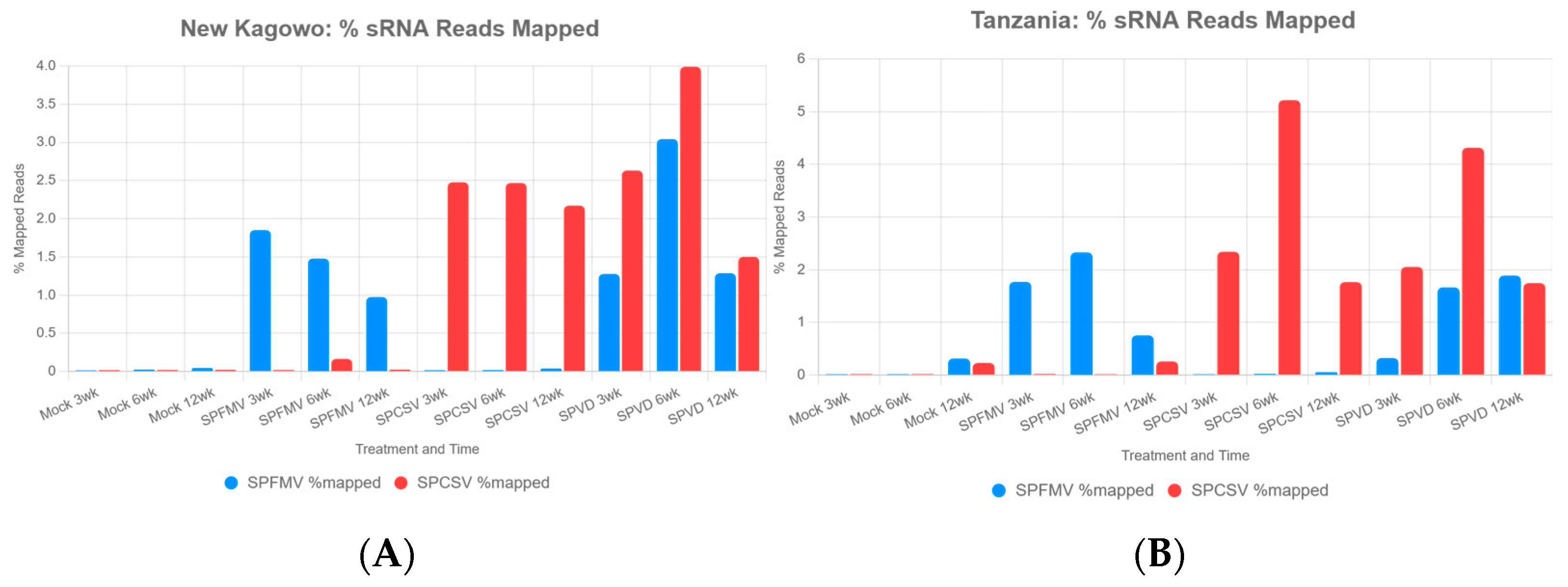
3.2. Small RNA Mapping to Viral Genomes in Sweetpotato
3.3. Gene Expression Following Infection
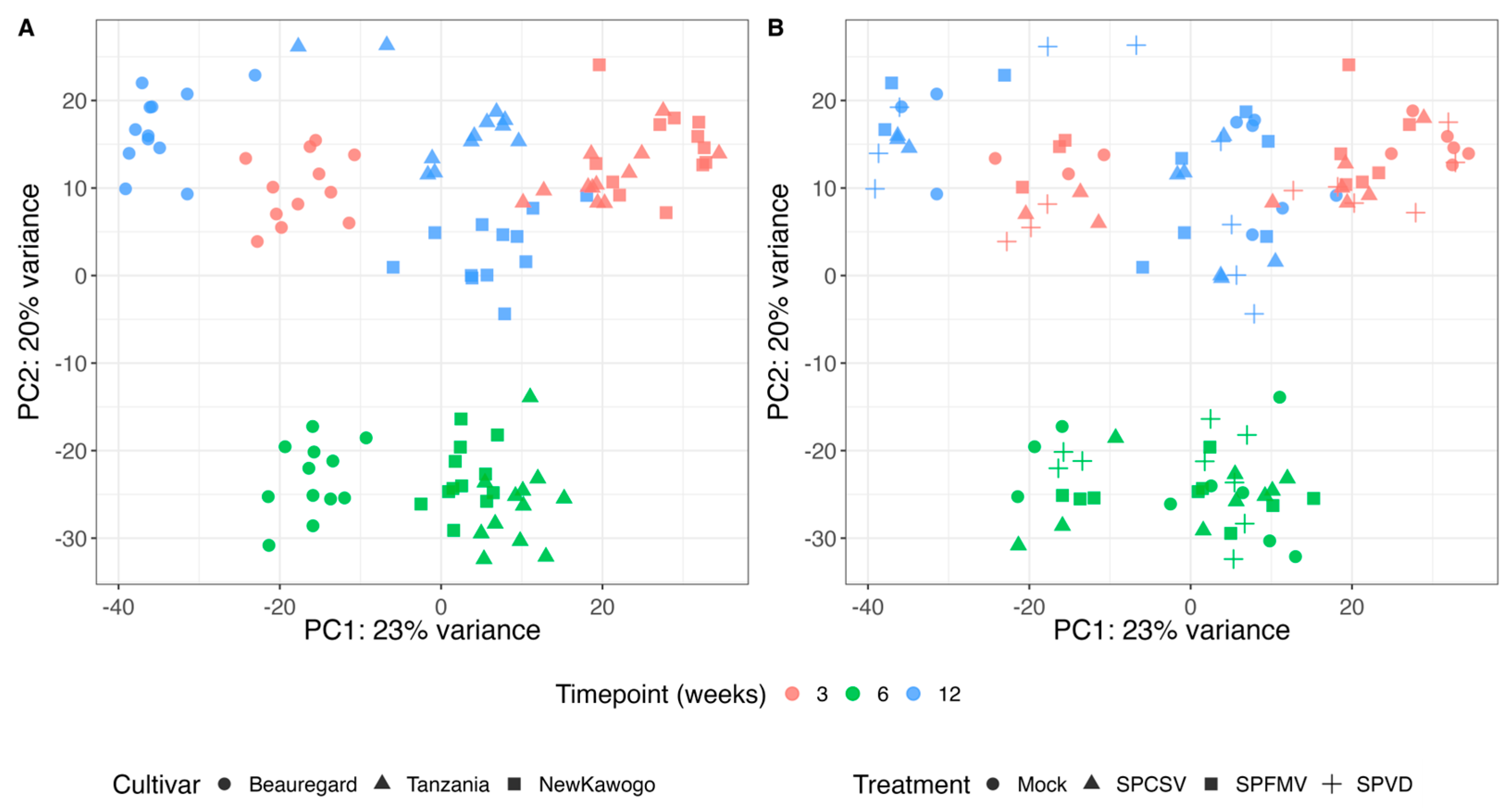
3.3.1. Principal Component Analysis (PCA)
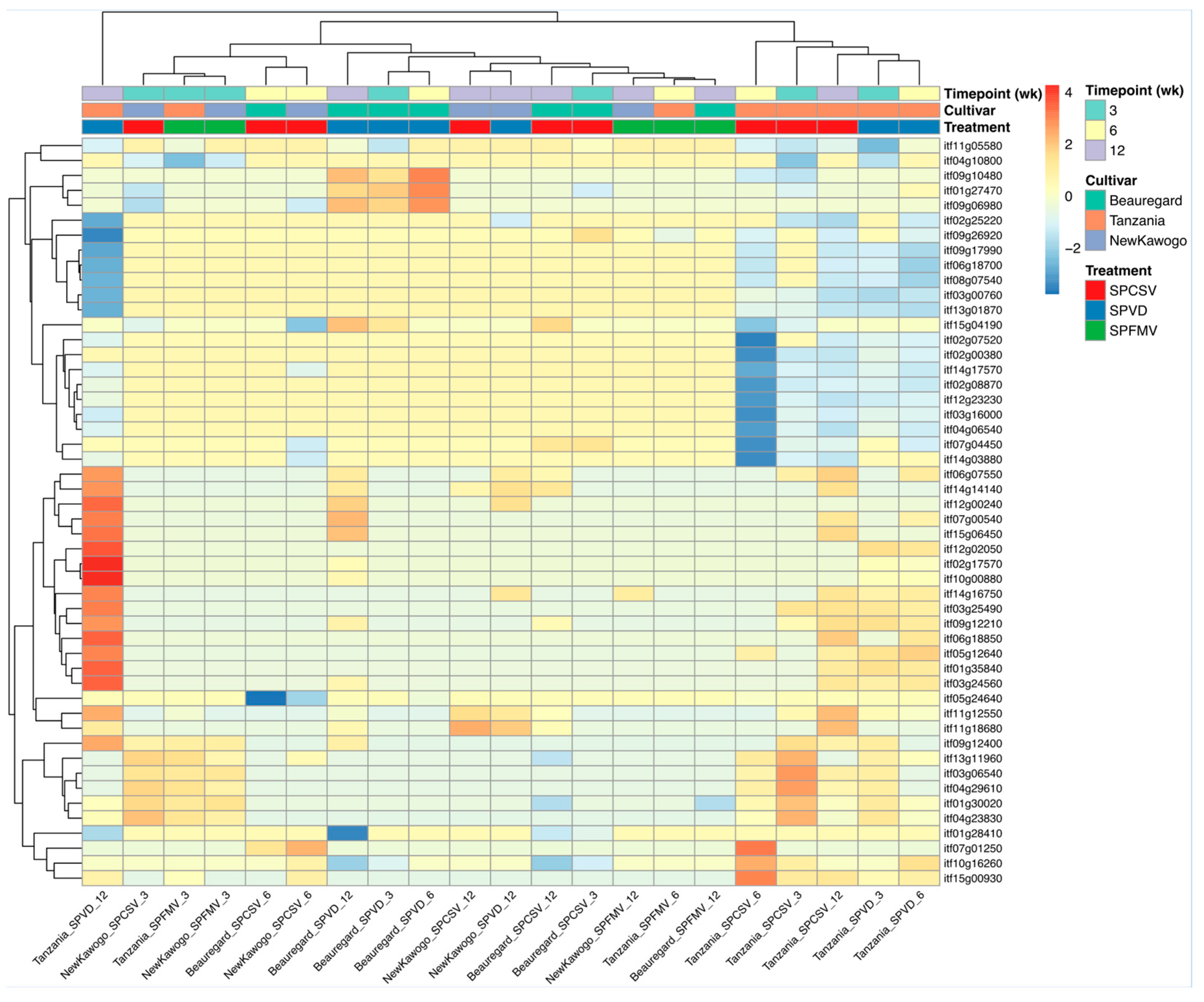
3.3.2. Hierarchical Clustering Identifies Discrete Transcriptional Response Modules
3.3.3. UpSet Analysis Reveals Complex DEG Sharing Patterns Across Experimental Conditions
3.3.4. Temporal Analysis Demonstrates Virus-Cultivar-Specific Responses
3.3.5. GO Enrichment Analysis Reveals Systematic Perturbation of Cellular Homeostasis
3.3.6. Defense Pathway Differential Expression Reveals Virus- and Cultivar-Specific Activation Signatures
3.3.7. Gene Expression in Viral Infections
3.3.8. Potential Molecular Markers for Viral Resistance and Susceptibility
Markers Associated with Moderate Tolerance in ‘New Kawogo’
Virus-Specific Immune Signatures in ‘Beauregard’
Markers Associated with Moderate Tolerance in ‘Tanzania’
Comparative Transcriptomic Analysis of ‘Beauregard’ and ‘New Kawogo’
Comparative Molecular Markers Underpinning Resistance and Susceptibility in Sweet Potato Cultivars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| et al. | And others |
| NGS | Next-generation sequencing |
| SPVD | Sweet potato virus disease |
| SPCSV | Sweet potato chlorotic stunt virus |
| SPFMV | Sweet potato feathery mottle virus |
| DEGs | Differentially expressed genes |
| miRNA | MicroRNA |
| SA | Salicylic acid |
| HSP pathways | Heat shock protein pathways |
| DNA | Deoxyribonucleic acid |
| WPI | Weeks post-inoculation or -infection |
| GO terms | Gene ontology terms |
| JA/ET | Jasmonic acid/ethylene |
| Redox | Reduction–oxidation balance |
| TCA cycle | Tricarboxylic acid cycle (also called the Krebs cycle) |
| PCA | Principal component analysis |
| SAR | Systemic acquired resistance |
| ABA | Abscisic acid signaling pathways |
| MAPK signaling | Mitogen-activated protein kinase signaling pathway |
| ATP | Adenosine triphosphate |
| NBS-LRR | Nucleotide-binding site leucine-rich repeat |
| CRISPR/Cas | Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein |
| QTLs | Quantitative trait loci |
| SUMOylation regulators | Enzymes that add small ubiquitin-like modifier (SUMO) proteins to other proteins, modifying their function, stability, or location |
References
- Low, J.W.; Mwanga, R.O.M.; Andrade, M.; Carey, E.; Ball, A.M. Tackling vitamin A deficiency with biofortified sweetpotato in sub-Saharan Africa. Glob. Food Secur. 2017, 14, 23–30. [Google Scholar] [CrossRef]
- Sugata, M.; Lin, C.Y.; Shih, Y.C. Anti-inflammatory and anticancer activities of Taiwanese purple-fleshed sweet potatoes (Ipomoea batatas L. Lam) Extracts. Biomed. Res. Int. 2015, 2015, 768093. [Google Scholar] [CrossRef] [PubMed]
- De los Ángeles Rosell, M.; Quizhpe, J.; Ayuso, P.; Peñalver, R.; Nieto, G. Proximate Composition, Health Benefits, and Food Applications in Bakery Products of Purple-Fleshed Sweet Potato (Ipomoea batatas L.) and Its By-Products: A Comprehensive Review. Antioxidants 2024, 13, 954. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO), Food and Agriculture Organization Statistical Databases (FAOSTAT), 2012 to 2024. Available online: http://www.fao.org/faostat/en/ (accessed on 1 March 2025).
- Adikini, S.; Mukasa, S.B.; Mwanga, R.O.M.; Gibson, R.W. Sweet potato cultivar degeneration rate under high and low sweet potato virus disease pressure zones in Uganda. Can. J. Plant Pathol. 2015, 37, 136–147. [Google Scholar] [CrossRef]
- Kreuze, J.F.; Perez, A.; Untiveros, M.; Quispe, D.; Fuentes, S.; Barker, I.; Simon, R. Complete viral genome sequence and discovery of novel viruses by deep sequencing of small RNAs: A generic method for diagnosis, discovery and sequencing of viruses. Virology 2009, 388, 1–7. [Google Scholar] [CrossRef]
- Adero, J.; Akongo, G.O.; Yada, B.; Byarugaba, D.K.; Kitavi, M.; Bua, B.; Yencho, G.C.; Otema, M.A. Sweet potato virus disease and its associated vectors: Farmers’ knowledge and management practices in Uganda. J. Agric. Sci. 2024, 16, 83. [Google Scholar] [CrossRef]
- Yada, B.; Alajo, A.; Ssemakula, G.N.; Mwanga, R.O.M.; Brown-Guedira, G.; Yencho, G.C. Selection of simple sequence repeat markers associated with inheritance of sweetpotato virus disease resistance in sweetpotato. Crop Sci. 2017, 57, 1421–1430. [Google Scholar] [CrossRef]
- Gurmu, F.; Hussein, S.; Laing, M. Self- and cross-incompatibilities in sweetpotato and their implications on breeding. Aust. J. Crop Sci. 2013, 7, 2074–2078. [Google Scholar]
- Gruner, K.; Griebel, T.; Návarová, H.; Attaran, E.; Zeier, J. Reprogramming of plants during systemic acquired resistance. Front. Plant Sci. 2013, 4, 252. [Google Scholar] [CrossRef]
- Pérez-de-Castro, A.M.; Vilanova, S.; Cañizares, J.; Pascual, L.; Blanca, J.M.; Díez, J.P.; Prohens, J.; Picó, B. Application of Genomic Tools in Plant Breeding. Curr. Genom. 2012, 13, 179–195. [Google Scholar] [CrossRef]
- Wu, S.; Lau, K.H.; Cao, Q.; Hamilton, J.P.; Sun, H.; Zhou, C.; Eserman, L.; Gemenet, D.C.; Olukolu, B.A.; Wang, H.; et al. Genome sequences of two diploid wild relatives of cultivated sweetpotato reveal targets for genetic improvement. Nat. Commun. 2018, 9, 4580. [Google Scholar] [CrossRef]
- Monden, Y.; Tahara, M. Genetic linkage analysis using DNA markers in sweetpotato. Breed. Sci. 2017, 67, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Ponniah, S.K.; Thimmapuram, J.; Bhide, K.; Kalavacharla, V.K.; Manoharan, M. Comparative analysis of the root transcriptomes of cultivated sweetpotato (Ipomoea batatas [L.] Lam) and its wild ancestor (Ipomoea trifida [Kunth] G. Don). BMC Plant Biol. 2017, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, R.; David, M.; Fuentes, S.; Kreuze, J.; Fei, Z. Transcriptome analysis provides insights into the responses of sweet potato to sweet potato virus disease (SPVD). Virus Res. 2021, 295, 198293. [Google Scholar] [CrossRef] [PubMed]
- McGregor, C.E.; Miano, D.W.; Labonte, D.R.; Hoy, M.; Clark, C.A.; Rosa, G.J.M. Differential gene expression of resistant and susceptible sweetpotato plants after infection with the causal agents of sweet potato virus disease. J. Am. Soc. Hortic. Sci. 2009, 134, 658–666. [Google Scholar] [CrossRef]
- Li, J.; He, L.; Zhang, Y.; Xue, C.; Cao, Y. A novel method for genome-wide profiling of dynamic host-pathogen interactions using 3′ end enriched RNA-seq. Sci. Rep. 2017, 7, 8681. [Google Scholar] [CrossRef]
- Heidary, M.; Kakhki, M.P. TRIzol-based RNA extraction: A reliable method for gene expression studies. J. Sci. Islam. Repub. Iran. 2014, 25, 13–17. [Google Scholar]
- Zhong, S.; Joung, J.G.; Zheng, Y.; Chen, Y.R.; Liu, B.; Shao, Y.; Xiang, J.Z.; Fei, Z.; Giovannoni, J.J. High-throughput illumina strand-specific RNA sequencing library preparation. Cold Spring Harb. Protoc. 2011, 6, 940–949. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, S.; Padmanabhan, C.; Li, R.; Galvez, M.; Gutierrez, D.; Fuentes, S.; Ling, K.S.; Kreuze, J.; Fei, Z. VirusDetect: An automated pipeline for efficient virus discovery using deep sequencing of small RNAs. Virology 2017, 500, 130–138. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2013, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 1 March 2025).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Bateman, A.; Marshall, M.; Khanna, A.; Eddy, S.R. Rfam: An RNA family database. Nucleic Acids Res. 2003, 31, 439–441. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Li, W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Conesa, A.; García-Alcalde, F. Qualimap 2: Advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics 2016, 32, 292–294. [Google Scholar] [CrossRef]
- Love, M.I.; Soneson, C.; Hickey, P.F.; Johnson, L.K.; Pierce, N.T.; Shepherd, L.; Morgan, M.; Patro, R. Tximeta: Reference sequence checksums for provenance identification in RNA-seq. PLoS Comput. Biol. 2020, 16, e1007664. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Schurch, N.J.; Schofield, P.; Gierliński, M.; Cole, C.; Sherstnev, A.; Singh, V.; Wrobel, N.; Gharbi, K.; Simpson, G.G.; Owen-Hughes, T.; et al. How many biological replicates are needed in an RNA-seq experiment and which differential expression tool should you use? RNA 2016, 22, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Vilo, J.; Peterson, H. gprofiler2—An R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000 Res. 2020, 9, 709. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, J.H.; Guo, H.S. Recent advances in understanding plant antiviral RNAi and viral suppressors of RNAi. Curr. Opin. Virol. 2021, 46, 65–72. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, H.; Jiang, M.; Zhong, Z.; Cheng, L. Recent Insights into Molecular Breeding for High Yield Sweet Potato Cultivars. Biosci. Methods 2025, 16, 23–32. [Google Scholar] [CrossRef]
- Mitra, A.; Skrzypczak, M.; Ginalski, K.; Rowicka, M. Strategies for achieving high sequencing accuracy for low diversity samples and avoiding sample bleeding using Illumina platform. PLoS ONE 2015, 10, e0120520. [Google Scholar] [CrossRef]
- Ssamula, A.; Okiror, A.; Avrahami-Moyal, L.; Tam, Y.; Gaba, V.; Gibson, R.W.; Gal-On, A.; Mukasa, S.B.; Wasswa, P. Factors influencing reversion from virus infection in sweetpotato. Ann. Appl. Biol. 2020, 176, 109–121. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, T. Unraveling the mechanisms of virus-induced symptom. Plants 2023, 12, 2830. [Google Scholar] [CrossRef]
- Bua, B.; Adipala, E.; Gibson, R. Reaction of sweetpotato landraces to sweetpotato virus disease in Uganda. Afr. Crop Sci. J. 2006, 14, 197–205. [Google Scholar]
- Gong, Q.; Wang, Y.; Jin, Z.; Hong, Y.; Liu, Y. Transcriptional and post-transcriptional regulation of RNAi-related gene expression during plant-virus interactions. Stress Biol. 2022, 2, 33. [Google Scholar] [CrossRef]
- Gasura, E.; Mukasa, S. Prevalence and implications of sweetpotato recovery from sweet potato virus disease in Uganda. Afr. Crop Sci. J. 2011, 18. [Google Scholar] [CrossRef]
- Baulcombe, D. RNA silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef]
- Kamthan, A.; Chaudhuri, A.; Kamthan, M.; Datta, A. Small RNAs in plants: Recent development and application for crop improvement. Front. Plant Sci. 2015, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, X.; Zhou, G.; Zhang, T. Engineering plant virus resistance: From RNA silencing to genome editing strategies. Plant Biotechnol. J. 2020, 18, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Mlotshwa, S.; Pruss, G.J.; Peragine, A.; Endres, M.W.; Li, J.; Chen, X.; Poethig, R.S.; Bowman, L.H.; Vance, V. Dicer-like2 plays a primary role in transitive silencing of transgenes in Arabidopsis. PLoS ONE 2008, 3, e1755. [Google Scholar] [CrossRef]
- Deleris, A.; Gallego-Bartolome, J.; Bao, J.; Kasschau, K.D.; Carrington, J.C.; Voinnet, O. Hierarchical Action and Inhibition of Plant Dicer-Like Proteins in Antiviral Defense. Science 2006, 313, 68–71. [Google Scholar] [CrossRef]
- Prins, M.; Laimer, M.; Noris, E.; Schubert, J.; Wassenegger, M.; Tepfer, M. Strategies for antiviral resistance in transgenic plants. Mol. Plant Pathol. 2008, 9, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Untiveros, M.; Quispe, D.; Kreuze, J. Analysis of complete genomic sequences of isolates of the Sweet potato feathery mottle virus strains C and EA: Molecular evidence for two distinct potyvirus species and two P1 protein domains. Arch. Virol. 2010, 155, 2059–2063. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, L.; Wu, W.; He, L.; Yang, X.; Pan, Q. Function and evolution of Magnaporthe oryzae avirulence gene AvrPib responding to the rice blast resistance gene Pib. Sci. Rep. 2015, 5, 11642. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liao, J.; Huang, W.; Qin, N. Salicylic acid protects sweet potato seedlings from drought stress by mediating abscisic acid-related gene expression and enhancing the antioxidant defense system. Int. J. Mol. Sci. 2022, 23, 14819. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Son, H.; Baek, J.H. Tricarboxylic acid (Tca) cycle intermediates: Regulators of immune responses. Life 2021, 11, 69. [Google Scholar] [CrossRef]
- Bolton, M. Primary Metabolism and Plant Defense—Fuel for the Fire. Mol. Plant-Microbe Interact. 2009, 22, 487–497. [Google Scholar] [CrossRef]
- He, W.Z.; Zhao, L.; Sun, K.; Feng, Z.; Zhou, G.; Rao, Q. Transcriptomic profiling reveals the complex interaction between a bipartite begomovirus and a cucurbitaceous host plant. BMC Genom. 2024, 25, 876. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, S.C.; Ji, C.Y.; Park, S.; Jeong, J.C.; Lee, H.S.; Kwak, S.S. Molecular characterization of biotic and abiotic stress-responsive MAP kinase genes, IbMPK3 and IbMPK6, in sweetpotato. Plant Physiol. Biochem. 2016, 108, 37–48. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N.S. Roles of plant hormones in the regulation of host-virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Du, X.; Duan, H.; Zhen, W.; Zhang, Y.; Shi, Z.; He, M.; Li, R. Transcriptomic and Metabolomic Analyses Reveal the Response to Short-Term Drought Stress in Bread Wheat (Triticum aestivum L.). Agronomy 2024, 14, 704. [Google Scholar] [CrossRef]
- Ma, P.; Guo, G.; Xu, X.; Luo, T.; Sun, Y.; Tang, X.; Heng, W.; Jia, B.; Liu, L. Transcriptome analysis reveals key genes involved in the response of Pyrus betuleafolia to drought and high-temperature stress. Plants 2024, 13, 309. [Google Scholar] [CrossRef]
- Li, F.; Wang, A. RNA decay is an antiviral defense in plants that is counteracted by viral RNA silencing suppressors. PLoS Pathog. 2018, 14, e1007228. [Google Scholar] [CrossRef]
- Ding, S.W.; Voinnet, O. Antiviral Immunity Directed by Small RNAs. Cell 2007, 130, 413–426. [Google Scholar] [CrossRef]
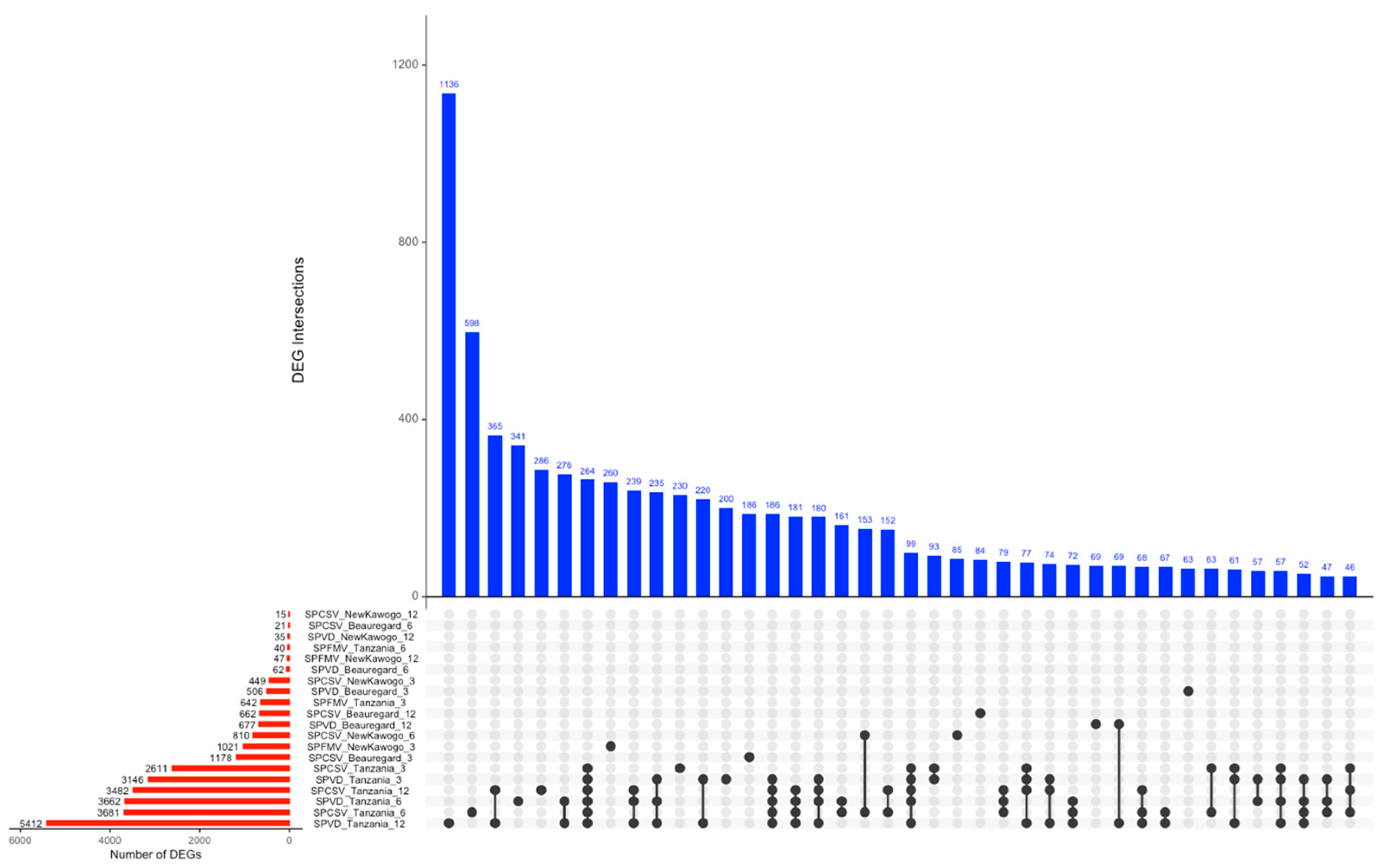
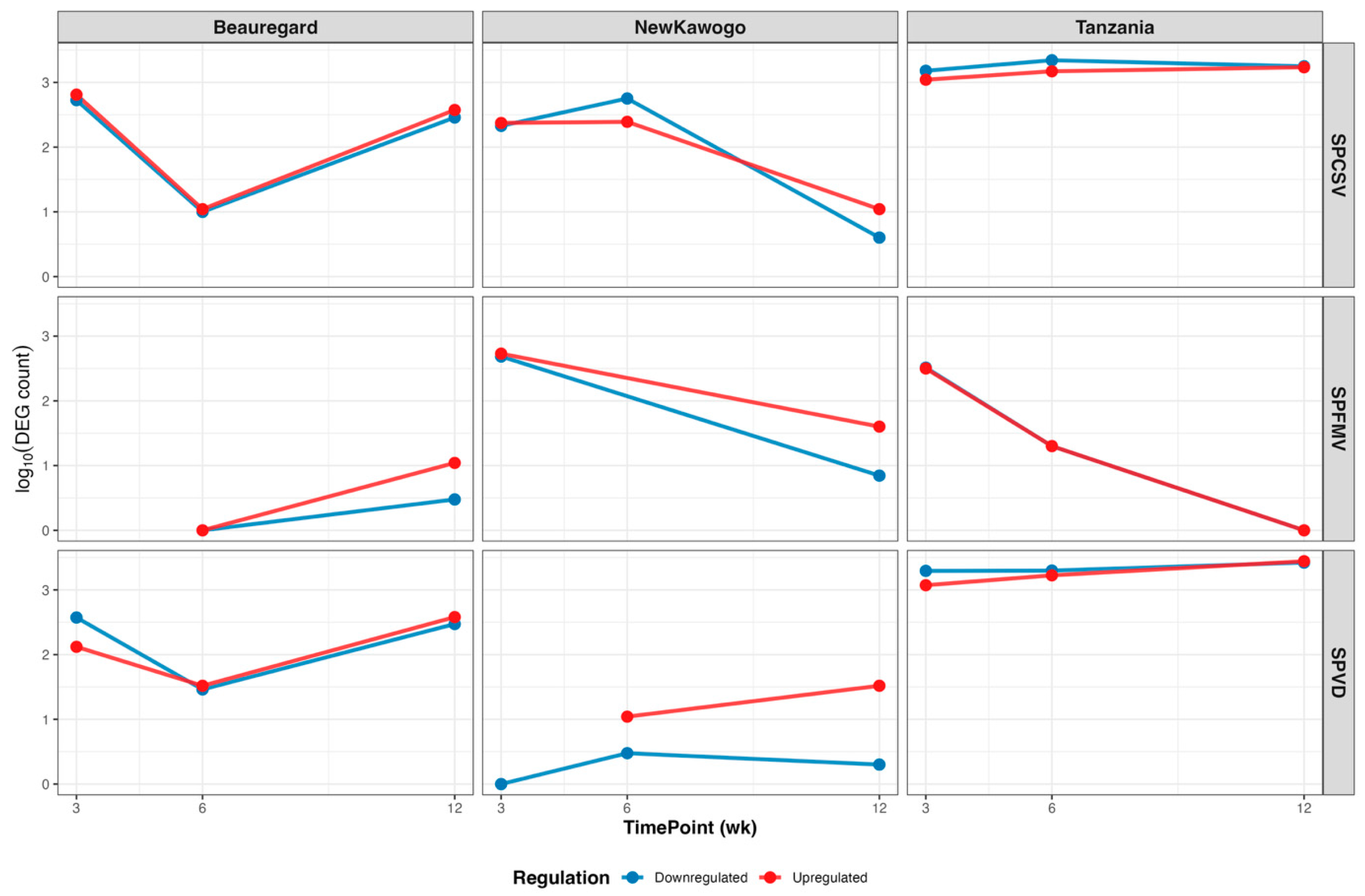
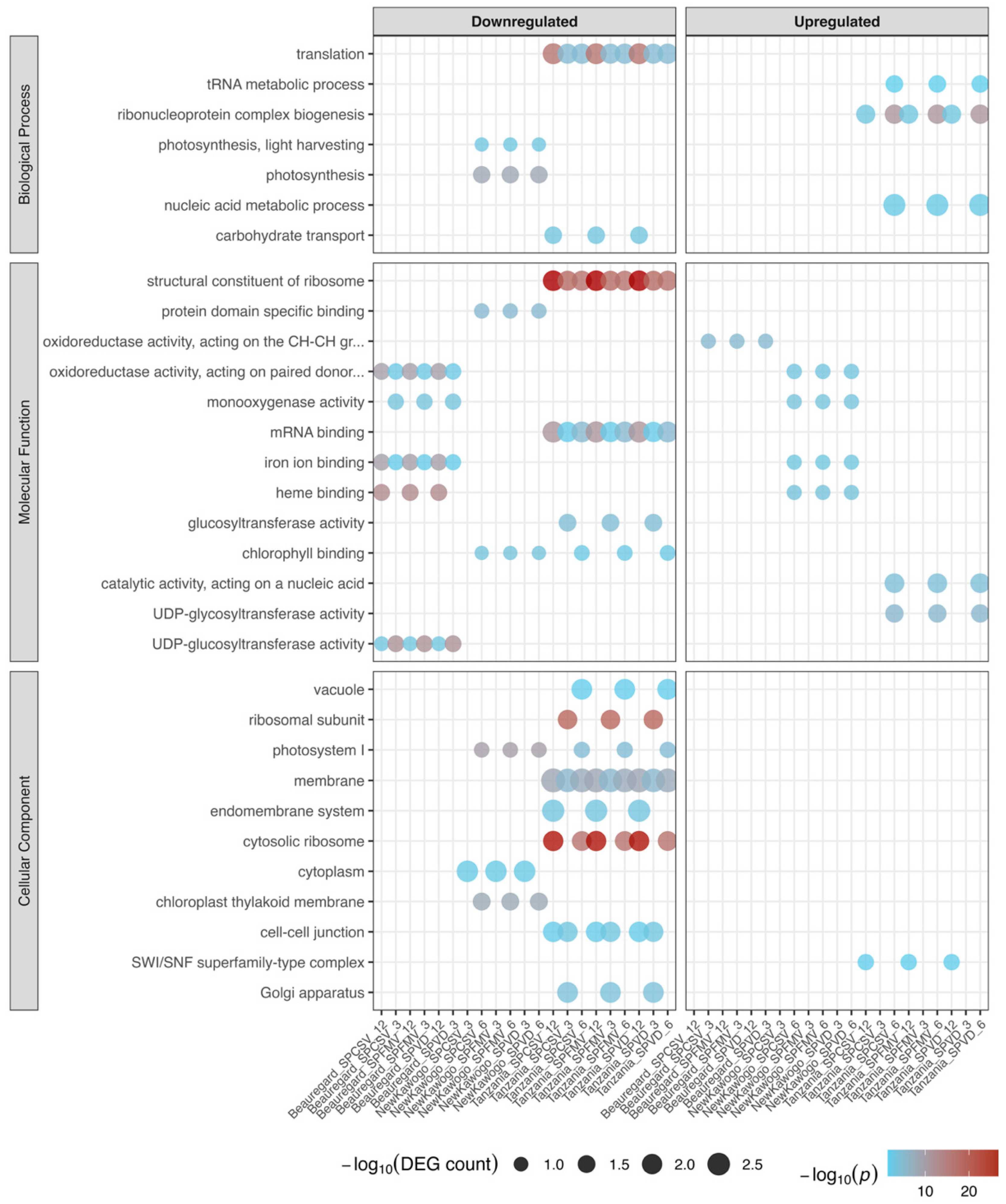
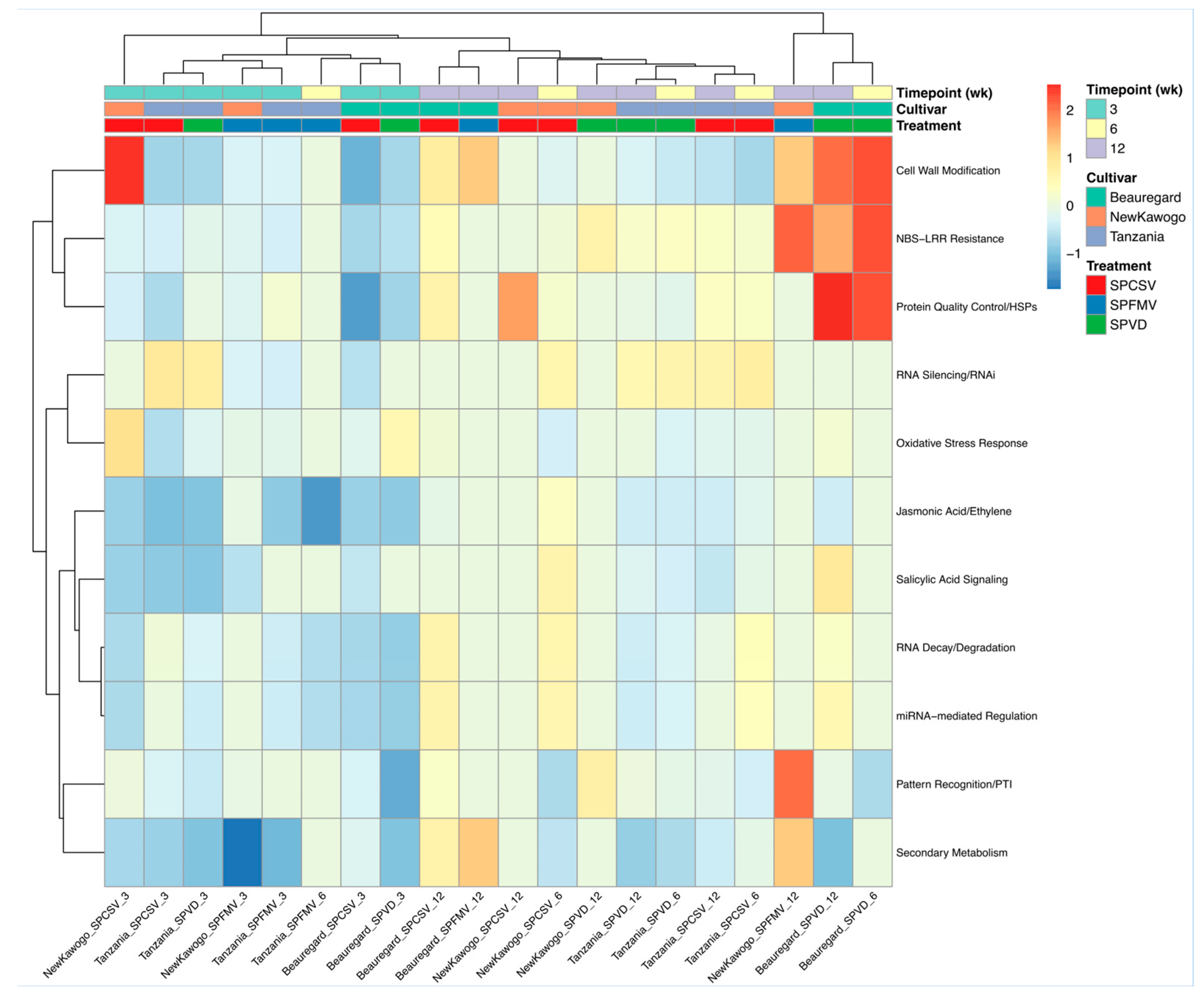
| Virus | Time (Week) | Cultivar | Downregulated Genes | Upregulated Genes |
|---|---|---|---|---|
| SPCSV | 3 | ‘Beauregard’ | 68 | 99 |
| SPCSV | 3 | ‘Tanzania’ | 149 | 82 |
| SPCSV | 3 | ‘New Kawogo’ | 33 | 37 |
| SPCSV | 6 | ‘Beauregard’ | 5 | 5 |
| SPCSV | 6 | ‘Tanzania’ | 514 | 168 |
| SPCSV | 6 | ‘New Kawogo’ | 108 | 40 |
| SPCSV | 12 | ‘Beauregard’ | 34 | 37 |
| SPCSV | 12 | ‘Tanzania’ | 120 | 42 |
| SPCSV | 12 | ‘New Kawogo’ | 1 | 9 |
| SPFMV | 3 | ‘Beauregard’ | 0 | 0 |
| SPFMV | 3 | ‘Tanzania’ | 13 | 6 |
| SPFMV | 3 | ‘New Kawogo’ | 42 | 52 |
| SPFMV | 6 | ‘Beauregard’ | 0 | 0 |
| SPFMV | 6 | ‘Tanzania’ | 0 | 1 |
| SPFMV | 6 | ‘New Kawogo’ | 0 | 0 |
| SPFMV | 12 | ‘Beauregard’ | 0 | 0 |
| SPFMV | 12 | ‘Tanzania’ | 0 | 0 |
| SPFMV | 12 | ‘New Kawogo’ | 0 | 0 |
| SPVD | 3 | ‘Beauregard’ | 93 | 31 |
| SPVD | 3 | ‘Tanzania’ | 277 | 43 |
| SPVD | 3 | ‘New Kawogo’ | 1 | 0 |
| SPVD | 6 | ‘Beauregard’ | 9 | 16 |
| SPVD | 6 | ‘Tanzania’ | 178 | 89 |
| SPVD | 6 | ‘New Kawogo’ | 1 | 0 |
| SPVD | 12 | ‘Beauregard’ | 52 | 73 |
| SPVD | 12 | ‘Tanzania’ | 659 | 577 |
| SPVD | 12 | ‘New Kawogo’ | 0 | 2 |
| # | Gene Function/GO Term | Timepoint | Pathogen | Resistance Function | Gene |
|---|---|---|---|---|---|
| 1 | DNA-binding TFs (RNA Pol II-specific) | 3–12 WPI | SPFMV/SPCSV | Sustained transcriptional activation of immune genes | itf02g15710 |
| 2 | ATP-binding proteins | 3–12 WPI | SPFMV/SPCSV | Energy-efficient signaling and stress response | itf12g25640, itf12g25640, itf05g04270 |
| 3 | mRNA-binding proteins | 3 WPI | SPFMV/SPCSV | Post-transcriptional regulation and immune priming | itf03g16440, itf09g14950 |
| 4 | Monooxygenases/oxidoreductases | 6 WPI | SPFMV/SPCSV | ROS detoxification and redox balance | itf06g16700 |
| 5 | Systemic acquired resistance (SAR) markers | 6 WPI | SPFMV/SPCSV | Long-range immune signaling | itf01g07140, itf12g22230 |
| 6 | Glyoxylate cycle enzymes (e.g., malate synthase) | 12 WPI | SPFMV/SPCSV | Energy metabolism adaptation and recovery | itf07g01900 |
| 7 | Protein kinases (MAPK, LRR-like) | 6–12 WPI | SPFMV/SPCSV | Immune signal transduction | itf03g07330 |
| 8 | Photoreceptor-related transcription factors | 12 WPI | SPFMV/SPCSV | Environmental sensing and late-phase regulation | itf04g14470 |
| 9 | Peroxisomal enzymes (e.g., catalase) | 6 WPI | SPFMV/SPCSV | ROS detoxification | itf12g02300 |
| 10 | DNA repair and chromatin regulators | 12 WPI | SPFMV/SPCSV | Genome integrity during stress recovery | itf02g21900 |
| # | Gene Function/GO Term | Timepoint | Pathogen | Susceptibility Indicator | Gene |
|---|---|---|---|---|---|
| 1 | DNA-binding transcription factors | 3 and 12 WPI | SPCSV/SPVD | Downregulated: impairs immune gene activation | itf02g15710 |
| 2 | Glycosyltransferase activity | 3, 6, 12 WPI | SPVD | Suppressed: limits structural defenses and metabolite biosynthesis | itf00g21310, itf06g06410 |
| 3 | 1-aminocyclopropane-1-carboxylate synthase | 3 WPI | SPVD | Suppressed: disrupts ethylene biosynthesis and stress signaling | itf12g18840 |
| 4 | Abscisic acid (ABA) binding and signaling | 12 WPI | SPVD | Downregulated: weakens hormonal regulation of stress responses | itf12g11260 |
| 5 | ATP-binding and ATP hydrolysis activity | 3 WPI | SPCSV | Suppressed: impairs energy management | itf12g25640 |
| 6 | SUMOylation enzymes | 12 WPI | SPVD | Repression: limits protein regulation under stress | itf08g08490 |
| 7 | Metal ion (iron, zinc, heme) binding | 3–12 WPI | SPCSV/SPVD | Suppressed: disrupts enzymatic activity and redox balance | itf03g17170, itf13g04600 |
| 8 | Sucrose synthase activity | 6 WPI | SPVD | Downregulated: disrupts sugar metabolism and energy provisioning | itf02g04900 |
| 9 | Monooxygenases/oxidoreductases | 12 WPI | SPVD | Suppressed: limits ROS detoxification and immune signaling | |
| 10 | Protein phosphatase inhibitors | 12 WPI | SPVD | Repressed: affects immune signal modulation | itf12g11260, itf15g22590 |
| # | Gene Function/GO Term | Timepoint | Pathogen | Susceptibility Indicator | Gene |
|---|---|---|---|---|---|
| 1 | DNA-binding transcription factors | 3 and 12 WPI | SPCSV/SPVD | Downregulated: impairs immune gene activation | itf02g15710 |
| 2 | Glycosyltransferase activity | 3, 6, 12 WPI | SPVD | Suppressed: limits structural defenses and metabolite biosynthesis | itf00g21310, itf06g06410 |
| 3 | 1-aminocyclopropane-1-carboxylate synthase | 3 WPI | SPVD | Suppressed: disrupts ethylene biosynthesis and stress signaling | itf12g18840 |
| 4 | Abscisic acid (ABA) binding and signaling | 12 WPI | SPVD | Downregulated: weakens hormonal regulation of stress responses | itf12g11260 |
| 5 | ATP-binding and ATP hydrolysis activity | 3 WPI | SPCSV | Suppressed: impairs energy management | itf12g25640 |
| 6 | SUMOylation enzymes | 12 WPI | SPVD | Repressed: limits protein regulation under stress | itf08g08490 |
| 7 | Metal ion (iron, zinc, heme) binding | 3–12 WPI | SPCSV/SPVD | Suppressed: disrupts enzymatic activity and redox balance | itf03g17170, itf13g04600 |
| 8 | Sucrose synthase activity | 6 WPI | SPVD | Downregulated: disrupts sugar metabolism and energy provisioning | itf02g04900 |
| 9 | Monooxygenases/oxidoreductases | 12 WPI | SPVD | Suppressed: limits ROS detoxification and immune signaling | itf06g16700 |
| 10 | Protein phosphatase inhibitors | 12 WPI | SPVD | Repressed: affects immune signal modulation | itf12g11260, itf15g22590 |
| 11 | Ubiquitin-mediated protein degradation | 3 WPI | SPVD | Downregulated: limits protein turnover and stress response | itf01g33310 |
| 12 | Photosystem stoichiometry adjustment | 12 WPI | SPVD | Upregulated; compensates energy conversion imbalance | itf03g10900 |
| 13 | Chromatin remodeling and gene regulation | 12 WPI | SPVD | Upregulated; enhances transcriptional flexibility under stress | itf05g18650 |
| 14 | Polycomb repressive complex activity | 12 WPI | SPVD | Upregulated: regulates chromatin structure and gene silencing | itf14g19980 |
| 15 | MAPK signaling cascade | 6 WPI | SPVD | Upregulated: facilitates stress signaling and response | itf12g20570 |
| 16 | Small GTPase activation | 12 WPI | SPVD | Upregulated: promotes cellular signaling and vesicle trafficking | itf09g25080 |
| Functional Category | ‘New Kawogo’ (Resistant) | ‘Beauregard’ (Susceptible) |
|---|---|---|
| Early Defense (3 WPI) | Selective suppression of lipids; ATP-binding and hypoxia response activated | Suppression of cell wall, lipid metabolism, and hormone signaling (panic) |
| Mid-Phase Response (6 WPI) | ROS detoxification, photosynthesis, SAR markers upregulated | Minimal recovery; SPVD suppresses growth and hormone pathways |
| Recovery Phase (12 WPI) | Full activation of DNA repair, glyoxylate cycle, mRNA surveillance | Delayed activation; chronic suppression of ABA, SUMO, sugar transport |
| Transcriptional Regulation | Early TFs and mRNA-binding proteins active | Late TF response; SUMOylation enzymes suppressed |
| Hormone Signaling | Maintains ABA and hypoxia responses, photoreceptors at recovery stag | ABA, JA, and Ca2+ signaling downregulated (especially under SPVD) |
| Energy Management | Glyoxylate/TCA cycle activated for efficient recovery | Sucrose metabolism genes suppressed |
| Redox and Detoxification | Monooxygenases and peroxisomal enzymes timely upregulated | Redox response delayed and incomplete |
| Transport and Structural Repair | Transporters, DNA repair, and membrane proteins reactivated by 12 WPI | Suppressed transporters, protein folding and phosphatase inhibitors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adero, J.; Ssali, R.; Segundo, F.; Maria, D.; Kitavi, M.; Yada, B.; Byarugaba, D.K.; Dube, F.; Aber, P.P.; Opiyo, S.O.; et al. Virus-Specific Defense Responses in Sweetpotato: Transcriptomic Insights into Resistance and Susceptibility to SPFMV, SPCSV, and SPVD. Biology 2025, 14, 1541. https://doi.org/10.3390/biology14111541
Adero J, Ssali R, Segundo F, Maria D, Kitavi M, Yada B, Byarugaba DK, Dube F, Aber PP, Opiyo SO, et al. Virus-Specific Defense Responses in Sweetpotato: Transcriptomic Insights into Resistance and Susceptibility to SPFMV, SPCSV, and SPVD. Biology. 2025; 14(11):1541. https://doi.org/10.3390/biology14111541
Chicago/Turabian StyleAdero, Joanne, Reuben Ssali, Fuentes Segundo, David Maria, Mercy Kitavi, Benard Yada, Denis Karuhize Byarugaba, Faruk Dube, Peace Proscovia Aber, Stephen Obol Opiyo, and et al. 2025. "Virus-Specific Defense Responses in Sweetpotato: Transcriptomic Insights into Resistance and Susceptibility to SPFMV, SPCSV, and SPVD" Biology 14, no. 11: 1541. https://doi.org/10.3390/biology14111541
APA StyleAdero, J., Ssali, R., Segundo, F., Maria, D., Kitavi, M., Yada, B., Byarugaba, D. K., Dube, F., Aber, P. P., Opiyo, S. O., Fei, Z., & Kreuze, J. F. (2025). Virus-Specific Defense Responses in Sweetpotato: Transcriptomic Insights into Resistance and Susceptibility to SPFMV, SPCSV, and SPVD. Biology, 14(11), 1541. https://doi.org/10.3390/biology14111541






