What Factors Shape the Flyability in Bats?—The Perspective from Bat’s Wing Development
Simple Summary
Abstract
1. Introduction
2. Fundamental Requirements for Powered Flight
2.1. Unique Powered Flight in Bats
2.2. Specialized Flight-Related Muscles in Bats
2.3. Efficient Energy Supply and Antioxidant Defense
2.4. Specialized Wings Adapted for Powered Flight
3. Wing Development: A Crucial Evolutionary Adaptation for Powered Flight
3.1. Wings Development During the Embryonic Stage Prepares for Powered Flight
3.2. Postnatal Wing Development Is Essential for the Full Realization of Powered Flight
4. Placing Wing and Powered Flight Development Within the Context of Echolocation Maturation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, T.K. On the origin of complex adaptive traits: Progress since the Darwin versus Mivart debate. J. Exp. Zool. Part B Mol. Dev. Evol. 2017, 328, 304–320. [Google Scholar] [CrossRef]
- Väinölä, R.; Johannesson, K. Genetic diversity and evolution. In Biological Oceanography of the Baltic Sea; Snoeijs-Leijonmalm, P., Schubert, H., Radziejewska, T., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 233–253. [Google Scholar]
- Stidsholt, L.; Hubancheva, A.; Greif, S.; Goerlitz, H.R.; Johnson, M.; Yovel, Y.; Madsen, P.T. Echolocating bats prefer a high risk-high gain foraging strategy to increase prey profitability. eLife 2023, 12, e84190. [Google Scholar] [CrossRef]
- Moss, C.F.; Sinha, S.R. Neurobiology of echolocation in bats. Curr. Opin. Neurobiol. 2003, 13, 751–758. [Google Scholar] [CrossRef]
- Reid, F.A.; Reid, F.A. Bats Order Chiroptera. In A Field Guide to the Mammals of Central America & Southeast Mexico; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Altringham, J.D.; Altringham, J.D. The evolution and diversity of bats. In Bats: Biology and Behaviour; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Domingos-Melo, A.; Albuquerque-Lima, S.; Diniz, U.M.; Lopes, A.V.; Machado, I.C. Bat pollination in the Caatinga: A review of studies and peculiarities of the system in the new world’s largest and most diverse seasonally dry tropical forest. Flora 2023, 305, 152332. [Google Scholar] [CrossRef]
- Petit, S.; Scanlon, A.T.; Naikatini, A.; Pukala, T.; Schumann, R. A novel bat pollination system involving obligate flower corolla removal has implications for global Dillenia conservation. PLoS ONE 2022, 17, e0262985. [Google Scholar] [CrossRef]
- Petit, S.; Scanlon, A.T.; Naikatini, A.; Pukala, T. Dillenia (Dilleniaceae) pollen heteromorphy and presentation, and implications for pollination by bats. Ecol. Evol. 2024, 14, e10997. [Google Scholar] [CrossRef]
- da Silveira, M.C.; Silveira, M.; Medeiros, L.S.; Aguiar, L.M.S. The role of feeding roosts in seed dispersal service bats provide in urban areas. Biotropica 2024, 56, e13291. [Google Scholar] [CrossRef]
- Regolin, A.L.; Muylaert, R.L.; Crestani, A.C.; Dáttilo, W.; Ribeiro, M.C. Seed dispersal by Neotropical bats in human-disturbed landscapes. Wildl. Res. 2021, 48, 1–6. [Google Scholar] [CrossRef]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, Y.; Papura, D.; Touzot, O.; Rhouy, N.; Sentenac, G.; Rusch, A. Pest control services provided by bats in vineyard landscapes. Agric. Ecosyst. Environ. 2021, 306, 107207. [Google Scholar] [CrossRef]
- Schäckermann, J.; Morris, E.J.; Alberdi, A.; Razgour, O.; Korine, C. The contribution of desert-dwelling bats to pest control in hyper-arid date agriculture. Diversity 2022, 14, 1034. [Google Scholar] [CrossRef]
- Almeida, F.C.; Amador, L.I.; Giannini, N.P. Explosive radiation at the origin of Old World fruit bats (Chiroptera, Pteropodidae). Org. Divers. Evol. 2021, 21, 231–243. [Google Scholar] [CrossRef]
- Simões, B.F.; Foley, N.M.; Hughes, G.M.; Zhao, H.; Zhang, S.; Rossiter, S.J.; Teeling, E.C. As blind as a bat? Opsin phylogenetics illuminates the evolution of color vision in bats. Mol. Biol. Evol. 2018, 36, 54–68. [Google Scholar] [CrossRef]
- Griffin, D.R.; Novick, A.; Kornfield, M. The sensitivity of echolocation in the fruit bat, Rousettus. Biol. Bull. 1958, 115, 107–113. [Google Scholar] [CrossRef]
- Smarsh, G.C.; Tarnovsky, Y.; Yovel, Y. Hearing, echolocation, and beam steering from day 0 in tongue-clicking bats. Proc. R. Soc. B Biol. Sci. 2021, 288, 20211714. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.R.; Galambos, R. The sensory basis of obstacle avoidance by flying bats. J. Exp. Zool. 1941, 86, 481–506. [Google Scholar] [CrossRef]
- Griffin, D.R. Listening in the Dark: The Acoustic Orientation of Bats and Men; Yale University Press: New Haven, CT, USA, 1958. [Google Scholar]
- Neuweiler, G. Evolutionary aspects of bat echolocation. J. Comp. Physiol. A 2003, 189, 245–256. [Google Scholar] [CrossRef]
- Shah, T.A.; Srinivasulu, C. Echolocation calls of some bats of Gujarat, India. Mammalia 2020, 84, 483–492. [Google Scholar] [CrossRef]
- Grinnell, A.D. Comparative auditory neurophysiology of neotropical bats employing different echolocation signals. Z. Vgl. Physiol. 1970, 68, 117–153. [Google Scholar] [CrossRef]
- Ito, T.; Yamamoto, R.; Furuyama, T.; Hase, K.; Kobayasi, K.I.; Hiryu, S.; Honma, S. Three forebrain structures directly inform the auditory midbrain of echolocating bats. Neurosci. Lett. 2019, 712, 134481. [Google Scholar] [CrossRef]
- Shen, Y.-Y.; Liang, L.; Li, G.-S.; Murphy, R.W.; Zhang, Y.-P. Parallel evolution of auditory genes for echolocation in bats and toothed whales. PLoS Genet. 2012, 8, e1002788. [Google Scholar] [CrossRef]
- Hechavarría, J.C.; Macías, S.; Vater, M.; Mora, E.C.; Kössl, M. Evolution of neuronal mechanisms for echolocation: Specializations for target-range computation in bats of the genus Pteronotus. J. Acoust. Soc. Am. 2013, 133, 570–578. [Google Scholar] [CrossRef]
- Moss, C.F. Auditory Mechanisms of Echolocation in Bats. 2018. Available online: https://oxfordre.com/neuroscience/display/10.1093/acrefore/9780190264086.001.0001/acrefore-9780190264086-e-102?print (accessed on 14 February 2024).
- Nnoka, C.; Hu, Y.; Grafe, U.; Bayandor, J.; Müller, R. An experimental array setup to study the integration of biosonar and maneuvering flight in bats. J. Acoust. Soc. Am. 2024, 155, A244. [Google Scholar] [CrossRef]
- Schnitzler, H.-U.; Kalko, E.K.V. Echolocation by insect-eating bats: We define four distinct functional groups of bats and find differences in signal structure that correlate with the typical echolocation tasks faced by each group. BioScience 2001, 51, 557–569. [Google Scholar] [CrossRef]
- Beetz, M.J.; Hechavarría, J.C.; Kössl, M. Temporal tuning in the bat auditory cortex is sharper when studied with natural echolocation sequences. Sci. Rep. 2016, 6, 29102. [Google Scholar] [CrossRef]
- Kohles, J.E.; Carter, G.G.; Page, R.A.; Dechmann, D.K.N. Socially foraging bats discriminate between group members based on search-phase echolocation calls. Behav. Ecol. 2020, 31, 1103–1112. [Google Scholar] [CrossRef]
- Sears, K.E. Molecular determinants of bat wing development. Cells Tissues Organs 2007, 187, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Eckalbar, W.L.; Schlebusch, S.A.; Mason, M.K.; Gill, Z.; Parker, A.V.; Booker, B.M.; Nishizaki, S.; Muswamba-Nday, C.; Terhune, E.; Nevonen, K.A.; et al. Transcriptomic and epigenomic characterization of the developing bat wing. Nat. Genet. 2016, 48, 528–536. [Google Scholar] [CrossRef]
- Cleland, T.P.; Wang, Z.; Wang, B.; Picu, C.R.; Vashishth, D. Mechano-chemical regulation of bat wing bones for flight. J. Mech. Behav. Biomed. Mater. 2021, 124, 104809. [Google Scholar] [CrossRef]
- Anderson, S.C.; Ruxton, G.D. The evolution of flight in bats: A novel hypothesis. Mammal Rev. 2020, 50, 426–439. [Google Scholar] [CrossRef]
- Maugoust, J.; Orliac, M.J. Anatomical correlates and nomenclature of the chiropteran endocranial cast. Anat. Rec. 2023, 306, 2791–2829. [Google Scholar] [CrossRef]
- Thiagavel, J.; Cechetto, C.; Santana, S.E.; Jakobsen, L.; Warrant, E.J.; Ratcliffe, J.M. Auditory opportunity and visual constraint enabled the evolution of echolocation in bats. Nat. Commun. 2018, 9, 98. [Google Scholar] [CrossRef]
- Nojiri, T.; Wilson, L.A.B.; López-Aguirre, C.; Tu, V.T.; Kuratani, S.; Ito, K.; Higashiyama, H.; Son, N.T.; Fukui, D.; Sadier, A.; et al. Embryonic evidence uncovers convergent origins of laryngeal echolocation in bats. Curr. Biol. 2021, 31, 1353–1365.e3. [Google Scholar] [CrossRef]
- Simmons, N.B.; Seymour, K.L.; Habersetzer, J.; Gunnell, G.F. Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature 2008, 451, 818–821. [Google Scholar] [CrossRef]
- Meng, J.; Hu, Y.; Wang, Y.; Wang, X.; Li, C. A Mesozoic gliding mammal from northeastern China. Nature 2006, 444, 889–893. [Google Scholar] [CrossRef]
- Simmons, N.B.; Seymour, K.L.; Habersetzer, J.; Gunnell, G.F. Inferring echolocation in ancient bats. Nature 2010, 466, E8. [Google Scholar] [CrossRef] [PubMed]
- Veselka, N.; McErlain, D.D.; Holdsworth, D.W.; Eger, J.L.; Chhem, R.K.; Mason, M.J.; Brain, K.L.; Faure, P.A.; Fenton, M.B. A bony connection signals laryngeal echolocation in bats. Nature 2010, 463, 939–942. [Google Scholar] [CrossRef]
- Giannini, N.P.; Cannell, A.; Amador, L.I.; Simmons, N.B. Palaeoatmosphere facilitates a gliding transition to powered flight in the Eocene bat, Onychonycteris finneyi. Commun. Biol. 2024, 7, 365. [Google Scholar] [CrossRef]
- Lauber, M.; Weymouth, G.D.; Limbert, G. Rapid flapping and fibre-reinforced membrane wings are key to high-performance bat flight. J. R. Soc. Interface 2023, 20, 20230466. [Google Scholar] [CrossRef]
- Riskin, D.K.; Bergou, A.; Breuer, K.S.; Swartz, S.M. Upstroke wing flexion and the inertial cost of bat flight. Proc. R. Soc. B Biol. Sci. 2012, 279, 2945–2950. [Google Scholar] [CrossRef]
- Gunnell, G.F.; Simmons, N.B. Fossil evidence and the origin of bats. J. Mamm. Evol. 2005, 12, 209–246. [Google Scholar] [CrossRef]
- Tokita, M.; Abe, T.; Suzuki, K. The developmental basis of bat wing muscle. Nat. Commun. 2012, 3, 1302. [Google Scholar] [CrossRef]
- Gardner, N.M.; Dececchi, T.A. Flight and echolocation evolved once in Chiroptera: Comments on ‘The evolution of flight in bats: A novel hypothesis’. Mammal Rev. 2022, 52, 284–290. [Google Scholar] [CrossRef]
- Feigin, C.Y.; Moreno, J.A.; Ramos, R.; Mereby, S.A.; Alivisatos, A.; Wang, W.; van Amerongen, R.; Camacho, J.; Rasweiler, J.J.; Behringer, R.R.; et al. Convergent deployment of ancestral functions during the evolution of mammalian flight membranes. Sci. Adv. 2023, 9, eade7511. [Google Scholar] [CrossRef]
- Anthwal, N.; Urban, D.J.; Sadier, A.; Takenaka, R.; Spiro, S.; Simmons, N.; Behringer, R.R.; Cretekos, C.J.; Rasweiler, J.J.; Sears, K.E. Insights into the formation and diversification of a novel chiropteran wing membrane from embryonic development. BMC Biol. 2023, 21, 101. [Google Scholar] [CrossRef]
- Ospina-Garcés, S.M.; Zamora-Gutierrez, V.; Lara-Delgado, J.M.; Morelos-Martínez, M.; Ávila-Flores, R.; Kurali, A.; Ortega, J.; Selem-Salas, C.I.; MacSwiney G., M.C. The relationship between wing morphology and foraging guilds: Exploring the evolution of wing ecomorphs in bats. Biol. J. Linn. Soc. 2024, 142, 481–498. [Google Scholar] [CrossRef]
- Pridmore, P.A.; Hoffmann, P.H. The aerodynamic performance of the feathertail glider, Acrobates pygmaeus (Marsupialia: Acrobatidae). Aust. J. Zool. 2014, 62, 80–99. [Google Scholar] [CrossRef]
- Panyutina, A.A.; Korzun, L.P.; Kuznetsov, A.N. Flight of Mammals: From Terrestrial Limbs to Wings; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Byrnes, G.; Spence, A.; Lim, N. Locomotor behavior of a free-ranging gliding mammal (Cynocephalus variegatus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, S143–S144. [Google Scholar] [CrossRef]
- Suzuki, K.; Yanagawa, H. Gliding patterns of Siberian flying squirrels in relation to forest structure. iForest-Biogeosci. For. 2019, 12, 114–117. [Google Scholar] [CrossRef]
- Bahlman, J.W.; Swartz, S.M.; Riskin, D.K.; Breuer, K.S. Glide performance and aerodynamics of non-equilibrium glides in northern flying squirrels (Glaucomys sabrinus). J. R. Soc. Interface 2013, 10, 20120794. [Google Scholar] [CrossRef]
- Johnson-Murray, J. The comparative myology of the gliding membranes of Acrobates, Petauroides and Petaurus contrasted with the cutaneous myology of Hemibelideus and Pseudocheirus (Marsupialia, Phalangeridae) and with selected gliding Rodentia (Sciuridae and Anamoluridae). Aust. J. Zool. 1987, 35, 101–113. [Google Scholar] [CrossRef]
- Burtner, A.E.; Grossnickle, D.M.; Santana, S.E.; Law, C.J. Gliding toward an understanding of the origin of flight in bats. PeerJ 2024, 12, e17824. [Google Scholar] [CrossRef]
- Gupta, B.B. The histology and musculature of plagiopatagium in bats. Mammalia 1967, 31, 313–321. [Google Scholar] [CrossRef]
- Runestad, J.A.; Ruff, C.B. Structural adaptations for gliding in mammals with implications for locomotor behavior in paromomyids. Am. J. Phys. Anthropol. 1995, 98, 101–119. [Google Scholar] [CrossRef]
- Meng, Q.-J.; Grossnickle, D.M.; Liu, D.; Zhang, Y.-G.; Neander, A.I.; Ji, Q.; Luo, Z.-X. New gliding mammaliaforms from the Jurassic. Nature 2017, 548, 291–296. [Google Scholar] [CrossRef]
- Swartz, S.M.; Groves, M.S.; Kim, H.D.; Walsh, W.R. Mechanical properties of bat wing membrane skin. J. Zool. 1996, 239, 357–378. [Google Scholar] [CrossRef]
- Taylor, G.K.; Carruthers, A.C.; Hubel, T.Y.; Walker, S.M. Wing morphing in insects, birds and bats: Mechanism and function. In Morphing Aerospace Vehicles and Structures; Wiley: Hoboken, NJ, USA, 2012; pp. 11–40. [Google Scholar]
- Fan, X.; Swartz, S.; Breuer, K. Power requirements for bat-inspired flapping flight with heavy, highly articulated and cambered wings. J. R. Soc. Interface 2022, 19, 20220315. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Dudchenko, O.; Feigin, C.Y.; Mereby, S.A.; Chen, Z.; Ramos, R.; Almet, A.A.; Sen, H.; Brack, B.J.; Johnson, M.R.; et al. Emx2 underlies the development and evolution of marsupial gliding membranes. Nature 2024, 629, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Feregrino, C.; Aldrovandi, S.; Lo, B.-W.; Monaco, A.A.; Ringel, A.R.; Morales, A.E.; Zehnder, T.; Behncke, R.Y.; Glaser, J.; et al. Comparative single-cell analyses reveal evolutionary repurposing of a conserved gene programme in bat wing development. Nat. Ecol. Evol. 2025, 9, 1626–1642. [Google Scholar] [CrossRef]
- Hubel, T.Y.; Hristov, N.I.; Swartz, S.M.; Breuer, K.S. Time-resolved wake structure and kinematics of bat flight. Exp. Fluids 2009, 46, 933–943. [Google Scholar] [CrossRef]
- Swartz, S.M.; Bishop, K.; Aguirre, M.-F.I.; Zubaid, A.; McCracken, G.F.; Kunz, T.H. Dynamic complexity of wing form in bats: Implications for flight performance. In Functionaland Evolutionary Ecology of Bats; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Tian, X.; Iriarte-Diaz, J.; Middleton, K.; Galvao, R.; Israeli, E.; Roemer, A.; Sullivan, A.; Song, A.; Swartz, S.; Breuer, K. Direct measurements of the kinematics and dynamics of bat flight. Bioinspir. Biomim. 2006, 1, S10. [Google Scholar] [CrossRef]
- Riskin, D.K.; Willis, D.J.; Iriarte-Díaz, J.; Hedrick, T.L.; Kostandov, M.; Chen, J.; Laidlaw, D.H.; Breuer, K.S.; Swartz, S.M. Quantifying the complexity of bat wing kinematics. J. Theor. Biol. 2008, 254, 604–615. [Google Scholar] [CrossRef]
- Swartz, S.M. Allometric patterning in the limb skeleton of bats: Implications for the mechanics and energetics of powered flight. J. Morphol. 1997, 234, 277–294. [Google Scholar] [CrossRef]
- Kawashima, T.; Thorington, R.W., Jr.; Bohaska, P.W.; Sato, F. Evolutionary transformation of the palmaris longus muscle in flying squirrels (Pteromyini: Sciuridae): An anatomical consideration of the origin of the uniquely specialized styliform cartilage. Anat. Rec. 2017, 300, 340–352. [Google Scholar] [CrossRef]
- Jackson, S.M.; Thorington, R.W. Gliding Mammals: Taxonomy of Living and Extinct Species; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2019. [Google Scholar]
- Zhao, F.; Wang, W.; Zhang, J.; Wyrwa, J.; Sun, F. Aerodynamic characteristics and pitching adjusting mechanism of the flying squirrel with deployed patagium. IEEE Access 2019, 7, 185554–185564. [Google Scholar] [CrossRef]
- Grossnickle, D.M.; Chen, M.; Wauer, J.G.A.; Pevsner, S.K.; Weaver, L.N.; Meng, Q.-J.; Liu, D.; Zhang, Y.-G.; Luo, Z.-X. Incomplete convergence of gliding mammal skeletons. Evolution 2020, 74, 2662–2680. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.M. Foraging Ecology, Behaviour and Management of the Mahogany Glider Petaurus gracilis; James Cook University: Douglas, QLD, Australia, 1998. [Google Scholar]
- Christian, N.G.; Geiser, F.; Koertner, G. Ecology, Energetics and Thermal Biology of Sugar Gliders. 2007. Available online: https://rune.une.edu.au/entities/publication/b222043f-baac-42ba-9db7-24357a1cab1b (accessed on 27 April 2024).
- Knipler, M.; Dowton, M.; Mikac, K. A Major Highway Acts to Genetically Structure a Sugar Glider (Petaurus breviceps) Population. 2021. Available online: https://www.researchgate.net/publication/352287636_A_Major_Highway_Acts_to_Genetically_Structure_a_Sugar_Glider_Petaurus_Breviceps_Population (accessed on 27 April 2024).
- Bishop, K.L. Aerodynamic force generation, performance and control of body orientation during gliding in sugar gliders (Petaurus breviceps). J. Exp. Biol. 2007, 210, 2593–2606. [Google Scholar] [CrossRef] [PubMed]
- Rummel, A.D.; Sierra, M.M.; Quinn, B.L.; Swartz, S.M. Hair, there and everywhere: A comparison of bat wing sensory hair distribution. Anat. Rec. 2023, 306, 2681–2692. [Google Scholar] [CrossRef]
- Hieronymus, T.L. Flight feather attachment in rock pigeons (Columba livia): Covert feathers and smooth muscle coordinate a morphing wing. J. Anat. 2016, 229, 631–656. [Google Scholar] [CrossRef]
- Cheney, J.A.; Konow, N.; Middleton, K.M.; Breuer, K.S.; Roberts, T.J.; Giblin, E.L.; Swartz, S.M. Membrane muscle function in the compliant wings of bats. Bioinspir. Biomim. 2014, 9, 025007. [Google Scholar] [CrossRef]
- Foehring, R.C.; Hermanson, J.W. Morphology and histochemistry of flight muscles in free-tailed bats, Tadarida brasiliensis. J. Mammal. 1984, 65, 388–394. [Google Scholar] [CrossRef]
- Hermanson, J.W.; Altenbach, J.S. Functional anatomy of the shoulder and arm of the fruit-eating bat Artibeus jamaicensis. J. Zool. 1985, 205, 157–177. [Google Scholar] [CrossRef]
- Swartz, S.M.; Allen, J.J. Structure and function of bat wings: A view from the Phyllostomidae. In Phyllostomid Bats: A Unique Mammalian Radiation; Fleming, T.H., Dávalos, L.M., A.R. Mello, M., Eds.; University of Chicago Press: Chicago, IL, USA, 2020. [Google Scholar]
- Hedenström, A.; Johansson, L.C. Bat flight: Aerodynamics, kinematics and flight morphology. J. Exp. Biol. 2015, 218, 653–663. [Google Scholar] [CrossRef]
- Jin, L.; Wu, J.; Bellusci, S.; Zhang, J.S. Fibroblast growth factor 10 and vertebrate limb development. Front. Genet. 2018, 9, 705. [Google Scholar] [CrossRef]
- Thewissen, J.G.M.; Babcock, S.K. Distinctive cranial and cervical innervation of wing muscles: New evidence for bat monophyly. Science 1991, 251, 934–936. [Google Scholar] [CrossRef]
- Bennett, A.F. Thermal dependence of muscle function. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1984, 247, R217–R229. [Google Scholar] [CrossRef]
- James, R.S. A review of the thermal sensitivity of the mechanics of vertebrate skeletal muscle. J. Comp. Physiol. B 2013, 183, 723–733. [Google Scholar] [CrossRef]
- Rummel, A.D.; Swartz, S.M.; Marsh, R.L. Low thermal dependence of the contractile properties of a wing muscle in the bat Carollia perspicillata. J. Exp. Biol. 2018, 221, jeb180166. [Google Scholar] [CrossRef]
- Thomas, S.P.; Suthers, R.A. The physiology and energetics of bat flight. J. Exp. Biol. 1972, 57, 317–335. [Google Scholar] [CrossRef]
- Voigt, C.C.; Borrisov, I.M.; Voigt-Heucke, S.L. Terrestrial locomotion imposes high metabolic requirements on bats. J. Exp. Biol. 2012, 215, 4340–4344. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.P. Metabolism during flight in two species of bats, Phyllostomus hastatus and Pteropus gouldii. J. Exp. Biol. 1975, 63, 273–293. [Google Scholar] [CrossRef]
- Shen, Y.-Y.; Liang, L.; Zhu, Z.-H.; Zhou, W.-P.; Irwin, D.M.; Zhang, Y.-P. Adaptive evolution of energy metabolism genes and the origin of flight in bats. Proc. Natl. Acad. Sci. USA 2010, 107, 8666–8671. [Google Scholar] [CrossRef]
- Cao, T.; Jin, J.P. Evolution of flight muscle contractility and energetic efficiency. Front. Physiol. 2020, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Rubalcaba, J.G.; Gouveia, S.F.; Villalobos, F.; Cruz-Neto, A.P.; Castro, M.G.; Amado, T.F.; Martinez, P.A.; Navas, C.A.; Dobrovolski, R.; Diniz-Filho, J.A.F.; et al. Physical constraints on thermoregulation and flight drive morphological evolution in bats. Proc. Natl. Acad. Sci. USA 2022, 119, e2103745119. [Google Scholar] [CrossRef] [PubMed]
- McNab, B.K. Evolutionary alternatives in the physiological ecology of bats. In Ecology of Bats; Kunz, T.H., Ed.; Springer: Boston, MA, USA, 1982; pp. 151–200. [Google Scholar]
- Guglielmo, C.G. Move that fatty acid: Fuel selection and transport in migratory birds and bats. Integr. Comp. Biol. 2010, 50, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, C.G. Obese super athletes: Fat-fueled migration in birds and bats. J. Exp. Biol. 2018, 221, jeb165753. [Google Scholar] [CrossRef]
- Voigt, C.C.; Sörgel, K.; Šuba, J.; Keišs, O.; Pētersons, G. The insectivorous bat Pipistrellus nathusii uses a mixed-fuel strategy to power autumn migration. Proc. R. Soc. B Biol. Sci. 2012, 279, 3772–3778. [Google Scholar] [CrossRef]
- Price, E.R.; McGuire, L.P.; Fenton, M.B.; Guglielmo, C.G. Flight muscle carnitine palmitoyl transferase activity varies with substrate chain length and unsaturation in the hoary bat (Lasiurus cinereus). Can. J. Zool. 2014, 92, 173–176. [Google Scholar] [CrossRef]
- Weber, J.-M. The physiology of long-distance migration: Extending the limits of endurance metabolism. J. Exp. Biol. 2009, 212, 593–597. [Google Scholar] [CrossRef]
- Landys, M.M.; Piersma, T.; Guglielmo, C.G.; Jukema, J.; Ramenofsky, M.; Wingfield, J.C. Metabolic profile of long–distance migratory flight and stopover in a shorebird. Proc. R. Soc. B Biol. Sci. USA 2005, 272, 295–302. [Google Scholar] [CrossRef]
- Gutiérrez, J.S.; Sabat, P.; Castañeda, L.E.; Contreras, C.; Navarrete, L.; Peña-Villalobos, I.; Navedo, J.G. Oxidative status and metabolic profile in a long-lived bird preparing for extreme endurance migration. Sci. Rep. 2019, 9, 17616. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Chetri, R.; Singh, N. Utilization of biomolecules as fuel energy and their physiological mechanism during migration in birds—A review. J. Environ. Biol. 2022, 43, 1–10. [Google Scholar] [CrossRef]
- Amaral, T.S.; Pinheiro, E.C.; Freitas, M.B.; Aguiar, L.M.S. Low energy reserves are associated with fasting susceptibility in Neotropical nectar bats Glossophaga soricina. Braz. J. Biol. 2019, 79, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.J.; Sommers, A.S.; McGuire, L.P. Seasonal dynamics of lipid metabolism and energy storage in the Brazilian free-tailed bat. Physiol. Biochem. Zool. 2019, 92, 386–395. [Google Scholar] [CrossRef]
- Voigt, C.C.; Rosner, E.; Guglielmo, C.G.; Currie, S.E. Fatty acid profiles of the European migratory common noctule bat (Nyctalus noctula). Sci. Nat. 2019, 106, 33. [Google Scholar] [CrossRef]
- Voigt, C.C.; Speakman, J.R. Nectar-feeding bats fuel their high metabolism directly with exogenous carbohydrates. Funct. Ecol. 2007, 21, 913–921. [Google Scholar] [CrossRef]
- Amitai, O.; Holtze, S.; Barkan, S.; Amichai, E.; Korine, C.; Pinshow, B.; Voigt, C.C. Fruit bats (Pteropodidae) fuel their metabolism rapidly and directly with exogenous sugars. J. Exp. Biol. 2010, 213, 2693–2699. [Google Scholar] [CrossRef]
- O’Mara, M.T.; Wikelski, M.; Voigt, C.C.; Ter Maat, A.; Pollock, H.S.; Burness, G.; Desantis, L.M.; Dechmann, D.K.N. Cyclic bouts of extreme bradycardia counteract the high metabolism of frugivorous bats. eLife 2017, 6, e26686. [Google Scholar] [CrossRef]
- Voigt, C.C.; Currie, S.E.; McGuire, L.P. Bat migration and foraging: Energy-demanding journeys on tight budgets. In A Natural History of Bat Foraging; Russo, D., Fenton, B., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 199–215. [Google Scholar]
- Cheney, J.A.; Konow, N.; Bearnot, A.; Swartz, S.M. A wrinkle in flight: The role of elastin fibres in the mechanical behaviour of bat wing membranes. J. R. Soc. Interface 2015, 12, 20141286. [Google Scholar] [CrossRef]
- Konow, N.; Cheney, J.A.; Roberts, T.J.; Waldman, J.R.S.; Swartz, S.M. Spring or string: Does tendon elastic action influence wing muscle mechanics in bat flight? Proc. R. Soc. B Biol. Sci. 2015, 282, 20151832. [Google Scholar] [CrossRef] [PubMed]
- Gilman, C. Tendon elasticity helps bats flap. J. Exp. Biol. 2016, 219, 612–613. [Google Scholar] [CrossRef]
- da Fonseca, R.R.; Johnson, W.E.; O’Brien, S.J.; Ramos, M.J.; Antunes, A. The adaptive evolution of the mammalian mitochondrial genome. BMC Genom. 2008, 9, 119. [Google Scholar] [CrossRef]
- Osada, N.; Akashi, H. Mitochondrial–nuclear interactions and accelerated compensatory evolution: Evidence from the primate cytochrome C oxidase complex. Mol. Biol. Evol. 2011, 29, 337–346. [Google Scholar] [CrossRef]
- Ten Chionh, Y.; Cui, J.; Koh, J.; Mendenhall, I.H.; Ng, J.H.J.; Low, D.; Itahana, K.; Irving, A.T.; Wang, L.-F. High basal heat-shock protein expression in bats confers resistance to cellular heat/oxidative stress. Cell Stress Chaperones 2019, 24, 835–849. [Google Scholar] [CrossRef]
- Elangovan, V.; Yuvana Satya Priya, E.; Raghuram, H.; Marimuthu, G. Wing morphology and flight development in the short-nosed fruit bat Cynopterus sphinx. Zoology 2007, 110, 189–196. [Google Scholar] [CrossRef]
- Jin, L.-R.; Lin, A.-Q.; Sun, K.-P.; Liu, Y.; Feng, J. Postnatal development of morphological features and vocalization in the pomona leaf-nosed bat Hipposideros pomona. Acta Theriol. 2011, 56, 13–22. [Google Scholar] [CrossRef]
- Wang, L.; Lin, A.; Xiao, Y.; Jiang, T.; Feng, J. Postnatal development in the big-footed bat, Myotis macrodactylus: Wing morphology, echolocation calls, and flight. Acta Theriol. 2014, 59, 435–441. [Google Scholar] [CrossRef]
- Kvist, A.; Lindström, Å.; Green, M.; Piersma, T.; Visser, G.H. Carrying large fuel loads during sustained bird flight is cheaper than expected. Nature 2001, 413, 730–732. [Google Scholar] [CrossRef]
- Jenni-Eiermann, S.; Jenni, L.; Kvist, A.; Lindström, A.; Piersma, T.; Visser, G.H. Fuel use and metabolic response to endurance exercise: A wind tunnel study of a long-distance migrant shorebird. J. Exp. Biol. 2002, 205, 2453–2460. [Google Scholar] [CrossRef]
- Tian, R.; Yang, C.; Chai, S.-M.; Guo, H.; Seim, I.; Yang, G. Evolutionary impacts of purine metabolism genes on mammalian oxidative stress adaptation. Zool. Res. 2022, 43, 241–254. [Google Scholar] [CrossRef]
- Genoud, M.; Christe, P. Thermal energetics and torpor in the common pipistrelle bat, Pipistrellus pipistrellus (Vespertilionidae: Mammalia). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 160, 252–259. [Google Scholar] [CrossRef]
- Luo, J.; Greif, S.; Ye, H.; Bumrungsri, S.; Eitan, O.; Yovel, Y. Flight rapidly modulates body temperature in freely behaving bats. Anim. Biotelemetry 2021, 9, 45. [Google Scholar] [CrossRef]
- Sifa, I.F.; Nisa, L.A.; Bahartin, W. Anatomical structure of large flying-fox bat’s (Pteropus vampyrus) forelimb skeleton. Proc. Int. Conf. Sci. Eng. 2020, 3, 91–93. [Google Scholar] [CrossRef]
- López-Aguirre, C.; Wilson, L.A.B.; Koyabu, D.; Tu, V.T.; Hand, S.J. Variation in cross-sectional shape and biomechanical properties of the bat humerus under Wolff’s law. Anat. Rec. 2021, 304, 1937–1952. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.S.; Carrizo, L.V. Forelimb bone morphology and its association with foraging ecology in four families of Neotropical bats. J. Mamm. Evol. 2021, 28, 99–110. [Google Scholar] [CrossRef]
- Sears, K.E.; Behringer, R.R.; Rasweiler, J.J.; Niswander, L.A. Development of bat flight: Morphologic and molecular evolution of bat wing digits. Proc. Natl. Acad. Sci. USA 2006, 103, 6581–6586. [Google Scholar] [CrossRef]
- Swartz, S.M.; Middleton, K.M. Biomechanics of the bat limb skeleton: Scaling, material properties and mechanics. Cells Tissues Organs 2007, 187, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.N.; Cretekos, C.J.; Sears, K.E. The evolution and development of mammalian flight. WIREs Dev. Biol. 2012, 1, 773–779. [Google Scholar] [CrossRef]
- Murphy, R.C. Fluorescence studies in the wing of the living bat. Anat. Rec. 1960, 136, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Cortese, T.A.; Nicoll, P.A. In vivo observations of skin appendages in the bat wing. J. Investig. Dermatol. 1970, 54, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, M.; Wang, Y.; Cooper, K.L.; Zhu, T.; Dong, D.; Zhang, J.; Zhang, S. Unique expression patterns of multiple key genes associated with the evolution of mammalian flight. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133133. [Google Scholar] [CrossRef]
- Cheney, J.A.; Allen, J.J.; Swartz, S.M. Diversity in the organization of elastin bundles and intramembranous muscles in bat wings. J. Anat. 2017, 230, 510–523. [Google Scholar] [CrossRef]
- Skulborstad, A.; Goulbourne, N.C. A chemo-mechanical constitutive model for muscle activation in bat wing skins. J. R. Soc. Interface 2024, 21, 20230593. [Google Scholar] [CrossRef]
- Song, A.; Tian, X.; Israeli, E.; Galvao, R.; Bishop, K.; Swartz, S.; Breuer, K. Aeromechanics of membrane wings with Implications for animal flight. AIAA J. 2008, 46, 2096–2106. [Google Scholar] [CrossRef]
- Tiomkin, S.; Raveh, D.E. A review of membrane-wing aeroelasticity. Prog. Aerosp. Sci. 2021, 126, 100738. [Google Scholar] [CrossRef]
- Qin, J.; Li, L.; Hao, Y.; Xu, J.; Bai, F.; Ye, J. Analysis of aerodynamic characteristics of flexible flapping flap with bidirectional fluid–structure interaction. AIP Adv. 2020, 10, 105108. [Google Scholar] [CrossRef]
- Lilong, C.; Yu, Y. Maneuvering characteristics of bilateral amplitude–asymmetric flapping motion based on a bat-inspired flexible wing. Biomimetics 2024, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. Progress in aerodynamics of bat flight. Kongqi Donglixue Xuebao/Acta Aerodyn. Sin. 2018, 36, 129–134. [Google Scholar]
- Gardiner, J.D.; Dimitriadis, G.; Codd, J.R.; Nudds, R.L. A potential role for bat tail membranes in flight control. PLoS ONE 2011, 6, e18214. [Google Scholar] [CrossRef]
- Bergou, A.J.; Swartz, S.M.; Vejdani, H.; Riskin, D.K.; Reimnitz, L.; Taubin, G.; Breuer, K.S. Falling with style: Bats perform complex aerial rotations by adjusting wing inertia. PLoS Biol. 2015, 13, e1002297. [Google Scholar] [CrossRef]
- Sekhar, S.; Windes, P.; Fan, X.; Tafti, D.K. Canonical description of wing kinematics and dynamics for a straight flying insectivorous bat (Hipposideros pratti). PLoS ONE 2019, 14, e0218672. [Google Scholar] [CrossRef]
- Singh, S.K.; Zhang, L.-B.; Zhao, J.-S. Direct measurements of the wing kinematics of a bat in straight flight. J. Biomech. Eng. 2020, 143, 041006. [Google Scholar] [CrossRef]
- Sihite, E.; Salagame, A.; Ghanem, P.; Ramezani, A. Actuation and flight control of high-DOF dynamic morphing wing flight by shifting structure response. In Proceedings of the 2023 62nd IEEE Conference on Decision and Control (CDC), Singapore, 13–15 December 2023; pp. 8824–8829. [Google Scholar]
- Hedenström, A.; Johansson, L.C. Bat flight. Curr. Biol. 2015, 25, R399–R402. [Google Scholar] [CrossRef] [PubMed]
- Windes, P.; Tafti, D.K.; Müller, R. Determination of spatial fidelity required to accurately mimic the flight dynamics of a bat. Bioinspir. Biomim. 2019, 14, 066011. [Google Scholar] [CrossRef] [PubMed]
- Giannini, N.; Goswami, A.; Sánchez-Villagra, M.R. Development of integumentary structures in Rousettus amplexicaudatus (Mammalia: Chiroptera: Pteropodidae) during late-embryonic and fetal stages. J. Mammal. 2006, 87, 993–1001. [Google Scholar] [CrossRef]
- Dai, M.; Wang, Y.; Fang, L.; Irwin, D.M.; Zhu, T.; Zhang, J.; Zhang, S.; Wang, Z. Differential expression of Meis2, Mab21l2 and Tbx3 during Limb development associated with diversification of limb morphology in mammals. PLoS ONE 2014, 9, e106100. [Google Scholar] [CrossRef]
- Booker, B.M.; Friedrich, T.; Mason, M.K.; VanderMeer, J.E.; Zhao, J.; Eckalbar, W.L.; Logan, M.; Illing, N.; Pollard, K.S.; Ahituv, N. Bat accelerated regions identify a bat forelimb specific enhancer in the HoxD locus. PLoS Genet. 2016, 12, e1005738. [Google Scholar] [CrossRef]
- Cretekos, C.J.; Weatherbee, S.D.; Chen, C.-H.; Badwaik, N.K.; Niswander, L.; Behringer, R.R.; Rasweiler, J.J., IV. Embryonic staging system for the short-tailed fruit bat, Carollia perspicillata, a model organism for the mammalian order Chiroptera, based upon timed pregnancies in captive-bred animals. Dev. Dyn. 2005, 233, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Scholz, G.M.; Sulaiman, N.S.; Al Baiiaty, S.; Kwa, M.Q.; Reynolds, E.C. A novel regulatory relationship between RIPK4 and ELF3 in keratinocytes. Cell. Signal. 2016, 28, 1916–1922. [Google Scholar] [CrossRef]
- Kalay, E.; Sezgin, O.; Chellappa, V.; Mutlu, M.; Morsy, H.; Kayserili, H.; Kreiger, E.; Cansu, A.; Toraman, B.; Abdalla, E.M.; et al. Mutations in RIPK4 cause the autosomal-recessive form of popliteal pterygium syndrome. Am. J. Hum. Genet. 2012, 90, 76–85. [Google Scholar] [CrossRef]
- Lyu, X.; Bai, J.; Jiang, J.-B.; Sun, C.-J.; Chen, P.; Liu, Q.; Ma, Y.-S.; Liu, Z. Single-cell expression profiling of bat wing development. Nat. Commun. 2025, 16, 6612. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, X.; Yu, H.; Wu, D.; Zheng, J. Wnt5a as a predictor in poor clinical outcome of patients and a mediator in chemoresistance of ovarian cancer. Int. J. Gynecol. Cancer 2011, 21, 280. [Google Scholar] [CrossRef]
- Yamaguchi, T.P.; Bradley, A.; McMahon, A.P.; Jones, S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999, 126, 1211–1223. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.L.; Li, D.H.; Wang, J.S.; Zhao, F. Dual role of Wnt5a in the progression of inflammatory diseases. Chin. Med. Sci. J. 2022, 37, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.R.M.; Raghoebir, L.; Franken, P.F.; Helvensteijn, W.; van Gurp, L.; Meijlink, F.; van der Valk, M.A.; Rottier, R.J.; Kuipers, E.J.; van Veelen, W.; et al. Induced Wnt5a expression perturbs embryonic outgrowth and intestinal elongation, but is well-tolerated in adult mice. Dev. Biol. 2012, 369, 91–100. [Google Scholar] [CrossRef]
- Eghbali, H.; Sharifi, M. Postnatal growth, age estimation, and wing development in Geoffroy’s bat Myotis emarginatus (Chiroptera: Vespertilionidae). Mammal Study 2018, 43, 153–165. [Google Scholar] [CrossRef]
- McLean, J.A.; Speakman, J.R. Morphological changes during postnatal growth and reproduction in the brown long-eared bat Plecotus auritus: Implications for wing loading and predicted flight performance. J. Nat. Hist. 2000, 34, 773–791. [Google Scholar] [CrossRef]
- Davies, K.T.J.; Yohe, L.R.; Almonte, J.; Sánchez, M.K.R.; Rengifo, E.M.; Dumont, E.R.; Sears, K.E.; Dávalos, L.M.; Rossiter, S.J. Foraging shifts and visual preadaptation in ecologically diverse bats. Mol. Ecol. 2020, 29, 1839–1859. [Google Scholar] [CrossRef] [PubMed]
- Power, M.L.; Foley, N.M.; Jones, G.; Teeling, E.C. Taking flight: An ecological, evolutionary and genomic perspective on bat telomeres. Mol. Ecol. 2022, 31, 6053–6068. [Google Scholar] [CrossRef]
- Carter, R.T.; Adams, R.A. Integrating ontogeny of echolocation and locomotion gives unique insights into the origin of bats. J. Mamm. Evol. 2016, 23, 413–421. [Google Scholar] [CrossRef]
- Halley, A.C.; Baldwin, M.K.L.; Cooke, D.F.; Englund, M.; Pineda, C.R.; Schmid, T.; Yartsev, M.M.; Krubitzer, L. Coevolution of motor cortex and behavioral specializations associated with flight and echolocation in bats. Curr. Biol. 2022, 32, 2935–2941.e3. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.E.; Grinnell, A.D. Echolocation ontogeny in bats. In Animal Sonar Systems; Busnel, R.-G., Fish, J.F., Eds.; Springer: Boston, MA, USA, 1980; pp. 355–377. [Google Scholar]
- Moss, C.F.; Surlykke, A. Auditory scene analysis by echolocation in bats. J. Acoust. Soc. Am. 2001, 110, 2207–2226. [Google Scholar] [CrossRef] [PubMed]
- Holderied, M.W.; Jones, G.; von Helversen, O. Flight and echolocation behaviour of whiskered bats commuting along a hedgerow: Range-dependent sonar signal design, Doppler tolerance and evidence for ‘acoustic focussing’. J. Exp. Biol. 2006, 209, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.T.; Adams, R.A. Ontogeny of the larynx and flight ability in Jamaican fruit bats (Phyllostomidae) with considerations for the evolution of echolocation. Anat. Rec. 2014, 297, 1270–1277. [Google Scholar] [CrossRef]
- Carter, R.T.; Adams, R.A. Postnatal ontogeny of the cochlea and flight ability in Jamaican fruit bats (Phyllostomidae) with implications for the evolution of echolocation. J. Anat. 2015, 226, 301–308. [Google Scholar] [CrossRef]
- Simmons, J.A.; Stein, R.A. Acoustic imaging in bat sonar: Echolocation signals and the evolution of echolocation. J. Comp. Physiol. 1980, 135, 61–84. [Google Scholar] [CrossRef]
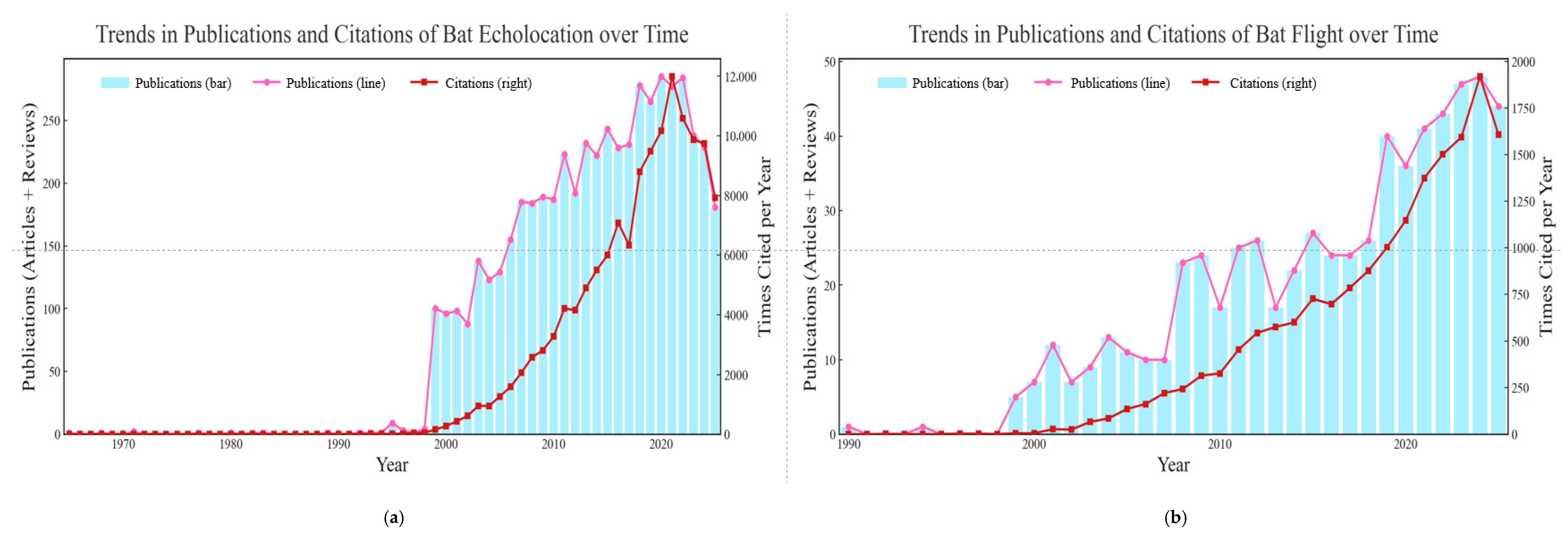
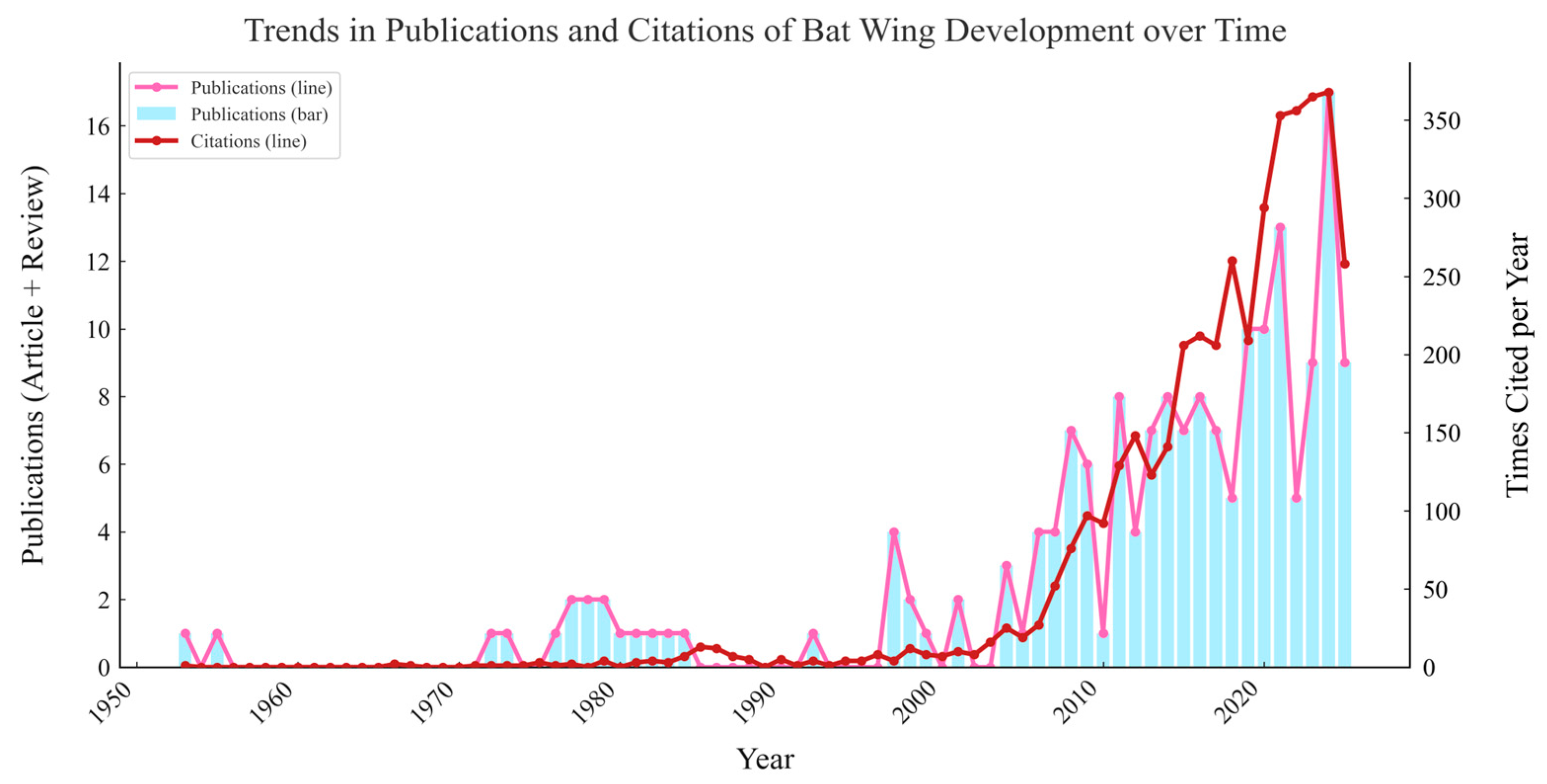

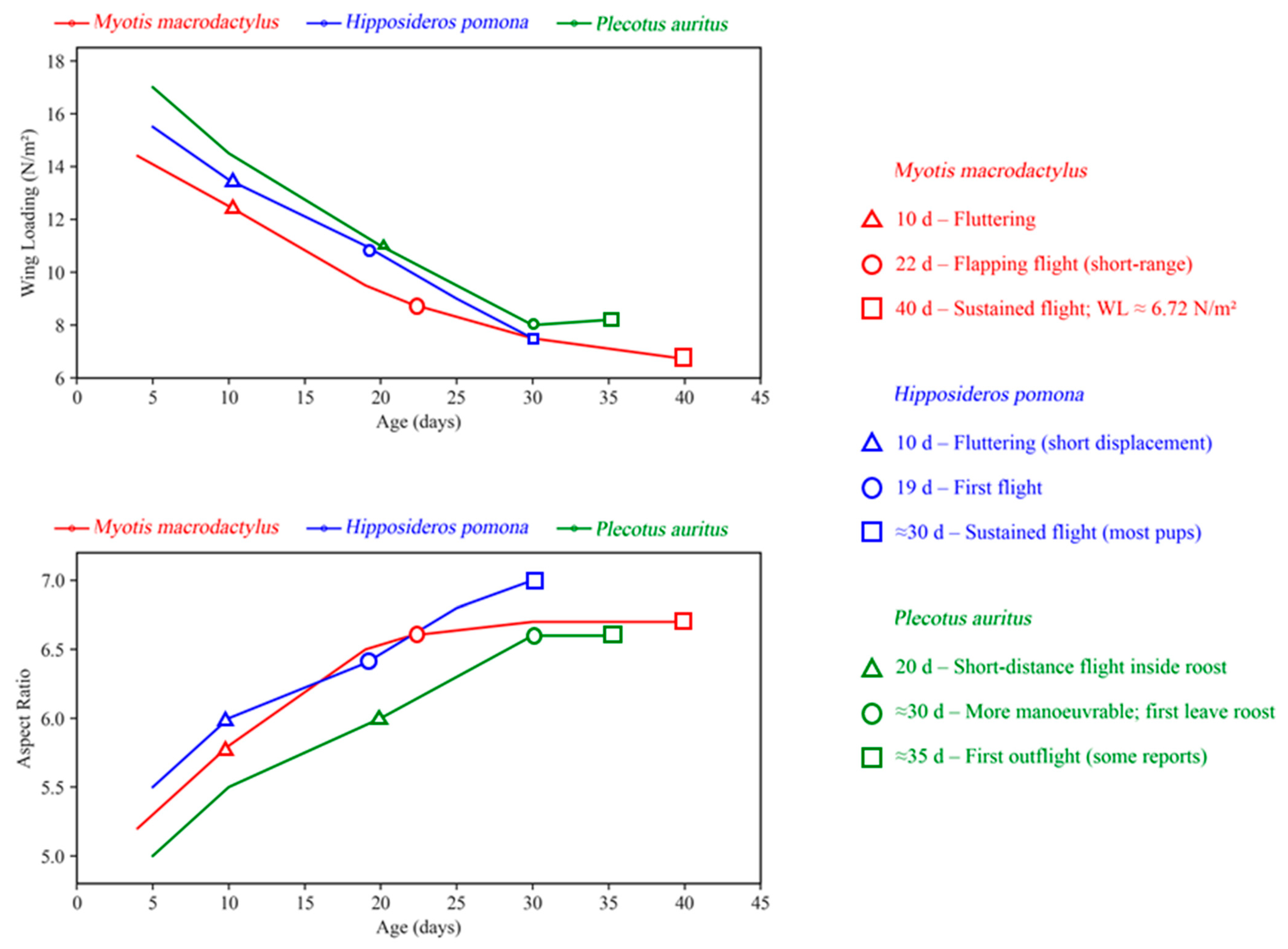
| Family | Species | Examples | Objectives | Method | Conclusion |
|---|---|---|---|---|---|
| Phyllostomidae | Carollia perspicillata | [32] | Molecular basis of bat wing morphogenesis | Comparative gene expression and functional enhancer replacement experiments | Gene expression shifts drive wing specialization |
| Miniopteridae | Miniopterus natalensis | [33] | Wing bone mineralization patterns for flight adaptation | Biomechanical and proteomic profiling | Reduced mineralization enhances wing flexibility |
| Pteropodidae | Pteropus poliocephalus | [34] | Molecular profiling of bat wing development | RNA-seq and ChIP-seq of developing limbs | Gene regulation drives forelimb specialization |
| Pteropus hypomelanus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, H.; Liu, Z.; Bao, M.; Li, X.; Wang, T.; Wang, R.; Feng, J. What Factors Shape the Flyability in Bats?—The Perspective from Bat’s Wing Development. Biology 2025, 14, 1524. https://doi.org/10.3390/biology14111524
Zhang M, Wang H, Liu Z, Bao M, Li X, Wang T, Wang R, Feng J. What Factors Shape the Flyability in Bats?—The Perspective from Bat’s Wing Development. Biology. 2025; 14(11):1524. https://doi.org/10.3390/biology14111524
Chicago/Turabian StyleZhang, Minjie, Hui Wang, Zhongzheng Liu, Mingyue Bao, Xintong Li, Tianhui Wang, Ruixue Wang, and Jiang Feng. 2025. "What Factors Shape the Flyability in Bats?—The Perspective from Bat’s Wing Development" Biology 14, no. 11: 1524. https://doi.org/10.3390/biology14111524
APA StyleZhang, M., Wang, H., Liu, Z., Bao, M., Li, X., Wang, T., Wang, R., & Feng, J. (2025). What Factors Shape the Flyability in Bats?—The Perspective from Bat’s Wing Development. Biology, 14(11), 1524. https://doi.org/10.3390/biology14111524






