The “Iron Gate” Outcompetes the “Enzymic Latch” as the Dominant Soil Organic Carbon Stabilization Mechanism in Permafrost Peatlands of the Great Hing’an Mountains
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Analysis of Soil Physicochemical Properties
2.3. Assay of Soil Extracellular Enzyme Activities
2.4. Measurement of Fe and Fe-SOC
2.5. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
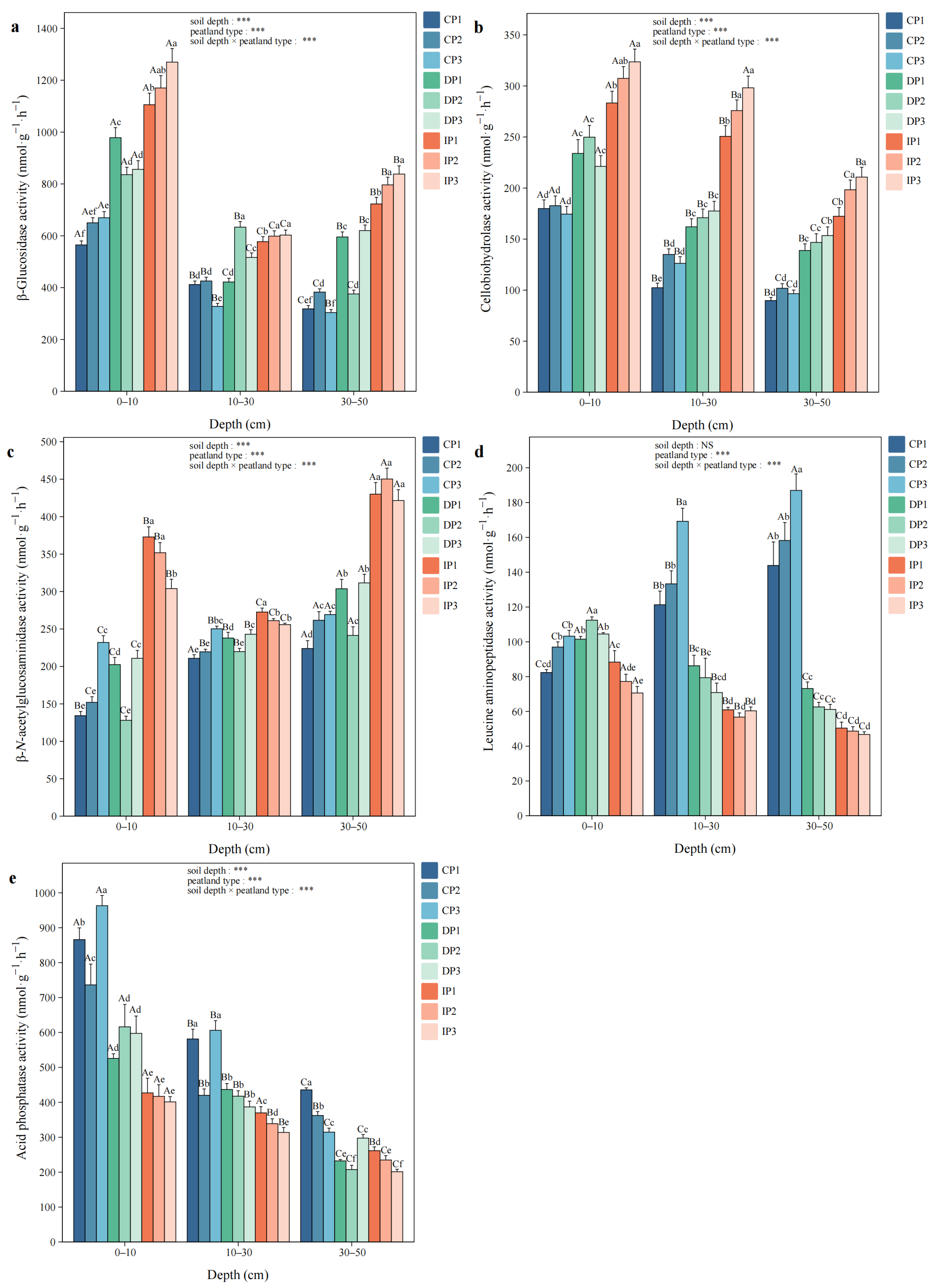
3.2. Soil Hydrolytic Enzyme Activities
3.3. Soil Oxidative Enzyme Activities
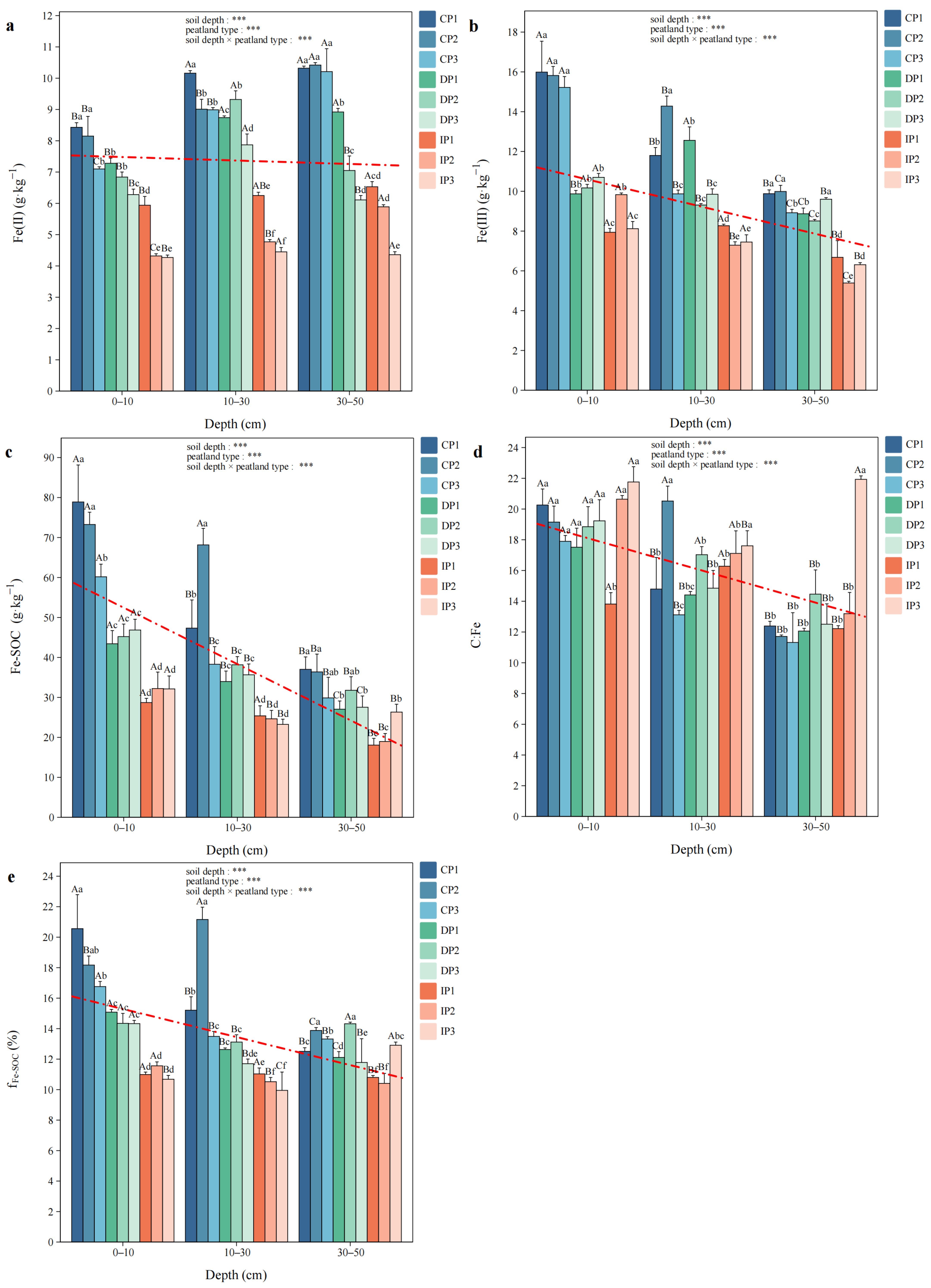
3.4. Fe and C Coupling
3.5. Relationships Among Fe Phases, Soil Physicochemical Properties and Soil Enzymes
4. Discussion
4.1. Enzyme Mechanisms
4.2. Preservation of SOC in Peatlands by Fe
4.3. Factors Regulating SOC Stability in Permafrost Peatlands
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.R.; Morris, P.J.; Liu, J.G.; Holden, J. PEATMAP: Refining estimates of global peatland distribution based on a meta-analysis. Catena 2018, 160, 134–140. [Google Scholar] [CrossRef]
- Geay, M.L.; Lauga, B.; Walcker, R.; Jassey, V.E.J. A meta-analysis of peatland microbial diversity and function responses to climate change. Soil Biol. Biochem. 2024, 189, 109287. [Google Scholar]
- Cong, J.X.; Gao, C.Y.; Han, D.X.; Li, Y.H.; Wang, G.P. Stability of the permafrost peatlands carbon pool under climate change and wildfires during the last 150 years in the northern Great Khingan Mountains, China. Sci. Total Environ. 2020, 712, 136476. [Google Scholar] [CrossRef]
- Smith, S.L.; O’Neill, H.B.; Isaksen, K.; Noetzli, J.; Romanovsky, V.E. The changing thermal state of permafrost. Nat. Rev. Earth Environ. 2022, 3, 10–23. [Google Scholar] [CrossRef]
- Miner, K.R.; Turetsky, M.R.; Malina, E.; Bartsch, A.; Tamminen, J.; McGuire, A.D.; Fix, A.; Sweeney, C.; Elder, C.D.; Miller, C.E. Permafrost carbon emissions in a changing Arctic. Nat. Rev. Earth Environ. 2022, 3, 55–67. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Gu, J.D. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef]
- Zhao, H.X.; Wang, Z.G.; Yang, W.; Yao, L.L.; Tan, Q. The role of water regime in soil organic carbon preservation in mangrove wetlands: "iron gate" vs. "enzyme latch". J. Hydrol. 2025, 663, 134088. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.; Kang, H. An enzymic ‘latch’ on a global carbon store. Nature 2001, 409, 149. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.J.; Treffkorn, J.; Silver, W.L. Breaking the enzymatic latch: Impacts of reducing conditions on hydrolytic enzyme activity in tropical forest soils. Ecology 2014, 95, 2964–2973. [Google Scholar] [CrossRef]
- Urbanová, Z.; Hájek, T. Revisiting the concept of ‘enzymic latch’ on carbon in peatlands. Sci. Total Environ. 2021, 779, 146384. [Google Scholar] [CrossRef] [PubMed]
- Li, R.X.; Luo, H.Q.; Yu, J.L.; Luo, L.; He, Y.; Deng, S.H.; Deng, O.P.; Shi, D.Z.; He, J.S.; Xiao, H.; et al. The importance of moisture in regulating soil organic carbon content based on a comparison of “enzymic latch” and “iron gate” in Zoige Plateau peatland. Catena 2023, 225, 107019. [Google Scholar] [CrossRef]
- Romanowicz, K.J.; Kane, E.S.; Potvin, L.R.; Daniels, A.L.; Kolka, R.K.; Lilleskov, E.A. Understanding drivers of peatland extracellular enzyme activity in the PEATcosm experiment: Mixed evidence for enzymic latch hypothesis. Plant Soil 2015, 397, 371–386. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, H.; He, J.S.; Feng, X.J. Iron-mediated soil carbon response to water-table decline in an alpine wetland. Nat. Commun. 2017, 8, 15972. [Google Scholar] [CrossRef]
- Chen, C.M.; Hall, S.J.; Coward, E.; Thompson, A. Iron-mediated organic matter decomposition in humid soils can counteract protection. Nat. Commun. 2020, 11, 2255. [Google Scholar] [CrossRef]
- Zhao, Q.; Adhikari, D.; Huang, R.X.; Patel, A.; Wang, X.L.; Tang, Y.Z.; Obrist, D.; Roden, E.E.; Yang, Y. Coupled dynamics of iron and iron-bound organic carbon in forest soils during anaerobic reduction. Chem. Geol. 2017, 464, 118–126. [Google Scholar] [CrossRef]
- Wan, D.; Ye, T.H.; Lu, Y.; Chen, W.L.; Cai, P.; Haung, Q.Y. Iron oxides selectively stabilize plant-derived polysaccharides and aliphatic compounds in agricultural soils. Eur. J. Soil Sci. 2019, 70, 1153–1163. [Google Scholar] [CrossRef]
- Feng, X.J.; Zhao, Y.P.; Wang, H.Q.; Liu, C.Z. Iron-organic carbon interactions in wetlands: Implications for wetland carbon preservation under global changes. Glob. Change Biol. 2025, 31, e70300. [Google Scholar] [CrossRef]
- Jin, H.J.; Yu, Q.H.; Lü, L.Z.; Guo, D.X.; He, R.X.; Yu, S.P.; Sun, G.Y.; Li, Y.W. Degradation of permafrost in the Xing’anling Mountains, Northeastern China. Permafrost. Periglac. 2007, 18, 245–258. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Abbott, B.W.; Jones, M.C.; Anthony, K.W.; Olefeldt, D.; Schuur, E.A.D.; Grosse, G.; Kuhry, P.; Hugelius, G.; Koven, C.; et al. Carbon release through abrupt permafrost thaw. Nat. Geosci. 2020, 13, 138–143. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.I.; Olefeldt, D.; Pelletier, N.; Blodau, C.; Knorr, K.H.; Talbot, J.; Heffernan, L.; Turetsky, M. Permafrost thaw causes large carbon loss in boreal peatlands while changes to peat quality are limited. Glob. Change Biol. 2023, 29, 5720–5735. [Google Scholar] [CrossRef]
- Wang, H.J.; River, M.; Richardson, C.J. Does an ‘iron gate’ carbon preservation mechanism exist in organic–rich wetlands? Soil Biol. Biochem. 2019, 135, 48–50. [Google Scholar] [CrossRef]
- Wang, C.Q.; Blagodatskaya, E.; Dippold, M.A.; Dorodnikov, M. Keep oxygen in check: Contrasting effects of short-term aeration on hydrolytic versus oxidative enzymes in paddy soils. Soil Biol. Biochem. 2022, 169, 108690. [Google Scholar] [CrossRef]
- Ren, H.X.; Ren, C.Y.; Wang, Z.M.; Jia, M.M.; Yu, W.S.; Liu, P.; Xia, C.Z. Continuous tracking of forest disturbance and recovery in the Greater Khingan mountains from annual Landsat Imagery. Remote Sens. 2023, 15, 5426. [Google Scholar] [CrossRef]
- Chang, X.L.; Jin, H.J.; He, R.X.; Zhang, Y.L.; Li, X.Y.; Jin, X.Y.; Li, G.Y. Permafrost changes in the northwestern Da Xing’anling Mountains, Northeast China, in the past decade. Earth Syst. Sci. Data 2022, 14, 3947–3959. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.T.; Sun, W.Y.; Zhang, Q.; Diao, M.M.; Sun, J. Extreme soil salinity reduces N and P metabolism and related microbial network complexity and community immigration rate. Environ. Res. 2025, 264, 120361. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, X.F.; Zheng, F.L.; Wilson, G.V.; Zhang, X.J.; Wang, Y.F.; Fu, H. The effects of freeze-thaw cycles at different initial soil water contents on soil erodibility in Chinese Mollisol region. Catena 2020, 193, 104615. [Google Scholar] [CrossRef]
- Box, J.D. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Jeewani, P.H.; Zwieten, L.V.; Zhu, Z.K.; Ge, T.; Guggenberger, G.; Luo, Y.; Xu, J. Abiotic and biotic regulation on carbon mineralization and stabilization in paddy soils along iron oxide gradients. Soil Biol. Biochem. 2021, 160, 108312. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef]
- Song, Y.Y.; Sun, L.; Song, C.C.; Li, M.T.; Liu, Z.D.; Zhu, M.Y.; Chen, S.; Yuan, J.B.; Gao, J.L.; Wang, X.W.; et al. Responses of soil microbes and enzymes to long-term warming incubation in different depths of permafrost peatland soil. Sci. Total Environ. 2023, 900, 165733. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Mooshammer, M.; Sauvadet, M.; Meng, C.; Alvarez, G.; Bernard, L.; Bertrand, I.; Blagodatskaya, E.; Bon, L.; Fontaine, S.; et al. Soil enzymes in response to climate warming: Mechanisms and feedbacks. Funct. Ecol. 2022, 36, 1378–1395. [Google Scholar] [CrossRef]
- Zuccarini, P.; Sardans, J.; Asensio, L.; Peñuelas, J. Altered activities of extracellular soil enzymes by the interacting global environmental changes. Glob. Change Biol. 2023, 29, 2067–2091. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Wang, G.S.; Post, W.M.; Mayes, M.A.; Frerichs, J.T.; Sindhu, J. Parameter estimation for models of ligninolytic and cellulolytic enzyme kinetics. Soil Biol. Biochem. 2012, 48, 28–38. [Google Scholar] [CrossRef]
- Brouns, K.; Verhoeven, J.T.; Hefting, M.M. Short period of oxygenation releases latch on peat decomposition. Sci. Total Environ. 2014, 481, 61–68. [Google Scholar] [CrossRef]
- Yao, J.Z.; Song, W.; Wang, C.Q.; Clough, T.; Qin, S.P. Rice root Fe plaque-induced hydroxyl radicals increase paddy soil CO2 emissions. Environ. Sci. Technol. 2025, 59, 15142–15150. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.Z.; Si, G.C.; Wang, J.; Zhang, G.X. Microbial communities and associated enzyme activities in alpine wetlands with increasing altitude on the Tibetan Plateau. Wetlands 2017, 37, 401–412. [Google Scholar] [CrossRef]
- Xin, P.Q.; Zhang, Y.L.; Jiang, N.; Chen, Z.H.; Chen, L.J. Neutral soil pH conditions favor the inhibition of phenol on hydrolase activities and soil organic carbon mineralization. Eur. J. Soil Biol. 2024, 121, 103621. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Ucros, J.M.; Wickings, K.; Wilhelm, R.C.; Sparks, J.; Buckley, D.H.; Bauerle, T.L. Prevalent root-derived phenolics drive shifts in microbial community composition and prime decomposition in forest soil. Soil Biol. Biochem. 2020, 145, 107797. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Daunoras, J.; Kacergius, A.; Gudiukaite, R. Role of soil microbiota enzymes in soil health and activity changes depending on climate change and the type of soil ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.W.; Zhang, T.Y.; Wang, S.Z.; Wang, Z.C. Soil pH and C/N ratio determines spatial variations in soil microbial communities and enzymatic activities of the agricultural ecosystems in northeast China: Jilin Province case. Appl. Soil Ecol. 2020, 155, 103629. [Google Scholar] [CrossRef]
- Tahvanainen, T.; Haraguchi, A. Effect of pH on phenol oxidase activity on decaying Sphagnum mosses. Eur. J. Soil Biol. 2013, 54, 41–47. [Google Scholar] [CrossRef]
- Jia, Z.X.; Huang, X.L.; Li, L.; Li, T.L.; Duan, Y.H.; Ling, N.; Yu, G.H. Rejuvenation of iron oxides enhances carbon sequestration by the ‘iron gate’ and ‘enzyme latch’ mechanisms in a rice-wheat cropping system. Sci. Total Environ. 2022, 839, 156209. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.L.; Peduruhewa, J.H.; Zwieten, L.V.; Gong, L.X.; Tan, B.C.; Zhang, G.L. The coupling between iron and carbon and iron reducing bacteria control carbon sequestration in paddy soils. Catena 2023, 223, 106937. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Liu, Y.Z.; Lin, J.D.; Mo, C.Y.; Yang, J.P.; Gunina, A. Conversion from double-rice to maize-rice increases iron-bound organic carbon by “iron gate” and “enzyme latch” mechanisms. Soil Tillage Res. 2021, 211, 105014. [Google Scholar] [CrossRef]
- Zhao, Q.; Poulson, S.R.; Obrist, D.; Sumaila, S.; Dynes, J.J.; McBeth, J.M.; Yang, Y. Iron-bound organic carbon in forest soils: Quantification and characterization. Biogeosciences 2016, 13, 4777–4788. [Google Scholar] [CrossRef]
- Chen, W.; Chen, W.X.; Dong, K.; Guo, Q.C.; Wang, G.X.; Han, J.G.; Zhang, X.H. Iron-bound organic carbon distribution in freshwater wetlands with varying vegetation and hydrological Regime. Wetlands 2024, 44, 71. [Google Scholar] [CrossRef]
- Fang, K.; Qin, S.Q.; Chen, L.Y.; Zhang, Q.W.; Yang, Y.H. Al/Fe mineral controls on soil organic carbon stock across Tibetan alpine grasslands. J. Geophys. Res. Biogeosci. 2019, 124, 247–259. [Google Scholar] [CrossRef]
- Su, M.L.; Zheng, L.K.; Zhang, X.T.; Hong, H.L.; Chu, T.; Shen, Z.Y.; Lu, H.L. Spatial distribution and environmental drivers of iron-bound organic carbon in coastal wetlands across climatic gradients. Catena 2025, 258, 109298. [Google Scholar] [CrossRef]
- Patzner, M.S.; Mueller, C.W.; Malusova, M.; Baur, M.; Nikeleit, V.; Scholten, T.; Hoeschen, C.; Byrne, J.M.; Borch, T.; Kappler, A. Iron mineral dissolution releases iron and associated organic carbon during permafrost thaw. Nat. Commun. 2020, 11, 6329. [Google Scholar] [CrossRef] [PubMed]
- Carneiro Barreto, M.S.; Wani, R.P.; Goranov, A.I.; Sowers, T.D.; Fischel, M.; Douglas, T.A.; Hatcher, P.G.; Sparks, D.L. Carbon fate, iron dissolution, and molecular characterization of dissolved organic matter in thawed Yedoma permafrost under varying redox conditions. Environ. Sci. Technol. 2024, 58, 4155–4166. [Google Scholar] [CrossRef]
- Yang, L.; Zou, Y.C.; Jiang, M.; Yu, Z.C. Permafrost enhances association of minerals with organic carbon of peatlands in anoxic environments. Environ. Res. 2025, 282, 3122100. [Google Scholar] [CrossRef] [PubMed]
- Joss, H.; Patzner, M.S.; Maisch, M.; Mueller, C.W.; Kappler, A.; Bryce, C. Cryoturbation impacts iron-organic carbon associations along a permafrost soil chronosequence in northern Alaska. Geoderma 2022, 413, 115738. [Google Scholar] [CrossRef]
- Feyissa, A.; Gurmesa, G.A.; Yang, F.; Long, C.Y.; Zhang, Q.A.; Cheng, X.L. Soil enzyme activity and stoichiometry in secondary grasslands along a climatic gradient of subtropical China. Sci. Total Environ. 2022, 825, 154019. [Google Scholar] [CrossRef]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Liu, C.Z.; Li, X.Q.; Ma, L.X.; Zhai, G.Q.; Feng, X.J. Sphagnum increases soil’s sequestration capacity of mineral-associated organic carbon via activating metal oxides. Nat. Commun. 2023, 14, 5052. [Google Scholar] [CrossRef]
- Curtinrich, H.J.; Sebestyen, S.D.; Griffiths, N.A.; Hall, S.J. Warming stimulates iron-mediated carbon and nutrient cycling in mineral-poor peatlands. Ecosystems 2022, 25, 44–60. [Google Scholar] [CrossRef]
- Zarov, E.A.; Lapshina, E.D.; Kuhlmann, I.; Schulze, E. Carbon accumulation and the possibility of carbon losses by vertical movement of dissolved organic carbon in Western Siberian peatlands. Forests 2023, 14, 2393. [Google Scholar] [CrossRef]
- Kanjana, N.; Li, Y.Y.; Shen, Z.J.; Mao, J.J.; Zhang, L.S. Effect of phenolics on soil microbe distribution, plant growth, and gall formation. Sci. Total Environ. 2024, 924, 171329. [Google Scholar] [CrossRef]
- Merino, C.; Kuzyakov, Y.; Godoy, K.; Jofré, I.; Nájera, F.; Matus, F. Iron-reducing bacteria decompose lignin by electron transfer from soil organic matter. Sci. Total Environ. 2021, 761, 143194. [Google Scholar] [CrossRef] [PubMed]
- Longman, J.; Faust, J.C.; Bryce, C.; Homoky, W.B.; März, C. Organic carbon burial with reactive iron across global environments. Glob. Biogeochem. Cycles 2022, 36, e2022GB007447. [Google Scholar] [CrossRef]
- Beillouin, D.; Corbeels, M.; Demenois, J.; Berre, D.; Boyer, A.; Fallot, A.; Feder, F.; Cardinael, R. A global meta-analysis of soil organic carbon in the Anthropocene. Nat. Commun. 2023, 14, 3700. [Google Scholar] [CrossRef]
- Chauhan, A.; Patzner, M.S.; Bhattacharyya, A.; Borch, T.; Fischer, S.; Obst, M.; ThomasArrigo, L.K.; Kretzschmar, R.; Mansor, M.; Bryce, C.; et al. Interactions between iron and carbon in permafrost thaw ponds. Sci. Total Environ. 2024, 946, 174321. [Google Scholar] [CrossRef]
- Villani, M.; Mauclet, E.; Agnan, Y.; Druel, A.; Jasinski, B.; Taylor, M.; Schuur, E.A.G.; Opfergelt, S. Mineral element recycling in topsoil following permafrost degradation and a vegetation shift in sub-Arctic tundra. Geoderma 2022, 421, 115915. [Google Scholar] [CrossRef]




| Sites | Soil Depth (cm) | SOC (g·kg−1) | DOC (mg·kg−1) | TN (g·kg−1) | TP (g·kg−1) | SWC (%) | pH | Phenolics (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|
| 0–10 | 385.29 ± 11.60 Aa | 435.85 ± 9.52 Aab | 7.79 ± 0.62 Cd | 3.26 ± 0.03 Abc | 66.25 ± 0.27 Ca | 5.32 ± 0.07 Bab | 15.18 ± 0.49 Af | |
| CP1 | 10–30 | 311.75 ± 8.29 Ba | 358.16 ± 8.90 Ba | 10.31 ± 0.27 Bc | 3.02 ± 0.04 Ba | 70.15 ± 0.69 Ba | 5.53 ± 0.05 Aa | 12.96 ± 0.25 Bh |
| 30–50 | 270.09 ± 12.77 Ba | 316.27±8.25 Ca | 12.08 ± 0.37 Ad | 2.11 ± 0.04 Ca | 72.36 ± 0.62 Aa | 5.29 ± 0.02 Bb | 15.22 ± 0.20 Ae | |
| 0–10 | 403.58 ± 14.51 Aa | 468.96 ± 14.07 Aa | 8.87 ± 0.25 Bcd | 3.01 ± 0.07 Ac | 61.85 ± 1.01 Bb | 5.45 ± 0.04 Aa | 15.81 ± 0.79 Ae | |
| CP2 | 10–30 | 321.88 ± 11.11 Ba | 363.05 ± 10.69 Ba | 10.33 ± 0.38 Bc | 2.61 ± 0.05 Bb | 67.44 ± 0.66 Ab | 5.21 ± 0.02 Bb | 15.75 ± 0.23 Ag |
| 30–50 | 262.91 ± 20.48 Ca | 300.54 ± 9.63 Cab | 15.94 ± 0.59 Abc | 1.97 ± 0.07 Ca | 70.85 ± 1.59 Aa | 5.65 ± 0.09 Aa | 11.33 ± 0.13 Bf | |
| 0–10 | 358.74 ± 13.18 Aa | 391.25 ± 10.48 Ab | 11.70 ± 0.43 Bb | 3.62 ± 0.08 Aa | 67.39 ± 0.87 Ba | 5.19 ± 0.01 Bb | 17.78 ± 0.39 ABd | |
| CP3 | 10–30 | 283.69 ± 8.36 Ba | 339.61 ± 9.58 Ba | 13.38 ± 0.93 ABab | 3.12 ± 0.03 Ba | 72.51 ± 0.72 Aa | 5.26 ± 0.02 Ab | 17.24 ± 0.24 Bf |
| 30–50 | 224.73 ± 12.24 Cab | 268.95 ± 11.70 Cb | 15.68 ± 0.55 Ac | 2.27 ± 0.06 Ca | 73.08 ± 1.45 Aa | 5.23 ± 0.01 ABb | 18.55 ± 0.16 Ad | |
| 0–10 | 288.61 ± 10.77 Ac | 328.74 ± 11.04 Ade | 11.52 ± 1.23 Bb | 2.62 ± 0.10 Ad | 61.58 ± 2.11 Bb | 5.02 ± 0.03 Acd | 20.02 ± 0.12 Bcd | |
| DP1 | 10–30 | 269.18 ± 9.24 Ab | 302.85 ± 13.82 ABb | 12.41 ± 0.44 ABa | 2.71 ± 0.04 Ab | 68.95 ± 0.43 Ab | 4.91 ± 0.03 ABd | 21.93 ± 0.32 Ad |
| 30–50 | 223.85 ± 5.24 Bab | 263.21 ± 9.79 Bb | 15.32 ± 0.44 Ac | 1.13 ± 0.17 Bc | 63.47 ± 0.36 Bb | 4.83 ± 0.02 Bcd | 20.17 ± 0.21 Bc | |
| 0–10 | 315.49 ± 7.51 Ab | 357.48 ± 13.53 Ac | 7.36 ± 0.26 Cd | 3.32 ± 0.03 Ab | 66.85 ± 0.52 Aa | 4.88 ± 0.03 Be | 21.81 ± 0.57 Abc | |
| DP2 | 10–30 | 291.05 ± 7.93 Aa | 330.92 ± 13.55 Aa | 10.79 ± 0.55 Bc | 2.75 ± 0.05 Bb | 69.01 ± 0.87 Ab | 5.09 ± 0.03 Ac | 21.09 ± 0.13 Ad |
| 30–50 | 221.56 ± 9.38 Bab | 258.72 ± 9.32 Bb | 12.88 ± 0.35 Ad | 1.21 ± 0.05 Cc | 63.78 ± 0.43 Bb | 5.01 ± 0.02 Ac | 20.23 ± 0.11 Bc | |
| 0–10 | 327.25 ± 14.35 Abc | 356.03 ± 11.60 Ac | 10.11 ± 0.44 Bbc | 2.61 ± 0.09 Ad | 66.43 ± 0.37 Aa | 4.82 ± 0.06 ABe | 23.95 ± 1.00 Ab | |
| DP3 | 10–30 | 304.68 ± 18.32 Aa | 349.66 ± 7.22 Aa | 11.35 ± 0.15 Bbc | 2.06 ± 0.07 Bc | 63.37 ± 0.73 Bc | 5.03 ± 0.02 Ac | 20.76 ± 0.22 Be |
| 30–50 | 233.67 ± 15.30 Ba | 285.19 ± 12.51 Ba | 14.26 ± 0.38 Acd | 1.58 ± 0.06 Cb | 62.17 ± 0.67 Bb | 4.72 ± 0.09 Bd | 24.62 ± 0.28 Ab | |
| 0–10 | 261.28 ± 6.77 Ad | 310.52 ± 7.70 Ae | 16.25 ± 1.01 Aa | 2.03 ± 0.03 Ae | 53.74 ± 0.49 Bc | 4.95 ± 0.03 Ade | 22.87 ± 0.21 Bb | |
| IP1 | 10–30 | 230.37 ± 11.15 Ac | 285.76 ± 6.78 Acd | 13.75 ± 0.18 Ba | 1.25 ± 0.04 Be | 58.62 ± 0.16 Ad | 4.67 ± 0.03 Ce | 24.05 ± 0.42 Ac |
| 30–50 | 167.55 ± 9.60 Bbc | 208.15 ± 12.10 Be | 18.37 ± 0.57 Aa | 1.06 ± 0.03 Ccd | 55.62 ± 0.84 Bc | 4.82 ± 0.02 Bcd | 24.19 ± 0.24 Ab | |
| 0–10 | 278.64 ± 17.71 Ad | 332.08 ± 8.26 Ad | 15.81 ± 0.76 Ba | 2.21 ± 0.04 Ae | 51.85 ± 0.66 Bc | 4.43 ± 0.02 Cf | 27.27 ± 0.79 Aa | |
| IP2 | 10–30 | 233.97 ± 11.19 ABc | 289.54 ± 11.64 Bc | 12.27 ± 0.41 Babc | 1.62 ± 0.05 Bd | 56.13 ± 0.89 Ad | 4.58 ± 0.04 Be | 27.09 ± 0.13 Ab |
| 30–50 | 182.43 ± 9.91 Bb | 231.15 ± 8.56 Cde | 18.85 ± 1.31 Aa | 0.98 ± 0.03 Ccd | 50.52 ± 0.87 Bd | 4.77 ± 0.03 Ad | 24.20 ± 0.25 Bb | |
| 0–10 | 300.82 ± 8.75 Ac | 375.86 ± 15.38 Ac | 14.42 ± 0.63 Ba | 2.15 ± 0.09 Ae | 47.55 ± 1.45 Bd | 4.51 ± 0.02 Bf | 27.79 ± 0.17 ABa | |
| IP3 | 10–30 | 223.95 ± 10.51 Bc | 274.65 ± 6.79 Bd | 11.39 ± 0.31 Cbc | 1.36 ± 0.10 Be | 52.38 ± 0.66 Ae | 4.40 ± 0.02 Cf | 28.38 ± 0.20 Aa |
| 30–50 | 203.81 ± 11.59 Bbc | 248.97 ± 9.90 Bc | 18.06 ± 0.67 Aa | 0.79 ± 0.06 Cd | 45.19 ± 0.49 Be | 4.63 ± 0.03 Ad | 27.22 ± 0.22 Ba |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kan, S.; Yin, W.; Li, Z.; Guo, X.; Ma, D.; Yu, H.; Zhao, Y. The “Iron Gate” Outcompetes the “Enzymic Latch” as the Dominant Soil Organic Carbon Stabilization Mechanism in Permafrost Peatlands of the Great Hing’an Mountains. Biology 2025, 14, 1504. https://doi.org/10.3390/biology14111504
Kan S, Yin W, Li Z, Guo X, Ma D, Yu H, Zhao Y. The “Iron Gate” Outcompetes the “Enzymic Latch” as the Dominant Soil Organic Carbon Stabilization Mechanism in Permafrost Peatlands of the Great Hing’an Mountains. Biology. 2025; 14(11):1504. https://doi.org/10.3390/biology14111504
Chicago/Turabian StyleKan, Shuping, Weiping Yin, Zhao Li, Xinmiao Guo, Dalong Ma, Huan Yu, and Yiting Zhao. 2025. "The “Iron Gate” Outcompetes the “Enzymic Latch” as the Dominant Soil Organic Carbon Stabilization Mechanism in Permafrost Peatlands of the Great Hing’an Mountains" Biology 14, no. 11: 1504. https://doi.org/10.3390/biology14111504
APA StyleKan, S., Yin, W., Li, Z., Guo, X., Ma, D., Yu, H., & Zhao, Y. (2025). The “Iron Gate” Outcompetes the “Enzymic Latch” as the Dominant Soil Organic Carbon Stabilization Mechanism in Permafrost Peatlands of the Great Hing’an Mountains. Biology, 14(11), 1504. https://doi.org/10.3390/biology14111504




