Photoperiod and Circadian Regulation in Plants: A Review of Insights from In Vitro Studies
Simple Summary
Abstract
1. Introduction
2. Circadian Rhythm in Plants
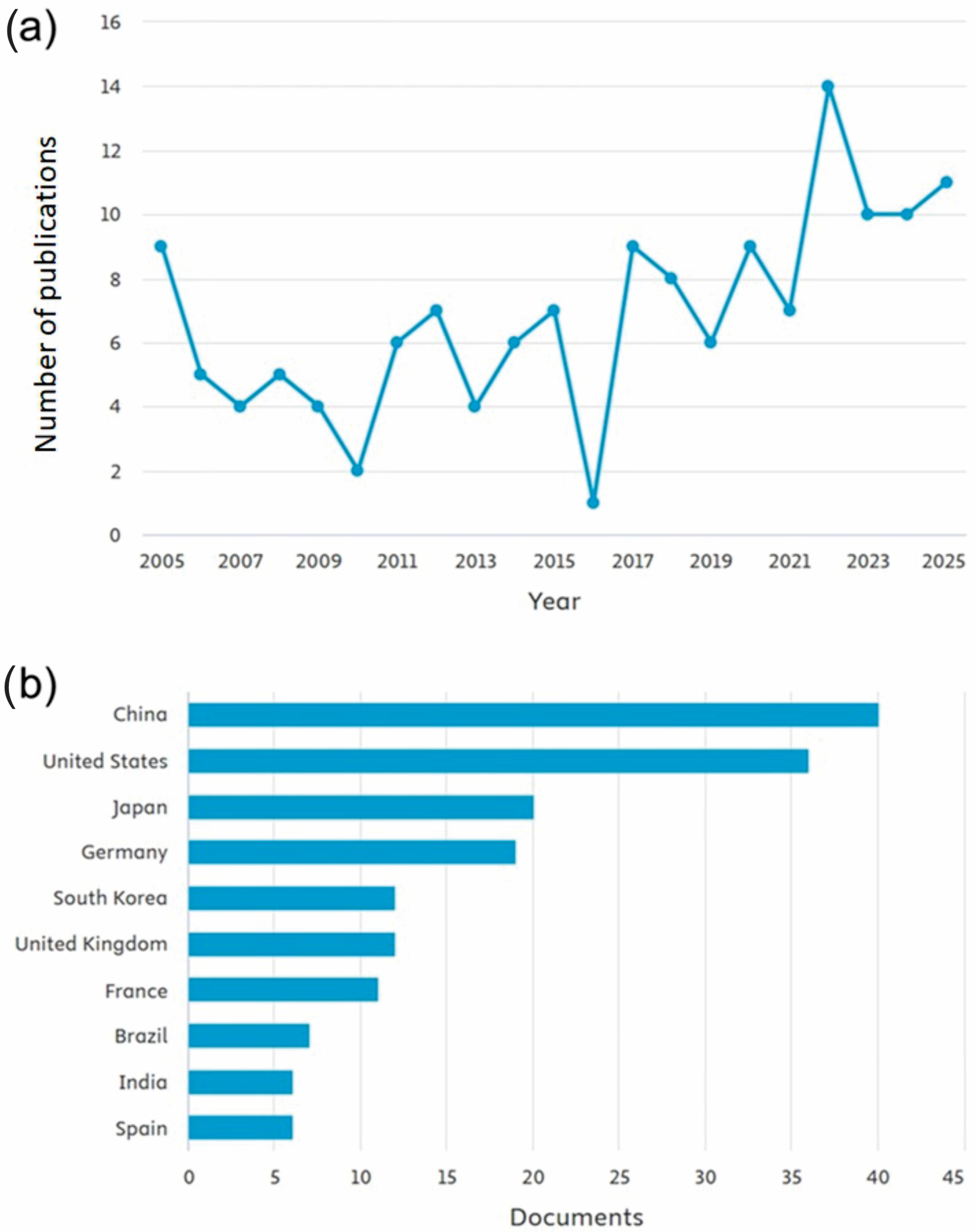
Publications on Circadian Rhythm in Plants In Vitro
3. Photoperiod in Plants
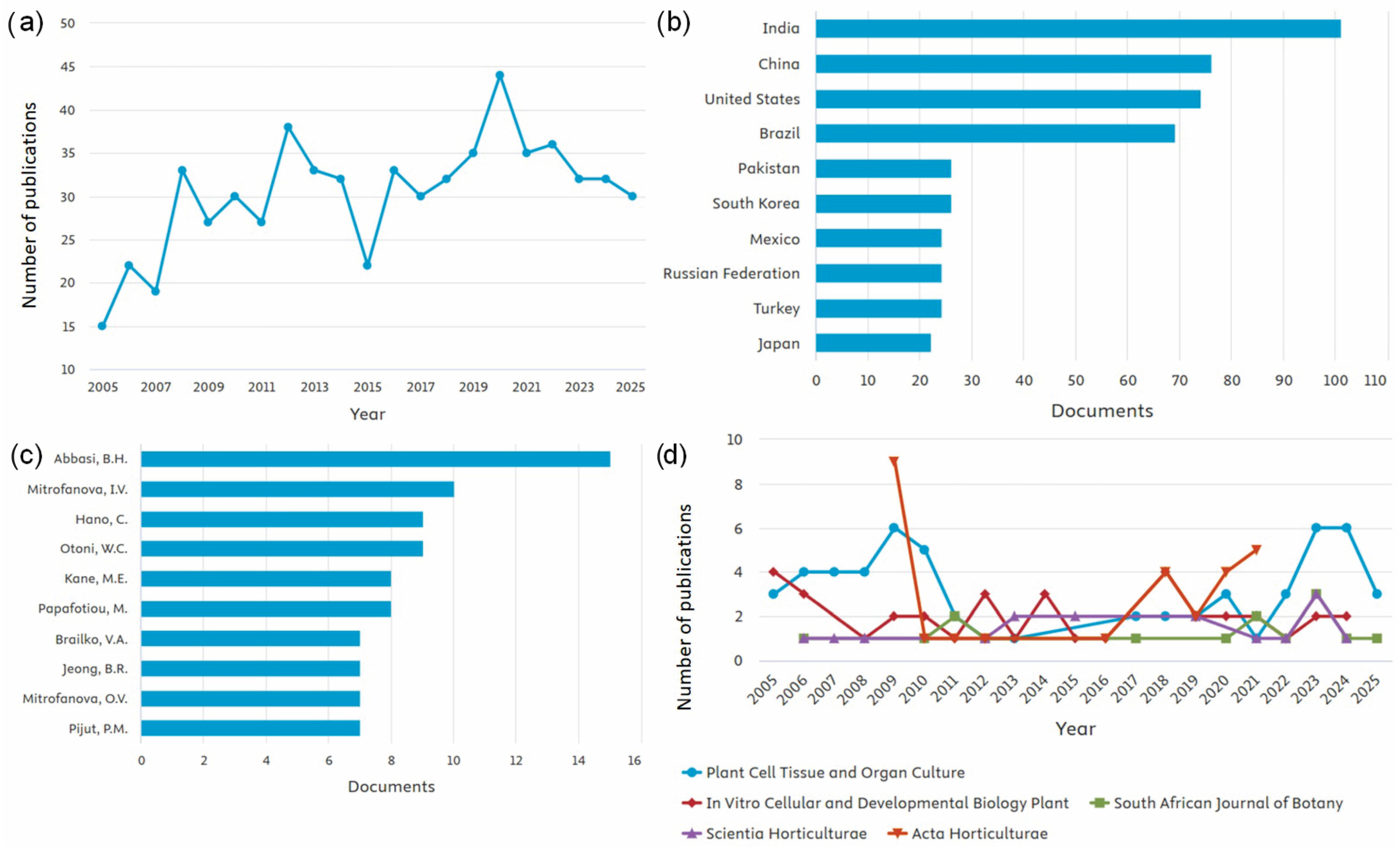
Publications on Photoperiod in In Vitro Plants
4. Response of In Vitro Grown Plants to Photoperiod and Variation in Circadian Rhythms
4.1. Plant Flowering Time Responses to Photoperiod
4.2. Responses on Primary Metabolism
4.3. Responses on Secondary Metabolism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.P.; Loh, C.S. Plant Tissue Culture for Biotechnology. In Plant Biotechnology and Agriculture; Elsevier: Amsterdam, The Netherlands, 2012; pp. 131–138. [Google Scholar] [CrossRef]
- Wijerathna-Yapa, A.; Ramtekey, V.; Ranawaka, B.; Basnet, B.R. Applications of in vitro tissue culture technologies in breeding and genetic improvement of wheat. Plants 2022, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L. Plant tissue culture. Biotechnology milestones. In Vitro Cell. Dev. Biol. Plant 2002, 38, 84–89. [Google Scholar] [CrossRef]
- Wijerathna-Yapa, A.; Hiti-Bandaralage, J. Tissue culture—A sustainable approach to explore plant stresses. Life 2023, 13, 780. [Google Scholar] [CrossRef]
- Geng, Y.; Gao, L.; Yang, J. Epigenetic flexibility underlying phenotypic plasticity. In Progress in Botany; Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 74, pp. 153–163. [Google Scholar] [CrossRef]
- Srivastava, D.; Shamim, M.D.; Kumar, M.; Mishra, A.; Maurya, R.; Sharma, D. Role of circadian rhythm in plant system: An update from development to stress response. Environ. Exp. Bot. 2019, 162, 256–271. [Google Scholar] [CrossRef]
- Man, A.W.C.; Xia, N.; Li, H. Circadian rhythm in adipose tissue: Novel antioxidant target for metabolic and cardiovascular diseases. Antioxidants 2020, 9, 968. [Google Scholar] [CrossRef]
- McClung, C.R. Plant circadian rhythms. Plant Cell 2006, 18, 792–803. [Google Scholar] [CrossRef]
- Creux, N.; Harmer, S. Circadian rhythms in plants. Cold Spring Harb. Perspect. Biol. 2019, 11, a034611. [Google Scholar] [CrossRef]
- Hotta, C.T. From crops to shops: How agriculture can use circadian clocks. J. Exp. Bot. 2021, 72, 7668–7679. [Google Scholar] [CrossRef]
- Greenwood, M.; Locke, J.C.W. The circadian clock coordinates plant development through specificity at the tissue and cellular level. Curr. Opin. Plant Biol. 2020, 53, 65–72. [Google Scholar] [CrossRef]
- Muranaka, T.; Oyama, T. Monitoring circadian rhythms of individual cells in plants. J. Plant Res. 2018, 131, 15–21. [Google Scholar] [CrossRef]
- Oravec, M.W.; Greenham, K. The adaptive nature of the plant circadian clock in natural environments. Plant Physiol. 2022, 190, 968–980. [Google Scholar] [CrossRef]
- Staiger, D.; Green, R. RNA-based regulation in the plant circadian clock. Trends Plant Sci. 2011, 16, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Gil, K.E.; Kim, W.Y.; Lee, H.J.; Faisal, M.; Saquib, Q.; Alatar, A.A.; Park, C.M. Contributes to a thermoresponsive protein quality control system in Arabidopsis. Plant Cell 2017, 29, 2882–2894. [Google Scholar] [CrossRef]
- Webb, A.A.R.; Seki, M.; Satake, A.; Caldana, C. Continuous dynamic adjustment of the plant circadian oscillator. Nat. Commun. 2019, 10, 550. [Google Scholar] [CrossRef]
- Taniguchi, T.; Murayama, N.; Ario, N.; Nakagawa, A.C.; Tanaka, S.; Tomoita, Y.; Ishibashi, Y. Photoperiod sensing of leaf regulates pod setting in soybean (Glycine max (L.) Merr.). Plant Prod. Sci. 2020, 23, 360–365. [Google Scholar] [CrossRef]
- Moher, M.; Jones, M.; Zheng, Y. Photoperiodic response of in vitro Cannabis sativa plants. HortScience 2020, 56, 108–113. [Google Scholar] [CrossRef]
- Serrano-Bueno, G.; Sanchez de Medina Hernandez, V.; Valverde, F. Photoperiodic signaling and senescence, an ancient solution to a modern problem? Front. Plant Sci. 2021, 12, 634393. [Google Scholar] [CrossRef]
- Rezvani, M.; Nadimi, S.; Zaefarian, F.; Chauhan, B.S. Environmental factors affecting seed germination and seedling emergence of three Phalaris species. Crop Prot. 2021, 148, 105743. [Google Scholar] [CrossRef]
- Asher, G.; Zhu, B. Beyond circadian rhythms: Emerging roles of ultradian rhythms in control of liver functions. Hepatology 2023, 77, 1022–1035. [Google Scholar] [CrossRef]
- Greenham, K.; McClung, C.R. Integrating circadian dynamics with physiological processes in plants. Nat. Rev. Genet. 2015, 16, 598–610. [Google Scholar] [CrossRef]
- Lal, H.; Verma, S.K.; Wang, Y.; Xie, M.; Young, M.E. Circadian rhythms in cardiovascular metabolism. Circ. Res. 2024, 134, 635–658. [Google Scholar] [CrossRef] [PubMed]
- Nohales, M.A.; Kay, S.A. Molecular mechanisms at the core of the plant circadian oscillator. Nat. Struct. Mol. Biol. 2016, 23, 1061–1069. [Google Scholar] [CrossRef]
- Inoue, K.; Araki, T.; Endo, M. Circadian clock during plant development. J. Plant Res. 2018, 131, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Dios, V.R.; Anderegg, W.R.L.; Li, X.; Tissue, D.T. Circadian regulation of photosynthesis and transpiration from genes to ecosystems. Environ. Exp. Bot. 2018, 152, 37–48. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, L.; Yang, X.; Zhang, X.; Wang, L.; Xie, Q. Circadian clock in plants: Linking timing to fitness. J. Integr. Plant Biol. 2022, 64, 792–811. [Google Scholar] [CrossRef]
- Harmer, S.L. The circadian system in higher plants. Annu. Rev. Plant Biol. 2009, 60, 357–377. [Google Scholar] [CrossRef]
- McClung, C.R. Wheels within wheels: New transcriptional feedback loops in the Arabidopsis circadian clock. F1000Prime Rep. 2014, 6, 2. [Google Scholar] [CrossRef]
- Bendix, C.; Marshall, C.M.; Harmon, F.G. Circadian clock genes universally control key agricultural traits. Mol. Plant 2015, 8, 1135–1152. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kiba, T.; Henriques, R.; Mizuno, T.; Chua, N.H.; Sakakibara, H. PSEUDO-RESPONSE REGULATORS 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell 2010, 22, 594–605. [Google Scholar] [CrossRef]
- Rawat, R.; Takahashi, N.; Hsu, P.Y.; Jones, M.A.; Schwartz, J.; Salemi, M.R.; Harmer, S.L. REVEILLE8 and PSEUDO-RESPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock. PLoS Genet. 2011, 7, e1001350. [Google Scholar] [CrossRef] [PubMed]
- Alabadi, D.; Oyama, T.; Yanovsky, M.J.; Harmon, F.G.; Mas, P.; Kay, S.A. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 2001, 293, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, L. Unraveling the circadian clock in Arabidopsis. Plant Signal. Behav. 2013, 8, e23014. [Google Scholar] [CrossRef]
- Seo, P.J.; Mas, P. STRESSing the role of the plant circadian clock. Trends Plant Sci. 2015, 20, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A. Entrainment of the Arabidopsis circadian clock. J. Plant Biol. 2009, 52, 202–209. [Google Scholar] [CrossRef]
- Weng, X.; Lovell, J.T.; Schwartz, S.L.; Cheng, C.; Haque, T.; Zhang, L. Complex interactions between day length and diurnal patterns of gene expression drive photoperiodic responses in a perennial C4 grass. Plant Cell Environ. 2019, 42, 2165–2182. [Google Scholar] [CrossRef]
- Michael, T.P.; Mockler, T.C.; Breton, G.; McEntee, C.; Byer, A.; Trout, J.D. Network discovery pipeline elucidates conserved time-of-day-specific cis-regulatory modules. PLoS Genet. 2008, 4, e14. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zuo, Y.; Wei, J.; Wang, L. The circadian clock coordinates the tradeoff between adaptation to abiotic stresses and yield in crops. Biology 2023, 12, 1364. [Google Scholar] [CrossRef]
- McClung, C.R. Circadian clock components offer targets for crop domestication and improvement. Genes 2021, 12, 374. [Google Scholar] [CrossRef]
- Ng, D.W.K.; Miller, M.; Yu, H.H.; Huang, T.Y.; Kim, E.D.; Lu, J.; Chen, Z.J. A role for CHH methylation in the parent-of-origin effect on altered circadian rhythms and biomass heterosis in Arabidopsis intraspecific hybrids. Plant Cell 2014, 26, 2430–2440. [Google Scholar] [CrossRef]
- Ko, D.K.; Rohozinski, D.; Song, Q.; Taylor, S.H.; Juenger, T.E.; Harmon, F.G.; Chen, Z.J. Temporal shift of circadian-mediated gene expression and carbon fixation contributes to biomass heterosis in maize hybrids. PLoS Genet. 2016, 12, e1006197. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, F.; Lin, Q.; Sheng, P.; Wu, Z.; Jin, X.; Wan, J. A regulatory loop establishes the link between the circadian clock and abscisic acid signaling in rice. Plant Physiol. 2023, 191, 1857–1870. [Google Scholar] [CrossRef]
- Fowler, S.; Lee, K.; Onouchi, H.; Samach, A.; Richardson, K.; Morris, B.; Putterill, J. GIGANTEA: A circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 1999, 18, 4679–4688. [Google Scholar] [CrossRef]
- Cao, S.; Ye, M.; Jiang, S. Involvement of GIGANTEA gene in the regulation of the cold stress response in Arabidopsis. Plant Cell Rep. 2005, 24, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.G.; Huh, J.S.; Lim, C.J.; Ahn, G.; Cha, J.Y.; Jeong, S.Y.; Kim, W.Y. GIGANTEA functions as a co-repressor of cold stress response with a histone-modifying complex. Plant Physiol. Biochem. 2025, 223, 109801. [Google Scholar] [CrossRef]
- Riboni, M.; Galbiati, M.; Tonelli, C.; Conti, L. GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS. Plant Physiol. 2013, 162, 1706–1719. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Kim, W.Y.; Cha, J.Y.; Park, H.J.; Shin, G.; Park, J.; Yun, D.J. The GIGANTEA-ENHANCED EM LEVEL complex enhances drought tolerance via regulation of abscisic acid synthesis. Plant Physiol. 2020, 184, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Ali, Z.; Park, H.J.; Park, S.J.; Cha, J.Y.; Perez-Hormaeche, J.; Quintero, F.J.; Shin, G.; Kim, M.R.; Qiang, Z.; et al. Release of SOS2 kinase from sequestration with GIGANTEA determines salt tolerance in Arabidopsis. Nat. Commun. 2013, 4, 1352. [Google Scholar] [CrossRef]
- Hajdu, A.; Nyári, D.; Terecskei, K.; Gyula, P.; Ádám, É.; Dobos, O.; Kozma-Bognár, L. LIP1 regulates the plant circadian oscillator by modulating the function of the clock component GIGANTEA. Cells 2024, 13, 1503. [Google Scholar] [CrossRef]
- Kim, J.A.; Jung, H.; Hong, J.K.; Hermand, V.; Robertson McClung, C.; Lee, Y.H.; Kim, J.Y.; Lee, S.I.; Jeong, M.J.; Kim, J.; et al. Reduction of GIGANTEA expression in transgenic Brassica rapa enhances salt tolerance. Plant Cell Rep. 2016, 35, 1943–1954. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; He, M.; Dong, L.; Huang, Z.; Chen, L.; Zhao, X. GIGANTEA orthologs, E2 members, redundantly determine photoperiodic flowering and yield in soybean. J. Integr. Plant Biol. 2023, 65, 188–202. [Google Scholar] [CrossRef]
- Jackson, S.D. Plant responses to photoperiod. New Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Roeber, V.M.; Schmülling, T.; Cortleven, A. The photoperiod: Handling and causing stress in plants. Front. Plant Sci. 2022, 12, 781988. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod control of plant growth: Flowering time genes beyond flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef]
- Hotta, C.T.; Gardner, M.J.; Hubbard, K.E.; Baek, S.J.; Dalchau, N.; Suhita, D.; Webb, A.A.R. Modulation of environmental responses of plants by circadian clocks. Plant Cell Environ. 2007, 30, 333–349. [Google Scholar] [CrossRef]
- Batista, D.S.; Felipe, S.H.S.; Silva, T.D.; de Castro, K.M.; Mamedes-Rodrigues, T.C.; Miranda, N.A.; Ríos-Ríos, A.M.; Faria, D.V.; Fortini, E.A. Light quality in plant tissue culture: Does it matter? Vitr. Cell. Dev. Biol. Plant 2018, 54, 195–215. [Google Scholar] [CrossRef]
- Garner, W.W.; Allard, H.A. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. Mon. Weather Rev. 1920, 48, 415. [Google Scholar] [CrossRef]
- Micallef, B.J. Circadian clocks/photoperiodism and crop quality. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Song, Y.H.; Shim, J.S.; Kinmonth-Schultz, H.A.; Imaizumi, T. Photoperiodic flowering: Time measurement mechanisms in leaves. Annu. Rev. Plant Biol. 2015, 66, 441–464. [Google Scholar] [CrossRef]
- Lavie, O.; Buxdorf, K.; Eshed Williams, L. Optimizing cannabis cultivation: An efficient in vitro system for flowering induction. Plant Methods 2024, 20, 141. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, Y.; Dong, H.; Shan, X.; Chen, H.; Li, X.; Ren, C. Comparative transcriptome analysis of oat varieties with different flowering performances under a short-day photoperiod. BMC Plant Biol. 2025, 25, 622. [Google Scholar] [CrossRef]
- Plantenga, F.D.; Heuvelink, E.; Rienstra, J.A.; Visser, R.G.; Bachem, C.W.; Marcelis, L.F. Coincidence of potato CONSTANS (StCOL1) expression and light cannot explain night-break repression of tuberization. Physiol. Plant. 2019, 167, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Svystun, T.; AlDahmash, B.; Jonsson, A.M.; Bhalerao, R.P. Photoperiod- and temperature-mediated control of phenology in trees: A molecular perspective. New Phytol. 2017, 213, 511–524. [Google Scholar] [CrossRef]
- Alter, H.; Sade, Y.; Sood, A.; Carmeli-Weissberg, M.; Shaya, F.; Kamenetsky-Goldstein, R.; Spitzer-Rimon, B. Inflorescence development in female cannabis plants is mediated by photoperiod and gibberellin. Hortic. Res. 2024, 11, uhae245. [Google Scholar] [CrossRef] [PubMed]
- Margay, A.R.; Ashraf, S.; Fatimah, N.; Jabeen, S.G.; Showkat, M.; Nayana R U, K.; Dilip, S.; Basu, S.R.; Aswathy, K.A. Plant Circadian Clocks: Unravelling the Molecular Rhythms of Nature. Int. J. Plant Soil Sci. 2024, 36, 596–617. [Google Scholar] [CrossRef]
- Roblin, G.; Moyen, C.; Fleurat-Lessard, P.; Dédaldéchamp, F. Rapid osmocontractile response of motor cells of Mimosa pudica pulvini induced by short light signals. Photochem. Photobiol. 2024, 101, 728–745. [Google Scholar] [CrossRef]
- González-Delgado, A.; Jiménez-Gómez, J.M.; Wabnik, K. Regulatory principles of photoperiod-driven clock function in plants. Trends Plant Sci. 2025, 30, 594–602. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Xu, M.; Wang, Y.; Zhang, Z.; Wai, M.H.; Rizwan, H.M.; Cheng, Y. A highly efficient organogenesis system based on 6-benzylaminopurine and indole-6-butyric acid in Suaeda glauca, a medicinal halophyte under varying photoperiods. Ind. Crops Prod. 2024, 216, 118672. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M. In vitro seed and clonal propagation of the Mediterranean bee-friendly plant Anthyllis hermanniae L. Sustainability 2023, 15, 4025. [Google Scholar] [CrossRef]
- Bajwa, M.N.; Khanum, M.; Zaman, G.; Ullah, M.A.; Farooq, U.; Waqas, M.; Abbasi, B.H. Effect of wide-spectrum monochromatic lights on growth, phytochemistry, nutraceuticals, and antioxidant potential of in vitro callus cultures of Moringa oleifera. Molecules 2023, 28, 1497. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, R.N.; Kalasnikova, E.A.; Bolotina, E.A.; Saleh, A.; Balakina, A.A.; Zaytseva, S.M. Localization of secondary metabolites in relict gymnosperms of the genus Sequoia in vivo and in cell cultures in vitro, and the biological activity of their extracts. Life 2024, 14, 1694. [Google Scholar] [CrossRef]
- Wojtania, A.; Markiewicz, M.; Waligórski, P. Growth cessation and dormancy induction in micropropagated plantlets of Rheum rhaponticum ‘Raspberry’ influenced by photoperiod and temperature. Int. J. Mol. Sci. 2022, 24, 607. [Google Scholar] [CrossRef]
- Zhu, T.T.; Xu, Y.L.; Ta, H.; Zhang, J.Z.; Xu, D.D.; Fu, J.; Lou, H.X. Reversible Glc-conjugation/hydrolysis modulates the homeostasis of lunularic acid in Marchantia polymorpha growth. Plant J. 2025, 121, e17166. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhang, S.; Ai, J.; Wang, Z.; Shi, G.; Guo, J.; Liu, Y. Physiological and molecular mechanisms of radicle development of somatic embryos in Schisandra chinensis cultured in the dark. Plant Cell Tissue Organ Cult. 2024, 157, 1. [Google Scholar] [CrossRef]
- Wang, D.; Hu, X.; Ye, H.; Wang, Y.; Yang, Q.; Liang, X.; Wang, K. Cell-specific clock-controlled gene expression program regulates rhythmic fiber cell growth in cotton. Genome Biol. 2023, 24, 49. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Li, Z.; Nulu, N.P.C.; Kalaipandian, S.; Biddle, J.M.; Bazrafshan, A.; Adkins, S.W. A photomixotrophic system to improve the growth of in vitro-cultured seedlings of coconut (Cocos nucifera L.). Horticulturae 2025, 11, 224. [Google Scholar] [CrossRef]
- Durul, M.S.; Aktaş, T.K. In vitro propagation of Cydonia oblonga cv. Esme. Turk. J. Agric. For. 2023, 47, 578–589. [Google Scholar] [CrossRef]
- Wang, E.; Zhou, T.; Jing, S.; Dong, L.; Sun, X.; Fan, Y.; Song, B. Leaves and stolons transcriptomic analysis provide insight into the role of phytochrome F in potato flowering and tuberization. Plant J. 2023, 113, 402–415. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Vlachou, G.; Trigka, M.; Papafotiou, M. In vitro studies on seed germination of the Mediterranean species Anthyllis barba-jovis to facilitate its introduction into the floriculture industry. Horticulturae 2022, 8, 889. [Google Scholar] [CrossRef]
- Takase, T.; Shimizu, M.; Takahashi, S.; Nemoto, K.; Goto, F.; Yoshida, C.; Nishihara, M. De novo transcriptome analysis reveals flowering-related genes that potentially contribute to flowering-time control in the Japanese cultivated gentian Gentiana triflora. Int. J. Mol. Sci. 2022, 23, 11754. [Google Scholar] [CrossRef]
- Zale, P.J.; Clayton, A.; Nix, J.; Taylor, M. Asymbiotic in vitro seed germination, in vitro seedling development, and ex vitro acclimatization of Spiranthes. Appl. Plant Sci. 2022, 10, e11494. [Google Scholar] [CrossRef]
- Ramos, S.M.; Berman-Bahena, S.; Alvarez, L.; Sánchez-Carranza, J.N.; Bernabé-Antonio, A.; Román-Guerrero, A.; Cruz-Sosa, F. Effect of plant growth regulators on different explants of Artemisia ludoviciana under photoperiod and darkness conditions and their influence on achillin production. Processes 2022, 10, 1439. [Google Scholar] [CrossRef]
- Amoo, S.O.; Hlatshwayo, N.A.; Doležal, K.; Olowoyo, J.O. Seed germination and in vitro propagation of three threatened endemic South African Aloe species. S. Afr. J. Bot. 2022, 147, 1214–1220. [Google Scholar] [CrossRef]
- Bohdanovych, T.A.; Matvieieva, N.A. Effect of phenylalanine and light on the growth of hairy roots of Artemisia tilesii Ledeb. Biotechnol. Acta 2023, 16, 61–69. [Google Scholar] [CrossRef]
- Istiqomah, N.; Indriani, H.; Wijaya, Y.I.F.; Yalapuspita, D.C.; Handini, E.; Diantina, S.; Semiarti, E. Clonal propagation of rare orchid species Paphiopedilum spp. (Orchidaceae) to save Indonesian biodiversity. S. Afr. J. Bot. 2024, 172, 779–785. [Google Scholar] [CrossRef]
- Pecheva, D.; Danova, K. Light and auxin treatments affect morphogenesis and polyphenolics productivity in Artemisia alba Turra cell aggregates in vitro. BioRisk 2022, 17, 213–225. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Injamum-Ul-Hoque, M.; Das, A.K.; Shaffique, S.; Hasan, M.; Kang, S.-M.; Lee, I.-J.; Choi, H.W. Tuning Up In Vitro Growth and Development of Cannabis sativa: Recent Advances in Micropropagational Approach. Appl. Biosc. 2025, 4, 12. [Google Scholar] [CrossRef]
- Shen, P.; Gao, S.; Hu, J.; Li, Y.; Lei, T.; Shi, L. In vitro flowering of the distylous plant Plumbago auriculata Lam. S. Afr. J. Bot. 2021, 137, 492–498. [Google Scholar] [CrossRef]
- Sarma, I.; Deka, A.C.; Sarma, T.C. A protocol for rapid clonal propagation and microrhizome production of Curcuma caesia Roxb. (Zingiberaceae): A critically endangered medicinal plant of North East India. Indian J. Agric. Res. 2021, 55, 13–22. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef]
- Rahimi Khonakdari, M.; Rezadoost, H.; Heydari, R.; Mirjalili, M.H. Effect of photoperiod and plant growth regulators on in vitro mass bulblet proliferation of Narcissus tazzeta L. (Amaryllidaceae), a potential source of galantamine. Plant Cell Tissue Organ Cult. 2020, 142, 187–199. [Google Scholar] [CrossRef]
- Kumar, S.S.; Arya, M.; Mahadevappa, P.; Giridhar, P. Influence of photoperiod on growth, bioactive compounds and antioxidant activity in callus cultures of Basella rubra L. J. Photochem. Photobiol. B 2020, 209, 111937. [Google Scholar] [CrossRef] [PubMed]
- Virdi, A.S.; Singh, N.; Bains, K.K.; Kaur, A. Effect of photoperiod and growth media on yield and antioxidant properties of wheatgrass juice of Indian wheat varieties. J. Food Sci. Technol. 2021, 58, 3019–3029. [Google Scholar] [CrossRef]
- Castro, K.M.; Batista, D.S.; Fortini, E.A.; Silva, T.D.; Felipe, S.H.S.; Fernandes, A.M.; Otoni, W.C. Photoperiod modulates growth, morphoanatomy, and linalool content in Lippia alba L. (Verbenaceae) cultured in vitro. Plant Cell Tissue Organ Cult. 2019, 139, 139–153. [Google Scholar] [CrossRef]
- El-Sayed, S.F.; Taha, S.S.; Darwish, O.S.; Mwessongo, S.Z. Effect of silver thiosulphate and photoperiod on in vitro tuberization of three potato (Solanum tuberosum L.) cultivars. Plant Arch. 2021, 21, 308–317. [Google Scholar] [CrossRef]
- Choirunnisa, J.P.; Wardana, R. Effect of photoperiod and KNO3 concentration on the induction and development of potato (Solanum tuberosum) microtuber in vitro. Cell Biol. Dev. 2021, 5, 2. [Google Scholar] [CrossRef]
- Wafa, A.; Fekry, W.; Hassan, A.; Khatab, I. In vitro microtuberization of some potato (Solanum tuberosum L.) cultivars as response to media constituents and photoperiod. J. Prod. Dev. 2024, 29, 81–98. [Google Scholar]
- Shofiyani, A.; Suwarto; Suprayogi; Yuniaty, A. Growth characteristics and production of bioactive compounds in aromatic ginger (Kaempferia galanga) callus under photoperiod and auxin treatments. Int. J. Agric. Biol. 2023, 29, 410–420. [Google Scholar]
- Al-Aizari, A.A.; Dewir, Y.H.; Al-Obeed, R.S.; Al-Saif, A.M.; Almutairi, K.F.; Murthy, H.N.; Hakiman, M. Study of shoot tip necrosis problems of Fegra Fig (Ficus palmata Forssk.) in vitro in Saudi Arabia. HortScience 2024, 59, 1127–1132. [Google Scholar] [CrossRef]
- Fortini, E.A.; Batista, D.S.; de Castro, K.M.; Silva, T.D.; Felipe, S.H.S.; Correia, L.N.F.; Otoni, W.C. Photoperiod modulates growth and pigments and 20-hydroxyecdysone accumulation in Brazilian ginseng [Pfaffia glomerata (Spreng.) Pedersen] grown in vitro. Plant Cell Tissue Organ Cult. 2020, 142, 595–611. [Google Scholar] [CrossRef]
- Guillén-Rodríguez, S.; Cruz-López, C.; Martínez-Ávalos, J.G.; Martínez-Palacios, A. Effect of N6-Benzyladenine and photoperiod on the flowering of in vitro protocorms of Bletia urbana (Orchidaceae). Rev. Fitotec. Mex. 2022, 45, 475–482. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Maloupa, E.; Grigoriadou, K. In vitro direct organogenesis of the Cretan dittany (Origanum dictamnus L.), an important threatened Greek endemic species. Notul. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 127–135. [Google Scholar] [CrossRef]
- Meneses, L.S.; Morillo, L.E.; Vásquez-Castillo, W. In vitro propagation of Vaccinium floribundum Kunth from seeds: Promissory technology for mortiño accelerated production. Can. J. Plant Sci. 2022, 102, 216–224. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Khan, T.; Khurshid, R.; Nadeem, M.; Drouet, S.; Hano, C. UV-C mediated accumulation of pharmacologically significant phytochemicals under light regimes in in vitro culture of Fagonia indica (L.). Sci. Rep. 2021, 11, 679. [Google Scholar] [CrossRef]
- Arcidiacono, M.; Catalano, C.; Motisi, A.; Sajeva, M.; Carimi, F.; Carra, A. Influence of culture conditions on in vitro asymbiotic germination of Anacamptis longicornu and Ophrys panormitana (Orchidaceae). Plants 2021, 10, 2543. [Google Scholar] [CrossRef]
- Kondhare, K.R.; Kumar, A.; Patil, N.S.; Malankar, N.N.; Saha, K.; Banerjee, A.K. Development of aerial and belowground tubers in potato is governed by photoperiod and epigenetic mechanism. Plant Physiol. 2021, 187, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Ricco, M.V.; Bari, M.L.; Catalano, A.V.; López, P.; Dobrecky, C.B.; Teves, S.A.; Álvarez, M.A. Dynamics of polyphenol bio-synthesis by calli cultures, suspension cultures and wild specimens of the medicinal plant Ligaria cuneifolia (Ruiz & Pav.) Tiegh. (Loranthaceae). Analysis of their biological activity. Plants 2021, 10, 1713. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Leung, C.C.; Tarté, D.A.; Gendron, J.M. Plants distinguish different photoperiods to independently control seasonal flowering and growth. Science 2024, 383, eadg9196. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kaya, H.; Goto, K.; Iwabuchi, M.; Araki, T. A pair of related genes with antagonistic roles in mediating flowering signals. Science 1999, 286, 1960–1962. [Google Scholar] [CrossRef]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef]
- Pittendrigh, C.S.; Minis, D.H. The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Am. Nat. 1964, 98, 261–294. [Google Scholar] [CrossRef]
- Dutta, M.; Mali, S.; Raturi, V.; Zinta, G. Transcriptional and post-transcriptional regulation of tuberization in potato (Solanum tuberosum L.). J. Plant Growth Reg. 2024, 43, 1–24. [Google Scholar] [CrossRef]
- Jo, E.A.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. Effect of photoperiod and light intensity on in vitro propagation of Alocasia amazonica. Plant Biotechnol. Rep. 2008, 2, 207–212. [Google Scholar] [CrossRef]
- Hussain, A.; Qarshi, I.A.; Nazir, H.; Ullah, I. Plant tissue culture: Current status and opportunities. In Recent Advances in Plant In Vitro Culture; IntechOpen: London, UK, 2012. [Google Scholar]
- Xiang, Y.; Sapir, T.; Rouillard, P. Interaction between photoperiod and variation in circadian rhythms in tomato. BMC Plant Biol. 2022, 22, 187. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.V.S.; de Castro, R.D.; da Silva Cunha, D.; Neto, V.G.; Carosio, M.G.A.; Ferreira, A.G.; Ribeiro, P.R. Stevia rebaudiana (Bert.) Bertoni cultivated under different photoperiod conditions: Improving physiological and biochemical traits for industrial applications. Ind. Crops Prod. 2021, 168, 113595. [Google Scholar] [CrossRef]
| Species | Propagation Method | Effects Studied | Response Type | Reference |
|---|---|---|---|---|
| Suaeda glauca | cotyledons, hypocotyls, and leaves | Explant type, plant growth regulators, and photoperiod | Molecular mechanisms and primary metabolism | Mohammadi et al. [69] |
| Marchantia polymorpha | buds | Light intensity | Molecular mechanisms and primary metabolism | Zhu et al. [74] |
| Schisandra chinensis | embryos | Light intensity | Primary metabolism | Sun et al. [75] |
| Gossypium hirsutum | ovules | Photoperiod | Primary metabolism | Wang et al. [76] |
| Cocos nucifera | embryos | CO2 concentrations, light intensities, light qualities, and photoperiod | Primary metabolism | Mu et al. [77] |
| Sequoia sempervirens | shoots | Culture medium and photoperiod | Secondary metabolism | Kirakosyan et al. [72] |
| Anthyllis hermanniae | shoots | Plant growth regulators and photoperiod | Primary metabolism | Martini et al. [70] |
| Moringa oleifera | callus | Light quality and photoperiod | Primary and secondary metabolism | Bajwa et al. [71] |
| Cydonia oblonga | shoots | Plant growth regulators and photoperiod | Primary metabolism | Durul et al. [78] |
| Rheum rhaponticum | shoots | Temperature and photoperiod | Primary and secondary metabolism | Wojtania et al. [73] |
| Solanum tuberosum | nodal segments | Gene expression | Molecular mechanisms and primary metabolism | Wang et al. [79] |
| Anthyllis barba-jovis | seeds | Temperature and photoperiod | Primary metabolism | Bertsouklis et al. [80] |
| Gentiana triflora | shoots | Gene expression | Molecular mechanisms | Takase et al. [81] |
| Spiranthes ochroleuca | seeds | Culture medium and photoperiod | Primary metabolism (germination) | Zale et al. [82] |
| Artemisia ludoviciana | nodal segments | Plant growth regulators and photoperiod | Primary and secondary metabolism | Ramos et al. [83] |
| Aloe sul-africana | seeds | Temperature and photoperiod | Primary metabolism (germination) | Amoo et al. [84] |
| Artemisia tilesii | roots | Phenylalanine concentrations and light | Primary and secondary metabolism | Bohdanovych et al. [85] |
| Paphiopedilum spp. | shoots | Plant growth regulators and photoperiod | Molecular mechanisms and primary metabolism | Istiqomah et al. [86] |
| Artemisia alba | shoots | Plant growth regulators and photoperiod | Primary and secondary metabolism | Pecheva et al. [87] |
| Cannabis sativa | stem segments | Culture medium and photoperiod | Flowering | Lavie et al. [88] |
| Plumbago auriculata | shoots | Temperature and photoperiod | Flowering | Shen et al. [89] |
| Curcuma caesia | shoots | Plant growth regulators and photoperiod | Primary metabolism | Sarma et al. [90] |
| Cannabis sativa | shoots | Photoperiod | Primary metabolism and flowering | Moher et al. [18] |
| Cunninghamia lanceolata | shoots | Photoperiod and light quality | Primary metabolism | Xu et al. [91] |
| Narcissus tazzeta | bulbs | Plant growth regulators and photoperiod | Primary and secondary metabolism | Rahimi et al. [92] |
| Basella rubra | callus | Plant growth regulators and photoperiod | Primary and secondary metabolism | Kumar et al. [93] |
| Triticum aestivum | seeds | Culture medium and photoperiod | Primary and secondary metabolism | Virdi et al. [94] |
| Lippia alba | nodal segments | Photoperiod | Primary and secondary metabolism | Castro et al. [95] |
| Solanum tuberosum | shoots | Photoperiod and silver thiosulfate concentrations | Primary metabolism | El-Sayed et al. [96] |
| Solanum tuberosum | stem segments | Photoperiod and KNO3 concentrations | Primary metabolism | Choirunnisa & Wardana. [97] |
| Solanum tuberosum | shoots | Culture medium and photoperiod | Primary metabolism | Wafa et al. [98] |
| Kaempferia galanga | rhizome shoots | Plant growth regulators and photoperiod | Primary and secondary metabolism | Shofiyani et al. [99] |
| Ficus palmata | axillary shoots | Culture medium and photoperiod | Primary metabolism | Ahmed et al. [100] |
| Pfaffia glomerata | nodal segments | Photoperiod | Primary and secondary metabolism | Fortini et al. [101] |
| Bletia urbana | protocorms | Plant growth regulators and photoperiod | Primary metabolism and flowering | Rodríguez et al. [102] |
| Origanum dictamnus | seeds | Plant growth regulators and photoperiod | Primary metabolism (germination) | Sarropoulou et al. [103] |
| Vaccinium floribundum | seeds and shoots | Medium, photoperiod, and temperature | Primary metabolism | Meneses et al. [104] |
| Fagonia indica | stem explants | UV-C regimes and photoperiod | Primary and secondary metabolism | Abbasi et al. [105] |
| Anacamptis longicornu e Ophrys panormitana | seeds | Culture medium, temperature, and photoperiod | Primary metabolism (germination) | Arcidiacono et al. [106] |
| Solanum tuberosum | cuttings | Gene expression, culture medium, and photoperiod | Molecular mechanisms and primary metabolism | Kondhare et al. [107] |
| Ligaria cuneifolia | embryos | Plant growth regulators and photoperiod | Primary and secondary metabolism | Ricco et al. [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Nascimento, A.S.M.; Henschel, J.M.; Felipe, S.H.S.; Rodrigues, A.A.C.; Figueiredo, F.A.M.M.d.A.; Ferraz, T.M.; Reis, F.d.O.; Corrêa, T.R.; Batista, D.S. Photoperiod and Circadian Regulation in Plants: A Review of Insights from In Vitro Studies. Biology 2025, 14, 1502. https://doi.org/10.3390/biology14111502
do Nascimento ASM, Henschel JM, Felipe SHS, Rodrigues AAC, Figueiredo FAMMdA, Ferraz TM, Reis FdO, Corrêa TR, Batista DS. Photoperiod and Circadian Regulation in Plants: A Review of Insights from In Vitro Studies. Biology. 2025; 14(11):1502. https://doi.org/10.3390/biology14111502
Chicago/Turabian Styledo Nascimento, Adriely Sá Menezes, Juliane Maciel Henschel, Sérgio Heitor Sousa Felipe, Antonia Alice Costa Rodrigues, Fábio Afonso Mazzei Moura de Assis Figueiredo, Tiago Massi Ferraz, Fabrício de Oliveira Reis, Thais Roseli Corrêa, and Diego Silva Batista. 2025. "Photoperiod and Circadian Regulation in Plants: A Review of Insights from In Vitro Studies" Biology 14, no. 11: 1502. https://doi.org/10.3390/biology14111502
APA Styledo Nascimento, A. S. M., Henschel, J. M., Felipe, S. H. S., Rodrigues, A. A. C., Figueiredo, F. A. M. M. d. A., Ferraz, T. M., Reis, F. d. O., Corrêa, T. R., & Batista, D. S. (2025). Photoperiod and Circadian Regulation in Plants: A Review of Insights from In Vitro Studies. Biology, 14(11), 1502. https://doi.org/10.3390/biology14111502








