Ecotone-Driven Vegetation Transitions Reshape Soil Nitrogen Cycling Functional Genes in Black Soils of Northeast China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Design
2.2. Soil Sampling
2.3. Soil Physicochemical Properties and Enzyme Activities
2.4. DNA Extraction and Quantification of Functional Genes
2.5. Microbial Community Profiling
2.6. Community Assembly and Network Analyses
2.7. Functional Prediction
2.8. Statistical Analyses
3. Results
3.1. Functional Gene Abundance and Multivariate Variation
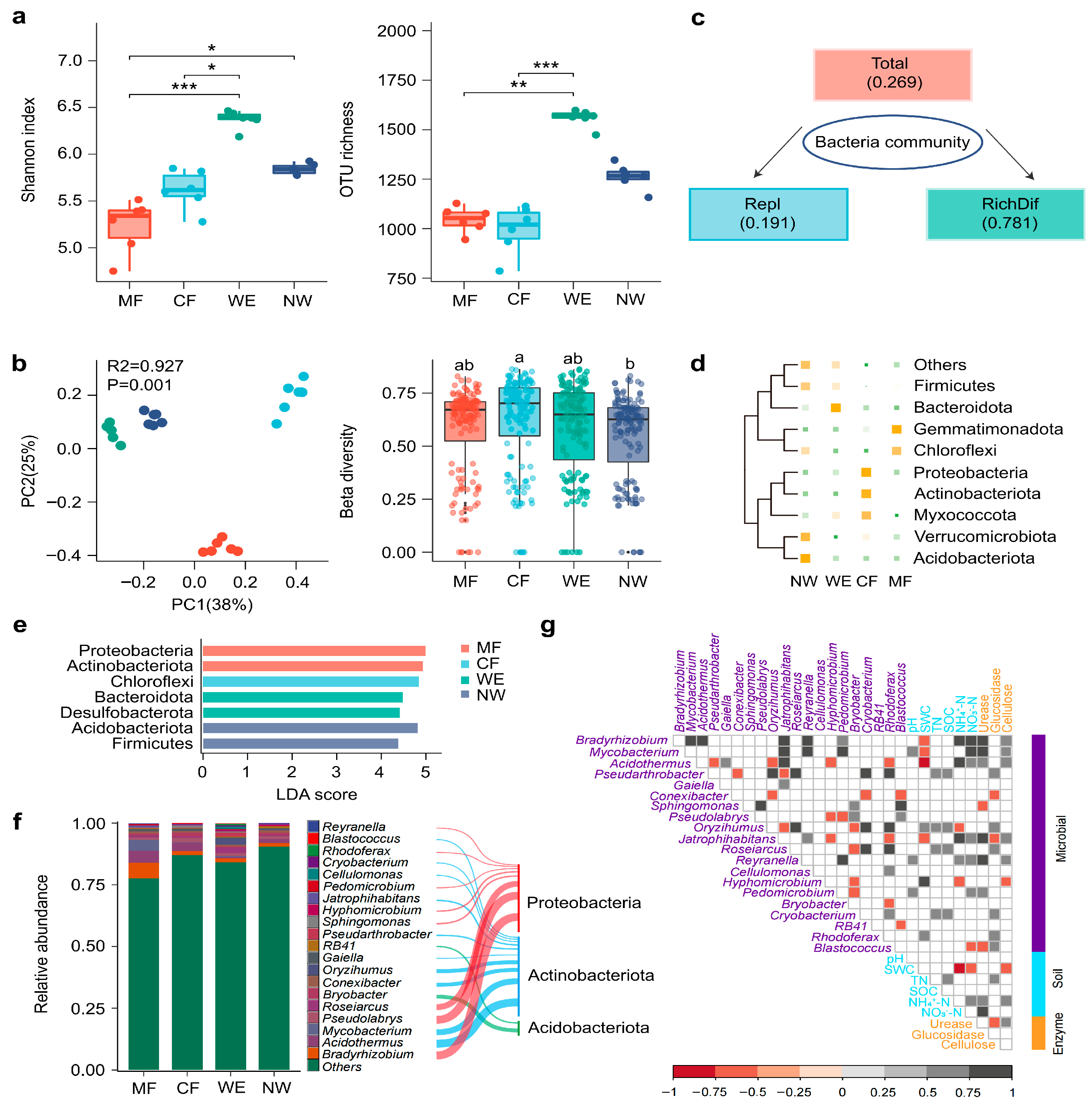
3.2. Microbial Diversity and Community Composition Along the Vegetation Transitions
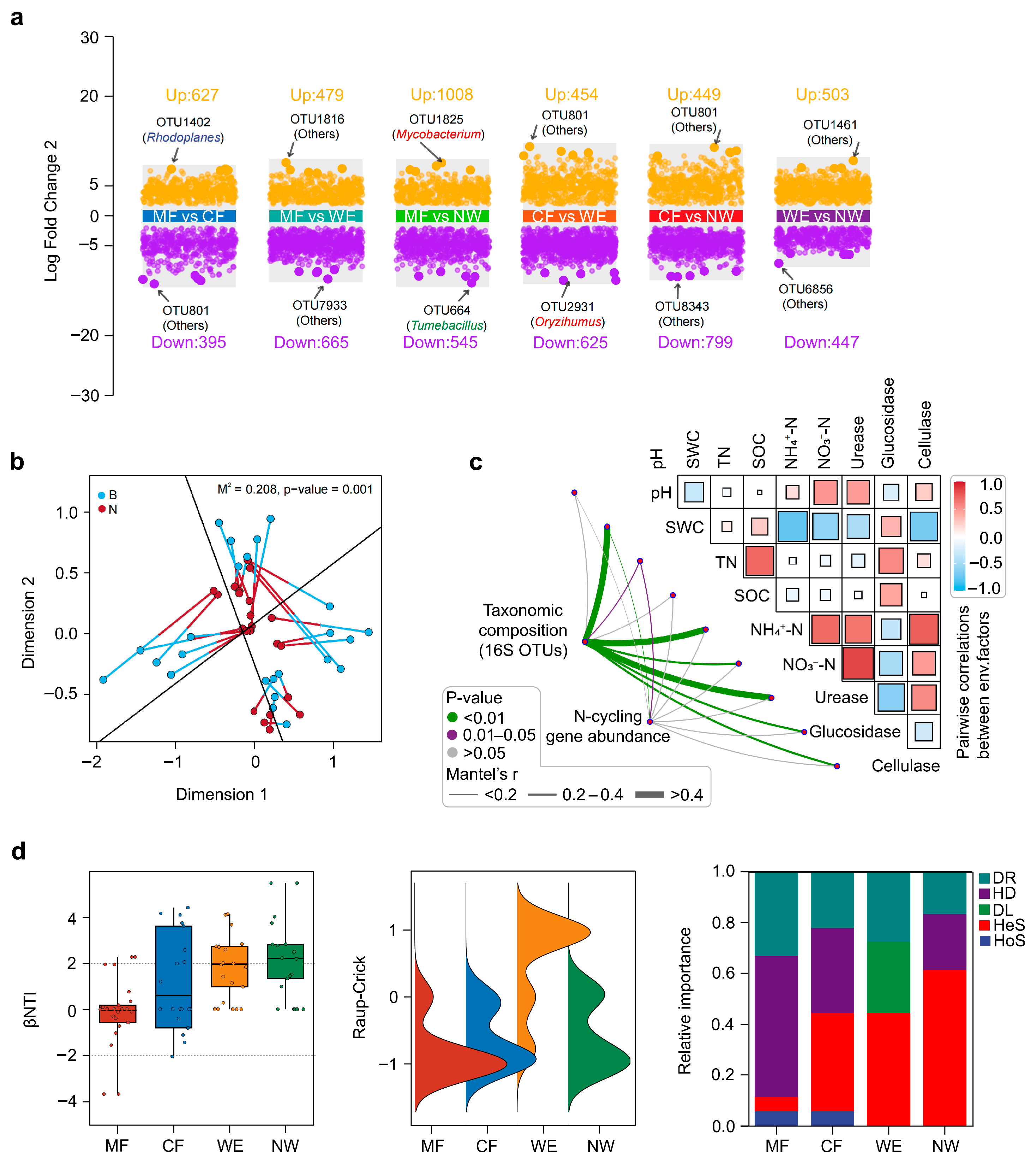
3.3. Microbial Community Differentiation and Assembly Patterns Across the Vegetation Transitions
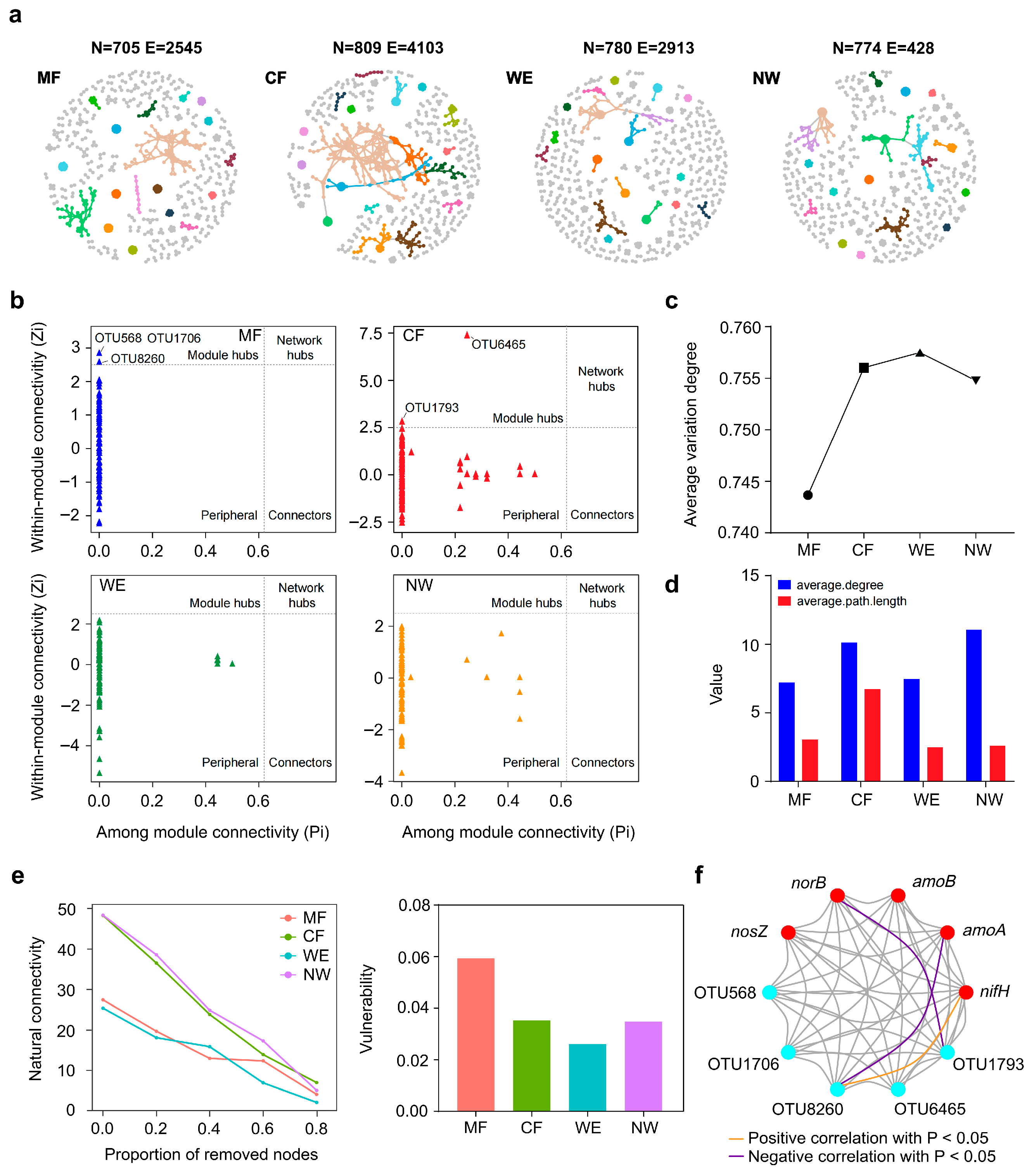
3.4. Microbial Co-Occurrence Networks and Functional Associations Across Vegetation Types
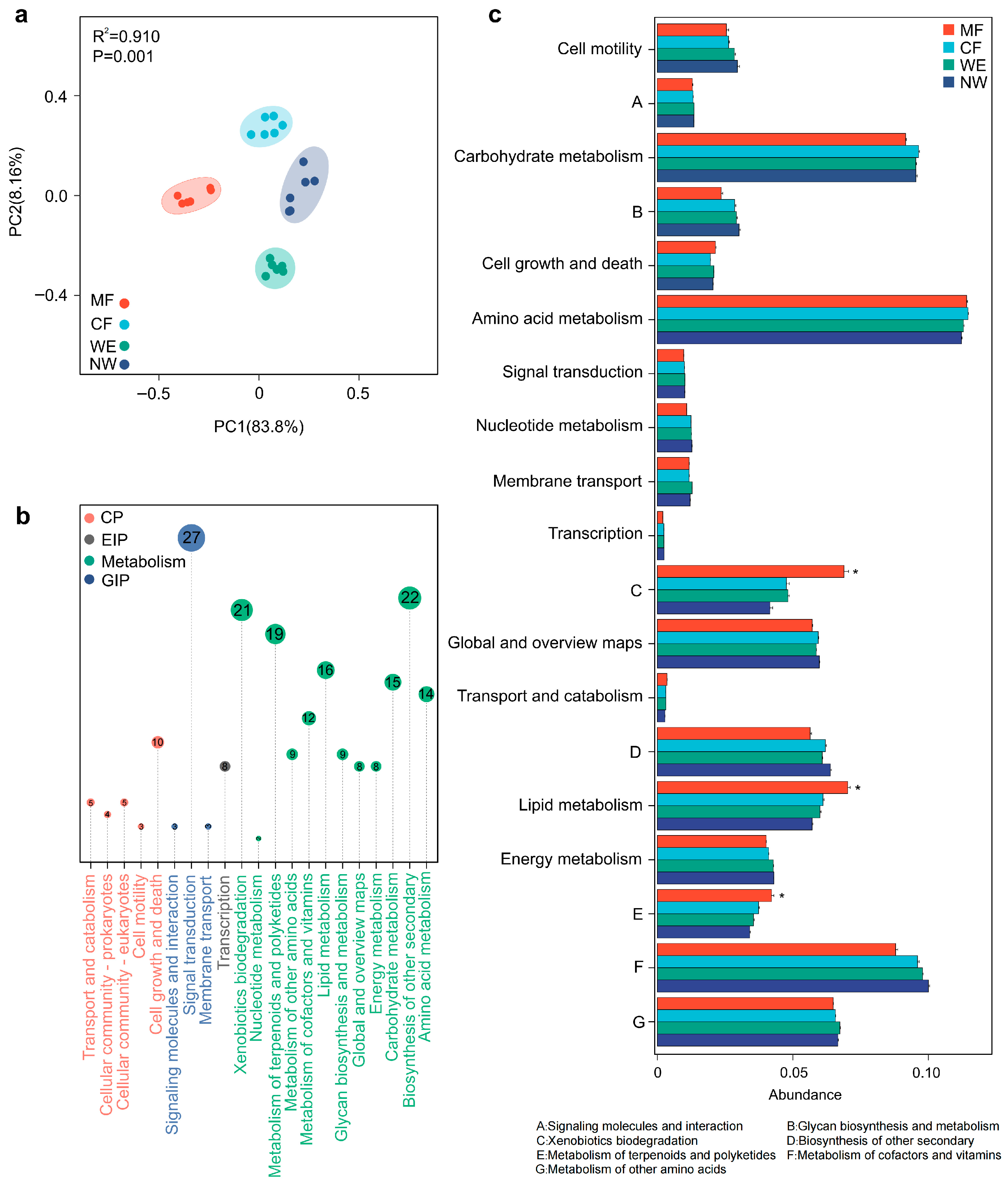
3.5. Functional Predictions of Microbial Communities Along the Forest–Wetland Transitions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AK | Available Potassium |
| amoA | Ammonia Monooxygenase Subunit A Gene |
| amoB | Ammonia Monooxygenase Subunit B Gene |
| AP | Available Phosphorus |
| ASV | Amplicon Sequence Variant |
| βNTI | Beta Nearest Taxon Index |
| BG | β-glucosidase |
| CEL | Cellulase |
| CF | Coniferous Forest |
| EC | Electrical Conductivity |
| LDA | Linear Discriminant Analysis |
| LEfSe | Linear Discriminant Analysis Effect Size |
| MF | Mixed Forest |
| NH4+-N | Ammonium Nitrogen |
| NO3−-N | Nitrate Nitrogen |
| norB | Nitric Oxide Reductase Subunit B Gene |
| nosZ | Nitrous Oxide Reductase Gene |
| NW | Natural Wetland |
| OTU | Operational Taxonomic Unit |
| PCoA | Principal Coordinate Analysis |
| pH | Potential of Hydrogen |
| QIIME2 | Quantitative Insights Into Microbial Ecology, version 2 |
| RDA | Redundancy Analysis |
| SOC | Soil Organic Carbon |
| SWC | Soil Water Content |
| TN | Total Nitrogen |
| URE | Urease |
| WE | Wetland Edge |
| nifH | Nitrogen Fixation Gene |
References
- Viceli, J.M.; Acosta, A.C.B.; Pocojeski, E.; Casali, C.A.; Kessler, N.C.H.; Tessaro, D. Influence of soil water content on chemical and microbiological characteristics of selected riparian forests in southern Brazil. Wetl. Ecol. Manag. 2025, 33, 26. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, Z.; Ouyang, L.; He, G.; Liu, W.; Cai, M. Macrohabitat and microhabitat mediate the relationships between wetland multifaceted biodiversity and multifunctionality. Catena 2024, 241, 108023. [Google Scholar] [CrossRef]
- Marzini, S.; Tasser, E.; Wellstein, C.; Albrich, K.; Rammer, W.; Mina, M. Future expansion of upper forest-grassland ecotone under land-use and climate change in the Eastern Alps. Landsc. Ecol. 2025, 40, 55. [Google Scholar] [CrossRef]
- Li, M.; Ye, W.; Li, Y.J.; Cui, C. Evaluation of the synergistic change in cultivated land and wetland in northeast China from 1990 to 2035. Sci. Rep. 2025, 15, 14973. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; López-Mondéjar, R.; Kohout, P. Forest microbiome and global change. Nat. Rev. Microbiol. 2023, 21, 487–501. [Google Scholar] [CrossRef]
- Chirilă Băbău, A.M.; Micle, V.; Damian, G.E.; Sur, I.M. Lead and copper removal from sterile dumps by phytoremediation with Robinia pseudoacacia. Sci. Rep. 2024, 14, 9842. [Google Scholar] [CrossRef]
- Pedrinho, A.; Mendes, L.W.; de Araujo Pereira, A.P.; Araujo, A.S.F.; Vaishnav, A.; Karpouzas, D.G.; Singh, B.K. Soil microbial diversity plays an important role in resisting and restoring degraded ecosystems. Plant Soil 2024, 500, 325–349. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, L.; Wang, Y.; Huang, Z. Impact of ecological restoration on the physicochemical properties and bacterial communities in alpine mining area soils. Microorganisms 2023, 12, 41. [Google Scholar] [CrossRef]
- Saud, S.; Wang, D.; Fahad, S. Improved nitrogen use efficiency and greenhouse gas emissions in agricultural soils as producers of biological nitrification inhibitors. Front. Plant Sci. 2022, 13, 854195. [Google Scholar] [CrossRef]
- Yan, B.; Ao, L.; Li, B.; Mao, L.; Sun, L.; Li, X. Response of Soil Nitrogen Cycle Microbial Functions to Ecological Reconstruction in Saline-Alkali Soils: A Dual Perspective of Natural Succession and Alfalfa Cropping. Land. Degrad. Dev. 2025, 30, 4753–4769. [Google Scholar] [CrossRef]
- Tian, D.F.; Lin, X.B.; Zheng, P.F.; Zhang, G.L.; Li, J.; Wang, M.R.; Liu, K.W.; Kong, T.T.; Fan, S.Y.; Guo, P.; et al. The influences of mangrove grown on sedimentary nitrate reduction activities are more pronounced in sandy coasts compared to muddy coasts. Plant Soil 2025, 511, 657–681. [Google Scholar] [CrossRef]
- Baskaran, V.; Prabavathy, V.R. Diverse key nitrogen cycling genes nifH, nirS and nosZ associated with Pichavaram mangrove rhizospheres as revealed by culture-dependent and culture-independent analyses. Arch. Microbiol. 2022, 204, 109. [Google Scholar] [CrossRef] [PubMed]
- Coban, O.; De Deyn, G.B.; van der Ploeg, M. Soil microbiota as game-changers in restoration of degraded lands. Science 2022, 375, abe0725. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, D.K.; Voigt, T.B.; Tian, G.; Yannarell, A.C. Agricultural practices of perennial energy crops affect nitrogen cycling microbial communities. Appl. Soil Ecol. 2022, 172, 104366. [Google Scholar] [CrossRef]
- Shan, J.; Sanford, R.A.; Chee-Sanford, J.; Ooi, S.K.; Löffler, F.E.; Konstantinidis, K.T.; Yang, W.H. Beyond denitrification: The role of microbial diversity in controlling nitrous oxide reduction and soil nitrous oxide emissions. Global. Change Biol. 2021, 27, 2669–2683. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Ji, M.M.; Yu, S.Y.; Li, J.; Wu, X.G.; Ju, X.T.; Liu, B.B.; Zhang, X.J. Distinct denitrifying phenotypes of predominant bacteria modulate nitrous oxide metabolism in two typical cropland soils. Micro. Ecol. 2023, 86, 509–520. [Google Scholar] [CrossRef]
- Ding, J.; Yu, S. Structural and Functional Characteristics of Soil Microbial Communities in Forest–Wetland Ecotones: A Case Study of the Lesser Khingan Mountains. Life 2025, 15, 570. [Google Scholar] [CrossRef]
- Naylor, D.; McClure, R.; Jansson, J. Trends in microbial community composition and function by soil depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Liu, Y.; Wei, Y.; Li, J.; Ding, G.C. Carbon amendment rather than nitrate fertilization dominated the reassembly of the total, denitrifying, and DNRA bacterial community in the anaerobic subsoil. J. Soil. Sediment 2023, 23, 1913–1926. [Google Scholar] [CrossRef]
- Liu, X.; Pang, L.; Yue, Y.; Li, H.; Chatzisymeon, E.; Lu, Y.; Yang, P. Insights into the shift of microbial community related to nitrogen cycle, especially N2O in vanadium-polluted soil. Environ. Pollut. 2023, 322, 121253. [Google Scholar] [CrossRef]
- Poblador, S.; Lupon, A.; Sabaté, S.; Sabater, F. Soil water content drives spatiotemporal patterns of CO2 and N2O emissions from a Mediterranean riparian forest soil. Biogeosciences 2017, 14, 4195–4208. [Google Scholar] [CrossRef]
- Graham, E.B.; Knelman, J.E. Implications of soil microbial community assembly for ecosystem restoration: Patterns, process, and potential. Micro. Ecol. 2023, 85, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Choudoir, M.J.; DeAngelis, K.M. A framework for integrating microbial dispersal modes into soil ecosystem ecology. Iscience 2022, 25, 103887. [Google Scholar] [CrossRef] [PubMed]
- Perring, M.P.; De Frenne, P.; Baeten, L.; Maes, S.L.; Depauw, L.; Blondeel, H.; Carón, M.M.; Verheyen, K. Global environmental change effects on ecosystems: The importance of land-use legacies. Glob. Change Biol. 2016, 22, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xie, R.; Ma, D.; Zhang, M.; Liu, L. Variations in soil microbial community structure and extracellular enzymatic activities along a forest–wetland ecotone in high-latitude permafrost regions. Ecol. Evolut. 2023, 13, e10205. [Google Scholar] [CrossRef]
- Yang, C.P.; Xia, D.A.; Xu, C.Q.; Qi, L.Z.; Liu, G.F.; Weng, Y.H.; Sheng, L.; Wei, F.Y.; Zhang, Z.S. Study on the provenance test of Dahurian Larch selection of best provenance. J. Northeast For. Univ. 1993, 4, 22–30. [Google Scholar]
- Tong, S.; Cao, G.; Zhang, Z.; Zhang, J. The spatial variation and driving factors of soil total carbon and nitrogen in the Heihe River source region. Environ. Monit. Assess. 2023, 195, 724. [Google Scholar] [CrossRef]
- Peng, M.; Qu, L.; Wang, Q.Y. Seed-specific identification of Larix gmelinii, Larix olgensis, and Larix principis-rupprechtii using sequence-characterised amplified region markers. Biochem. Syst. Ecol. 2014, 55, 231–235. [Google Scholar] [CrossRef]
- Levipan, H.A.; Opazo, L.F.; Arenas-Uribe, S.; Wicki, H.; Marchant, F.; Florez-Leiva, L.; Avendaño-Herrera, R. Estimating taxonomic and functional structure along a tropical estuary: Linking metabolic traits and aspects of ecosystem functioning. Microbiol. Spectr. 2024, 12, e03886-23. [Google Scholar] [CrossRef]
- Mao, X.L.; Zheng, J.Y.; Yu, W.; Guo, X.W.; Xu, K.; Zhao, R.Y.; Xiao, L.J.; Wang, M.M.; Jiang, Y.F.; Zhang, S.; et al. Climate-induced shifts in composition and protection regulate temperature sensitivity of carbon decomposition through soil profile. Soil Biol. Biochem. 2022, 172, 108743. [Google Scholar] [CrossRef]
- Gao, W.; Ma, T.; Shi, B.; Yang, Z.; Li, Y.; Zhu, J.; He, J.S. Effects of nitrogen and phosphorus addition on the mineralization potential of soil organic carbon and the corresponding regulations in the Tibetan alpine grassland. Appl. Soil. Ecol. 2024, 196, 105314. [Google Scholar] [CrossRef]
- Shi, F.Y.; Fang, H.J.; Cheng, S.L.; Guo, Y.F.; Wang, H.; Chen, L.; Pu, H.G.; Liu, B.P. Cadmium accumulation suppresses rice nitrogen use efficiency by inhibiting rhizosphere nitrification and promoting nitrate reduction. J. Hazard. Mater. 2025, 496, 139298. [Google Scholar] [CrossRef]
- Shi, H.; Wang, L.; Wu, Y.; Lv, Y.; Cai, B. Molecular regulation of hyphosphere soil dissolved organic matter degradation by arbuscular mycorrhizal fungi under tetracycline stress. Chem. Eng. J. 2025, 521, 167081. [Google Scholar] [CrossRef]
- Šebesta, M.; Nemček, L.; Urík, M.; Kolenčík, M.; Bujdoš, M.; Vávra, I.; Dobročka, E.; Matúš, P. Partitioning and stability of ionic, nano-and microsized zinc in natural soil suspensions. Sci. Total Environ. 2020, 700, 134445. [Google Scholar] [CrossRef] [PubMed]
- Sveen, T.R.; Hannula, S.E.; Bahram, M. Microbial regulation of feedbacks to ecosystem change. Trends Microbiol. 2024, 32, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Lucia, Z.; Giulio, G.; Matteo, G.; Stefano, C.; Irene, P.L.; Paolo, P.; Giorgio, B.; Hauffe, H.C. More Than Meets the Eye: Unraveling the Interactions Between Skin Microbiota and Habitat in an Opportunistic Amphibian. Microb. Ecol. 2024, 87, 176. [Google Scholar] [CrossRef] [PubMed]
- Asch, J.; Johnson, K.; Mondal, S.; Asch, F. Comprehensive assessment of extraction methods for plant tissue samples for determining sodium and potassium via flame photometer and chloride via automated flow analysis. J. Plant Nutr. Soil Sci. 2022, 185, 308–316. [Google Scholar] [CrossRef]
- Ahmad, S.; Nadeem, M.Y.; Gao, S.; Li, Q.X.; Ding, Y.F.; Liu, Z.H.; Jiang, Y.; Li, G.H. Subsurface placement of controlled-release blended fertilizers mitigates ammonia volatilization by promoting nitrogen transformation in rice fields. Agr. Ecosyst. Environ. 2025, 386, 109624. [Google Scholar] [CrossRef]
- Lee, S.; Jung, Y.J.; Moon, J.; Lee, J.Y.; Kim, H.; Yang, J.E.; Lee, H.; Jung, J.; Kim, H.R. Comparison and selection of conventional PCR primer sets for studies associated with nitrogen cycle microorganisms in surface soil. Appl. Sci. 2022, 12, 10314. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Z.; Liu, C.; Sun, J.; Song, J.; Li, X.; Liu, Y. Effects of different manures in combination with fulvic acid on the abundance of N-cycling functional genes in greenhouse soils. Agriculture 2023, 13, 2224. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://ropensci.org/blog/2021/11/16/how-to-cite-r-and-r-packages (accessed on 15 July 2022).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Wu, J.; Mauricio, B.; Tan, Y.J.; Deng, H.Z. Natural Connectivity of Complex Networks. Chin. Phys. Lett. 2010, 27, 078902. [Google Scholar] [CrossRef]
- Chen, D.B.; Gao, H.; Lü, L.; Zhou, T. Identifying influential nodes in large-scale directed networks: The role of clustering. PLoS ONE 2013, 8, e77455. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.M.; Moreira, A.A.; Andrade, J.S.; Havlin, S.; Herrmann, H.J. Mitigation of malicious attacks on networks. Proc. Natl. Acad. Sci. USA 2011, 108, 3838–3841. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Yan, B.; Liu, G. The responses of soil nitrogen transformation to nitrogen addition are mainly related to the changes in functional gene relative abundance in artificial Pinus tabulaeformis forests. Sci. Total Environ. 2020, 723, 137679. [Google Scholar] [CrossRef]
- Roose, J.J.; Stribling, J.M.; Owens, M.S.; Cornwell, J.C. The development of denitrification and of the denitrifying community in a newly-created freshwater wetland. Wetlands 2020, 40, 1005–1016. [Google Scholar] [CrossRef]
- Lee, K.K.; Liu, S.; Crocker, K.; Wang, J.; Huggins, D.R.; Tikhonov, M.; Mani, M.; Kuehn, S. Functional regimes define soil microbiome response to environmental change. Nature 2025, 644, 1028–1038. [Google Scholar] [CrossRef]
- Yousaf, A.; Khalid, N.; Aqeel, M.; Noman, A.; Naeem, N.; Sarfraz, W.; Ejaz, U.; Qaiser, Z.; Khalid, A. Nitrogen dynamics in wetland systems and its impact on biodiversity. Nitrogen 2021, 2, 196–217. [Google Scholar] [CrossRef]
- Mattoo, R.; Mallikarjuna, S.B.; Hemachar, N. Ecosystem and Climate Change Impacts on the Nitrogen Cycle and Biodiversity. Nitrogen 2025, 6, 78. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Y.; Xu, L.; Ye, J.; Zhang, X.; Xu, X.; Meng, H.; Xie, W.; He, H.; Wang, G. NosZ II/nosZ I ratio regulates the N2O reduction rates in the eutrophic lake sediments. Sci. Total Environ. 2024, 951, 175852. [Google Scholar] [CrossRef]
- Wilson, S.J.; Megonigal, J.P. Nitrate reduction across soils transitioning from coastal forest to wetland are hotspots for denitrification. Soil Biol. Biochem. 2025, 209, 109904. [Google Scholar] [CrossRef]
- Roque-Malo, S.; Woo, D.K.; Kumar, P. Modeling the role of root exudation in critical zone nutrient dynamics. Water Resour. Res. 2020, 56, e2019WR026606. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Chiu, C.Y. Biogeochemical processes of C and N in the soil of mangrove forest ecosystems. Forests 2020, 11, 492. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Kou, Y.; Liu, Y.; He, H.; Liu, Q. Secondary forest succession drives differential responses of bacterial communities and interactions rather than bacterial functional groups in the rhizosphere and bulk soils in a subalpine region. Plant Soil 2023, 484, 293–312. [Google Scholar] [CrossRef]
- Dai, T.; Liu, R.; Zhou, X.; Zhang, J.; Song, M.; Zou, P.; Bi, X.; Li, S. Role of lake aquatic–terrestrial ecotones in the ecological restoration of eutrophic water bodies. Toxics 2023, 11, 560. [Google Scholar] [CrossRef]
- Lim, J.; Wehmeyer, H.; Heffner, T.; Aeppli, M.; Gu, W.; Kim, P.J.; Horn, M.A.; Ho, A. Resilience of aerobic methanotrophs in soils; spotlight on the methane sink under agriculture. FEMS Microbiol. Ecol. 2024, 100, fiae008. [Google Scholar] [CrossRef]
- Han, X.; Luo, Q.; Chen, Y.; Xuan, Y.; Huang, C.; Liu, B.; Zhang, Y.; Wu, X.; Chen, Y.; Guo, J. Dynamic changes in soil characteristics, enzyme activity, and microbial communities during montane riparian forest succession. Appl. Soil. Ecol. 2025, 211, 106158. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, Q.; Wang, J.; Deng, Y.; Yan, Z.; Tian, L.; Jiang, H. Water level fluctuations modulate the microbiomes involved in biogeochemical cycling in floodplains. Micro. Ecol. 2024, 87, 24. [Google Scholar] [CrossRef]
- Neubauer, S.C.; Megonigal, J.P. Biogeochemistry of wetland carbon preservation and flux. Wetl. Carbon Environ. Manag. 2021, 33–71. [Google Scholar]
- Liu, S.; García-Palacios, P.; Tedersoo, L.; Guirado, E.; van der Heijden, M.G.; Wagg, C.; Chen, D.; Wang, Q.; Wang, J.; Singh, B.K. Phylotype diversity within soil fungal functional groups drives ecosystem stability. Nat. Ecol. Evol. 2020, 6, 900–909. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, K.; Li, K.; Jin, Y.; He, X. Deciphering the diversity patterns and community assembly of rare and abundant bacterial communities in a wetland system. Sci. Total Environ. 2022, 838, 156334. [Google Scholar] [CrossRef]
- Wu, D.; Bai, H.; Zhao, C.; Peng, M.; Chi, Q.; Dai, Y.; Gao, F.; Zhang, Q.; Huang, M.; Niu, B. The characteristics of soil microbial co-occurrence networks across a high-latitude forested wetland ecotone in China. Front. Microbiol. 2023, 14, 1160683. [Google Scholar] [CrossRef]
- Beringer, J.; Moore, C.E.; Cleverly, J.; Campbell, D.I.; Cleugh, H.; De Kauwe, M.G.; Kirschbaum, M.U.; Griebel, A.; Grover, S.; Huete, A. Bridge to the future: Important lessons from 20 years of ecosystem observations made by the OzFlux network. Global Change Biol. 2022, 28, 3489–3514. [Google Scholar] [CrossRef]
- Qiu, X.; Cao, G.; Han, G.; Zhao, Q.; Cao, S.; Ji, S. Impact of Vegetation Type on Taxonomic and Functional Composition of Soil Microbial Communities in the Northeastern Qinghai–Tibet Plateau. Microorganisms 2025, 13, 2075. [Google Scholar] [CrossRef]
- Daniel, J.; Gleason, J.E.; Cottenie, K.; Rooney, R.C. Stochastic and deterministic processes drive wetland community assembly across a gradient of environmental filtering. Oikos 2019, 128, 1158–1169. [Google Scholar] [CrossRef]
- Van De Koppel, J.; Van Der Heide, T.; Altieri, A.H.; Eriksson, B.K.; Bouma, T.J.; Olff, H.; Silliman, B.R. Long-distance interactions regulate the structure and resilience of coastal ecosystems. Annu. Rev. Mar. Sci. 2015, 7, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Bai, J.; Wang, J.; Zhang, G.; Wang, W.; Wang, X.; Zhang, L.; Wang, Y.; Liu, X.; Cui, B. Different stochastic processes regulate bacterial and fungal community assembly in estuarine wetland soils. Soil Biol. Biochem. 2022, 167, 108586. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Zheng, H.; Hu, B.; Dai, X.; Meng, N.; Zhu, J.; Yan, D. Environmental changes drive soil microbial community assembly across arid alpine grasslands on the Qinghai-Tibetan Plateau, China. Catena 2023, 228, 107175. [Google Scholar] [CrossRef]
- Ciafré, C.M.; Gienger, C.M.; Rehm, E.M.; Estes, L.D. Deterministic and stochastic factors jointly drive plant community composition and diversity in isolated wetlands. Wetlands 2022, 42, 71. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Onet, A.; Grenni, P.; Onet, C.; Stoian, V.; Crisan, V. Forest soil microbiomes: A review of key research from 2003 to 2023. Forests 2025, 16, 148. [Google Scholar] [CrossRef]
- Gui, H.; Hou, L.; Wang, J.; Dong, X.; Han, S. Flood changed the community composition and increased the importance of stochastic process of vegetation and seed bank in a riparian ecosystem of the Yellow River. Ecol. Indic. 2023, 154, 110505. [Google Scholar] [CrossRef]
- Pan, X.; Raaijmakers, J.M.; Carrión, V.J. Importance of Bacteroidetes in host–microbe interactions and ecosystem functioning. Trends Microbiol. 2023, 31, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Liu, Y.; Wang, H.; Wang, C.; Xia, M.; Wang, N.; Cui, W.; Xiao, D.; Wang, H. Plant litter decomposition in wetlands is closely associated with phyllospheric fungi as revealed by microbial community dynamics and co-occurrence network. Sci. Total Environ. 2021, 753, 142194. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, K.T.; Hynson, N.A. Networks as tools for defining emergent properties of microbiomes and their stability. Microbiome 2024, 12, 184. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, D.; Li, Y.; Chen, Z.; Li, J.; Dong, Y.; Yang, C.; Miao, Y.; Yuan, J.; Ding, W. Wetland restoration suppresses microbial carbon metabolism by altering keystone species interactions. Front. Microbiol. 2025, 16, 1570703. [Google Scholar] [CrossRef]
- Niu, Y.; Kang, E.; Li, Y.; Zhang, X.; Yan, Z.; Li, M.; Yan, L.; Zhang, K.; Wang, X.; Yang, A. Non-flooding conditions caused by water table drawdown alter microbial network complexity and decrease multifunctionality in alpine wetland soils. Environ. Res. 2024, 254, 119152. [Google Scholar] [CrossRef]
- Hu, M.; Sardans, J.; Sun, D.; Yan, R.; Wu, H.; Ni, R.; Peñuelas, J. Microbial diversity and keystone species drive soil nutrient cycling and multifunctionality following mangrove restoration. Environ. Res. 2024, 251, 118715. [Google Scholar] [CrossRef]
- Yan, L.; Kuang, Y.; Xie, X.; Peng, K.; Deng, Y.; Gan, Y.; Li, Q.; Zhang, Y. Insights into nitrogen biogeochemical cycling in mangrove wetland from genome-resolved metagenomic sequencing. J. Hydrol. 2024, 640, 131741. [Google Scholar] [CrossRef]
- Li, W.; Lu, Q.; Alharbi, S.A.; Soromotin, A.V.; Kuzyakov, Y.; Lei, Y. Plant–soil–microbial interactions mediate vegetation succession in retreating glacial forefields. Sci. Total Environ. 2023, 873, 162393. [Google Scholar] [CrossRef]
- Naughton, H.R.; Keiluweit, M.; Tfaily, M.M.; Dynes, J.J.; Regier, T.; Fendorf, S. Development of energetic and enzymatic limitations on microbial carbon cycling in soils. Biogeochemistry 2021, 153, 191–213. [Google Scholar] [CrossRef]
- Pattnaik, S.; Mohapatra, B.; Gupta, A. Plant growth-promoting microbe mediated uptake of essential nutrients (Fe, P, K) for crop stress management: Microbe–soil–plant continuum. Front. Agron. 2021, 3, 689972. [Google Scholar] [CrossRef]
- Luo, M.; Liu, J.; Qu, F.; Sun, B.; Yu, Y.; Guan, B. Ecological Stoichiometric Characteristics and Adaptive Strategies of Herbaceous Plants in the Yellow River Delta Wetland, China. Biology 2025, 14, 1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hao, Q.; Xu, S.; Li, Y.; Zhang, W.; Liang, Z.; Jiang, C. Optimizing nitrogen removal in constructed wetlands for low C/N ratio wastewater treatment: Insights from fermentation liquid utilization. Water Res. 2024, 262, 122124. [Google Scholar] [CrossRef]
- López, J.C.; Quijano, G.; Souza, T.S.; Estrada, J.M.; Lebrero, R.; Muñoz, R. Biotechnologies for greenhouse gases (CH4, N2O, and CO2) abatement: State of the art and challenges. Appl. Microbiol. Biotechnol. 2013, 97, 2277–2303. [Google Scholar] [CrossRef]
- Telo da Gama, J. The role of soils in sustainability, climate change, and ecosystem services: Challenges and opportunities. Ecologies 2023, 4, 552–567. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, W.; Hou, X.; Li, Y.; Tong, J. How nutrient loads influence microbial-derived carbon accumulation in wetlands: A new insight from microbial metabolic investment strategies. Environ. Res. 2023, 217, 114981. [Google Scholar] [CrossRef]
- Sepp, S.-K.; Vasar, M.; Davison, J.; Oja, J.; Anslan, S.; Al-Quraishy, S.; Bahram, M.; Bueno, C.G.; Cantero, J.J.; Fabiano, E.C. Global diversity and distribution of nitrogen-fixing bacteria in the soil. Front. Plant Sci. 2023, 14, 1100235. [Google Scholar] [CrossRef]
- Naz, F.; Arif, M.; Xue, T.; Chen, Y.; Khan, S.U.; Changxiao, L. Bacterial communities and soil functionality in artificially remediated vegetation of the three gorges reservoir zone. Front. Plant Sci. 2025, 16, 1550306. [Google Scholar] [CrossRef]
- Ortiz-Colin, P.; Hulshof, C.M. Ecotones as windows into organismal-to-biome scale responses across neotropical forests. Plants 2024, 17, 2396. [Google Scholar] [CrossRef]
- Costa, D.; Sutter, C.; Shepherd, A.; Jarvie, H.; Wilson, H.; Elliott, J.; Liu, J.; Macrae, M. Impact of climate change on catchment nutrient dynamics: Insights from around the world. Environ. Rev. 2022, 31, 4–25. [Google Scholar] [CrossRef]
- Liang, X.; Yu, S.; Ju, Y.; Wang, Y.; Yin, D. Integrated management practices foster soil health, productivity, and agroecosystem resilience. Agronomy 2025, 15, 1816. [Google Scholar] [CrossRef]
- Bahram, M.; Espenberg, M.; Pärn, J.; Lehtovirta-Morley, L.; Anslan, S.; Kasak, K.; Kõljalg, U.; Liira, J.; Maddison, M.; Moora, M. Structure and function of the soil microbiome underlying N2O emissions from global wetlands. Nat. Commun. 2022, 13, 1430. [Google Scholar] [CrossRef]
- Gufwan, L.A.; Peng, L.; Gufwan, N.M.; Lan, S.; Wu, L. Enhancing soil health through biocrusts: A microbial ecosystem approach for degradation control and restoration. Microb. Ecol. 2025, 88, 8. [Google Scholar] [CrossRef]
- Jyoti, P.; Bhardwaj, J.; Kaushal, G.; Yadav, S.K. Advancing Our Understanding of Plant–Microbe Interactions through Integrating Multiomics and Stable Isotopes for Sustainable Agriculture. ACS Agric. Sci. Technol. 2025, 5, 1225–1237. [Google Scholar] [CrossRef]
- Candry, P.; Abrahamson, B.; Stahl, D.A.; Winkler, M.K.H. Microbially mediated climate feedbacks from wetland ecosystems. Global Change Biol. 2023, 29, 5169–5183. [Google Scholar] [CrossRef]
- Sheng, X.; Zhou, J.; Lu, M.; Jin, H.; Wang, W.; Zhang, Z.; Chen, L.; Liu, W.; Wang, X.; La, Q. Precipitation rather than temperature dominates microbial necromass accumulation by regulating soil physicochemical properties in alpine wetlands. Soil Biol. Biochem. 2025, 211, 109987. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Wang, Y.; Yu, S. Ecotone-Driven Vegetation Transitions Reshape Soil Nitrogen Cycling Functional Genes in Black Soils of Northeast China. Biology 2025, 14, 1474. https://doi.org/10.3390/biology14111474
Ding J, Wang Y, Yu S. Ecotone-Driven Vegetation Transitions Reshape Soil Nitrogen Cycling Functional Genes in Black Soils of Northeast China. Biology. 2025; 14(11):1474. https://doi.org/10.3390/biology14111474
Chicago/Turabian StyleDing, Junnan, Yingjian Wang, and Shaopeng Yu. 2025. "Ecotone-Driven Vegetation Transitions Reshape Soil Nitrogen Cycling Functional Genes in Black Soils of Northeast China" Biology 14, no. 11: 1474. https://doi.org/10.3390/biology14111474
APA StyleDing, J., Wang, Y., & Yu, S. (2025). Ecotone-Driven Vegetation Transitions Reshape Soil Nitrogen Cycling Functional Genes in Black Soils of Northeast China. Biology, 14(11), 1474. https://doi.org/10.3390/biology14111474






