Comparative Photosynthetic Induction Reveals Stomatal Limitation and Reduced Efficiency in Digitalis purpurea Versus Cucumis sativus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Measurement of Photosynthetic Induction
2.3. Quantification of Limitations During Photosynthetic Induction
2.4. Photosynthetic Induction Kinetics, Carbon Gain, and Potential Carbon Loss
2.5. Statistical Analysis
3. Results
3.1. Variations in Photosynthetic Parameters During Induction
3.2. Photosynthetic Traits at Steady State
3.3. Dynamic Changes in Water Use Efficiency
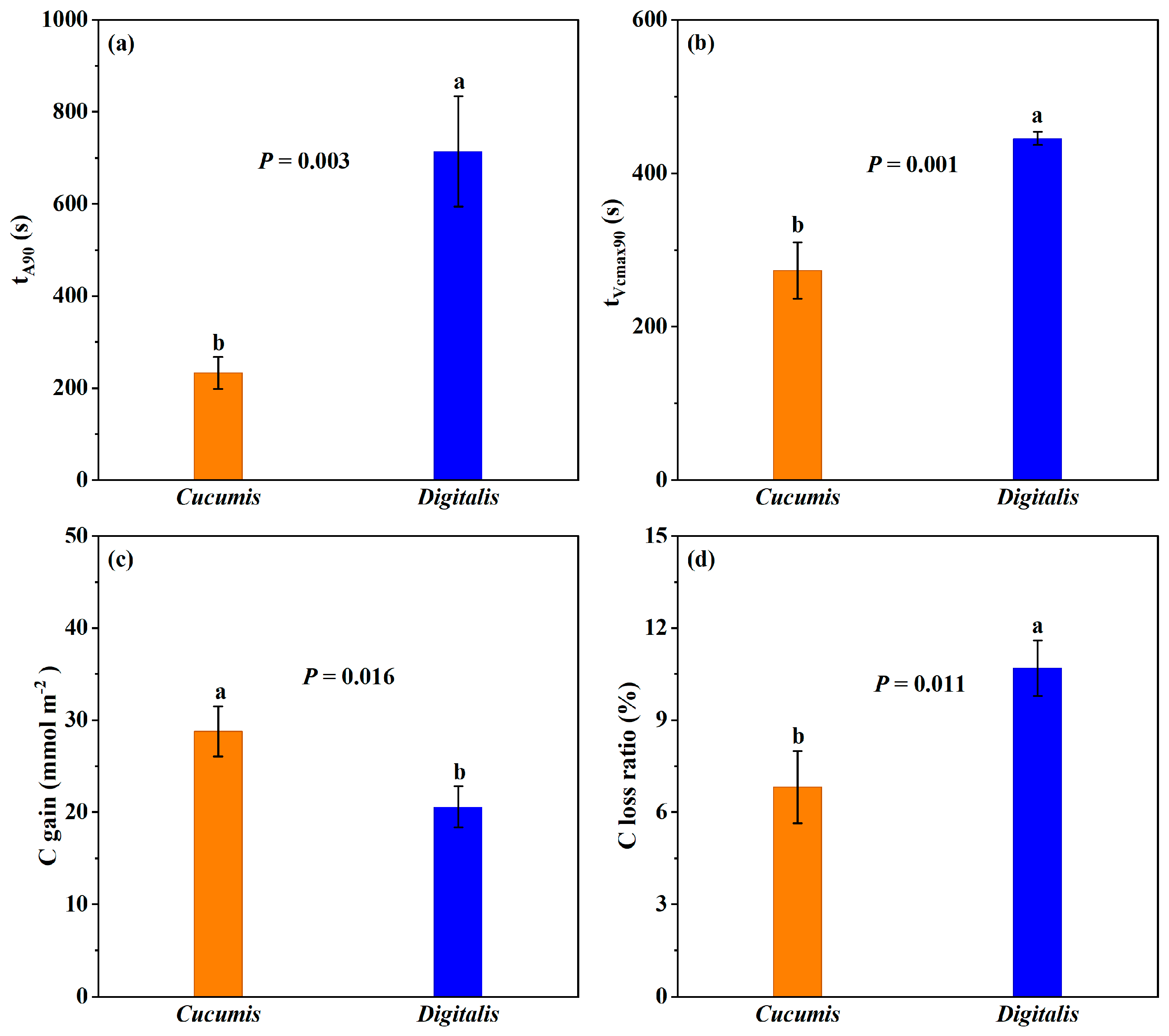
3.4. Response Speed to Reach 90% Induction State
3.5. Dynamic and Time-Integrated Photosynthetic Limitations
4. Discussion
4.1. Slower Induction Rates in D. purpurea Compared with Those of Cucumis Result in Greater Carbon Loss
4.2. Stomatal Limitation as the Primary Constraint on Photosynthetic Induction in D. purpurea
4.3. Cultivation Recommendations for D. purpurea
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, S.R.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.J.; Brown, E.; Thornton, R.; Shiva, T.; Hubbard, J.; Reddy, K.; Doherty, J.; Cardello, F.; Fast, A.; Radford, M.; et al. The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med. 1997, 336, 525–533. [Google Scholar]
- Prassas, I.; Diamandis, E.P. Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef]
- Glaser, V. Billion-dollar market blossoms as botanicals take root. Nat. Biotechnol. 1999, 17, 17–18. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef]
- Buchanan, B.B. The carbon (formerly dark) reactions of photosynthesis. Photosynth. Res. 2016, 128, 215–217. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Tichá, I. Ontogeny of leaf morphology and anatomy. In Photosynthesis During Leaf Development; Šesták, Z., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 16–50. [Google Scholar]
- Kolodziejek, J. Effects of microhabitat on leaf traits in Digitalis grandiflora L. (Veronicaceae) growing at forest edge and interior. Arch. Biol. Sci. 2014, 66, 615–627. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Y.; Kopp, K.; Oki, L.; Jones, S.; Hipps, L. Physiological and canopy temperature responses to drought of four Penstemon species. Hort. Sci. 2023, 58, 539–549. [Google Scholar] [CrossRef]
- Leuschner, C. Air humidity as an ecological factor for woodland herbs: Leaf water status, nutrient uptake, leaf anatomy, and productivity of eight species grown at low or high VPD levels. Flora 2002, 197, 262–274. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbó, M.; Diaz-Espejo, A.; Galmés, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Carriquí, M.; Cabrera, H.; Conesa, M.A.; Coopman, R.E.; Douthe, C.; Gago, J.; Gallé, A.; Galmés, J.; Ribas-Carbo, M.; Tomás, M.; et al. Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant Cell Environ. 2015, 38, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Harley, P.C.; Sharkey, T.D. An improved model of C3 photosynthesis at high CO2: Reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynth. Res. 1991, 27, 169–178. [Google Scholar] [CrossRef]
- Verma, S.; Gantait, S.; Jeong, B.; Hwang, S. Enhanced growth and cardenolides production in Digitalis purpurea under the influence of different LED exposures in the plant factory. Sci. Rep. 2018, 8, 18009. [Google Scholar] [CrossRef]
- Pearcy, R.W.; Roden, J.S.; Gamon, J.A. Sunfleck dynamics in relation to canopy structure in a soybean (Glycine max (L.) Merr.) canopy. Agric. For. Meteorol. 1990, 52, 359–372. [Google Scholar] [CrossRef]
- Durand, M.; Robson, T.M. Fields of a thousand shimmers: Canopy architecture determines high-frequency light fluctuations. New Phytol. 2023, 238, 2000–2015. [Google Scholar] [CrossRef]
- Acevedo-Siaca, L.G.; McAusland, L. A guide to understanding and measuring photosynthetic induction: Considerations and recommendations. New Phytol. 2025, 247, 450–469. [Google Scholar] [CrossRef]
- Taylor, S.H.; Long, S.P. Slow induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160543. [Google Scholar] [CrossRef]
- Wang, Y.; Burgess, S.J.; de Becker, E.M.; Long, S.P. Photosynthesis in the fleeting shadows: An overlooked opportunity for increasing crop productivity? Plant J. 2020, 101, 874–884. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J. Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiol. 2018, 176, 977–989. [Google Scholar] [CrossRef]
- Salter, W.T.; Merchant, A.; Richards, R.A.; Trethowan, R.M.; Buckley, T.N. Rate of photosynthetic induction in fluctuating light varies widely among genotypes of wheat. J. Exp. Bot. 2019, 70, 2787–2796. [Google Scholar] [CrossRef]
- Sakoda, K.; Yamori, W.; Groszmann, M.; Evans, J.R. Stomatal, mesophyll conductance, and biochemical limitations to photosynthesis during induction. Plant Physiol. 2021, 185, 146–160. [Google Scholar] [CrossRef]
- De Souza, A.P.; Wang, Y.; Orr, D.J.; Carmo-Silva, E.; Long, S.P. Photosynthesis across African cassava germplasm is limited by Rubisco and mesophyll conductance at steady state, but by stomatal conductance in fluctuating light. New Phytol. 2020, 225, 2498–2512. [Google Scholar] [CrossRef] [PubMed]
- McAusland, L.; Vialet-Chabrand, S.; Davey, P.; Baker, N.R.; Brendel, O.; Lawson, T. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol. 2016, 211, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Makino, A.; Shikanai, T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef]
- Yamori, W.; Masumoto, C.; Fukayama, H.; Makino, A. Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 2012, 71, 871–880. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef]
- Acevedo-Siaca, L.G.; Coe, R.A.; Wang, Y.; Kromdijk, J.; Quick, W.P.; Long, S.P. Variation in photosynthetic induction between rice accessions and its potential for improving productivity. New Phytol. 2020, 227, 1097–1108. [Google Scholar] [CrossRef]
- Eyland, D.; van Wesemael, J.; Lawson, T.; Carpentier, S.C. The impact of slow stomatal kinetics on photosynthesis and water use efficiency under fluctuating light. Plant Physiol. 2021, 186, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Peng, S.; Li, Y. Increase rate of light-induced stomatal conductance is related to stomatal size in the genus Oryza. J. Exp. Bot. 2019, 70, 5259–5269. [Google Scholar] [CrossRef] [PubMed]
- Deans, R.M.; Farquhar, G.D.; Busch, F.A. Estimating stomatal and biochemical limitations during photosynthetic induction. Plant Cell Environ. 2019, 42, 3227–3240. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Jin, W.; Li, L.; Xu, K.; Wei, Y. Potassium-mediated variations in the photosynthetic induction characteristics of Phaseolus vulgaris L. Plants 2025, 14, 1623. [Google Scholar] [CrossRef]
- Mott, K.A.; Woodrow, I.E. Modelling the role of Rubisco activase in limiting non-steady-state photosynthesis. J. Exp. Bot. 2000, 51, 399–406. [Google Scholar] [CrossRef]
- Pfitsch, W.A.; Pearcy, R.W. Steady-state and dynamic photosynthetic response of Adenocaulon bicolor (Asteraceae) in its redwood forest habitat. Oecologia 1989, 80, 471–476. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Scholes, J.D.; Press, M.C. Physiological and ecological significance of sunflecks for dipterocarp seedlings. J. Exp. Bot. 2005, 56, 469–482. [Google Scholar] [CrossRef]
- Tanaka, Y.; Adachi, S.; Yamori, W. Natural genetic variation of the photosynthetic induction response to fluctuating light environment. Curr. Opin. Plant Biol. 2019, 49, 52–59. [Google Scholar] [CrossRef]
- Taylor, T.C.; Andersson, I. Structural transitions during activation and ligand binding in hexadecameric Rubisco inferred from the crystal structure of the activated unliganded spinach enzyme. Nat. Struct. Biol. 1996, 3, 95–101. [Google Scholar] [CrossRef]
- Soleh, M.A.; Tanaka, Y.; Nomoto, Y.; Iwahashi, Y.; Nakashima, K.; Fukuda, Y.; Long, S.P.; Shiraiwa, T. Factors underlying genotypic differences in the induction of photosynthesis in soybean (Glycine max (L.) Merr.). Plant Cell Environ. 2016, 39, 685–693. [Google Scholar] [CrossRef]
- Pan, Y.; Du, H.; Meng, X.; Guo, S. Variation in photosynthetic induction between super hybrid rice and inbred super rice. Plant Physiol. Biochem. 2022, 178, 105–115. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Wakabayashi, Y.; Mercer, K.; Kawabata, S.; Kobayashi, T.; Tabuchi, T.; Yamori, W. Natural genetic variation in dynamic photosynthesis is correlated with stomatal anatomical traits in diverse tomato species across geographical habitats. J. Exp. Bot. 2024, 75, 6762–6777. [Google Scholar] [CrossRef]
- Papanatsiou, M.; Petersen, J.; Henderson, L.; Wang, Y.; Christie, J.M.; Blatt, M.R. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 2019, 363, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhao, J.; Zhou, J.; Zhu, Q.; Sheng, X.; Yue, C. Elevated CO2 shifts photosynthetic constraint from stomatal to biochemical limitations during induction in Populus tomentosa and Eucalyptus robusta. Plants 2025, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Tomimatsu, H.; Tang, Y. Effects of high CO2 levels on dynamic photosynthesis: Carbon gain, mechanisms, and environmental interactions. J. Plant Res. 2016, 129, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Scheibner, H.; Björk, L.; Schulz, U.; Diettrich, B.; Luckner, M. Influence of light on cardenolide accumulation in somatic embryos of Digitalis lanata. J. Plant Physiol. 1987, 130, 211–219. [Google Scholar] [CrossRef]
- Yao, S.; Kang, Y.; Liu, H. Effects of sprinkler irrigation on photosynthesis features of winter wheat. In Proceedings of the Land and Water Management: Decision Tools and Practices, Beijing, China, 11–14 October 2004; China Agriculture Press: Beijing, China, 2004; Volumes 1–2, pp. 454–459. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Xiang, X.; Jin, W.; Xiong, H.; Tan, L. Comparative Photosynthetic Induction Reveals Stomatal Limitation and Reduced Efficiency in Digitalis purpurea Versus Cucumis sativus. Biology 2025, 14, 1445. https://doi.org/10.3390/biology14101445
Wei Y, Xiang X, Jin W, Xiong H, Tan L. Comparative Photosynthetic Induction Reveals Stomatal Limitation and Reduced Efficiency in Digitalis purpurea Versus Cucumis sativus. Biology. 2025; 14(10):1445. https://doi.org/10.3390/biology14101445
Chicago/Turabian StyleWei, Yunmin, Xiaohong Xiang, Wei Jin, Haifeng Xiong, and Lihong Tan. 2025. "Comparative Photosynthetic Induction Reveals Stomatal Limitation and Reduced Efficiency in Digitalis purpurea Versus Cucumis sativus" Biology 14, no. 10: 1445. https://doi.org/10.3390/biology14101445
APA StyleWei, Y., Xiang, X., Jin, W., Xiong, H., & Tan, L. (2025). Comparative Photosynthetic Induction Reveals Stomatal Limitation and Reduced Efficiency in Digitalis purpurea Versus Cucumis sativus. Biology, 14(10), 1445. https://doi.org/10.3390/biology14101445





