Tiny Fish, Big Hope: Zebrafish Unlocking Secrets to Fight Parkinson’s Disease
Simple Summary
Abstract
1. Introduction
2. History of Zebrafish as a Model Organism
3. Applications in Development and Disease Research
4. Advances in Neurobiology and Imaging
5. Zebrafish as a Model Organism to Study Parkinson’s Disease
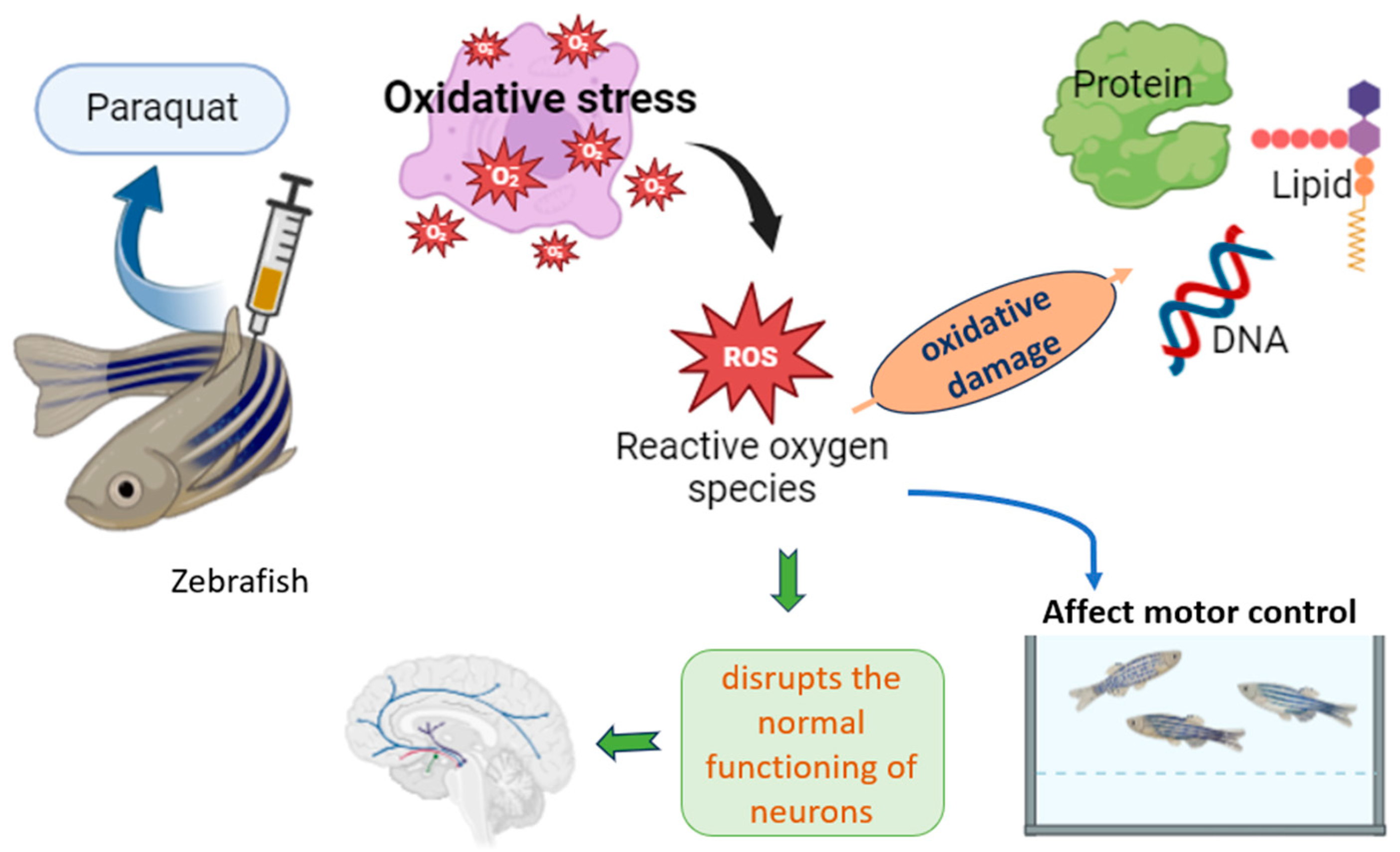
6. Inducing Parkinson’s Symptoms in Zebrafish Using Parquat
6.1. MPTP Exposure
6.2. Rotenone Exposure
6.3. Genetic Studies
6.4. Paraquat-Induced Symptoms
6.5. Shared Pathological Mechanisms
6.6. Behavioral Assessments in Practice
6.7. Neurochemical Analyses
7. Advantages of Zebrafish as a PD Model
Success Stories
8. Limitations and Considerations
9. Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s disease: Etiology, neuropathology, and pathogenesis. Exon Publ. 2018, 21, 3–26. [Google Scholar]
- Kumar, S.; Goyal, L.; Singh, S. Tremor and rigidity in patients with Parkinson’s disease: Emphasis on epidemiology, pathophysiology and contributing factors. CNS Neurol. Disord. Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2022, 21, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Chao, Y.X.; West, A.; Chan, L.L.; Poewe, W.; Jankovic, J. Parkinson disease and the immune system—Associations, mechanisms and therapeutics. Nat. Rev. Neurol. 2020, 16, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Le, W. Recent advances and perspectives of metabolomics-based investigations in Parkinson’s disease. Mol. Neurodegener. 2019, 14, 3. [Google Scholar] [CrossRef]
- Xi, Y.; Noble, S.; Ekker, M. Modelling neurodegeneration in zebrafish. Curr. Neurol. Neurosci. Rep. 2011, 11, 274–282. [Google Scholar] [CrossRef]
- Mazzolini, L.; Cerri, S.; Blandini, F.; Simola, N. Alpha-synuclein pathology in zebrafish is linked to neurodegeneration and visual deficits. Cell Death Dis. 2020, 11, 1–14. [Google Scholar]
- Najib, N.H.; Nies, Y.H.; Abd Halim, S.A.; Yahaya, M.F.; Das, S.; Lim, W.L.; Teoh, S.L. Modeling Parkinson’s disease in zebrafish. CNS Neurol. Disord. Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2020, 19, 386–399. [Google Scholar] [CrossRef]
- Prabhudesai, S.; Sinha, S.; Attar, A.; Kotagiri, A.; Fitzmaurice, A.G.; Lakshmana, M.K.; Maiti, P. LRRK2 knockdown in zebrafish causes Parkinsonism-like loss of neurons and locomotor deficits. Neuroreport 2016, 27, 1335–1340. [Google Scholar]
- Sun, Y.; Choi, J.; Lee, S.-J. MPTP-induced dopaminergic neurodegeneration and α-synuclein expression in zebrafish. J. Chem. Neuroanat. 2020, 105, 101753. [Google Scholar]
- Ulloa, P.E.; Iturra, P.; Neira, R.; Araneda, C. Zebrafish as a model organism for nutrition and growth: Towards comparative studies of nutritional genomics applied to aquacultured fishes. Rev. Fish Biol. Fish. 2011, 21, 649–666. [Google Scholar] [CrossRef]
- Ali, M.S.; Anuradha, V.; Yogananth, N. Zebrafish: A Model Organism for Regeneration Studies; Darshan Publishers: Rasipuram, India, 2021. [Google Scholar]
- Meunier, R. Stages in the development of a model organism as a platform for mechanistic models in developmental biology: Zebrafish, 1970–2000. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2012, 43, 522–531. [Google Scholar] [CrossRef]
- Poon, K.L.; Brand, T. The zebrafish model system in cardiovascular research: A tiny fish with mighty prospects. Glob. Cardiol. Sci. Pract. 2013, 2013, 9–28. [Google Scholar] [CrossRef]
- Brittijn, S.A.; Duivesteijn, S.J.; Belmamoune, M.; Bertens, L.F.; Bitter, W.; De Bruijn, J.D.; Champagne, D.L.; Cuppen, E.; Flik, G.; Vandenbroucke-Grauls, C.M.; et al. Zebrafish development and regeneration: New tools for biomedical research. Int. J. Dev. Biol. 2009, 53, 835–850. [Google Scholar] [CrossRef]
- González-Rosa, J.M. Zebrafish models of cardiac disease: From fortuitous mutants to precision medicine. Circ. Res. 2022, 130, 1803–1826. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Adhish, M.; Manjubala, I. Effectiveness of zebrafish models in understanding human diseases—A review of models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef]
- Briñez-Gallego, P.; da Costa Silva, D.G.; Cordeiro, M.F.; Horn, A.P.; Hort, M.A. Experimental models of chemically induced Parkinson’s disease in zebrafish at the embryonic larval stage: A systematic review. J. Toxicol. Environ. Health Part B 2023, 26, 201–237. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, P. Calcium imaging in the zebrafish. Adv. Exp. Med. Biol. 2012, 740, 1039–1071. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, P. Calcium imaging in the zebrafish. Adv. Exp. Med. Biol. 2020, 1131, 901–942. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, K.; Handa, H.; Kawakami, K. Illuminating ALS motor neurons with optogenetics in zebrafish. Front. Cell Dev. Biol. 2021, 9, 640414. [Google Scholar] [CrossRef]
- Chai, Y.; Qi, K.; Wu, Y.; Li, D.; Tan, G.; Guo, Y.; Chu, J.; Mu, Y.; Shen, C.; Wen, Q. All-optical interrogation of brain-wide activity in freely swimming larval zebrafish. iScience 2024, 27, 108385. [Google Scholar] [CrossRef]
- Antinucci, P.; Hindges, R. A crystal-clear zebrafish for in vivo imaging. Sci. Rep. 2016, 6, 29490. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Sakai, C.; Ijaz, S.; Hoffman, E.J. Zebrafish models of neurodevelopmental disorders: Past, present, and future. Front. Mol. Neurosci. 2018, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Kannan, R.R. Zebrafish: A promising real-time model system for nanotechnology-mediated neurospecific drug delivery. Nanoscale Res. Lett. 2021, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Gao, J.M.; Huang, C.J.; Li, C.Q. Zebrafish models for assessing developmental and reproductive toxicity. Neurotoxicol. Teratol. 2014, 42, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Javitch, J.A.; D’Amato, R.J.; Strittmatter, S.M.; Snyder, S.H. Parkinsonism-inducing neurotoxin, MPTP: Up-take of the metabolite MPP+ by dopamine neurons explains selective toxicity. Proc. Natl. Acad. Sci. USA 1985, 82, 2173–2177. [Google Scholar] [CrossRef]
- Hare, D.J.; Adlard, P.A.; Doble, P.A.; Finkelstein, D.I. Metallobiology of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Metallomics 2013, 5, 91–109. [Google Scholar] [CrossRef]
- Lange, K.W. Bedeutung des Neurotoxins MPTP für Ätiologie und Therapie der idiopathischen Parkin-sonkrankheit. Fortschr. Neurol. Psychiatr. 1989, 57, 142–148. [Google Scholar] [CrossRef]
- Drechsel, D.A.; Patel, M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic. Biol. Med. 2008, 44, 1873–1886. [Google Scholar] [CrossRef]
- Liu, B.; Gao, H.M.; Hong, J.S. Role of microglia in inflammation-mediated neurodegeneration. Glia 2003, 41, 180–190. [Google Scholar]
- Razali, K.; Mohd Nasir, M.H.; Kumar, J.; My Mohamed, W. Mitophagy: A Bridge Linking HMGB1 and Parkinson’s Disease Using Adult Zebrafish as a Model Organism. Brain Sci. 2023, 13, 1076. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Weston, L.J.; Cook, Z.T.; Stackhouse, T.L.; Sal, M.K.; Schultz, B.I.; Tobias, Z.J.; Osterberg, V.R.; Brockway, N.L.; Pizano, S.; Glover, G.; et al. In vivo aggregation of presynaptic alpha-synuclein is not influenced by its phosphorylation at serine-129. Neurobiol. Dis. 2021, 152, 105291. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, Y.; Wan, F.; Ma, K.; Guo, X.; Kou, L.; Yin, S.; Han, C.; Liu, L.; Huang, J.; et al. New perspectives on roles of alpha-synuclein in Parkinson’s disease. Front. Aging Neurosci. 2018, 10, 370. [Google Scholar] [CrossRef]
- Wasel, O.; Freeman, J.L. Chemical and Genetic Zebrafish Models to Define Mechanisms of and Treatments for Dopaminergic Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5981. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, L.; Caldarazzo-Ienco, E.; Fornai, F. MPTP neurotoxicity: Actions, mechanisms, and animal modeling of Parkinson’s disease. In Handbook of Neurotoxicity; Springer: New York, NY, USA, 2014; pp. 237–275. [Google Scholar]
- Mohamed, W. Induction and Validation of Zebrafish Model of Parkinson’s Disease. In Zebrafish as a Model for Parkinson’s Disease; CRC Press: Boca Raton, FL, USA, 2024; pp. 204–214. [Google Scholar] [CrossRef]
- Athauda, D.; Foltynie, T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat. Rev. Neurol. 2015, 11, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.I.; Rojo, A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s disease: Mechanisms and therapeutic implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A common feature of pesticides: Oxidative stress—The role of oxidative stress in pesticide-induced toxicity. Oxidative Medicine and Cellular Longevity. Oxidative Med. Cell. Longev. 2022, 5563759. [Google Scholar] [CrossRef]
- Chen, J.; Su, Y.; Lin, F.; Iqbal, M.; Mehmood, K.; Zhang, H.; Shi, D. Effect of paraquat on cytotoxicity involved in oxidative stress and inflammatory reaction: A review of mechanisms and ecological implications. Ecotoxicol. Environ. Saf. 2021, 224, 112711. [Google Scholar] [CrossRef]
- Wang, X.H.; Souders, C.L., II; Zhao, Y.H.; Martyniuk, C.J. Paraquat affects mitochondrial bioenergetics, dopamine system expression, and locomotor activity in zebrafish (Danio rerio). Chemosphere 2018, 191, 106–117. [Google Scholar] [CrossRef]
- Vaz, R.L.; Outeiro, T.F.; Ferreira, J.J. Zebrafish as an animal model for drug discovery in Parkinson’s dis-ease and other movement disorders: A systematic review. Front. Neurol. 2018, 9, 357994. [Google Scholar] [CrossRef]
- Doyle, J.M.; Croll, R.P. A critical review of zebrafish models of Parkinson’s disease. Front. Pharmacol. 2022, 13, 835827. [Google Scholar] [CrossRef]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative stress factors in Parkinson’s disease. Neural Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef]
- Omar, N.A.; Kumar, J.; Teoh, S.L. Neuroprotective effects of Neurotrophin-3 in MPTP-induced zebrafish Parkinson’s disease model. Front. Pharmacol. 2023, 14, 1307447. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar]
- Czerniczyniec, A.; Karadayian, A.G.; Bustamante, J.; Cutrera, R.A.; Lores-Arnaiz, S. Paraquat induces behavioral changes and cortical and striatal mitochondrial dysfunction. Free Radic. Biol. Med. 2011, 51, 142836. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Niu, Y.; Zhang, R.; Guo, H.; Gao, Y.; Li, Y.; Liu, R. The toxic influence of paraquat on hippocampus of mice: Involvement of oxidative stress. Neurotoxicology 2010, 31, 310–316. [Google Scholar] [CrossRef]
- Harini, V.S.; Marimuthu, R.; Tantry, M.S.A.; Santhakumar, K. Induction of Paraquat-Mediated Parkinsonian Phenotype in Zebrafish. Curr. Protoc. 2024, 4, e990. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.E.M.; Müller, T.E.; Braga, M.M.; Fontana, B.D.; Quadros, V.A.; Marins, A.T.; Rodrigues, C.C.R.; Menezes, C.; Rosemberg, D.B.; Loro, V.L. Chronic Treatment with Paraquat Induces Brain Injury, Changes in Antioxidant Defence System, and Modulates Behavioural Functions in Zebrafish. Mol. Neurobiol. 2017, 54, 3925–3934. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.C.B. The Parkinson-Associated Toxin Paraquat Shifts Physiological α-Synuclein Tetramers to-ward Monomers That Can Be Calpain-Truncated and Form Oligomers. Am. J. Pathol. 2023, 193, 520–531. [Google Scholar] [CrossRef]
- Bagwell, E.; Larsen, J. A review of MPTP-induced Parkinsonism in adult zebrafish to explore pharmacological interventions for human Parkinson’s disease. Front. Neurosci. 2024, 18, 1451845. [Google Scholar] [CrossRef]
- Razali, K.; Mohd Nasir, M.H.; Othman, N.; Doolaanea, A.A.; Kumar, J.; Nabeel Ibrahim, W.; Mohamed, W.M. Characterization of neurobehavioral pattern in a zebrafish 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced model: A 96-hour behavioral study. PLoS ONE 2022, 17, e0274844. [Google Scholar] [CrossRef]
- Tagkalidou, N.; Stevanović, M.; Romero-Alfano, I.; Elizalde-Velázquez, G.A.; Herrera-Vázquez, S.E.; Prats, E.; Gómez-Canela, C.; Gómez-Oliván, L.M.; Raldúa, D. Motor and non-motor effects of acute MPTP in adult zebrafish: Insights into Parkinson’s disease. Int. J. Mol. Sci. 2025, 26, 1674. [Google Scholar] [CrossRef]
- Christensen, C.; Þorsteinsson, H.; Maier, V.H.; Karlsson, K.Æ. Multi-parameter behavioral phenotyping of the MPP+ model of Parkinson’s disease in zebrafish. Front. Behav. Neurosci. 2020, 14, 623924. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, P.; Niyangoda, S.S.; Jarosova, R.; Johnson, M.A. Dopamine release impairments accompany locomotor and cognitive deficiencies in rotenone-treated Parkinson’s disease model zebrafish. Chem. Res. Toxicol. 2022, 35, 1974–1982. [Google Scholar] [CrossRef]
- Ilie, O.D.; Duta, R.; Jijie, R.; Nita, I.B.; Nicoara, M.; Faggio, C.; Dobrin, R.; Mavroudis, I.; Ciobica, A.; Doroftei, B. Assessing anti-social and aggressive be-havior in a zebrafish (Danio rerio) model of Parkinson’s disease chronically exposed to rotenone. Brain Sci. 2022, 12, 898. [Google Scholar] [CrossRef]
- Khalili, A.; Safarian, N.; van Wijngaarden, E.; Zoidl, G.S.; Zoidl, G.R.; Rezai, P. Loss of Panx1 function in zebrafish alters motor behavior in a lab-on-chip model of Parkinson’s disease. J. Neurosci. Res. 2023, 101, 1814–1825. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Yang, J.; Wang, F.; Sima, Y.; Zhong, Z.M.; Wang, H.; Hu, L.F.; Liu, C.F. Parkinson’s disease-like motor and non-motor symptoms in rote-none-treated zebrafish. Neurotoxicology 2017, 58, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Nukala, S.B.; Murthy, C.L.; Kakara, S.; Sharma, R.; Swamy, C.V.B.; Idris, M.M. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s disease in zebrafish. Proteomics 2016, 16, 1407–1420. [Google Scholar] [CrossRef]
- Ranasinghe, T.; Seo, Y.; Park, H.; Choe, S.; Cha, S. Rotenone exposure causes features of Parkinson’s disease pathology linked with muscle atrophy in developing zebrafish embryo. J. Hazard. Mater. 2024, 480, 136215. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, L.M.; Murata, H.; Bronstein, J.M. Studying the Pathophysiology of Parkinson’s Disease Using Zebrafish. Biomedicines 2020, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mittal, P. Paraquat (herbicide) as a cause of Parkinson’s Disease. Park. Relat. Disord. 2024, 119, 105932. [Google Scholar] [CrossRef]
- Colle, D.; Farina, M. Oxidative stress in paraquat-induced damage to nervous tissues. In Toxicology; Academic Press: Cambridge, MA, USA, 2021; pp. 69–78. [Google Scholar]
- See, W.Z.; Naidu, R.; San Tang, K. Paraquat and Parkinson’s disease: The molecular crosstalk of up-stream signal transduction pathways leading to apoptosis. Curr. Neuropharmacol. 2024, 22, 140–151. [Google Scholar]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R. Rotenone, paraquat, and Parkinson’s disease. Envrion. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef]
- Bassett, S.S. Cognitive impairment in Parkinson’s disease. Prim. Psychiatry 2005, 12, 50–55. [Google Scholar]
- Xie, A.; Gao, J.; Xu, L.; Meng, D. Shared mechanisms of neurodegeneration in Alzheimer’s disease and Parkinson’s disease. Biomed. Res. Int. 2014, 2014, 648740. [Google Scholar] [CrossRef]
- Moustafa, A.A.; Chakravarthy, S.; Phillips, J.R.; Crouse, J.J.; Gupta, A.; Frank, M.J.; Hall, J.M.; Jahanshahi, M. Interrelations between cognitive dysfunction and motor symptoms of Parkinson’s disease: Behavioral and neural studies. Rev. Neurosci. 2016, 27, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Monzio Compagnoni, G.; Di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The role of mitochondria in neurodegenerative diseases: The lesson from Alzheimer’s disease and Parkinson’s disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef]
- Tembhurnikar, H.J.; Thool, N.D.; Patil, R.J.; Das, R.K. Review on various factors responsible for neurodegenerative disorders. GSC Biol. Pharm. Sci. 2021, 16, 126–132. [Google Scholar] [CrossRef]
- Adamson, K.I.; Sheridan, E.; Grierson, A.J. Use of zebrafish models to investigate rare human disease. J. Med. Genet. 2018, 55, 641–649. [Google Scholar] [CrossRef]
- Trollope, L. Zebrafish as a Translational Model of Parkinson’s Disease—A Study of Micro RNAs. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2015. Available online: https://etheses.whiterose.ac.uk/id/eprint/11971/1/Lisa%20Trollope%20final%20PhD%20thesis.pdf (accessed on 1 January 2020).
- Sager, J.J.; Bai, Q.; Burton, E.A. Transgenic zebrafish models of neurodegenerative diseases. Brain Struct. Funct. 2010, 214, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, K.J.; Bandres-Ciga, S.; Saez-Atienzar, S.; Singleton, A.B. Genetic risk factors in Parkinson’s 2disease. Cell Tissue Res. 2018, 373, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 2006, 443, 780–786. [Google Scholar] [CrossRef]
- Hewitt, V.L.; Whitworth, A.J. Mechanisms of Parkinson’s disease: Lessons from Drosophila. Curr. Top. Dev. Biol. 2017, 121, 173–200. [Google Scholar]
- Fontana, B.D.; Mezzomo, N.J.; Kalueff, A.V.; Rosemberg, D.B.; Miller, G.W.; Chandrasekaran, V.; Yaghoobi, B.; Lein, P.J. The developing utility of zebrafish models of Neurological and neuropsychiatric disorders: A critical review. Exp. Neurol. 2018, 299, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Wen, Z.H.; Lin, C.S.; Chakraborty, C. The zebrafish model: Use in studying cellular mechanisms for a spectrum of clinical disease entities. Curr. Neurovascular Res. 2007, 4, 111–120. [Google Scholar] [CrossRef]
- Miller, G.W.; Chandrasekaran, V.; Yaghoobi, B.; Lein, P.J. Opportunities and challenges for using the zebrafish to study neuronal connectivity as an endpoint of developmental neurotoxicity. Neurotoxicology 2018, 67, 102–111. [Google Scholar] [CrossRef]
- Marchesan, E. Calcineurin Regulates Parkin-Translocation to Mitochondria and Mitophagy. Ph.D. Thesis, University of Padua, Padua, Italy, 2019. [Google Scholar]
- d’Alençon, C.A.; Peña, O.A.; Wittmann, C.; Gallardo, V.E.; Jones, R.A.; Loosli, F.; Liebel, U.; Grabher, C.; Allende, M.L. A high-throughput chemically induced inflammation assay in zebrafish. BMC Biol. 2010, 8, 151. [Google Scholar] [CrossRef]
- Turrini, L.; Roschi, L.; de Vito, G.; Pavone, F.S.; Vanzi, F. Imaging Approaches to Investigate Pathophysiological Mechanisms of brain disease in zebrafish. Int. J. Mol. Sci. 2023, 24, 9833. [Google Scholar] [CrossRef]
- Colón-Rodríguez, A.; Uribe-Salazar, J.M.; Weyenberg, K.B.; Sriram, A.; Quezada, A.; Kaya, G.; Jao, E.; Radke, B.; Lein, P.J.; Dennis, M.Y. Assessment of autism zebrafish mutant models using a high-throughput larval phenotyping platform. Front. Cell Dev. Biol. 2020, 8, 586296. [Google Scholar] [CrossRef]
- Gibert, Y.; Trengove, M.C.; Ward, A.C. Zebrafish as a genetic model in pre-clinical drug testing and screening. Curr. Med. Chem. 2013, 20, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Chia, K.; Klingseisen, A.; Sieger, D.; Priller, J. Zebrafish as a model organism for neurodegenerative disease. Front. Mol. Neurosci. 2022, 15, 940484. [Google Scholar] [CrossRef] [PubMed]
- Trumon, K. Zebrafish: A potential preclinical model for neurological research. In Modern Biology; Springer: Singapore, 2022; pp. 321–345. [Google Scholar] [CrossRef]
- Noor, S.M.; Norazit, A. Fishing for synucleinopathy models. Fish. Aquat. Sci. 2022, 25, 117–139. [Google Scholar] [CrossRef]
- Yamanaka, T.; Matsui, H. Modeling familial and sporadic Parkinson’s diseases in small fishes. Dev. Growth Differ. 2024, 66, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, B.; Asrar, S.S.; Revanth, B.; Gautam, P.; Sapkota, B.; Reddy, K.S.; Pasala, P.K. Advancing Neuroscience through Zebrafish: Challenges, Innovations, and Future Directions. Uttar Pradesh J. Zool. 2024, 45, 164–176. [Google Scholar] [CrossRef]
- Zeng, X.S.; Geng, W.S.; Jia, J.J. Neurotoxin-induced animal models of Parkinson disease: Pathogenic mechanism and assessment. ASN Neuro 2018, 10, 1759091418777438. [Google Scholar] [CrossRef]
- Keow, J. Physiological and Behavioral Changes in a Rotenone Model of Dopamine Neurotoxicity and Neurodegeneration in Zebrafish. Ph.D. Thesis, Université d’Ottawa/University of Ottawa, Ottawa, ON, Canada, 2016. Available online: https://ruor.uottawa.ca/items/644ae441-f7fb-4e61-b47d-09aaaa0310f9 (accessed on 1 January 2020).
- Grillner, S.; El Manira, A. Current principles of motor control, with special reference to vertebrate loco-motion. Physiol. Rev. 2020, 100, 271–320. [Google Scholar] [CrossRef]
- Bretaud, S.; MacRaild, S.; Ingham, P.W.; Bandmann, O. The influence of the zebrafish genetic background on Parkinson’s disease-related aspects. Zebrafish 2011, 8, 103–108. [Google Scholar] [CrossRef]
- Meshalkina, D.A.; Kizlyk, M.N.; Kysil, E.V.; Collier, A.D.; Echevarria, D.J.; Abreu, M.S.; Barcellos, L.J.G.; Song, C.; Kalueff, A.V. Understanding zebrafish cognition. Behav. Process. 2017, 141, 229–241. [Google Scholar] [CrossRef]
- Latif, S.; Jahangeer, M.; Razia, D.M.; Ashiq, M.; Ghaffar, A.; Akram, M.; Allam, A.E.; Bouyahya, A.; Garipova, L.; Shariati, M.A.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Hou, L.; Chen, W.; Liu, X.; Qiao, D.; Zhou, F.M. Exercise-induced Neuroprotection of the nigrostriatal dopamine system in Parkinson’s disease. Front. Aging Neurosci. 2017, 9, 358. [Google Scholar] [CrossRef]
- Trigo-Damas, I.; Del Rey, N.L.; Blesa, J. Novel models for Parkinson’s disease and their impact on future drug discovery. Expert Opin. Drug Discov. 2018, 13, 229–239. [Google Scholar] [CrossRef]
- Saponjic, J.; Mejías, R.; Nikolovski, N.; Dragic, M.; Canak, A.; Papoutsopoulou, S.; Gürsoy-Özdemir, Y.; Fladmark, K.E.; Ntavaroukas, P.; Muluk, N.B.; et al. Experimental models to study immune dysfunction in the Pathogenesis of Parkinson’s disease. Int. J. Mol. Sci. 2024, 25, 4330. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Mc Cormick, L.; Thapa, P.; Karki, R.; Evans, T. Use of zebrafish in chemical biology and drug discovery. Future Med. Chem. 2013, 5, 2103–2116. [Google Scholar] [CrossRef]
- Gelders, G.; Baekelandt, V.; Van der Perren, A. Linking neuroinflammation and neurodegeneration in Parkinson’s disease. J. Immunol. Res. 2018, 16, 4784268. [Google Scholar] [CrossRef]
- Goodwin, N.; Karp, N.A.; Blackledge, S.; Clark, B.; Keeble, R.; Kovacs, C.; Murray, K.N.; Price, M.; Thompson, P.; Bussell, J. Standardized welfare terms for the zebrafish Community. Zebrafish 2016, 13 (Suppl. S1), S164–S168. [Google Scholar] [CrossRef] [PubMed]
- Vorberg, I.; Chiesa, R. Experimental models to study prion disease pathogenesis and identify potential therapeutic compounds. Curr. Opin. Pharmacol. 2019, 44, 28–38. [Google Scholar] [CrossRef]
- Ochenkowska, K.; Herold, A.; Samarut, É. Zebrafish is a powerful tool for precision medicine ap-proaches to neurological disorders. Front. Mol. Neurosci. 2022, 15, 944693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.-B.; He, K.-J.; Wang, F.; Liu, C.-F. Advances of Zebrafish in Neurodegener-ative Disease: From Models to Drug Discovery. Front. Pharmacol. 2021, 12, 713963. [Google Scholar] [CrossRef]
- Hernández, T.D.R.; Gore, S.V.; Kreiling, J.A.; Creton, R. Drug repurposing for neurodegenerative diseases using Zebrafish behavioural profiles. Biomed. Pharmacother. 2024, 171, 116096. [Google Scholar] [CrossRef]
- Dey, S.; Thamaraikani, T.; Vellapandian, C. Advancing Alzheimer’s Research With Zebrafish Models: Current Insights, Addressing Challenges, and Charting Future Courses. Cureus 2024, 16, e66935. [Google Scholar] [CrossRef] [PubMed]

| Model Category | Typical Stage Used | Application |
|---|---|---|
| Neurotoxin (MPTP, Rotenone, Paraquat, 6-OHDA) | Larvae for throughput; Adults for chronic/behavioral | Larvae: imaging & high throughput; Adults: complex motor assays |
| Genetic (SNCA, PINK1, Parkin, LRRK2, DJ-1) | Embryo/larva → adult (depending on phenotype) | Early developmental effects in larvae; adult lines for progressive phenotypes |
| Environmental (Mn, Pb, pesticide mixtures) | Larvae and adults (dose/time dependent) | Models cumulative, low-dose or chronic exposures; behavioural impact in adults |
| Category | Method | Protocol Overview | Key Parameters | Typical Equipment | Validation/Notes |
|---|---|---|---|---|---|
| Motor | Open-field locomotor assay (adult) | Single fish in arena, recorded 0–96 h post-toxin (MPTP, rotenone) | Distance, velocity, immobility, turn angle, meander | Video camera, arena, tracking software | Validated against DA depletion and TH staining [46,55,56,57,58] |
| Motor | Automated larval swimming tracking | Larvae exposed to MPP+ or rotenone; monitored under light/dark cycles | Swim distance, bout counts, thigmotaxis, transitions | Multi-well plates, automated imaging/tracking | Dose–response and drug rescue shown [46,56,57,58] |
| Motor | Maze & reward latency tests | Fish trained to reach reward after toxin exposure | Latency, errors, path efficiency, learning curve | Custom maze, video tracking | Cognitive impairments linked to DA release deficits [55,59,60] |
| Motor | Kinematic analysis & acoustic startle | High-speed capture of escape/startle; acoustic pulses | Turn duration, angular velocity, startle latency/habituation | High-speed camera, acoustic stimulator | Sensitive to subtle sensorimotor + deficits [46,56,61]. |
| Motor | Electrical stimulation (microfluidic) | 6-OHDA larvae; electrical pulses in lab-on-chip | Evoked locomotor amplitude, response frequency | Microfluidic chip, electrodes, video | Validated with Panx1 mutants & TH analysis [58,61] |
| Non-motor | Light–dark preference | Fish explore divided tank | Time in zones, transitions, latency | Light/dark box, tracker | Anxiety-like phenotypes observed [55,57,60,62] |
| Non-motor | Thigmotaxis | Open field with center/periphery zones | Wall-following, time in center vs. periphery | Arena, tracker | Reliable anxiety measure in PD models [46,58,62] |
| Non-motor | Sleep & circadian monitoring | 24 h continuous recording | Sleep duration, latency, fragmentation, circadian phase | Infrared cameras, automated software | Melatonin rescue of sleep deficits shown [55,56,57,62] |
| Non-motor | Social interaction/shoaling | Paired/group assays or mirror tests | Interaction time, aggression, shoaling | Dual chamber, video | Rotenone reduces sociality, increases aggression [60,62] |
| Non-motor | Olfactory response testing | Odor choice/gradient assays | Latency, preference index, discrimination | Olfactometer, airflow, video | Limited but reported in MPTP/rotenone [46,56] |
| Non-motor | Cognitive (conditioning) assays | Classical/operant learning, memory retention | Acquisition, retention, reversal learning | Conditioning chambers, stimulus system | MPTP/rotenone impair memory; rescued by drugs [55,59,60] |
| Modeling Approach | Key Phenotypes in Zebrafish | Comparative Advantages vs. Rodent Systems | Limitations | References |
|---|---|---|---|---|
| Neurotoxin: MPTP, 6-OHDA | Dopaminergic neuron loss; reduced locomotion; erratic swimming; altered gene/protein expression in neurological pathways | Rapid induction of PD-like symptoms; transparent larvae allow real-time imaging; cost-effective and scalable for drug screening | May not fully capture chronic or late-onset features of PD | [24,27,28,31,32,37] |
| Neurotoxin: Rotenone, Paraquat | Oxidative stress; mitochondrial dysfunction; progressive dopaminergic neurodegeneration; motor impairments | Mimics environmental toxin exposure in humans; models oxidative stress mechanisms effectively | Toxicity profiles differ from mammals; long-term exposure studies are limited | [31,32,37] |
| α-Synuclein transgenic lines | Protein aggregation; Lewy body-like inclusions; dopaminergic cell loss | Directly models hallmark human PD pathology; optical transparency allows tracking of aggregation in vivo | Zebrafish lack endogenous α-synuclein homolog, requiring transgenic approaches | [6,7,34,35,36,91,92] |
| PINK1/Parkin knockdown or mutants | Defective mitophagy; dopaminergic neuron vulnerability; motor dysfunction | Conserved mitochondrial pathways; faster assessment of mitophagy compared to rodents | Early-onset PD mutations may not model late-onset disease well | [33,37,46] |
| DJ-1 knockdown | Increased susceptibility to oxidative stress; dopaminergic cell loss | Mechanistic insight into oxidative stress pathways in PD | Partial phenotype compared to human PD | [37,91,92] |
| LRRK2 mutant lines | Synaptic dysfunction, altered vesicle trafficking, impaired autophagy | Models familial PD mutations; allows rapid in vivo functional assays | Some phenotypes are less pronounced than in mammalian models | [46] |
| Other genetic knockdowns (dj1, pink1, prkn) | Familial PD-like phenotypes; altered dopaminergic pathways | Stronger phenotypic expression than rodents in some cases; genetic tractability | Require validation against human disease heterogeneity | [37,91] |
| Drug screening/high-throughput assays | Behavioural rescue; reduced aggregation; restored mitochondrial function | Transparent embryos allow in vivo pharmacology; a scalable, cost-effective alternative to rodent models | Differences in metabolism and lifespan limit direct translation | [93,94,95,96] |
| Comparative advantages | Real-time imaging; rapid development; high-throughput screening | Cost-effective, ethically favourable, and genetically tractable | Short lifespan, lack of some human-specific proteins (e.g., α-synuclein) | [7,46,89,97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bangeppagari, M.; Manjunath, A.; Srinivasa, A.; Lee, S.J. Tiny Fish, Big Hope: Zebrafish Unlocking Secrets to Fight Parkinson’s Disease. Biology 2025, 14, 1397. https://doi.org/10.3390/biology14101397
Bangeppagari M, Manjunath A, Srinivasa A, Lee SJ. Tiny Fish, Big Hope: Zebrafish Unlocking Secrets to Fight Parkinson’s Disease. Biology. 2025; 14(10):1397. https://doi.org/10.3390/biology14101397
Chicago/Turabian StyleBangeppagari, Manjunatha, Akshatha Manjunath, Anusha Srinivasa, and Sang Joon Lee. 2025. "Tiny Fish, Big Hope: Zebrafish Unlocking Secrets to Fight Parkinson’s Disease" Biology 14, no. 10: 1397. https://doi.org/10.3390/biology14101397
APA StyleBangeppagari, M., Manjunath, A., Srinivasa, A., & Lee, S. J. (2025). Tiny Fish, Big Hope: Zebrafish Unlocking Secrets to Fight Parkinson’s Disease. Biology, 14(10), 1397. https://doi.org/10.3390/biology14101397







