Unlocking Antioxidant Potential: Interactions Between Cyanidin-3-Glucoside and Corbicula fluminea Protein
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CFP
2.3. Preparation of C3G-CFP Complexes
2.4. Determination of Particle Size and Zeta Potential
2.5. Determination of Endogenous Fluorescence Spectrum
2.6. Synchronous Fluorescence Spectrum
2.7. Ultraviolet-Visible (UV-Vis) Absorption Spectrum
2.8. Antioxidant Properties Determination
2.8.1. ABTS Method
2.8.2. DPPH Method
2.8.3. FRAP Method
2.8.4. Definition of Synergistic Effect
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fluorescence Spectroscopy
3.2. Fluorescence Quenching Mechanism
3.3. Thermodynamic Analysis
3.4. Synchronous Fluorescence Spectroscopy
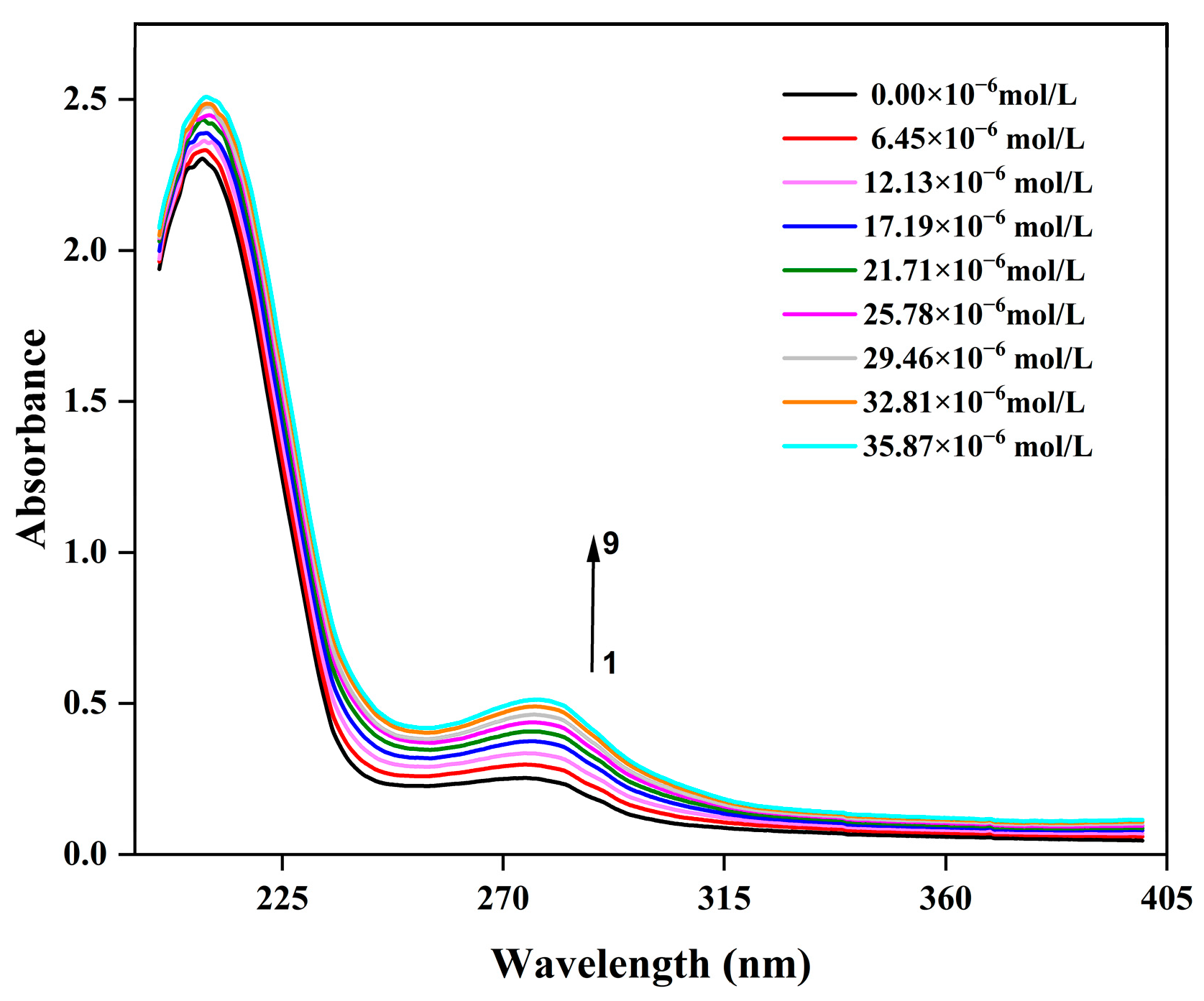
3.5. UV-Vis Absorption Spectral Analysis
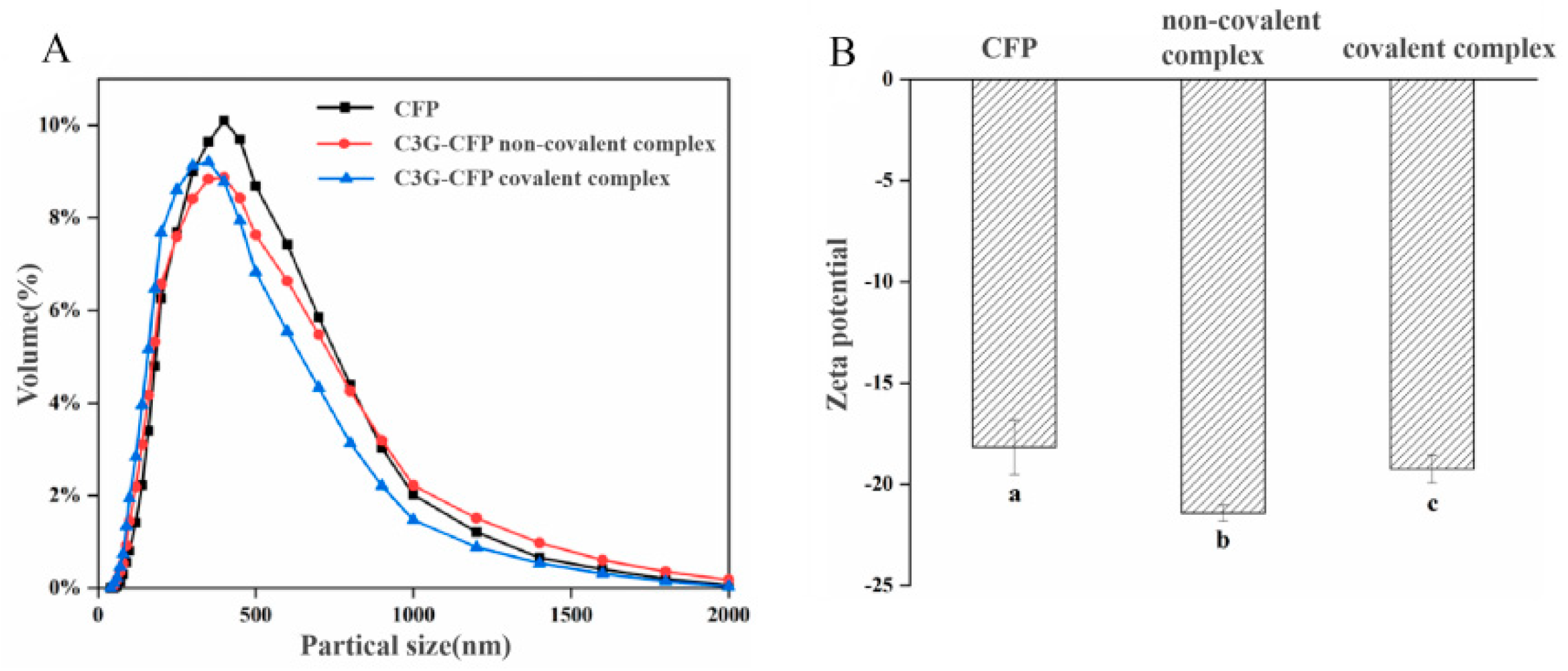
3.6. Particle Size and Zeta Potential
3.7. Antioxidant Properties Determination
3.7.1. ABTS Radical Scavenging Activity
3.7.2. DPPH Radical Scavenging Capacity
3.7.3. Reducing Power of C3G-CFP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFP | Corbicula fluminea protein |
| C3G | Cyanidin-3-O-glucoside |
| UV-Vis | Ultraviolet-visible |
| SFS | Synchronous fluorescence spectroscopy |
| ABTS | 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonate) |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| FRAP | Ferric ion-reducing antioxidant power |
| TPTZ | 2,4,6-Tri(2-pyridyl)-s-triazine |
| Trp | Tryptophan |
| Tyr | Tyrosine |
| TEAC | Trolox equivalent antioxidant capacity |
References
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Bouhadana, D.; Godin Pagé, M.H.; Montjean, D.; Bélanger, M.C.; Benkhalifa, M.; Miron, P.; Petrella, F. The Role of Antioxidants in Male Fertility: A Comprehensive Review of Mechanisms and Clinical Applications. Antioxidants 2025, 14, 1013. [Google Scholar] [CrossRef]
- Ren, J.; Li, Z.; Li, X.; Yang, L.; Bu, Z.; Wu, Y.; Li, Y.; Zhang, S.; Meng, X. Exploring the Mechanisms of the Antioxidants BHA, BHT, and TBHQ in Hepatotoxicity, Nephrotoxicity, and Neurotoxicity from the Perspective of Network Toxicology. Foods 2025, 14, 1095. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Kerasioti, E.; Skaperda, Z.; Papapostolou, P.A.; Nepka, C.; Spandidos, D.A.; Asprodini, E.; Taitzoglou, I.; Kouretas, D. Whey protein boosts the antioxidant profile of rats by enhancing the activities of crucial antioxidant enzymes in a tissue-specific manner. Food Chem. Toxicol. 2020, 142, 111508. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Jin, C.; Lv, S.; Zhang, H.; Ren, F.; Wang, J. Molecular Mechanisms and Applications of Polyphenol-Protein Complexes with Antioxidant Properties: A Review. Antioxidants 2023, 12, 1577. [Google Scholar] [CrossRef]
- McCann, S.; Roe, W.E.; Agnew, H.E.; Knipe, P.C. Non-Covalent Interactions Enforce Conformation in Switchable and Water-Soluble Diketopiperazine-Pyridine Foldamers. Angew. Chem. Int. Ed. Engl. 2023, 62, e202307180. [Google Scholar] [CrossRef]
- Joshi, T.; Deepa, P.R.; Sharma, P.K. Effect of Different Proportions of Phenolics on Antioxidant Potential: Pointers for Bioactive Synergy/Antagonism in Foods and Nutraceuticals. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 92, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Sanjay, N.; Park, M.; Lee, H.J. Cyanidin-3-O-glucoside protects the brain and improves cognitive function in APPswe/PS1ΔE9 transgenic mice model. J. Neuroinflamm. 2023, 20, 268. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, F.; Hu, Z.; Zhou, Y.; Guo, T.; Yan, S.; Xie, Q.; Xia, X.; Yuan, H.; Li, G.; et al. Cyanidin-3-O-Glucoside Alleviates Alcoholic Liver Injury via Modulating Gut Microbiota and Metabolites in Mice. Nutrients 2024, 16, 694. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Wu, Y.; Chen, L.; Yuan, M.; Jiang, Y.; Zhao, L.; Bai, C. Physical modification of corbicula fluminea protein hydrolysates by interacting with chlorogenic acid: Impacts on antioxidant activity and digestion behaviors. Food Chem. 2025, 490, 145075. [Google Scholar] [CrossRef]
- Dai, T.; Chen, J.; McClements, D.J.; Hu, P.; Ye, X.; Liu, C.; Li, T. Protein-polyphenol interactions enhance the antioxidant capacity of phenolics: Analysis of rice glutelin-procyanidin dimer interactions. Food Funct. 2019, 10, 765–774. [Google Scholar] [CrossRef]
- Tan, C.; Zhu, J.; Shi, C.; Zhang, X.; Lu, S.; Wang, S.; Guo, C.; Ning, C.; Xue, Y. Interactions with peanut protein isolate regulate the bioaccessibility of cyanidin-3-O-glucoside: Multispectral analysis, simulated digestion, and molecular dynamic simulation. Food Chem. 2025, 464, 141586. [Google Scholar] [CrossRef]
- Huizenga, J.M.; Semprini, L. Fluorescent spectroscopy paired with parallel factor analysis for quantitative monitoring of phenanthrene biodegradation and metabolite formation. Chemosphere 2023, 316, 137771. [Google Scholar] [CrossRef]
- Sunuwar, S.; Haddad, A.; Acheson, A.; Manzanares, C.E. Synchronous Fluorescence as a Sensor of Trace Amounts of Polycyclic Aromatic Hydrocarbons. Sensors 2024, 24, 3800. [Google Scholar] [CrossRef]
- Vogt, C.; Wondergem, C.S.; Weckhuysen, B.M. Ultraviolet-visible (UV-Vis) spectroscopy. In Springer Handbook of Advanced Catalyst Characterization; Springer: Berlin/Heidelberg, Germany, 2023; pp. 237–264. [Google Scholar]
- Wang, Y.; Zhou, Q.; Zheng, J.; Xiong, H.; Zhao, L.; Xu, Y.; Bai, C. Fabricating pectin and chitosan double layer coated liposomes to improve physicochemical stability of beta-carotene and alter its gastrointestinal fate. Int. J. Biol. Macromol. 2023, 247, 125780. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Ghadamgahi, Z.; Motavalizadehkakhky, A.; Mehrzad, J.; Amiri-Tehranizadeh, Z.; Chamani, J. Probing the interaction behavior of Nano-Resveratrol with α-lactalbumin in the presence of β-lactoglobulin and β-casein: Spectroscopy and molecular simulation studies. J. Biomol. Struct. Dyn. 2024, 391, 123205. [Google Scholar]
- Ren, C.; Xiong, W.; Li, B. Binding interaction between β-conglycinin/glycinin and cyanidin-3-O-glucoside in acidic media assessed by multi-spectroscopic and thermodynamic techniques. Int. J. Biol. Macromol. 2019, 137, 366–373. [Google Scholar] [CrossRef]
- Wei, J.; Xu, D.; Yang, J.; Zhang, X.; Mu, T.; Wang, Q. Analysis of the interaction mechanism of Anthocyanins (Aronia melanocarpa Elliot) with β-casein. Food Hydrocoll. 2018, 84, 276–281. [Google Scholar] [CrossRef]
- Deepika; Sharma, S.; Yadav, D.; Pandey, S. Anomalous Fluorescence Quenching in Fluorous Solvent-Added Media. J. Phys. Chem. B 2024, 128, 8194–8206. [Google Scholar]
- Zhang, R.-J.; Kou, S.-B.; Hu, L.; Li, L.; Shi, J.-H.; Jiang, S.-L. Exploring binding interaction of baricitinib with bovine serum albumin (BSA): Multi-spectroscopic approaches combined with theoretical calculation. J. Mol. Liq. 2022, 354, 118831. [Google Scholar] [CrossRef]
- Ma, Z.; Prasanna, G.; Jiang, L.; Jing, P. Molecular interaction of cyanidin-3-O-glucoside with ovalbumin: Insights from spectroscopic, molecular docking and in vitro digestive studies. J. Biomol. Struct. Dyn. 2020, 38, 1858–1867. [Google Scholar] [CrossRef]
- Precupas, A.; Sandu, R.; Leonties, A.R.; Anghel, D.-F.; Popa, V.T. Complex interaction of caffeic acid with bovine serum albumin: Calorimetric, spectroscopic and molecular docking evidence. New J. Chem. 2017, 41, 15003–15015. [Google Scholar] [CrossRef]
- Bai, J.; Sun, X.; Geng, B.; Ma, X. Interaction mechanism of Cu+/Cu2+ on bovine serum albumin: Vitro simulation experiments by spectroscopic methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 293, 122491. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, J.H.; Prasanna, G.; Jing, P. Spectrofluorimetric and molecular docking studies on the interaction of cyanidin-3-O-glucoside with whey protein, β-lactoglobulin. Int. J. Biol. Macromol. 2017, 105, 965–972. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, Y.; Qi, X.; Yan, H.; Zhao, Y.; Wu, Y.; Zhang, N.; Ding, Z.; Yuan, L.; Liu, M. Noncovalent interaction of chlorogenic acid and/or gallocatechin gallate with β-lactoglobulin: Effect on stability and bioaccessibility of complexes and nanoparticles. LWT 2023, 175, 114493. [Google Scholar] [CrossRef]
- Yang, M.; Shi, D.; Wang, Y.; Ebadi, A.G.; Toughani, M. Study on interaction of coomassie brilliant blue g-250 with bovine serum albumin by multispectroscopic. Int. J. Pept. Res. Ther. 2021, 27, 421–431. [Google Scholar]
- Pan, F.; Li, J.; Zhao, L.; Tuersuntuoheti, T.; Mehmood, A.; Zhou, N.; Hao, S.; Wang, C.; Guo, Y.; Lin, W. A molecular docking and molecular dynamics simulation study on the interaction between cyanidin-3-O-glucoside and major proteins in cow’s milk. J. Food Biochem. 2021, 45, e13570. [Google Scholar] [CrossRef]
- Banaś, J.; Banaś, M. Combined application of fluorescence spectroscopy and principal component analysis in characterisation of selected herbhoneys. Molecules 2024, 29, 749. [Google Scholar] [CrossRef]
- Han, S.; Cui, F.; McClements, D.J.; Xu, X.; Ma, C.; Wang, Y.; Liu, X.; Liu, F. Structural Characterization and Evaluation of Interfacial Properties of Pea Protein Isolate-EGCG Molecular Complexes. Foods 2022, 11, 2895. [Google Scholar] [CrossRef]
- Biter, A.B.; Pollet, J.; Chen, W.H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. A method to probe protein structure from UV absorbance spectra. Anal. Biochem. 2019, 587, 113450. [Google Scholar] [CrossRef]
- Hamzi, H.; Rajabpour, A.; Roldán, É.; Hassanali, A. Learning the Hydrophobic, Hydrophilic, and Aromatic Character of Amino Acids from Thermal Relaxation and Interfacial Thermal Conductance. J. Phys. Chem. B 2022, 126, 670–678. [Google Scholar] [CrossRef]
- Shamsi, A.; Anwar, S.; Shahbaaz, M.; Mohammad, T.; Alajmi, M.F.; Hussain, A.; Hassan, I.; Ahmad, F.; Islam, A. Evaluation of binding of rosmarinic acid with human transferrin and its impact on the protein structure: Targeting polyphenolic Acid-Induced protection of neurodegenerative disorders. Oxidative Med. Cell. Longev. 2020, 2020, 1245875. [Google Scholar] [CrossRef]
- Bekdeşer, B.; Apak, R. Colorimetric Sensing of Antioxidant Capacity via Auric Acid Reduction Coupled to ABTS Oxidation. ACS Omega 2024, 9, 11738–11746. [Google Scholar] [CrossRef]
- Nagaoka, S.i.; Nomoto, N.; Tasaka, T.; Nagashima, U.; Matsumoto, T.; Ohara, K. Solvent effect on activities of aryloxyl-radical scavenging and singlet-oxygen quenching reactions by vitamin E: Addition of water to ethanol solution. Int. J. Chem. Kinet. 2022, 54, 570–576. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. Synergistic and antagonistic antioxidant effects in the binary cannabinoids mixtures. Fitoterapia 2021, 153, 104992. [Google Scholar] [CrossRef]
- Rana, M.S.; Rayhan, N.M.A.; Emon, M.S.H.; Islam, M.T.; Rathry, K.; Hasan, M.M.; Islam Mansur, M.M.; Srijon, B.C.; Islam, M.S.; Ray, A.; et al. Antioxidant activity of Schiff base ligands using the DPPH scavenging assay: An updated review. RSC Adv. 2024, 14, 33094–33123. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
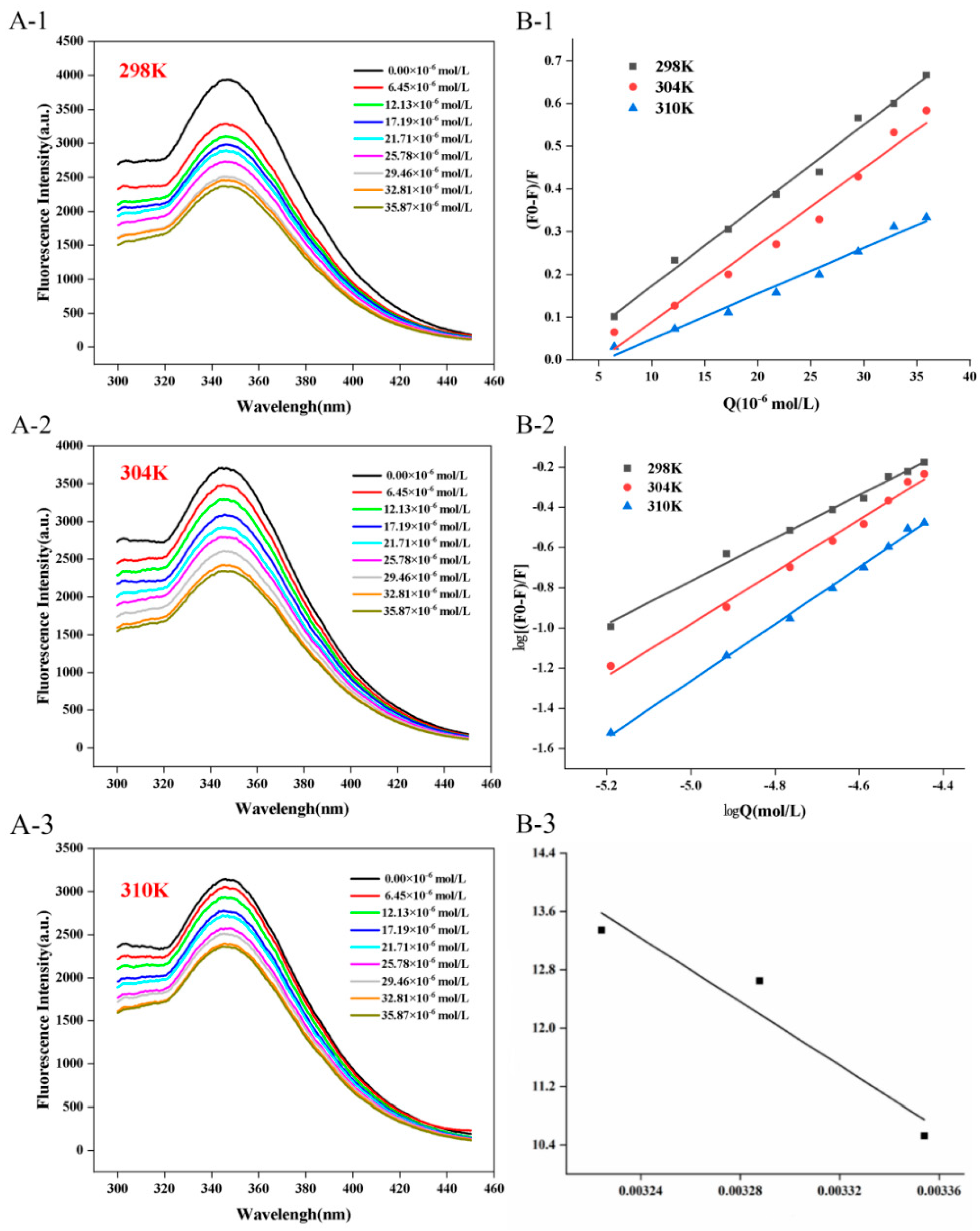


| Complexes | T(K) | Ksv (×104 L∙ mol−1) | KQ (×1012 L∙ mol−1∙S−1) | Ra2 | Ka (×104 L∙mol−1) | n | Rb2 |
|---|---|---|---|---|---|---|---|
| C3G-CFP | 298 | 1.89 ± 0.07 A | 1.89 ± 0.07 A | 0.99 | 3.72 C | 1.07 ± 0.04 C | 0.99 |
| 304 | 1.80 ± 0.12 B | 1.80 ± 0.12 B | 0.97 | 31.21 B | 1.29 ± 0.05 B | 0.99 | |
| 310 | 1.07 ± 0.06 C | 1.07 ± 0.06 C | 0.98 | 62.55 A | 1.41 ± 0.03 A | 0.99 |
| Complexes | T(K) | ∆G (kJ∙mol−1) | ∆S (kJ∙mol−1∙K−1) | ∆H (kJ∙mol−1) |
|---|---|---|---|---|
| C3G-CFP | 298 | −26.09 | 0.70 | 181.45 |
| 304 | −31.99 | |||
| 310 | −34.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Liu, X.; Wang, F.; Jiang, Y.; Chen, L.; Yuan, M.; Zhao, L.; Bai, C. Unlocking Antioxidant Potential: Interactions Between Cyanidin-3-Glucoside and Corbicula fluminea Protein. Biology 2025, 14, 1392. https://doi.org/10.3390/biology14101392
Guo S, Liu X, Wang F, Jiang Y, Chen L, Yuan M, Zhao L, Bai C. Unlocking Antioxidant Potential: Interactions Between Cyanidin-3-Glucoside and Corbicula fluminea Protein. Biology. 2025; 14(10):1392. https://doi.org/10.3390/biology14101392
Chicago/Turabian StyleGuo, Sifan, Xuemei Liu, Fei Wang, Yong Jiang, Lili Chen, Meilan Yuan, Li Zhao, and Chunqing Bai. 2025. "Unlocking Antioxidant Potential: Interactions Between Cyanidin-3-Glucoside and Corbicula fluminea Protein" Biology 14, no. 10: 1392. https://doi.org/10.3390/biology14101392
APA StyleGuo, S., Liu, X., Wang, F., Jiang, Y., Chen, L., Yuan, M., Zhao, L., & Bai, C. (2025). Unlocking Antioxidant Potential: Interactions Between Cyanidin-3-Glucoside and Corbicula fluminea Protein. Biology, 14(10), 1392. https://doi.org/10.3390/biology14101392







