Changes in Gonadal Sex Differentiation, Digestive Enzymes, and Growth-Related Hormone Contents in the Larval and Juvenile Black Scraper, Thamnaconus modestus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Observation of Gonadal Histology
2.2.2. Detection of Sex Hormone Content
2.2.3. Detection of Digestive Enzyme Activity and Hormone Content
2.3. Data Analysis
3. Results
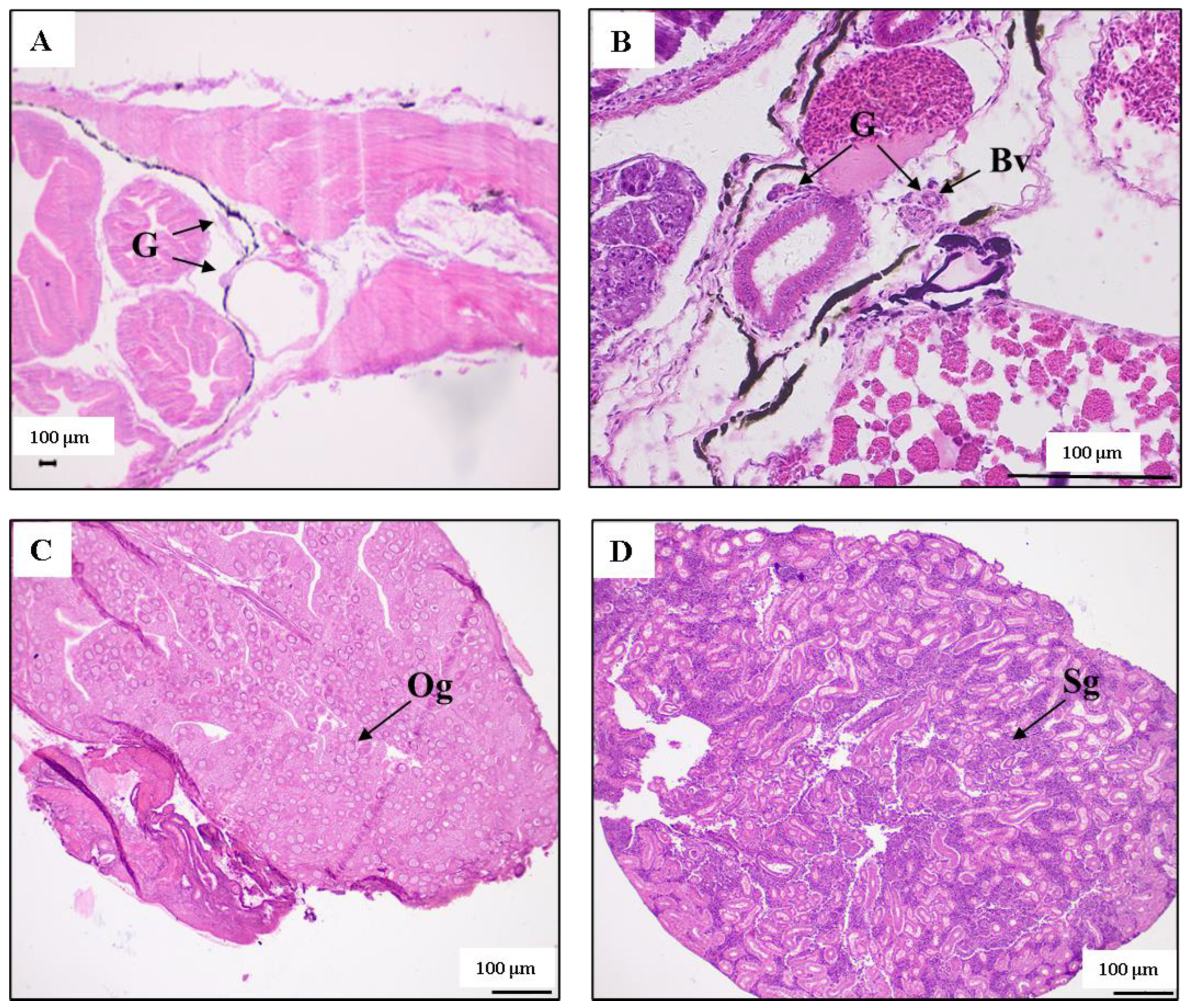
3.1. Sex Differentiation of Female and Male Juvenile Fish
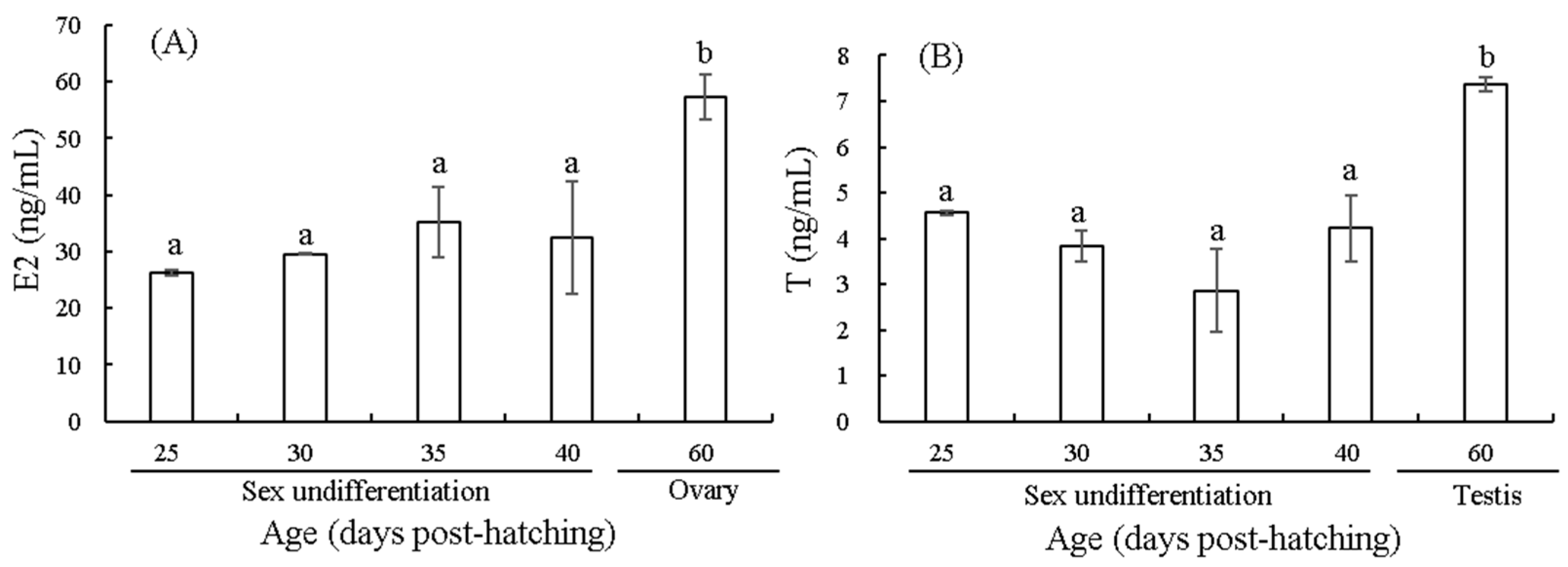
3.2. Changes in E2 and T Contents Before and After Sex Differentiation
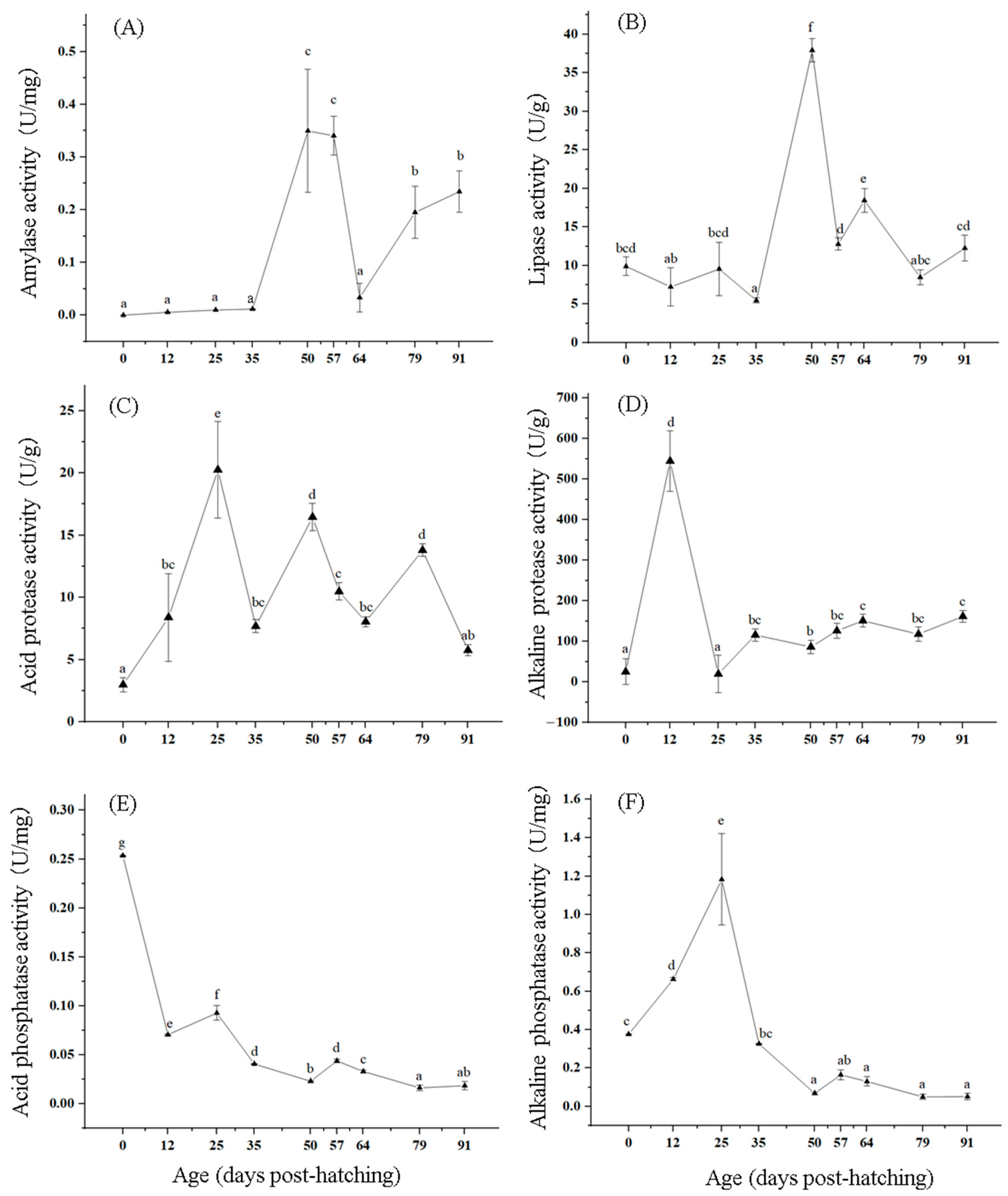
3.3. Changes in Digestive Enzyme Activity of T. modestus
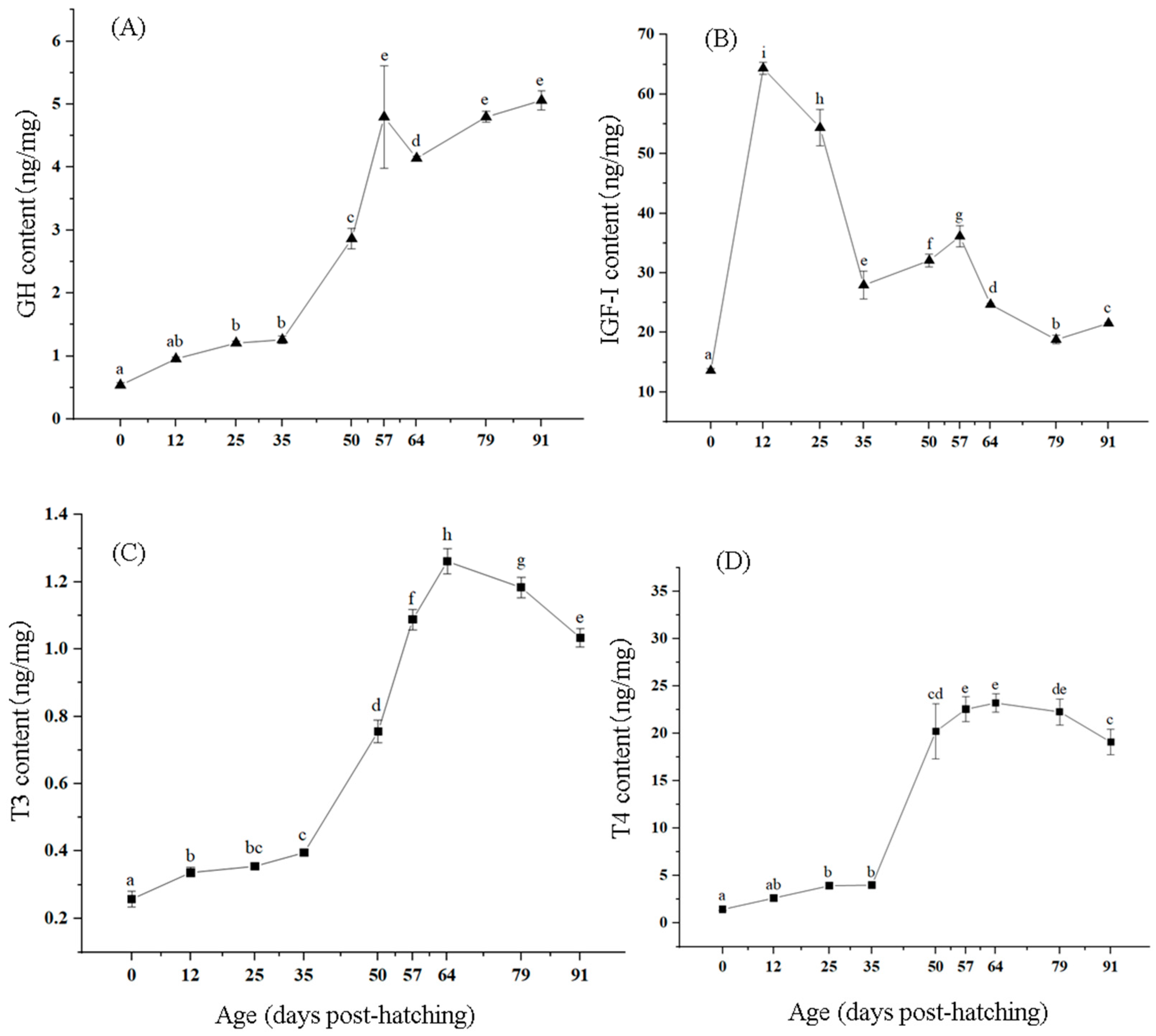
3.4. Changes in Growth-Related Hormone Levels
4. Discussion
4.1. The Size and Timing of Sex Differentiation in T. modestus
4.2. Effects of E2 and T on Sex Differentiation
4.3. Effects of Digestive Enzymes on Growth and Development
4.4. Effects of Growth-Related Hormones on Growth and Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishimura, T.; Tanaka, M. Gonadal development in fish. Sex. Dev. 2014, 8, 252–261. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocr. 2010, 165, 367–389. [Google Scholar] [CrossRef]
- Piprek, R.P.; Kloc, M.; Kubiak, J.Z. Early Development of the Gonads: Origin and Differentiation of the Somatic Cells of the Genital Ridges. In Molecular Mechanisms of Cell Differentiation in Gonad Development; Results and Problems in Cell Differentiation; Springer: Cham, Switzerland, 2016; Volume 58, pp. 1–22. [Google Scholar] [CrossRef]
- Parenti, L.R.; Grier, H.J. Evolution and phylogeny of gonad morphology in bony fishes. Integr. Comp. Biol. 2004, 44, 333–348. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Pronsato, L.; Ronda, A.C.; Boland, R.; Milanesi, L. Role of 17β-estradiol and testosterone in apoptosis. Steroids 2011, 76, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.G.; Manabe, S.; Mushirobira, Y.; Nagae, M. Changes in expression of reproduction-related hormones in the brain and pituitary during early ovarian differentiation and development in the red spotted grouper Epinephelus akaara, with emphasis on FSHβ and LHβ. Aquaculture 2020, 514, 734497. [Google Scholar] [CrossRef]
- Wahbi, O.; Shalaby, S. Oral administration of testosterone in fish diet affect sex differentiation and testis development in tilapia. Res. J. Agric. Biol. Sci. 2010, 6, 946–952. Available online: https://api.semanticscholar.org/CorpusID:31399656 (accessed on 10 September 2024).
- Tanaka, M. Studies on the structure and function of the digestive system in teleost larvae-v. epithelial changes in the posterior-gut and protein ingestion. Ichthyological. Res. 1972, 19, 172–180. [Google Scholar] [CrossRef]
- Cahu, C.L.; Infante, J.Z. Early weaning of sea bass (Dicentrarchus labrax) larvae with a compound diet: Effect on digestive enzymes. Comp. Biochem. Phys. A 1994, 109, 213–222. [Google Scholar] [CrossRef]
- Chen, B.N.; Qin, J.G.; Kumar, M.S.; Hutchinson, W.; Clarke, S. Ontogenetic development of the digestive system in yellowtail kingfish Seriola lalandi larvae. Aquaculture 2006, 256, 489–501. [Google Scholar] [CrossRef]
- Pipe, R.K. Hydrolytic enzymes associated with the granular haemocytes of the marine mussel Mytilus edulis. Histochem. J. 1990, 22, 595–603. [Google Scholar] [CrossRef]
- MacKenzie, D.S.; VanPutte, C.M.; Leiner, K.A. Nutrient regulation of endocrine function in fish. Aquaculture 1998, 161, 3–25. [Google Scholar] [CrossRef]
- Wilson, C.M.; McNabb, F.A. Maternal thyroid hormones in Japanese quail eggs and their influence on embryonic development. Gen. Comp. Endocrinol. 1997, 107, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Power, D.M.; Llewellyn, L.; Faustino, M.; Nowell, M.A.; Björnsson, B.T.; Einarsdóttir, I.E.; Sweeney, G.E. Thyroid hormones in growth and development of fish. Comp. Biochem. Phys. C 2001, 130, 447–459. [Google Scholar] [CrossRef]
- Brown, C.L.; Urbinati, E.C.; Zhang, W.; Brown, S.B.; Mccomb-Kobza, M. Maternal thyroid and glucocorticoid hormone interactions in larval fish development, and their applications in aquaculture. Rev. Fish. Sci. 2014, 22, 207–220. [Google Scholar] [CrossRef]
- Bartke, A.; Sun, L.Y.; Longo, V. Somatotropic signaling: Trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 2013, 93, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Choi, J.H.; Park, W.G. Vertical distribution and feeding ecology of the black scraper, Thamnaconus modestus, in the Southern Sea of Korea. Turk. J. Fish. Aquat. Sci. 2013, 13, 249–259. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.Y.; Jung, J.W.; Kim, M.J.; Lee, W.S.; An, C.M.; Kang, J.H.; Hwang, S.Y. Species identification of filefishes (Monacanthidae) using DNA microarray in Korean marketplace. Bio. Chip. J. 2011, 5, 229–235. [Google Scholar] [CrossRef]
- Ying, N.; Wang, Y.; Qin, B.; Chen, H.; Song, X.; Yang, L. Report of Amyloodinium ocellatum in farmed black scraper (Thamnaconus modestus) in China. Aquaculture 2022, 561, 738722. [Google Scholar] [CrossRef]
- Wang, D.; Wu, F.X. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2013. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. 2023. Available online: https://www.iucnredlist.org (accessed on 1 September 2025).
- Lauff, M.; Hofer, R. Proteolytic enzymes in fish development and the importance of dietary enzymes. Aquaculture 1984, 37, 335–346. [Google Scholar] [CrossRef]
- Govoni, J.J.; Boehlert, G.W.; Watanabe, Y. The physiology of digestion in fish larvae. Environ. Biol. Fish. 1986, 16, 59–77. [Google Scholar] [CrossRef]
- Bisbal, G.A.; Bengtson, D.A. Development of the digestive tract in larval summer flounder. J. Fish Biol. 1995, 47, 277–291. [Google Scholar] [CrossRef]
- de Jesus, E.G.T.; Ayson, F.G.; Amemiya, Y.; Moriyama, S.; Hyodo, S.; Hirano, T.; Kawauchi, H. Milkfish (Chanos chanos) growth hormone cDNA cloning and mRNA expression in embryos and early larval stages. Aquaculture 2002, 208, 177–188. [Google Scholar] [CrossRef]
- Picha, M.E.; Turano, M.J.; Beckman, B.R.; Borski, R.J. Endocrine biomarkers of growth and applications to aquaculture: A minireview of growth hormone, insulin-like growth factor (IGF)-I, and IGF-binding proteins as potential growth indicators in fish. N. Am. J. Aquacult. 2008, 70, 196–211. [Google Scholar] [CrossRef]
- Weber, R.A.; Peleteiro, J.B.; García Martín, L.O.; Aldegunde, M. The efficacy of 2-phenoxyethanol, metomidate, clove oil and MS-222 as anaesthetic agents in the Senegalese sole (Solea senegalensis Kaup 1858). Aquaculture 2009, 288, 147–150. [Google Scholar] [CrossRef]
- Xu, W.; Soyano, K.; Manabe, S. High water temperature triggers early sexual maturation in the juvenile red spotted grouper Epinephelus akaara: Via regulation of reproduction-related hormones in the brain-pituitary-gonadal axis. Anim. Reprod. Sci. 2024, 268, 107546. [Google Scholar] [CrossRef]
- Goto, R.; Mori, T.; Kawamata, K.; Matsubara, T.; Mizuno, S.; Adachi, S.; Yamauchi, K. Effects of temperature on gonadal sex determination in barfin flounder Verasper moseri. Fish. Sci. 1999, 65, 884–887. [Google Scholar] [CrossRef]
- Lee, Y.D.; Lee, T.Y. Sex differentiation and development of the gonad in the flounder, Paralichthys olivaceus. Bull. Mar. Res. Inst. Cheju Natl. Univ. 1990, 149, 61–86. [Google Scholar]
- Xu, W.G.; Liu, L.M.; Wang, J.L. Immunohistochemistry of FSHβ and LHβ cells and their gene expression in the pituitary around gonadal differentiation in red spotted grouper Epinephelus akaara and blacktip grouper E. fasciatus. J. Dalian Fish Univ. 2021, 36, 554–562. [Google Scholar] [CrossRef]
- Sao, P.N.; Hur, S.W.; Lee, C.H. Gonadal sex differentiation of hatchery reared longtooth grouper (Epinephelus bruneus). Dev. Rerprod. 2012, 16, 185–193. Available online: https://api.semanticscholar.org/CorpusID:86311149 (accessed on 10 September 2024).
- Hu, P.; Liu, X.; Liu, B. Histological differentiation of gonads in Takifugu rubripes. Peri. Ocean Univ. China 2015, 45, 25–30. [Google Scholar] [CrossRef]
- Zhao, W.J.; Fan, W.T.; Zhang, F.C. Histological observation of gonadal differentiation in Takifugu flavidus. Fish. Sci. 2012, 31, 12–17. [Google Scholar] [CrossRef]
- Murata, R.; Karimata, H.; Alam, M.A.; Nakamura, M. Gonadal sex differentiation in the malabar grouper, Epinephelus malabaricus. Aquaculture 2009, 293, 286–289. [Google Scholar] [CrossRef]
- Goto-Kazeto, R.; Abe, Y.; Masai, K. Temperature-dependent sex differentiation in goldfish: Establishing the temperature-sensitive period and effect of constant and fluctuating water temperatures. Aquaculture 2006, 254, 617–624. [Google Scholar] [CrossRef]
- Bláquez, M.; Zanuy, S.; Carillo, M.; Piferrer, F. Effects of rearing temperature on sex differentiation in the European sea bass (Dicentrarchus labrax L.). J. Exp. Zool. 1998, 281, 207–216. [Google Scholar] [CrossRef]
- Li, M.; Sun, L.; Wang, D. Roles of estrogens in fish sexual plasticity and sex differentiation. Gen. Comp. Endocrinol. 2019, 277, 9–16. [Google Scholar] [CrossRef]
- Sun, L.N.; Jiang, X.L.; Xie, Q.P.; Yuan, J.; Huang, B.F.; Tao, W.J.; Wang, D.S. Transdifferentiation of differentiated ovary into functional testis by long-term treatment of aromatase inhibitor in Nile tilapia. Endocrinology 2014, 155, 1476–1488. [Google Scholar] [CrossRef]
- Rougeot, C.; Krim, A.; Mandiki, S.N. Sex steroid dynamics during embryogenesis and sexual differentiation in Eurasian perch, Perca fluviatilis. Theriogenology 2007, 67, 1046–1052. [Google Scholar] [CrossRef]
- Qiang, J.; He, J.; Zhu, J.H. Optimal combination of temperature and photoperiod for sex steroid hormone secretion and egg development of Oreochromis niloticus as determined by response surface methodology. J. Therm. Biol. 2021, 97, 102889. [Google Scholar] [CrossRef]
- Buddington, R.K. Digestive secretions of lake sturgeon, Acipenser fulvescens, during early development. J. Fish Biol. 1985, 26, 715–723. [Google Scholar] [CrossRef]
- Comabella, Y.; Mendoza, R.; Aguilera, C.; Carrillo, O.; Hurtado, A.; García-Galano, T. Digestive enzyme activity during early larval development of the Cuban gar Atractosteus tristoechus. Fish Physiol. Biochem. 2006, 32, 147–157. [Google Scholar] [CrossRef]
- Morais, S.; Cahu, C.; Zambonino-Infante, J.L.; Robin, J.; Rønnestad, I.; Dinis, M.T.; Conceição, L.E.C. Dietary TAG source and level affect performance and lipase expression in larval sea bass (Dicentrarchus labrax). Lipids 2004, 39, 449–458. [Google Scholar] [CrossRef]
- Alvarez-González, C.A.; Moyano-López, F.J.; Civera-Cerecedo, R.; Carrasco-Chávez, V.; Ortiz-Galindo, J.L.; Dumas, S. Development of digestive enzyme activity in larvae of spotted sand bass Paralabrax maculatofasciatus. 1. Biochemical analysis. Fish Physiol. Biochem. 2008, 34, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Cousin, J.C.B.; Baudin-Laurencin, F.; Gabaudan, J. Ontogeny of enzymatic activities in fed and fasting turbot, Scophthalmus maximus L. J. Fish Biol. 1987, 30, 15–33. [Google Scholar] [CrossRef]
- Martínez-Lagos, R.; Tovar-Ramírez, D.; Gracia-López, V.; Lazo, J.P. Changes in digestive enzyme activities during larval development of leopard grouper (Mycteroperca rosacea). Fish Physiol. Biochem. 2014, 40, 773–785. [Google Scholar] [CrossRef]
- Oozeki, Y.; Bailey, K.M. Ontogenetic development of digestive enzyme activities in larval walleye pollock, Theragra chalcogramma. Mar. Biol. 1995, 122, 177–186. [Google Scholar] [CrossRef]
- Gisbert, E.; Giménez, G.; Fernández, I.; Kotzamanis, Y.; Estévez, A. Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture 2009, 287, 381–387. [Google Scholar] [CrossRef]
- López-Ramírez, G.; Cuenca-Soria, C.A.; Álvarez-González, C.A.; Tovar-Ramírez, D.; Ortiz-Galindo, J.L.; Perales-García, N.; Moyano, F.J. Development of digestive enzymes in larvae of Mayan cichlid Cichlasoma urophthalmus. Fish Physiol. Biochem. 2011, 37, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Walford, J.; Lam, T.J. Development of digestive tract and proteolytic enzyme activity in seabass. Aquaculture 1993, 109, 187–205. [Google Scholar] [CrossRef]
- Yongshi, L.; Yonghai, S.; Genyu, Z.; Yongde, X.; Jiabo, X.; Pingping, D.; Zongfeng, Z. Growth, digestive enzyme and antioxidant enzyme activities of tawny puffer (Takifugu flavidus) larvae. J. Zhejiang Univ. 2014, 40, 688–696. [Google Scholar] [CrossRef]
- Tian, X.L.; Ren, X.W.; Dong, S.L.; Wang, G.D.; Fang, J.H. Studies on the specific activities of digestive enzymes of Cynoglossus semilaevis Günther at different salinities and temperatures. J. Ocean Univ. China 2008, 38, 895–901. [Google Scholar] [CrossRef]
- Cuvier-Péres, A.; Kestemont, P. Development of some digestive enzymes in Eurasian perch larvae Perca fluviatilis. Fish Physiol. Biochem. 2001, 24, 279–285. [Google Scholar] [CrossRef]
- Babaei, S.S.; Kenari, A.A.; Nazari, R.; Gisbert, E. Developmental changes of digestive enzymes in Persian sturgeon (Acipenser persicus) during larval ontogeny. Aquaculture 2011, 318, 138–144. [Google Scholar] [CrossRef]
- Martinez, I.; Moyano, F.J.; Fernández-Díaz, C.; Yúfera, M. Digestive enzyme activity during larval development of the Senegal sole (Solea senegalensis). Fish Physiol. Biochem. 1999, 21, 317–323. [Google Scholar] [CrossRef]
- Chin, B.S.; Nakagawa, M.; Tagawa, M.; Masuda, R.; Yamashita, Y. Ontogenetic changes of habitat selection and thyroid hormone levels in black rockfish (Sebastes schlegelii) reared in captivity. Ichthyol. Res. 2010, 57, 278–285. [Google Scholar] [CrossRef]
- Miwa, S.; Tagawa, M.; Inui, Y.; Hirano, T. Thyroxine surge in metamorphosing flounder larvae. Gen. Comp. Endocrinol. 1988, 70, 158–163. [Google Scholar] [CrossRef]
- de Jesus, E.G.; Hirano, T.; Inui, Y. Changes in cortisol and thyroid hormone concentrations during early development and metamorphosis in the Japanese flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 1991, 82, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ayson, F.G.; Lam, T.J. Thyroxine injection of female rabbitfish (Siganus guttatus) broodstock: Changes in thyroid hormone levels in plasma, eggs, and yolk-sac larvae, and its effect on larval growth and survival. Aquaculture 1993, 109, 83–93. [Google Scholar] [CrossRef]
- Leatherland, J.F.; Reddy, P.K.; Yong, A.N.; Leatherland, A.; Lam, T.J. Hepatic 5′-monodeiodinase activity in teleosts in vitro: A survey of thirty-three species. Fish Physiol. Biochem. 1990, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zeng, J.; Zhang, Z.; Wang, J.; Mei, W.; Wang, C.; Xu, W. Changes in growth, morphology, and levels of digestive enzymes and growth-related hormones in early ontogeny of black scraper, Thamnaconus modestus. Front. Mar. Sci. 2014, 11, 1344844. [Google Scholar] [CrossRef]
- Yada, T. Growth hormone and fish immune system. Gen. Comp. Endocrinol. 2007, 152, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Zhang, J.; Shi, Z.; Fu, Y. Identification and expression analysis of IGFBP-1 gene from Japanese flounder (Paralichthys olivaceus). Comp. Biochem. Phys. B 2012, 161, 413–420. [Google Scholar] [CrossRef] [PubMed]
| Days Post-Hatching | Sampling Number | Body Weight (mg) | Total Length (mm) | Body Length (mm) |
|---|---|---|---|---|
| 10 | 30 | 0.45 ± 0.09 a | 3.11 ± 0.17 a | 2.90 ± 0.16 a |
| 15 | 30 | 0.62 ± 0.11 a | 4.62 ± 0.15 a | 3.71 ± 0.16 a |
| 20 | 30 | 2.90 ± 0.62 a | 5.71 ± 0.23 a | 4.42 ± 0.21 a |
| 25 | 30 | 41.10 ± 9.89 b | 11.39 ± 0.73 b | 10.07 ± 0.56 b |
| 30 | 30 | 113.33 ± 15.80 c | 19.20 ± 1.16 c | 17.27 ± 0.90 c |
| 35 | 30 | 225.67 ± 97.10 d | 33.21 ± 1.14 d | 30.21 ± 0.84 d |
| 40 | 30 | 644.21 ± 151.11 e | 41.81 ± 0.97 e | 40.20 ± 0.90 e |
| 45 | 30 | 1014.42 ± 286.08 f | 45.40 ± 3.11 f | 44.28 ± 2.70 f |
| 50 | 30 | 1356.86 ± 461.81 g | 51.97 ± 3.61 g | 49.89 ± 2.83 g |
| 55 | 30 | 1869.34 ± 461.81 h | 57.53 ± 5.46 h | 55.23 ± 5.33 h |
| 60 | 30 | 2460.56 ± 109.89 i | 62.19 ± 0.05 i | 60.04 ± 0.05 i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Liu, Y.; Wang, J.; Yang, P.; Wu, Y.; Liu, L. Changes in Gonadal Sex Differentiation, Digestive Enzymes, and Growth-Related Hormone Contents in the Larval and Juvenile Black Scraper, Thamnaconus modestus. Biology 2025, 14, 1385. https://doi.org/10.3390/biology14101385
Xu W, Liu Y, Wang J, Yang P, Wu Y, Liu L. Changes in Gonadal Sex Differentiation, Digestive Enzymes, and Growth-Related Hormone Contents in the Larval and Juvenile Black Scraper, Thamnaconus modestus. Biology. 2025; 14(10):1385. https://doi.org/10.3390/biology14101385
Chicago/Turabian StyleXu, Wengang, Yan Liu, Jiulong Wang, Pei Yang, Yanqing Wu, and Liming Liu. 2025. "Changes in Gonadal Sex Differentiation, Digestive Enzymes, and Growth-Related Hormone Contents in the Larval and Juvenile Black Scraper, Thamnaconus modestus" Biology 14, no. 10: 1385. https://doi.org/10.3390/biology14101385
APA StyleXu, W., Liu, Y., Wang, J., Yang, P., Wu, Y., & Liu, L. (2025). Changes in Gonadal Sex Differentiation, Digestive Enzymes, and Growth-Related Hormone Contents in the Larval and Juvenile Black Scraper, Thamnaconus modestus. Biology, 14(10), 1385. https://doi.org/10.3390/biology14101385






