Distribution Changes in Lichen: A Staple Fallback Food for Yunnan Snub-Nosed Monkey and Their Implications for the Species
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Occurrence Collection
2.3. Environmental Parameters
2.4. Model Settings and Assessment
2.5. Spatial Correlation Analysis of the Distribution of Yunnan Snub-Nosed Monkey and Lichen
3. Results
3.1. Model Results and Performance
3.2. Importance of Environmental Variables
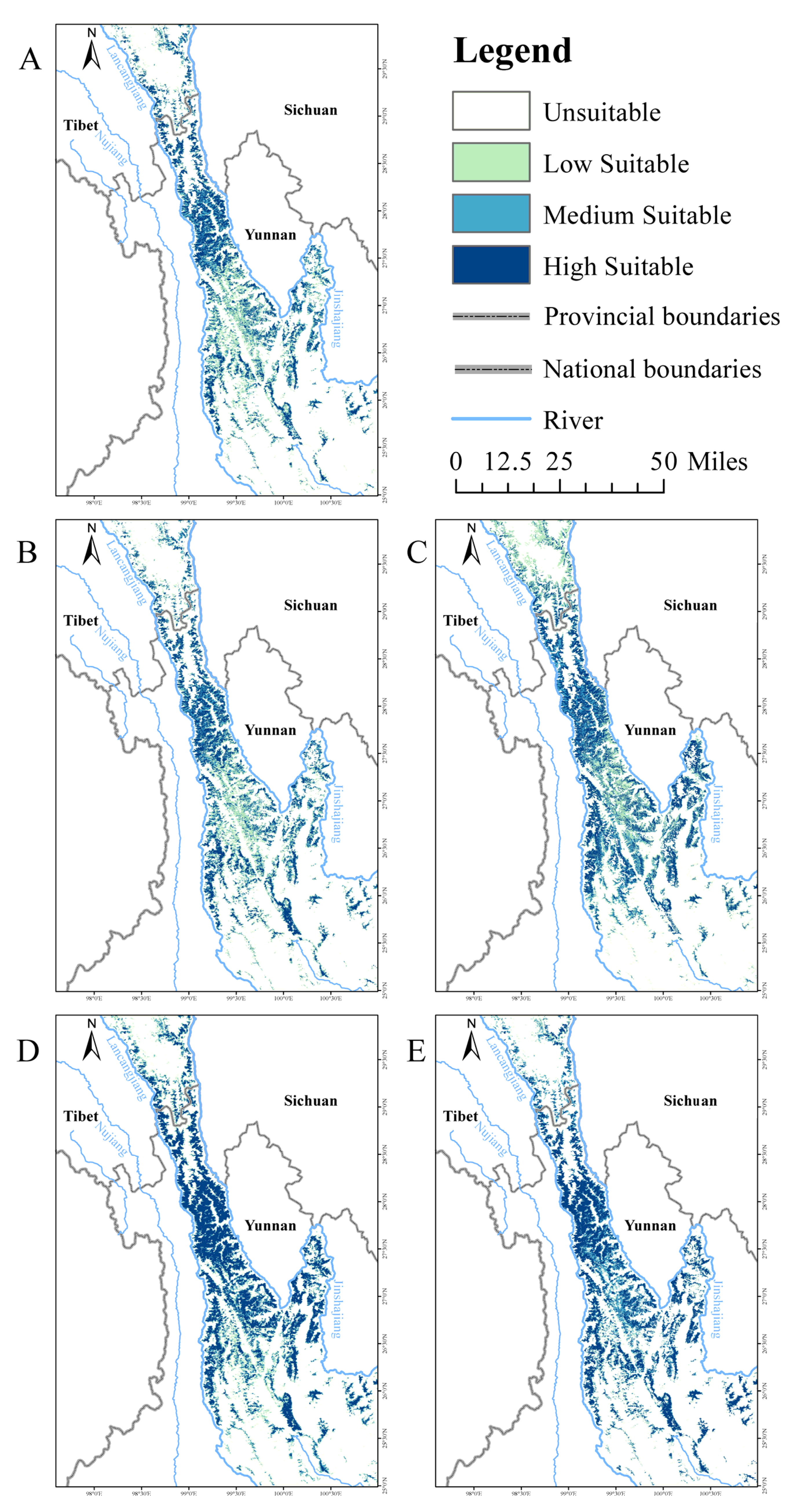
3.3. Potential Suitable Habitat of Lichen in Present and Future
3.3.1. Potential Suitable Habitat of Lichen
3.3.2. Potential Suitable Habitat of Lichen in the Future
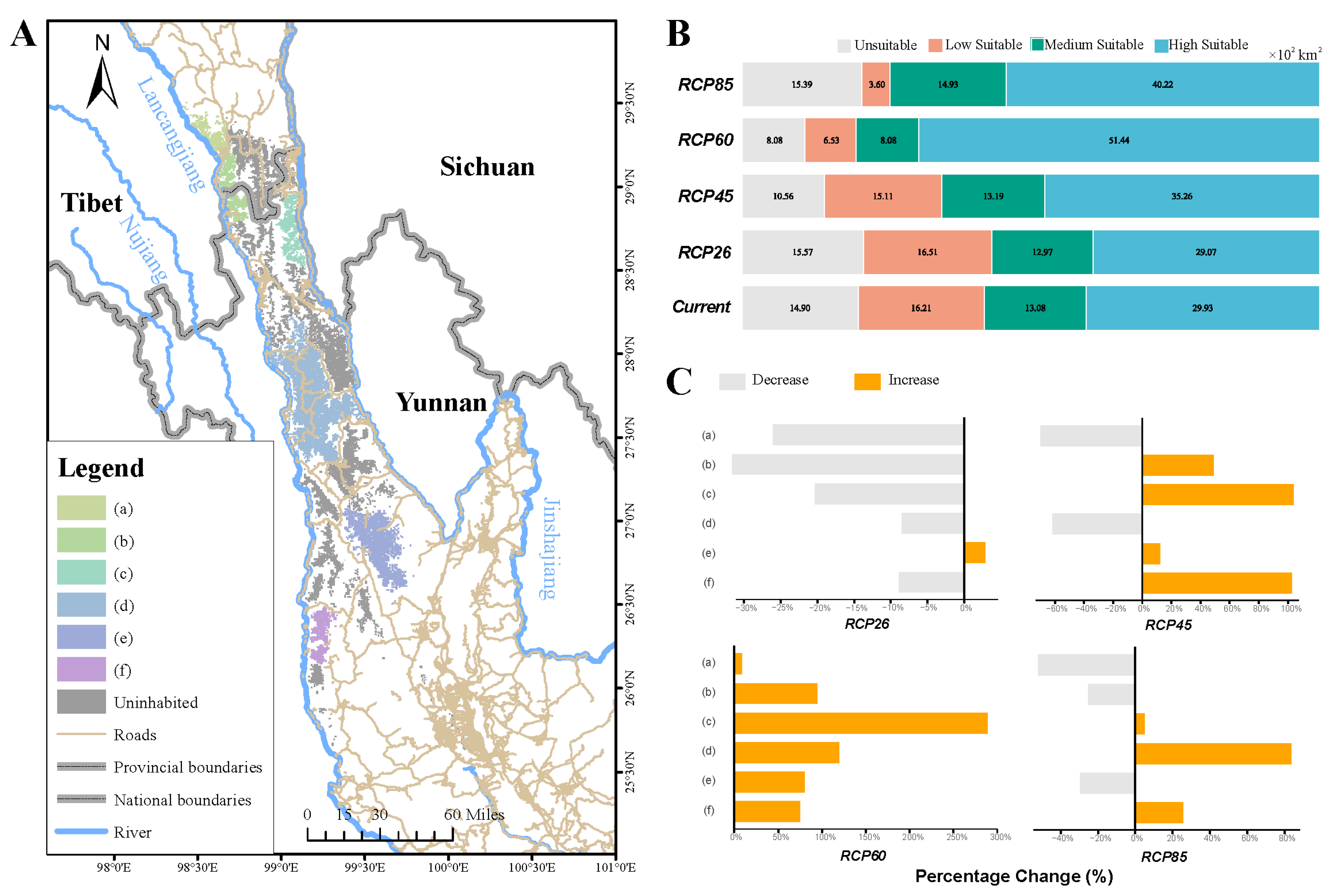
3.4. Correlation Between the Distribution of Lichen and Yunnan Snub-Nosed Monkey
3.5. Effect of Lichen Distribution on Yunnan Snub-Nosed Monkey in the Future
4. Discussion
4.1. Model Prediction Accuracy
4.2. Temporal and Spatial Variation and Driving Factors of Suitable Habitat of Lichen
4.3. Food Security Potential and Sustainable Utilization Challenge of Yunnan Snub-Nosed Monkey
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Intergovernmental Panel on Climate. Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Bongaarts, J. IPCC, 2023: Climate Change 2023: Synthesis Report; IPCC: Geneva, Switzerland, 2024; 184p. [Google Scholar] [CrossRef]
- Kumar, R. Climatic shifts in the Beas Basin: A spatio-temporal analysis of time series of temperature and precipitation of TerraClimate dataset. Sci. Total Environ. 2025, 984, 179712. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Yue, C.; Wang, W.; Luo, H.; Zhang, L.; Chen, T.; Shen, J.; Teng, Z. Distribution patterns of suitable habitat for Cycas tanqingii in the China-Laos-Vietnam region under the interaction of climate change and pest factors. Ecol. Indic. 2025, 177, 113794. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future threats to biodiversity and pathways to their prevention. Nature 2017, 546, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T.; Hudson, L.N.; Arnell, A.P.; Contu, S.; De Palma, A.; Ferrier, S.; Hill, S.L.L.; Hoskins, A.J.; Lysenko, I.; Phillips, H.R.P.; et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 2016, 353, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Goldewijk, K.K.; Beusen, A.; van Drecht, G.; de Vos, M. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Glob. Ecol. Biogeogr. 2011, 20, 73–86. [Google Scholar] [CrossRef]
- Hermy, M.; Verheyen, K. Legacies of the past in the present-day forest biodiversity: A review of past land-use effects on forest plant species composition and diversity. Ecol. Res. 2007, 22, 361–371. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Sandel, B.; Arge, L.; Dalsgaard, B.; Davies, R.G.; Gaston, K.J.; Sutherland, W.J.; Svenning, J.-C. The Influence of Late Quaternary Climate-Change Velocity on Species Endemism. Science 2011, 334, 660–664. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Wilting, A.; Cord, A.; Hearn, A.J.; Hesse, D.; Mohamed, A.; Traeholdt, C.; Cheyne, S.M.; Sunarto, S.; Jayasilan, M.-A.; Ross, J.; et al. Modelling the Species Distribution of Flat-Headed Cats (Prionailurus planiceps), an Endangered South-East Asian Small Felid. PLoS ONE 2010, 5, e9612. [Google Scholar] [CrossRef]
- Guo, L.; Gao, Y.; He, P.; He, Y.; Meng, F. Modeling for Predicting the Potential Geographical Distribution of Three Ephedra Herbs in China. Plants 2023, 12, 787. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Chao, C.; Jing, D.; Lyu, Y.; Zheng, X. Spatiotemporal responses of bird populations to forest dynamics: A case study of Yunnan Province, China. J. Environ. Manag. 2025, 393, 127205. [Google Scholar] [CrossRef] [PubMed]
- Montauban, C.; Budinski, I.; Webala, P.W.; Laverty, T.M.; Tanshi, I.; Torrent, L.; Bakwo-Fils, E.; Taylor, P.J.; Kane, A.; Monadjem, A. Underrepresentation of bats in Africa’s protected areas. Conserv. Biol. 2025, 15, e70108. [Google Scholar] [CrossRef]
- Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B.; Peterson, A.T.; Soberón, J.; Pearson, R.G. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Wu, R.; Qi, J.; Li, W.; Wang, L.; Shen, Y.; Liu, J.; Teng, Y.; Roos, C.; Li, M. Landscape genomics analysis provides insights into future climate change-driven risk in rhesus macaque. Sci. Total Environ. 2023, 899, 165746. [Google Scholar] [CrossRef]
- Xia, W.; Grueter, C.C.; Zhang, C.; Zhuang, H.; Hu, J.; Krzton, A.; Li, D. Distribution range contractions and identification of conservation priority areas for canids in Sichuan Province, China. Glob. Ecol. Conserv. 2023, 44, e02499. [Google Scholar] [CrossRef]
- Kabir, M.; Hameed, S.; Ali, H.; Bosso, L.; Din, J.U.; Bischof, R.; Redpath, S.; Nawaz, M.A. Habitat suitability and movement corridors of grey wolf (Canis lupus) in Northern Pakistan. PLoS ONE 2017, 12, e0187027. [Google Scholar] [CrossRef]
- Olsson, O.; Rogers, D.J. Predicting the distribution of a suitable habitat for the white stork in Southern Sweden: Identifying priority areas for reintroduction and habitat restoration. Anim. Conserv. 2009, 12, 62–70. [Google Scholar] [CrossRef]
- Yang, J.; Fu, Z.; Xiao, K.; Dong, H.; Zhou, Y.; Zhan, Q. Climate Change Potentially Leads to Habitat Expansion and Increases the Invasion Risk of Hydrocharis (Hydrocharitaceae). Plants 2023, 12, 4124. [Google Scholar] [CrossRef]
- Araujo, M.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2008, 15, 59–69. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Carvalheiro, L.G.; Polce, C.; Van Loon, E.E.; Raes, N.; Reemer, M.; Biesmeijer, J.C. Fit-for-Purpose: Species Distribution Model Performance Depends on Evaluation Criteria—Dutch Hoverflies as a Case Study. PLoS ONE 2013, 8, e63708. [Google Scholar] [CrossRef]
- Thuiller, W. Editorial commentary on ‘BIOMOD—Optimizing predictions of species distributions and projecting potential future shifts under global change’. Glob. Change Biol. 2014, 20, 3591–3592. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.J.; Boyko, C.M.; Feilen, K.L.; Boyko, R.H.; Leighton, M. Defining fallback foods and assessing their importance in primate ecology and evolution. Am. J. Phys. Anthropol. 2009, 140, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.J.; Leighton, M. How does food availability limit the population density of agile gibbons? Feed. Ecol. Apes Other Primates 2006, 48, 313. [Google Scholar]
- Marshall, A.J.; Wrangham, R.W. Evolutionary Consequences of Fallback Foods. Int. J. Primatol. 2007, 28, 1219–1235. [Google Scholar] [CrossRef]
- Knott, C.D. Changes in Orangutan Caloric Intake, Energy Balance, and Ketones in Response to Fluctuating Fruit Availability. Int. J. Primatol. 1998, 19, 1061–1079. [Google Scholar] [CrossRef]
- Doran-Sheehy, D.; Mongo, P.; Lodwick, J.; Conklin-Brittain, N. Male and female western gorilla diet: Preferred foods, use of fallback resources, and implications for ape versus old world monkey foraging strategies. Am. J. Phys. Anthropol. 2009, 140, 727–738. [Google Scholar] [CrossRef]
- Xiang, Z.; Huo, S.; Xiao, W.; Quan, R.; Grueter, C.C. Diet and feeding behavior of Rhinopithecus bieti at Xiaochangdu, Tibet: Adaptations to a marginal environment. Am. J. Primatol. 2007, 69, 1141–1158. [Google Scholar] [CrossRef]
- Grueter, C.C.; Li, D.; Ren, B.; Wei, F.; van Schaik, C.P. Dietary Profile of Rhinopithecus bieti and Its Socioecological Implications. Int. J. Primatol. 2009, 30, 601–624. [Google Scholar] [CrossRef]
- Das, A.K.; Sarkar, S.; Devi, P. Overview of Lichen. In Chemistry, Biology and Pharmacology of Lichen; Wiley: Hoboken, NJ, USA, 2024; pp. 1–12. [Google Scholar] [CrossRef]
- Li, H.; Xia, W.; Liu, X.; Wang, X.; Liu, G.; Chen, H.; Zhu, L.; Li, D. Food provisioning results in functional, but not compositional, convergence of the gut microbiomes of two wild Rhinopithecus species: Evidence of functional redundancy in the gut microbiome. Sci. Total Environ. 2023, 858, 159957. [Google Scholar] [CrossRef]
- Xia, W.; Liu, G.; Wang, D.; Chen, H.; Zhu, L.; Li, D. Functional convergence of Yunnan snub-nosed monkey and bamboo-eating panda gut microbiomes revealing the driving by dietary flexibility on mammal gut microbiome. Comput. Struct. Biotechnol. J. 2022, 20, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kromer, B.; Schukraft, G.; Bubenzer, O.; Huang, M.-R.; Wang, Z.-M.; Bian, L.-G.; Li, C.-S. Growth rate of Usnea aurantiacoatra (Jacq.) Bory on Fildes Peninsula, Antarctica and its climatic background. PLoS ONE 2014, 9, e100735. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Hou, R.; Zhang, H.; Li, Y.; Huang, Z.; Cui, L.; Xiao, W. Surviving at the highest and coldest: Nutritional and chemical components of fallback foods for Yunnan snub-nosed monkeys. Ecol. Evol. 2024, 14, e11219. [Google Scholar] [CrossRef] [PubMed]
- Austin, M. Species distribution models and ecological theory: A critical assessment and some possible new approaches. Ecol. Model. 2007, 200, 1–19. [Google Scholar] [CrossRef]
- Hanya, G.; Chapman, C.A. Linking feeding ecology and population abundance: A review of food resource limitation on primates. Ecol. Res. 2013, 28, 183–190. [Google Scholar] [CrossRef]
- Grueter, C.C.; Li, D.; Ren, B.; Wei, F.; Xiang, Z.; van Schaik, C.P. Fallback foods of temperate-living primates: A case study on snub-nosed monkeys. Am. J. Phys. Anthropol. 2009, 140, 700–715. [Google Scholar] [CrossRef]
- Esseen, P.-A.; Ericson, L.; Lindström, H.; Zackrisson, O. Occurrence and Ecology of Usnea Longissima in Central Sweden. Lichenologist 1981, 13, 177–190. [Google Scholar] [CrossRef]
- Seaward, M.R.D. Effects of Quantitative and Qualitative Changes in Air Pollution on the Ecological and Geographical Performance of Lichens. In Effects of Atmospheric Pollutants on Forests, Wetlands and Agricultural Ecosystems; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Smith, S.J.; Pitcher, H.; Wigley, T. Global and regional anthropogenic sulfur dioxide emissions. Glob. Planet. Change 2001, 29, 99–119. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Wang, S.-J.; Guo, A.-W.; Chen, F.-F.; Cui, L.-W.; Xiao, W. Spring food selection by Rhinopithecus bieti at Mt. Lasha in relation to phytochemical components. Dongwuxue Yanjiu 2013, 34, 152–159. [Google Scholar] [PubMed]
- Xia, W.; Zhang, C.; Zhuang, H.; Ren, B.; Zhou, J.; Shen, J.; Krzton, A.; Luan, X.; Li, D. The potential distribution and disappearing of Yunnan snub-nosed monkey: Influences of habitat fragmentation. Glob. Ecol. Conserv. 2020, 21, e00835. [Google Scholar] [CrossRef]
- Wong, M.H.G.; Li, R.; Xu, M.; Long, Y. An integrative approach to assessing the potential impacts of climate change on the Yunnan snub-nosed monkey. Biol. Conserv. 2013, 158, 401–409. [Google Scholar] [CrossRef]
- Ni, L.; Li, W.; Zhao, Z.; Gaawe, D.; Liu, T. Migration patterns of Gentiana crassicaulis, an alpine gentian endemic to the Himalaya–Hengduan Mountains. Ecol. Evol. 2022, 12, e8703. [Google Scholar] [CrossRef]
- Clements, G.R.; Rayan, D.M.; Aziz, S.A.; Kawanishi, K.; Traeholt, C.; Magintan, D.; Yazi, M.F.A.; Tingley, R. Predicting the distribution of the Asian tapir in Peninsular Malaysia using maximum entropy modeling. Integr. Zool. 2012, 7, 400–406. [Google Scholar] [CrossRef]
- Xu, L.; Fan, Y.; Zheng, J.; Guan, J.; Lin, J.; Wu, J.; Liu, L.; Wu, R.; Liu, Y. Impacts of climate change and human activity on the potential distribution of Aconitum leucostomum in China. Sci. Total Environ. 2024, 912, 168829. [Google Scholar] [CrossRef]
- Huang, Y.; Li, T.; Chen, W.; Zhang, Y.; Xu, Y.; Guo, T.; Wang, S.; Liu, J.; Qin, Y. Analysis of the Distribution Pattern of Phenacoccus manihoti in China under Climate Change Based on the Biomod2 Model. Biology 2024, 13, 538. [Google Scholar] [CrossRef]
- McGarigal, K.; Marks, B.J. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; Forest Service, Pacific Northwest Research Station: Corvallis, OR, USA, 1995. [Google Scholar] [CrossRef]
- Olsoy, P.J.; Zeller, K.A.; Hicke, J.A.; Quigley, H.B.; Rabinowitz, A.R.; Thornton, D.H. Quantifying the effects of deforestation and fragmentation on a range-wide conservation plan for jaguars. Biol. Conserv. 2016, 203, 8–16. [Google Scholar] [CrossRef]
- Pavlović, L.; Stojanović, D.; Mladenović, E.; Lakićević, M.; Orlović, S. Potential Elevation Shift of the European Beech Stands (Fagus sylvatica L.) in Serbia. Front. Plant Sci. 2019, 10, 849. [Google Scholar] [CrossRef]
- Adão, F.; Campos, J.C.; Santos, J.A.; Malheiro, A.C.; Fraga, H. Relocation of bioclimatic suitability of Portuguese grapevine varieties under climate change scenarios. Front. Plant Sci. 2023, 14, 974020. [Google Scholar] [CrossRef]
- Stanton, D.E.; Ormond, A.; Koch, N.M.; Colesie, C. Lichen ecophysiology in a changing climate. Am. J. Bot. 2023, 110, e16131. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Bosso, L.; Fasola, M.; Santicchia, F.; Aloise, G.; Lioy, S.; Tricarico, E.; Ruggieri, L.; Bovero, S.; Mori, E.; et al. Different facets of the same niche: Integrating citizen science and scientific survey data to predict biological invasion risk under multiple global change drivers. Glob. Change Biol. 2023, 29, 5509–5523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, Y.; Yue, J.; Wang, Z.; Zou, H.; Ji, X.; Zhang, S.; Liu, Z. Prediction of Potential Suitable Areas and Priority Protection for Cupressus gigantea on the Tibetan Plateau. Plants 2024, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.-Q.; Zhang, M.-X.; Chen, Y.; Wang, A.-X.; Li, M.-H. Applying Biomod2 for modeling of species suitable habitats:a case study of Paeonia lactiflora in China. Zhongguo Zhong Yao Za Zhi 2022, 47, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.E.; Villella, J.; Stone, D.; Hardman, A. Using lichen communities as indicators of forest stand age and conservation value. For. Ecol. Manag. 2020, 475, 118436. [Google Scholar] [CrossRef]
- Wallace, J.M.; Hobbs, P.V. Atmospheric Science: An Introductory Survey, 2nd ed.; Bulletin of the American Meteorological Society; Elsevier: Amsterdam, The Netherlands, 2007; p. 88. [Google Scholar] [CrossRef]
- Gasulla, F.; del Campo, E.M.; Casano, L.M.; Guéra, A. Advances in Understanding of Desiccation Tolerance of Lichens and Lichen-Forming Algae. Plants 2021, 10, 807. [Google Scholar] [CrossRef]
- Jonsson, A.V.; Moen, J.; Palmqvist, K. Predicting lichen hydration using biophysical models. Oecologia 2008, 156, 259–273. [Google Scholar] [CrossRef]
- Kraus, J.B.; Huang, Z.; Li, Y.; Cui, L.; Wang, S.; Li, J.; Liu, F.; Wang, Y.; Strier, K.B.; Xiao, W. Variation in monthly and seasonal elevation use impacts behavioral and dietary flexibility in Rhinopithecus bieti. Am. J. Primatol. 2024, 86, e23627. [Google Scholar] [CrossRef]
- Thomas, H.; Nash, I. Lichen Biology; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar] [CrossRef]
- Gauslaa, Y. Rain, dew, and humid air as drivers of morphology, function and spatial distribution in epiphytic lichens. Lichenologist 2014, 46, 1–16. [Google Scholar] [CrossRef]
- Zhao, Q.; He, S.; Wu, B.; Nash, L.T. Excrement distribution and habitat use in Rhinopithecus bieti in winter. Am. J. Primatol. 1988, 16, 275–284. [Google Scholar] [CrossRef]
- Norberg, R.A. An Ecological Theory on Foraging Time and Energetics and Choice of Optimal Food-Searching Method. J. Anim. Ecol. 1977, 46, 511–529. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregaard, R.O.; Laurance, S.G.; Sampaio, E. Ecosystem Decay of Amazonian Forest Fragments: A 22-Year Investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef]


| Variable Type | Unit | Description | Selection |
|---|---|---|---|
| Climatic variables | bio1 | Annual mean temperature (°C) | |
| bio2 | Mean diurnal temperature range (°C) | √ | |
| bio3 | Isothermality (bio2/bio7) (×100) | √ | |
| bio4 | Temperature seasonality (standard deviation × 100) | ||
| bio5 | Maximum temperature of warmest month (°C) | ||
| bio6 | Minimum temperature of coldest month (°C) | ||
| bio7 | Temperature annual range (°C) | ||
| bio8 | Mean temperature of wettest quarter (°C) | ||
| bio9 | Mean temperature of driest quarter (°C) | ||
| bio10 | Mean temperature of warmest quarter (°C) | ||
| bio11 | Mean temperature of coldest quarter (°C) | ||
| bio12 | Annual precipitation (mm) | √ | |
| bio13 | Precipitation of wettest month (mm) | ||
| bio14 | Precipitation of driest month (mm) | ||
| bio15 | Precipitation seasonality (mm) | √ | |
| bio16 | Precipitation of wettest quarter (mm) | ||
| bio17 | Precipitation of driest quarter (mm) | ||
| bio18 | Precipitation of warmest quarter (mm) | ||
| bio19 | Precipitation of coldest quarter (mm) | ||
| Topographical variables | Asp | Aspect | √ |
| Ele | Elevation | √ | |
| Slo | Slope | √ | |
| Vegetation variables | Vege | Vegetation type | √ |
| Model Variable | TSS | AUC | Model Selection | |
|---|---|---|---|---|
| Single model | GLM | 0.766 ± 0.02 | 0.919 ± 0.01 | √ |
| GBM | 0.806 ± 0.03 | 0.932 ± 0.02 | √ | |
| GAM | N/A | N/A | ||
| CTA | 0.746 ± 0.03 | 0.877 ± 0.02 | ||
| ANN | 0.621 ± 0.01 | 0.835 ± 0.03 | ||
| SRE | 0.488 ± 0.05 | 0.744 ± 0.04 | ||
| FDA | 0.730 ± 0.03 | 0.901 ± 0.01 | ||
| MARS | 0.750 ± 0.03 | 0.912 ± 0.02 | √ | |
| RF | 0.809 ± 0.03 | 0.938 ± 0.01 | √ | |
| MAXENT | 0.728 ± 0.05 | 0.918 ± 0.03 | √ | |
| Integrated model | EMmean | 0.867 ± 0.03 | 0.969 ± 0.03 | |
| EMca | 0.912 ± 0.03 | 0.993 ± 0.01 | √ | |
| Emcv | N/A | N/A | ||
| EMwmean | 0.869 ± 0.03 | 0.971 ± 0.02 | ||
| Variable | Aspect | bio2 | bio3 | bio12 | bio15 | Ele | Slo | Vege |
|---|---|---|---|---|---|---|---|---|
| Contributions | 0.039 | 0.037 | 0.113 | 0.118 | 0.081 | 0.664 | 0.041 | 0.338 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhu, H.; Huang, L.; He, X.; Ge, S.; Lai, J.; Zhaba, D.; Li, D.; Xia, W. Distribution Changes in Lichen: A Staple Fallback Food for Yunnan Snub-Nosed Monkey and Their Implications for the Species. Biology 2025, 14, 1369. https://doi.org/10.3390/biology14101369
Zhang Y, Zhu H, Huang L, He X, Ge S, Lai J, Zhaba D, Li D, Xia W. Distribution Changes in Lichen: A Staple Fallback Food for Yunnan Snub-Nosed Monkey and Their Implications for the Species. Biology. 2025; 14(10):1369. https://doi.org/10.3390/biology14101369
Chicago/Turabian StyleZhang, Yuan, Hanyu Zhu, Lianghua Huang, Xinming He, Sang Ge, Jiandong Lai, Duji Zhaba, Dayong Li, and Wancai Xia. 2025. "Distribution Changes in Lichen: A Staple Fallback Food for Yunnan Snub-Nosed Monkey and Their Implications for the Species" Biology 14, no. 10: 1369. https://doi.org/10.3390/biology14101369
APA StyleZhang, Y., Zhu, H., Huang, L., He, X., Ge, S., Lai, J., Zhaba, D., Li, D., & Xia, W. (2025). Distribution Changes in Lichen: A Staple Fallback Food for Yunnan Snub-Nosed Monkey and Their Implications for the Species. Biology, 14(10), 1369. https://doi.org/10.3390/biology14101369





