Luopan Mountain Pig Bone Marrow Mesenchymal Stem Cells Promote Liver Regeneration in D-Galactosamine-Induced Acute Liver Failure Rats by Regulating the PTEN-PI3K/Akt/mTOR Pathway
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of pBMSCs
2.2. Immunofluorescence Detection of pBMSCs
2.3. pBMSCs Multidirectional Differentiation Ability Detection
2.4. Construction of a Rat Model of Acute Liver Failure
2.4.1. Serum Biochemical Indicator Testing
2.4.2. Serum Cytokine Testing
2.4.3. Liver Tissue Pathology Testing
2.4.4. Detection of Apoptosis in Liver Tissue Cells
2.4.5. Liver Tissue Glycogen Staining
2.4.6. Immunohistochemical Testing
2.4.7. Real-Time Fluorescent Quantitative PCR Analysis
2.4.8. Western Blot Analysis
2.5. Statistical Analysis
3. Results
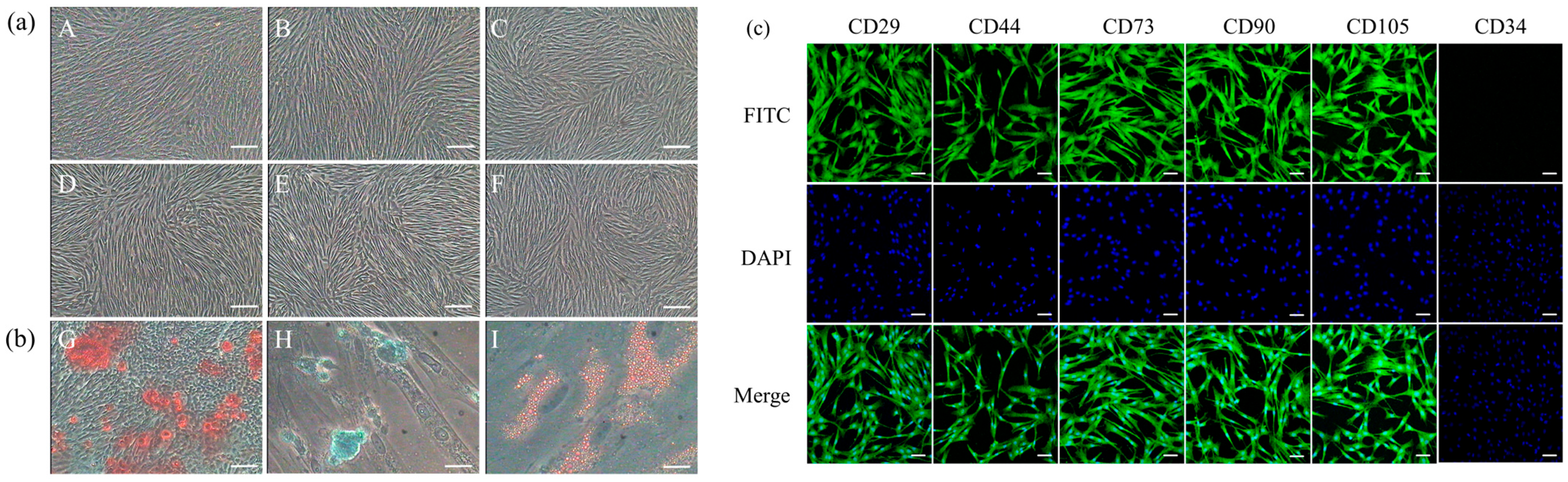
3.1. Biological Characteristics of Luopan Mountain Pig pBMSCs
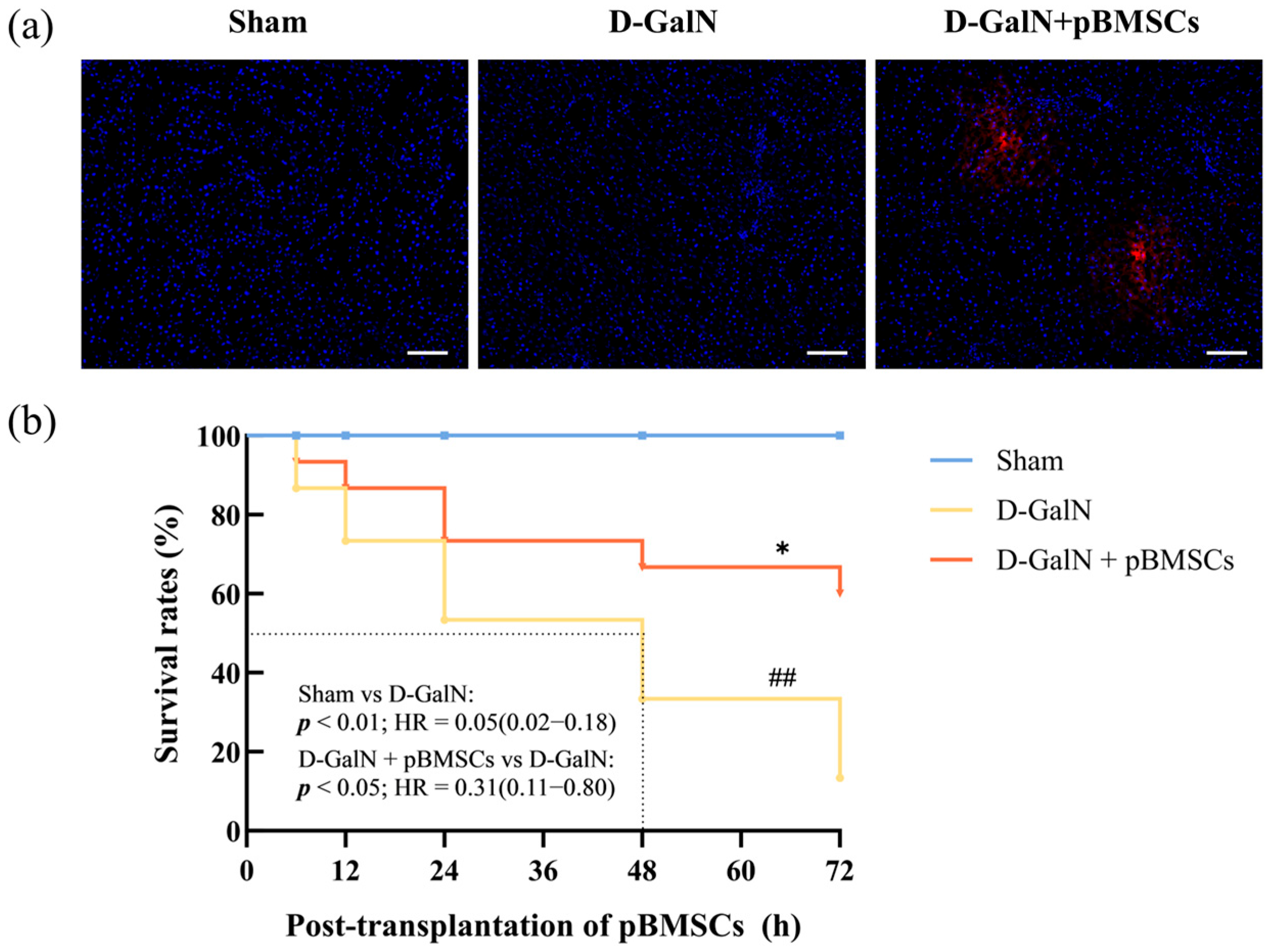
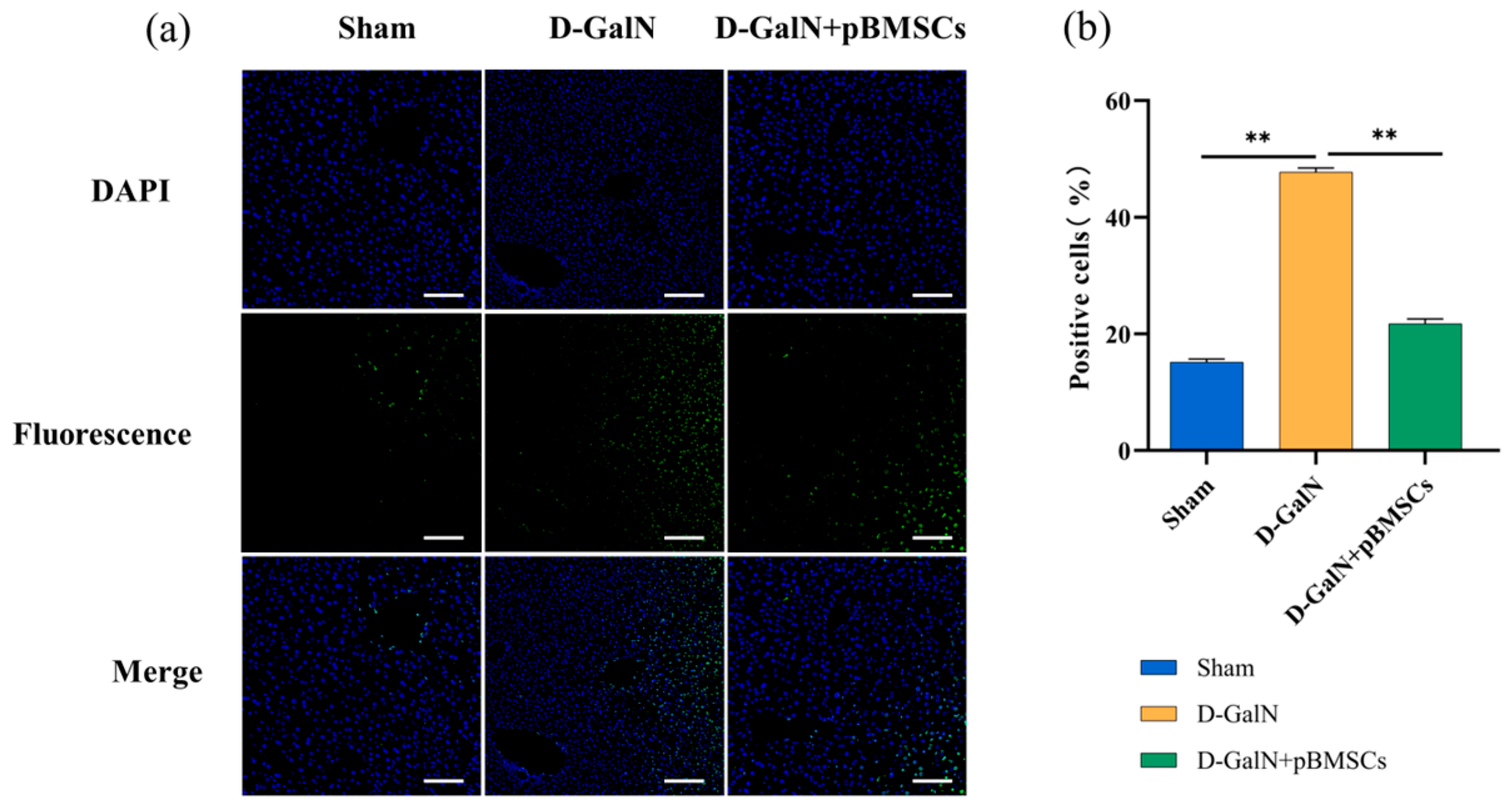
3.2. Liver-Targeted Homing and Improved Survival Rate of pBMSCs
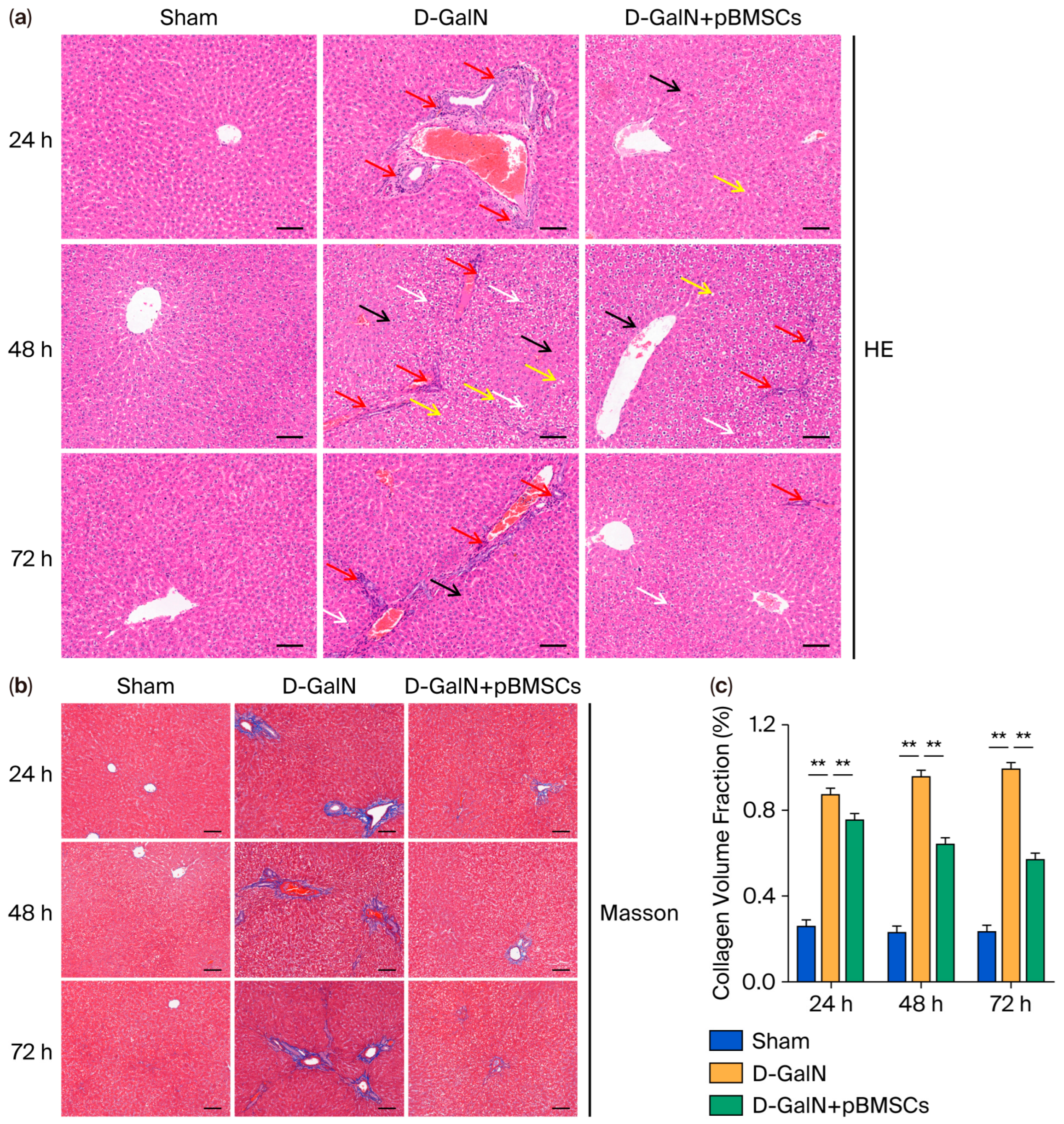
3.3. pBMSC Transplantation Alleviates Histopathological Damage in the Liver Tissue of ALF Rats
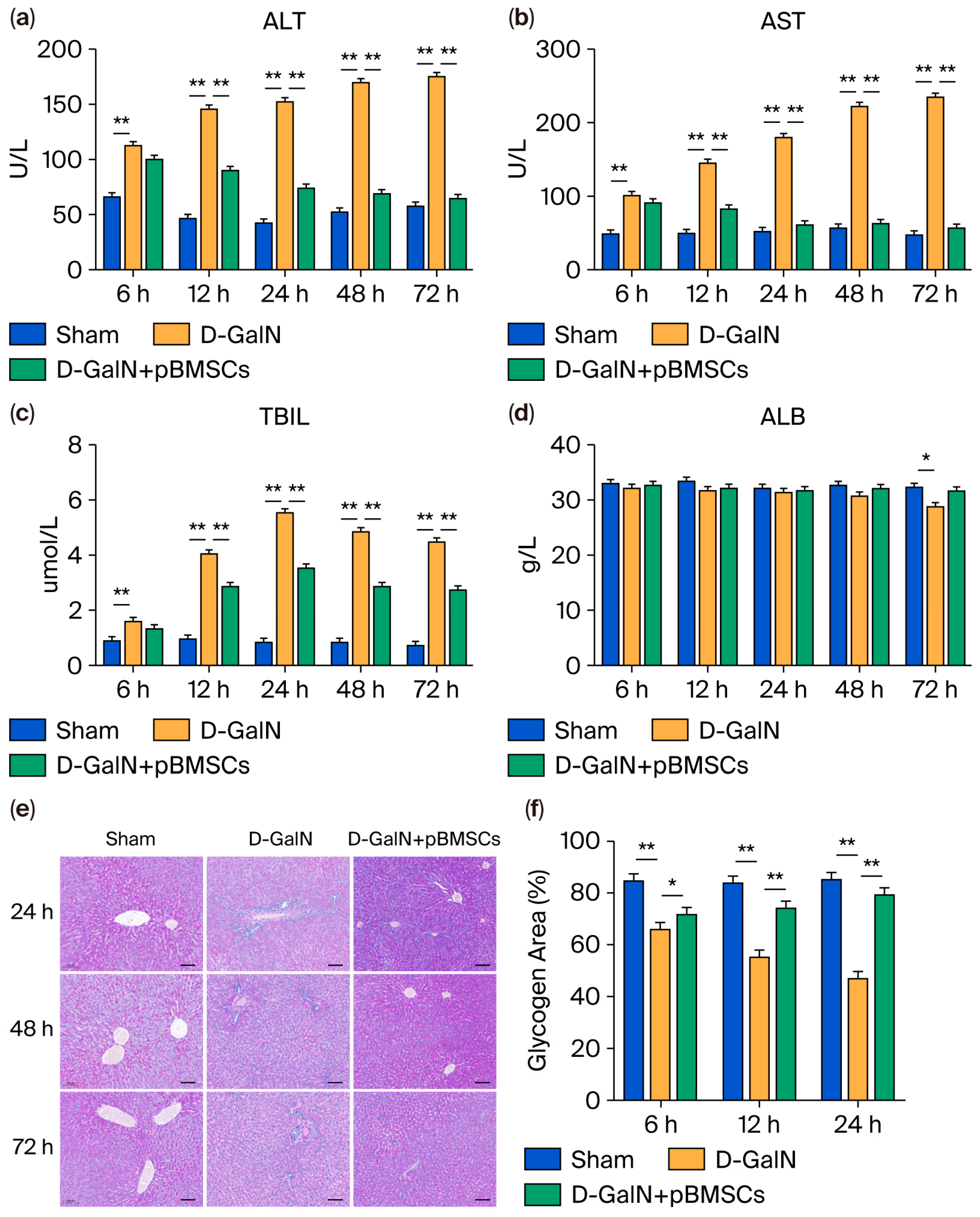
3.4. pBMSC Transplantation Improves Liver Function and Metabolic Marker Levels in ALF Rats
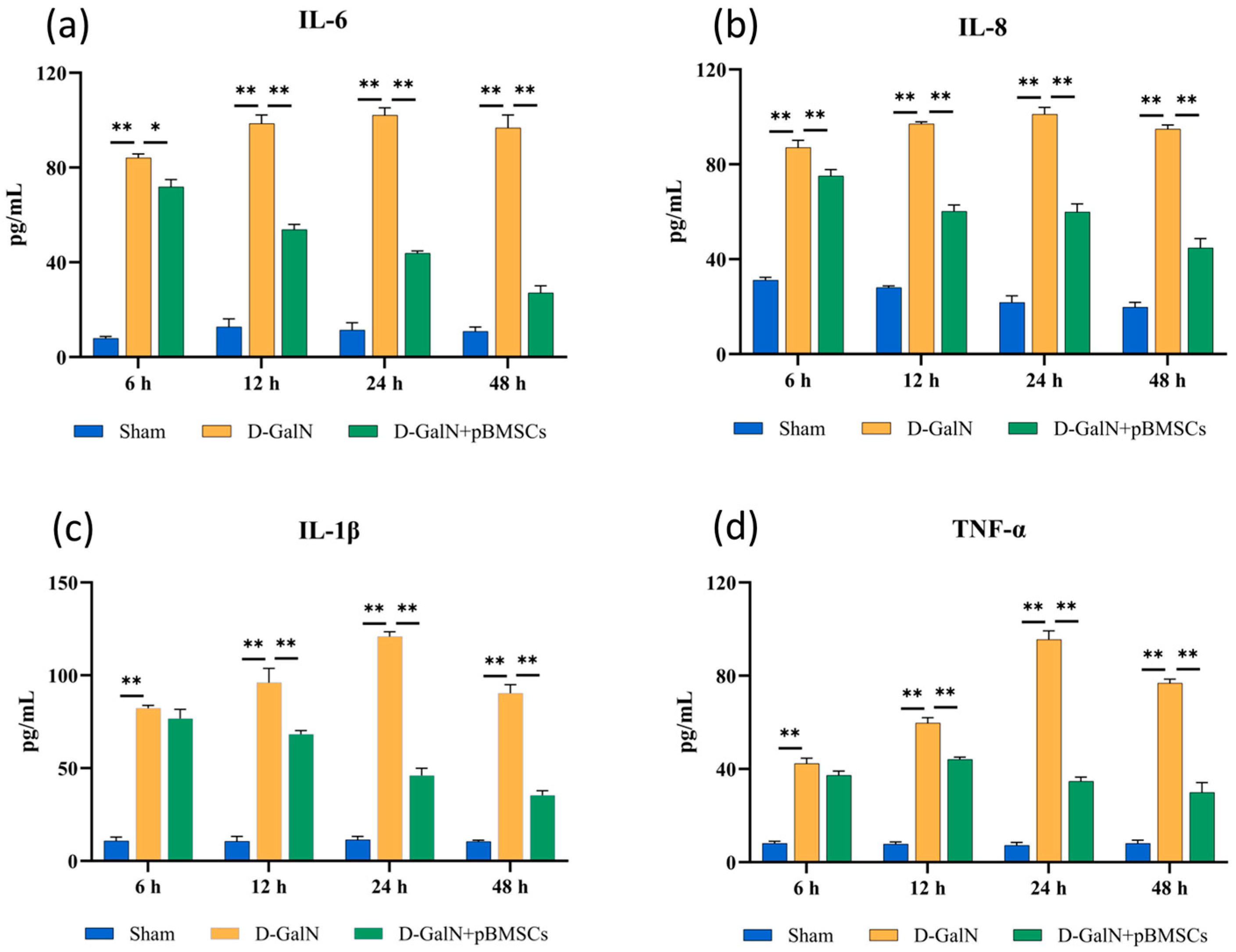
3.5. pBMSC Transplantation Inhibits Proinflammatory Cytokine Release
3.6. pBMSC Transplantation Inhibits Apoptosis of Liver Cells in ALF Rats
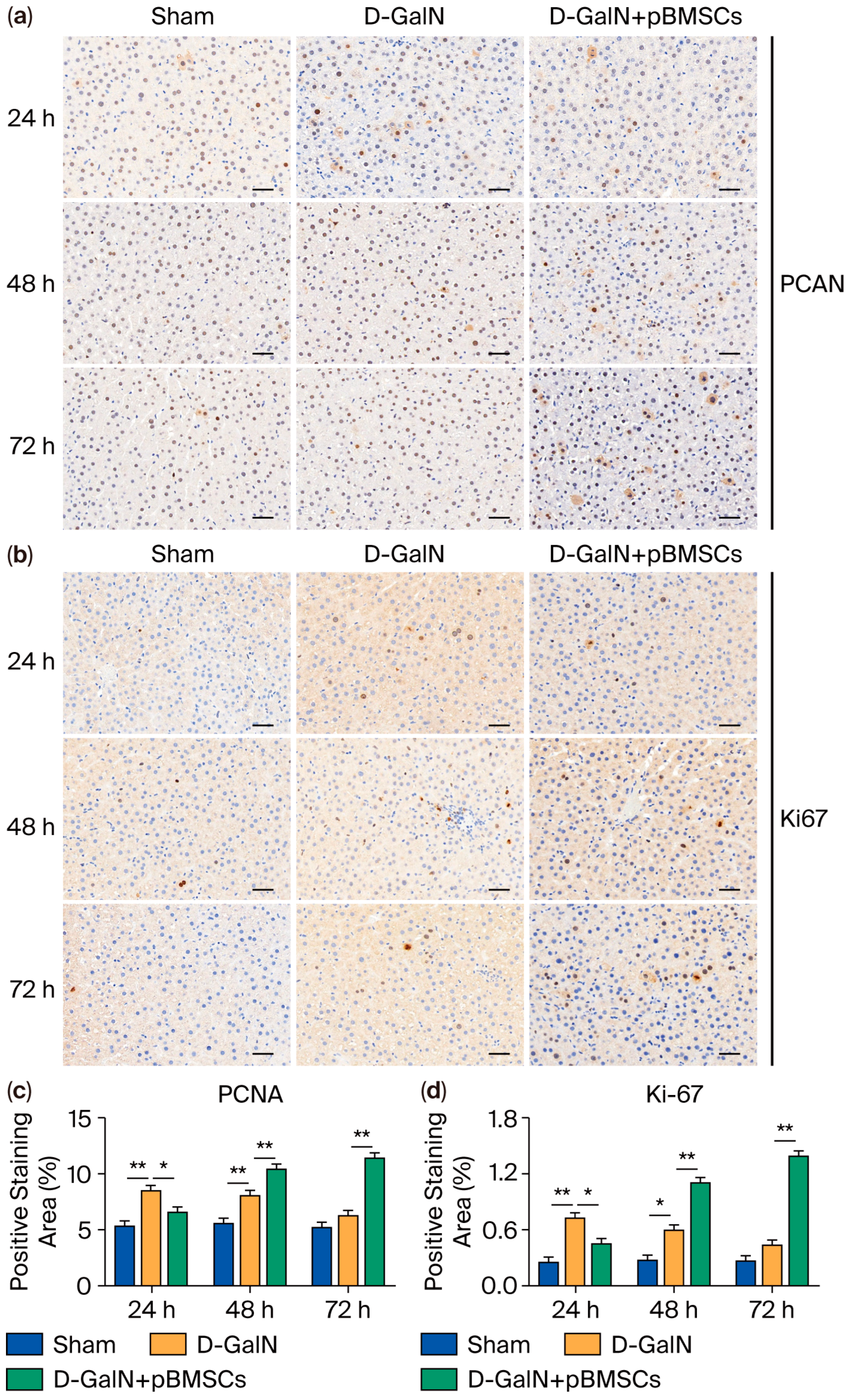
3.7. Regulation of Liver Cell Proliferation in ALF Rats by pBMSC Transplantation
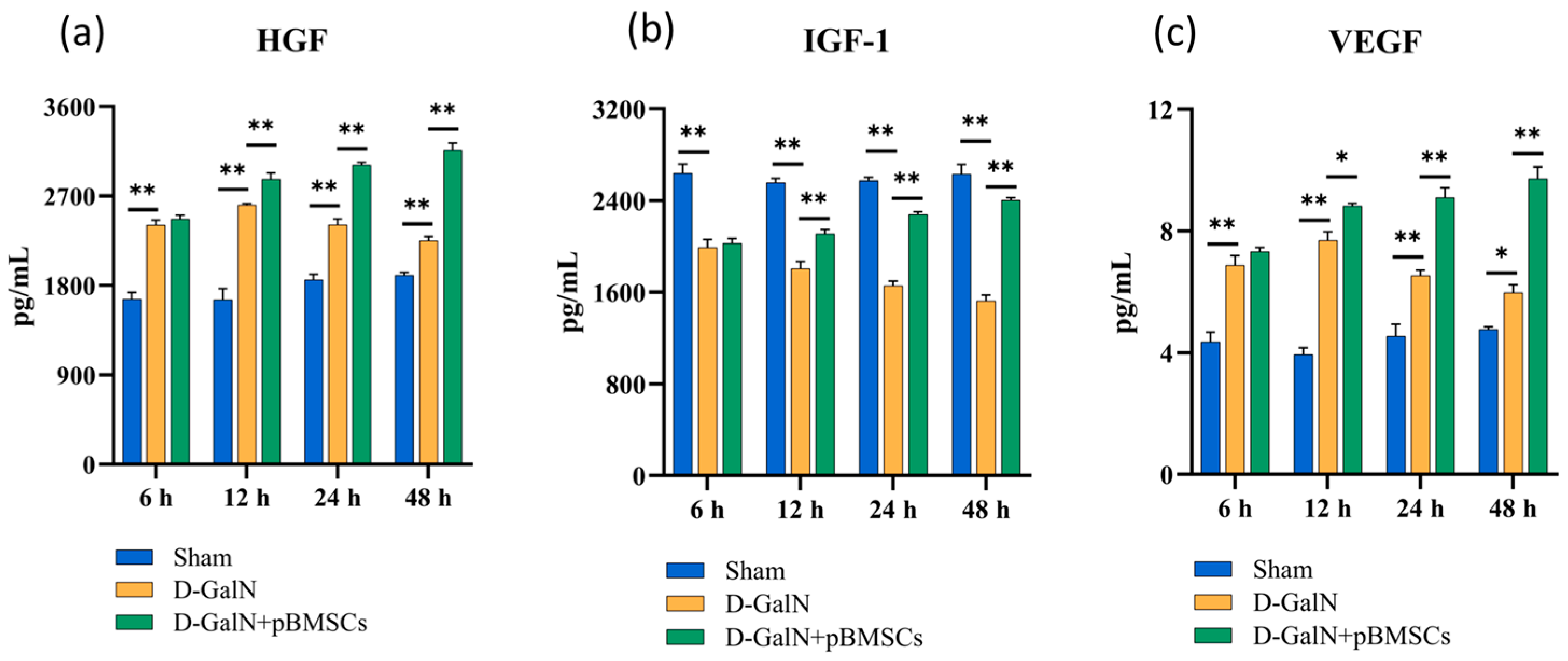
3.8. pBMSC Transplantation Upregulates Serum Growth Factor Levels in ALF Rats
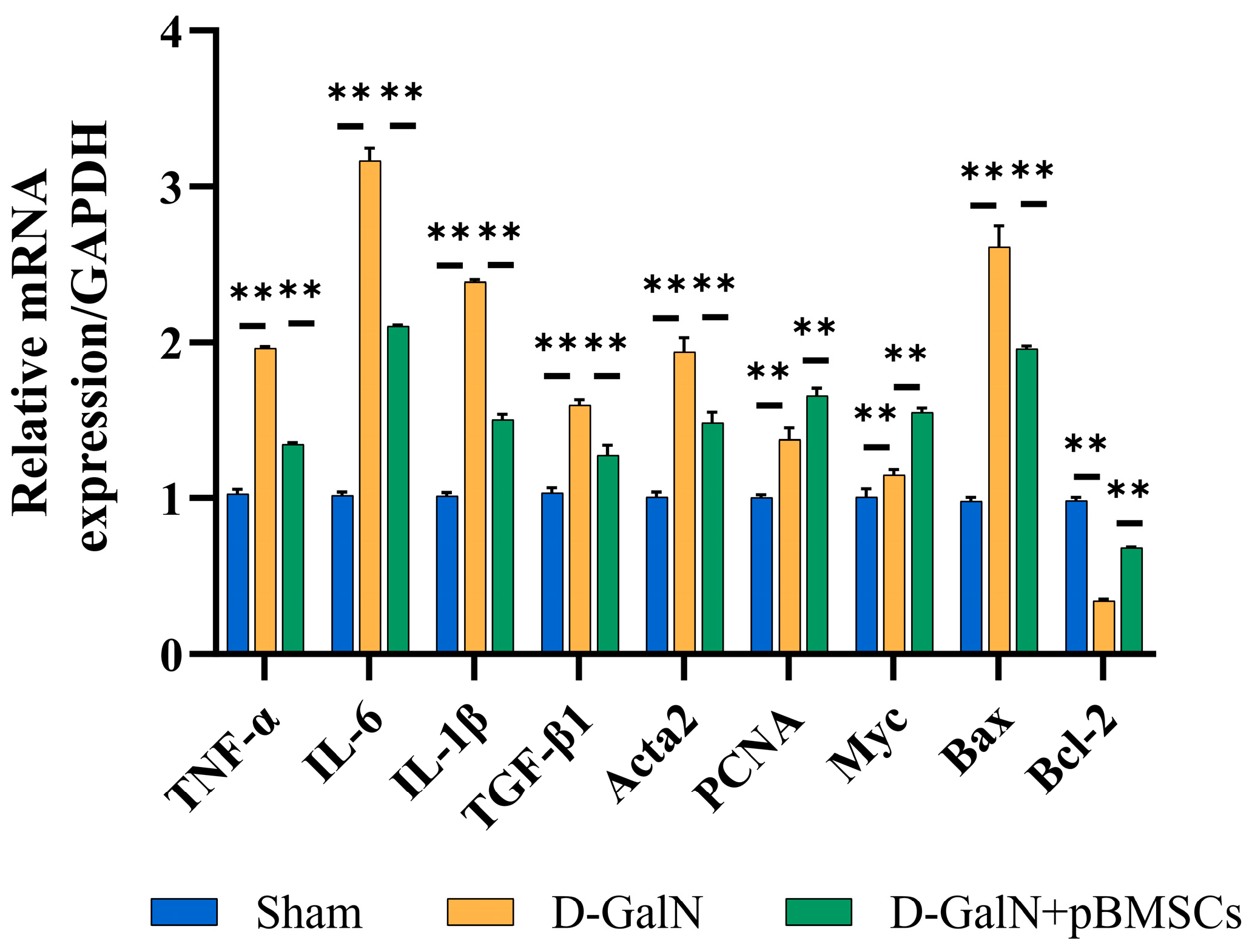
3.9. Regulation of Inflammatory, Fibrotic, and Apoptotic Gene Expression in Liver Tissue of ALF Rats Following pBMSC Transplantation
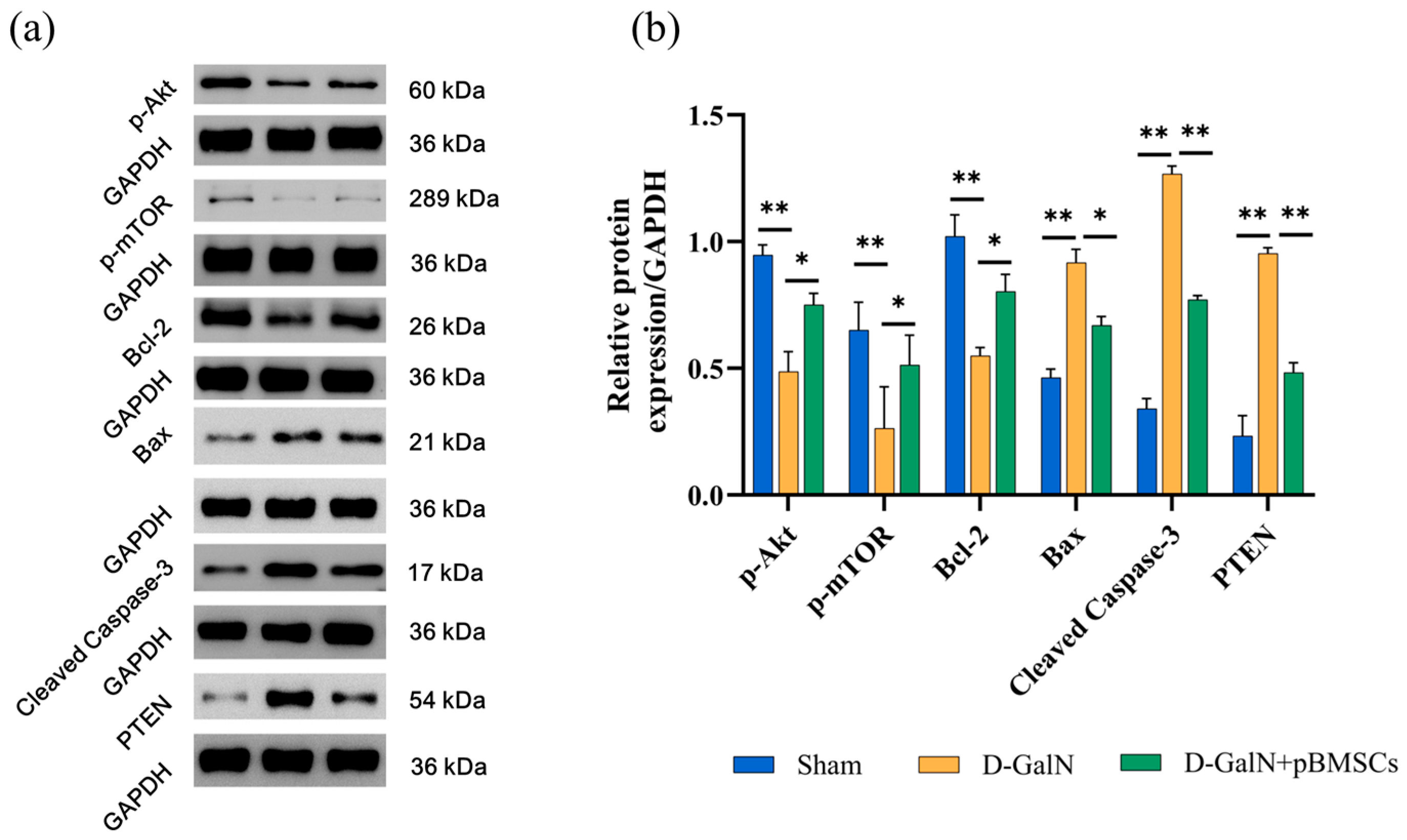
3.10. pBMSC Transplantation Alleviates Liver Damage by Activating the PI3K/Akt/mTOR Pathway and Modulating the Expression of Proteins Associated with Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALF | Acute Liver Failure |
| pBMSCs | Porcine Bone Marrow Mesenchymal Stem Cells |
| D-GalN | D-galactosamine |
References
- Maiwall, R.; Kulkarni, A.V.; Arab, J.P.; Piano, S. Acute Liver Failure. Lancet 2024, 404, 789–802. [Google Scholar] [CrossRef]
- Vento, S.; Cainelli, F. Acute Liver Failure in Low-Income and Middle-Income Countries. Lancet Gastroenterol. Hepatol. 2023, 8, 1035–1045. [Google Scholar] [CrossRef]
- Vasques, F.; Cavazza, A.; Bernal, W. Acute Liver Failure. Curr. Opin. Crit. Care 2022, 28, 198–207. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, H.S.; Cho, H.I.; Jin, Y.W.; Lee, E.K.; Ahn, J.Y.; Lee, S.M. Protective Effect of Wild Ginseng Cambial Meristematic Cells on D-Galactosamine-Induced Hepatotoxicity in Rats. J. Ginseng Res. 2015, 39, 376–383. [Google Scholar] [CrossRef]
- Luan, Y.; Kong, X.; Feng, Y. Mesenchymal Stem Cells Therapy for Acute Liver Failure: Recent Advances and Future Perspectives. Liver Res. 2021, 5, 53–61. [Google Scholar] [CrossRef]
- Ding, Y.; Tan, R.; Gu, J.; Gong, P. Herpetin Promotes Bone Marrow Mesenchymal Stem Cells to Alleviate Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Molecules 2023, 28, 3842. [Google Scholar] [CrossRef]
- He, X.; Wang, S.; Yu, X.; Zhou, X. Bone Marrow Mesenchymal Stem Cells Response on Collagen/Hyaluronan/Chondroitin Scaffold Enriched with Gentamicin-Loaded Gelatin Microparticles for Skin Tissue Engineering. J. Biomater. Appl. 2023, 38, 134–145. [Google Scholar] [PubMed]
- Ashour, A.A.; El-Kamel, A.H.; Mehanna, R.A.; Mourad, G.; Heikal, L.A. Luteolin-Loaded Exosomes Derived from Bone Marrow Mesenchymal Stem Cells: A Promising Therapy for Liver Fibrosis. Drug Deliv. 2022, 29, 3270–3280. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ni, B.; Wang, L.; Shan, J.; Pan, L.; He, Y.; Lv, G.; Lin, H.; Chen, W.; Zhang, Q. CCR2-Overexpressing Mesenchymal Stem Cells Targeting Damaged Liver Enhance Recovery of Acute Liver Failure. Stem Cell Res. Ther. 2022, 13, 55. [Google Scholar] [CrossRef]
- Jiang, J.; Xin, J.; Ding, W.; Shi, D.; Sun, S.; Guo, B.; Zhou, X.; Zheng, C.; Li, J. MicroRNA Profile of Human Bone Marrow Mesenchymal Stem Cells during Hepatic Differentiation and Therapy. Int. J. Med. Sci. 2022, 19, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Seo, K.; Wi, H.; Kim, Y.; Lee, S.; Ock, S.A. Induction of the Differentiation of Porcine Bone Marrow Mesenchymal Stem Cells into Premature Hepatocyte-Like Cells in an Indirect Coculture System with Primary Hepatocytes. Anim. Cells Syst. 2020, 24, 289–298. [Google Scholar] [CrossRef]
- Liu, S.; Guo, R.; Hou, X.; Zhang, Y.; Jiang, X.; Wang, T.; Wu, X.; Xu, K.; Pan, X.; Qiao, L. Adipose-Tissue Derived Porcine Mesenchymal Stem Cells Efficiently Ameliorate CCl4-Induced Acute Liver Failure in Mice. Cytotechnology 2020, 72, 327–341. [Google Scholar] [CrossRef]
- Scarano, A.; Crincoli, V.; Di Benedetto, A.; Cozzolino, V.; Lorusso, F.; Podaliri Vulpiani, M.; Grano, M.; Kalemaj, Z.; Mori, G.; Grassi, F.R. Bone Regeneration Induced by Bone Porcine Block with Bone Marrow Stromal Stem Cells in a Minipig Model of Mandibular “Critical Size” Defect. Stem Cells Int. 2017, 2017, 9082869. [Google Scholar] [CrossRef]
- Lohmann, S.; Pool, M.B.F.; Rozenberg, K.M.; Keller, A.K.; Moers, C.; Møldrup, U.; Møller, B.K.; Lignell, S.J.M.; Krag, S.; Sierra-Parraga, J.M.; et al. Mesenchymal Stromal Cell Treatment of Donor Kidneys during Ex Vivo Normothermic Machine Perfusion: A Porcine Renal Autotransplantation Study. Am. J. Transplant. 2021, 21, 2348–2359. [Google Scholar] [CrossRef]
- Thäte, C.; Woischwill, C.; Brandenburg, G.; Müller, M.; Böhm, S.; Baumgart, J. Non-Clinical Assessment of Safety, Biodistribution and Tumorigenicity of Human Mesenchymal Stromal Cells. Toxicol. Rep. 2021, 8, 1960–1969. [Google Scholar] [CrossRef]
- Chin, S.P.; Saffery, N.S.; Then, K.Y.; Cheong, S.K. Preclinical Assessments of Safety and Tumorigenicity of Very High Doses of Allogeneic Human Umbilical Cord Mesenchymal Stem Cells. In Vitr. Cell. Dev. Biol. Anim. 2024, 60, 307–319. [Google Scholar] [CrossRef]
- Cotten, C.M.; Fisher, K.; Malcolm, W.; Gustafson, K.E.; Cheatham, L.; Marion, A.; Greenberg, R.; Kurtzberg, J. A Pilot Phase I Trial of Allogeneic Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells in Neonates with Hypoxic-Ischemic Encephalopathy. Stem Cells Transl. Med. 2023, 12, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hao, X.; Lin, Z.; Li, L.; Jiang, H.; Zhang, H.; Geng, X.; Zhu, H.; Wen, H. Bio-Distribution and Toxicity Potential of Human Umbilical Cord Mesenchymal Stem Cells in Cynomolgus Monkeys. Sci. Rep. 2024, 14, 12251. [Google Scholar] [CrossRef]
- Dai, W.; Ni, Z.; Zhang, G.; Xu, J.; Qin, X.; Cao, J.; Liu, L. JDHY3 Inhibits Hypopharyngeal Carcinoma Cell Proliferation and Promotes Apoptosis by Inhibiting the PI3K/AKT Pathway. Anticancer Agents Med. Chem. 2023, 23, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Z.; Wang, Y.; Shi, B.; Jin, Y.; Wang, Y.; Jiang, X.; Song, M.; Yu, W. Grape Seed Extract Proanthocyanidin Antagonizes Aristolochic Acid I-Induced Liver Injury in Rats by Activating PI3K-AKT Pathway. Toxicol. Mech. Methods 2023, 33, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Lv, M.; Cao, S.; Yang, G.; Zhang, Z.; Yu, Q. Fructus Lycii Oligosaccharide Alleviates Acute Liver Injury via PI3K/Akt/mTOR Pathway. Immunol. Res. 2024, 72, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Zhao, Q.; Qin, Y.; Tong, H.; Wang, Q.; Yu, M.; Mao, C.; Lu, T.; Qiu, J.; Jiang, C. Germacrone Improves Liver Fibrosis by Regulating the PI3K/AKT/mTOR Signalling Pathway. Cell Biol. Int. 2021, 45, 1866–1875. [Google Scholar] [CrossRef]
- Fathi, A.M.; Waz, S.; Alaaeldin, E.; Toni, N.D.M.; El-Sheikh, A.A.K.; Sayed, A.M.; Abdelmohsen, U.R.; Nazmy, M.H. Blackberry-Loaded AgNPs Attenuate Hepatic Ischemia/Reperfusion Injury via PI3K/Akt/mTOR Pathway. Metabolites 2023, 13, 419. [Google Scholar] [CrossRef]
- Lu, M.M.; Ren, Y.; Zhou, Y.W.; Xu, L.L.; Zhang, M.M.; Ding, L.P.; Cheng, W.X.; Jin, X. Antagonizing Adipose Tissue-Derived Exosome miR-103-Hepatocyte Phosphatase and Tensin Homolog Pathway Alleviates Autophagy in Non-Alcoholic Steatohepatitis: A Trans-Cellular Crosstalk. World J. Gastroenterol. 2023, 29, 4528–4541. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Y.; Zheng, Q.; Zhao, H.; Wei, X.; Li, X. Itaconate Attenuates Autoimmune Hepatitis via PI3K/AKT/mTOR Pathway-Mediated Inhibition of Dendritic Cell Maturation and Autophagy. Heliyon 2023, 9, e17551. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Z.; Zhang, Z.; Liu, S.; Wang, M.; Zhang, X.; Shi, J.; Gao, H.; Gu, J.; Han, H.; et al. Comprehensive Genomic Analysis and Selection Signature Detection in Endangered Beigang Pigs Using Whole-Genome Sequencing Data. Anim. Genet. 2025, 56, e13502. [Google Scholar] [CrossRef]
- Sivananthan, S.; Bhakta, V.; Chaechi Tehrani, N.; Sheffield, W.P. Prolonging the Circulatory Half-Life of C1 Esterase Inhibitor via Albumin Fusion. PLoS ONE 2024, 19, e0305719. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guo, X.; Lan, T.; Xia, J.; Wang, J.; Li, B.; Peng, C.; Chen, Y.; Hu, X.; Meng, Z. Correction to: Human Umbilical Cord-Derived Mesenchymal Stem Cells Improve the Function of Liver in Rats with Acute-on-Chronic Liver Failure via Downregulating Notch and Stat1/Stat3 Signaling. Stem Cell Res. Ther. 2022, 13, 65. [Google Scholar] [CrossRef]
- Abdallah, M.; Lin, L.; Styles, I.K.; Mörsdorf, A.; Grace, J.L.; Gracia, G.; Nowell, C.; Quinn, J.F.; Landersdorfer, C.B.; Whittaker, M.R.; et al. Functionalisation of Brush Polyethylene Glycol Polymers with Specific Lipids Extends Their Elimination Half-Life Through Association with Natural Lipid Trafficking Pathways. Acta Biomater. 2024, 174, 191–205. [Google Scholar] [CrossRef]
- Lu, C.; Li, X.; Fang, C.; Li, C.; Xu, Y.; Guo, Y. Pretreatment of Artesunate Promoted Hepatocyte Proliferation by Activating the PI3K/Akt/mTOR Signaling Pathway in Mice. Acta Cir. Bras. 2024, 39, e394324. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Lu, S.; Tian, Y.; Ma, H.; Wang, Y.; Zhang, X.; Sun, G.; Luo, Y.; Sun, X. Bavachinin Protects the Liver in NAFLD by Promoting Regeneration via Targeting PCNA. J. Adv. Res. 2024, 55, 131–144. [Google Scholar] [PubMed]
- Ghosh, A.; Ihsan, A.U.; Nandi, M.; Cloutier, M.; Khan, M.G.M.; Ramanathan, S.; Ilangumaran, S. Application of EdU-Based DNA Synthesis Assay to Measure Hepatocyte Proliferation In Situ During Liver Regeneration. Methods Mol. Biol. 2022, 2544, 195–206. [Google Scholar] [PubMed]
- Anghinoni, M.; Toderke, E.L.; Nakadomari, T.S.; Oliveira, T.K.M.; Locatelli, F.P.; Matias, J.E.F. Liver Regeneration after Extensive Hepatectomy in Rats: Effect of Preoperative Chemotherapy with Intravenous 5-Fluorouracil. Acta Cir. Bras. 2022, 37, e370901. [Google Scholar] [CrossRef]
- Yuan, Z.; Georgescu, R.; Yao, N.Y.; Yurieva, O.; O’Donnell, M.E.; Li, H. Mechanism of PCNA Loading by Ctf18-RFC for Leading-Strand DNA Synthesis. Science 2024, 385, eadk5901. [Google Scholar]
- Wang, J.; Ding, H.; Zhou, J.; Xia, S.; Shi, X.; Ren, H. Transplantation of Mesenchymal Stem Cells Attenuates Acute Liver Failure in Mice via an Interleukin-4-Dependent Switch to the M2 Macrophage Anti-Inflammatory Phenotype. J. Clin. Transl. Hepatol. 2022, 10, 669–679. [Google Scholar] [CrossRef]
- Wu, H.; Fan, F.; Liu, Z.; Shen, C.; Wang, A.; Lu, Y. Norcantharidin Combined with EGFR-TKIs Overcomes HGF-Induced Resistance to EGFR-TKIs in EGFR Mutant Lung Cancer Cells via Inhibition of Met/PI3k/Akt Pathway. Cancer Chemother. Pharmacol. 2015, 76, 307–315. [Google Scholar] [PubMed]
- Gesualdi, L.; Leonetti, E.; Cucina, A.; Scicchitano, B.M.; Sorrentino, S.; Tarsitano, M.G.; Isidori, A.; Bizzarri, M.; Filippini, A.; Riccioli, A.; et al. The PI3K/AKT Pathway Is Activated by HGF in NT2D1 Non-Seminoma Cells and Has a Role in the Modulation of Their Malignant Behavior. Int. J. Mol. Sci. 2020, 21, 8669. [Google Scholar] [CrossRef]
- Wahyuni, T.; Kobayashi, A.; Tanaka, S.; Miyake, Y.; Yamamoto, A.; Bahtiar, A.; Mori, S.; Kametani, Y.; Tomimatsu, M.; Matsumoto, K.; et al. Maresin-1 Induces Cardiomyocyte Hypertrophy Through IGF-1 Paracrine Pathway. Am. J. Physiol. Cell Physiol. 2021, 321, C82–C93. [Google Scholar]
- Calderone, V.; Gallego, J.; Fernandez-Miranda, G.; Garcia-Pras, E.; Maillo, C.; Berzigotti, A.; Mejias, M.; Bava, F.A.; Angulo-Urarte, A.; Graupera, M.; et al. Sequential Functions of CPEB1 and CPEB4 Regulate Pathologic Expression of Vascular Endothelial Growth Factor and Angiogenesis in Chronic Liver Disease. Gastroenterology 2016, 150, 982–997. [Google Scholar] [CrossRef]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/mTOR Signaling Pathway for Targeted Therapeutic Treatment in Human Cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar]
- Tian, L.Y.; Smit, D.J.; Jücker, M. The Role of PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma Metabolism. Int. J. Mol. Sci. 2023, 24, 2652. [Google Scholar] [PubMed]
- Bang, J.; Jun, M.; Lee, S.; Moon, H.; Ro, S.W. Targeting EGFR/PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma. Pharmaceutics 2023, 15, 2130. [Google Scholar]
- Jung, K.; Kim, M.; So, J.; Lee, S.H.; Ko, S.; Shin, D. Farnesoid X Receptor Activation Impairs Liver Progenitor Cell-Mediated Liver Regeneration via the PTEN-PI3K-AKT-mTOR Axis in Zebrafish. Hepatology 2021, 74, 397–410. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Choudhury, A.D. PTEN-PI3K Pathway Alterations in Advanced Prostate Cancer and Clinical Implications. Prostate 2022, 82, S60–S72. [Google Scholar] [CrossRef]
- Subbiah, V.; Coleman, N.; Piha-Paul, S.A.; Tsimberidou, A.M.; Janku, F.; Rodon, J.; Pant, S.; Dumbrava, E.E.I.; Fu, S.; Hong, D.S.; et al. Phase I Study of mTORC1/2 Inhibitor Sapanisertib (CB-228/TAK-228) in Combination with Metformin in Patients with mTOR/AKT/PI3K Pathway Alterations and Advanced Solid Malignancies. Cancer Res. Commun. 2024, 4, 378–387. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Xia, X.; Wang, S.; Wu, L.; Li, G.; Hou, K.; Yu, A.; Yang, Z. TIPE2 Attenuates Neuroinflammation and Brain Injury through Bcl-2/Bax/Cleaved Caspase-3 Apoptotic Pathways after Intracerebral Hemorrhage in Mice. Brain Res. Bull. 2022, 191, 1–8. [Google Scholar] [CrossRef]
- Tian, H.; Liu, L.; Li, Z.; Liu, W.; Sun, Z.; Xu, Y.; Wang, S.; Liang, C.; Hai, Y.; Feng, Q.; et al. Chinese Medicine CGA Formula Ameliorates Liver Fibrosis Induced by Carbon Tetrachloride Involving Inhibition of Hepatic Apoptosis in Rats. J. Ethnopharmacol. 2019, 232, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, F.; Li, S.; Wu, B.; Gu, Y.; Yuan, Y. Salvianolic Acid A Attenuates CCl4-Induced Liver Fibrosis by Regulating the PI3K/AKT/mTOR, Bcl-2/Bax and Caspase-3/Cleaved Caspase-3 Signaling Pathways. Drug Des. Devel Ther. 2019, 13, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ji, Z.; Wu, D.; Wei, S.; Zhu, W.; Peng, G.; Hu, M.; Zhao, Y.; Wu, H. MYBL2 Promotes Cell Proliferation and Inhibits Cell Apoptosis via PI3K/AKT and BCL2/BAX/Cleaved-Caspase-3 Signaling Pathway in Gastric Cancer Cells. Sci. Rep. 2025, 15, 9148. [Google Scholar]
- Naso, F.; Gandaglia, A.; Sturaro, G.; Galli, C.; Melder, R.J. The α-Gal KO Mouse Animal Model is a Reliable and Predictive Tool for the Immune-Mediated Calcification Assessment of Heart Valve Bioprostheses. Front. Biosci. (Landmark Ed.) 2024, 29, 181. [Google Scholar] [CrossRef]
- Saleh, F.M.; Chandra, P.K.; Lin, D.; Robinson, J.E.; Izadpanah, R.; Mondal, D.; Bollensdorff, C.; Alt, E.U.; Zhu, Q.; Marasco, W.A.; et al. A New Humanized Mouse Model Mimics Humans in Lacking α-Gal Epitopes and Secreting Anti-Gal Antibodies. J. Immunol. 2020, 204, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Kogata, S.; Toyama, C.; Lo, P.C.; Okamatsu, C.; Yamamoto, R.; Masahata, K.; Kamiyama, M.; Eguchi, H.; Watanabe, M.; et al. The Innate Cellular Immune Response in Xenotransplantation. Front. Immunol. 2022, 13, 858604. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, A.; Nguyen, H.; Li, J.; Burnette, K.; Ayares, D.; Cooper, D.K.C.; Hara, H. Does Expression of a Human Complement-Regulatory Protein on Xenograft Cells Protect Them from Systemic Complement Activation? Int. J. Surg. 2020, 83, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Min, Y.; Park, C.; Kim, D.; Heo, Y.; Kim, M.; Son, E.; Ghosh, M.; Son, Y.O.; Hur, C.G. Innate Immune Response Analysis in Meniscus Xenotransplantation Using Normal and Triple Knockout Jeju Native Pigs. Int. J. Mol. Sci. 2022, 23, 10416. [Google Scholar] [CrossRef]
- Niu, D.; Ma, X.; Yuan, T.; Niu, Y.; Xu, Y.; Sun, Z.; Ping, Y.; Li, W.; Zhang, J.; Wang, T.; et al. Porcine Genome Engineering for Xenotransplantation. Adv. Drug Deliv. Rev. 2021, 168, 229–245. [Google Scholar] [CrossRef]
- Ko, N.; Shim, J.; Kim, H.J.; Lee, Y.; Park, J.K.; Kwak, K.; Lee, J.W.; Jin, D.I.; Kim, H.; Choi, K. A Desirable Transgenic Strategy Using GGTA1 Endogenous Promoter-Mediated Knock-In for Xenotransplantation Model. Sci. Rep. 2022, 12, 9611. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, Z.; Yan, X.; Liu, Y.; He, Y.; Zhang, B.; Guan, W. Luopan Mountain Pig Bone Marrow Mesenchymal Stem Cells Promote Liver Regeneration in D-Galactosamine-Induced Acute Liver Failure Rats by Regulating the PTEN-PI3K/Akt/mTOR Pathway. Biology 2025, 14, 1363. https://doi.org/10.3390/biology14101363
Li M, Wang Z, Yan X, Liu Y, He Y, Zhang B, Guan W. Luopan Mountain Pig Bone Marrow Mesenchymal Stem Cells Promote Liver Regeneration in D-Galactosamine-Induced Acute Liver Failure Rats by Regulating the PTEN-PI3K/Akt/mTOR Pathway. Biology. 2025; 14(10):1363. https://doi.org/10.3390/biology14101363
Chicago/Turabian StyleLi, Minjuan, Zhongfa Wang, Xingxing Yan, Yanchen Liu, Yunan He, Bianying Zhang, and Weijun Guan. 2025. "Luopan Mountain Pig Bone Marrow Mesenchymal Stem Cells Promote Liver Regeneration in D-Galactosamine-Induced Acute Liver Failure Rats by Regulating the PTEN-PI3K/Akt/mTOR Pathway" Biology 14, no. 10: 1363. https://doi.org/10.3390/biology14101363
APA StyleLi, M., Wang, Z., Yan, X., Liu, Y., He, Y., Zhang, B., & Guan, W. (2025). Luopan Mountain Pig Bone Marrow Mesenchymal Stem Cells Promote Liver Regeneration in D-Galactosamine-Induced Acute Liver Failure Rats by Regulating the PTEN-PI3K/Akt/mTOR Pathway. Biology, 14(10), 1363. https://doi.org/10.3390/biology14101363




