Rap1 Guanosine Triphosphate Hydrolase (GTPase) Regulates Shear Stress-Mediated Adhesion of Mesenchymal Stromal Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies, Reagents, and Cells
2.2. Cell Transfection and siRNA-Mediated Rap1 Regulation
2.3. Flow Cytometry and Immunohistochemistry
2.4. Analysis of MSC Adhesion Under Shear Stress
2.5. Animal Experiments
2.6. Statistical Analysis
3. Results
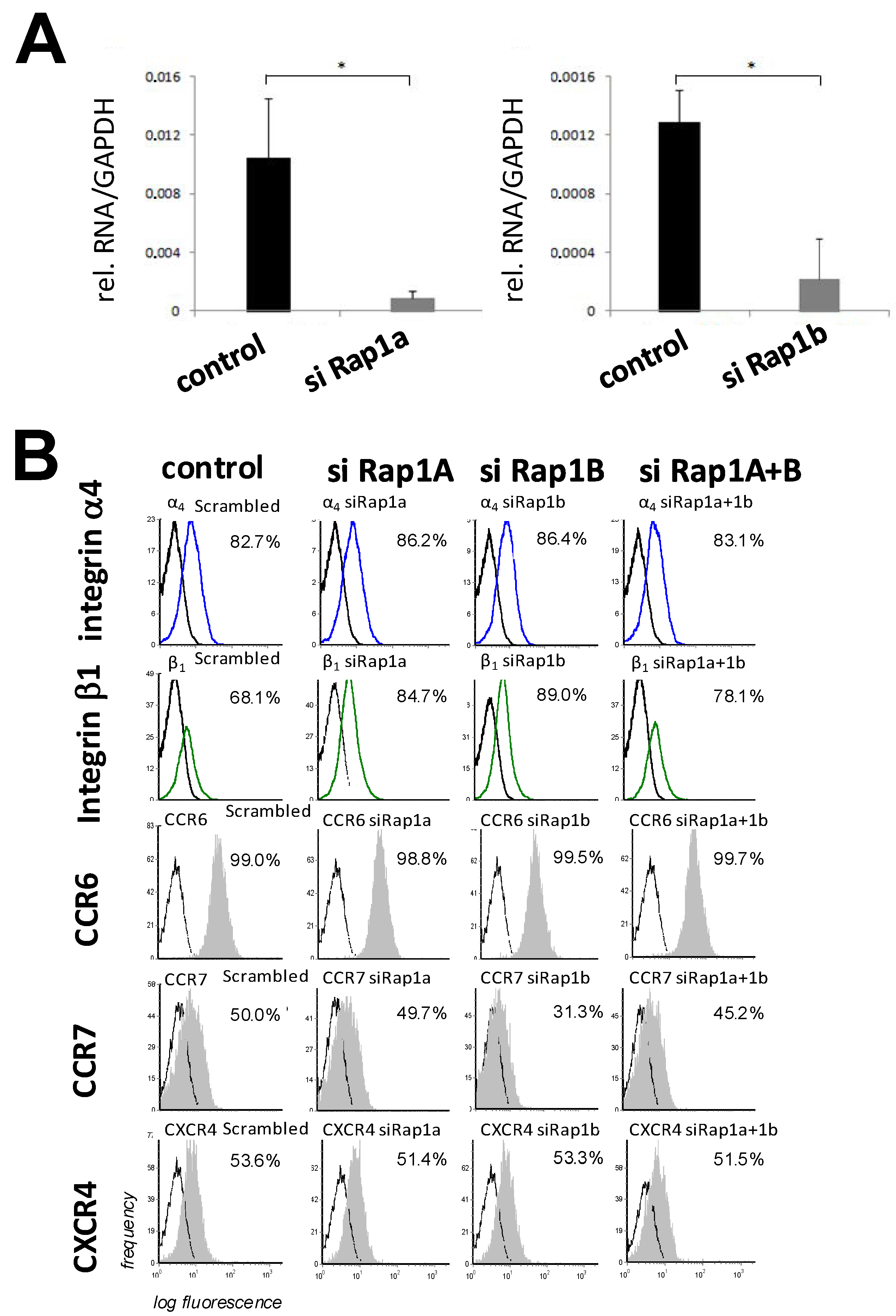
3.1. Expression and Regulation of GTPase Rap1 in MSCs
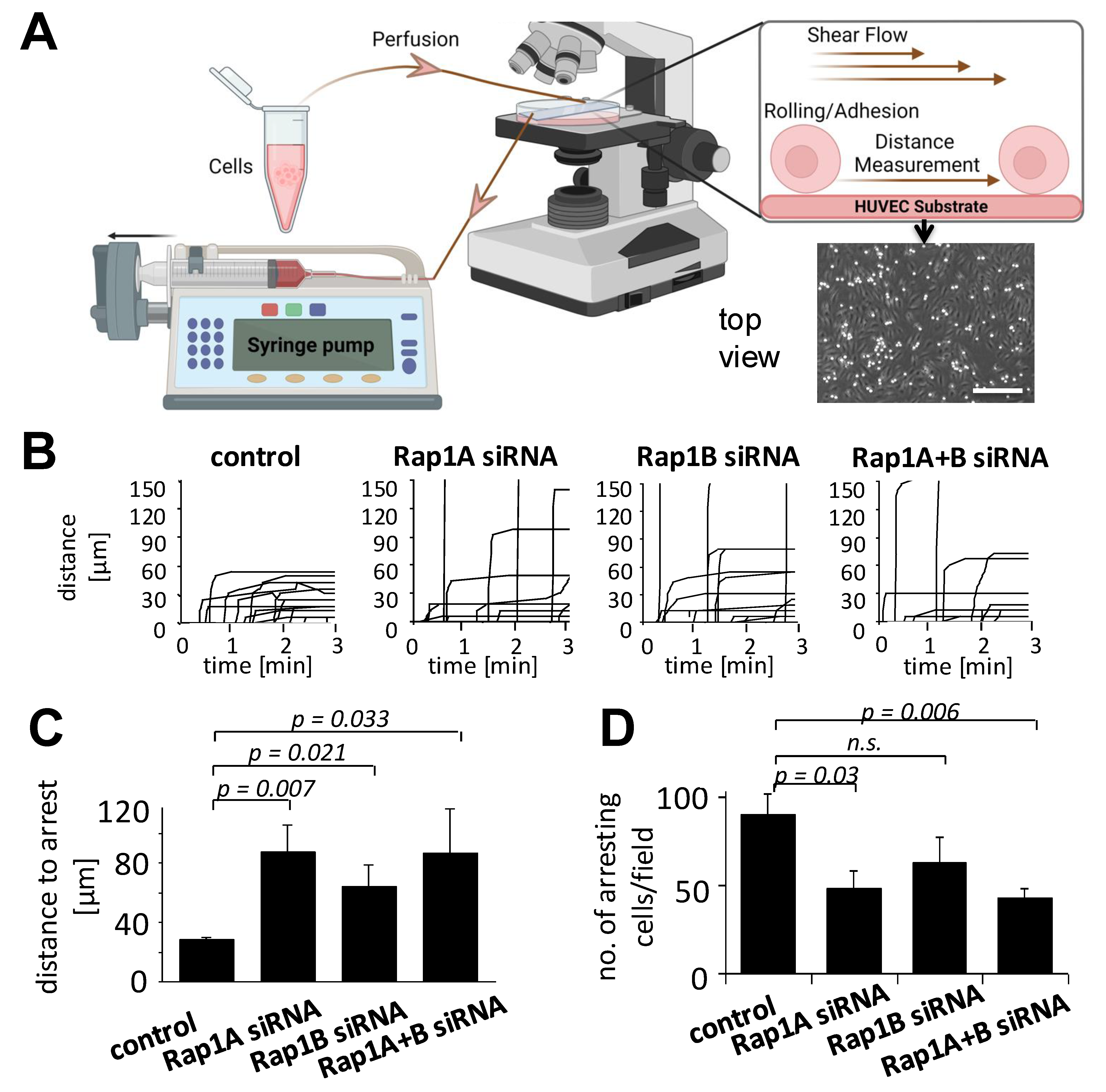
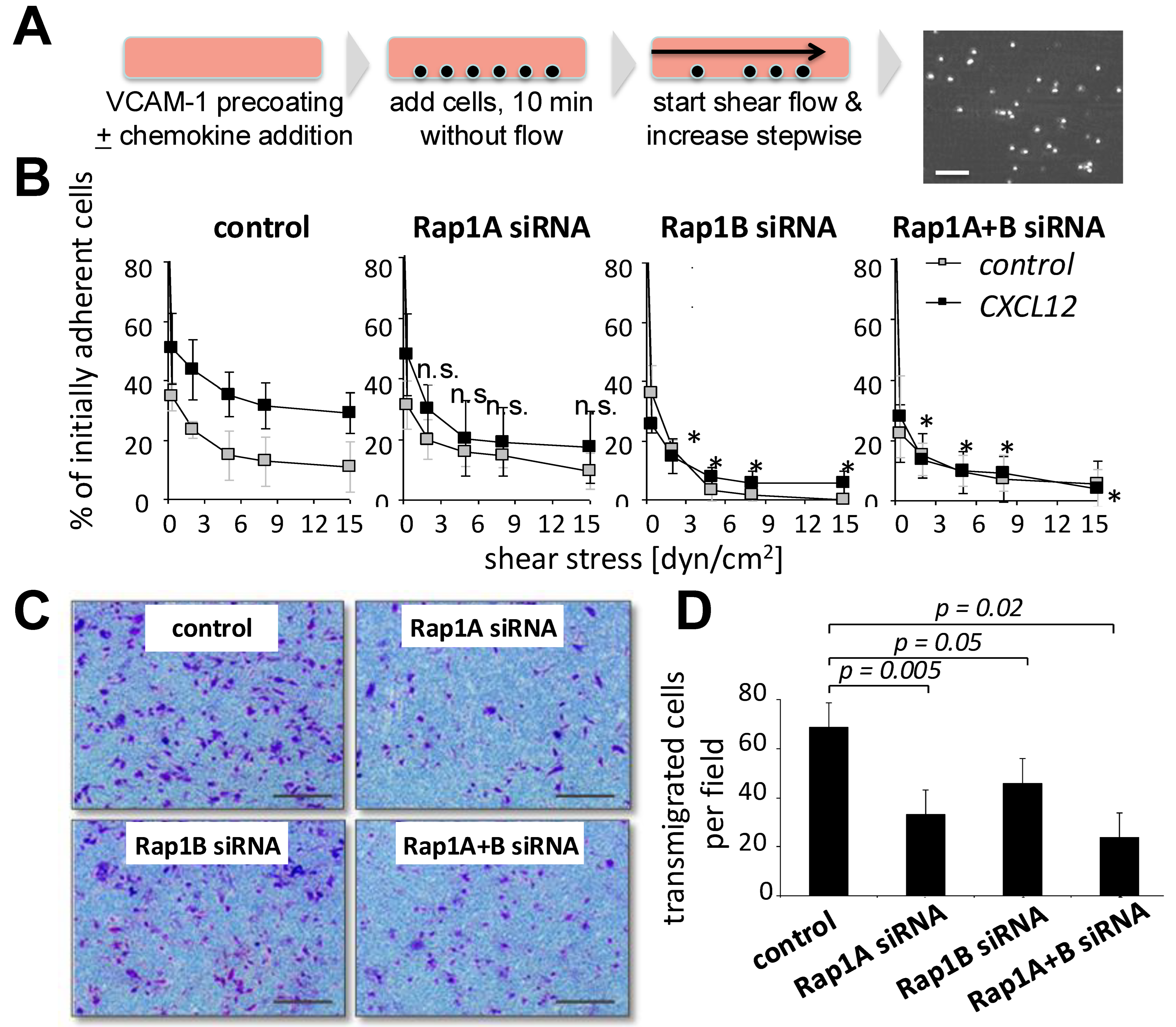
3.2. Downregulation of Rap1 GTPase Alters Adhesion Behavior of MSCs on HUVECs
3.3. Rap1 Regulates Migration and Firm Arrest of MSCs
3.4. Downregulation of Rap1A+B Affects Tissue Distribution of Fluorescence-Marked MSCs in Immune-Deficient Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barmada, A.; Sharan, J.; Band, N.; Rumschlag, T.; Yaqub, A.; Liebman, E.; Prodromos, C. Review of the Published Literature Confirms the Safety of Intravenous Infusion of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2023, 18, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Rüster, B.; Göttig, S.; Ludwig, R.J.; Bistrian, R.; Müller, S.; Seifried, E.; Gille, J.; Henschler, R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006, 108, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.M.; Teo, G.S.L. Mesenchymal Stem Cell Homing: The Devil Is in the Details. Cell Stem Cell 2008, 4, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Teo, G.S.; Ankrum, J.A.; Martinelli, R.; Boetto, S.E.; Simms, K.; Sciuto, T.E.; Dvorak, A.M.; Karp, J.M.; Carman, C.V. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells 2012, 30, 2472–2486. [Google Scholar] [CrossRef]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise Review: MSC Adhesion Cascade-Insights into Homing and Transendothelial Migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef]
- Gao, Q.; Xia, Y.; Liu, L.; Huang, L.; Liu, Y.; Zhang, X.; Xu, K.; Wei, J.; Hu, Y.; Mu, Y.; et al. Galectin-3 Enhances Migration of Minature Pig Bone Marrow Mesenchymal Stem Cells Through Inhibition of RhoA-GTP Activity. Sci. Rep. 2016, 24, 26577. [Google Scholar] [CrossRef]
- Suila, H.; Hirvonen, T.; Kotovuori, A.; Ritamo, I.; Kerkelä, E.; Anderson, H.; Natunen, S.; Tuimala, J.; Laitinen, S.; Nystedt, J.; et al. Human umbilical cord blood-derived mesenchymal stromal cells display a novel interaction between P-selectin and galectin-1. Scand J. Immunol. 2014, 80, 12–21. [Google Scholar] [CrossRef]
- Sackstein, R. Glycoengineering of HCELL, the human bone marrow homing receptor: Sweetly programming cell migration. Ann. Biomed. Eng. 2012, 40, 766–776. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, R.C. The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Rev. 2012, 8, 243–250. [Google Scholar] [CrossRef]
- Ciuculescu, F.; Giesen, M.; Deak, E.; Lang, V.; Seifried, E.; Henschler, R. Variability in chemokine-induced adhesion of human mesenchymal stromal cells. Cytotherapy 2011, 13, 1172–1179. [Google Scholar] [CrossRef]
- Semon, J.A.; Nagy, L.H.; Llamas, C.B.; Tucker, H.A.; Lee, R.H.; Prockop, D.J. Integrin expression and integrin-mediated adhesion in vitro of human multipotent stromal cells (MSCs) to endothelial cells from various blood vessels. Cell Tissue Res. 2010, 341, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Simmons, P.J.; Graves, S.E.; Robey, P.G. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone 2001, 28, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zwolanek, D.; Flicker, M.; Kirstätter, E.; Zaucke, F.; van Osch, G.J.; Erben, R.G. β1 Integrins Mediate Attachment of Mesenchymal Stem Cells to Cartilage Lesions. Biores. Open Access 2015, 4, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, P.; Guo, F.; Zhang, M.; Yan, Y.; Huang, M.; Chen, Y.; Zhang, L.; Zhang, L. mDia1 and Cdc42 Regulate Activin B-Induced Migration of Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells 2019, 37, 150–162. [Google Scholar] [CrossRef]
- Ge, J.; Burnier, L.; Adamopoulou, M.; Kwa, M.Q.; Schaks, M.; Rottner, K.; Brakebusch, C. RhoA, Rac1, and Cdc42 differentially regulate αSMA and collagen I expression in mesenchymal stem cells. J. Biol. Chem. 2018, 293, 9358–9369. [Google Scholar] [CrossRef]
- Melzer, M.; Niebert, S.; Heimann, M.; Ullm, F.; Pompe, T.; Scheiner-Bobis, G.; Burk, J. Differential Smad2/3 linker phosphorylation is a crosstalk mechanism of Rho/ROCK and canonical TGF-β3 signaling in tenogenic differentiation. Sci. Rep. 2024, 14, 10393. [Google Scholar] [CrossRef]
- Li, C.; Zhen, G.; Chai, Y.; Xie, L.; Crane, J.L.; Farber, E.; Farber, C.R.; Luo, X.; Gao, P.; Cao, X.; et al. RhoA determines lineage fate of mesenchymal stem cells by modulating CTGF-VEGF complex in extracellular matrix. Nat. Commun. 2016, 7, 11455. [Google Scholar] [CrossRef]
- Jang, M.W.; Yun, S.P.; Park, J.H.; Ryu, J.M.; Lee, J.H.; Han, H.J. Cooperation of Epac1/Rap1/Akt and PKA in prostaglandin E(2) -induced proliferation of human umbilical cord blood derived mesenchymal stem cells: Involvement of c-Myc and VEGF expression. J. Cell Physiol. 2012, 227, 3756–3767. [Google Scholar] [CrossRef]
- Zhang, Y.; Chiu, S.; Liang, X.; Gao, F.; Zhang, Z.; Liao, S.; Liang, Y.; Chai, Y.H.; Low, D.J.; Tse, H.F.; et al. Rap1-mediated nuclear factor-kappaB (NF-κB) activity regulates the paracrine capacity of mesenchymal stem cells in heart repair following infarction. Cell Death Discov. 2015, 1, 15007. [Google Scholar] [CrossRef]
- Lin, F.; Xue, D.; Xie, T.; Pan, Z. HMGB1 promotes cellular chemokine synthesis and potentiates mesenchymal stromal cell migration via Rap1 activation. Mol. Med. Rep. 2016, 14, 1283–1289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leibacher, J.; Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res. Ther. 2016, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, B.G.; Ruester, B.; Dressel, L.; Stein, S.; Grez, M.; Seifried, E.; Henschler, R. Rho inhibition induces migration of mesenchymal stromal cells. Stem Cells 2007, 25, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Duchniewicz, M.; Zemojtel, T.; Kolanczyk, M.; Grossmann, S.; Scheele, J.S.; Zwartkruis, F.J. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol. Cell. Biol. 2006, 26, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Leibacher, J.; Dauber, K.; Ehser, S.; Brixner, V.; Kollar, K.; Vogel, A.; Spohn, G.; Schäfer, R.; Seifried, E.; Henschler, R. Human mesenchymal stromal cells undergo apoptosis and fragmentation after intravenous application in immune-competent mice. Cytotherapy 2017, 19, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.L.; Deng, R.; Chung, S.K.; Chan, G.C. Epac Activation Regulates Human Mesenchymal Stem Cells Migration and Adhesion. Stem Cells 2016, 34, 948–959. [Google Scholar] [CrossRef]
- Bos, J.L.; de Rooij, J.; Reedquist, K.A. Rap1 signaling: Adhering to new models. The small GTPase Rap1 is a potent activation signal for beta1, beta2, and beta3 integrins and enhances cellular adhesion in both immune and nonimmune cells. Nat. Rev. Mol. Cell Biol. 2001, 2, 369–377. [Google Scholar] [CrossRef]
- Bivona, T.G.; Wiener, H.H.; Ahearn, I.M.; Silletti, J.; Chiu, V.K.; Philips, M.R. Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J. Cell Biol. 2004, 164, 461–470. [Google Scholar] [CrossRef]
- Ebisuno, Y.; Katagiri, K.; Katakai, T.; Ueda, Y.; Nemoto, T.; Inada, H.; Nabekura, J.; Okada, T.; Kannagi, R.; Tanaka, T.; et al. Rap1 controls lymphocyte adhesion cascade and interstitial migration within lymph nodes in RAPL-dependent and -independent manners. Blood 2010, 115, 804–814. [Google Scholar] [CrossRef]
- Carmona, G.; Göttig, S.; Orlandi, A.; Scheele, J.; Bäuerle, T.; Jugold, M.; Kiessling, F.; Henschler, R.; Zeiher, A.M.; Dimmeler, S.; et al. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood 2009, 113, 488–497. [Google Scholar] [CrossRef]
- Teo, G.S.; Yang, Z.; Carman, C.V.; Karp, J.M.; Lin, C.P. Intravital imaging of mesenchymal stem cell trafficking and association with platelets and neutrophils. Stem Cells 2015, 33, 265–277. [Google Scholar] [CrossRef]
- Masterson, C.H.; Tabuchi, A.; Hogan, G.; Fitzpatrick, G.; Kerrigan, S.W.; Jerkic, M.; Kuebler, W.M.; Laffey, J.G.; Curley, G.F. Intra-vital imaging of mesenchymal stromal cell kinetics in the pulmonary vasculature during infection. Sci. Rep. 2021, 11, 5265. [Google Scholar] [CrossRef] [PubMed]
- Alon, R.; Rose, D.M.; Ginsberg, M.H. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol. Rev. 2007, 218, 126–134. [Google Scholar]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Mazo, I.B.; Gutierrez-Ramos, J.C.; Frenette, P.S.; Hynes, R.O.; Wagner, D.D.; von Andrian, U.H. Hematopoietic progenitor cell rolling in bone marrow microvessels: Parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J. Exp. Med. 1998, 188, 465–474. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giesen, M.; Fleck, E.; Scheele, J.; Hartmann, T.N.; Henschler, R. Rap1 Guanosine Triphosphate Hydrolase (GTPase) Regulates Shear Stress-Mediated Adhesion of Mesenchymal Stromal Cells. Biology 2025, 14, 96. https://doi.org/10.3390/biology14010096
Giesen M, Fleck E, Scheele J, Hartmann TN, Henschler R. Rap1 Guanosine Triphosphate Hydrolase (GTPase) Regulates Shear Stress-Mediated Adhesion of Mesenchymal Stromal Cells. Biology. 2025; 14(1):96. https://doi.org/10.3390/biology14010096
Chicago/Turabian StyleGiesen, Melanie, Erika Fleck, Jürgen Scheele, Tanja Nicole Hartmann, and Reinhard Henschler. 2025. "Rap1 Guanosine Triphosphate Hydrolase (GTPase) Regulates Shear Stress-Mediated Adhesion of Mesenchymal Stromal Cells" Biology 14, no. 1: 96. https://doi.org/10.3390/biology14010096
APA StyleGiesen, M., Fleck, E., Scheele, J., Hartmann, T. N., & Henschler, R. (2025). Rap1 Guanosine Triphosphate Hydrolase (GTPase) Regulates Shear Stress-Mediated Adhesion of Mesenchymal Stromal Cells. Biology, 14(1), 96. https://doi.org/10.3390/biology14010096






