Morphological, Physiological, and Transcriptional Changes in Crocus sativus L. Under In Vitro Polyethylene Glycol-Induced Water Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Induction of Drought Stress
2.3. Analysis of Plant Biomass
2.4. Assessment of RWC
2.5. Electrolyte Leakage
2.6. Measurement of Chlorophylls, Carotenoids, and Chlorophyll Fluorescence
Chl b—(22.9 × A645 − 4.68 × A663) × V × W/1000
Carotenoids—1000 × A470 − 3.29 × Chl a − (104 × Chl b)/198
V = volume of extracted solution in mL, W = weight of fresh sample (g)
2.7. Proline Estimation
2.8. H2O2 and Lipid Peroxidation Estimation
2.9. Enzymatic and Non-Enzymatic Antioxidant Activity
2.10. RNA Isolation and PCR Evaluation
2.11. Data Analysis
3. Results
3.1. Establishment of Multiple Shoot Culture
3.2. Assessing Morphological and Physiological Adaptations to Drought Stress
3.3. Evaluating the Effects on Lipid Peroxidation and Membrane Damage
3.4. H2O2 Determination
3.5. Effect of Proline Content
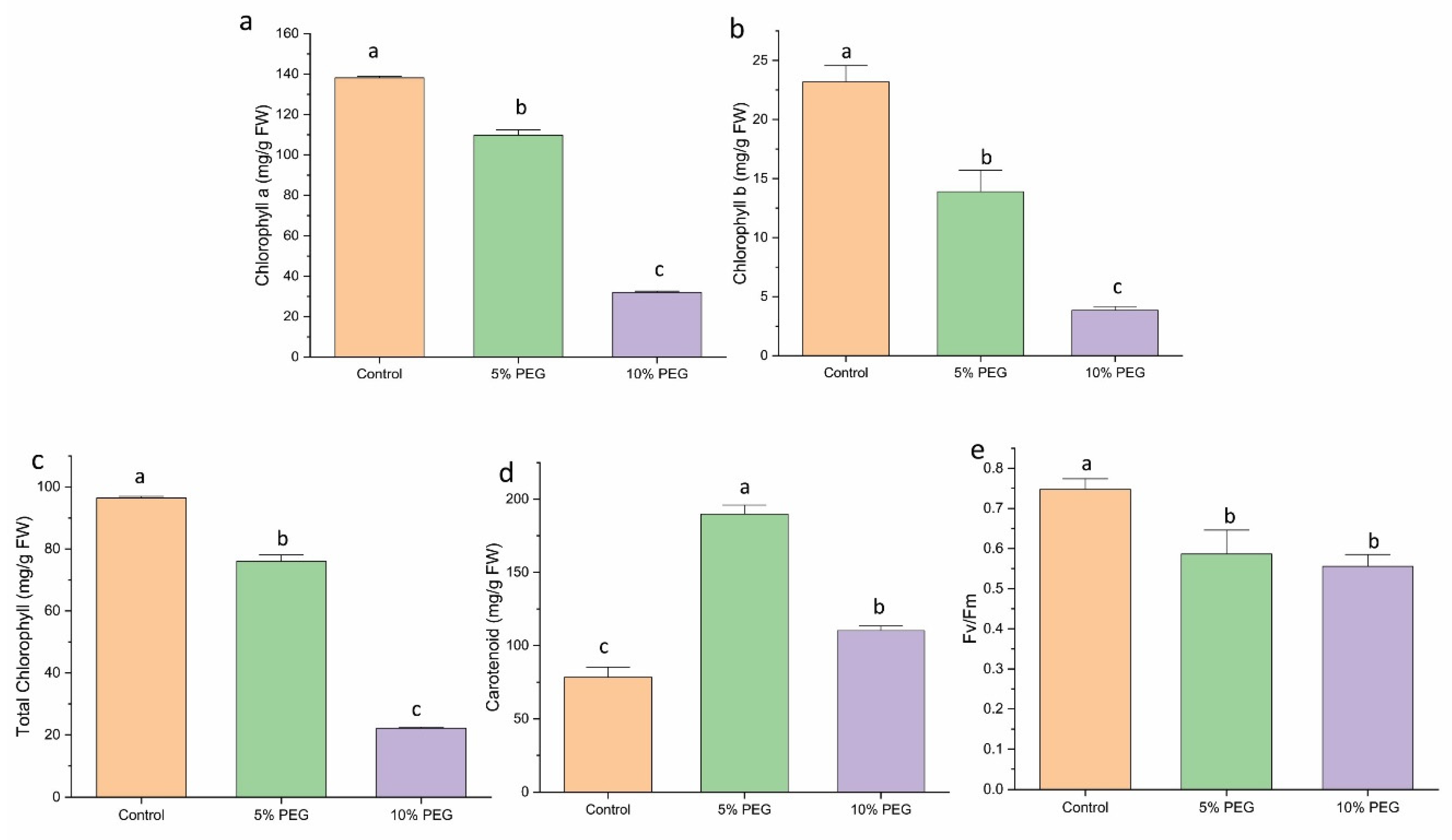
3.6. Effect of PEG on Chlorophyll, Carotenoid, and Chlorophyll Fluorescence
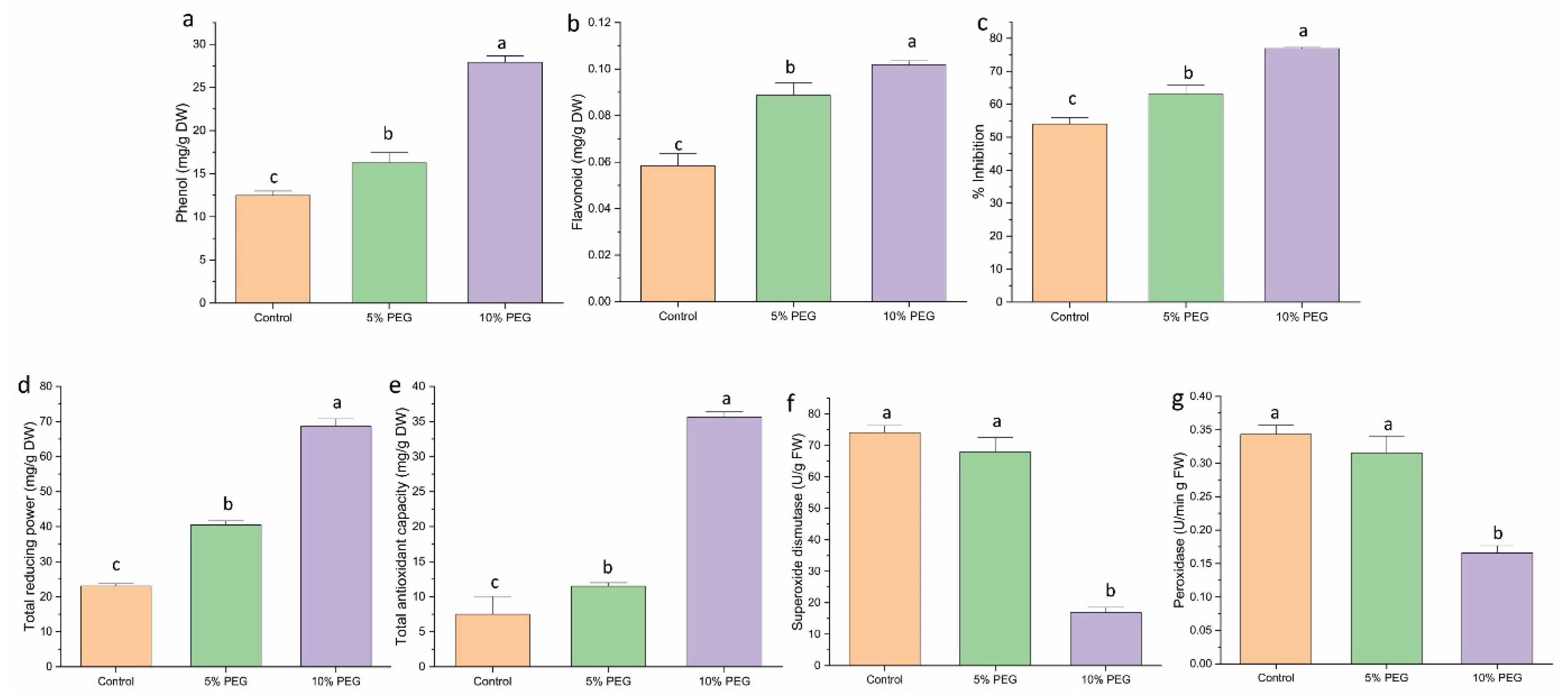
3.7. Effect on Antioxidant Activity
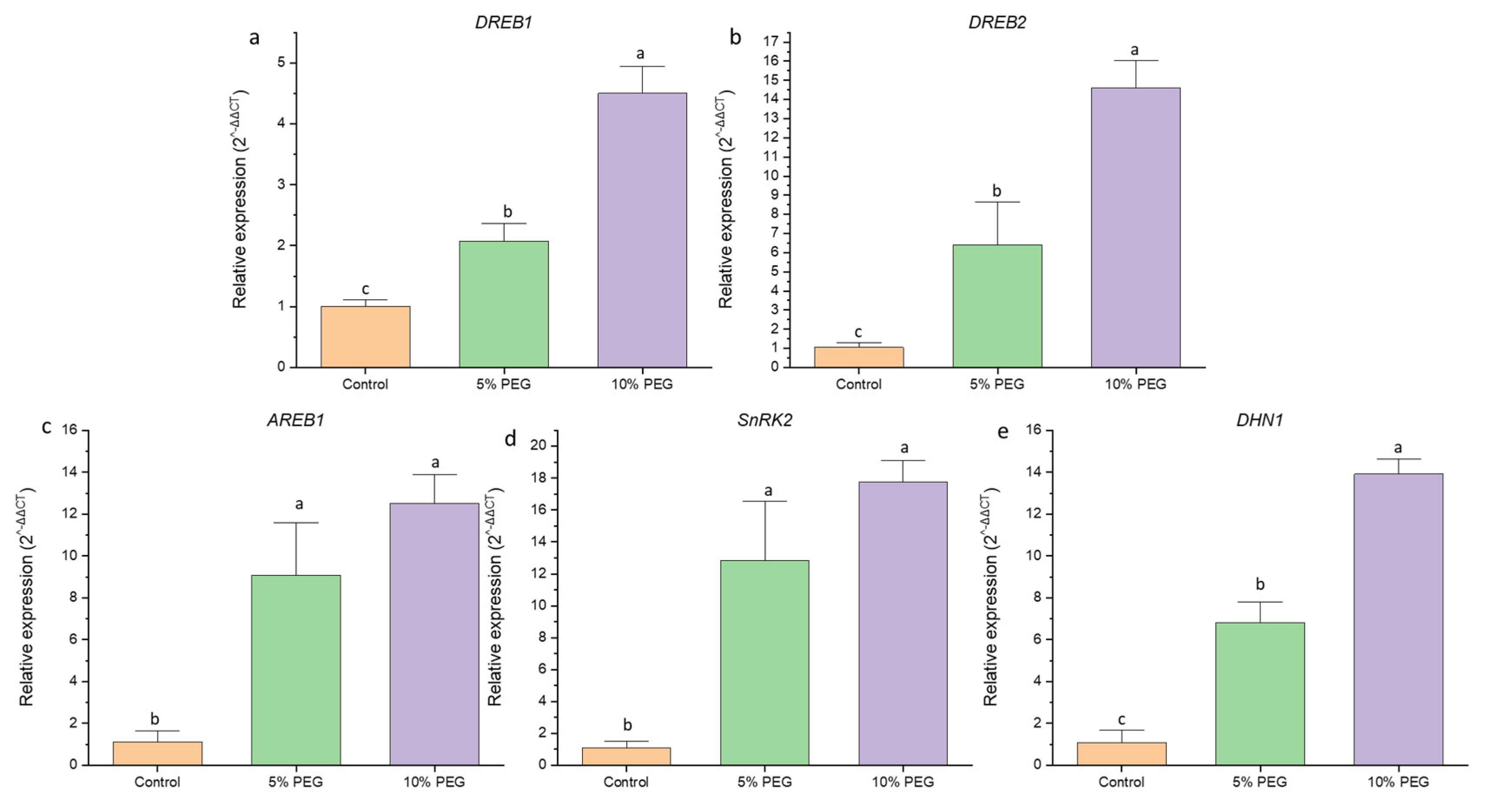
3.8. Alteration in Gene Expression Under Drought Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbasi, T.; Abbasi, S.A. Biomass energy and the environmental impacts associated with its production and utilization. Renew. Sustain. Energy Rev. 2010, 14, 919–937. [Google Scholar] [CrossRef]
- Bajji, M.; Lutts, S.; Kinet, J.M. Physiological changes after exposure to and recovery from polyethylene glycol-induced water deficit in callus cultures issued from durum wheat (Triticum durum Desf.) cultivars differing in drought resistance. J. Plant. Physiol. 2000, 156, 75–83. [Google Scholar] [CrossRef]
- Mokhtari, N.; Majidi, M.M.; Mirlohi, A. Physiological and antioxidant responses of synthetic hexaploid wheat germplasm under drought. BMC. Plant. Biol. 2024, 24, 747. [Google Scholar] [CrossRef]
- Koocheki, A.; Karbasi, A.; Seyyedi, M. Some reasons for saffron yield loss over the last 30 years period. Saffron Agron. Technol. 2017, 5, 107–122. [Google Scholar]
- Sojasi Qidari, H.; Behrooz, Z. Analysis of the effects of change in cropping pattern due to drought on saffron production in rural areas of the Zebarkhan district villages. Rural Dev. Strateg. 2017, 4, 40–59. [Google Scholar]
- Feroze, S.M.; Baba, S.H.; Laitonjam, N.; Singh, R.; Thangjam, D. Saffron production depends on rainfall: Empirical evidence from Jammu & Kashmir. SKUAST J. Res. 2021, 23, 160–165. [Google Scholar]
- Maleki, M.; Ebrahimzade, H.; Gholami, M.; Niknam, V. The effect of drought stress and exogenous abscisic acid on growth, protein content and antioxidative enzyme activity in saffron (Crocus sativus L.). Afr. J. Biotechnol. 2011, 10, 9068–9075. [Google Scholar]
- Tavakoli, F.; Rafieiolhossaini, M.; Ravash, R. Effects of PEG and Nano-Silica Elicitors on Secondary Metabolites Production in Crocus sativus L. Russ. J. Plant Physiol. 2021, 68, 931–940. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Barker, A.V.; Hashemi, M. Physiology of medicinal and aromatic plants under drought stress. Crop J. 2024, 12, 330–339. [Google Scholar] [CrossRef]
- Devi, K.; Sharma, M.; Singh, M.; Singh Ahuja, P. In vitro cormlet production and growth evaluation under greenhouse conditions in saffron (Crocus sativus L.)—A commercially important crop. Eng. Life Sci. 2011, 11, 189–194. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Sergiev, I.; Alexieva, V.; Karanov, E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt. Rend. Acad. Bulg. Sci. 1997, 51, 121–124. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Kalita, P.; Tapan, B.K.; Pal, T.K.; Kalita, R. Estimation of total flavonoids content (TFC) and antioxidant activities of methanolic whole plant extract of Biophytum sensitivum Linn. J. Drug Deliv. Ther. 2013, 3, 33–37. [Google Scholar]
- Susanti, D.; Sirat, H.M.; Ahmad, F.; Ali, R.M.; Aimi, N.; Kitajima, M. Antioxidant and cytotoxic flavonoids from the flowers of Melastoma malabathricum L. Food Chem. 2007, 103, 710–716. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidant activities of products of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Pathi, K.M.; Tula, S.; Huda, K.M.K.; Srivastava, V.K.; Tuteja, N. An efficient and rapid regeneration via multiple shoot induction from mature seed derived embryogenic and organogenic callus of Indian maize (Zea mays L.). Plant Signal. Behav. 2013, 8, e25891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, S.; Wang, B.; Zhao, J. Physiological and biochemical changes in different drought-tolerant alfalfa (Medicago sativa L.) varieties under PEG-induced drought stress. Acta Physiol. Plant. 2018, 40, 25. [Google Scholar] [CrossRef]
- Aghaie, P.; Tafreshi, S.A.H.; Ebrahimi, M.A.; Haerinasab, M. Tolerance evaluation and clustering of fourteen tomato cultivars grown under mild and severe drought conditions. Sci. Hortic. 2018, 232, 1–12. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, S.; Liu, Z.; Yang, F.; Yin, G. Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. J. Plant Physiol. 2019, 232, 226–240. [Google Scholar] [CrossRef]
- Iwuala, E.; Odjegba, V.; Sharma, V.; Alam, A. Drought stress modulates expression of aquaporin gene and photosynthetic efficiency in Pennisetum glaucum (L.) R. Br. genotypes. Curr. Plant Biol. 2020, 21, 100131. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, S.T.; He, N.Y.; Wang, Q.L.; Zhao, Y.; Gao, W.; Guo, F.Q. Nuclear-encoded synthesis of the D1 subunit of photosystem II increases photosynthetic efficiency and crop yield. Nat. Plants 2020, 6, 570–580. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhuang, Y.; Wang, X.G.; Wang, X.D. Evaluation of growth, physiological response, and drought resistance of different flue-cured tobacco varieties under drought stress. Front. Plant Sci. 2024, 15, 1442618. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Yang, Y. PEG 6000-stimulated drought stress improves the attributes of in vitro growth, steviol glycosides production, and antioxidant activities in Stevia rebaudiana Bertoni. Plants 2020, 9, 1552. [Google Scholar] [CrossRef]
- Fan, H.F.; Ding, L.; Xu, Y.L.; Du, C.X. Antioxidant system and photosynthetic characteristics responses to short-term PEG-induced drought stress in cucumber seedling leaves. Russ. J. Plant Physiol. 2017, 64, 162–173. [Google Scholar] [CrossRef]
- Bondok, A.E.T.; Mousa, W.M.; Rady, A.M.; Saad-Allah, K.M. Phenotypical, physiological and molecular assessment of drought tolerance of five Egyptian teosinte genotypes. J. Plant Interact. 2022, 17, 656–673. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Gu, W.; Chen, Z.; Gu, Y.; Pei, L.; Tian, R. Effect of drought on photosynthesis, total antioxidant capacity, bioactive component accumulation, and the transcriptome of Atractylodes lancea. BMC Plant Biol. 2021, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Thayale Purayil, F.; Rajashekar, B.S.; Kurup, S.; Cheruth, A.J.; Subramaniam, S.; Hassan Tawfik, N.; MA Amiri, K. Transcriptome profiling of Haloxylon persicum (Bunge ex Boiss and Buhse) an endangered plant species under PEG-induced drought stress. Genes 2020, 11, 640. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Wu, Y.; Luo, Q.; Zhang, Y.; Qiu, D.; He, G. TaSnRK2. 9, a sucrose non-fermenting 1-related protein kinase gene, positively regulates plant response to drought and salt stress in transgenic tobacco. Front. Plant Sci. 2019, 9, 2003. [Google Scholar] [CrossRef]

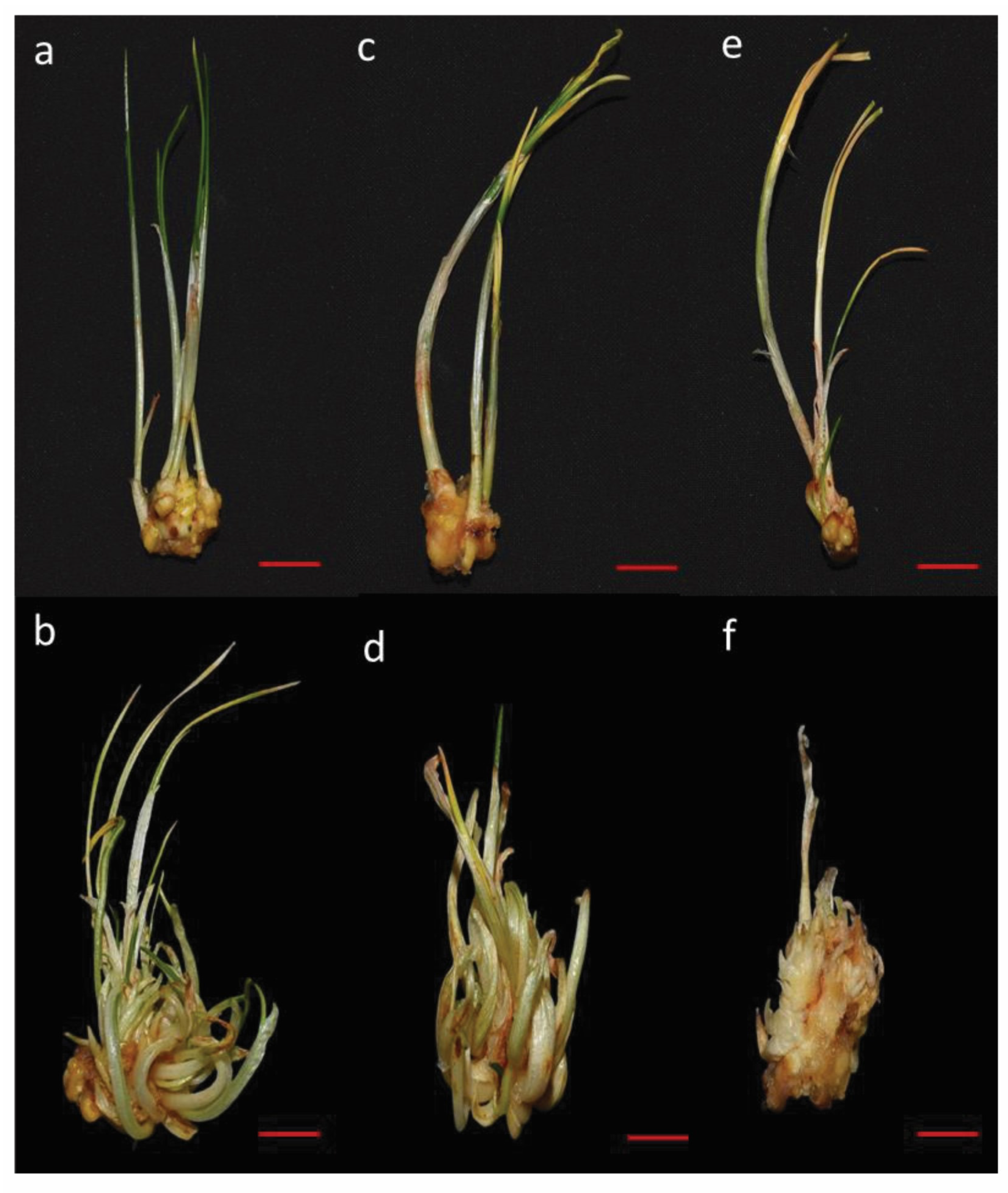

| Medium | BAP (mg/L) | NAA (mg/L) | Metatopolin (mg/L) | Response After 1 Month |
|---|---|---|---|---|
| M1 | 1 | - | - | Delayed growth and browning of shoots |
| M2 | 2 | - | - | Delayed growth and browning of shoots |
| M3 | 3 | - | - | Delayed growth and browning of shoots |
| M4 | 4 | - | - | Delayed growth and browning of shoots |
| M5 | 5 | - | - | Delayed growth and browning of shoots |
| M6 | 6 | - | - | Healthy multiple shoots |
| M7 | 6 | 0.2 | - | Healthy multiple shoots |
| M8 | 6 | 0.4 | - | Healthy multiple shoots |
| M9 | 6 | 0.6 | - | Healthy multiple shoots |
| M10 | 6 | 0.8 | - | Healthy multiple shoots |
| M11 | 6 | 1 | - | Healthy multiple shoots with increased leaf length |
| M12 | - | - | 0.25 | No response |
| M13 | - | - | 0.50 | No response |
| M14 | - | - | 0.75 | No response |
| M15 | - | - | 1 | No response |
| Gene | Sequence | Length (bp) | Product Size | Annealing Temperature (°C) |
|---|---|---|---|---|
| AREB1_F | TTCGACGAGTTCCAGAGCAC | 20 | 87 | 60 |
| AREB1_R | CGTCCACACGTTCCGTAGAA | 20 | ||
| DHN1_F | GGTGGCCACAAGTCGGA | 17 | 50 | 60 |
| DHN1_R | TCTTGTCCGTAGTCGTATCTGT | 22 | ||
| DREB1_F | TCCTCCTACATGACCGTCTC | 20 | 70 | 60 |
| DREB1_R | GGGTCTCGTGGAACTTGGT | 19 | ||
| DREB2_F | CACAATGCCGTCGACAAGAAG | 20 | 79 | 60 |
| DREB2_R | AGCCCTTTCTTGATTTCCGC | 20 | ||
| SnRK2_F | CTACGTGCTCCGTCACCTTT | 20 | 87 | 60 |
| SnRK2_R | TTGACGAGGCACGAGAACAG | 20 | ||
| Tubulin_F | CGTGCGTTTGTTCACTGGTA | 20 | 104 | 60 |
| Tubulin_R | CCCACCTCTTCGTAATCCTTC | 21 | ||
| 18srRNA_F | TGTTATTGCCTCAGCCTTCC | 20 | 133 | 60 |
| 18srRNA_R | GCGGTTTCTCTGGTTAATTCC | 21 |
| Biomass | Shoot FW (g) | Shoot DW (g) | FW/DW | |
|---|---|---|---|---|
| Control | 2.44 ± 0.13 a | 2.69 ± 0.15 a | 0.25 ± 0.02 a | 10.65 ± 0.69 a |
| 5% PEG | 2.29 ± 0.05 a | 2.57 ± 0.09 a | 0.28 ± 0.04 a | 9.49 ± 1.2 ab |
| 10% PEG | 1.48 ± 0.19 b | 1.80 ± 0.19 b | 0.32 ± 0.02 a | 5.65 ± 0.62 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusain, S.; Joshi, R. Morphological, Physiological, and Transcriptional Changes in Crocus sativus L. Under In Vitro Polyethylene Glycol-Induced Water Stress. Biology 2025, 14, 78. https://doi.org/10.3390/biology14010078
Gusain S, Joshi R. Morphological, Physiological, and Transcriptional Changes in Crocus sativus L. Under In Vitro Polyethylene Glycol-Induced Water Stress. Biology. 2025; 14(1):78. https://doi.org/10.3390/biology14010078
Chicago/Turabian StyleGusain, Suman, and Rohit Joshi. 2025. "Morphological, Physiological, and Transcriptional Changes in Crocus sativus L. Under In Vitro Polyethylene Glycol-Induced Water Stress" Biology 14, no. 1: 78. https://doi.org/10.3390/biology14010078
APA StyleGusain, S., & Joshi, R. (2025). Morphological, Physiological, and Transcriptional Changes in Crocus sativus L. Under In Vitro Polyethylene Glycol-Induced Water Stress. Biology, 14(1), 78. https://doi.org/10.3390/biology14010078







