The Combination Effect of Purpureocillium lilacinum Strain (AUMC 10620) and Avermectin (B1a and B1b) on Control Citrus Nematode Tylenchulus semipenetrans (Cobb) Under Laboratory and Field Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Extraction of Citrus Nematode Second-Stage Juveniles (J2)
2.3. Preparation of Root Samples for Nematode Egg Extraction
2.4. Preparation of Avermectin and P. lilacinum for Testing on J2 and Eggs
2.5. The Field Experiment
2.6. Statistic Analysis
3. Results
3.1. Efficacy of the Avermectin and P. lilacinum Mixture on Citrus Nematode (J2) In Vitro
3.2. The Effect of the Tested Mixtures on the Egg Hatching of Citrus Nematodes In Vitro
3.3. The Efficacy of Combination Treatment (Avermectin and P. lilacinum) Against T. semipenetrans Under Field Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd-Elgawad, M.M.M.; Koura, F.F.H.; Montasser, S.A.; Hammam, M.M.A. Distribution and losses of Tylenchulus semipenetrans in citrus orchards on reclaimed land in Egypt. Nematology 2016, 18, 1141–1150. [Google Scholar] [CrossRef]
- El-Marzoky, A.; Salem, A.; Mahrous, M.; Basha, A. Plant parasitic nematodes infesting Citrus orchards in Sharkia Governorate, east delta, Egypt with special reference to Taylenchulus semipenetrans COBB. Zagazig J. Agric. Res. 2009, 36, 195–211. [Google Scholar]
- Zoubi, B.; Mokrini, F.; Rafya, M.; Benkebboura, A.; Akachoud, O.; Ghoulam, C.; Housseini, A.I.; Qaddoury, A. Citrus rootstocks vs. nematodes: A battle for resistance against Tylenchulus semipenetrans. Sci. Hortic. 2024, 331, 113115. [Google Scholar] [CrossRef]
- Afzal, M.U. Synergism of citrus nematode (Tylenchulus semipenetrans Cobb.) and Colletotrichum gloeosporioides plays a major role in citrus dieback. Pak. J. Agric. Sci. 2021, 58, 1291–1299. [Google Scholar] [CrossRef]
- Sasser, J.N. Plant-Parasitic Nematodes: The Farmer’s Hidden Enemy; North Carolina State University: Raleigh, NC, USA, 1989. [Google Scholar]
- Hagag, E.S. Pathogenicity of Citrus Nematode (Tylenchulus semipenetrans). In Nematode-Plant Interactions and Controlling Infection; IGI Global: Hershey, PA, USA, 2023; pp. 142–164. [Google Scholar]
- Wilson, M.J.; Jackson, T.A. Progress in the commercialisation of bionematicides. BioControl 2013, 58, 715–722. [Google Scholar] [CrossRef]
- Ahmed, S.; Monjil, M.S. Effect of Paecilomyces lilacinus on tomato plants and the management of root knot nematodes. J. Bangladesh Agric. Univ. 2019, 17, 9–13. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Liu, F.; Pan, C. Enhancing the virulence of Paecilomyces lilacinus against Meloidogyne incognita eggs by overexpression of a serine protease. Biotechnol. Lett. 2010, 32, 1159–1166. [Google Scholar] [CrossRef]
- Isaac, G.; El-Deriny, M.; Taha, R. Efficacy of Purpureocillium lilacinum AUMC 10149 as biocontrol agent against root-knot nematode Meloidogyne incognita infecting tomato plant. Braz. J. Biol. 2021, 84, e253451. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.S.; Leong, S.C.T.; Pau, C.G.; Beattie, G.A.C. In Vitro Bioassay of Purpureocillium lilacinum and Bacillus thuringiensis for Control of Meloidogyne incognita on Black Pepper (Piper nigrum L.) in Sarawak, Malaysia, Northern Borneo. Gazi Entomolojik Arastirmalar Dern. 2021, 1, 41–59. [Google Scholar] [CrossRef]
- Youssef, M.; El-Ghonaimy, A.; El-Nagdi, W. Evaluation of some commercial bacterial biofertilizers and isolates against root knot nematode, Meloidogyne incognita infesting green bean, Phaseolus vulgaris. Sci. Agric. 2015, 10, 49–54. [Google Scholar]
- Sasanelli, N.; Toderas, I.; Veronico, P.; Iurcu-Straistaru, E.; Rusu, S.; Melillo, M.T.; Caboni, P. Abamectin Efficacy on the Potato Cyst Nematode Globodera pallida. Plants 2020, 9, 12. [Google Scholar] [CrossRef]
- Khalil, M.S. Abamectin and azadirachtin as eco-friendly promising biorational tools in integrated nematodes management programs. J. Plant Pathol. Microbiol. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- da Silva, M.P.; Tylka, G.L.; Munkvold, G.P. Seed Treatment Effects on Maize Seedlings Coinfected with Rhizoctonia solani and Pratylenchus penetrans. Plant Dis. 2017, 101, 957–963. [Google Scholar] [CrossRef]
- El-Marzoky, A.M.; Abdel-Hafez, S.H.; Sayed, S.; Salem, H.M.; El-Tahan, A.M.; El-Saadony, M.T. The effect of abamectin seeds treatment on plant growth and the infection of root-knot nematode Meloidogyne incognita (Kofoid and White) chitwood. Saudi J. Biol. Sci. 2022, 29, 970–974. [Google Scholar] [CrossRef]
- Ravichandra, N. Horticultural Nematology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 411. [Google Scholar]
- Hooper, D.J.; Hallmann, J.; Subbotin, S.A. Methods for extraction, processing and detection of plant and soil nematodes. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; CABI Publishing: Wallingford, UK, 2005; pp. 53–86. [Google Scholar]
- Van Bezooijen, J. Extraction from soil and other substrates. Methods Tech. Nematol. 2006, 118. [Google Scholar]
- Siddiqi, M.R. Tylenchida: Parasites of Plants and Insects; CABI: Delémont, Switzerland, 2000. [Google Scholar]
- Püntener, W. Manual for Field Trials in Plant Protection; Ciba-Geigy: Dover Township, NJ, USA, 1981. [Google Scholar]
- Colby, S. Calculating synergistic and antagonistic responses of herbicide combinations. Weeds 1967, 15, 20–22. [Google Scholar] [CrossRef]
- Hussey, R.; Barker, K. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Report. 1973, 57, 1025–1028. [Google Scholar]
- Sun, M.-H.; Gao, L.; Shi, Y.-X.; Li, B.-J.; Liu, X.-Z. Fungi and actinomycetes associated with Meloidogyne spp. eggs and females in China and their biocontrol potential. J. Invertebr. Pathol. 2006, 93, 22–28. [Google Scholar] [CrossRef]
- El-Marzoky, A.M.; Elnahal, A.S.; Jghef, M.M.; Abourehab, M.A.; El-Tarabily, K.A.; Ali, M.A. Purpureocillium lilacinum strain AUMC 10620 as a biocontrol agent against the citrus nematode Tylenchulus semipenetrans under laboratory and field conditions. Eur. J. Plant Pathol. 2023, 167, 59–76. [Google Scholar] [CrossRef]
- Castaño Zapata, J. Prácticas De Laboratorio De Fitopatología; Universidad de Caldas: Caldas, Colombia, 1998. [Google Scholar]
- Esparza-Diaz, G.; Villanueva, R.T.; Badillo-Vargas, I.E. A novel interaction of Nesidiocoris tenuis (hemiptera: Miridae) as a biological control agent of Bactericera cockerelli (hemiptera: Triozidae) in potato. Insects 2024, 15, 261. [Google Scholar] [CrossRef]
- Pertot, I.; Alabouvette, C.; Esteve, E.H.; França, S. Mini-paper—The use of microbial biocontrol agents against soil-borne diseases. Eip-Agri Focus Group Soil-Borne Dis. 2015, 1–11. [Google Scholar]
- Kepenekci, İ.; Toktay, H.; Oksal, E.; Buzboğa, R.; İmren, M. Effect of Purpureocillium lilacinum on root lesion nematode, Pratylenchus thornei. J. Agric. Sci. 2018, 24, 323–328. [Google Scholar] [CrossRef]
- Sarven, M.S.; Aminuzzaman, F.M.; Huq, M.E. Dose-response relations between Purpureocillium lilacinum PLSAU-1 and Meloidogyne incognita infecting brinjal plant on plant growth and nematode management: A greenhouse study. Egypt. J. Biol. Pest Control 2019, 29, 26. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Sabir, N.; El-Sheikh, M.A.; Alyemeni, M. Impact assessment of Karanja deoiled cake and sundried biogas slurry as a mixed substrate on the nematicidal potential of Purpureocillium lilacinum. J. King Saud Univ.–Sci. 2021, 33, 101399. [Google Scholar] [CrossRef]
- El-shahaat, M. Efficacy of three Bio-pesticide products and oxamyl against citrus nematode (Tylenchulus semipenetrans) and on productivity of Washington navel orange trees. Egypt. J. Hortic. 2018, 45, 275–287. [Google Scholar]
- El-Saedy, M.; Hammad, S.E.; Awd Allah, S. Nematicidal effect of abamectin, boron, chitosan, hydrogen peroxide and Bacillus thuringiensis against citrus nematode on Valencia orange trees. Plant Sci. Phytopathol. 2019, 3, 111–117. [Google Scholar]
- Khalil, M.S.; Darwesh, D.M. Avermectins: The promising solution to control plant parasitic nematodes. J. Plant Sci. Phytopathol. 2019, 3, 081–089. [Google Scholar] [CrossRef]
- Sasanelli, N.; Konrat, A.; Migunova, V.; Toderas, I.; Iurcu-Straistaru, E.; Rusu, S.; Bivol, A.; Andoni, C.; Veronico, P. Review on Control Methods against Plant Parasitic Nematodes Applied in Southern Member States (C Zone) of the European Union. Agriculture 2021, 11, 602. [Google Scholar] [CrossRef]
- El-Ashry, R.M.; Ali, M.A.S.; Elsobki, A.E.A.; Aioub, A.A.A. Integrated management of Meloidogyne incognita on tomato using combinations of abamectin, Purpureocillium lilacinum, rhizobacteria, and botanicals compared with nematicide. Egypt. J. Biol. Pest Control. 2021, 31, 93. [Google Scholar] [CrossRef]
- Khairy, D.; Refaei, A.; Mostafa, F. Management of Meloidogyne incognita infecting Eggplant Using Moringa Extracts, Vermicompost, and Two Commercial Bio-products. Egypt. J. Agronematol. 2021, 20, 1–16. [Google Scholar] [CrossRef]
- Kumar, K.K.; Arthurs, S. Recent advances in the biological control of citrus nematodes: A review. Biol. Control 2021, 157, 104593. [Google Scholar] [CrossRef]
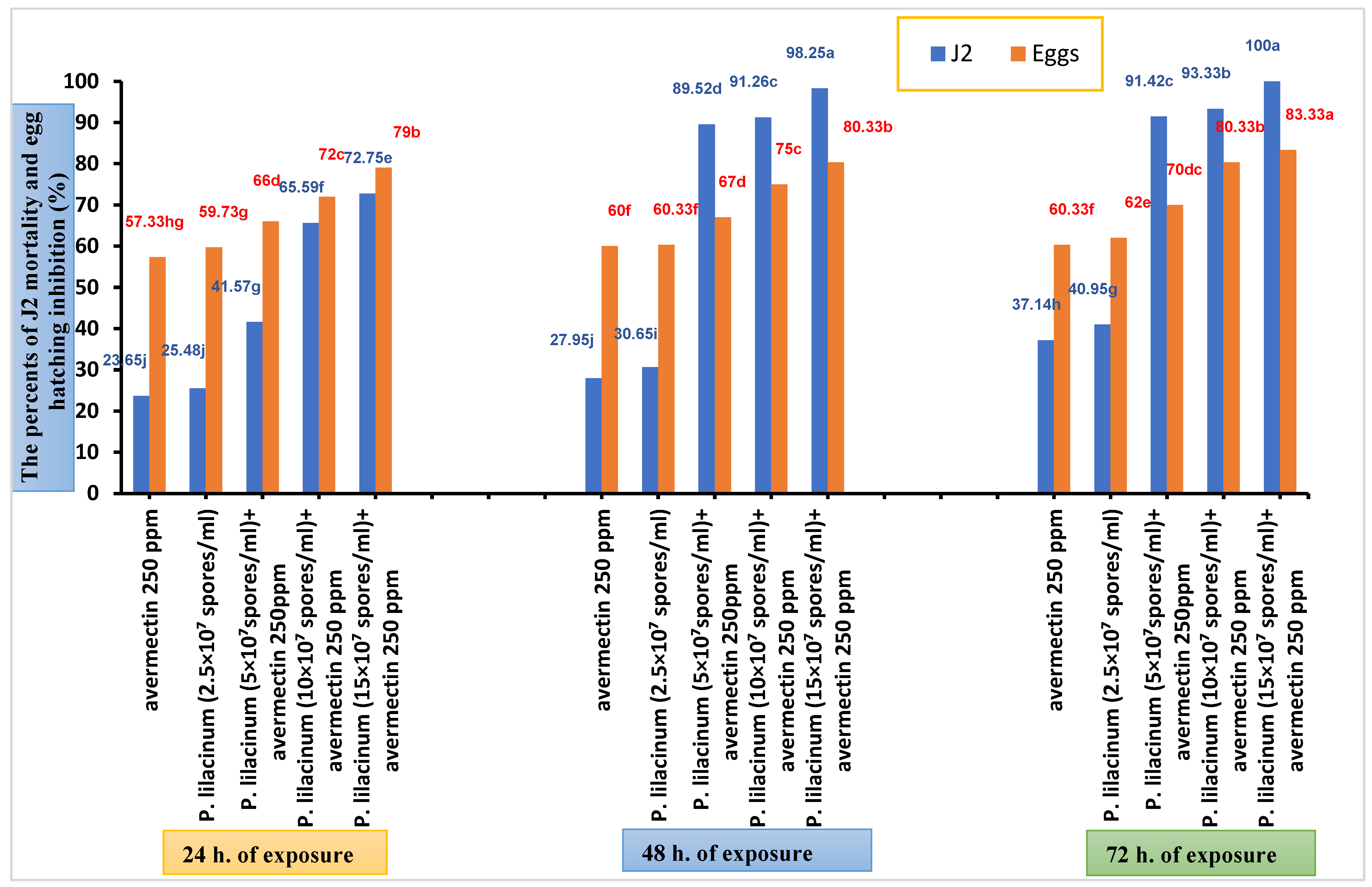
| Treatments | No. of Dead J2 After 24 h | No. of Dead J2 After 48 h | No. of Dead J2 After 72 h |
|---|---|---|---|
| Negative control (nematodes + distilled water) | 35.00 e ± 2.88 | 118.33 d ± 4.41 | 150.00 d ± 2.88 |
| Mortality percentage (%) | 0 | 0 | 0 |
| Nematodes + avermectin 250 ppm | 145.00 d ± 2.88 | 225.00 c ± 2.88 | 280.00 c ± 5.77 |
| Mortality percentage (%) | 23.65 | 27.95 | 37.14 |
| Nematodes + P. lilacinum (2.5 × 107 spores/mL) | 152.00 d ± 2.78 | 235.33 c ± 5.68 | 293.33 c ± 3.77 |
| Mortality percentage (%) | 25.48 | 30.65 | 40.95 |
| Nematodes + P. lilacinum (5 × 107spores/mL) + avermectin 250 ppm | 228.33 c ± 4.41 | 460.00 ab ± 23.09 | 470.00 ab ±2 0.81 |
| Mortality percentage (%) (SF) | 41.57 (0.96) | 89.52 (1.78) | 91.42 (1.45) |
| Nematodes + P. lilacinum (10 × 107 spores/mL) + avermectin 250 ppm | 340.00 b ± 5.77 | 466.67 ab ± 14.53 | 476.67 ab ± 17.63 |
| Mortality percentage (%) (SF) | 65.59 (1.52) | 91.26 (1.82) | 93.33 (1.48) |
| Nematodes + P. lilacinum (15 × 107 spores/mL) + avermectin 250 ppm | 373.33 a ± 18.55 | 493.33 a ± 3.33 | 500.00 a ± 0.00 |
| Mortality percentage (%) (SF) | 72.75 (1.68) | 98.25 (1.96) | 100.00 (1.59) |
| Treatments | No. of Hatched Eggs After 24 h | No. of Hatched Eggs After 48 h | No. of Hatched Eggs After 72 h |
|---|---|---|---|
| Negative control (Nematode eggs + distilled water) | 215.00 a ± 2.88 | 218.33 a ± 4.41 | 270.00 a ± 5.77 |
| Egg hatching rate (%) | 43.00 | 43.66 | 54.00 |
| Nematode eggs + avermectin 250 ppm | 213.33 b ± 17.63 | 200.00 b ± 5.77 | 198.33 a ± 6.00 |
| Egg hatching rate (%) | 42.66 | 40.00 | 39.66 |
| Nematode eggs + P. lilacinum (2.5 × 107 spores/ml | 201.33 b ± 7.63 | 198.33 b ± 7.00 | 190.00 a ± 4.33 |
| Egg hatching rate (%) | 40.26 | 39.66 | 38.00 |
| Nematode eggs + P. lilacinum (5 × 107spores/mL) + avermectin 250ppm | 170.00 c ± 5.77 | 165.00 c ± 2.88 | 150.00 b ± 5.77 |
| Egg hatching rate (%) | 34.00 | 33.00 | 30.00 |
| Nematode eggs + P. lilacinum (10 × 107 spores/mL) + avermectin 250 ppm | 140.00 cd ± 5.77 | 125.00 d ± 2.88 | 98.33 c ± 4.41 |
| Egg hatching rate (%) | 28.00 | 25.00 | 19.66 |
| Nematode eggs + P. lilacinum (15 × 107 spores/mL) + avermectin 250 ppm | 105.00 e ± 2.88 | 98.33 e ± 6.00 | 83.33 cd ± 4.41 |
| Egg hatching rate (%) | 21.00 | 19.66 | 16.66 |
| Treatments | Number of J2 in 250 g/Soil | ||
|---|---|---|---|
| One Week After Treatment | Two Weeks After Treatment | Three Weeks After Treatment | |
| Control | 2363.40 a | 2427.80 a | 2471.40 a |
| (0%) | (0%) | (0%) | |
| Oxamyl (10% G) | 1163.40 e | 802.00 e | 405.40 e |
| (50.77%) | (66.96%) | (83.59%) | |
| Avermectin (1000 ppm) | 2105.40 a | 1627.80 b | 1327.40 b |
| (10.92%) | (32.95%) | (46.29%) | |
| P. lilacinum (15 × 107 spores/mL) | 1963.40 ab | 1377.80 c | 1050.00 c |
| (16.92%) | (43.24%) | (57.51%) | |
| Avermectin + P. lilacinum (in the abovementioned concentrations) | 1623.40 d | 1127.80 d | 720.40 d |
| (31.31%) | (53.55%) | (70.85%) | |
| Treatments | Number of J2 in 250 g/Soil | ||
|---|---|---|---|
| One Week After Treatment | Two Weeks After Treatment | Three Weeks After Treatment | |
| Control | 2466.40 a | 2530.8 a | 2574.40 a |
| (0%) | (0%) | (0%) | |
| Oxamyl (10% G) | 1227.40 e | 866.00 e | 469.40 e |
| (50.33%) | (65.78%) | (81.76%) | |
| Avermectin (1000 ppm) | 2148.40 a | 1670.80 b | 1370.40 b |
| (12.89%) | (33.98%) | (46.76%) | |
| P. lilacinum (15 × 107 spores/mL) | 2014.40 ab | 1428.80 c | 1101.00 c |
| (18.33%) | (43.54%) | (57.23%) | |
| Avermectin + P. lilacinum (in the abovementioned concentrations) | 1655.40 d | 1159.80 d | 752.40 d |
| (32.88%) | (54.17%) | (70.77%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Marzoky, A.M.; Ali, M.A.M.S.; Elnahal, A.S.M.; Abuljadayel, D.A.; Alkherb, W.A.H.; Moustafa, M.; Alshaharni, M.O.; Abd El-Aal, E.M. The Combination Effect of Purpureocillium lilacinum Strain (AUMC 10620) and Avermectin (B1a and B1b) on Control Citrus Nematode Tylenchulus semipenetrans (Cobb) Under Laboratory and Field Conditions. Biology 2025, 14, 60. https://doi.org/10.3390/biology14010060
El-Marzoky AM, Ali MAMS, Elnahal ASM, Abuljadayel DA, Alkherb WAH, Moustafa M, Alshaharni MO, Abd El-Aal EM. The Combination Effect of Purpureocillium lilacinum Strain (AUMC 10620) and Avermectin (B1a and B1b) on Control Citrus Nematode Tylenchulus semipenetrans (Cobb) Under Laboratory and Field Conditions. Biology. 2025; 14(1):60. https://doi.org/10.3390/biology14010060
Chicago/Turabian StyleEl-Marzoky, Amr M., Mohamed A. M. S. Ali, Ahmed S. M. Elnahal, Dalia A. Abuljadayel, Wafa A. H. Alkherb, Mahmoud Moustafa, Mohammed O. Alshaharni, and Elsayed M. Abd El-Aal. 2025. "The Combination Effect of Purpureocillium lilacinum Strain (AUMC 10620) and Avermectin (B1a and B1b) on Control Citrus Nematode Tylenchulus semipenetrans (Cobb) Under Laboratory and Field Conditions" Biology 14, no. 1: 60. https://doi.org/10.3390/biology14010060
APA StyleEl-Marzoky, A. M., Ali, M. A. M. S., Elnahal, A. S. M., Abuljadayel, D. A., Alkherb, W. A. H., Moustafa, M., Alshaharni, M. O., & Abd El-Aal, E. M. (2025). The Combination Effect of Purpureocillium lilacinum Strain (AUMC 10620) and Avermectin (B1a and B1b) on Control Citrus Nematode Tylenchulus semipenetrans (Cobb) Under Laboratory and Field Conditions. Biology, 14(1), 60. https://doi.org/10.3390/biology14010060






