The History of the Brazilian Sardine (Sardinella brasiliensis) Between Two Fishery Collapses: An Ecosystem Modeling Approach to Study Its Life Cycle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
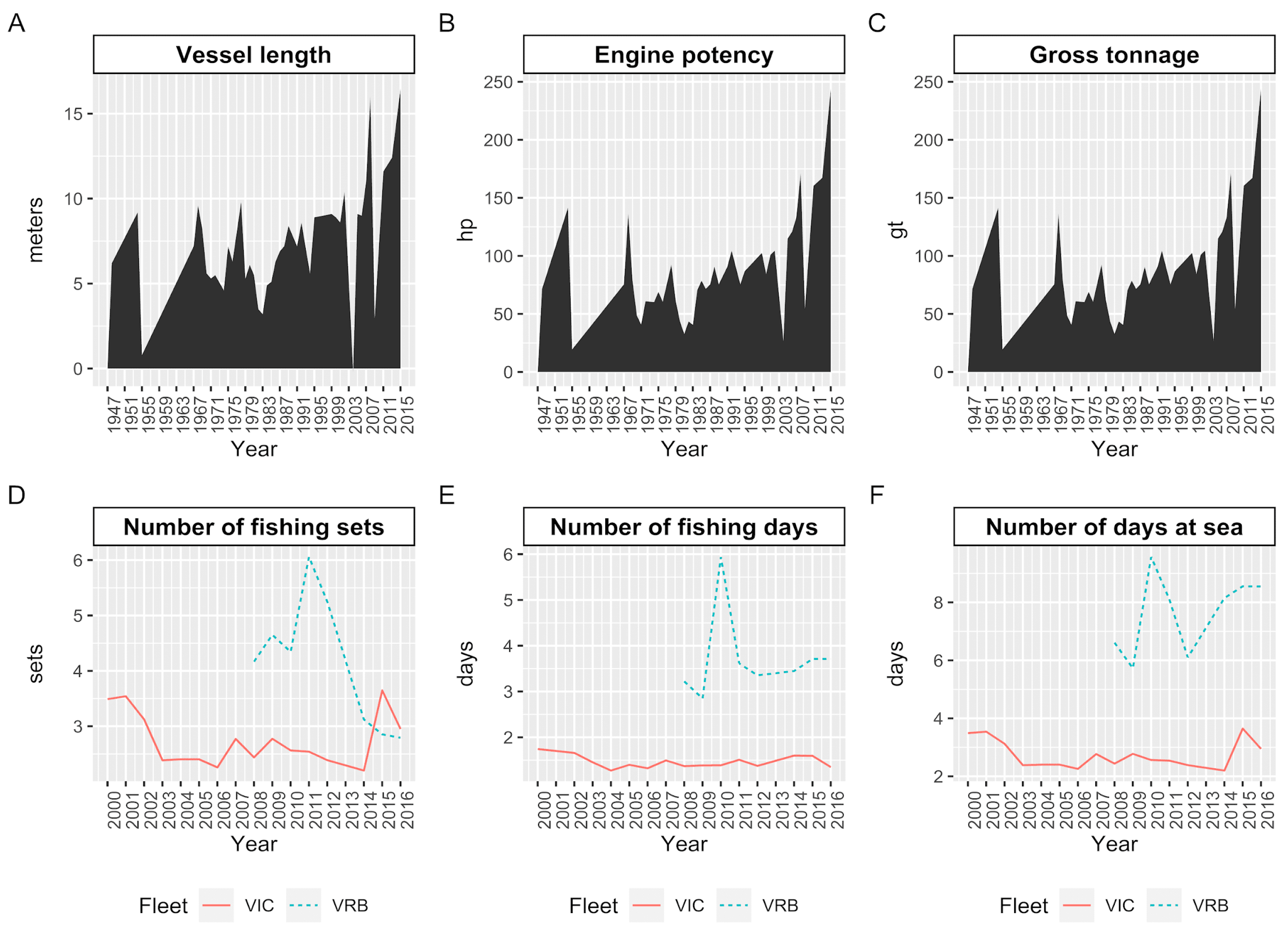
2.1. Study Area, Biological, and Fishing Data
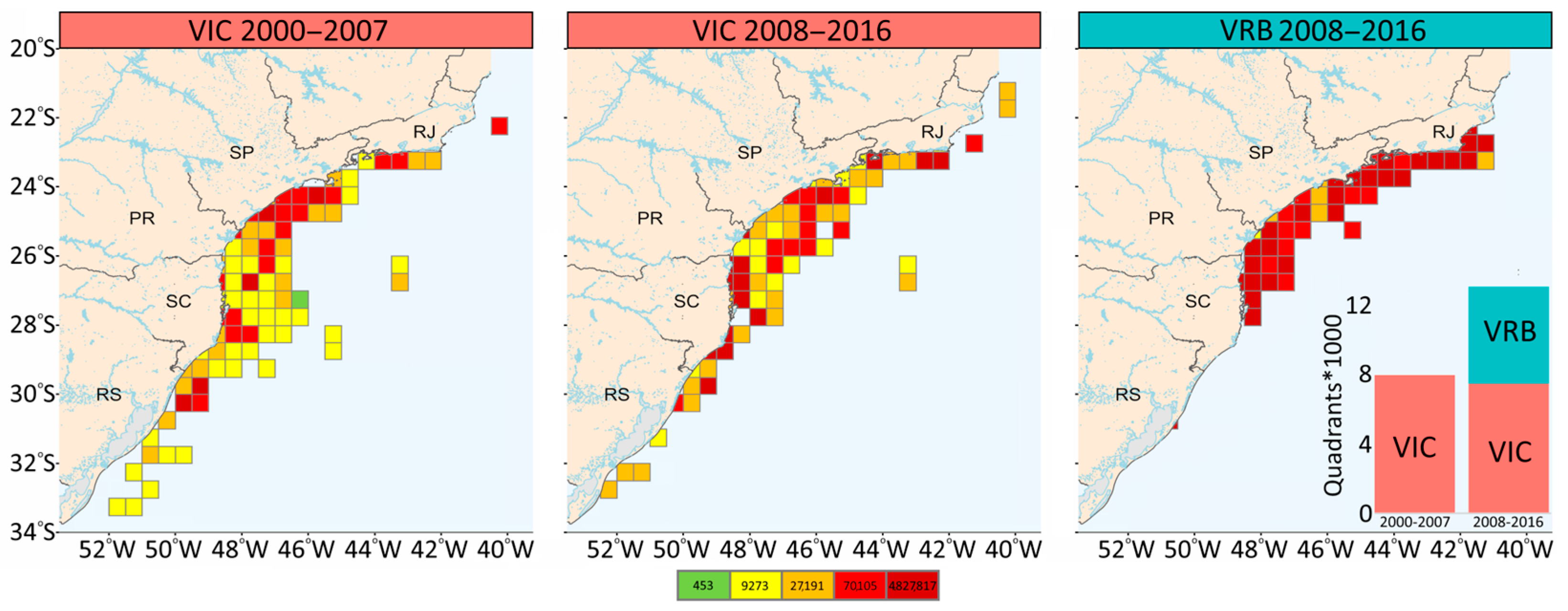
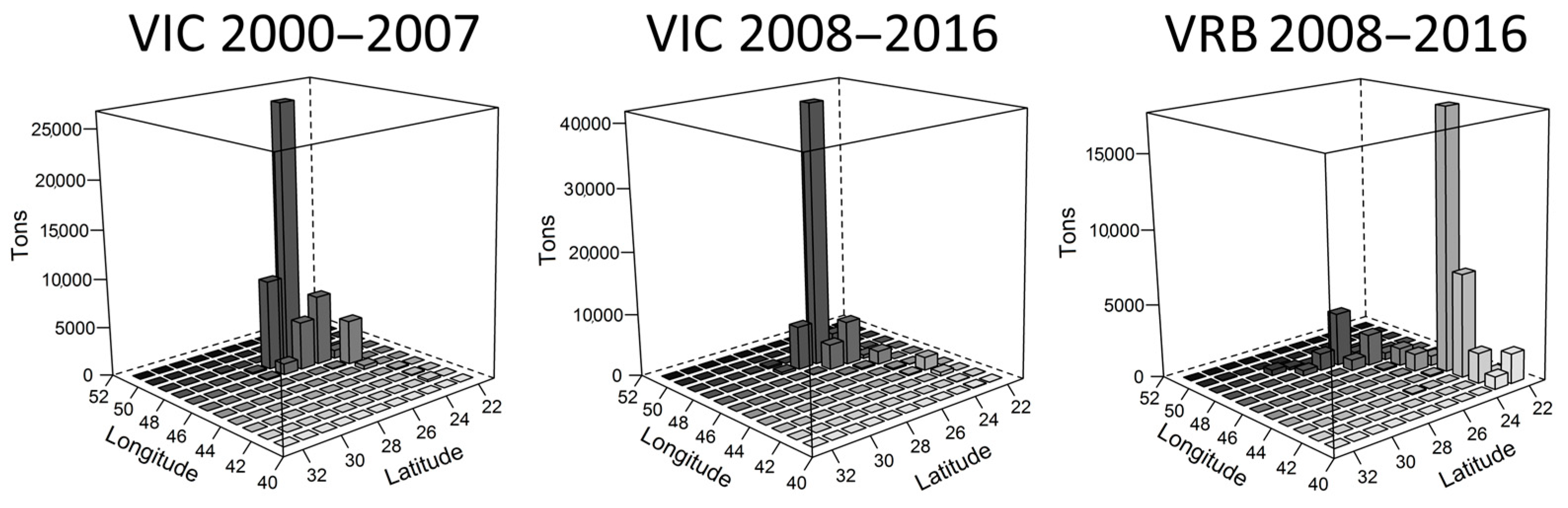
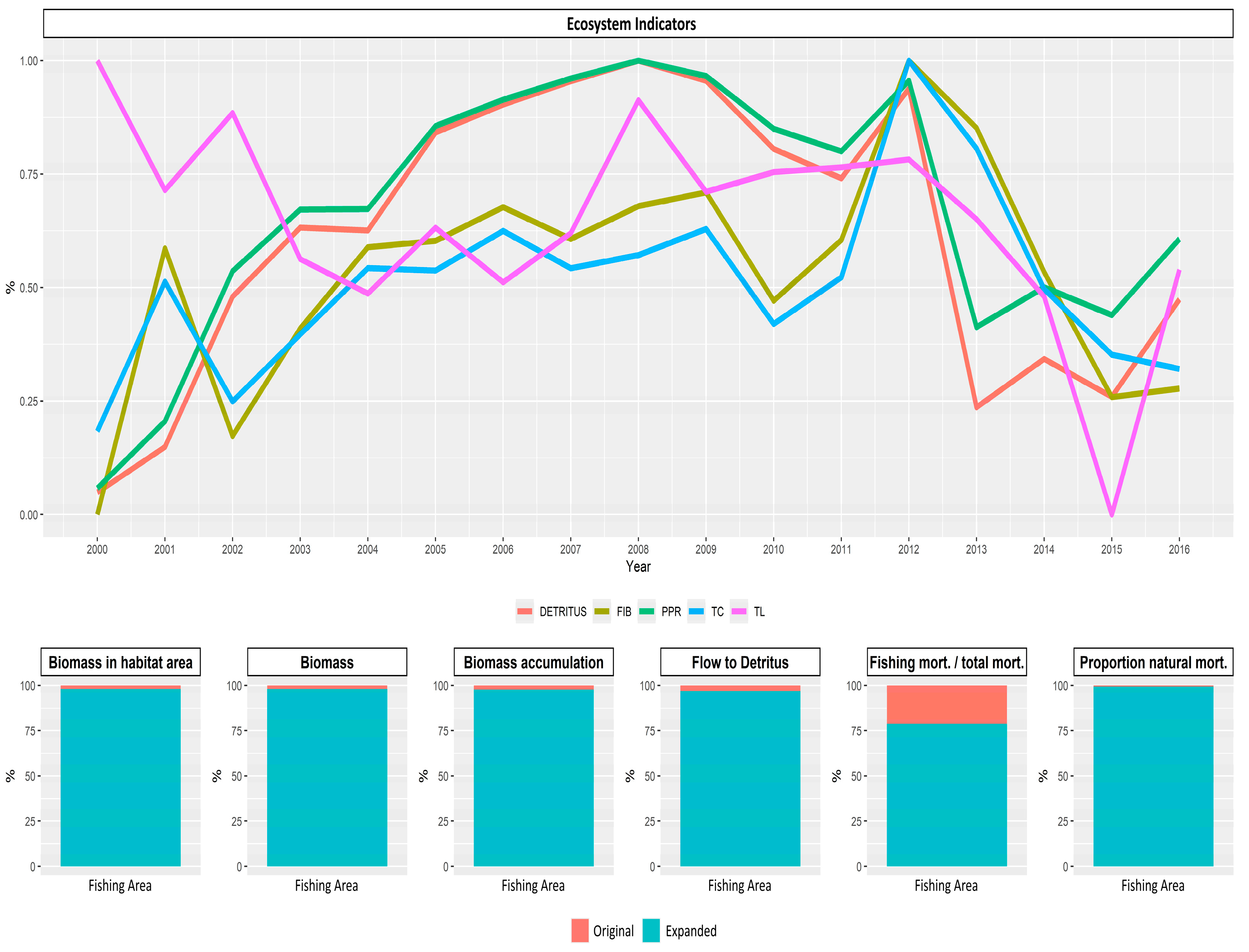
2.2. Variability of the Trophic Level of the Catches on a Spatial and Temporal Scale
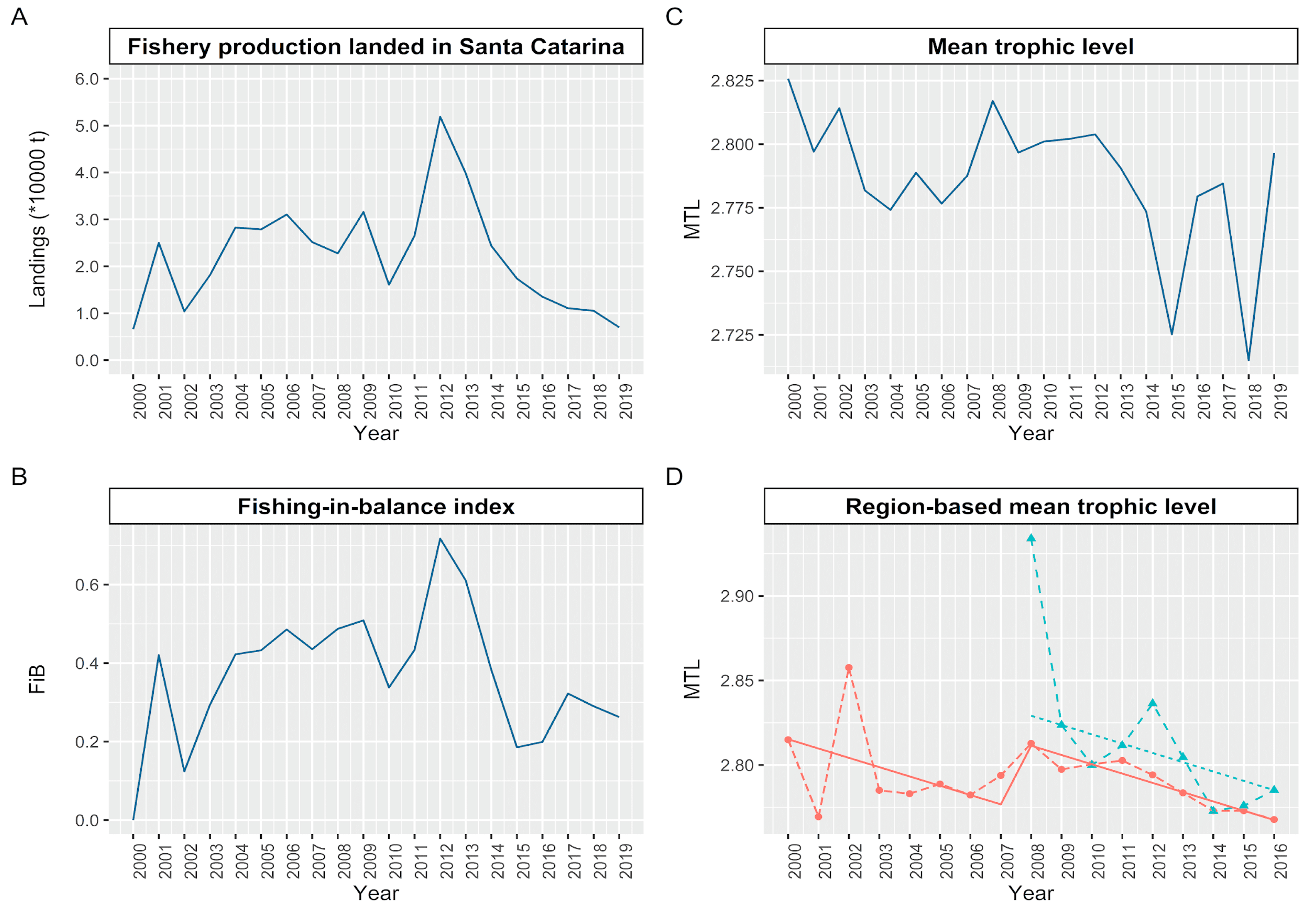
2.2.1. Mean Trophic Level of the Catches
2.2.2. Mean Trophic Level of the Catches
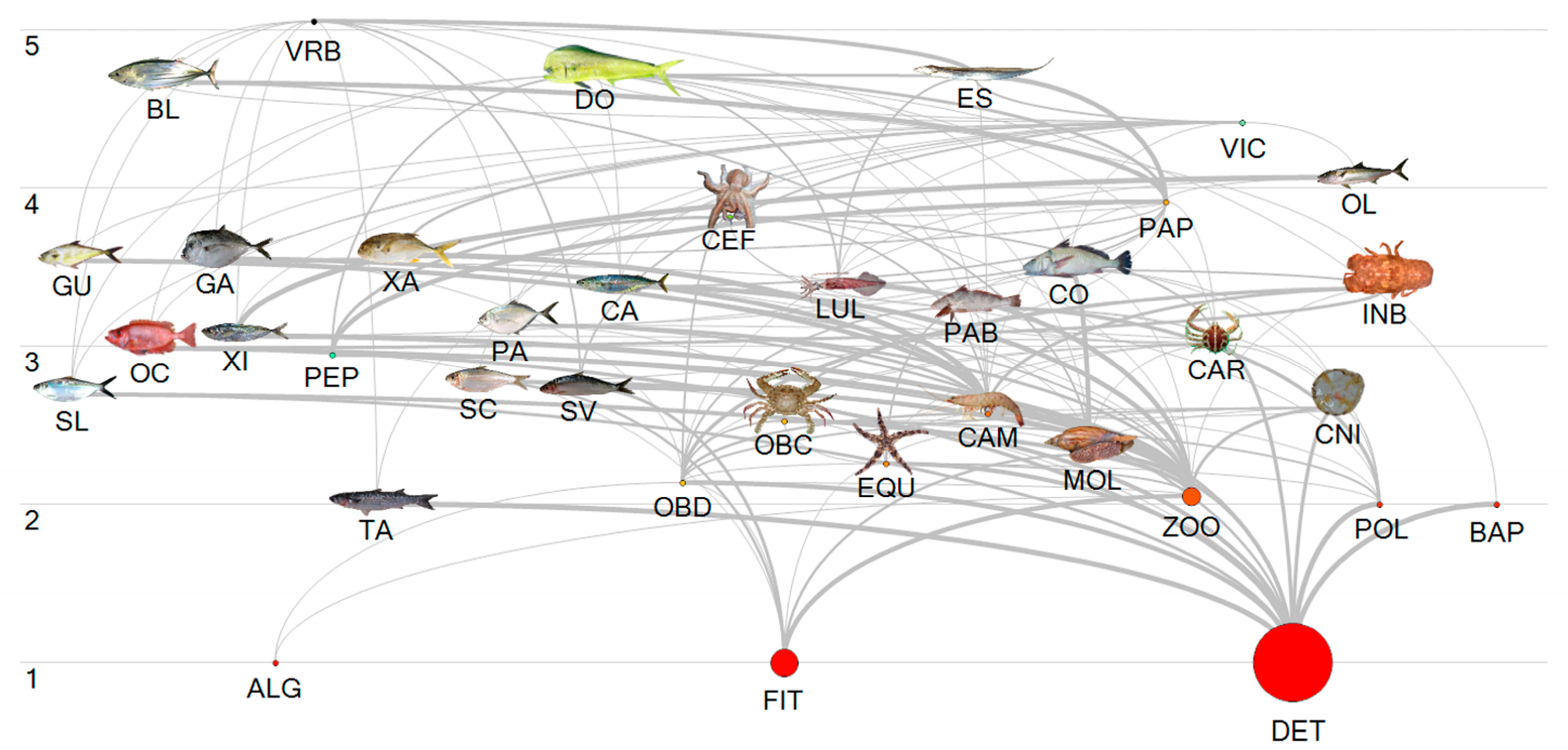
2.3. Modeling Trophic Relationships
2.3.1. Model Input Parameters
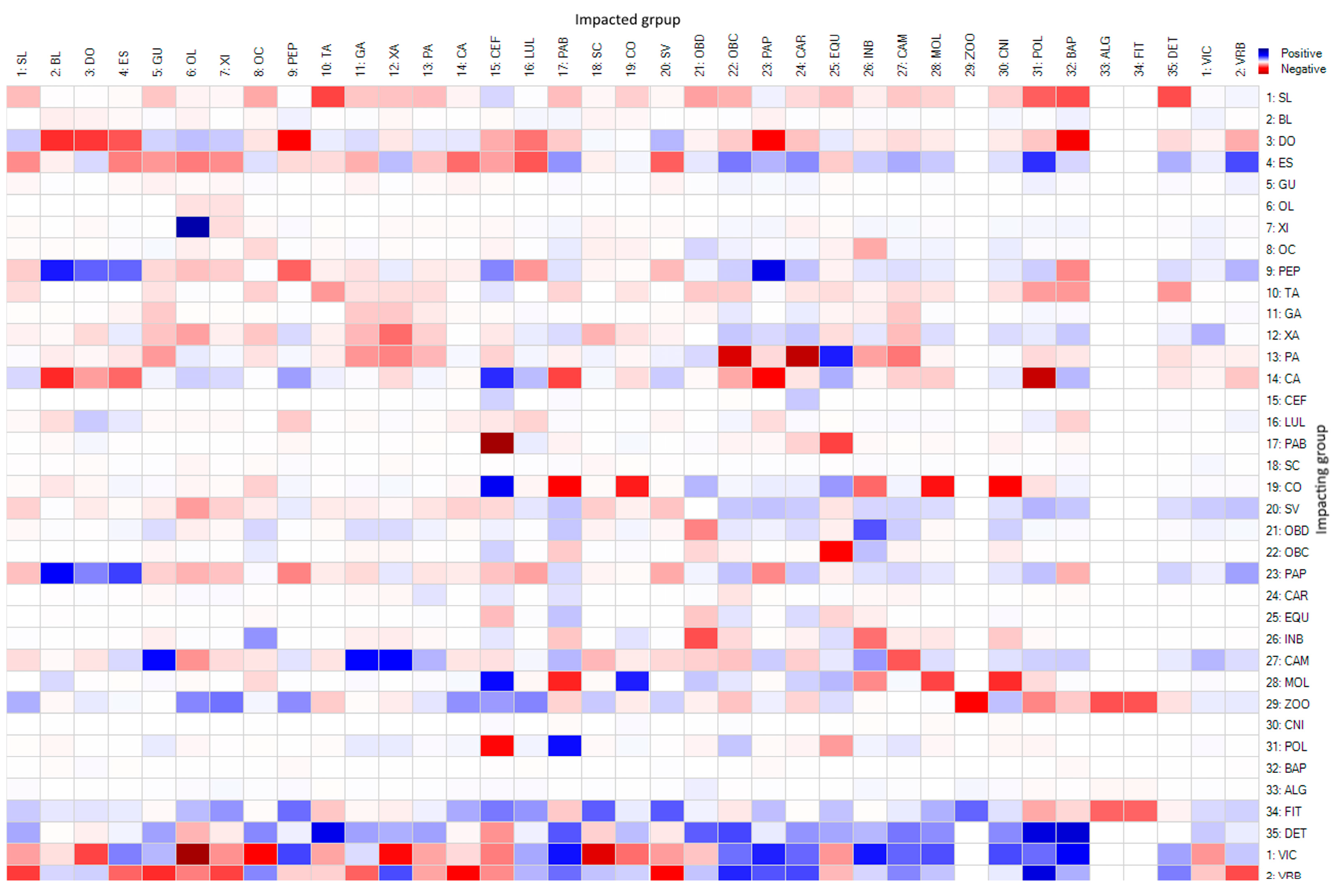
2.3.2. Impact on Trophic Relations
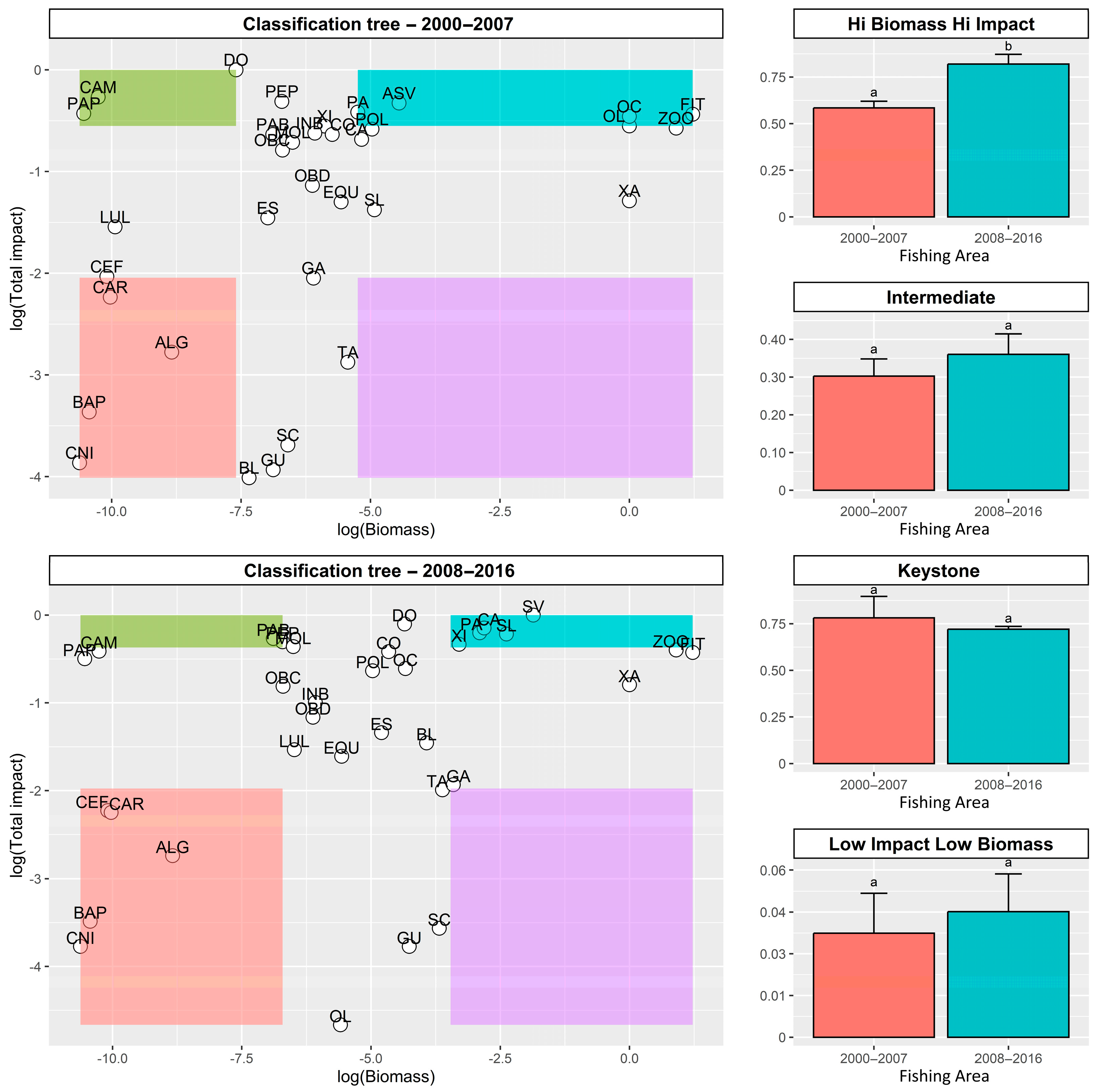
2.4. Identification of Keystone Species
2.5. Statistical Analyses
3. Results
3.1. Variability of the Trophic Level of the Catches on a Spatial and Temporal Scale
3.2. Modeling Trophic Relationships
3.3. Identification of Keystone Species
3.4. Influences on the Trophic Web
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colloca, F.; Scarcella, G.; Libralato, S. Recent Trends and Impacts of Fisheries Exploitation on Mediterranean Stocks and Ecosystems. Front. Mar. Sci. 2017, 4, 244. [Google Scholar] [CrossRef]
- Pikitch, E.K.; Rountos, K.J.; Essington, T.E.; Santora, C.; Pauly, D.; Watson, R.; Sumaila, U.R.; Boersma, P.D.; Boyd, I.L.; Conover, D.O.; et al. The global contribution of forage fish to marine fisheries and ecosystems. Fish Fish. 2014, 15, 43–64. [Google Scholar] [CrossRef]
- Piroddi, C.; Coll, M.; Liquete, C.; Macias, D.; Greer, K.; Buszowski, J.; Steenbeek, J.; Danovaro, R.; Christensen, V. Historical changes of the Mediterranean Sea ecosystem: Modelling the role and impact of primary productivity and fisheries changes over time. Sci. Rep. 2017, 7, 44491. [Google Scholar] [CrossRef]
- Peck, M.A.; Alheit, J.; Bertrand, A.; Catalán, I.A.; Garrido, S.; Moyano, M.; Rykaczewski, R.R.; Takasuka, A.; van der Lingen, C.D. Small pelagic fish in the new millennium: A bottom-up view of global research effort. Prog. Oceanogr. 2021, 191, 102494. [Google Scholar] [CrossRef]
- Piroddi, C.; Coll, M.; Steenbeek, L.; Moy, D.M.; Christensen, V. Modelling the Mediterranean marine ecosystem as a whole: Addressing the challenge of complexity. Mar. Ecol. Prog. Ser. 2015, 533, 47–65. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Bornatowski, H.; Angelini, R.; Coll, M.; Barreto, R.R.P.; Amorim, A.F. Ecological role and historical trends of large pelagic predators in a subtropical marine ecosystem of the South Atlantic. Rev. Fish. Biol. Fisher. 2017, 28, 241–259. [Google Scholar] [CrossRef]
- Endrédi, A.; Patonai, K.; Podani, J.; Libralato, S.; Jordán, F. Who Is Where in Marine Food Webs? A Trait-Based Analysis of Network Positions. Front. Mar. Sci. 2021, 8, 636042. [Google Scholar] [CrossRef]
- Hunsicker, M.E.; Essington, T.E. Evaluating the potential for trophodynamic control of fish by the longfin inshore squid (Loligo pealeii) in the Northwest Atlantic Ocean. Can. J. Fish. Aquat. Sci. 2008, 65, 754–765. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Keramidas, I.; Sylaios, G.; Tsikliras, A.C. Ecotrophic Effects of Fishing across the Mediterranean Sea. Water 2021, 13, 482. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Walters, C. Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impacts of fisheries. ICES J. Mar. Sci. 2000, 57, 697–706. [Google Scholar] [CrossRef]
- Valls, A.; Coll, M.; Christensen, V. Keystone species: Toward an operational concept for marine biodiversity conservation. Ecol. Monogr. 2015, 85, 29–47. [Google Scholar] [CrossRef]
- Taghavimotlagh, S.A.; Vahabnezhad, A.; Shojaei, M.G. A trophic model of the coastal fisheries ecosystem of the northern Persian Gulf using a mass balance Ecopath model. Reg. Stud. Mar. Sci. 2021, 42, 101639. [Google Scholar] [CrossRef]
- Vasconcellos, M.; Gasalla, M.A. Fisheries catches and the carrying capacity of marine ecosystems in southern Brazil. Fish. Res. 2001, 50, 279–295. [Google Scholar] [CrossRef]
- Gasalla, M.A.; Rossi-Wongtschowski, C.L.D.B. Contribution of ecosystem analysis to investigating the effects of changes in fishing strategies in the South Brazil Bight coastal ecosystem. Ecol. Modell. 2004, 172, 283–306. [Google Scholar] [CrossRef]
- Veiga-Malta, T.; Szalaj, D.; Angélico, M.M.; Azevedo, M.; Farias, I.; Garrido, S.; Lourenço, S.; Marçalo, A.; Marques, V.; Moreno, A.; et al. First representation of the trophic structure and functioning of the Portuguese continental shelf ecosystem: Insights into the role of sardine. Mar. Ecol. Prog. Ser. 2019, 617–618, 323–340. [Google Scholar] [CrossRef]
- Matsuura, Y. Brazilian sardine (Sardinella brasiliensis) spawing in the southeast Brazilian Bight over period 1976–1993. Rev. Bras. Oceanog. 1998, 46, 33–43. [Google Scholar] [CrossRef]
- Cergole, M.C.; Saccardo, S.A.; Rossi-Wongtschowski, C.L.D.B. Fluctuations in the spawning stock biomass and recruitment of the Brazilian sardine (Sardinella brasiliensis) 1977–1997. Rev. Bras. Oceanogr. 2002, 50, 13–26. [Google Scholar] [CrossRef]
- Schroeder, R.; Petermann, A.; Schwingel, P.R.; Correia, A.T. Biological patterns of reproduction of the Brazilian sardine Sardinella brasiliensis in the purse seine fishery of Southwest Atlantic Ocean: A long-term assessment. Mar. Environ. Res. 2024, 197, 106457. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.; Schwingel, P.R.; Pinto, E.; Almeida, A.; Correia, A.T. Stock structure of the Brazilian sardine Sardinella brasiliensis from Southwest Atlantic Ocean inferred from otolith elemental signatures. Fish. Res. 2022, 248, 106192. [Google Scholar] [CrossRef]
- Rossi-Wongtschowski, C.L.D.B. Estudo das variações da relação peso total/comprimento total em função do ciclo reprodutivo e do comportamento de Sardinella brasilinesis (Steindachner, 1879) da costa do Brasil entre 23°S e 28°S. Bol. Inst. Oceanogr. 1977, 26, 131–180. [Google Scholar] [CrossRef]
- Matsuura, Y.; Spach, H.L.; Katsuragawa, M. Comparison of swning patterns of the Brazilian sardine (Sardinella brasiliensis) and anchoita (Engraulis anchoita) in Ubatuba region, southern Brazil during 1985 through 1988. Bol. Inst. Oceanog. 1992, 40, 101–115. [Google Scholar] [CrossRef]
- Matsuura, Y. A probable cause of recruitment failure of the Brazilian sardine Sardinella aurita population during the 1974/75 spawning season. S. Afr. J. Mar. Sci. 1996, 17, 29–35. [Google Scholar] [CrossRef]
- Matsuura, Y. Large scale fluctuations of small pelagic fish populations and climate change: A review. Bull. Tohoku. Natl. Fish. Res. Inst. 1999, 62, 195–205. [Google Scholar]
- Jablonski, S.; Legey, L.F.L. Environmental effects on the recruitment of the Brazilian sardine (Sardinella brasiliensis) (1977–1993). Sci. Mar. 2004, 68, 385–398. [Google Scholar] [CrossRef]
- Araújo, F.G.; Teixeira, T.P.; Guedes, A.P.P.; Azevedo, M.C.C.; Pessanha, A.L.M. Shifts in the abundance and distribution of shallow water fish fauna on the southeastern Brazilian coast: A response to climate change. Hydrobiologia 2018, 814, 205–218. [Google Scholar] [CrossRef]
- Kurtz, F.W. Dinâmica Larval de Sardinella brasiliensis (Steindachner, 1879) (Teleostei, Clupeidae) na Região Sudeste do Brasil e Implicações no Recrutamento. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 1999. [Google Scholar]
- Schroeder, R.; Correia, A.T.; Medeiros, S.D.; Pessatti, M.L.; Schwingel, P.R. Spatiotemporal variability of the catch composition and discards estimates of the different methods of onboard preservation for the Brazilian sardine fishery in the southwest Atlantic Ocean. Thalassas 2022, 38, 573–597. [Google Scholar] [CrossRef]
- Magro, M.; Moreira, L.H.A.; Cardoso, L.C.C. Estrutura e dinâmica da frota pesqueira de cerco atuante em Angra dos Reis e Cabo Frio (Rio de Janeiro, Brasil). In Dinâmica das Frotas Pesqueiras Comerciais da Região Sudeste-Sul do Brasil; Rossi-Wongtchowski, C.L.D.B., Bernardes, R.A., Cergole, M.C., Eds.; Instituto Oceanográfico: São Paulo, Brazil, 2007; pp. 165–198. [Google Scholar]
- Imoto, R.D.; Carneiro, M.H.; Ávila-da-Silva, A.O. Spatial patterns of fishing fleets on the Southeastern Brazilian Bight. Lat. Am. J. Aquat. Res. 2016, 44, 1005–1018. [Google Scholar] [CrossRef]
- Branch, T.A.; Watson, R.; Fulton, E.A.; Jennings, S.; McGilliard, C.R.; Pablico, G.T.; Ricard, D.; Tracey, S.R. The trophic fingerprint of marine fisheries. Nature 2010, 468, 431–435. [Google Scholar] [CrossRef]
- Palomares, M.L.D.; Pauly, D. On the creeping increase of vessels’ fishing power. Ecol. Soc. 2019, 24, 2–7. [Google Scholar] [CrossRef]
- Pauly, D.; Watson, R. Background and interpretation of the “Marine Trophic Index” as a measure of biodiversity. Philos. Trans. R. Soc. B Sci. 2005, 360, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Kleisner, K.; Mansour, H.; Pauly, D. Region-based MTI: Resolving geographic expansion in the Marine Trophic Index. Mar. Ecol. Prog. Ser. 2015, 512, 185–199. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Moore, A.B.M.; Brander, K.; Evans, S.; Holm, P.; Hiddink, J.G. Century-scale loss and change in the fishes and fisheries of a temperate marine ecosystem revealed by qualitative historical sources. Fish Fish. 2024, 25, 876–894. [Google Scholar] [CrossRef]
- Stergiou, K.I.; Tsikliras, A.C. Fishing down, fishing through and fishing up: Fundamental process versus technical details. Mar. Ecol. Prog. Ser. 2011, 441, 295–301. [Google Scholar] [CrossRef]
- Babouri, K.; Pennino, M.G.; Bellido, J.M. A trophic indicator toolbox for implementing an ecosystem approach in data-poor fisheries: The Algerian and Bou-Ismael Bay example. Sci. Mar. 2014, 78, 37–51. [Google Scholar] [CrossRef]
- Shannon, J.L.; Coll, M.; Bundy, A.; Gascuel, D.; Heymans, J.J.; Kleisner, K.; Lynam, C.P.; Piroddi, C.; Tam, J.; Travers-Trolet, M.; et al. Trophic level-based indicators to track fishing impacts across marine ecosystems. Mar. Ecol. Prog. Ser. 2014, 512, 115–140. [Google Scholar] [CrossRef]
- Morato, T.; Watson, R.; Pitcher, T.J.; Pauly, D. Fishing down the deep. Fish Fish. 2006, 7, 23–33. [Google Scholar] [CrossRef]
- Swartz, W.; Sala, E.; Tracey, S.; Watson, R.; Pauly, D. The spatial expansion and ecological footprint of fisheries (1950 to present). PLoS ONE 2010, 5, e15143. [Google Scholar] [CrossRef]
- Watson, R.A.; Morato, T. Fishing down the deep: Accounting for within-species changes in depth of fishing. Fish. Res. 2013, 140, 63–65. [Google Scholar] [CrossRef]
- Bhathal, B.; Pauly, D. Fishing down marine food webs and spatial expansion of coastal fisheries in India, 1950–2000. Fish. Res. 2008, 91, 26–34. [Google Scholar] [CrossRef]
- Kleisner, K.; Pauly, D. The Marine Trophic Index (MTI), the Fishing in Balance (FiB) Index and the spatial expansion of fisheries. In The State of Biodiversity and Fisheries in Regional Seas; Christensen, V., Lai, S., Palomares, M.L.D., Zeller, D., Pauly, D., Eds.; Fisheries Centre Research Reports: Vancouver, BC, Canada, 2011; pp. 41–44. [Google Scholar]
- Libralato, S.; Christensen, V.; Pauly, D. A method for identifying keystone species in food web models. Ecol. Model. 2006, 195, 153–171. [Google Scholar] [CrossRef]
- Schroeder, R.; Schwingel, P.R.; Schwarz, R.; Daros, F.A.; Franco, T.P.; Hoff, N.T.; Méndez Vicente, A.; Castro, J.P.; Vaz-dos-Santos, A.M.; Correia, A.T. Contribution of the nursery areas to the major fishing grounds of the Brazilian sardine (Sardinella brasiliensis) in Southeastern Brazilian Bight inferred from otolith fingerprints. Fish. Res. 2023, 267, 106825. [Google Scholar] [CrossRef]
- de Macedo-Soares, L.C.P.; Garcia, C.A.E.; Freire, A.S.; Muelbert, J.H. Large-scale ichthyoplankton and water mass distribution along the south Brazil shelf. PLoS ONE 2014, 9, e91241. [Google Scholar] [CrossRef]
- Rossi-Wongtschowski, C.L.D.B.; Madureira, L.S.-P. O ambiente Marinho Oceanográfico da Plataforma Continental e do Talude na Região Sudeste-Sul do Brasil; Edusp: São Paulo, Brazil, 2006. [Google Scholar]
- Möller, O.O., Jr.; Piola, A.R.; Freitas, A.C.; Campos, E.J.D. The effects of river discharge and seasonal winds on the shelf off Southeastern South America. Cont. Shelf Res. 2008, 28, 1607–1624. [Google Scholar] [CrossRef]
- Braga, E.S.; Chiozzini, V.C.; Berbel, G.B.B.; Maluf, J.C.C.; Aguiar, V.M.C. Nutrient distributions over the Southwestern South Atlantic continental shelf from Mar del Plata (Argentina) to Itajaí (Brazil): Winter–summer aspects. Cont. Shelf Res. 2008, 28, 1649–1661. [Google Scholar] [CrossRef]
- UNIVALI/LEMA, Estatística Pesqueira de Santa Catarina, Consulta On-Line, 2020. Projeto de Monitoramento da Atividade Pesqueira do Estado de Santa Catarina, disponível em: Laboratório de Estudos Marinhos Aplicados (LEMA) da Universidade do Vale do Itajaí (UNIVALI). Available online: http://pmap-sc.acad.univali.br/sistema.html?id=597b7b77d8597d4a00e6f9c1 (accessed on 1 April 2019).
- Schroeder, R.; Schwingel, P.R.; Correia, A.T. Population structure of the Brazilian sardine (Sardinella brasiliensis) in the Southwest Atlantic inferred from body morphology and otolith shape signatures. Hydrobiologia 2021, 849, 1367–1381. [Google Scholar] [CrossRef]
- Freire, K.M.F.; Pauly, D. Fishing down Brazilian marine food webs, with emphasis on the east Brazil large marine ecosystem. Fish. Res. 2010, 105, 57–62. [Google Scholar] [CrossRef]
- Pauly, D.; Palomares, M.L.; Froese, R.; Sa-a, P.; Vakily, M.; Preikshot, D.; Wallace, S. Fishing down Canadian aquatic food webs. Can. J. Fish. Aquat. Sci. 2001, 58, 51–62. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V. Primary production required to sustain global fisheries. Nature 1995, 374, 255–257. [Google Scholar] [CrossRef]
- Masi, M.; Ainsworth, C.H.; Chagaris, D.D. A probabilistic representation of fish diet compositions from multiple data sources: A Gulf of Mexico case study. Ecol. Model. 2014, 284, 60–74. [Google Scholar] [CrossRef]
- Rocha, G.R.A.; Rossi-Wongtschowski, C.L.D.B.; Pires-Vanin, A.M.S.; Soares, L.S.H. Trophic models of São Sebastião Channel and continental shelf systems, SE Brazil. Pan-Am. J. Aquat. Sci. 2007, 2, 149–162. [Google Scholar]
- Shephard, S.; Reid, D.G.; Gerritsen, H.D.; Farnsworth, K.D. Estimating biomass, fishing mortality, and “total allowable discards” for surveyed non-target fish. Mar. Biol. 2014, 72, 458–466. [Google Scholar] [CrossRef]
- Pauly, D. On the interrelationship between natural mortality, growth parameters and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish Stock Assessment, Part 1-Manual; FAO Fisheries Technical Paper 306; FAO: Rome, Italy, 1998. [Google Scholar]
- Hoenig, J.M. Empirical Use of Longevity Data to Estimate Mortality Rates; SEDAR: Supply, NC, USA, 1983. [Google Scholar]
- Palomares, M.L.D.; Pauly, D. Predicting food consumption of fish populations as functions of mortality, food type, morphometrics, temperature, and salinity. Mar. Freshw. Res. 1998, 49, 447–453. [Google Scholar] [CrossRef]
- Stantipoulos, M.D.; Rigby, R.A.; Heller, G.Z.; Voudouris, V.; De Bastiani, F. Flexible Regression and Smoothing: Using GAMLSS in R; Chapman and Hall/CRC: London, UK, 2017. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Essington, T.E.; Moriarty, P.M.; Froehlich, H.E.; Hodgson, E.E.; Koehn, L.E.; Oken, K.L.; Siple, M.C.; Christine, C.; Stawitz, C.C. Fishing amplifies forage fish population collapses. Proc. Nat. Acad. Sci. USA 2015, 112, 6648–6652. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.M.R. Management implications of fishing up, down, or through the marine food web. Mar. Pol. 2013, 37, 176–182. [Google Scholar] [CrossRef]
- Murawski, S.A. Definitions of overfishing from an ecosystem perspective. ICES J. Mar. Sci. 2000, 57, 649–658. [Google Scholar] [CrossRef]
- Hornborg, S.; Belgrano, A.; Bartolino, V.; Valentinsson, D.; Ziegler, F. Trophic indicators in fisheries: A call for re-evaluation. Biol. Lett. 2013, 9, 20121050. [Google Scholar] [CrossRef]
- Pauly, D.; Belhabib, D.; Blomeyer, R.; Cheung, W.W.W.L.; Cisneros-Montemayor, A.M.; Copeland, D.; Harper, S.; Lam, V.W.Y.; Mai, Y.; Le Manach, F.; et al. China’s distant-water fisheries in the 21st century. Fish Fish. 2014, 15, 474–488. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Dinouli, A.; Tsiros, V.-Z.; Tsalkou, E. The Mediterranean and Black Sea fisheries at risk from overexploitation. PLoS ONE 2015, 10, e0121188. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Quaas, M.; Matz-Lück, N. Status and rebuilding of European fisheries. Mar. Pol. 2018, 93, 159–170. [Google Scholar] [CrossRef]
- BRASIL. 2007 Portaria IBAMA N°43, de 24 de setembro de 2007. IBAMA. Diário Oficial da União, 24 September 2007.
- Rossi-Wongtschowski, C.L.D.B.; Vazzoler, A.E.A.M.; Braga, F.M.S. Estudos sobre estrutura, ciclo de vida e comportamento de Sardinella brasiliensis (Steindachner, 1879), na área entre 22°S e 28°S, Brasil. I. Morfologia dos otólitos. Bol. Inst. Oceanogr. 1982, 31, 57–76. [Google Scholar] [CrossRef]
- Braga, F.M.S. Estudo da diversidade de Sardinella braslliensis (Steindachner, 1879), na área entre Macaé (22◦23’S) e Ilha de Santa Catarina (27◦35’S). 1. Crescimento de Dimensões Corporais. Rev. Bras. Zool. 1987, 4, 235–250. [Google Scholar] [CrossRef]
- Franco, B.C.; Defeo, O.; Piola, A.R.; Barreiro, M.; Yang, H.; Ortega, L.; Gianelli, I.; Castello, J.P.; Vera, C.; Buratti, C.; et al. Climate change impacts on the atmospheric circulation, ocean, and fisheries in the southwest South Atlantic Ocean: A review. Clim. Chang. 2020, 162, 2359–2377. [Google Scholar] [CrossRef]
- Gianelli, I.; Ortega, L.; Marín, Y.; Piola, A.R.; Defeo, O. Evidence of ocean warming in Uruguay’s fisheries landings: The mean temperature of the catch approach. Mar. Ecol. Prog. Ser. 2019, 625, 115–125. [Google Scholar] [CrossRef]
- Perez, J.A.A.; Sant’Ana, R. Tropicalization of demersal megafauna in the western South Atlantic since 2013. Commun. Earth Environ. 2022, 3, 227. [Google Scholar] [CrossRef]
- Hobday, A.J.; Pecl, G.T. Identification of global marine hotspots: Sentinels for change and vanguards for adaptation action. Rev. Fish Biol. Fish. 2014, 24, 415–425. [Google Scholar] [CrossRef]
- Popova, E.; Yool, A.; Byfield, V.; Cochrane, K.; Coward, A.C.; Salim, S.; Gasalla, M.A.; Henson, S.A.; Hobday, A.; Pecl, G.; et al. From global to regional and back again: Common climate stressors of marine ecosystems relevant for adaptation across five ocean warming hotspots. Glob. Chang. Biol. 2016, 22, 2038–2053. [Google Scholar] [CrossRef]
- Lumpkin, R.; Garzoli, S. Interannual to decadal changes in the western South Atlantic’s surface circulation. J. Geophys. Res. 2011, 116, C01014. [Google Scholar] [CrossRef]
- Artana, C.; Provost, C.; Lellouche, J.-M.; Rio, M.-H.; Ferrari, R.; Sennéchael, N. The Malvinas current at the confluence with the Brazil current: Inferences from 25 years of mercator ocean reanalysis. J. Geophys. Res. Oceans 2019, 124, 7178–7200. [Google Scholar] [CrossRef]
- Perez, J.A.A.; Sant’Ana, R. A pesca Demersal nas Regiões Sudeste e Sul do Brasil: Síntese Espacial e Modelo de Gestão Baseada no Ecossistema. Relatório Final do Projeto MEEE PDSES—Subsídios Científicos para o Manejo Espacial e com Enfoque Ecossistêmico da Pesca Demersal nas Regiões Sul e Sudeste do Brasil—Chamada MCTI/ MPA/ CNPq no. 22/ 2015, Ordenamento da Pesca Marinha Brasileira. Processo 445782/2015-3; Itajaí: Santa Catarina, Brazil, 2022. [Google Scholar]

| Class | Order | Family | Species | Common Name | TL |
|---|---|---|---|---|---|
| Cephalopoda | Teuthida | Loliginidae | Doryteuthis plei | Slender inshore squid | 3.30 |
| Elasmobranchii | Squaliformes | Squalidae | Squalus spp. | Dogfish | 3.22 |
| Lamniformes | Odontaspididae | Carcharias taurus | Sand tiger shark | 4.08 | |
| Carchariniformes | Carcharinidae | Carcharhinus brevipinna | Spinner shark | 3.87 | |
| Carcharhinus leucas | Bull shark | 5.00 | |||
| Prionace glauca | Blue shark | 4.24 | |||
| Rhizoprionodon porosus | Carribean sharpnose shark | 3.26 | |||
| Sphyrnidae | Sphyrna lewini | Scalloped hammerhead | 3.82 | ||
| Rajiformes | Arhynchobatidae | Atlantoraja castelnaui | Spotback skate | 4.28 | |
| Not discriminated | Rays | 4.28 | |||
| Actinopterygii | Clupeiformes | Engraulidae | Anchoviella lepidentostole | Broadband anchovy | 2.88 |
| Engraulis anchoita | Argentine anchovy | 2.83 | |||
| Clupeidae | Brevoortia spp | Menhaden | 3.10 | ||
| Harengula clupeola | False herring | 3.05 | |||
| Opisthonema oglinum | Atlantic thread herring | 2.61 | |||
| Pellona harroweri | American coastal pellona | 3.33 | |||
| Sardinella brasiliensis | Brazilian sardine | 2.77 | |||
| Siluriformes | Ariidae | Family Ariidae | Catfish | 3.13 | |
| Mugiliformes | Mugilidae | Mugil liza | Lebranche mullet | 2.00 | |
| Beloniformes | Belonidae | Ablennes hians | Flat needlefish | 5.00 | |
| Zeiformes | Zeidae | Zenopsis conchifer | Silvery John dory | 3.10 | |
| Scorpaeniformes | Scorpaenidae | Helicolenus lahillei | Rosefish | 4.17 | |
| Scorpaeniformes | Triglidae | Prionotus punctatus | Bluewing searobin | 3.72 | |
| Perciformes | Centropomidae | Centropomus spp, | Snook | 3.19 | |
| Serranidae | Epinephelus marginatus | Dusky grouper | 3.94 | ||
| Priacanthidae | Priacanthus arenatus | Atlantic bigeye | 2.99 | ||
| Pomatomidae | Pomatomus saltatrix | Bluefish | 3.60 | ||
| Coryphaenidae | Coryphaena hippurus | Common Dolphinfish | 4.44 | ||
| Carangidae | Caranx crysos | Blue runner | 3.18 | ||
| Caranx hippos | Crevalle jack | 3.24 | |||
| Caranx latus | Horse-eye jack | 3.59 | |||
| Chloroscombrus chrysurus | Atlantic bumper | 2.49 | |||
| Oligoplites saliens | Castin leatherjacket | 3.28 | |||
| Parona signata | Parona leatherjacket | 3.10 | |||
| Selene setapinnis | Atlantic moonfish | 3.28 | |||
| Seriola dumerili | Greater amberjack | 3.22 | |||
| Trachurus lathami | Rough scad | 2.77 | |||
| Seriola lalandi | Yellowtail amberjack | 3.39 | |||
| Actinopterygii | Perciformes | Carangidae | Trachinotus carolinus | Florida pompano | 2.75 |
| Lutjanidae | Rhomboplites aurorubens | Vermilion snapper | 3.47 | ||
| Gerreidae | Diapterus rhombeus | Caitipa mojarra | 2.82 | ||
| Eucinostomus spp. | Mojarra | 3.04 | |||
| Haemulidae | Conodon nobilis | Bared grunt | 3.29 | ||
| Haemulon aurolineatum | Tomtate grunt | 2.82 | |||
| Sparidae | Archosargus probatocephalus | Sheepshead | 2.88 | ||
| Pagrus pagrus | Red porgy | 3.83 | |||
| Sciaenidae | Cynoscion acoupa | Acoupa weakfish | 3.80 | ||
| Cynoscion guatucupa | Stripped weakfish | 3.64 | |||
| Cynoscion jamaicensis | Jamaica weakfish | 3.06 | |||
| Cynoscion leiarchus | Smooth weakfish | 3.12 | |||
| Cynoscion microlepidotus | Smallscale weakfish | 3.32 | |||
| Cynoscion virescens | Green weakfish | 3.60 | |||
| Larimus breviceps | Shorthead drum | 3.26 | |||
| Sciaenidae | Macrodon atricauda | Southern king weakfish | 3.14 | ||
| Mentichirrhus americanus | Southern kingcroaker | 3.23 | |||
| Micropogonias furnieri | Whitemouth croaker | 3.11 | |||
| Paralonchurus brasiliensis | Banded croaker | 3.14 | |||
| Pogonias cromis | Black drum | 3.34 | |||
| Stellifer rastrifer | Rake stardrum | 3.12 | |||
| Umbrina canosai | Argentine croaker | 3.11 | |||
| Ephippidae | Chaetodipterus faber | Atlantic spadefish | 2.96 | ||
| Trichiuridae | Trichiurus lepturus | Largehead hairtail | 3.21 | ||
| Scombridae | Auxis thazard | Frigate tuna | 3.50 | ||
| Euthynnus alletteratus | Little tuny | 3.90 | |||
| Katsuwonus pelamis | Skipjack tuna | 3.24 | |||
| Non discriminated | Tuna fish | 3.19 | |||
| Scomber colias | Atlantic chub mackerel | 2.99 | |||
| Scomberomorus brasiliensis | Serra Spanish mackerel | 3.04 | |||
| Thunnus albacares | Yellowfin tuna | 4.27 | |||
| Xiphiidae | Xiphias gladius | Swordfish | 3.93 | ||
| Istiophoridae | Istiophorus albicans | Atlantic sailfish | 4.19 | ||
| Stromateidae | Peprilus paru | American harvestfish | 2.77 | ||
| Tetraodontiformes | Balistidae | Balistes capriscus | Grey triggerfish | 3.40 |
| Code | Common Name | Fishing Area | TL | B | PB | QB | EE | PQ | OI | VIC | VRB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. SL | Atlantic thread herring | Original | 2.69 | 1.18 × 10−02 | 0.580 | 1.619 | 0.868 | 3.58 × 10−01 | 0.249 | 3.74 × 10−03 | - |
| 2. BL | Skipjack tuna | 4.67 | 4.52 × 10−05 | 0.340 | 0.749 | 0.772 | 4.53 × 10−01 | 0.324 | 3.19 × 10−05 | - | |
| 3. DO | Dolphinfish | 4.72 | 2.52 × 10−05 | 0.180 | 1.212 | 0.772 | 1.48 × 10−01 | 0.480 | 2.50 × 10−05 | - | |
| 4. ES | Largehead hairtail | 4.71 | 1.04 × 10−04 | 0.360 | 0.661 | 0.772 | 5.44 × 10−01 | 0.177 | 8.27 × 10−06 | - | |
| 5. GU | Castin leatherjacket | 3.54 | 1.32 × 10−04 | 0.480 | 0.661 | 0.868 | 7.26 × 10−01 | 0.079 | 4.61 × 10−05 | - | |
| 6. OL | Yellowtail amberjack | 4.07 | 1.92 × 10−06 | 0.180 | 1.212 | 0.772 | 1.48 × 10−01 | 0.001 | 0 | - | |
| 7. XI | Rough scad | 3.06 | 8.93 × 10−05 | 0.430 | 1.092 | 0.868 | 3.93 × 10−01 | 0.011 | 3.74 × 10−04 | - | |
| 8. OC | Atlantic bigeye | 2.99 | 4.62 × 10−05 | 0.570 | 5.300 | 0.648 | 1.07 × 10−01 | 1.000 | 0 | - | |
| 9. PEP | Pelagic fish | 2.95 | 1.93 × 10−07 | 2.500 | 25.000 | 0.742 | 1.00 × 10−01 | 0.100 | 0 | - | |
| 10. TA | Lebranche mullet | 2.00 | 3.64 × 10−03 | 0.320 | 1.296 | 0.648 | 2.46 × 10−01 | 0 | 5.90 × 10−04 | - | |
| 11. GA | Atlantic moonfish | 3.55 | 7.93 × 10−04 | 0.750 | 0.987 | 0.868 | 7.59 × 10−01 | 0.067 | 1.36 × 10−04 | - | |
| 12. XA | Crevalle jack | 3.58 | 1.62 × 10−07 | 0.570 | 5.300 | 0.868 | 1.07 × 10−01 | 0.0 | 3.20 × 10−04 | - | |
| 13. PA | Atlantic bumper | 3.13 | 5.66 × 10−03 | 0.400 | 0.943 | 0.868 | 4.24 × 10−01 | 0.462 | 8.65 × 10−04 | - | |
| 14. CA | Atlantic chub mackerel | 3.38 | 6.73 × 10−03 | 0.480 | 0.950 | 0.868 | 5.05 × 10−01 | 0.492 | 4.51 × 10−04 | - | |
| 15. CEF | Cephalopods | 3.82 | 8.10 × 10−11 | 3.000 | 10.000 | 0.858 | 3.00 × 10−01 | 0.282 | 0 | - | |
| 16. LUL | Squids | 3.39 | 1.16 × 10−07 | 0.680 | 5.155 | 0.858 | 1.31 × 10−01 | 0.476 | 1.16 × 10−07 | - | |
| 17. PAB | Benthic feeding fish | 3.25 | 1.28 × 10−07 | 0.960 | 7.600 | 0.648 | 1.26 × 10−01 | 0.144 | 0 | - | |
| 18. SC | False herring | 2.77 | 2.09 × 10−04 | 0.930 | 1.500 | 0.868 | 6.20 × 10−01 | 0.288 | 0 | - | |
| 19. CO | Whitemouth croaker | 3.48 | 1.82 × 10−03 | 0.340 | 0.801 | 0.800 | 4.24 × 10−01 | 0.149 | 6.41 × 10−04 | - | |
| 20. SV | Brazilian sardine | 2.74 | 2.53 × 10−04 | 0.810 | 1.714 | 0.868 | 4.72 × 10−01 | 0.231 | 2.57 × 10−04 | - | |
| 21. OBD | Detritivorous benthic organisms | 2.14 | 7.53 × 10−07 | 7.860 | 13.450 | 0.501 | 5.84 × 10−01 | 0.130 | 0 | - | |
| 22. OBC | Carnivorous benthic organisms | 2.53 | 1.99 × 10−07 | 6.630 | 12.220 | 0.711 | 5.42 × 10−01 | 0.354 | 0 | - | |
| 23. PAP | Pelagic feeding fish | 3.91 | 2.90 × 10−11 | 1.300 | 2.800 | 0.868 | 4.64 × 10−01 | 0.018 | 0 | - | |
| 24. CAR | Crabs | 3.01 | 9.40 × 10−11 | 4.420 | 25.800 | 0.729 | 1.71 × 10−01 | 0.905 | 0 | - | |
| 25. EQU | Echinoderms | 2.26 | 2.70 × 10−06 | 1.580 | 2.860 | 0.137 | 5.52 × 10−01 | 0.214 | 0 | - | |
| 26. INB | Benthic invertebrates | 3.37 | 8.38 × 10−07 | 2.680 | 3.894 | 0.950 | 6.88 × 10−01 | 0.044 | 0 | - | |
| 27. CAM | Shrimps | 2.58 | 5.50 × 10−11 | 3.930 | 19.130 | 0.622 | 2.05 × 10−01 | 0.297 | 0 | - | |
| 28. MOL | Mollusks | 2.32 | 3.12 × 10−07 | 5.290 | 8.210 | 0.950 | 6.44 × 10−01 | 0.233 | 0 | - | |
| 29. ZOO | Zooplankton | 2.05 | 8.00 × 100 | 40.000 | 324.600 | 0.429 | 1.23 × 10−01 | 0.053 | 0 | - | |
| 30. CNI | Cnidaria | 2.61 | 2.40 × 10−11 | 1.000 | 2.000 | 0.551 | 5.00 × 10−01 | 0.311 | 0 | - | |
| 31. POL | Polychaeta | 2.00 | 1.07 × 10−05 | 6.320 | 11.190 | 0.205 | 5.64 × 10−01 | 0 | 0 | - | |
| 32. BAP | Bacterioplankton | 2.00 | 3.70 × 10−11 | 250.000 | 500.000 | 0.689 | 5.00 × 10−01 | 0 | 0 | - | |
| 33. ALG | Algae | 1.00 | 1.44 × 10−09 | 41.660 | − | 0.950 | − | 0 | 0 | - | |
| 34. FIT | Fitoplankton | 1.00 | 1.67 × 10+01 | 100,000.000 | − | 0.965 | − | 0 | 0 | - | |
| 35. DET | Detritus | 1.00 | 1.50 × 10+02 | − | − | 0.000 | 0.013 | 0 | - | ||
| 1. SL | Atlantic thread herring | Expanded | 2.69 | 4.16 × 10−03 | 0.580 | 1.619 | 0.868 | 3.58 × 10−01 | 0.249 | 4.09 × 10−03 | 6.88 × 10−04 |
| 2. BL | Skipjack tuna | 4.67 | 1.18 × 10−04 | 0.340 | 0.749 | 0.772 | 4.53 × 10−01 | 0.324 | 1.02 × 10−04 | 5.49 × 10−06 | |
| 3. DO | Dolphinfish | 4.72 | 4.44 × 10−05 | 0.180 | 1.212 | 0.772 | 1.48 × 10−01 | 0.480 | 2.92 × 10−05 | 1.69 × 10−06 | |
| 4. ES | Largehead hairtail | 4.71 | 1.59 × 10−05 | 0.360 | 0.661 | 0.772 | 5.44 × 10−01 | 0.177 | 8.69 × 10−06 | 1.25 × 10−05 | |
| 5. GU | Castin leatherjacket | 3.54 | 5.50 × 10−05 | 0.480 | 0.661 | 0.868 | 7.26 × 10−01 | 0.079 | 6.29 × 10−06 | 1.08 × 10−04 | |
| 6. OL | Yellowtail amberjack | 4.07 | 2.56 × 10−06 | 0.180 | 1.212 | 0.772 | 1.48 × 10−01 | 0.001 | 2.56 × 10−06 | 0 | |
| 7. XI | Rough scad | 3.06 | 5.04 × 10−04 | 0.430 | 1.092 | 0.868 | 3.93 × 10−01 | 0.011 | 3.74 × 10−04 | 8.93 × 10−05 | |
| 8. OC | Atlantic bigeye | 2.99 | 4.62 × 10−05 | 0.570 | 5.300 | 0.648 | 1.07 × 10−01 | 1.000 | 4.60 × 10−05 | 0 | |
| 9. PEP | Pelagic fish | 2.95 | 1.93 × 10−07 | 2.500 | 25.000 | 0.742 | 1.00 × 10−01 | 0.100 | 0 | 0 | |
| 10. TA | Lebranche mullet | 2.00 | 2.40 × 10−04 | 0.320 | 1.296 | 0.648 | 2.46 × 10−01 | 0 | 1.42 × 10−04 | 3.50 × 10−05 | |
| 11. GA | Atlantic moonfish | 3.55 | 3.89 × 10−04 | 0.750 | 0.987 | 0.868 | 7.59 × 10−01 | 0.067 | 3.72 × 10−04 | 2.21 × 10−04 | |
| 12. XA | Crevalle jack | 3.58 | 6.13 × 10−04 | 0.570 | 5.300 | 0.868 | 1.07 × 10−01 | 0 | 0 | 0 | |
| 13. PA | Atlantic bumper | 3.13 | 1.26 × 10−03 | 0.400 | 0.943 | 0.868 | 4.24 × 10−01 | 0.462 | 1.35 × 10−03 | 1.55 × 10−04 | |
| 14. CA | Atlantic chub mackerel | 3.38 | 1.53 × 10−03 | 0.480 | 0.950 | 0.868 | 5.05 × 10−01 | 0.492 | 7.66 × 10−04 | 9.00 × 10−04 | |
| 15. CEF | Cephalopods | 3.82 | 8.10 × 10−11 | 3.000 | 10.000 | 0.858 | 3.00 × 10−01 | 0.282 | 0 | 0 | |
| 16. LUL | Squids | 3.39 | 3.29 × 10−07 | 0.680 | 5.155 | 0.858 | 1.31 × 10−01 | 0.476 | 3.29 × 10−07 | 0 | |
| 17. PAB | Benthic feeding fish | 3.25 | 1.28 × 10−07 | 0.960 | 7.600 | 0.648 | 1.26 × 10−01 | 0.144 | 0 | 0 | |
| 18. SC | False herring | 2.77 | 2.09 × 10−04 | 0.930 | 1.500 | 0.868 | 6.20 × 10−01 | 0.288 | 2.09 × 10−04 | 0 | |
| 19. CO | Whitemouth croaker | 3.48 | 2.19 × 10−05 | 0.340 | 0.801 | 0.800 | 4.24 × 10−01 | 0.149 | 9.35 × 10−06 | 1.00 × 10−05 | |
| 20. SV | Brazilian sardine | 2.74 | 1.37 × 10−02 | 0.810 | 1.714 | 0.868 | 4.72 × 10−01 | 0.231 | 1.24 × 10−02 | 5.02 × 10−03 | |
| 21. OBD | Detritivorous benthic organisms | 2.14 | 7.53 × 10−07 | 7.860 | 13.450 | 0.501 | 5.84 × 10−01 | 0.130 | 0 | 0 | |
| 22. OBC | Carnivorous benthic organisms | 2.53 | 1.99 × 10−07 | 6.630 | 12.220 | 0.711 | 5.42 × 10−01 | 0.354 | 0 | 0 | |
| 23. PAP | Pelagic feeding fish | 3.91 | 2.90 × 10−11 | 1.300 | 2.800 | 0.868 | 4.64 × 10−01 | 0.018 | 0 | 0 | |
| 24. CAR | Crabs | 3.01 | 9.40 × 10−11 | 4.420 | 25.800 | 0.729 | 1.71 × 10−01 | 0.905 | 0 | 0 | |
| 25. EQU | Echinoderms | 2.26 | 2.70 × 10−06 | 1.580 | 2.860 | 0.137 | 5.52 × 10−01 | 0.214 | 0 | 0 | |
| 26. INB | Benthic invertebrates | 3.37 | 8.38 × 10−07 | 2.680 | 3.894 | 0.950 | 6.88 × 10−01 | 0.044 | 0 | 0 | |
| 27. CAM | Shrimps | 2.58 | 5.50 × 10−11 | 3.930 | 19.130 | 0.622 | 2.05 × 10−01 | 0.297 | 0 | 0 | |
| 28. MOL | Mollusks | 2.32 | 3.12 × 10−07 | 5.290 | 8.210 | 0.950 | 6.44 × 10−01 | 0.233 | 0 | 0 | |
| 29. ZOO | Zooplankton | 2.05 | 8.00 × 100 | 40.000 | 324.600 | 0.429 | 1.23 × 10−01 | 0.053 | 0 | 0 | |
| 30. CNI | Cnidaria | 2.61 | 2.40 × 10−11 | 1.000 | 2.000 | 0.551 | 5.00 × 10−01 | 0.311 | 0 | 0 | |
| 31. POL | Polychaeta | 2.00 | 1.07 × 10−05 | 6.320 | 11.190 | 0.205 | 5.64 × 10−01 | 0 | 0 | 0 | |
| 32. BAP | Bacterioplankton | 2.00 | 3.70 × 10−11 | 250.000 | 500.000 | 0.689 | 5.00 × 10−01 | 0 | 0 | 0 | |
| 33. ALG | Algae | 1.00 | 1.44 × 10−09 | 41.660 | − | 0.950 | − | 0 | 0 | 0 | |
| 34. FIT | Fitoplankton | 1.00 | 1.67 × 10+01 | 100,000.000 | − | 0.965 | − | 0 | 0 | 0 | |
| 35. DET | Detritus | 1.00 | 1.50 × 10+02 | 67.500 | − | − | 5.24 × 10−08 | 0.013 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroeder, R.; Petermann, A.; Correia, A.T. The History of the Brazilian Sardine (Sardinella brasiliensis) Between Two Fishery Collapses: An Ecosystem Modeling Approach to Study Its Life Cycle. Biology 2025, 14, 13. https://doi.org/10.3390/biology14010013
Schroeder R, Petermann A, Correia AT. The History of the Brazilian Sardine (Sardinella brasiliensis) Between Two Fishery Collapses: An Ecosystem Modeling Approach to Study Its Life Cycle. Biology. 2025; 14(1):13. https://doi.org/10.3390/biology14010013
Chicago/Turabian StyleSchroeder, Rafael, Angélica Petermann, and Alberto Teodorico Correia. 2025. "The History of the Brazilian Sardine (Sardinella brasiliensis) Between Two Fishery Collapses: An Ecosystem Modeling Approach to Study Its Life Cycle" Biology 14, no. 1: 13. https://doi.org/10.3390/biology14010013
APA StyleSchroeder, R., Petermann, A., & Correia, A. T. (2025). The History of the Brazilian Sardine (Sardinella brasiliensis) Between Two Fishery Collapses: An Ecosystem Modeling Approach to Study Its Life Cycle. Biology, 14(1), 13. https://doi.org/10.3390/biology14010013







