Influence of Grassland Habitats on Acridoidea (Orthoptera) Species Diversity in Different Divisions of the Xinjiang Production and Construction Corps
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Methodology
2.1.1. Survey Methodology and Study Period
2.1.2. Setting Up Investigation Sites for Trekking
2.1.3. Description of Study Site
2.2. Collection Methods
2.3. Specimen Preparation and Identification
2.4. Data Processing
3. Results
3.1. Survey of Grassland Acridoidea Species in Each Division
3.2. Comparison of Grassland Acridoidea Species Diversity
3.3. Distribution of Grassland Acridoidea in Different Grassland Types
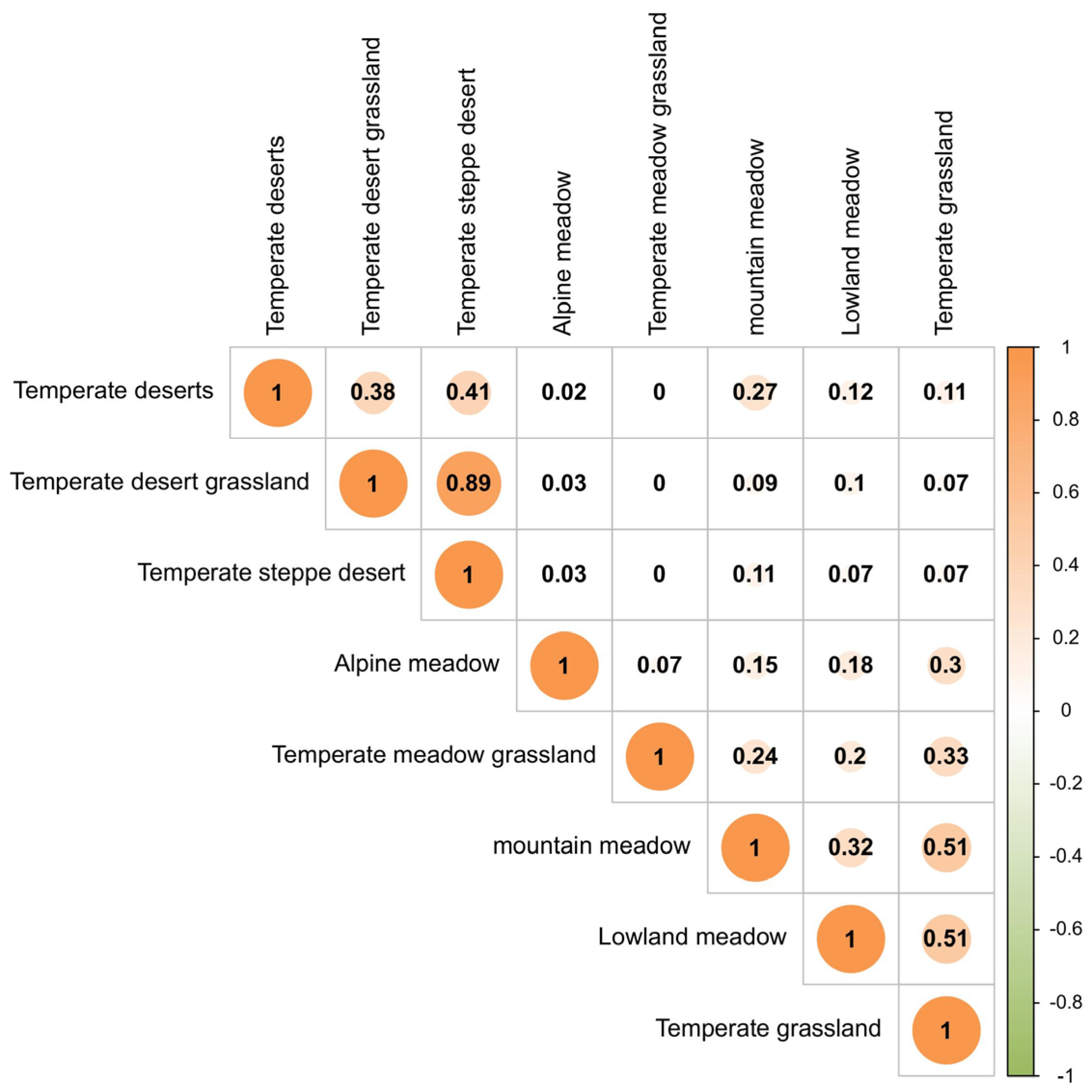
3.4. Relationship Between Grassland Acridoidea Species Diversity and Grassland Types
3.5. The Relationship Between Dominant Grassland Acridoidea Species and Soil Erodibility (K) Values
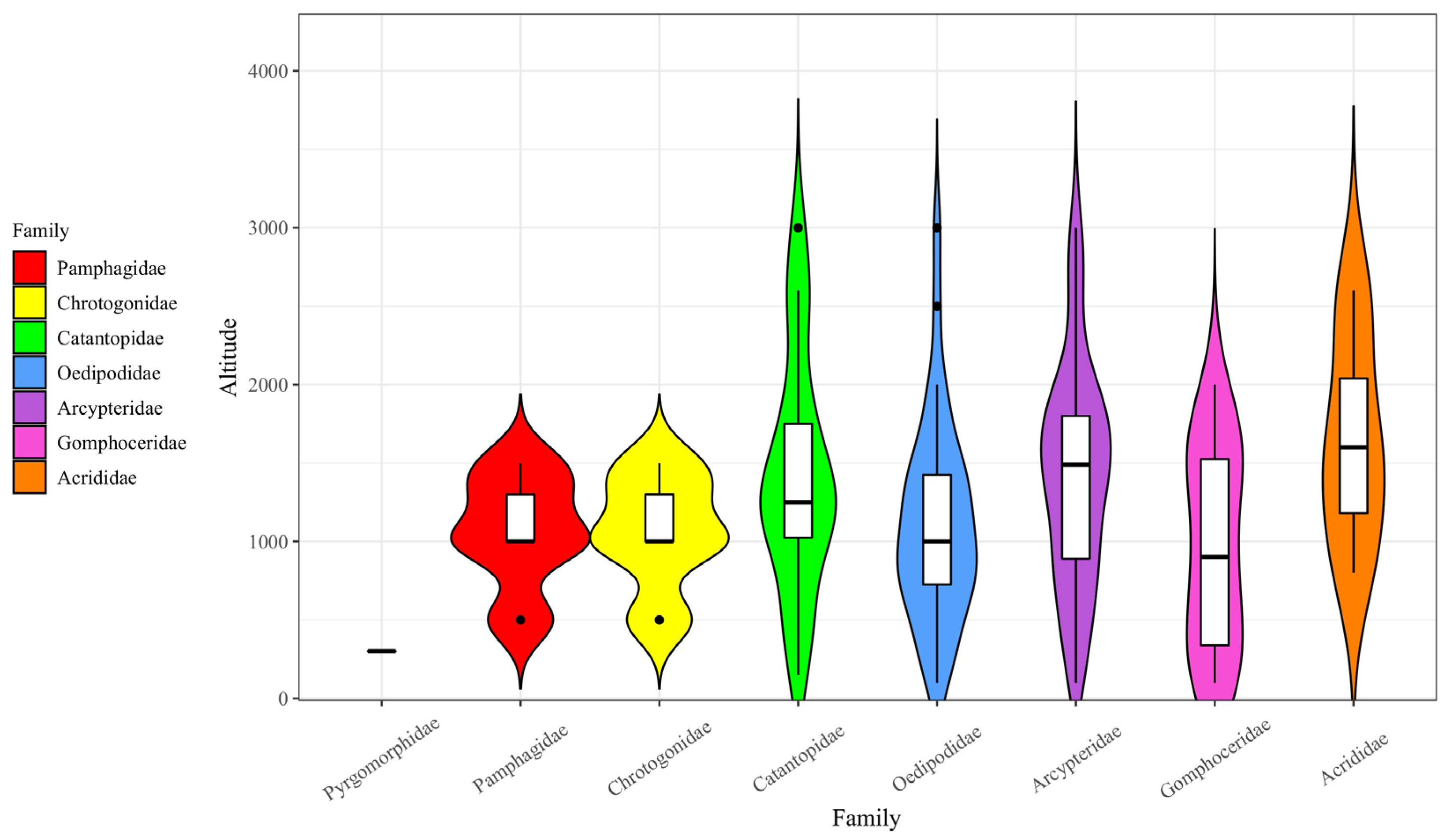
3.6. The Relationship Between Grassland Acridoidea Species Diversity and Altitude
3.7. The Relationships of Acridoidea Species Diversity with Temperature and Rainfall
4. Discussion
4.1. Comparison of Acridoidea Species Diversity Across Corps Divisions
4.2. Comparison of the Distribution and Diversity of Acridoidea in Different Grassland Types
4.3. Species Diversity of Acridoidea at Different Altitudes
4.4. Effects of Temperature and Rainfall on Species Diversity of Acridoidea
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, L. Application of locust integrated control technology based on biological control in Xinjiang grassland. Livest. Husb. Xinjiang 2011, 3, 59–63. [Google Scholar]
- Li, M.F.; Ma, Y.C.; Liu, Y.H.; Sheng, J.D.; Chen, J.H. Pattern and drivers of phylogenetic diversity in Xinjiang grassland. Acta Ecol. Sin. 2020, 40, 2285–2299. [Google Scholar]
- Zhang, D.L.; Feng, Z.D. Holocene climate variations in the Altai Mountains and the surrounding areas:A synthesis of pollen records. Earth-Sci. Rev. 2018, 185, 847–869. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Li, W.; Liu, Z.; Wei, C.; Tang, J. Understanding the complexity of temperature dynamics in Xinjiang, China, from multitemporal scale and spatial perspectives. Sci. World J. 2013, 2013, 259248. [Google Scholar] [CrossRef]
- Luo, J.T.; Fan, Y.M.; Wu, H.Q.; Cheng, J.H.; Sheng, J.D.; Yang, R.; Yuan, B.B. Analysis of Soil Diversity Characteristics and Main Influencing Factors in Different Geomorphologic Regions of Northern Xinjiang. Chin. J. Soil Sci. 2023, 54, 1261–1270. [Google Scholar]
- Mendonça, R.; Müller, R.A.; Clow, D.; Verpoorter, C.; Raymond, P.; Tranvik, L.J.; Sobek, S. Organic carbon burial in global lakes and reservoirs. Nat. Commun. 2017, 8, 1694. [Google Scholar] [CrossRef]
- Unsicker, S.B.; Franzke, A.; Specht, J.; Köhler, G.; Linz, J.; Renker, C.; Stein, C.; Weisser, W.W. Plant species richness in montane grasslands affects the fitness of a generalist Acridoidea species. Ecology 2010, 91, 1083–1091. [Google Scholar] [CrossRef]
- Bugaev, G.S. Habitat distribution of Acridoidea in the zone of band pine forests in North-East Kazakhstan. Vestn. Selskokhoziastvennoj Nauk. Kazakhstana 1977, 6, 37–40. (In Russian) [Google Scholar]
- Zhao, L.; Wang, D.Y.; Zhao, L.; Xiao, W.H. Research progress of Acridoidea in Xinjiang. Xinjiang Agric. Sci. 2012, 49, 1212–1223. [Google Scholar]
- Li, J.X.; Jin, X.; Guan, T.X.; Li, R.C.; Zhou, D.L.; Dorhong, B.; Bu, R.D.; Zhang, X.Y.; Ren, J.L.; Zhao, L. Diversity and Community Structure Characteristics of Grassland Locusts in Bortala Mongol Autonomous Prefecture, Xinjiang. Chin. J. Biol. Control 2022, 38, 1213–1222. [Google Scholar]
- Tiede, Y.; Hemp, C.; Schmidt, A.; Nauss, T.; Farwig, N.; Brandl, R. Beyond body size: Consistent decrease of traits within orthopteran assemblages with elevation. Ecology 2018, 99, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, K.R.; Habel, J.C.; Gossner, M.M.; Loxdale, H.D.; Köhler, G.; Schneider, A.R.; Tiedemann, R.; Weisser, W.W. Effects of habitat structure and land-use intensity on the genetic structure of the Acridoidea species Chorthippus parallelus. R. Soc. Open Sci. 2014, 1, 140133. [Google Scholar] [CrossRef] [PubMed]
- Cherrill, A. Large-scale spatial patterns in species richness of Orthoptera in the Greater London Area, United Kingdom: Relationships with land cover. Landsc. Res. 2015, 40, 476–485. [Google Scholar] [CrossRef]
- Jakab, D.A.; Nagy, A. How can an intensively used agricultural landscape preserve diversity of Orthoptera assemblages? J. Insect Conserv. 2022, 26, 947–958. [Google Scholar] [CrossRef]
- Liu, C.; Fischer, G.; Hita Garcia, F.; Yamane, S.; Liu, Q.; Peng, Y.Q.; Economo, E.P.; Guénard, B.; Pierce, N.E. Ants of the Hengduan Mountains: A new altitudinal survey and updated checklist for Yunnan Province highlight an understudied insect biodiversity hotspot. ZooKeys 2020, 978, 1–171. [Google Scholar]
- Sergeev, M.G. Distribution Patterns of Acridoidea and Their Kin over the Eurasian Steppes. Insects 2021, 12, 77. [Google Scholar] [CrossRef]
- Zheng, Z.M. Taxonomy of Locusts; Shaanxi Normal University Press: Xi’an, China, 1993. [Google Scholar]
- Zheng, Z.M. Zoology of China: Insecta, Institute of Zoology; Chinese Academy of Sciences: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Chen, Y.L. Chinese Locust Research; Hubei Science and Technology Press: Wuhan, China, 2019. (In Chinese) [Google Scholar]
- Wang, J.G.; Zhao, L.; Lei, Y.H. Identification of Locusts in Xinjiang; Corps Press: Urumqi, China, 2014. (In Chinese) [Google Scholar]
- Wang, M.; Tian, Y.; Zhang, N.; Nong, X.; Tu, X.; Zhang, Z.; Huang, Y.; Wang, Y.; Zhuang, L.; Cha, G.; et al. Molecular identification and related functional characterization of the FKBP52 gene in immunity of Locusta migratoria manilensis (Orthoptera: Oedipodidae). J. Econ. Entomol. 2024, 117, 1130–1140. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Bukero, A.A.; Shu, J.P.; Zhuo, F.Y.; Liu, L.Y.; Zhang, A.H. An Evaluation of the Crop Preference and Phenotypic Characteristics of Ceracris kiangsu Tsai (Orthoptera: Arcypteridae) under Different Temperatures. Biology 2023, 12, 1377. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Xue, X.; Qin, G.; Gao, Y.; Li, K.; Zhang, Y.; Li, X.J. Comparative analysis of mitogenomes among three species of grasshoppers (Orthoptera: Acridoidea: Gomphocerinae) and their phylogenetic implications. PeerJ 2023, 11, e16550. [Google Scholar] [CrossRef]
- Qian, H.; Altanchimeg, D.; Naizab, N.; Wang, S.; Wen, S.; Lin, C. The complete mitochondrial genome of Eclipophleps carinata (Orthoptera: Acridoidea: Gomphoceridae). Mitochondrial DNA B Resour. 2021, 6, 1310–1312. [Google Scholar] [CrossRef]
- Nakano, M.; Morgan-Richards, M.; Trewick, S.A.; Clavijo-McCormick, A. Chemical Ecology and Olfaction in Short-Horned Grasshoppers (Orthoptera: Acrididae). J. Chem. Ecol. 2022, 48, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.H.; Shen, W.X.; Jia, C.Y.; Yin, Z. A new species and key to known species of the genus Conophymacris Willemse, 1933 (Orthoptera, Acridoidea, Catantopidae, Podisminae) from China. Zootaxa 2021, 5047, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.Y.; Shi, Q.Y.; Ling, Y.; Chen, J.Y.; Zhang, B.F.; Li, X.J. Comparative Analysis of Mitogenomes among Five Species of Filchnerella (Orthoptera: Acridoidea: Pamphagidae) and Their Phylogenetic and Taxonomic Implications. Insects 2021, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Dong, L.; Yin, H.; Zhang, D. The complete mitochondrial genome of Mekongiella kingdoni (Uvarov, 1937) (Orthoptera: Acridoidea: Chrotogonidae). Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016, 27, 187–188. [Google Scholar] [CrossRef]
- Nankivell, R.N. Karyotype differences in the crenaticeps-group of Atractomorpha (Orthoptera, Acridoidea, Pyrgomorphidae). Chromosoma 1976, 56, 127–142. [Google Scholar] [CrossRef]
- Hu, J.; Qian, X.J.; Liu, C.Z. Responses of the Acridoidea community biodiversity and pattern intensity to the plant community. J. Plant Prot. 2021, 48, 202–211. [Google Scholar]
- Jawuoro, S.; Koech, O.; Karuku, G.; Mbau, J. Plant species composition and diversity depending on piospheres and seasonality in the southern rangelands of Kenya. Ecol. Process 2017, 6, 16. [Google Scholar] [CrossRef]
- Foldi, J.; Blenman, K.R.M.; Marczyk, M.; Gunasekharan, V.; Polanska, A.; Gee, R.; Davis, M.; Kahn, A.M.; Silber, A.; Pusztai, L. Peripheral blood immune parameters, response, and adverse events after neoadjuvant chemotherapy plus durvalumab in early-stage triple-negative breast cancer. Breast Cancer Res. Treat. 2024, 208, 369–377. [Google Scholar] [CrossRef]
- Biodiversity Committee; Chinese Academy of Sciences. Principles and Methods of Biodiversity Research; China Science and Technology Press: Beijing, China, 1994. [Google Scholar]
- Ding, J.Y.; Zhao, W.W.; Daryanto, S.; Wang, L.X.; Fan, H.; Feng, Q.; Wang, Y.P. The spatial distribution and temporal variation of desert riparian forests and their influencing factors in the downstream Heihe River Basin, China. Hydrol. Earth Syst. Sci. 2017, 21, 2405–2419. [Google Scholar] [CrossRef]
- Miao, Q.; Yuan, Y.J.; Luo, G.M.; Wei, C.H.; Rao, Y.Q.; Gong, Y.H.; Zhang, L.; Shao, J.; Dong, Y.K. Study on ecological suitability of Gardenia jasminoides based on ArcGIS and Maxent model. Zhongguo Zhongyao Zazhi 2016, 41, 3181–3185. [Google Scholar]
- Williams, J.R.; Renard, K.G.; Dyke, P.T. EPIC: A new method for assessing erosion’s effect on soil productivity. J. Soil Water Conserv. 1983, 38, 381–383. [Google Scholar]
- Yan, D.J.; Zhou, Q.W.; Lu, H.; Wu, C.C.; Zhao, B.Y.; Cao, D.D.; Ma, F.; Liu, X.X. The Disaster, Ecological Distribution and Control of Poisonous Weeds in Natural Grasslands of Xinjiang Uygur Autonomous Region. Sci. Agric. Sin. 2015, 48, 565–582. [Google Scholar]
- Zhong, J.L.; Guo, Z.X. Methods of Calculating and Mapping Soil Erodibility K in Xinjiang. Xinjiang Environ. Prot. 2014, 36, 1–4+10. [Google Scholar]
- Welti, E.A.R.; Roeder, K.A.; de Beurs, K.M.; Joern, A.; Kaspari, M. Nutrient dilution and climate cycles underlie declines in a dominant insect herbivore. Proc. Natl. Acad. Sci. USA 2020, 117, 7271–7275. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L. Studies on the acridoids of xinjiang uighur autonomous region: Distribution of acridoids. Acta Entomol. Sin. 1981, 24, 17–26. [Google Scholar]
- Cease, A.J.; Elser, J.J.; Ford, C.F.; Hao, S.; Kang, L.; Harrison, J.F. Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science 2012, 335, 467–469. [Google Scholar] [CrossRef]
- Veran, S.; Simpson, S.J.; Sword, G.A.; Deveson, E.; Piry, S.; Hines, J.E.; Berthier, K. Modeling spatiotemporal dynamics of outbreaking species: Influence of environment and migration in a locust. Ecology 2015, 96, 737–748. [Google Scholar] [CrossRef]
- Guo, X.; Yu, Q.; Chen, D.; Wei, J.; Yang, P.; Yu, J.; Wang, X.; Kang, L. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 2020, 584, 584–588. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Guo, X.J.; Kang, L. Phenotypic Plasticity in Locusts: Trade-Off Between Migration and Reproduction. Annu. Rev. Entomol. 2024; In Press. [Google Scholar]
- Joern, A. Context-dependent foraging and enemy-free space: Acridoidea sparrows (Ammodramus savannarum) searching for Acridoidea (Acrididae). Écoscience 2016, 9, 231–240. [Google Scholar] [CrossRef]
- Word, M.L.; Hall, S.J.; Robinson, B.E.; Manneh, B.; Beye, A.; Cease, A.J. Soil-targeted interventions could alleviate locust and grasshopper pest pressure in West Africa. Sci. Total Environ. 2019, 663, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Herzog, S.K.; Fjeldså, J. Species richness and endemism of plant and bird communities along two gradients of elevation, humidity and land use in the Bolivian Andes. Divers. Distrib. 2001, 7, 61–77. [Google Scholar] [CrossRef]
- Zhang, L.B.; Feng, D.; Yang, J.Y.; Xie, C.H.; Zhou, Z.H.; Cao, D.H.; Chen, P. Flight Path and Habitat Distribution of Immigration Typeyellow Spined Bamboo Locusts (Ceracris kiangsu Tsai) in Yunnan, China. J. West China For. Sci. 2023, 52, 103–109+127. [Google Scholar]
- Rahbek, C. The role of spatial scale and the perception of large scale species-richness patterns. Ecol. Lett. 2005, 8, 224–239. [Google Scholar] [CrossRef]
- Wan Zaki, W.M.; Yahya, M.S.; Norhisham, A.R.; Sanusi, R.; van der Meer, P.J.; Azhar, B. Agroforestry orchards support greater butterfly diversity than monoculture plantations in the tropics. Oecologia 2023, 201, 863–875. [Google Scholar] [CrossRef]
- Qiao, Y.M.; Lian, Z.M.; Hu, Y.Q. Vertical distribution of Acridoidea in Balikun of Xinjang. J. Shaanxi Norm. Univ. (Nat. Sci. Ed.) 2001, 29, 71–74. [Google Scholar]
- Liang, J.; Ding, Z.; Lie, G. Species richness patterns of vascular plants and their drivers along an elevational gradient in the central Himalayas. Glob. Ecol. Conserv. 2020, 24, e01279. [Google Scholar] [CrossRef]
- de Souza Amorim, D.; Brown, B.V.; Boscolo, D.; Ale-Rocha, R.; Alvarez-Garcia, D.M.; Balbi, M.I.P.A.; de Marco Barbosa, A.; Capellari, R.S.; de Carvalho, C.J.B.; Couri, M.S.; et al. Vertical stratification of insect abundance and species richness in an Amazonian tropical forest. Sci. Rep. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Zergoun, Y.; Guezoul, O.; Sekour, M. Effects of temperatures and rainfall variability on the abundance and diversity of Caelifera (Insecta, Orthoptera) in three natural environments in the Mzab Valley, Septentrional Sahara (Algeria). Tunis. J. Plant Prot. 2018, 13, 217–228. [Google Scholar]
- Cease, A.J. How Nutrients Mediate the Impacts of Global Change on Locust Outbreaks. Annu. Rev. Entomol. 2024, 69, 527–550. [Google Scholar] [CrossRef]
- Gardiner, T.; Hassall, M. Does microclimate affect Acridoidea populations after cutting of hay in improved grassland? J. Insect Conserv. 2009, 13, 97–102. [Google Scholar] [CrossRef]
- Youngblood, J.P.; Cease, A.J.; Talal, S.; Copa, F.; Medina, H.E.; Rojas, J.E.; Trumper, E.V. Climate change expected to improve digestive rate and trigger range expansion in outbreaking locusts. Ecol. Monogr. 2022, 93, e1550. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J. Mountain biodiversity, species distribution and ecosystem functioning in a changing world. Diversity 2023, 15, 799. [Google Scholar] [CrossRef]
- Sergeev, M.G. Ecogeographical distribution of Orthoptera. In The Bionomics of Acridoidea, Katydids and Their Kin; Gangwere, S.K., Muralirangan, M.C., Muralirangan, M., Eds.; CAB International: Oxon, UK; New York, NY, USA, 1997; pp. 129–146. [Google Scholar]
- Joern, A.; Gaines, S.B. Population dynamics and regulation in grasshoppers. In Biology of Grasshoppers; Chapman, R.F., Joern, A., Eds.; John Wiley and Sons: New York, NY, USA, 1990; pp. 415–482. [Google Scholar]
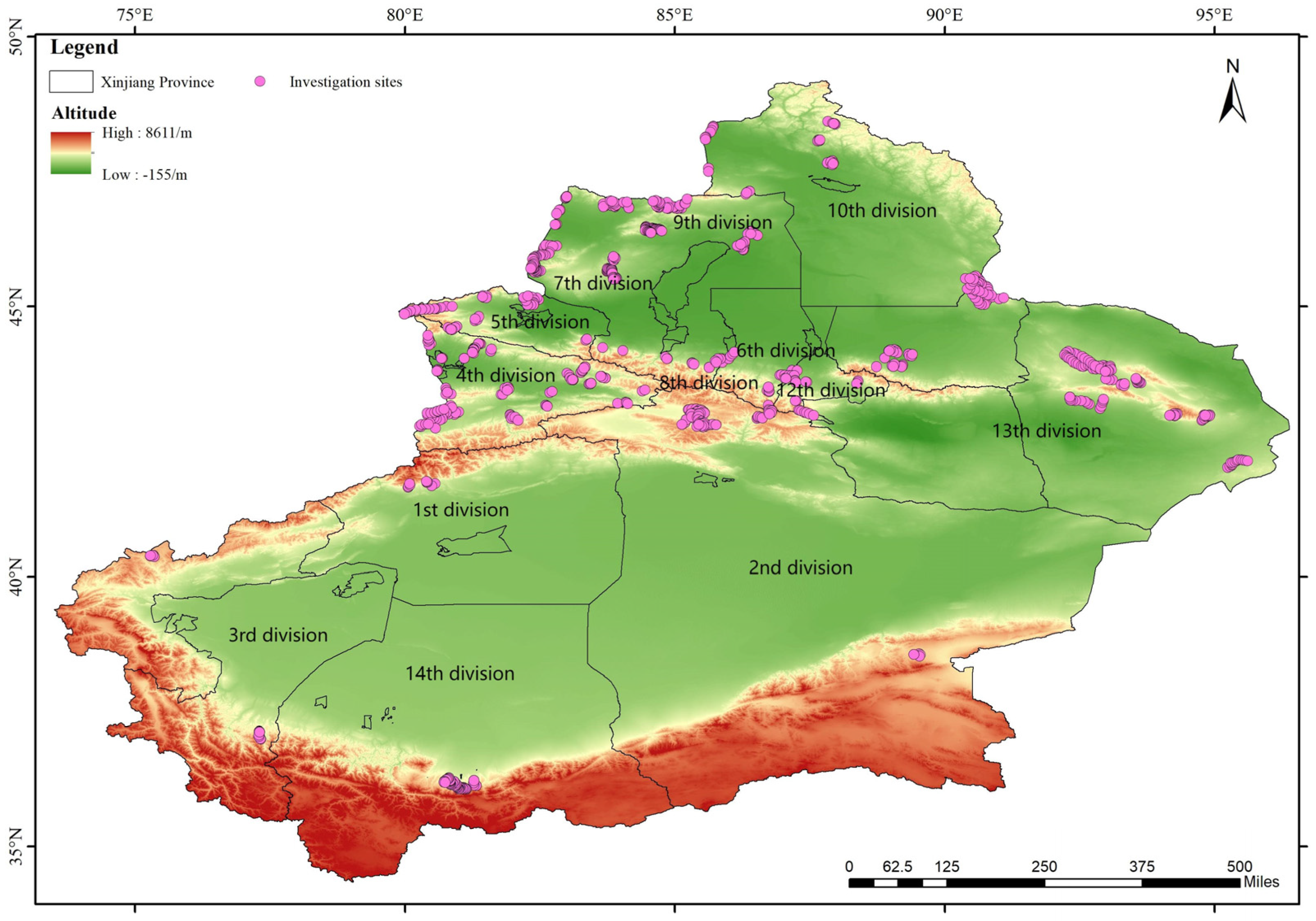


| Division Headquarter | Area | Grassland Type | Grassland Area | Regiment Field Distribution |
|---|---|---|---|---|
| 1st division | Aksu prefecture | Temperate grasslands, alpine meadows, lowland meadows, and mountain meadows | 2.3373 | 4th and 5th regiments |
| 2nd division | Bayin’guoleng Mongol autonomous prefecture of Xinjiang | Temperate grasslands, alpine meadows, and lowland meadows | 18.4 | 21st, 22nd, 24th, 25th, 27th, 29th, 30th, 36th, and 223rd regiments |
| 3rd division | Kashgar or Kāshí prefecture | Alpine meadows, temperate desert grasslands, temperate meadow grasslands, temperate deserts, and lowland meadows | 11.31 | Toyun Ranch and Yecheng Second Ranch |
| 4th division | Ili Kazakh autonomous prefecture | Temperate grasslands, temperate meadow grasslands, mountain meadows, lowland meadows, alpine meadows, temperate deserts, and temperate desert grasslands | 19.17 | 61st, 63rd, 64th, 66th, 67th, 70th, 71st, 72nd, 73rd, 74th, 76th, 77th, 78th, and 79th regiments |
| 5th division | Börtala Mongol autonomous prefecture | Temperate grasslands, temperate meadow grasslands, mountain meadows, lowland meadows, alpine meadows, temperate deserts, and temperate desert grasslands | 13.16 | 83rd, 84th, 86th, 87th, 88th, 89th, 90th, and 91st regiments |
| 6th division | Changji prefecture | Temperate grasslands, temperate deserts, temperate desert grasslands, and temperate steppe deserts | 26.59 | Beitashan Ranch, Hongqi Ranch, and Qitai Ranch |
| 8th division | Temperate grasslands, temperate deserts, mountain meadows, temperate desert grasslands, lowland meadows, and alpine meadows | 7.36 | 142nd, 143rd, and 151st regiments | |
| 12th division | Temperate grasslands, temperate meadow grasslands, mountain meadows, temperate desert grasslands, lowland meadows, alpine meadows, temperate deserts, and temperate steppe deserts | 19.92 | 104th regiment | |
| 7th division | Tacheng prefecture | Temperate grasslands, temperate deserts, mountain meadows, lowland meadows, temperate meadow grasslands, temperate desert grasslands, and alpine meadows | 10.44 | 124th, 131st, and 137th regiments |
| 9th division | Lowland meadows, temperate grasslands, temperate deserts, temperate desert grasslands, mountain meadows, temperate meadow grasslands, alpine meadows, and temperate steppe deserts | 13.65 | 161st, 162nd, 163rd, 164th, 165th, 166th, 167th, 168th, and 170th regiments | |
| 10th division | Altay prefecture | Temperate meadow grasslands, temperate grasslands, temperate deserts, temperate desert grasslands, mountain meadows, lowland meadows, alpine meadows, and temperate steppe deserts | 9.29 | 181st, 182nd, 183rd, 184th, 185th, 186th, 187th, and 188th regiments |
| 13th division | Kumul prefecture | Temperate grasslands, temperate desert grasslands, alpine meadows, temperate deserts, lowland meadows, and temperate steppe deserts | 18.59 | Red Hill Farm, Red Star Farm, Yellowfield Farm, and Willow Springs Farm |
| 14th division | Khotan prefecture | Temperate grasslands, temperate desert grasslands, alpine meadows, temperate deserts, lowland meadows, and temperate steppe deserts | 7.61 | I Ranch, 47th regiment |
| Division | Number of Families | Number of Genera | Number of Species | Number of Specimens | Dominant Species |
|---|---|---|---|---|---|
| 1st division | 2 | 2 | 2 | 7 | Chorthippus biguttulus Gomphocerus sibiricus |
| 2nd division | 2 | 4 | 7 | 68 | Myrmeleotettix palpalis |
| 3rd division | 2 | 3 | 7 | 13 | Omocestus rufipes |
| 4th division | 6 | 27 | 60 | 3130 | Dociostaurus brevicollis |
| 5th division | 5 | 21 | 39 | 616 | Calliptamus barbarus Calliptamus italicus |
| 6th division | 4 | 9 | 15 | 49 | Sphingonotus nebulosus Stauroderus scalaris |
| 7th division | 4 | 12 | 22 | 224 | Gomphocerus sibiricus Omocestus haemorrhoidalis |
| 8th division | 4 | 12 | 20 | 772 | Calliptamus italicus Omocestus haemorrhoidalis Notostaurus albicornis |
| 9th division | 4 | 16 | 30 | 235 | Calliptamus barbarus Calliptamus italicus Oedaleus decorus |
| 10th division | 3 | 7 | 11 | 55 | Bryodemella zaisanica ferrugineums Sphingonotus eurasius Myrmeleotettix brachypterus |
| 12th division | 3 | 10 | 12 | 82 | Omocestus haemorrhoidalis Oedaleus decorus |
| 13th division | 1 | 3 | 7 | 19 | Sphingonotus obscuratus |
| 14th division | 1 | 3 | 6 | 20 | Chorthippus maritimushuabeiensis |
| Division | H | E |
|---|---|---|
| 1st division | 0.598 | 0.863 |
| 2nd division | 1.491 | 0.766 |
| 3rd division | 1.692 | 0.87 |
| 4th division | 2.883 | 0.704 |
| 5th division | 2.979 | 0.813 |
| 6th division | 2.169 | 0.800 |
| 7th division | 2.349 | 0.759 |
| 8th division | 2.167 | 0.730 |
| 9th division | 2.795 | 0.822 |
| 10th division | 2.17 | 0.905 |
| 12th division | 2.025 | 0.815 |
| 13th division | 1.837 | 0.944 |
| 14th division | 1.657 | 0.925 |
| Grassland Types | Lowland Meadow | Alpine Meadow | Mountain Meadow | Temperate Grassland | Temperate Meadow Grassland | Temperate Desert | Temperate Desert Grassland | Temperate Steppe Desert |
|---|---|---|---|---|---|---|---|---|
| Arcypteridae | 14 | 10 | 29 | 28 | 14 | 8 | 2 | 2 |
| Oedipodidae | 7 | 1 | 10 | 5 | 0 | 24 | 12 | 12 |
| Gomphoceridae | 3 | 3 | 1 | 5 | 0 | 0 | 0 | 0 |
| Acrididae | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 |
| Catantopidae | 3 | 3 | 0 | 3 | 0 | 3 | 3 | 3 |
| Pamphagidae | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Chrotogonidae | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pyrgomorphidae | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of genera | 16 | 10 | 18 | 22 | 3 | 16 | 8 | 7 |
| Number of species | 28 | 18 | 42 | 43 | 14 | 38 | 17 | 17 |
| Grassland Type | Number of Species | Number of Specimens | H | E |
|---|---|---|---|---|
| Lowland meadow | 28 | 361 | 2.505 | 0.752 |
| Alpine meadow | 18 | 86 | 2.421 | 0.837 |
| Mountain meadow | 42 | 1257 | 2.563 | 0.686 |
| Temperate grassland | 43 | 1351 | 3.053 | 0.812 |
| Temperate meadow grassland | 14 | 53 | 2.055 | 0.778 |
| Temperate deserts | 38 | 1527 | 2.437 | 0.670 |
| Temperate desert grassland | 17 | 447 | 1.887 | 0.666 |
| Temperate steppe desert | 17 | 208 | 1.703 | 0.601 |
| Altitude (Meters) | Number of Species | Number of Specimens | H | E |
|---|---|---|---|---|
| 0–500 | 40 | 771 | 2.906 | 0.788 |
| 500–1000 | 52 | 1368 | 2.976 | 0.743 |
| 1000–1500 | 66 | 1831 | 3.237 | 0.725 |
| 1500–2000 | 35 | 917 | 2.867 | 0.806 |
| 2000–2500 | 26 | 185 | 2.634 | 0.806 |
| 2500–3000 | 20 | 132 | 2.568 | 0.815 |
| 3000–3500 | 18 | 86 | 2.421 | 0.837 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, S.; He, Y.; Yuan, G.; Pu, X.; Zhou, C. Influence of Grassland Habitats on Acridoidea (Orthoptera) Species Diversity in Different Divisions of the Xinjiang Production and Construction Corps. Biology 2025, 14, 14. https://doi.org/10.3390/biology14010014
Liu Y, Wang S, He Y, Yuan G, Pu X, Zhou C. Influence of Grassland Habitats on Acridoidea (Orthoptera) Species Diversity in Different Divisions of the Xinjiang Production and Construction Corps. Biology. 2025; 14(1):14. https://doi.org/10.3390/biology14010014
Chicago/Turabian StyleLiu, Yuxian, Shaoshan Wang, Yuheng He, Guanzheng Yuan, Xingyu Pu, and Chao Zhou. 2025. "Influence of Grassland Habitats on Acridoidea (Orthoptera) Species Diversity in Different Divisions of the Xinjiang Production and Construction Corps" Biology 14, no. 1: 14. https://doi.org/10.3390/biology14010014
APA StyleLiu, Y., Wang, S., He, Y., Yuan, G., Pu, X., & Zhou, C. (2025). Influence of Grassland Habitats on Acridoidea (Orthoptera) Species Diversity in Different Divisions of the Xinjiang Production and Construction Corps. Biology, 14(1), 14. https://doi.org/10.3390/biology14010014




