Simple Summary
CKD is a common outcome of the progressive deterioration caused by various primary and secondary kidney diseases. It is characterized by renal interstitial fibrosis and a progressive decline in renal function. As CKD advances, patients often experience disturbances in iron and lipid metabolism, along with inflammation and oxidative stress, leading to tubular epithelial cell death. Ferroptosis, an iron-dependent form of cell death driven by lipid peroxidation, plays a crucial role in tissue and organ fibrosis. This review highlights the potential mechanisms by which ferroptosis promotes renal fibrosis and explores the therapeutic potential of targeting ferroptosis to treat kidney fibrosis.
Abstract
Chronic kidney disease (CKD) is a global health concern caused by conditions such as hypertension, diabetes, hyperlipidemia, and chronic nephritis, leading to structural and functional kidney injury. Kidney fibrosis is a common outcome of CKD progression, with abnormal fatty acid oxidation (FAO) disrupting renal energy homeostasis and leading to functional impairments. This results in maladaptive repair mechanisms and the secretion of profibrotic factors, and exacerbates renal fibrosis. Understanding the molecular mechanisms of renal fibrosis is crucial for delaying CKD progression. Ferroptosis is a type of discovered an iron-dependent lipid peroxidation-regulated cell death. Notably, Ferroptosis contributes to tissue and organ fibrosis, which is correlated with the degree of renal fibrosis. This study aims to clarify the complex mechanisms of ferroptosis in renal parenchymal cells and explore how ferroptosis intervention may help alleviate renal fibrosis, particularly by addressing the gap in CKD mechanisms related to abnormal lipid metabolism under the ferroptosis context. The goal is to provide a new theoretical basis for clinically delaying CKD progression.
1. Introduction
CKD is a leading cause of mortality worldwide, affecting 15–20% of adults worldwide [1]. Primary and secondary nephropathies are the major contributors of chronic kidney injury, with the persistent synthesis and accumulation of fibrous matrix in the interstitial space leading to extensive scarring of the kidneys. This results in tubular interstitial fibrosis and glomerulosclerosis, accelerating the progression to end-stage renal disease (ESRD) [2]. CKD also causes a range of complications, including cardiovascular diseases, anemia, metabolic acidosis, and disruptions in bone mineral metabolism. Under chronic stimulation, several types of programmed cell death are triggered, including ferroptosis. The cellular release of damage-associated molecular patterns (DAMPs) is promoted by ferroptosis, which contributes to the progression of CKD (Figure 1).
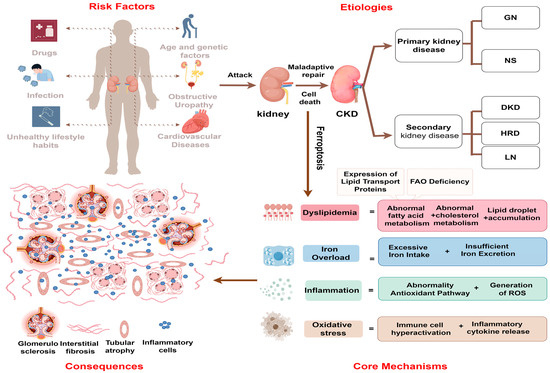
Figure 1.
The causes and consequences of ferroptosis in cells during CKD. Various risk factors such as aging, infection, and the prolonged toxic effects of nephrotoxic drugs disrupt renal energy metabolism, damage the immune barrier, and lead to metabolic disorders in renal parenchymal cells. This results in iron overload, lipid accumulation, inflammation, and oxidative stress, collectively activating ferroptosis, which spreads among renal parenchymal cells, damaging tubular structure and function. The maladaptive repair process leads to glomerulosclerosis, interstitial fibrosis, and increased hyperfiltration pressure. GN, glomerular nephritis; NS, nephrosis syndrome; DN, diabetic nephropathy; HRD, hypertensive renal disease; LN, lupus nephritis.
Ferroptosis is an iron-dependent, nonapoptotic form of regulated cell death (RCD) [3]. It is characterized by decreased activity of glutathione peroxidase 4 (GPX4), reduced antioxidant capacity, the accumulation of lipid reactive oxygen species (ROS), and the consumption of polyunsaturated fatty acids (PUFAs) in the plasma membrane. Morphologically, ferroptotic cells exhibit smaller mitochondria, an increased membrane density, and reduced cristae morphologically [3]. Ferroptosis is involved in the pathogenesis of fibrotic diseases. The imbalance of intracellular iron and redox homeostasis during ferroptosis disrupts the secretion of transforming growth factor-beta (TGF-β) in the fibrotic process. Simultaneously, ferroptosis increases intracellular free iron, which generates more ROS and exacerbates fibrosis [4]. Iron levels are correlated with the extent of fibrosis and kidney atrophy, and early noninvasive methods, such as magnetic resonance imaging (MRI), can be used to assess iron content in fibrotic kidneys, enhancing CKD diagnosis [5]. In fibrotic kidney injury, proximal tubule cells express proinflammatory and profibrotic factors, with ferroptosis acting as a critical susceptibility pathway [6]. Furthermore, pharmacologically inducing ferroptosis can hinder the progression of renal fibrosis and promote adaptive repair in damaged cells [7,8,9,10]. These findings highlight the pivotal role of ferroptosis in the progression in renal fibrosis. This review aims to elucidate the molecular mechanisms underlying ferroptosis, focusing on the links between ectopic lipid deposition, lipotoxicity, and ferroptosis in CKD, and provides new insights for future CKD treatment strategies.
2. General Mechanisms of Ferroptosis
This section provides an overview of the oxidative and antioxidant pathways involved in the mechanisms of ferroptosis, highlighting the key regulatory molecules involved (Figure 2). Research into the mechanisms of ferroptosis has enhanced our understanding of this process.
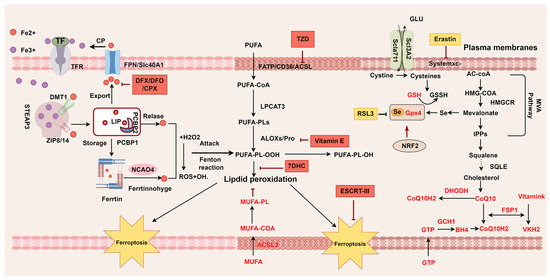
Figure 2.
Mechanisms of ferroptosis. Ferroptosis is characterized by imbalances between oxidative and antioxidant pathways. Iron overload and PUFAs drive the production of oxidized lipids, which are key contributors to ferroptotic cell death. Several regulatory factors and monounsaturated fatty acids (MUFAs) play crucial roles in counteracting oxidative stress and providing antioxidant protection. TF transferrin; TBI transferrin-bound iron; TfR transferrin receptor; ZIP Zrt- and Irt-like family proteins; DMT1 divalent metal transporter 1; FTH ferritin heavy; FTL ferritin light; STEAP3 endosomal ferrireductase 3; PCBP iron chaperones of the poly-rC-binding protein family; FPN ferroportin; NCOA4 nuclear coactivator 4; NRF2 nuclear factor erythroid2-related factor 2; LIP labile iron pool; MUFA monounsaturated fatty acid; PUFA polyunsaturated fatty acid; FATP fatty acid transport protein; CD36 CD36 molecule; ACSL acyl-CoA synthetase long-chain family member; LPCAT3 Lysophosphatidylcholine Acyltransferase 3; NOXs NADPH oxidases; ALOX Lipoxygenase; SLC7A11 solute carrier family 7 member 11; SLC3A12 solute carrier family 3 member 2; Glu glutamic acid; GPX4 glutathione peroxide 4; GSH glutathione; GSSG glutathione disulfide; GTP guanosine triphosphate; DHODH dihydroorotate dehydrogenase; LOOH lipid hydroperoxides; LOH lipid alcohols; FSP1 ferroptosis suppressor protein 1; GTP Guanosine triphosphate;BH4 tetrahydrobioptrin; GCH1 GTP cyclohydroxylase1; IPP isopentenyl-pyrophosphate; MVA; mevalonatepath; HMG-CoA 3-hydroxy-3-methylglutaryl CoA; DFX Deferasirox; DFO Deferoxamine; CPX ciclopirox; TZD Thiazolidinediones; 7-DHC 7-dehydrocholesterol. This picture was drawn by Figdraw 2.0.
2.1. Driving Ferroptosis: Iron Overload
Iron overload initiates ferroptosis by driving redox reactions under pathological conditions. Even subtle changes in intracellular iron levels can affect iron homeostasis. Transferrin (TF) captures extracellular iron and binds to transferrin receptors (TFRs) to deliver transferrin-bound iron (TBI) into cells [11,12]. TFR1 serves as a biomarker that distinguishes ferroptosis from other forms of cell death [13]. When the endosomal pH decreases to 5.5–6.5, Fe3+-TF is converted to Fe2+ by six-transmembrane epithelial antigen of prostate 3 (STEAP3) and transported to the cytoplasm via metal-ion transporter-1 (DMT1/SLC11A2) or ZRT/IRT-like protein (ZIP) 8 or 14, forming a labile iron pool (LIP). Chaperones like Poly (rC)-binding protein (PCBP) regulate Fe2+ in the LIP and bind glutathione with low affinity to maintain iron homeostasis [14,15,16]. Excess iron is stored in ferritin, which consists of two subunits, FTH (which oxidizes Fe2+ to Fe3+) and FTL (which aids in iron mineralization and core formation) [3]. Nuclear coactivator 4 (NCOA4) selectively mediates ferritinophagy in the lysosome, releasing Fe2+ and triggering ferroptosis [17,18,19,20]. The only cellular iron export pathway is regulated by hepcidin through Ferroportin (FPN/SLC40A1) [21,22]. Hepcidin, a hormone synthesized by the liver, binds to and degrades Ferroportin (FPN), reducing intracellular iron efflux [23,24]. The exported Fe2+ is then oxidized to Fe3+ by ceruloplasmin (CP), allowing it to re-enter the intracellular iron cycle.
2.2. Ferroptosis Execution: Formation of Lipid Peroxidation Substrates
PUFAs are the primary targets of lipid peroxidation, and their accumulation of lipid peroxides ultimately triggers ferroptosis [25]. The intake, metabolism, and synthesis of fatty acids, phospholipids, and cholesterol are critical processes, with arachidonic acid (AA) and adrenic acid (AdA) being key contributors to ferroptosis [26]. AA and AdA are acylated by acyl-CoA synthetase long-chain family member 4 (ACSL4) and esterified by lysophosphatidylcholine acyltransferase 3 (LPCAT3) to form AA/AdA-PE [27]. These molecules are then oxidized by lipoxygenase (LOX) or cytochrome P450 reductase (POR) to generate AA/AdA-PE-OOH [28,29]. In contrast, ACSL3 acylates monounsaturated fatty acids (MUFAs), displacing PUFAs in cell membranes and reducing lipid peroxidation [30]. The balance between ACSL4-PUFAs and ACSL3-MUFAs plays a crucial role in determining membrane stability [31,32,33].
2.3. Accelerating Ferroptosis: Imbalance in Antioxidant Pathways
2.3.1. Classical Antioxidant Pathway: System Xc-GSH—GPX4
The antioxidant system is crucial for cellular redox reaction balance, with the system Xc-GSH-GPX4 axis serving as the earliest and key regulator in halting ferroptosis. This system is fundamental to antioxidant defense. System Xc- consists of two subunits: SLC7A11 (also known as xCT) and SLC3A2. Its activity and expression are influenced by external factors. For instance, the activation of the TP53 gene downregulates SLC7A11 expression, while elevated extracellular glutamate levels inhibit cysteine uptake via System Xc [34,35,36,37]. L-cysteine, a key amino acid, limits the synthesis of GSH and GPX4 [3], with selenocysteine being an essential amino acid for the active site of GPX4. The inhibition of the mevalonate pathway impairs selenocysteine tRNA maturation, disrupting GPX4 synthesis [38]. GSH acts as a reducing substrate for GPX4 and a free radical scavenger. GPX4 is critical for protecting cells from ferroptosis. NRF2 regulates antioxidant gene expression to maintain redox balance. It can upregulate GPX4 expression, directly or indirectly, protecting cells from ferroptosis [39,40,41,42]. NRF2 also activates System Xc- to increase intracellular cysteine levels and modulates the mTORC1/4EBP1/GPX4 pathway to enhance GPX4 synthesis and reduce its degradation [43]. Focusing on NRF2 agonists, which activate the Nrf2/GPX4 signaling pathway to inhibit ferroptosis, could be a promising future therapeutic target in CKD. The subcellular localization of different GPX4 isoforms (cytoplasm, nucleus, and mitochondria) determines their specific functions. In conclusion, the system Xc-GSH-GPX4 pathway is intricately involved in the sophisticated antioxidant defense system that regulates ferroptosis.
2.3.2. Parallel Antioxidant Pathway: FSP1-CoQ10-NAD(P)H
Metabolic pathways parallel to GPX4 also contribute to the mitigation of ferroptosis. Ferroptosis suppressor protein 1 (FSP1) plays a crucial role in preventing ferroptosis independently of GPX4 via the FSP1-CoQ10-NAD(P)H pathway [44,45]. FSP1, located in lipid droplets and the plasma membrane, utilizes NADPH to convert coenzyme Q10 (CoQ10) into ubiquinol, which neutralizes oxygen free radicals and reduces ferroptosis [46]. CoQ10, essential for mitochondrial electron transfer, regenerates α-tocopherol, which scavenges free radicals and prevents ferroptosis.
Research also shows that FSP1 protects against ferroptosis by enhancing cell membrane repair through an ESCRT-III-FSP1 mechanism, independent of CoQ10 [47,48,49]. Additionally, various forms of vitamin K, including phylloquinone, MK4, and menadione, inhibit ferroptosis in mouse embryonic fibroblasts and protect against ischemia–reperfusion injury in liver and kidney models [50,51].
GTP cyclohydrolase I (GTPCH-I), the rate-limiting enzyme in the synthesis of BH4, plays a protective role by scavenging oxidants [52,53]. BH4 strongly counteracts ferroptosis induced by inducers and GPX4 knockout [27], making the GCH1-BH4 pathway a key anti-ferroptosis by selectively preventing phospholipid depletion. The pathways described above are involved in ferroptosis, and a deeper investigation into the intricate molecular mechanisms underlying these processes is warranted.
2.3.3. Mitochondrial Antioxidant Pathway
Mitochondrial dysfunction, due to impaired antioxidant pathways, is one of the earliest observed organelle changes observed during ferroptosis, underscoring its critical role in this process. DHODH catalyzes the reduction in ubiquinone (CoQ) to dihydroubiquinone (CoQH2), which acts as an antioxidant by preventing the formation of lipid peroxides in the cell membrane, thus inhibiting ferroptosis. Recent research indicated that mitochondrial DHODH and GPX4 collaborate to suppress ferroptosis. Inhibiting DHODH with Brequinar (BQR) induces ferroptosis in cells with low GPX4 expression, increasing their susceptibility to ferroptosis [54,55]. Moreover, combining BQR with the system Xc- inhibitor sulfasalazine effectively halts cancer cell proliferation, irrespective of their GPX4 levels [56]. The DHODH-CoQH2 pathway operates independently of cytoplasmic GPX4 or FSP1, suggesting that further investigation into other subcellular compartments may reveal additional mechanisms for ferroptosis defense.
3. The Foundation of Ferroptosis in CKD
The activation of ferroptosis is closely associated with key pathophysiological processes in CKD, including inflammation, immune cell accumulation, and lipid metabolism disorders. These contribute to sustained renal damage and dysfunction, facilitating the transition from AKI to CKD and ultimately leading to irreversible fibrosis [57]. Preventing fibrosis is crucial for slowing CKD progression. The pathogenic mechanisms underlying fibrosis in CKD are sophisticated, and emerging research suggests a strong link between ferroptosis and the development of renal fibrosis.
3.1. Inflammation Trigger for CKD
Inflammation is crucial in kidney repair, leading to tubular damage, fibrosis, and CKD progression [58,59]. Continuous damage to immune cells and renal parenchymal cells attracts inflammatory cells and drives the release of cytokines, chemokines, and adhesion molecules [60,61]. This section explores how immune and renal parenchymal cell interactions during inflammation trigger CKD mechanisms, highlighting the potential pathways of ferroptosis in CKD.
3.1.1. Activate of Innate Immune Cells
Within the first 48 h, necrotic renal tubular epithelial cells (TECs) release DAMPs and ROS, which are recognized by macrophage receptors, leading to M1 polarization and the production of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6. After 48 h, macrophages shift to the M2 phenotype, where they secrete repair-promoting cytokines (IL-10 and IL-1Rα) and profibrotic factors (TGF-β1, FGF-2, PDGF, and MMP-9) that activate fibroblasts during renal injury. This shift fosters myofibroblast proliferation in the renal interstitium, contributing to glomerular and tubular capillary damage [62,63]. Renal fibrosis is largely driven by imbalanced M1/M2 macrophage polarization and persistent inflammation.
Dendritic cells (DCs) play a dual role in renal inflammation. On one hand, they recruit T cells and mediate the release of toxic mediators, leading to acute tubular interstitial damage and chronic glomerulonephritis. On the other hand, they promote the activation of regulatory T cells, resolving the immune response and facilitating renal repair [64].
Neutrophils respond swiftly to renal injury by interacting with endothelial chemokines and adhesion molecules during the initial injury stages. In a unilateral ureteral obstruction (UUO) mouse model, neutrophil depletion reduced inflammatory factor expression and inhibited kidney fibrosis [65].
T cells are central to inflammation, and the pathogenic role of CD4+ T cells has been identified in the UUO model [66]. In mouse models of ischemia and nephrotoxicity, CD8+ T cell deficiency provided less protection compared to CD4+ T cell deficiency [67,68].Activated CD4+ T cells differentiate into various subsets, each with distinct effects [69]. Treg cells stimulated by IL-2 and IL-33 help mitigate persistent renal injury [70]. Furthermore, immunosuppressants such as sirolimus and mycophenolate mofetil have been demonstrated to reduce renal fibrosis in animal models of obstructive nephropathy [71,72,73,74].
B cells are involved in various immune processes, and research on B cells in renal fibrosis remains limited. The initial studies highlighted B cell interactions with other immune cells during renal injury, potentially driving the progression from AKI to CKD. Early B cell infiltration is linked to macrophage accumulation and acute inflammation in UUO. Depleting B cells reduces macrophage infiltration and renal fibrosis, highlighting the significant role of B cells in renal fibrosis development [75]. Prolonged B cell targeting could improve renal function and impede the progression of CKD [76].
3.1.2. Maladaptive Renal Repair
TECs are particularly vulnerable to external injury. While mild damage is often repairable, severe or persistent injury activates pattern recognition receptors in TECs. This triggers the release of high mobility group box protein 1 (HMGB1), which stimulates Toll-like receptor 4 (TLR4) in TECs, leading to NF-κB pathway activation and the production of inflammatory factors such as IL-6, TNF-α, and MCP-1 [77]. TECs also secrete chemokines like MCP-1, recruiting macrophages and mesangial cells to produce TGF-β1 in renal failure [78]. If TECs lose their regenerative, irreversible basement membrane, thickening and interstitial fibrosis occur.
During injury, endothelial cells recruit leukocytes, promoting immune cell adhesion, which exacerbates ischemia and tubular injury [79,80,81]. Acute inflammation can transition to chronic inflammation [82], prompting endothelial cells to express chemokines and adhesion molecules in response to the local inflammatory environment [82,83]. Persistent inflammation causes local endothelial cell proliferation, and some undergo endothelial-to-mesenchymal transition, driving renal fibrosis progression [80].
Mesangial cells release proinflammatory factors, including TNF-α and IL-6, along with oxidants and growth factors in kidney injury [84]. This promotes mesangial cell proliferation, alters endothelial permeability, and increases extracellular matrix (ECM) production, ultimately leading to glomerulosclerosis [85,86]. Damage to podocytes not only triggers inflammatory responses but also disrupts the filtration barrier, leading to proteinuria and tubular degeneration, which further worsen kidney dysfunction [87].
3.2. Ectopic Lipid Deposition
Understanding the process of lipid metabolism in a healthy kidney is crucial for exploring lipid-related kidney damage (Figure 3). Excessive and unprocessed lipids accumulation, characterized by elevated triglycerides (TGs) and decreased FAO, leads to organelle dysfunction, cell injury, oxidative stress, autophagy dysregulation, and inflammation—a condition known as lipid nephrotoxicity [88]. This condition disrupts energy metabolism and is a key driver of renal fibrosis. The following sections examine the impact of fatty acid and cholesterol toxicity on CKD.
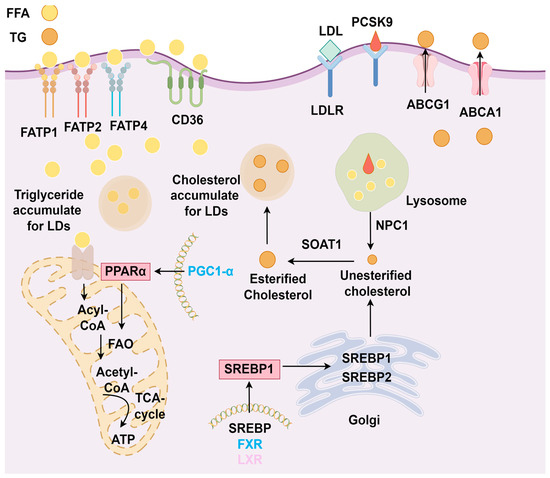
Figure 3.
Physiological mechanisms of lipid metabolism in the kidney. In proximal tubular cells, FATP and CD36 on podocytes take up free fatty acids (FFAs) from the bloodstream for fatty acid oxidation (FAO) to produce ATP. Peroxisome proliferator-activated receptor α (PPARα), PPARγ coactivator 1α (PGC-1α), and AMP-activated protein kinase (AMPK) regulate FAO. Excess FAs accumulate and are stored as lipid droplets. Liver X receptor (LXR) and farnesoid X receptor (FXR) regulate the expression of sterol regulatory element-binding protein 1 (SREBP1). SREBP1 and SREBP2 are transported from the endoplasmic reticulum to the Golgi apparatus, where they are cleaved and then transported to the nucleus to synthesize unesterified cholesterol. This cholesterol is subsequently converted into esterified cholesterol (CE) by sterol O-acyltransferase 1 (SOAT1) or transported to the plasma membrane by ATP-binding cassette transporters A1 (ABCA1) and G1 (ABCG1) to form low-density lipoprotein (LDL) and high-density lipoprotein (HDL). Proprotein convertase subtilisin/kexin type 9 (PCSK9) binds and degrades LDL to maintain cholesterol metabolism balance. The accumulation of free cholesterol (FC) results in the formation of lipid droplets, maintaining normal levels of fatty acids and cholesterol in the kidney.
3.2.1. Fatty Acid Toxicity
Fatty acid homeostasis is an important component in kidney lipid metabolism. Renal tubules, rich in mitochondria, rely on FAs for energy under physiological conditions. Under low-glucose conditions, endothelial and mesangial cells demand FAO for energy.
Defective FAO contributes to the progression of renal fibrosis. The overexpression of the key upstream FAO transcription factor PGC-1α in TECs restores the FAO rate-limiting enzyme CPT1, reducing fibrosis induced by folic acid [89].The PPARα agonist fenofibrate protects against interstitial fibrosis in a mouse model of ischemia–reperfusion injury [90]. AMP-activated protein kinase (AMPK), a key upstream signaling molecule, also regulates the expression of CPT-1α. Studies have shown that AMPK activity is associated with kidney lipid accumulation and lipotoxicity. In a rat model of non-diabetic renal interstitial fibrosis, AMPK activation corrects FAO dysfunction in TECs and improves kidney fibrosis [91].
FAs are transported into cells mainly through fatty acid transport proteins (FATP1, FATP2, and FATP4), while epithelial and mesangial cells utilize CD36 for FA uptake. Podocytes primarily take up FA through CD36. Palmitic acid treatment increases CD36 levels in podocytes, causing intracellular lipid accumulation and ROS production, mitochondrial dysfunction, and elevated profibrotic factors, ultimately contributing to glomerular injury [92]. Proximal tubules depend on FAO for energy, while CD36 and FATP2 facilitate FA uptake and transport. In a UUO mouse model, the inhibition or knockout of FATP2 in proximal TECs (PTECs) prevents lipotoxicity [93]. In 8-week-old mice, FA accumulation is observed, and by 20 weeks, a fibrotic phenotype emerges, indicating that CD36 accelerates disease progression through early lipid accumulation and that CD36 overexpression in PTECs promotes inflammation and fibrosis [94]. These mechanisms highlight the role of various kidney cells in FA uptake abnormalities, linking kidney fibrosis and fatty acid toxicity to the development of CKD.
3.2.2. Cholesterol Toxicity
Dysregulation of cholesterol metabolism is one of the hallmarks of CKD. Early studies showed that a high-cholesterol diet caused lipid deposition in rabbit kidneys [95]. Prospective cohort studies have also shown that familial hypercholesterolemia is linked to a heightened risk of CKD [96], highlighting cholesterol toxicity as a significant factor in CKD progression [97]. In CKD patients, increased SREBP expression accelerates cholesterol synthesis, leading to increased sterol O-acyltransferase 1 (SOAT1) activity and reduced neutral cholesterol ester hydrolase (NCEH) activity. Additionally, the nuclear translocation of ABCA1 and ABCG1 is inhibited, reducing their activity and preventing the elimination of excess cholesterol, causing cholesterol to accumulate in lipid droplets (LDs) [98,99].
Renal biopsies have revealed cholesterol accumulation in damaged podocytes of CKD patients [100,101]. Studies on diabetic nephropathy [102] and focal segmental glomerulosclerosis [103] have provided evidence that decreased ABCA1 expression in podocytes leads to significant cholesterol buildup and lipid droplet formation in the glomeruli. eGFR correlates positively with cholesterol metabolism, indicating that podocytes are key sites of cholesterol buildup. Cholesterol metabolism is disrupted in the TECs of fibrotic kidneys. In the TECs of fibrotic kidneys, excessive cholesterol induces endoplasmic reticulum stress and apoptosis [104], underscoring the role of cholesterol toxicity in kidney disease progression.
4. Key Mechanism of Ferroptosis in Advancement of CKD
Renal parenchymal cell damage in CKD is often accompanied by micronutrient imbalances, ectopic lipid deposition, immune cell activation inducing inflammation, and an imbalance in the antioxidant system. These factors accelerate lipid peroxidation and promote renal parenchymal cell death. Ferroptosis, which involves these processes, plays a role in the pathogenesis of CKD, presenting a potential target for therapeutic intervention. (Figure 4).
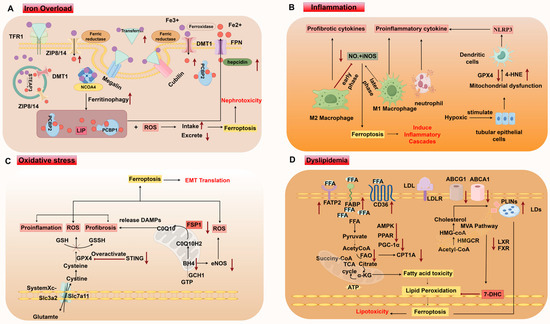
Figure 4.
Mechanisms of ferroptosis in CKD. (A) Iron overload. (B) Inflammation. (C) Oxidative Stress. (D) Dyslipidemia. LDL low-density lipoproteins; LDLR LDL receptor; ABCG1 ATP-binding cassette subfamily G member 1; ABCA1 ATP-binding cassette subfamily A member 1; LXR liver X receptor; FXR farnesoid X receptor; PPAR peroxisome proliferator-activated receptor; PGC-1α peroxisome proliferator-activated receptor-gamma coactivator 1α; AMPK; AMP-activated protein kinase; SREBP sterol regulatory binding proteins; PLINS perilipins; LD lipid droplets; FFA free fatty acids; FAO fatty acid oxidation; CPTA1 carnitine palmitoyltransferase 1A; HMGCR 3-hydroxy-3-methylglutaryl-CoA reductase; 7-DHC 7-dehydrocholesterol; STING stimulator of interferon genes; DAMPs damage-associated molecular patterns; TECs tubular epithelial cells. This picture is drawn in Figure 2.
4.1. Iron Overload Induces Nephrotoxicity
The kidneys meticulously regulate iron metabolism using various iron transport proteins and regulatory pathways throughout different segments of the nephron. Iron overload within the kidney can both cause and result from kidney injury, with ferroptosis linked to disruptions in iron metabolism, suggesting its role as a key etiological factor in CKD pathogenesis.
Iron overload induces nephrotoxicity [5]. In a 5/6 nephrectomy-induced CKD rat model, reducing iron intake disrupts iron metabolism and mitigates glomerular and tubular injury and fibrosis [105]. Elevated levels of TF and iron have been observed in the urine of patients with diabetic nephropathy and glomerulonephritis [106,107]. TFR1 and the Megalin–Cubilin complex are essential for iron uptake in PTEC, with differential transcriptional regulation affecting iron levels. Therefore, proximal tubules are capable of reabsorbing iron [108]. Additionally, ZIP8/14 promotes the involvement of both proximal and distal tubules in non-transferrin-bound iron (NTBI) uptake, accelerating iron absorption [109]. An increase in iron absorption could theoretically lead to intracellular iron overload, particularly in the distal tubules where ferroportin is absent, increasing susceptibility to ferroptosis.
Ferritin, abundant in the kidneys, affects the intracellular LIP. Therapeutic iron supplementation or FTH-specific knockout in renal macrophages can mitigate renal fibrosis and slow CKD progression [110,111]. This finding suggests that imbalances in FTH expression can lead to extremes in intracellular iron levels, either overload or deficiency.
PCBPs are essential regulators that collaborate with iron incorporated into ferritin [112]. A higher PCBP1 expression in tubules helps protect cells from ROS, whereas the specific mechanisms of PCBP2 are not fully understood [113]. PCBP1 potentially reverses alcohol-induced fibrosis in hepatic stellate cells by inhibiting proinflammatory and profibrotic signals. However, the precise roles of PCBPs in iron-induced ferroptosis during CKD need to be further investigated.
CKD patients often exhibit impaired hepcidin excretion due to decreased GFR. Inflammation increases hepcidin production, leading to high intracellular hepcidin levels and excessive Fe2+ storage, inhibiting heme synthesis, and disrupting iron utilization. This imbalance causes extracellular iron deficiency and intracellular iron overload. In models of adriamycin-induced focal segmental glomerulosclerosis, increased Fe2+ levels and elevated hepcidin levels drive tubular atrophy and interstitial fibrosis [114]. Similarly, ochratoxin A-induced renal toxicity involves intracellular iron accumulation driven by FPN downregulation, increased hepcidin, and disrupted iron homeostasis, leading to ferroptosis [115]. Taken together, these studies reveal a lurking link between iron overload-induced ferroptosis and CKD progression.
4.2. Inflammation
The increase in inflammatory markers suggests the onset of CKD, with key participants being cells of the innate immune response. Ferroptotic cells release DAMPs, triggering the innate immune system and sustaining the low-grade inflammatory response observed in CKD. The interplay between inflammation and ferroptosis in CKD is observed from an immune cell perspective.
Macrophages differentiate into proinflammatory M1 macrophages, which have high nitric oxide (NO•) and inducible nitric oxide synthase (iNOS) levels and are more resistant to ferroptosis, whereas anti-inflammatory M2 macrophages, which produce less iNOS and NO•, are more susceptible [116]. In CKD, early macrophage polarization is predominantly M1, with M1 macrophages producing ROS and inflammatory factors. Later, M2 macrophage levels increase, releasing pro-fibrotic factors like TGF-β. Understanding the dynamics of macrophage polarization and its impact on ferroptosis during CKD progression is crucial, but further research is needed to fully elucidate this relationship [117].
Ferroptosis in cancer cells can impair dendritic cell-mediated antitumor immunity [118]. The activation of inflammasomes in DCs is a key driver of tubular interstitial fibrosis and inflammation in the kidneys. In CKD models, hypoxic renal tubular epithelial cells exhibit characteristics of ferroptosis, such as mitochondrial dysfunction, reduced GPX4, and increased 4-HNE, driving inflammation and fibrosis through the activation of the NLRP3 inflammasome in CD1+ DCs of the myeloid cell population, leading to elevated levels of IL-1β and IL-18 [119]. In the tumor microenvironment, tumor-associated neutrophils (PMN-MDSCs) undergo spontaneous cell death due to ferroptosis, which limits T cell activity [120], and neutrophils accumulate during renal fibrosis [121]. In ACSL4 knockout mice, the ferroptosis inhibitor Fer-1 decreases neutrophil infiltration. In CKD, neutrophils may exacerbate the ferroptosis process by releasing inflammatory mediators and ROS, thereby promoting kidney damage. Ferroptosis-induced neutrophil death may further release inflammatory cytokines, creating a vicious cycle that intensifies renal inflammation and fibrosis [122]. In patients with hyperhomocysteinemia, B cell-derived antibodies are deposited in glomerular endothelial cells, increasing membrane phospholipids and promoting iron accumulation, which causes ferroptosis [123]. CD20 monoclonal antibodies and ferroptosis inhibitors such as liproxstatin-1 can effectively improve kidney injury [124].
In brief, persistent kidney injury in CKD releases many endogenous DAMPs, provoking immunocytes and triggering inflammatory cascades. Further research is needed to elucidate the relationships between different immune cells and ferroptosis in CKD.
4.3. Oxidative Stress
The kidneys, as a high-energy metabolic organ, are sensitive to oxidative stress-induced damage. High levels of oxidative stress are observed in the early stages of CKD and increase as the disease progression to ESRD [125]. ROS in the kidneys are primary generated by enzymes such as the mitochondrial respiratory chain and NADPH oxidase (NOX). In renal tubular epithelial cells, H₂O₂ leads to decreased GPX4 and GSH levels, increased iron content, and elevated lipid peroxide levels, triggering ferroptosis. Ferrostatin-1, a ferroptosis inhibitor, effectively counteracts this oxidative damage [126].Antioxidant pathways help safeguard renal tissue from oxidative stress-related damage, including ferroptosis, by suppressing synthesis, removing excess ROS, and repairing injuries. In the context of ferroptosis, the accumulation of excess iron in chronic kidney injury cells triggers the Fenton reaction, generating large amounts of ROS and catalyzing their spread, leading to severe oxidative stress. GPX4 is a key antioxidant molecule in ferroptosis, with mitochondrial GPX4 being the primary form that limits ROS production in the kidneys. In diabetic nephropathy models, inhibiting GPX4 ubiquitination reduces oxidative stress, slows ferroptosis, and improves kidney function [127]. In cisplatin-induced acute kidney injury model, mitochondria autophagy in renal cells helps remove intracellular ROS and protect the kidneys from ferroptosis by upregulating GPX4 [128]. Overactivation of the STING1 pathway depletes GPX4, thereby increasing oxidative stress and ferroptosis susceptibility [129]. GPX4 inactivation enhances the susceptibility of renal tissue to ferroptosis, possibly because the physiological functions and cellular characteristics of the kidney make it more prone to oxidative stress.
FSP1 collaborates with coenzyme Q10 to scavenge lipid radicals, and its absence increases ferroptosis sensitivity in renal tubular epithelial cells [130]. In the fibrotic stage, of CKD, ferroptotic renal tubular cells rupture and release DAMPs, accelerating ferroptosis in adjacent cells through oxidative stress and lipid peroxidation [131]. FSP1 prevents ferroptosis by reducing oxidized phospholipids, and has been shown to repair sepsis-induced ferroptosis caused by renal injury [132].
Tetrahydrobiopterin (BH4) stabilizes endothelial nitric oxide synthase (eNOS). A deficiency in BH4 uncouples eNOS, leading to increased production of inflammatory factors and ROS, which accelerates oxidative stress and fibrosis in CKD. Ferroptosis inhibitors such as Fer-1 counteract this effect by upregulating eNOS and reducing oxidative damage [133]. Mycotoxins, which impair mitochondrial coenzyme Q binding, also alter cell morphology in tubular epithelial cells, generate a large amount of ROS and worsening to CKD progression [134]. Abnormalities in antioxidant pathways further exacerbate ferroptosis in CKD, highlighting the complex interplay between oxidative stress, ferroptosis, and kidney fibrosis.
4.4. Dyslipidemia
Dyslipidemia in CKD disrupts renal energy metabolism, damages cellular membranes, and induces mitochondrial and endoplasmic reticulum stress. These effects lead to lipid peroxidation, triggering ferroptosis, which contributes to renal dysfunction and fibrosis. Moreover, ferroptosis has also been identified as a critical link between lipid metabolism disorders and CKD development.
4.4.1. Abnormal Fatty Acid Metabolism
Fatty acid metabolism varies across kidney regions, with specific transport proteins and enzymes playing crucial roles in CKD-related dyslipidemia. Ferroptosis is closely associated with abnormal lipid metabolism, driven by disruptions in these proteins and enzymes.
The key molecules involved in FAO play an important role in ferroptosis, as they regulate the metabolism of fatty acids and directly or indirectly influence the occurrence and progression of ferroptosis. CPT1A, an enzyme that regulates long-chain fatty acid entry into mitochondria for β-oxidation, plays a central role. Inhibiting CPT1A induces ferroptosis in HK-2 cells [135], while upregulating CPT1A expression in diabetic mouse models alleviates ferroptosis and improves kidney injury [136]. Ferroptosis has been observed in calcium oxalate-induced TEC injury, where PGC-1α recruits NRF2 to the GPX4 promoter region, collaboratively activating GPX4 to delay cell death [137]. In kidney ischemia–reperfusion injury associated with ferroptosis, the inactivation of AMPK diminishes some of the resistance to ferroptosis [138], and using AMPK agonists to promote AMPK activation and NRF2 activation can prevent the occurrence of ferroptosis in diabetic nephropathy [139]. By regulating the activity of these molecules, the ferroptosis process in CKD can be intervened
The abnormal expression of lipid transport proteins in CKD also contributes to ferroptosis. CD36 is crucial for non-esterified fatty acid uptake in podocytes. Inhibiting CD36 reduces lipid buildup and ROS production [92,140]. CD36 exacerbates tubular injury in CKD by increasing inflammation, oxidative stress, and fibrosis in TECs [141]. In high-fat and high-glucose conditions, excess fatty acids transported by CD36 promote renal fibrosis in HK-2 cells [142]. CD36 also plays a role in the tumor microenvironment, mediating FA uptake by CD8+ T cells and inducing lipid peroxidation and ferroptosis [143]. In AKI, CD36 specifically binds to and degrades FSP1, exacerbating renal injury [144]. Increasing levels of soluble CD36 (sCD36) in the circulation and tissue indicate disease severity. Targeting CD36 can prevent renal fibrosis and reduce CKD progression [92]. CD36 modification effects vary by cell type, and the knockout of CD36 in macrophages retards interstitial fibrosis in obstructive nephropathy, whereas in proximal tubular cells, it mainly reduces proteinuria [145]. These findings underscore the role of CD36 in promoting lipid peroxidation to support ferroptosis during CKD.
FATPs (SLC27a1-6) transport long-chain fatty acids. FATP1, FATP2, and FATP4 are highly expressed in proximal renal tubular cells, with FATP2 being one of the most abundant [93,146]. Consequently, FATP2 is a significant factor in FAO impairment, tubular atrophy, and interstitial fibrosis. Inhibiting FATP2 decreases lipotoxicity in tubular cells and alleviates tubulointerstitial fibrosis [147]. Targeting FATPs for inhibition may play a critical role in regulating ferroptosis [148,149,150,151,152,153,154,155]. In the tumor microenvironment, where macrophages and CD8+ T cells take up lipids via CD36, the knockout of CD36 in CD8+ T cells in mice diminishes lipid peroxidation and inhibits ferroptosis [143,156,157]. The selective upregulation of fatty acid transport protein 2 (FATP2) in tumor-associated neutrophils increases AA uptake and induces ferroptosis [120,158]. Exploring FATP as a regulator of lipotoxicity-driven ferroptosis could be valuable.
FATP2 facilitates the uptake of PUFA and converts AA into AA-CoA. Lipidomic analysis revealed that FATP2 knockout cells have lower AA-PE levels, whereas ACSL4 knockout does not significantly affect AA-PE levels. FATP2 and ACSL4 independently provide substrates for peroxidation, with FATP2 potentially replacing or cooperating with ACSL4 in ferroptosis, influencing CKD development. In tumor neutrophils, the elevated intake of AA during differentiation is upregulated, and increased FATP2 activity leads to excessive lipid peroxides and worsens CKD through ferroptosis [158].
Fatty acid-binding proteins (FABPs) are membrane proteins that enhance the absorption of abnormal FA uptake in CKD patients. The overexpression of FABP3 (heart-type FABP) in podocytes is linked to increased fatty acid-induced lipotoxic effects [159]. Ferroptosis inducers such as RSL3 and FIN56 can upregulate FABP3 in tumor cells activated by hypoxia-inducible factor-1α (HIF1α) [160]. These findings indicate that FABP3 might be involved in ferroptosis. The deletion of FABP4 in obese mice reduces the synthesis of proinflammatory factors and inhibits FA breakdown [161]. Increased FABP4 levels in nondialysis CKD patients indicate its potential as a therapeutic target for reducing fibrosis [162]. In tumors, increased FABP4 enhances PUFAs, increasing ferroptosis [163]. L-FABP, or liver-type fatty acid binding protein, serves as a sensitive biomarker for CKD [164]. Higher levels of L-FABP have been associated with antioxidant properties and protective effects on renal function [165]. L-FABP binds long-chain fatty acids, facilitates lipid signaling, accelerates FA metabolism, and reduces inflammation, alleviating tubular interstitial injury [166]. Its role as an endogenous antioxidant in CKD underscores its potential as a target for inhibiting ferroptosis and providing renal protection.
4.4.2. Abnormal Cholesterol Metabolism
Cellular cholesterol homeostasis is vital for renal lipid metabolism. The dysregulation of reverse cholesterol transport and hypercholesterolemia is commonly observed in various stages of CKD [100,167,168]. Numerous lines of evidence suggest that cholesterol plays an important role in ferroptosis. However, in HEK293T cells (human embryonic kidney 293 cells), cholesterol does not directly induce ferroptosis. Several genes involved in distal cholesterol biosynthesis have been identified as ferroptosis inhibitors. Specifically, 7-dehydrocholesterol (7-DHC), an intermediate in the distal cholesterol biosynthesis pathway, is converted to cholesterol by DHCR7. This conversion suppresses ferroptosis by preventing membrane lipid peroxidation. In renal ischemia-reperfusion injury models, targeting DHCR7 to increase 7-DHC levels in renal tissue reduces blood urea nitrogen and creatinine levels, mitigating renal injury and inhibiting ferroptosis [169].
Liver X receptor (LXR) and farnesoid X receptor (FXR) are key regulators of renal cholesterol synthesis. FXR and LXR overexpression in a diabetic nephropathy mouse model triggers AMPK-induced inflammatory signaling pathways, which mitigate lipid accumulation, inflammation, oxidative stress, and fibrosis [170]. FXR and RXR also upregulate key ferroptosis-regulating proteins, including FSP1, GPX4, and enzymes such as ACSL3, which contribute to antioxidant activity and ferroptosis defense [171]. In contrast, ferroptosis inducers increase sensitivity to ferroptosis by promoting cholesterol ester production through the ACSL4-SOAT1 pathway [172,173]. Additionally, GPX4 deficiency in macrophages reduces ABCA1 and ABCG1 expression, leading to increased modified low-density lipoprotein uptake. This suggests that these proteins may help resist ferroptosis in CKD [174].
PCSK9 increases low-density lipoprotein cholesterol (LDL-C) levels in the bloodstream [175]. In LDLR+/− mice fed a high-cholesterol diet, PCSK9 worsens renal fibrosis [176]. Recent studies have linked PCSK9 to ferroptosis in abdominal aortic aneurysms [177]. PCSK9 affects ferroptosis by altering lipid metabolism, suggesting that it could be a potential therapeutic target for cholesterol-related diseases and ferroptosis.
4.4.3. Lipid Droplet Accumulation
Lipid droplets (LDs) are cellular organelles responsible for this process [178]. They are involved in membrane transport, protein storage and degradation, signal transduction, and detoxification [179,180,181]. In CKD, LDs accumulate notably in podocytes and renal tubular cells, reflecting disruptions in lipid balance and LD quality and function [182,183]. LD deposition in fibrotic kidneys is closely linked to changes in FAO proteins, cholesterol intake, and lipid autophagy [184]. LD numbers increase considerably during ferroptosis [185]. Lipid degradation and autophagy break down LDs into FFAs through selective degradation mediated by molecular chaperones such as the PLIN family [186]. For example, PLIN5 suppresses lipotoxicity and ferroptosis in cardiomyocytes by modulating the PIR-NF-κB axis [187]. In sepsis-related acute kidney injury, lipid autophagy promotes ferroptosis and renal injury in tubular epithelial cells [188], although its specific role in CKD requires further investigation.
5. Specific Drug Targets for Ferroptosis in CKD
Ferroptosis plays a crucial in CKD progression. Pathogenic factors can stimulate ferroptosis through various mechanisms, making it a novel research target for CKD research. Ferroptosis inhibitors offer protective effects by reducing labile iron, preventing lipid peroxidation, and eliminating lipid peroxides, thereby providing anti-inflammatory and antioxidant benefits. Additionally, regulating abnormal lipid metabolism proteins and enzymes also can impede ferroptosis. This section summarizes recent advancements in CKD treatment through ferroptosis inhibition and related agents (Table 1).

Table 1.
Molecular modulators of ferroptosis in CKD UUO, unilateral ureteral obstruction; IRI ischemia–reperfusion injury; DN, diabetic nephropathy; TECs, tubular epithelial cells, HO-1, heme oxygenase-1; AKI, acute kidney injury; UIR, unilateral renal ischemia-reperfusion; GSH, glutathione; GSSG, glutathione disulfide; NRF2, nuclear factor erythroid 2-related factor 2; GPX4, glutathione peroxidase 4; KEAP1, Kelch-like ECH-associated protein 1; PGE2, prostaglandin E2; PMN-MDSCs, polymorphonuclear myeloid-derived suppressor cells, PKD, polycystic kidney disease.
Iron chelators such as deferoxamine (DFO), deferiprone (DFP), deferasirox (DFX), and ciclopirox (CPX) prevent ferroptosis by binding excess iron, inhibiting ROS and lipid peroxide production, and treating renal fibrosis [213,214]. In CKD rat models, DFX inhibits the TGFβ-Smad3 pathway, reducing inflammation and oxidative stress while attenuating renal fibrosis [189]. DFO alleviates renal fibrosis and iron accumulation in adenine-induced CKD models by modulating iron metabolism [190]. CPX lowers ferritin levels through autophagy, improving kidney function in polycystic kidney disease (PKD) mice [191]. A novel 3-hydroxypyridin-4(1H)-one chelates divalent free iron, scavenges ROS, and promotes cell repair in kidney injury [192]. In addition to the use of ferroptosis inhibitors, a low-iron diet in CKD mouse models can reduce redox-active levels, delaying the deterioration of renal function [215]. Activated carbon adsorbents also mitigate iron deposition, reducing renal injury and fibrosis resulting from ferroptosis [190].
Treatments targeting lipid peroxidation can be categorized into two main types: radical scavengers and enzyme-based inhibitors of lipid peroxidation [197,216]. Ferrostatin-1, liproxstatin-1, and their analogs are the most researched ferroptosis inhibitors easing fibrosis. Ferrostatin-1 is capable of inhibiting the 15LOX–PEBP1 complex, preventing ferroptotic PE oxidation in vivo [217]. Ferrostatin-1 inhibits the regulatory factor HIF-1α/HO-1 and reduces lipid peroxidation in diabetic mice [194,195,196]. Liproxstatin-1 mitigates inflammation and renal fibrosis in UUO and IRI mouse models [197].
ACSL4 is a key target for the enzyme-based inhibition of oxidation during ferroptosis, particularly in renal injury. The novel ACSL4-targeting inhibitor AS-252424 (AS), a furan-2-ylmethylene thiazolidinedione, effectively reduces swelling and damage in renal tubular cells, decreases oxidized phospholipid fatty acids and inflammation, and suppresses ferroptosis in AKI [198]. Dexmedetomidine downregulates ACSL4 expression, reducing ferroptosis-mediated inflammatory responses and protecting renal function [199]. XJB-5-131, a mitochondrial-targeted nitric oxide donor, exerts dual antioxidant effects by specifically inhibiting ferroptosis in TECs, alleviating injury and inflammation in renal ischemia-reperfusion, and slowing CKD progression [200]. Fisetin inhibits ACSL4-mediated ferroptosis in renal tubular cells, improving renal fibrosis in CKD mice [8]. Other ACSL4 inhibitors, such as thiazolidinediones, including rosiglitazone, pioglitazone, and troglitazone, can reduce ferroptosis and lipid peroxidation induced by RSL3 and GPX4 knockout, similarly reducing oxidative stress and inflammation in CKD to protect the kidneys [201,202,203]. Additionally, inhibiting LOX enzymes, such as baicalein, has also hindered the pathophysiology of AKI and CKD, reducing neutrophil infiltration and peroxide substrate production and decreasing ferroptosis in CKD [204,205]. NADPH oxidase 4 (NOX4) promotes the production of intracellular ROS, enhancing oxidative stress, lipid peroxidation accumulation, and ferroptosis. The natural flavonoid glycoside kaempferitrin inhibits NOX4-mediated ferroptosis, alleviates renal fibrosis and inflammation, and protects the kidneys [206]. Hederagenin inhibits ferroptosis induced by the TGF-β/Smad3 signaling pathway in renal tubular cells by reducing NOX4 expression and increasing GPX4 expression, thereby improving fibrosis in diabetic nephropathy mouse models [207].
Inhibiting the degradation of antioxidant factors has emerged as a potential therapeutic approach to halt radical chain reactions in various pathological conditions. The combination of melatonin and zileuton upregulates the AKT-mTOR-NRF2 signaling pathway, which inhibits ferroptosis and synergistically decreases fibrosis in UUO models [210]. Formononetin inhibits the Smad3-ATF3-SLC7A11 pathway, increases SLC7A11 and GPX4 expression, promotes NRF2 nuclear accumulation, and ameliorates renal fibrosis and ferroptosis [208]. Celastrol modulates NRF2 to upregulate GPX4, reducing intracellular iron accumulation and lipid peroxidation [211]. Vitexin activates the KEAP1-NRF2-OH-1 pathway, leading to increased GPX4 expression, the inhibition of lipid peroxidation and ferroptosis, and improvements in renal tubular injury, interstitial fibrosis, and inflammation [209].
Abnormal activities of transport proteins or enzymes involved in renal lipid metabolism can accelerate ferroptosis in CKD patients. Targeting these proteins or enzymes may help reduce the likelihood of ferroptosis and mitigate renal injury. Astragaloside IV (AS-IV) inhibits CD36 expression, reduces lipid ROS, downregulates ACSL4 and P53, and upregulates GPX4, reducing the risk of myocardial cell death in diabetic mice [212]. The FATP2-specific inhibitor lipofermata reduces AA accumulation and PGE2 production, thereby decreasing ferroptosis-related gene expression and slowing tumor progression [158]. Lipofermata also regulates profibrotic factor secretion and endoplasmic reticulum stress, improving renal fibrosis [147]. Canagliflozin influences lipid metabolism by upregulating the expression of the transcription factor FOXA1, which in turn increases CPT1A expression, promoting fatty acid oxidation and reducing ferroptosis in TECs in DN [136]. Similarly, empagliflozin prevents ferroptosis in diabetic nephropathy by promoting the AMPK-mediated NRF2 activation pathway [139]. However, the role of lipid metabolism enzymes in ferroptosis remains largely unexplored.
6. Frontiers and Prospects of Ferroptosis in CKD
Increasing evidence indicates a significant association between ferroptosis and CKD. This review systematically elucidates the mechanisms underlying the interplay between renal pathology and ferroptosis, providing a novel perspective on CKD. Ferroptosis contributes to CKD through abnormal iron deposition, ectopic lipid accumulation, and imbalances in antioxidant pathways, which lead to oxidative stress and inflammatory cascades. These conditions create an environment conducive to the progression of CKD. Timely and multifaceted interventions targeting ferroptosis could emerge as novel approaches for controlling CKD progression, alleviating kidney injury, and managing complications. However, current research remains at an early stage, with targeted clinical transition and development still in progress. Furthermore, this review is limited by its focus on classical ferroptosis regulatory mechanisms, and future research must address the following issues:
Interaction with other types of cell death: Understanding how ferroptosis interacts with other cell death mechanisms—such as autophagy, apoptosis, necrosis, cuproptosis, and disulfidptosis—is essential. This involves determining whether these interactions facilitate or impede CKD progression, which could unveil novel treatment opportunities.
Interaction and transmission mechanisms between ferroptosis and abnormal lipid metabolism in CKD: It is crucial to explore whether key fatty acid signals trigger ferroptosis during the process of abnormal lipid metabolism in CKD, including the pathways through which ferroptosis influences these conditions. Additionally, the ways in which ferroptosis propagates in renal parenchymal cells should be clarified.
Biomarker identification: Identifying specific biomarkers for ferroptosis could facilitate the early and accurate detection of CKD progression. The development of such biomarkers will be crucial for monitoring disease status and assessing the effectiveness of new treatments.
7. Conclusions
CKD remains a global public health issue with high morbidity and mortality rates, and persistent kidney fibrosis is a key factor leading to ESRD. Ferroptosis is closely linked to CKD. In this review, we systematically summarize the pathological mechanisms of ferroptosis in CKD and discuss potential therapeutic drugs to delay renal fibrosis, opening new avenues for future drug development.
In conclusion, the full extent of ferroptosis in the pathological process of CKD remains unclear. Despite the challenges, in-depth studies on the regulatory mechanisms of ferroptosis in CKD hold great potential for discovering effective biomarkers and therapeutic strategies. We firmly believe that focusing on ferroptosis research could bring new insights and directions for the diagnosis and treatment of CKD.
Author Contributions
R.J. conceptualized and wrote the manuscript. Y.D. developed the design and revised the manuscript. Z.W. and Q.H. searched and analyzed the literature. C.Z. was in charge of visualization and validation. Q.Y. was responsible for conceptualization and editing. H.G. was responsible for funding acquisition and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by grants from the National Natural Science Foundation of China (NSFC) (No. 82171575) and Hubei Provincial Clinical Medical Research Center for Nephropathy (No. OIR202402Z) to Qi Yan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We want to express our gratitude for the drawing materials provided by Figdraw 2.0.
Conflicts of Interest
The authors declare no conflicts of interests. No new data were created or analyzed in this study.
Abbreviations
CKD chronic kidney disease; AKI acute kidney injury; DAMPs damage-associated molecular patterns; PUFAs polyunsaturated fatty acids; MUFAs monounsaturated fatty acids; ROS reactive oxygen species; TGF-β transforming growth factor-β; TBI transferrin-bound iron; NTBI non-transferrin-bound iron; TF transferrin; TFR transferrin receptor; TFR1/C transferrin receptor1/C; FATPs fatty acid transport proteins; FABPs fatty acid-binding protein; TECs tubular epithelial cells; TLR toll-like receptor; IL interleukin; IRI ischemia–reperfusion injury; HMGB1 high-mobility group box 1; UUO unilateral ureteral obstruction; DN diabetic nephropathy; Th T helper cells; FAO fatty acid oxidation; TG triglycerides; TNF tumor necrosis factor; MMP 9 matrix metalloproteinase 9; FGF-2 fibroblast growth factor 2; PDGF Platelet-Derived Growth Factor; DCs dendritic cells; CCL chemokine ligand; MCP monocyte chemotactic protein; 7-DHC 7-dehydrocholesterol; FA fatty acid; LDs lipid droplets; NOX NADPH oxidase.
References
- Obrador, G.T.; Levin, A. CKD Hotspots: Challenges and Areas of Opportunity. Semin. Nephrol. 2019, 39, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Tang, B.; Zhang, C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.H.; Tian, H.Y.; Gao, X.; Lei, W.W.; Hu, Y.; Wang, D.M.; Pan, X.C.; Yu, M.L.; Xu, G.J.; Zhao, F.K.; et al. Ferritin heavy chain-mediated iron homeostasis and subsequent increased reactive oxygen species production are essential for epithelial-mesenchymal transition. Cancer Res. 2009, 69, 5340–5348. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.; López-Polo, V.; Mateo, L.; Lafarga, M.; Aguilera, M.; De Lama, E.; Meyer, K.; Sola, A.; Lopez-Martinez, C.; López-Alonso, I.; et al. Iron accumulation drives fibrosis, senescence and the senescence-associated secretory phenotype. Nat. Metab. 2023, 5, 2111–2130. [Google Scholar] [CrossRef]
- Balzer, M.S.; Doke, T.; Yang, Y.W.; Aldridge, D.L.; Hu, H.; Mai, H.; Mukhi, D.; Ma, Z.; Shrestha, R.; Palmer, M.B.; et al. Single-cell analysis highlights differences in druggable pathways underlying adaptive or fibrotic kidney regeneration. Nat. Commun. 2022, 13, 4018. [Google Scholar] [CrossRef]
- Zhou, L.; Xue, X.; Hou, Q.; Dai, C. Targeting Ferroptosis Attenuates Interstitial Inflammation and Kidney Fibrosis. Kidney Dis. 2022, 8, 57–71. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.N.; Yang, L.T.; Liang, Y.; Guo, F.; Fu, P.; Ma, L. Fisetin ameliorates fibrotic kidney disease in mice via inhibiting ACSL4-mediated tubular ferroptosis. Acta Pharmacol. Sin. 2024, 45, 150–165. [Google Scholar] [CrossRef]
- Chen, T.; Liang, L.; Wang, Y.; Li, X.; Yang, C. Ferroptosis and cuproptposis in kidney Diseases: Dysfunction of cell metabolism. Apoptosis 2024, 29, 289–302. [Google Scholar] [CrossRef]
- Lai, W.; Huang, R.; Wang, B.; Shi, M.; Guo, F.; Li, L.; Ren, Q.; Tao, S.; Fu, P.; Ma, L. Novel aspect of neprilysin in kidney fibrosis via ACSL4-mediated ferroptosis of tubular epithelial cells. MedComm 2023, 4, e330. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell. Rep. 2020, 30, 3411–3423.e7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zheng, H.; Ishida, M.; Lyu, Q.; Akatsuka, S.; Motooka, Y.; Sato, K.; Sekido, Y.; Nakamura, K.; Tanaka, H.; et al. Elaborate cooperation of poly(rC)-binding proteins 1/2 and glutathione in ferroptosis induced by plasma-activated Ringer’s lactate. Free Radic. Biol. Med. 2024, 214, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Protchenko, O.; Baratz, E.; Jadhav, S.; Li, F.; Shakoury-Elizeh, M.; Gavrilova, O.; Ghosh, M.C.; Cox, J.E.; Maschek, J.A.; Tyurin, V.A.; et al. Iron Chaperone Poly rC Binding Protein 1 Protects Mouse Liver from Lipid Peroxidation and Steatosis. Hepatology 2021, 73, 1176–1193. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Xie, M.; Kang, R.; Fan, Y.; Niu, X.; Wang, H.; Cao, L.; Tang, D. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene 2015, 34, 5617–5625. [Google Scholar] [CrossRef]
- Fujimaki, M.; Furuya, N.; Saiki, S.; Amo, T.; Imamichi, Y.; Hattori, N. Iron Supply via NCOA4-Mediated Ferritin Degradation Maintains Mitochondrial Functions. Mol Cell. Biol. 2019, 39, e00010-19. [Google Scholar] [CrossRef]
- Liu, J.; Kuang, F.; Kroemer, G.; Klionsky, D.J.; Kang, R.; Tang, D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem. Biol. 2020, 27, 420–435. [Google Scholar] [CrossRef]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, X.; Li, S.; Liu, W.; Yan, J.; Wang, S.; Cui, F.; Li, D.; Li, J. DMT1 differentially regulates mitochondrial complex activities to reduce glutathione loss and mitigate ferroptosis. Free Radic. Biol. Med. 2023, 207, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Luo, X.; Bai, X.; Lv, Y.; Weng, X.; Zhang, S.; Leng, Y.; Huang, J.; Dai, X.; Wang, Y.; et al. Cigarette tar mediates macrophage ferroptosis in atherosclerosis through the hepcidin/FPN/SLC7A11 signaling pathway. Free Radic. Biol. Med. 2023, 201, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, J.; Ma, H.; Han, Y.; Xu, W.; Wang, J.; Cai, Y.; Jia, X.; Jia, Q.; Yang, Q. High Hepcidin Levels Promote Abnormal Iron Metabolism and Ferroptosis in Chronic Atrophic Gastritis. Biomedicines 2023, 11, 2338. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Huang, Y.; Wang, C. 1,25(OH)(2)D(3) Inhibited Ferroptosis in Zebrafish Liver Cells (ZFL) by Regulating Keap1-Nrf2-GPx4 and NF-κB-hepcidin Axis. Int. J. Mol. Sci. 2021, 22, 11334. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Kapralov, A.A.; Tyurin, V.A.; Shurin, G.; Amoscato, A.A.; Rajasundaram, D.; Tian, H.; Bunimovich, Y.L.; Nefedova, Y.; Herrick, W.G.; et al. Redox phospholipidomics discovers pro-ferroptotic death signals in A375 melanoma cells in vitro and in vivo. Redox Biol. 2023, 61, 102650. [Google Scholar] [CrossRef]
- Soula, M.; Weber, R.A.; Zilka, O.; Alwaseem, H.; La, K.; Yen, F.; Molina, H.; Garcia-Bermudez, J.; Pratt, D.A.; Birsoy, K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020, 16, 1351–1360. [Google Scholar] [CrossRef]
- Anthonymuthu, T.S.; Tyurina, Y.Y.; Sun, W.Y.; Mikulska-Ruminska, K.; Shrivastava, I.H.; Tyurin, V.A.; Cinemre, F.B.; Dar, H.H.; VanDemark, A.P.; Holman, T.R.; et al. Resolving the paradox of ferroptotic cell death: Ferrostatin-1 binds to 15LOX/PEBP1 complex, suppresses generation of peroxidized ETE-PE, and protects against ferroptosis. Redox Biol. 2021, 38, 101744. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Magtanong, L.; Ko, P.J.; To, M.; Cao, J.Y.; Forcina, G.C.; Tarangelo, A.; Ward, C.C.; Cho, K.; Patti, G.J.; Nomura, D.K.; et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019, 26, 420–432.e9. [Google Scholar] [CrossRef]
- Dixon, S.J.; Winter, G.E.; Musavi, L.S.; Lee, E.D.; Snijder, B.; Rebsamen, M.; Superti-Furga, G.; Stockwell, B.R. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem. Biol. 2015, 10, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Klasson, T.D.; LaGory, E.L.; Zhao, H.; Huynh, S.K.; Papandreou, I.; Moon, E.J.; Giaccia, A.J. ACSL3 regulates lipid droplet biogenesis and ferroptosis sensitivity in clear cell renal cell carcinoma. Cancer Metab. 2022, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Henry, W.S.; Ricq, E.L.; Graham, E.T.; Phadnis, V.V.; Maretich, P.; Paradkar, S.; Boehnke, N.; Deik, A.A.; Reinhardt, F.; et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 2020, 585, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Hong, T.; Zhang, X.; Liu, X.; Mao, C.; Yan, Y.; Koppula, P.; Cheng, W.; Sood, A.K.; et al. Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity. Oncogene 2021, 40, 3533–3547. [Google Scholar] [CrossRef]
- Shin, D.; Lee, J.; Roh, J.L. Pioneering the future of cancer therapy: Deciphering the p53-ferroptosis nexus for precision medicine. Cancer Lett. 2024, 585, 216645. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Chai, D.; Peng, J.; Xia, Y.; Hu, R.; Jiang, H. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging 2020, 12, 12943–12959. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, K.; Sun, L.; Yin, X.; Zhang, J.; Liu, C.; Li, B. SLC7A11 regulated by NRF2 modulates esophageal squamous cell carcinoma radiosensitivity by inhibiting ferroptosis. J. Transl. Med. 2021, 19, 367. [Google Scholar] [CrossRef]
- Liu, J.; Huang, C.; Liu, J.; Meng, C.; Gu, Q.; Du, X.; Yan, M.; Yu, Y.; Liu, F.; Xia, C. Nrf2 and its dependent autophagy activation cooperatively counteract ferroptosis to alleviate acute liver injury. Pharmacol. Res. 2023, 187, 106563. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Chen, P.; Bao, H.; Zhang, L.; Sun, K.; Song, S.; Li, T. Dimethyl fumarate protects against hepatic ischemia-reperfusion injury by alleviating ferroptosis via the NRF2/SLC7A11/HO-1 axis. Cell Cycle 2023, 22, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Swanda, R.V.; Nie, L.; Liu, X.; Wang, C.; Lee, H.; Lei, G.; Mao, C.; Koppula, P.; Cheng, W.; et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 2021, 12, 1589. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, A.; Li, L.; Liang, Q.; Wang, S.; Dong, Q.; Fu, M.; Lan, Z.; Li, Y.; Liu, X.; et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022, 102, 1259–1275. [Google Scholar] [CrossRef]
- Dai, E.; Zhang, W.; Cong, D.; Kang, R.; Wang, J.; Tang, D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem. Biophys. Res. Commun. 2020, 523, 966–971. [Google Scholar] [CrossRef]
- Larios, J.; Mercier, V.; Roux, A.; Gruenberg, J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 2020, 219, e201904113. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Tang, D. ESCRT-III-mediated membrane repair in cell death and tumor resistance. Cancer Gene Ther. 2021, 28, 1–4. [Google Scholar] [CrossRef]
- Kolbrink, B.; von Samson-Himmelstjerna, F.A.; Messtorff, M.L.; Riebeling, T.; Nische, R.; Schmitz, J.; Bräsen, J.H.; Kunzendorf, U.; Krautwald, S. Vitamin K1 inhibits ferroptosis and counteracts a detrimental effect of phenprocoumon in experimental acute kidney injury. Cell. Mol. Life Sci. CMLS 2022, 79, 387. [Google Scholar] [CrossRef]
- Mishima, E.; Wahida, A.; Seibt, T.; Conrad, M. Diverse biological functions of vitamin K: From coagulation to ferroptosis. Nat. Metab. 2023, 5, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wei, W.; Wu, D.; Huang, F.; Li, M.; Li, W.; Yin, J.; Peng, Y.; Lu, Y.; Zhao, Q.; et al. Blockade of GCH1/BH4 Axis Activates Ferritinophagy to Mitigate the Resistance of Colorectal Cancer to Erastin-Induced Ferroptosis. Front. Cell Dev. Biol. 2022, 10, 810327. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Kang, R.; Yang, N.; Pan, X.; Yang, J.; Yu, H.; Deng, W.; Jia, Z.; Zhang, J.; Shen, Q. Tetrahydrobiopterin inhibitor-based antioxidant metabolic strategy for enhanced cancer ferroptosis-immunotherapy. J. Colloid Interface Sci. 2024, 658, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef]
- Zhou, T.J.; Zhang, M.M.; Liu, D.M.; Huang, L.L.; Yu, H.Q.; Wang, Y.; Xing, L.; Jiang, H.L. Glutathione depletion and dihydroorotate dehydrogenase inhibition actuated ferroptosis-augment to surmount triple-negative breast cancer. Biomaterials 2024, 305, 122447. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Y.; Wang, L.; Guo, Z.; Ma, L.; Yang, R.; Wu, Y.; Li, X.; Niu, J.; Chu, Q.; et al. De novo pyrimidine biosynthetic complexes support cancer cell proliferation and ferroptosis defence. Nat. Cell Biol. 2023, 25, 836–847. [Google Scholar] [CrossRef]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef]
- Liu, Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int. 2006, 69, 213–217. [Google Scholar] [CrossRef]
- Yang, K.; Fan, B.; Zhao, Q.; Ji, Y.; Liu, P.; Gao, S.; Ren, T.; Dou, Y.; Pei, M.; Yang, H. Hirudin Ameliorates Renal Interstitial Fibrosis via Regulating TGF-β1/Smad and NF-κB Signaling in UUO Rat Model. Evid.-Based Complement. Altern. Med. eCAM 2020, 2020, 7291075. [Google Scholar] [CrossRef]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. A J. Virtual Libr. 1997, 2, d12–d26. [Google Scholar] [CrossRef]
- Scotton, C.J.; Chambers, R.C. Molecular targets in pulmonary fibrosis: The myofibroblast in focus. Chest 2007, 132, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xiang, Y.; Li, H.; Chen, A.; Dong, Z. Inflammation in kidney repair: Mechanism and therapeutic potential. Pharmacol. Ther. 2022, 237, 108240. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yanagita, M. Immune cells and inflammation in AKI to CKD progression. Am. J. Physiol. Ren. Physiol. 2018, 315, F1501–F1512. [Google Scholar] [CrossRef]
- Kurts, C.; Ginhoux, F.; Panzer, U. Kidney dendritic cells: Fundamental biology and functional roles in health and disease. Nat. Rev. Nephrol. 2020, 16, 391–407. [Google Scholar] [CrossRef]
- Wang, H.; Gao, M.; Li, J.; Sun, J.; Wu, R.; Han, D.; Tan, J.; Wang, J.; Wang, B.; Zhang, L.; et al. MMP-9-positive neutrophils are essential for establishing profibrotic microenvironment in the obstructed kidney of UUO mice. Acta Physiol. 2019, 227, e13317. [Google Scholar] [CrossRef]
- Tapmeier, T.T.; Fearn, A.; Brown, K.; Chowdhury, P.; Sacks, S.H.; Sheerin, N.S.; Wong, W. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010, 78, 351–362. [Google Scholar] [CrossRef]
- Burne, M.J.; Daniels, F.; El Ghandour, A.; Mauiyyedi, S.; Colvin, R.B.; O’Donnell, M.P.; Rabb, H. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J. Clin. Investig. 2001, 108, 1283–1290. [Google Scholar] [CrossRef]
- Liu, M.; Chien, C.C.; Burne-Taney, M.; Molls, R.R.; Racusen, L.C.; Colvin, R.B.; Rabb, H. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J. Am. Soc. Nephrol. JASN 2006, 17, 765–774. [Google Scholar] [CrossRef]
- Mehrotra, P.; Patel, J.B.; Ivancic, C.M.; Collett, J.A.; Basile, D.P. Th-17 cell activation in response to high salt following acute kidney injury is associated with progressive fibrosis and attenuated by AT-1R antagonism. Kidney Int. 2015, 88, 776–784. [Google Scholar] [CrossRef]
- do Valle Duraes, F.; Lafont, A.; Beibel, M.; Martin, K.; Darribat, K.; Cuttat, R.; Waldt, A.; Naumann, U.; Wieczorek, G.; Gaulis, S.; et al. Immune cell landscaping reveals a protective role for regulatory T cells during kidney injury and fibrosis. JCI Insight 2020, 5, e130651. [Google Scholar] [CrossRef]
- Badid, C.; Vincent, M.; McGregor, B.; Melin, M.; Hadj-Aissa, A.; Veysseyre, C.; Hartmann, D.J.; Desmouliere, A.; Laville, M. Mycophenolate mofetil reduces myofibroblast infiltration and collagen III deposition in rat remnant kidney. Kidney Int. 2000, 58, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Bayazit, A.K.; Bayazit, Y.; Noyan, A.; Gonlusen, G.; Anarat, A. Comparison of mycophenolate mofetil and azathioprine in obstructive nephropathy. Pediatr. Nephrol. 2003, 18, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.G.; Biato, M.A.; Colosimo, R.D.; Martinusso, C.A.; Pecly, I.D.; Farias, E.K.; Cardoso, L.R.; Takiya, C.M.; Ornellas, J.F.; Leite, M., Jr. Effects of mycophenolate mofetil and lisinopril on collagen deposition in unilateral ureteral obstruction in rats. Am. J. Nephrol. 2004, 24, 527–536. [Google Scholar] [CrossRef]
- Wu, M.J.; Wen, M.C.; Chiu, Y.T.; Chiou, Y.Y.; Shu, K.H.; Tang, M.J. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int. 2006, 69, 2029–2036. [Google Scholar] [CrossRef]
- Han, H.; Zhu, J.; Wang, Y.; Zhu, Z.; Chen, Y.; Lu, L.; Jin, W.; Yan, X.; Zhang, R. Renal recruitment of B lymphocytes exacerbates tubulointerstitial fibrosis by promoting monocyte mobilization and infiltration after unilateral ureteral obstruction. J. Pathol. 2017, 241, 80–90. [Google Scholar] [CrossRef]
- Fleig, S.V.; Konen, F.F.; Schröder, C.; Schmitz, J.; Gingele, S.; Bräsen, J.H.; Lovric, S.; Schmidt, B.M.W.; Haller, H.; Skripuletz, T.; et al. Long-term B cell depletion associates with regeneration of kidney function. Immun. Inflamm. Dis. 2021, 9, 1479–1488. [Google Scholar] [CrossRef]
- Wu, H.; Ma, J.; Wang, P.; Corpuz, T.M.; Panchapakesan, U.; Wyburn, K.R.; Chadban, S.J. HMGB1 contributes to kidney ischemia reperfusion injury. J. Am. Soc. Nephrol. JASN 2010, 21, 1878–1890. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Chen, L.; Tay, Y.C.; Rangan, G.K.; Harris, D.C. Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J. Am. Soc. Nephrol. JASN 1997, 8, 1537–1545. [Google Scholar] [CrossRef]
- Basile, D.P. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007, 72, 151–156. [Google Scholar] [CrossRef]
- Basile, D.P.; Donohoe, D.; Roethe, K.; Osborn, J.L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Ren. Physiol. 2001, 281, F887–F899. [Google Scholar] [CrossRef]
- De Greef, K.E.; Ysebaert, D.K.; Persy, V.; Vercauteren, S.R.; De Broe, M.E. ICAM-1 expression and leukocyte accumulation in inner stripe of outer medulla in early phase of ischemic compared to HgCl2-induced ARF. Kidney Int. 2003, 63, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Enis, D.R.; Koh, K.P.; Shiao, S.L.; Pober, J.S. T lymphocyte-endothelial cell interactions. Annu. Rev. Immunol. 2004, 22, 683–709. [Google Scholar] [CrossRef] [PubMed]
- Austrup, F.; Vestweber, D.; Borges, E.; Löhning, M.; Bräuer, R.; Herz, U.; Renz, H.; Hallmann, R.; Scheffold, A.; Radbruch, A.; et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature 1997, 385, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Schlöndorff, D.; Banas, B. The mesangial cell revisited: No cell is an island. J. Am. Soc. Nephrol. JASN 2009, 20, 1179–1187. [Google Scholar] [CrossRef]
- Johnson, R.J.; Iida, H.; Alpers, C.E.; Majesky, M.W.; Schwartz, S.M.; Pritzi, P.; Gordon, K.; Gown, A.M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J. Clin. Investig. 1991, 87, 847–858. [Google Scholar] [CrossRef]
- Meng, X.M. Inflammatory Mediators and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 381–406. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Wang, Z.V.; Park, A.S.; Zhang, J.; Zhang, D.; Hu, M.C.; Moe, O.W.; Susztak, K.; Scherer, P.E. Adiponectin promotes functional recovery after podocyte ablation. J. Am. Soc. Nephrol. JASN 2013, 24, 268–282. [Google Scholar] [CrossRef]
- Emma, F.; Montini, G.; Parikh, S.M.; Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat. Rev. Nephrol. 2016, 12, 267–280. [Google Scholar] [CrossRef]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Jao, T.M.; Nangaku, M.; Wu, C.H.; Sugahara, M.; Saito, H.; Maekawa, H.; Ishimoto, Y.; Aoe, M.; Inoue, T.; Tanaka, T.; et al. ATF6α downregulation of PPARα promotes lipotoxicity-induced tubulointerstitial fibrosis. Kidney Int. 2019, 95, 577–589. [Google Scholar] [CrossRef]
- Satriano, J.; Sharma, K.; Blantz, R.C.; Deng, A. Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2013, 305, F727–F733. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Okamura, D.M.; Lu, X.; Chen, Y.; Moorhead, J.; Varghese, Z.; Ruan, X.Z. CD36 in chronic kidney disease: Novel insights and therapeutic opportunities. Nat. Rev. Nephrol. 2017, 13, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cabral, P.D.; Schilling, W.P.; Schmidt, Z.W.; Uddin, A.N.; Gingras, A.; Madhavan, S.M.; Garvin, J.L.; Schelling, J.R. Kidney Proximal Tubule Lipoapoptosis Is Regulated by Fatty Acid Transporter-2 (FATP2). J. Am. Soc. Nephrol. JASN 2018, 29, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Okamura, D.M.; Pennathur, S.; Pasichnyk, K.; López-Guisa, J.M.; Collins, S.; Febbraio, M.; Heinecke, J.; Eddy, A.A. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J. Am. Soc. Nephrol. JASN 2009, 20, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Ho, K.J. Cholesterol fatty kidney: Morphological changes in the course of its development in rabbits. Exp. Mol. Pathol. 1978, 29, 412–425. [Google Scholar] [CrossRef]
- Emanuelsson, F.; Nordestgaard, B.G.; Benn, M. Familial Hypercholesterolemia and Risk of Peripheral Arterial Disease and Chronic Kidney Disease. J. Clin. Endocrinol. Metab. 2018, 103, 4491–4500. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Zhao, K.; Gao, L.; Zhao, J. Cholesterol-induced toxicity: An integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021, 33, 1911–1925. [Google Scholar] [CrossRef]
- Levy, E.; Spahis, S.; Sinnett, D.; Peretti, N.; Maupas-Schwalm, F.; Delvin, E.; Lambert, M.; Lavoie, M.A. Intestinal cholesterol transport proteins: An update and beyond. Curr. Opin. Lipidol. 2007, 18, 310–318. [Google Scholar] [CrossRef]
- Ducasa, G.M.; Mitrofanova, A.; Mallela, S.K.; Liu, X.; Molina, J.; Sloan, A.; Pedigo, C.E.; Ge, M.; Santos, J.V.; Hernandez, Y.; et al. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J. Clin. Investig. 2019, 129, 3387–3400. [Google Scholar] [CrossRef]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef]
- Lee, H.S.; Kruth, H.S. Accumulation of cholesterol in the lesions of focal segmental glomerulosclerosis. Nephrology 2003, 8, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Zhang, J.; Zhang, R.; Wang, Y.; Liu, F. ABCA1 deficiency-mediated glomerular cholesterol accumulation exacerbates glomerular endothelial injury and dysfunction in diabetic kidney disease. Metab. Clin. Exp. 2023, 139, 155377. [Google Scholar] [CrossRef] [PubMed]
- Fornoni, A.; Merscher, S.; Kopp, J.B. Lipid biology of the podocyte--new perspectives offer new opportunities. Nat. Rev. Nephrol. 2014, 10, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Li, S.; Jin, L.; Feng, P.; Kong, Y.; Zhao, X.; Lin, Y.; Xu, Y.; Li, C.; Wang, W. Combination of Chymostatin and Aliskiren attenuates ER stress induced by lipid overload in kidney tubular cells. Lipids Health Dis. 2018, 17, 183. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Liu, Y.; Cai, X.; Huang, X.; Fu, W.; Wang, L.; Qiu, L.; Li, J.; Sun, L. Ferroptosis, a new target for treatment of renal injury and fibrosis in a 5/6 nephrectomy-induced CKD rat model. Cell Death Discov. 2022, 8, 127. [Google Scholar] [CrossRef]
- Zhao, P.; Lv, X.; Zhou, Z.; Yang, X.; Huang, Y.; Liu, J. Indexes of ferroptosis and iron metabolism were associated with the severity of diabetic nephropathy in patients with type 2 diabetes mellitus: A cross-sectional study. Front. Endocrinol. 2023, 14, 1297166. [Google Scholar] [CrossRef]
- Alli, A.A.; Desai, D.; Elshika, A.; Conrad, M.; Proneth, B.; Clapp, W.; Atkinson, C.; Segal, M.; Searcy, L.A.; Denslow, N.D.; et al. Kidney tubular epithelial cell ferroptosis links glomerular injury to tubulointerstitial pathology in lupus nephritis. Clin. Immunol. 2023, 248, 109213. [Google Scholar] [CrossRef]
- van Raaij, S.; van Swelm, R.; Bouman, K.; Cliteur, M.; van den Heuvel, M.C.; Pertijs, J.; Patel, D.; Bass, P.; van Goor, H.; Unwin, R.; et al. Tubular iron deposition and iron handling proteins in human healthy kidney and chronic kidney disease. Sci. Rep. 2018, 8, 9353. [Google Scholar] [CrossRef]
- Fujishiro, H.; Kambe, T. Manganese transport in mammals by zinc transporter family proteins, ZNT and ZIP. J. Pharmacol. Sci. 2022, 148, 125–133. [Google Scholar] [CrossRef]
- Patino, E.; Bhatia, D.; Vance, S.Z.; Antypiuk, A.; Uni, R.; Campbell, C.; Castillo, C.G.; Jaouni, S.; Vinchi, F.; Choi, M.E.; et al. Iron therapy mitigates chronic kidney disease progression by regulating intracellular iron status of kidney macrophages. JCI insight 2023, 8, e159235. [Google Scholar] [CrossRef]
- Hanudel, M.R. Filling the pool: Possible renoprotective effects of repleting the kidney macrophage labile iron pool in CKD? Kidney Int. 2023, 104, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.G.; Nandal, A.; Park, J.H.; Smith, P.M.; Yabe, T.; Ryu, M.S.; Ghosh, M.C.; Lee, J.; Rouault, T.A.; Park, M.H.; et al. Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase. Proc. Natl. Acad. Sci. USA 2014, 111, 8031–8036. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C.; Patel, S.J.; Protchenko, O. Management versus miscues in the cytosolic labile iron pool: The varied functions of iron chaperones. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118830. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shi, X.; Zhao, M.; Zhang, Y.; Zhang, Q.; Liu, J.; Duan, H.; Yang, B.; Zhang, Y. Ferroptosis is involved in focal segmental glomerulosclerosis in rats. Sci. Rep. 2023, 13, 22250. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, H.; Fan, C.; Lin, Q.; Liu, M.; Tian, J. Ochratoxin A Induces Renal Cell Ferroptosis by Disrupting Iron Homeostasis and Increasing ROS. J. Agric. Food Chem. 2024, 72, 1734–1744. [Google Scholar] [CrossRef]
- Dar, H.H.; Anthonymuthu, T.S.; Ponomareva, L.A.; Souryavong, A.B.; Shurin, G.V.; Kapralov, A.O.; Tyurin, V.A.; Lee, J.S.; Mallampalli, R.K.; Wenzel, S.E.; et al. A new thiol-independent mechanism of epithelial host defense against Pseudomonas aeruginosa: iNOS/NO(•) sabotage of theft-ferroptosis. Redox Biol. 2021, 45, 102045. [Google Scholar] [CrossRef]
- Lo, T.H.; Tseng, K.Y.; Tsao, W.S.; Yang, C.Y.; Hsieh, S.L.; Chiu, A.W.; Takai, T.; Mak, T.W.; Tarng, D.C.; Chen, N.J. TREM-1 regulates macrophage polarization in ureteral obstruction. Kidney Int. 2014, 86, 1174–1186. [Google Scholar] [CrossRef]
- Wiernicki, B.; Maschalidi, S.; Pinney, J.; Adjemian, S.; Vanden Berghe, T.; Ravichandran, K.S.; Vandenabeele, P. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat. Commun. 2022, 13, 3676. [Google Scholar] [CrossRef]
- Giuliani, K.T.K.; Grivei, A.; Nag, P.; Wang, X.; Rist, M.; Kildey, K.; Law, B.; Ng, M.S.; Wilkinson, R.; Ungerer, J.; et al. Hypoxic human proximal tubular epithelial cells undergo ferroptosis and elicit an NLRP3 inflammasome response in CD1c(+) dendritic cells. Cell Death Dis. 2022, 13, 739. [Google Scholar] [CrossRef]
- Kim, R.; Hashimoto, A.; Markosyan, N.; Tyurin, V.A.; Tyurina, Y.Y.; Kar, G.; Fu, S.; Sehgal, M.; Garcia-Gerique, L.; Kossenkov, A.; et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 2022, 612, 338–346. [Google Scholar] [CrossRef]
- Ryu, S.; Shin, J.W.; Kwon, S.; Lee, J.; Kim, Y.C.; Bae, Y.S.; Bae, Y.S.; Kim, D.K.; Kim, Y.S.; Yang, S.H.; et al. Siglec-F-expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis. J. Clin. Investig. 2022, 132, e156876. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, J.; Ye, K.; Zhao, J.; Huang, S.; He, S.; Qin, Y.; Meng, L.; Wang, J.; Song, J. Neutrophil-Derived IL-6 Potentially Drives Ferroptosis Resistance in B Cells in Lupus Kidney. Mediat. Inflamm. 2023, 2023, 9810733. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Ma, X.; Tan, Y.; Shao, F.; Li, C.; Zhao, Y.; Miao, Y.; Han, L.; Dang, G.; Song, Y.; et al. B cell-derived anti-beta 2 glycoprotein I antibody mediates hyperhomocysteinemia-aggravated hypertensive glomerular lesions by triggering ferroptosis. Signal Transduct. Target. Ther. 2023, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xiang, M.; Gao, Z.; Lvu, F.; Sun, Z.; Wang, Y.; Shi, X.; Xu, J.; Wang, J.; Liang, J. The role of B-cell ferroptosis in the pathogenesis of systemic lupus erythematosus. Clin. Immunol. 2023, 256, 109778. [Google Scholar] [CrossRef]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2006, 48, 752–760. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Lv, C.; Liu, L.; Miao, S.; Xu, Y.; Li, K.; Zhao, Y.; Zhao, J. PGE2 pathway mediates oxidative stress-induced ferroptosis in renal tubular epithelial cells. FEBS J. 2023, 290, 533–549. [Google Scholar] [CrossRef]
- Chen, J.; Ou, Z.; Gao, T.; Yang, Y.; Shu, A.; Xu, H.; Chen, Y.; Lv, Z. Ginkgolide B alleviates oxidative stress and ferroptosis by inhibiting GPX4 ubiquitination to improve diabetic nephropathy. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 156, 113953. [Google Scholar] [CrossRef]
- Lin, Q.; Li, S.; Jin, H.; Cai, H.; Zhu, X.; Yang, Y.; Wu, J.; Qi, C.; Shao, X.; Li, J.; et al. Mitophagy alleviates cisplatin-induced renal tubular epithelial cell ferroptosis through ROS/HO-1/GPX4 axis. Int. J. Biol. Sci. 2023, 19, 1192–1210. [Google Scholar] [CrossRef]
- Dai, E.; Han, L.; Liu, J.; Xie, Y.; Zeh, H.J.; Kang, R.; Bai, L.; Tang, D. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat. Commun. 2020, 11, 6339. [Google Scholar] [CrossRef]
- Tonnus, W.; Meyer, C.; Steinebach, C.; Belavgeni, A.; von Mässenhausen, A.; Gonzalez, N.Z.; Maremonti, F.; Gembardt, F.; Himmerkus, N.; Latk, M.; et al. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat. Commun. 2021, 12, 4402. [Google Scholar] [CrossRef]
- Belavgeni, A.; Meyer, C.; Stumpf, J.; Hugo, C.; Linkermann, A. Ferroptosis and Necroptosis in the Kidney. Cell Chem. Biol. 2020, 27, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, L.; Ma, M. Ginsenoside Rg1 Suppresses Ferroptosis of Renal Tubular Epithelial Cells in Sepsis-induced Acute Kidney Injury via the FSP1-CoQ(10)-NAD(P)H Pathway. Curr. Med. Chem. 2024, 31, 2119–2132. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Q.; Sun, Q.; Kong, R.; Liu, H.; Yi, X.; Liang, Z.; Letcher, R.J.; Liu, C. Ustiloxin A inhibits proliferation of renal tubular epithelial cells in vitro and induces renal injury in mice by disrupting structure and respiratory function of mitochondria. J. Hazard. Mater. 2023, 448, 130791. [Google Scholar] [CrossRef]
- Chen, J.; Wu, K.; Lei, Y.; Huang, M.; Cheng, L.; Guan, H.; Lin, J.; Zhong, M.; Wang, X.; Zheng, Z. Inhibition of Fatty Acid β-Oxidation by Fatty Acid Binding Protein 4 Induces Ferroptosis in HK2 Cells Under High Glucose Conditions. Endocrinol. Metab. 2023, 38, 226–244. [Google Scholar] [CrossRef]
- Gan, T.; Wang, Q.; Song, Y.; Shao, M.; Zhao, Y.; Guo, F.; Wei, F.; Fan, X.; Zhang, W.; Luo, Y.; et al. Canagliflozin improves fatty acid oxidation and ferroptosis of renal tubular epithelial cells via FOXA1-CPT1A axis in diabetic kidney disease. Mol. Cell. Endocrinol. 2024, 582, 112139. [Google Scholar] [CrossRef]
- Duan, C.; Li, B.; Liu, H.; Zhang, Y.; Yao, X.; Liu, K.; Wu, X.; Mao, X.; Wu, H.; Xu, Z.; et al. Sirtuin1 Suppresses Calcium Oxalate Nephropathy via Inhibition of Renal Proximal Tubular Cell Ferroptosis Through PGC-1α-mediated Transcriptional Coactivation. Adv. Sci. 2024, 2408945. [Google Scholar] [CrossRef]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, L.; Xiao, J.J.; Liu, Q.; Ni, L.; Hu, J.W.; Yu, H.; Wu, X.; Zhang, B.F. Empagliflozin attenuates the renal tubular ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway. Free Radic. Biol. Med. 2023, 195, 89–102. [Google Scholar] [CrossRef]
- Byun, J.H.; Lebeau, P.F.; Platko, K.; Carlisle, R.E.; Faiyaz, M.; Chen, J.; MacDonald, M.E.; Makda, Y.; Yousof, T.; Lynn, E.G.; et al. Inhibitory Antibodies against PCSK9 Reduce Surface CD36 and Mitigate Diet-Induced Renal Lipotoxicity. Kidney360 2022, 3, 1394–1410. [Google Scholar] [CrossRef]
- Su, K.; Zhao, S.L.; Yang, W.X.; Lo, C.S.; Chenier, I.; Liao, M.C.; Pang, Y.C.; Peng, J.Z.; Miyata, K.N.; Cailhier, J.F.; et al. NRF2 Deficiency Attenuates Diabetic Kidney Disease in Db/Db Mice via Down-Regulation of Angiotensinogen, SGLT2, CD36, and FABP4 Expression and Lipid Accumulation in Renal Proximal Tubular Cells. Antioxidants 2023, 12, 1715. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, T.; Geng, J.; Wu, Z.; Xu, L.; Liu, J.; Tian, J.; Zhou, Z.; Nie, J.; Bai, X. Advanced Oxidation Protein Products Promote Lipotoxicity and Tubulointerstitial Fibrosis via CD36/β-Catenin Pathway in Diabetic Nephropathy. Antioxid. Redox Signal. 2019, 31, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021, 33, 1001–1012.e5. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, L.; Zhang, Z.; Yang, P.; Chen, Q.; Zeng, X.; Tan, F.; Wang, C.; Ruan, X.; Liao, X. CD36 promotes tubular ferroptosis by regulating the ubiquitination of FSP1 in acute kidney injury. Genes Dis. 2024, 11, 449–463. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, X.G.; Zhang, S. Druggability of lipid metabolism modulation against renal fibrosis. Acta Pharmacol. Sin. 2022, 43, 505–519. [Google Scholar] [CrossRef]
- Khan, S.; Gaivin, R.; Abramovich, C.; Boylan, M.; Calles, J.; Schelling, J.R. Fatty acid transport protein-2 regulates glycemic control and diabetic kidney disease progression. JCI Insight 2020, 5, e136845. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Q.; Lv, M.; Song, K.; Dai, Y.; Huang, Y.; Zhang, L.; Zhang, C.; Gao, H. Involvement of FATP2-mediated tubular lipid metabolic reprogramming in renal fibrogenesis. Cell Death Dis. 2020, 11, 994. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef]
- Son, N.H.; Basu, D.; Samovski, D.; Pietka, T.A.; Peche, V.S.; Willecke, F.; Fang, X.; Yu, S.Q.; Scerbo, D.; Chang, H.R.; et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J. Clin. Investig. 2018, 128, 4329–4342. [Google Scholar] [CrossRef]
- Hao, J.W.; Wang, J.; Guo, H.; Zhao, Y.Y.; Sun, H.H.; Li, Y.F.; Lai, X.Y.; Zhao, N.; Wang, X.; Xie, C.; et al. CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat. Commun. 2020, 11, 4765. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, F.; Chen, M.; Li, Y.; You, M.; Zhang, Y.; Yang, P.; Wei, L.; Ruan, X.Z.; Zhao, L.; et al. Inhibition of Fatty Acid Translocase (FAT/CD36) Palmitoylation Enhances Hepatic Fatty Acid β-Oxidation by Increasing Its Localization to Mitochondria and Interaction with Long-Chain Acyl-CoA Synthetase 1. Antioxid. Redox Signal. 2022, 36, 1081–1100. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Ring, A.; Hermann, T.; Stremmel, W. Role of FATP in parenchymal cell fatty acid uptake. Biochim. Biophys. Acta 2004, 1686, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Harasim, E.; Kalinowska, A.; Chabowski, A.; Stepek, T. The role of fatty-acid transport proteins (FAT/CD36, FABPpm, FATP) in lipid metabolism in skeletal muscles. Postepy Hig. Med. Dosw. 2008, 62, 433–441. [Google Scholar]
- Black, P.N.; Sandoval, A.; Arias-Barrau, E.; DiRusso, C.C. Targeting the fatty acid transport proteins (FATP) to understand the mechanisms linking fatty acid transport to metabolism. Immunol. Endocr. Metab. Agents Med. Chem. 2009, 9, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Shetty, S.S.; Kumari, N.S. Fatty acid transport proteins (FATPs) in cancer. Chem. Phys. Lipids 2023, 250, 105269. [Google Scholar] [CrossRef]
- Su, P.; Wang, Q.; Bi, E.; Ma, X.; Liu, L.; Yang, M.; Qian, J.; Yi, Q. Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages. Cancer Res. 2020, 80, 1438–1450. [Google Scholar] [CrossRef]
- Ubellacker, J.M.; Tasdogan, A.; Ramesh, V.; Shen, B.; Mitchell, E.C.; Martin-Sandoval, M.S.; Gu, Z.; McCormick, M.L.; Durham, A.B.; Spitz, D.R.; et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 2020, 585, 113–118. [Google Scholar] [CrossRef]
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.K.J.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019, 569, 73–78. [Google Scholar] [CrossRef]
- Yu, T.H.; Hsuan, C.F.; Wu, C.C.; Hung, W.C.; Lee, T.L.; Tsai, I.T.; Wei, C.T.; Houng, J.Y.; Chung, F.M.; Lee, Y.J.; et al. Association of plasma fatty acid-binding protein 3 with estimated glomerular filtration rate in patients with type 2 diabetes mellitus. Int. J. Med. Sci. 2022, 19, 82–88. [Google Scholar] [CrossRef]
- Yang, M.; Chen, P.; Liu, J.; Zhu, S.; Kroemer, G.; Klionsky, D.J.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci. Adv. 2019, 5, eaaw2238. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Y.; Song, K.; Huang, Y.; Zhang, L.; Zhang, C.; Yan, Q.; Gao, H. Pre-emptive pharmacological inhibition of fatty acid-binding protein 4 attenuates kidney fibrosis by reprogramming tubular lipid metabolism. Cell Death Dis. 2021, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Miyoshi, T.; Doi, M.; Takeda, K.; Kajiya, M.; Nosaka, K.; Nakayama, R.; Hirohata, S.; Usui, S.; Kusachi, S.; et al. Elevated serum adipocyte fatty acid-binding protein concentrations are independently associated with renal dysfunction in patients with stable angina pectoris. Cardiovasc. Diabetol. 2012, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Luis, G.; Godfroid, A.; Nishiumi, S.; Cimino, J.; Blacher, S.; Maquoi, E.; Wery, C.; Collignon, A.; Longuespée, R.; Montero-Ruiz, L.; et al. Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol. 2021, 43, 102006. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: A novel biomarker of kidney disease. Clin. Chim. Acta 2015, 445, 85–90. [Google Scholar] [CrossRef]
- Ichikawa, D.; Kamijo-Ikemori, A.; Sugaya, T.; Shibagaki, Y.; Yasuda, T.; Katayama, K.; Hoshino, S.; Igarashi-Migitaka, J.; Hirata, K.; Kimura, K. Renoprotective effect of renal liver-type fatty acid binding protein and angiotensin II type 1a receptor loss in renal injury caused by RAS activation. Am. J. Physiol. Ren. Physiol. 2014, 306, F655–F663. [Google Scholar] [CrossRef]
- Yamamoto, T.; Noiri, E.; Ono, Y.; Doi, K.; Negishi, K.; Kamijo, A.; Kimura, K.; Fujita, T.; Kinukawa, T.; Taniguchi, H.; et al. Renal L-type fatty acid--binding protein in acute ischemic injury. J. Am. Soc. Nephrol. JASN 2007, 18, 2894–2902. [Google Scholar] [CrossRef]
- Alaupovic, P.; Attman, P.O.; Knight-Gibson, C.; Mulec, H.; Weiss, L.; Samuelsson, O. Effect of fluvastatin on apolipoprotein-defined lipoprotein subclasses in patients with chronic renal insufficiency. Kidney Int. 2006, 69, 1865–1871. [Google Scholar] [CrossRef]
- Joven, J.; Villabona, C.; Vilella, E.; Masana, L.; Albertí, R.; Vallés, M. Abnormalities of lipoprotein metabolism in patients with the nephrotic syndrome. N. Engl. J. Med. 1990, 323, 579–584. [Google Scholar] [CrossRef]
- Li, Y.; Ran, Q.; Duan, Q.; Jin, J.; Wang, Y.; Yu, L.; Wang, C.; Zhu, Z.; Chen, X.; Weng, L.; et al. 7-Dehydrocholesterol dictates ferroptosis sensitivity. Nature 2024, 626, 411–418. [Google Scholar] [CrossRef]
- Proctor, G.; Jiang, T.; Iwahashi, M.; Wang, Z.; Li, J.; Levi, M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 2006, 55, 2502–2509. [Google Scholar] [CrossRef]
- Tschuck, J.; Theilacker, L.; Rothenaigner, I.; Weiß, S.A.I.; Akdogan, B.; Lam, V.T.; Müller, C.; Graf, R.; Brandner, S.; Pütz, C.; et al. Farnesoid X receptor activation by bile acids suppresses lipid peroxidation and ferroptosis. Nat. Commun. 2023, 14, 6908. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, J.; Long, F.; Kang, R.; Kroemer, G.; Tang, D.; Yang, M. The lipid flippase SLC47A1 blocks metabolic vulnerability to ferroptosis. Nat. Commun. 2022, 13, 7965. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Schick, J.A. PUFAs dictate the balance of power in ferroptosis. Cell Calcium 2023, 110, 102703. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, S.; Chen, X.; Hou, L.; Zhong, Q.; Luo, W.; Dai, C.; Dai, X. Macrophage Gpx4 deficiency aggravates foam cell formation by regulating the expression of scavenger receptors, ABCA1, and ABCG1. Cell Biol. Int. 2023, 47, 1589–1599. [Google Scholar] [CrossRef]
- Sucajtys-Szulc, E.; Szolkiewicz, M.; Swierczynski, J.; Rutkowski, B. Up-regulation of liver Pcsk9 gene expression as a possible cause of hypercholesterolemia in experimental chronic renal failure. Mol. Cell. Biochem. 2016, 411, 281–287. [Google Scholar] [CrossRef][Green Version]
- Skeby, C.K.; Hummelgaard, S.; Gustafsen, C.; Petrillo, F.; Frederiksen, K.P.; Olsen, D.; Kristensen, T.; Ivarsen, P.; Madsen, P.; Christensen, E.I.; et al. Proprotein convertase subtilisin/kexin type 9 targets megalin in the kidney proximal tubule and aggravates proteinuria in nephrotic syndrome. Kidney Int. 2023, 104, 754–768. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhu, H.; Cheng, Z.; Hu, X.; Yu, X.; Li, J.; Liu, H.; Tang, P.; Zhang, Y.; Xiong, X.; et al. PCSK9, a novel immune and ferroptosis related gene in abdominal aortic aneurysm neck. Sci. Rep. 2023, 13, 6054. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Q.Y.; Wang, L.M.; Luo, J.; Chen, K.H.; He, Y.N. Proteomics reveals defective peroxisomal fatty acid oxidation during the progression of acute kidney injury and repair. Heliyon 2023, 9, e18134. [Google Scholar] [CrossRef]
- Listenberger, L.; Townsend, E.; Rickertsen, C.; Hains, A.; Brown, E.; Inwards, E.G.; Stoeckman, A.K.; Matis, M.P.; Sampathkumar, R.S.; Osna, N.A.; et al. Decreasing Phosphatidylcholine on the Surface of the Lipid Droplet Correlates with Altered Protein Binding and Steatosis. Cells 2018, 7, 230. [Google Scholar] [CrossRef]
- Smolič, T.; Zorec, R.; Vardjan, N. Pathophysiology of Lipid Droplets in Neuroglia. Antioxidants 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Luo, Y.; Yang, S.; Zeng, M.; Zhang, S.; Liu, J.; Han, Y.; Liu, Y.; Zhu, X.; Wu, H.; et al. Ectopic lipid accumulation: Potential role in tubular injury and inflammation in diabetic kidney disease. Clin. Sci. 2018, 132, 2407–2422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shrestha, R.; Yang, X.; Wu, X.; Jia, J.; Chiba, H.; Hui, S.P. Oxidative Stress and Lipid Dysregulation in Lipid Droplets: A Connection to Chronic Kidney Disease Revealed in Human Kidney Cells. Antioxidants 2022, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Minami, S.; Yamamoto, T.; Takabatake, Y.; Takahashi, A.; Namba, T.; Matsuda, J.; Kimura, T.; Kaimori, J.Y.; Matsui, I.; Hamano, T.; et al. Lipophagy maintains energy homeostasis in the kidney proximal tubule during prolonged starvation. Autophagy 2017, 13, 1629–1647. [Google Scholar] [CrossRef]
- Chen, R.; Li, Z.; Peng, C.; Wen, L.; Xiao, L.; Li, Y. Rational Design of Novel Lipophilic Aggregation-Induced Emission Probes for Revealing the Dynamics of Lipid Droplets during Lipophagy and Ferroptosis. Anal. Chem. 2022, 94, 13432–13439. [Google Scholar] [CrossRef]
- Itabe, H.; Yamaguchi, T.; Nimura, S.; Sasabe, N. Perilipins: A diversity of intracellular lipid droplet proteins. Lipids Health Dis. 2017, 16, 83. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Zhou, Z.; Yu, R. PLIN5 Suppresses Lipotoxicity and Ferroptosis in Cardiomyocyte via Modulating PIR/NF-κB Axis. Int. Heart J. 2024, 65, 537–547. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Q.; Zhu, X.; Shao, X.; Li, S.; Li, J.; Wu, J.; Jin, H.; Qi, C.; Jiang, N.; et al. Activation of lipophagy is required for RAB7 to regulate ferroptosis in sepsis-induced acute kidney injury. Free Radic. Biol. Med. 2024, 218, 120–131. [Google Scholar] [CrossRef]
- Naito, Y.; Fujii, A.; Sawada, H.; Oboshi, M.; Iwasaku, T.; Okuhara, Y.; Morisawa, D.; Eguchi, A.; Hirotani, S.; Masuyama, T. Association between renal iron accumulation and renal interstitial fibrosis in a rat model of chronic kidney disease. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2015, 38, 463–470. [Google Scholar] [CrossRef]
- Tsai, L.T.; Weng, T.I.; Chang, T.Y.; Lan, K.C.; Chiang, C.K.; Liu, S.H. Inhibition of Indoxyl Sulfate-Induced Reactive Oxygen Species-Related Ferroptosis Alleviates Renal Cell Injury In Vitro and Chronic Kidney Disease Progression In Vivo. Antioxidants 2023, 12, 1931. [Google Scholar] [CrossRef]
- Radadiya, P.S.; Thornton, M.M.; Puri, R.V.; Yerrathota, S.; Dinh-Phan, J.; Magenheimer, B.; Subramaniam, D.; Tran, P.V.; Zhu, H.; Bolisetty, S.; et al. Ciclopirox olamine induces ferritinophagy and reduces cyst burden in polycystic kidney disease. JCI Insight 2021, 6, e141299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, Y.; Zhang, J.; Zang, A.; Ren, J.; Zheng, Z.; Fan, M.; Xie, Y. Novel 3-hydroxypyridin-4(1H)-One derivatives as ferroptosis inhibitors with iron-chelating and reactive oxygen species scavenging activities and therapeutic effect in cisplatin-induced cytotoxicity. Eur. J. Med. Chem. 2024, 263, 115945. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, B.; Fan, Y.; Liu, M.; Han, B.; Meng, Y.; Xu, X.; Song, Z.; Liu, X.; Hao, Q.; et al. Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br. J. Pharmacol. 2021, 178, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Skouta, R.; Himmerkus, N.; Mulay, S.R.; Dewitz, C.; De Zen, F.; Prokai, A.; Zuchtriegel, G.; Krombach, F.; Welz, P.S.; et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 16836–16841. [Google Scholar] [CrossRef]
- Wiggin, T.D.; Kretzler, M.; Pennathur, S.; Sullivan, K.A.; Brosius, F.C.; Feldman, E.L. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology 2008, 149, 4928–4937. [Google Scholar] [CrossRef][Green Version]
- Li, L.X.; Fan, L.X.; Zhou, J.X.; Grantham, J.J.; Calvet, J.P.; Sage, J.; Li, X. Lysine methyltransferase SMYD2 promotes cyst growth in autosomal dominant polycystic kidney disease. J. Clin. Investig. 2017, 127, 2751–2764. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Ru, F.; Gan, Y.; Li, B.; Xia, W.; Dai, G.; He, Y.; Chen, Z. Liproxstatin-1 attenuates unilateral ureteral obstruction-induced renal fibrosis by inhibiting renal tubular epithelial cells ferroptosis. Cell Death Dis. 2021, 12, 843. [Google Scholar] [CrossRef]
- Huang, Q.; Ru, Y.; Luo, Y.; Luo, X.; Liu, D.; Ma, Y.; Zhou, X.; Linghu, M.; Xu, W.; Gao, F.; et al. Identification of a targeted ACSL4 inhibitor to treat ferroptosis-related diseases. Sci. Adv. 2024, 10, eadk1200. [Google Scholar] [CrossRef]
- Tao, W.H.; Shan, X.S.; Zhang, J.X.; Liu, H.Y.; Wang, B.Y.; Wei, X.; Zhang, M.; Peng, K.; Ding, J.; Xu, S.X.; et al. Dexmedetomidine Attenuates Ferroptosis-Mediated Renal Ischemia/Reperfusion Injury and Inflammation by Inhibiting ACSL4 via α2-AR. Front. Pharmacol. 2022, 13, 782466. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, J.; Xu, H.; Zhou, C.; Han, B.; Zhu, H.; Hu, Z.; Ma, Z.; Ming, Z.; Yao, Y.; et al. XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death Dis. 2020, 11, 629. [Google Scholar] [CrossRef]
- Setti, G.; Hayward, A.; Dessapt, C.; Barone, F.; Buckingham, R.; White, K.; Bilous, R.; Hiroshi, K.; Gruden, G.; Viberti, G.; et al. Peroxisome proliferator-activated receptor-γ agonist rosiglitazone prevents albuminuria but not glomerulosclerosis in experimental diabetes. Am. J. Nephrol. 2010, 32, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Gumieniczek, A. Effect of the new thiazolidinedione-pioglitazone on the development of oxidative stress in liver and kidney of diabetic rabbits. Life Sci. 2003, 74, 553–562. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Bi, R.; Su, Y.; Quan, F.; Lin, Y.; Yue, C.; Cui, X.; Zhao, Q.; Liu, S.; et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022, 51, 102262. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Cuzzocrea, S.; Chatterjee, P.K.; Di Paola, R.; Sautebin, L.; Britti, D.; Thiemermann, C. Reduction of renal ischemia-reperfusion injury in 5-lipoxygenase knockout mice and by the 5-lipoxygenase inhibitor zileuton. Mol. Pharmacol. 2004, 66, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhou, L.; Liu, X.; Gao, L.; Li, Y.; Wu, Y. Baicalein alleviates cisplatin-induced acute kidney injury by inhibiting ALOX12-dependent ferroptosis. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 130, 155757. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, J.; Xian, Q.; Su, H.; Ni, Y.; Wang, L. Kaempferitrin attenuates unilateral ureteral obstruction-induced renal inflammation and fibrosis in mice by inhibiting NOX4-mediated tubular ferroptosis. Phytother. Res. PTR 2024, 38, 2656–2668. [Google Scholar] [CrossRef]
- Jia, J.; Tan, R.; Xu, L.; Wang, H.; Li, J.; Su, H.; Zhong, X.; Liu, P.; Wang, L. Hederagenin improves renal fibrosis in diabetic nephropathy by regulating Smad3/NOX4/SLC7A11 signaling-mediated tubular cell ferroptosis. Int. Immunopharmacol. 2024, 135, 112303. [Google Scholar] [CrossRef]
- Zhu, B.; Ni, Y.; Gong, Y.; Kang, X.; Guo, H.; Liu, X.; Li, J.; Wang, L. Formononetin ameliorates ferroptosis-associated fibrosis in renal tubular epithelial cells and in mice with chronic kidney disease by suppressing the Smad3/ATF3/SLC7A11 signaling. Life Sci. 2023, 315, 121331. [Google Scholar] [CrossRef]
- Song, J.; Wang, H.; Sheng, J.; Zhang, W.; Lei, J.; Gan, W.; Cai, F.; Yang, Y. Vitexin attenuates chronic kidney disease by inhibiting renal tubular epithelial cell ferroptosis via NRF2 activation. Mol. Med. 2023, 29, 147. [Google Scholar] [CrossRef]
- Jung, K.H.; Kim, S.E.; Go, H.G.; Lee, Y.J.; Park, M.S.; Ko, S.; Han, B.S.; Yoon, Y.C.; Cho, Y.J.; Lee, P.; et al. Synergistic Renoprotective Effect of Melatonin and Zileuton by Inhibition of Ferroptosis via the AKT/mTOR/NRF2 Signaling in Kidney Injury and Fibrosis. Biomol. Ther. 2023, 31, 599–610. [Google Scholar] [CrossRef]
- Pan, M.; Wang, Z.; Wang, Y.; Jiang, X.; Fan, Y.; Gong, F.; Sun, Y.; Wang, D. Celastrol alleviated acute kidney injury by inhibition of ferroptosis through Nrf2/GPX4 pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 166, 115333. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Dong, X.; Wu, Y.; Li, B.; Kuang, B.; Chen, G.; Zhang, L. Astragaloside IV attenuates myocardial dysfunction in diabetic cardiomyopathy rats through downregulation of CD36-mediated ferroptosis. Phytother. Res. PTR 2023, 37, 3042–3056. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Hamano, H.; Horinouchi, Y.; Miyamoto, L.; Hirayama, T.; Nagasawa, H.; Tamaki, T.; Tsuchiya, K. Role of ferroptosis in cisplatin-induced acute nephrotoxicity in mice. J. Trace Elem. Med. Biol. 2021, 67, 126798. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Ozono, I.; Tajima, S.; Imao, M.; Horinouchi, Y.; Izawa-Ishizawa, Y.; Kihira, Y.; Miyamoto, L.; Ishizawa, K.; Tsuchiya, K.; et al. Iron chelation by deferoxamine prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. PLoS ONE 2014, 9, e89355. [Google Scholar] [CrossRef]
- Naito, Y.; Fujii, A.; Sawada, H.; Hirotani, S.; Iwasaku, T.; Eguchi, A.; Ohyanagi, M.; Tsujino, T.; Masuyama, T. Effect of iron restriction on renal damage and mineralocorticoid receptor signaling in a rat model of chronic kidney disease. J. Hypertens. 2012, 30, 2192–2201. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Skouta, R.; Dixon, S.J.; Wang, J.; Dunn, D.E.; Orman, M.; Shimada, K.; Rosenberg, P.A.; Lo, D.C.; Weinberg, J.M.; Linkermann, A.; et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 2014, 136, 4551–4556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).