Mesenchymal Stem Cells: What We Have Learned and How to Manage Them
- Conclusions
Funding
Conflicts of Interest
References
- Tavakoli, S.; Ghaderi Jafarbeigloo, H.R.; Shariati, A.; Jahangiryan, A.; Jadidi, F.; Jadidi Kouhbanani, M.A.; Hassanzadeh, A.; Zamani, M.; Javidi, K.; Naimi, A. Mesenchymal stromal cells; a new horizon in regenerative medicine. J. Cell Physiol. 2020, 235, 9185–9210. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R.; Markov, A.; Zekiy, A.O.; Hamza, M.U.; Al-Dabbagh, K.A.; Al-Zubaidi, S.H.; Hameed, N.M.; Ahmad, I.; Sivaraman, R.; Kzar, H.H.; et al. Clinical application of mesenchymal stem cell in regenerative medicine: A narrative review. Stem Cell Res. Ther. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Baghaei, K.; Hashemi, S.M.; Tokhanbigli, S.; Asadi Rad, A.; Assadzadeh-Aghdaei, H.; Sharifian, A.; Zali, M.R. Isolation, Differentiation, and Characterization of Mesenchymal Stem Cells from Human Bone Marrow. Gastroenterol. Hepatol. Bed Bench 2017, 10, 208–213. [Google Scholar] [PubMed]
- Trivanović, D.; Kocić, J.; Mojsilović, S.; Krstić, A.; Ilić, V.; Djordjević, I.O.; Santibanez, J.F.; Jovcić, G.; Terzić, M.; Bugarski, D. Mesenchymal Stem Cells Isolated from Peripheral Blood and Umbilical Cord Wharton’s Jelly. Srp. Arh. Celok. Lek. 2013, 141, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Zeddou, M.; Briquet, A.; Relic, B.; Josse, C.; Malaise, M.G.; Gothot, A.; Lechanteur, C.; Beguin, Y. The Umbilical Cord Matrix Is a Better Source of Mesenchymal Stem Cells (MSC) than the Umbilical Cord Blood. Cell Biol. Int. 2010, 34, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Megaloikonomos, P.D.; Panagopoulos, G.N.; Bami, M.; Igoumenou, V.G.; Dimopoulos, L.; Milonaki, A.; Kyriakidou, M.; Mitsiokapa, E.; Anastassopoulou, J.; Mavrogenis, A.F. Harvesting, Isolation and Differentiation of Rat Adipose-Derived Stem Cells. Curr. Pharm. Biotechnol. 2018, 19, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental issues vs. hose from their sources: Their biology and role in regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Zhidu, S.; Ying, T.; Rui, J.; Chao, Z. Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: Challenges and opportunities. Stem Cell Res. Ther. 2024, 15, 266. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Wang, H. Emerging Landscape of Mesenchymal Stem Cell Senescence Mechanisms and Implications on Therapeutic Strategies. ACS Pharmacol. Transl. Sci. 2024, 7, 2306–2325. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 1–24. [Google Scholar] [CrossRef] [PubMed]
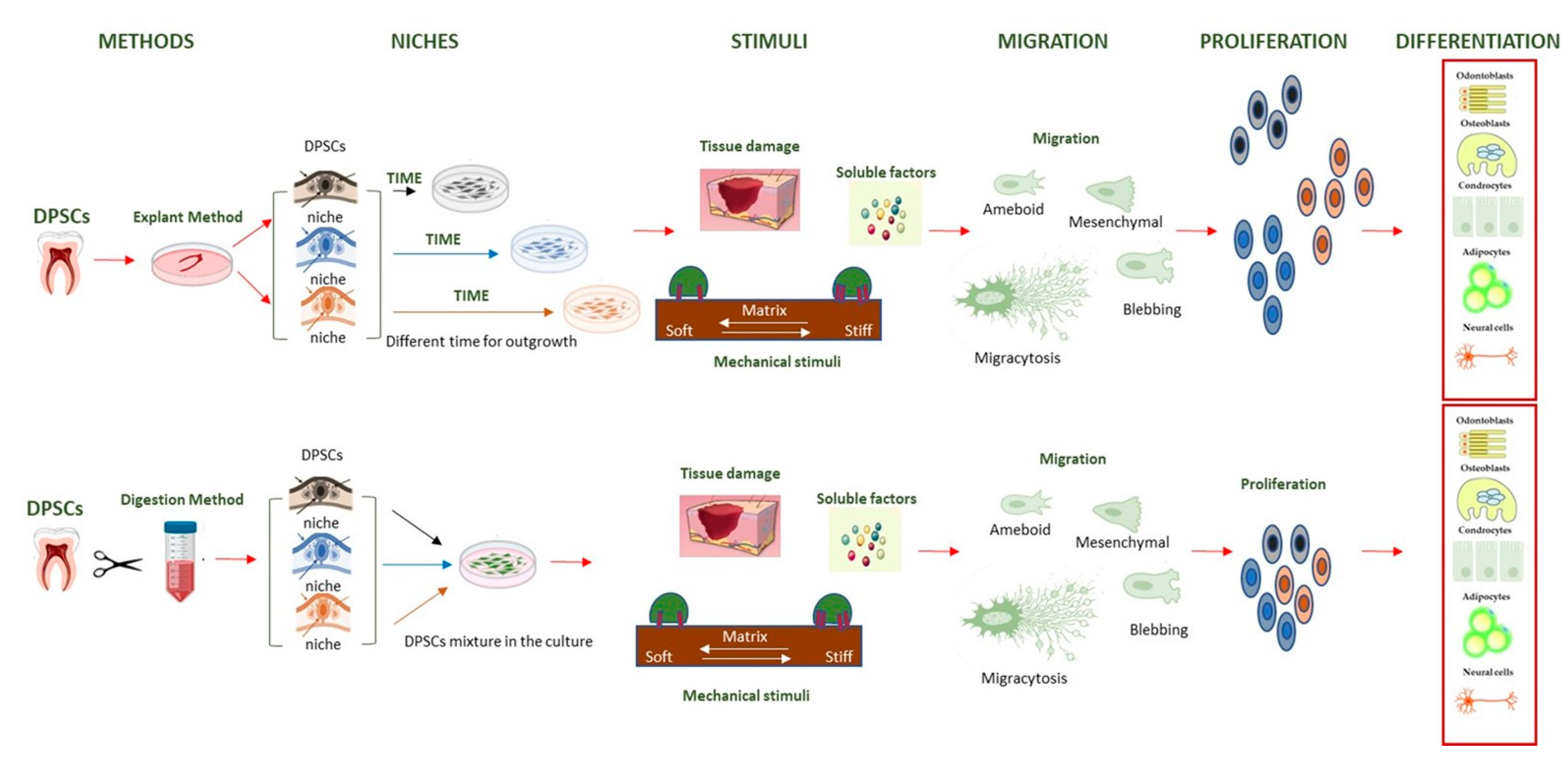
- Lampiasi, N. The Migration and the Fate of Dental Pulp Stem Cells. Biology 2023, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, I.; Barrett, J.C. Transcriptional Regulation of the Telomerase hTERT Gene as a Target for Cellular and Viral Oncogenic Mechanisms. Carcinogenesis 2003, 24, 1167–1176. [Google Scholar] [CrossRef]
- Shu, Y.; Yang, C.; Ji, X.; Zhang, L.; Bi, Y.; Yang, K.; Gong, M.; Liu, X.; Guo, Q.; Su, Y.; et al. Reversibly Immortalized Human Umbilical Cord-Derived Mesenchymal Stem Cells (UC-MSCs) Are Responsive to BMP9-Induced Osteogenic and Adipogenic Differentiation. J. Cell Biochem. 2018, 119, 8872–8886. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-J.; Chen, X.-J.; Yang, D.-H.; Huang, S.-M.; Sun, G.-D.; Chen, Y.-P. Differentiation of Human Umbilical Cord Mesenchymal Stem Cells into Hepatocyte-like Cells by hTERT Gene Transfection In Vitro. Cell Biol. Int. 2012, 36, 215–221. [Google Scholar] [CrossRef]
- Pino-Barrio, M.J.; García-García, E.; Menéndez, P.; Martínez-Serrano, A. V-Myc Immortalizes Human Neural Stem Cells in the Absence of Pluripotency-Associated Traits. PLoS ONE 2015, 10, e0118499. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific Association of Human Telomerase Activity with Immortal Cells and Cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
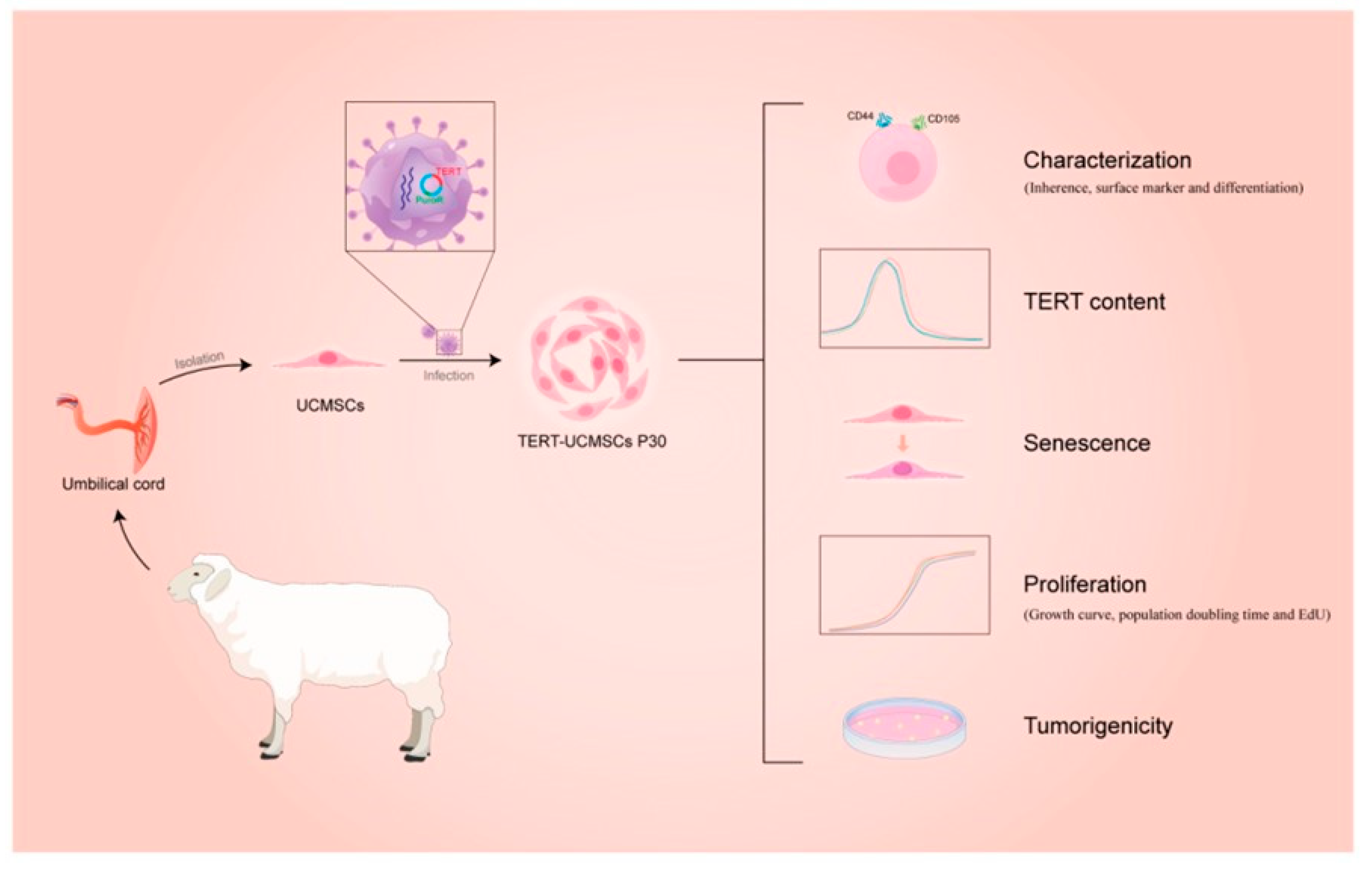
- Yang, J.; Dong, Y.; Hu, L.; Wang, W.; Li, Y.; Wang, S.; Wang, C. Immortalization of Mesenchymal Stem Cell Lines from Sheep Umbilical Cord Tissue. Biology 2024, 13, 551. [Google Scholar] [CrossRef]
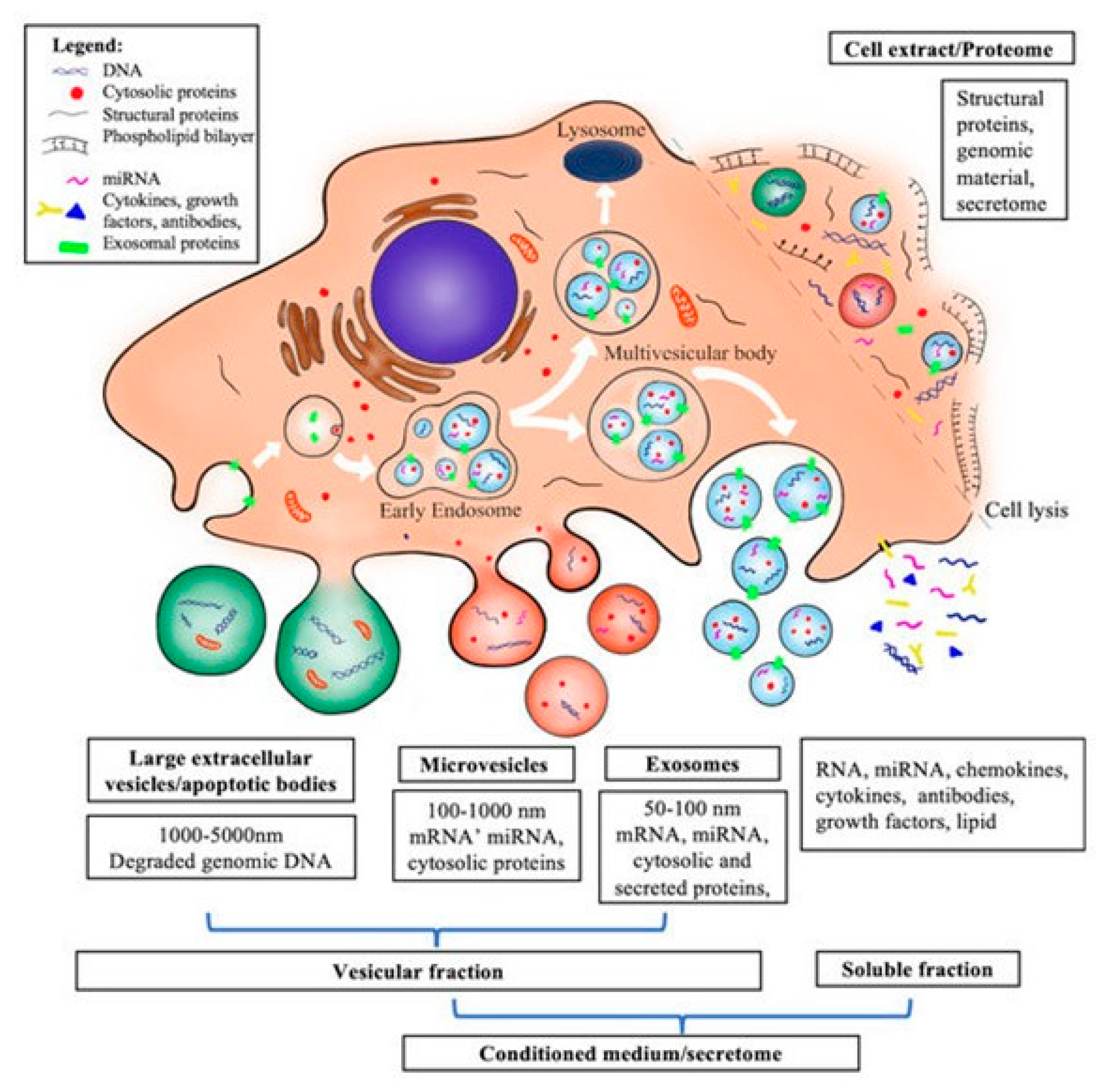
- Shimizu, Y.; Ntege, E.H.; Inoue, Y.; Matsuura, N.; Sunami, H.; Sowa, Y. Optimizing mesenchymal stem cell extracellular vesicles for chronic wound healing: Bioengineering, standardization, and safety. Regen. Ther. 2024, 26, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Clua-Ferré, L.; Suau, R.; Vañó-Segarra, I.; Ginés, I.; Serena, C.; Manyé, J. Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles: A focus on inflammatory bowel disease. Clin. Transl. Med. 2024, 14, e70075. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, X.; Long, Y.; Chen, Y. Emerging Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Chronic Respiratory Diseases: An Overview of Recent Progress. Front. Bioeng. Biotechnol. 2022, 10, 845042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, P.; Liang, Q.; Zhou, J.; Wu, B.; Xu, W. Conditioned Medium Derived From Human Dental Follicle Mesenchymal Stem Cells Alleviates Macrophage Proinflammatory Responses Through MAPK-ERK-EGR1 Axis. Stem Cells Int. 2024, 2024, 5514771. [Google Scholar] [CrossRef]
- Su, X.; Upadhyay, A.; Tran, S.D.; Lin, Z. Cell-Free Therapies: The Use of Cell Extracts to Mitigate Irradiation-Injured Salivary Glands. Biology 2023, 12, 305. [Google Scholar] [CrossRef]
- Kamprom, W.; Tangporncharoen, R.; Vongthaiwan, N.; Tragoonlugkana, P.; Phetfong, J.; Pruksapong, C.; Supokawej, A. Enhanced potent immunosuppression of intracellular adipose tissue-derived stem cell extract by priming with three-dimensional spheroid formation. Sci. Rep. 2024, 14, 9084. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Iwasaki, K.; Taguchi, Y.; Ishikawa, I.; Umeda, M. Mesenchymal stem cell-derived protein extract induces periodontal regeneration. Cytotherapy 2024. preprint. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, T.; Xie, J.; Wu, H.; Hu, W.; Yuan, X. MSC-derived mitochondria promote axonal regeneration via Atf3 gene up-regulation by ROS induced DNA double strand breaks at transcription initiation region. Cell Commun. Signal. 2024, 22, 240. [Google Scholar] [CrossRef]
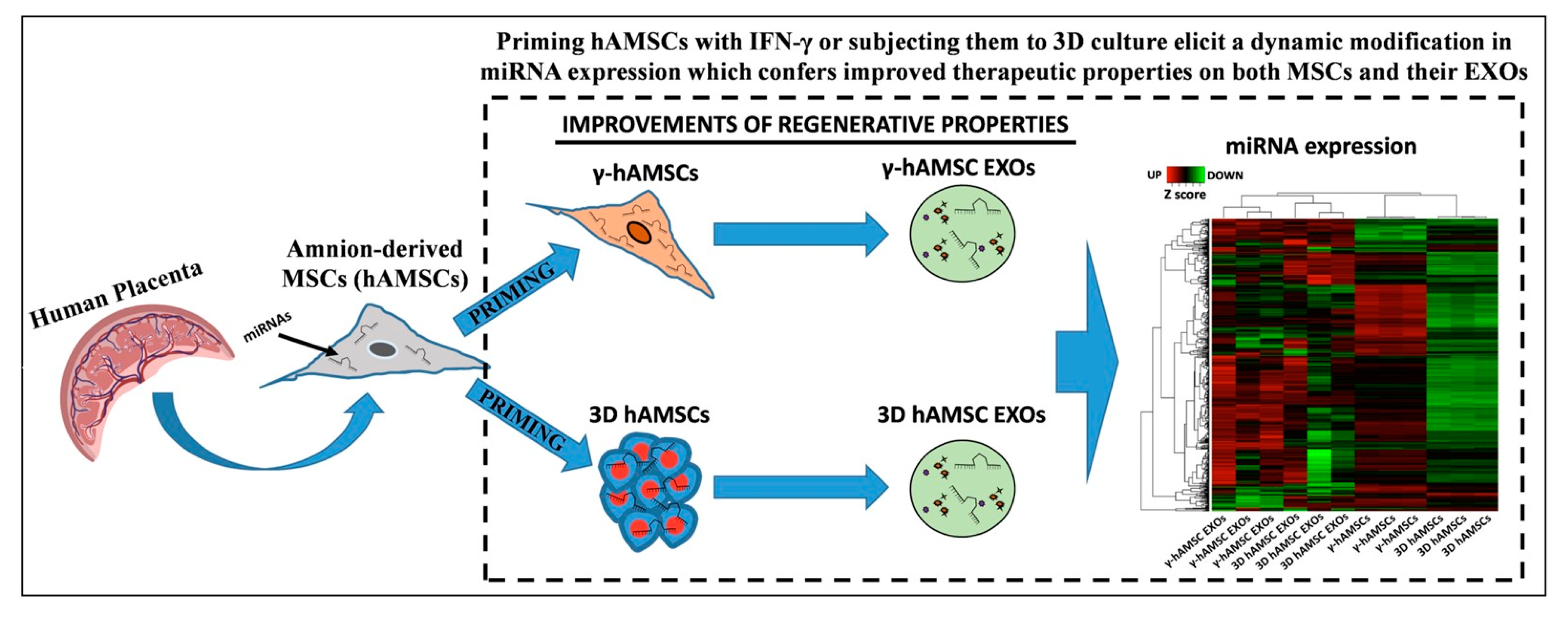
- Bulati, M.; Gallo, A.; Zito, G.; Busà, R.; Iannolo, G.; Cuscino, N.; Castelbuono, S.; Carcione, C.; Centi, C.; Martucci, G.; et al. 3D Culture and Interferon-Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal microRNAs. Biology 2023, 12, 1063. [Google Scholar] [CrossRef]
- Firouzabadi, S.R.; Mohammadi, I.; Ghafourian, K.; Mofidi, S.A.; Firouzabadi, R.S. Mesenchymal stem cell-derived extracellular vesicles therapy for primary ovarian insufficiency: A systematic review and meta-analysis of pre-clinical studies. J. Ovarian Res. 2024, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, Y.; Wang, X.; Lu, W.; Tang, X.; Jin, Y.; Ye, J. Single and combined strategies for mesenchymal stem cell exosomes alleviate liver fibrosis: A systematic review and meta-analysis of preclinical animal models. Front. Pharmacol. 2024, 15, 1432683. [Google Scholar] [CrossRef]
- Agosti, E.; Antonietti, S.; Ius, T.; Fontanella, M.M.; Zeppieri, M.; Panciani, P.P. A Systematic Review of Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Potential Treatment for Glioblastoma. Brain Sci. 2024, 14, 1058. [Google Scholar] [CrossRef]
- Wang, L.; Wei, J.; Da Fonseca Ferreira, A.; Wang, H.; Zhang, L.; Zhang, Q.; Bellio, M.A.; Chu, X.M.; Khan, A.; Jayaweera, D.; et al. Rejuvenation of Senescent Endothelial Progenitor Cells by Extracellular Vesicles Derived From Mesenchymal Stromal Cells. JACC Basic. Transl. Sci. 2020, 5, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Fraile, M.; Eiro, N.; Costa, L.A.; Arancha, M.; Vizoso, F.J. Aging and Mesenchymal Stem Cells: Basic Concepts, Challenges and Strategies. Biology 2022, 11, 1678. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampiasi, N. Mesenchymal Stem Cells: What We Have Learned and How to Manage Them. Biology 2025, 14, 1. https://doi.org/10.3390/biology14010001
Lampiasi N. Mesenchymal Stem Cells: What We Have Learned and How to Manage Them. Biology. 2025; 14(1):1. https://doi.org/10.3390/biology14010001
Chicago/Turabian StyleLampiasi, Nadia. 2025. "Mesenchymal Stem Cells: What We Have Learned and How to Manage Them" Biology 14, no. 1: 1. https://doi.org/10.3390/biology14010001
APA StyleLampiasi, N. (2025). Mesenchymal Stem Cells: What We Have Learned and How to Manage Them. Biology, 14(1), 1. https://doi.org/10.3390/biology14010001




