Dynamic Metabolic Responses of Resistant and Susceptible Poplar Clones Induced by Hyphantria cunea Feeding
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Insect and Rearing Conditions
2.3. Treatment of Poplar Trees with H. cunea
2.4. Determination of the Enzymatic Activity of PPO and POD
2.5. Metabolomic Analysis of Leaf Tissues
2.6. Data Processing and Statistical Analyses
3. Results
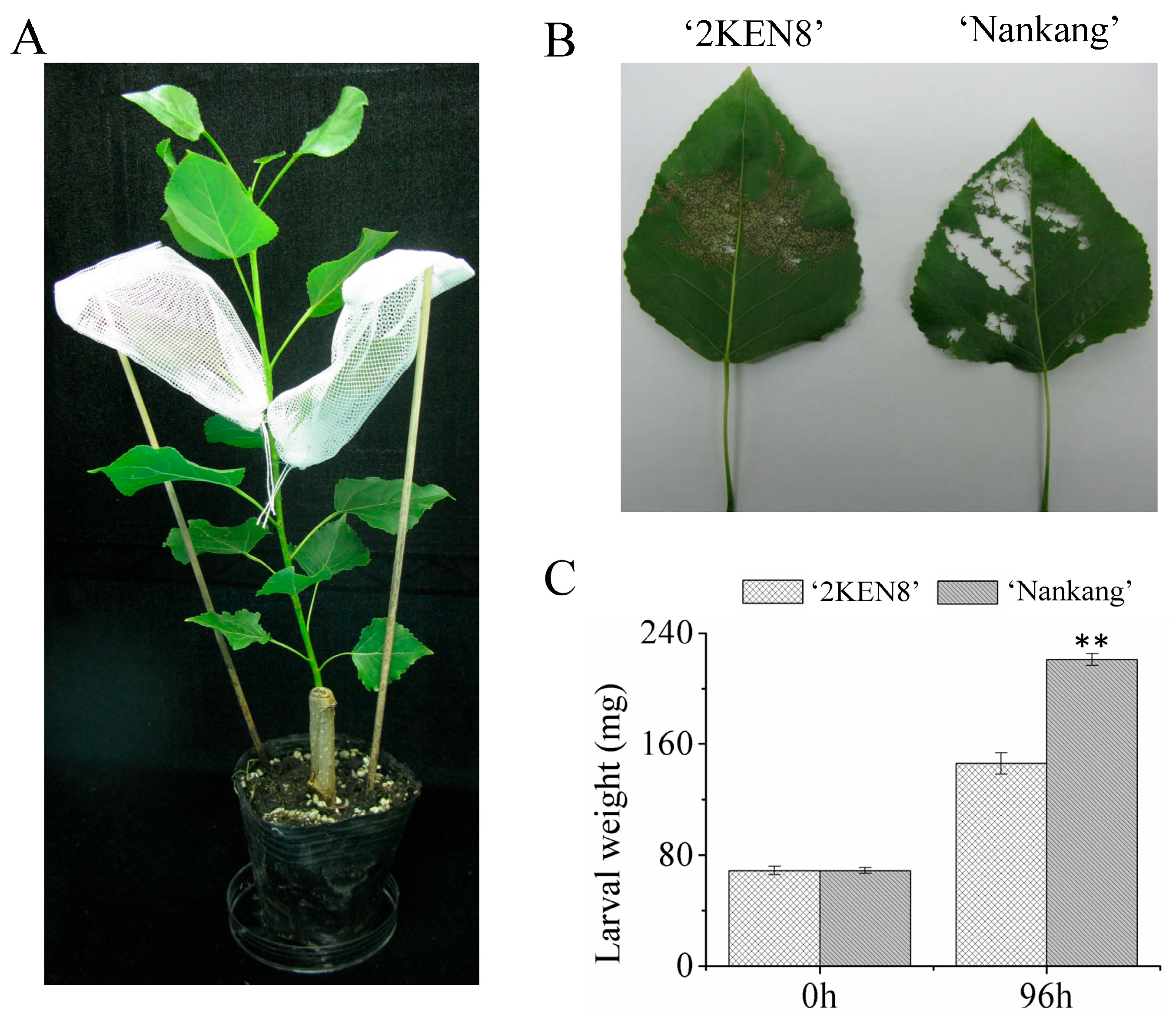
3.1. Quantification of Leaf Damage in Resistant and Susceptible Poplar Clone
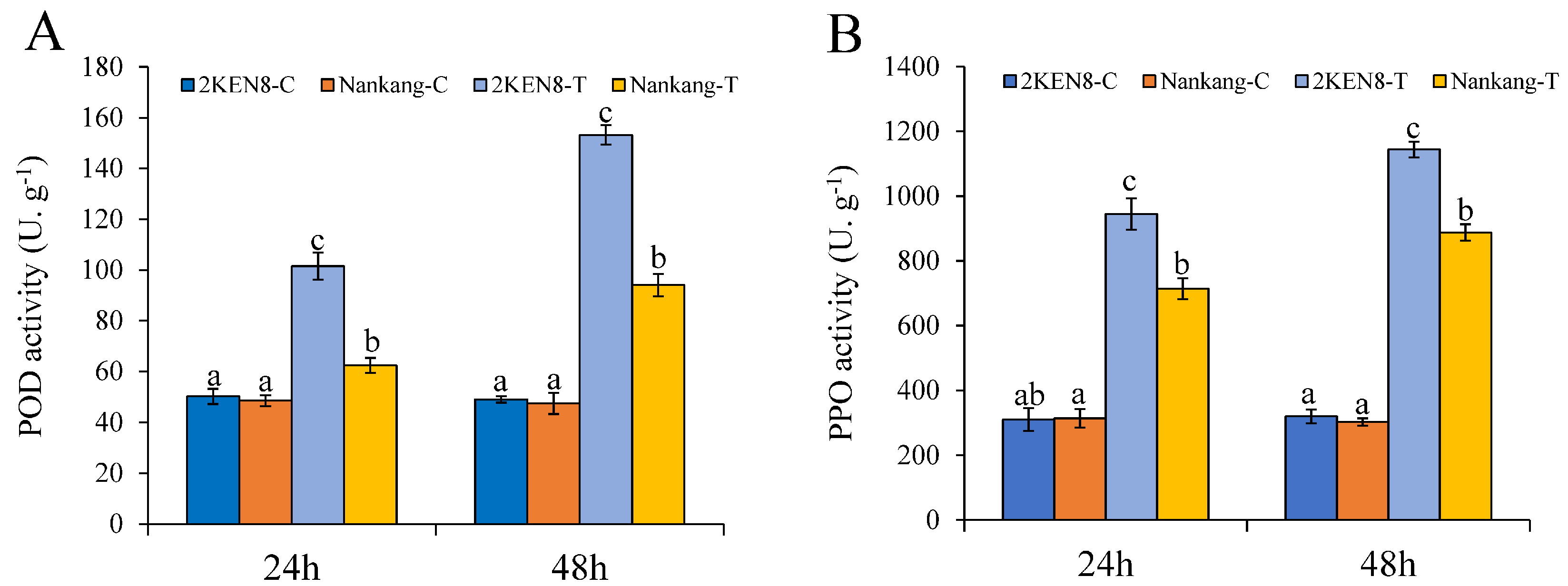
3.2. Changes in the Activity of Defensive Enzymes
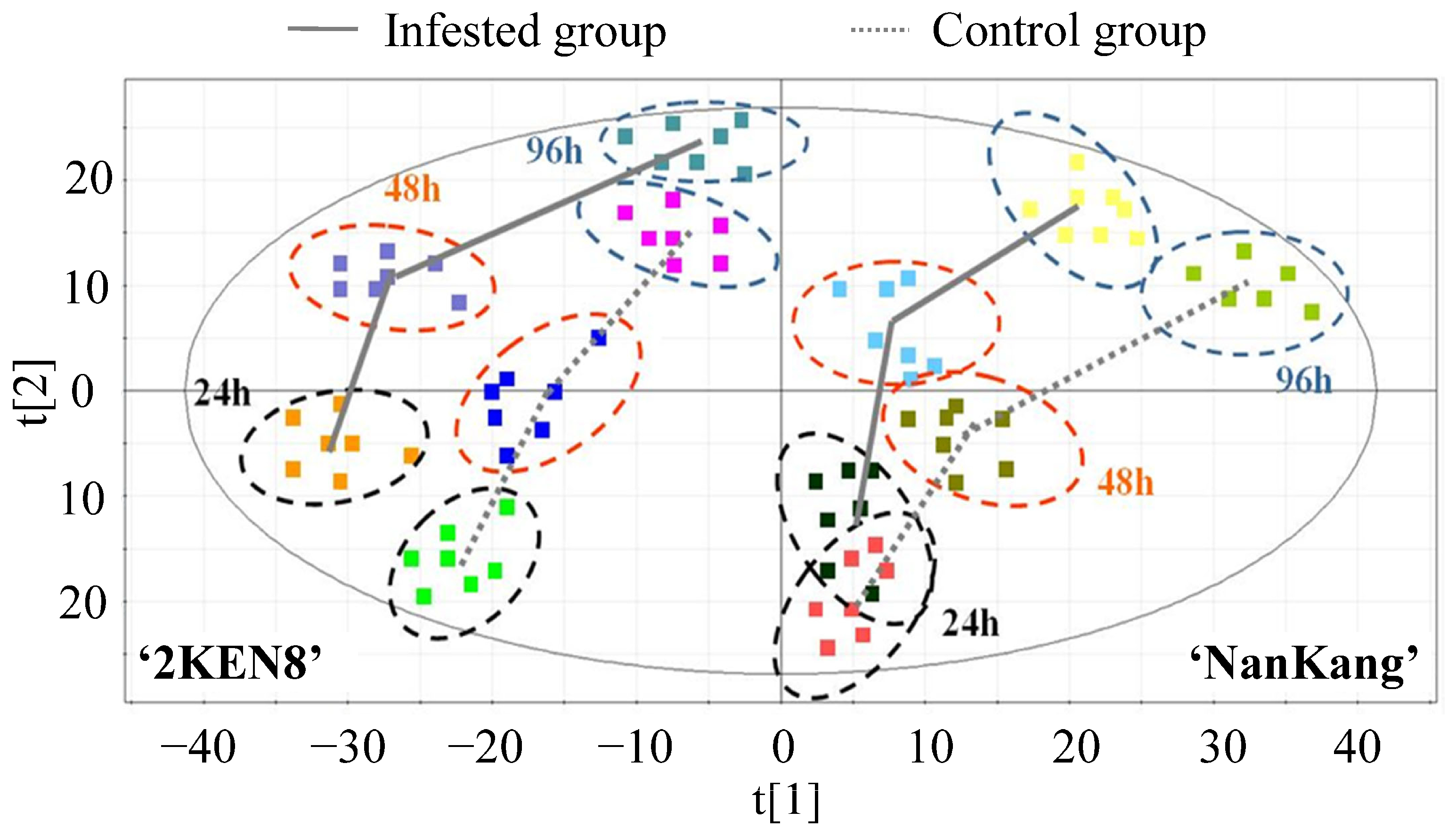
3.3. Dynamic Metabolic Responses in Resistant and Susceptible Cultivar Induced by H. cunea
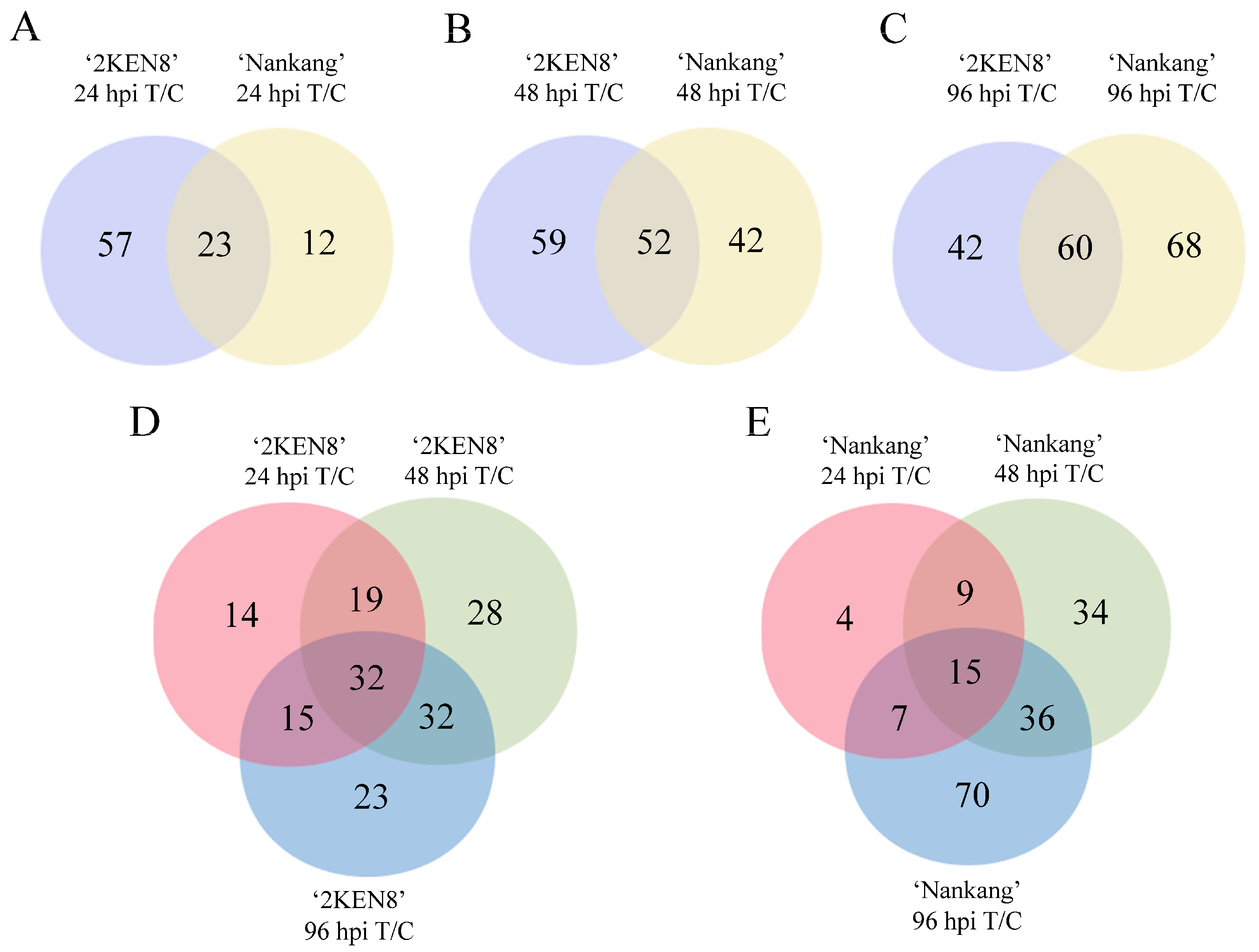
3.4. Differential Metabolites Induced by H. cunea in ‘2KEN8’ and ‘Nankang’
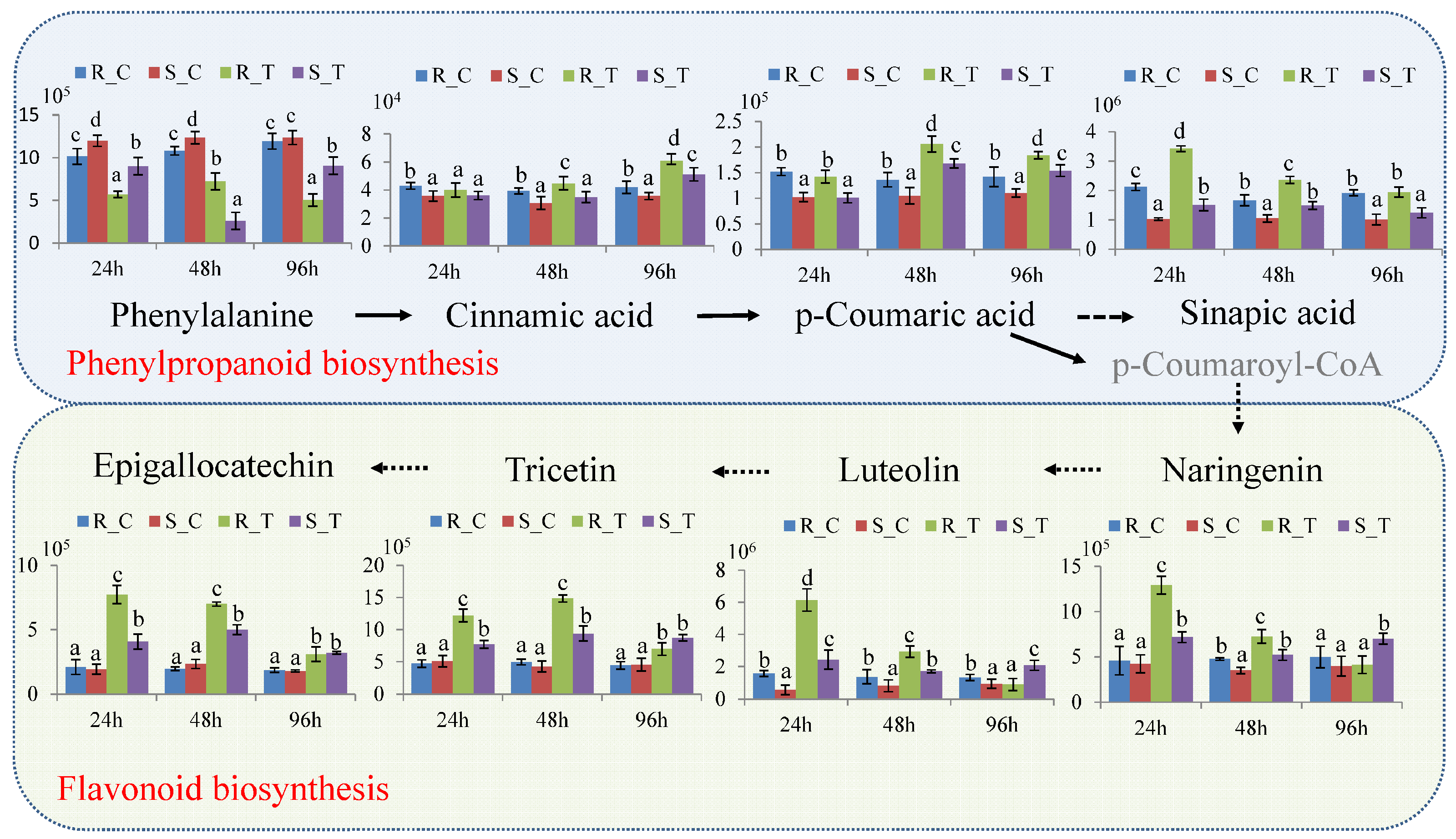
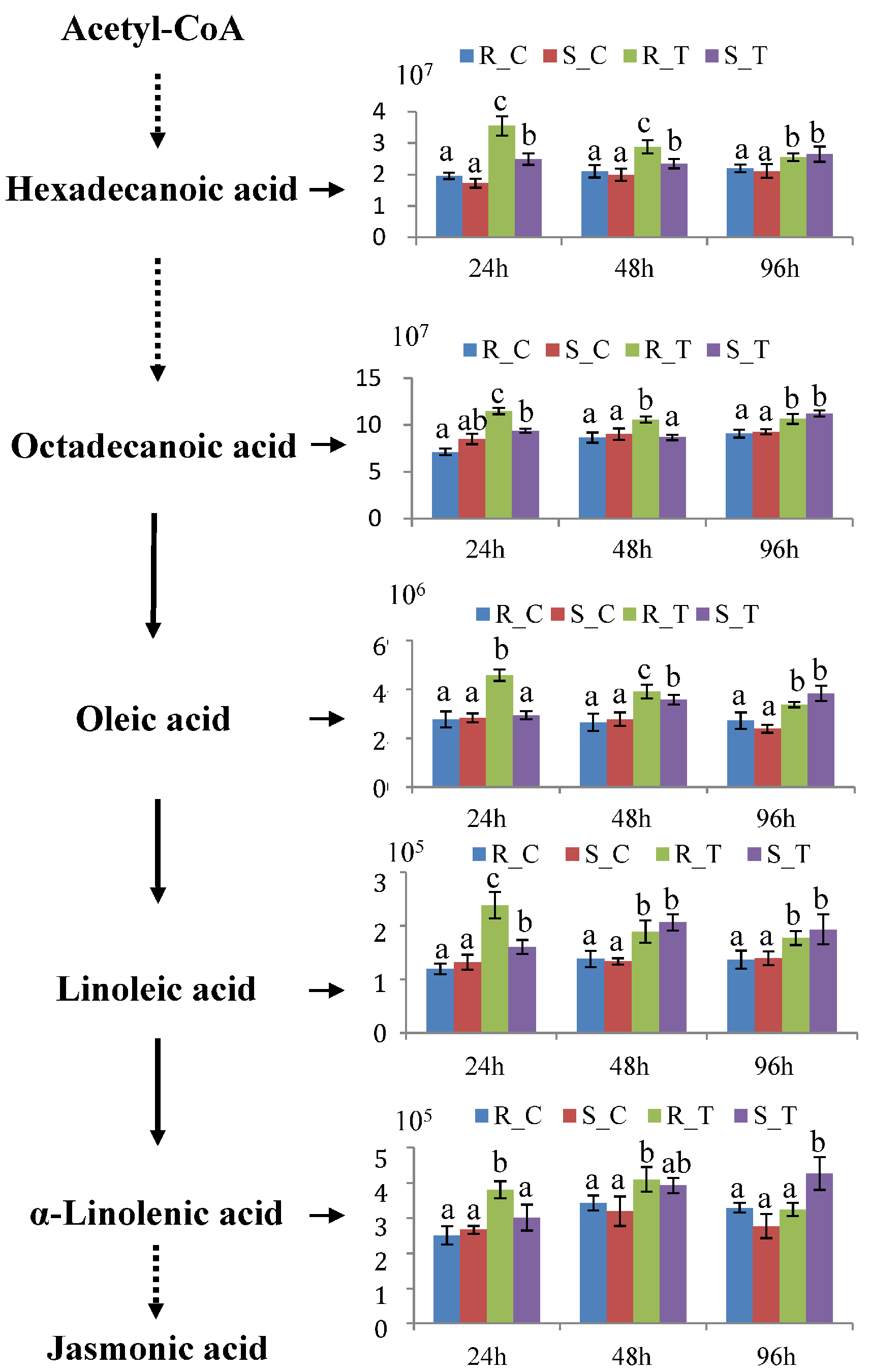
3.5. Pathways Associated with Resistance to H. cunea in Poplar
4. Discussion
4.1. The Resistant ‘2KEN8’ Exhibited Higher Levels of Defensive Enzymes after Infestation with H. cunea
4.2. Feeding of H. cunea Triggered Stronger Metabolic Responses in the Resistant ‘2KEN8’
4.3. The Phenylpropanoid Pathway Is Associated with Induced Poplar Resistance to H. cunea
4.4. Flavonoid and Unsaturated Fatty Acids Contribute to the Earlier Defensive Responses in ‘2KEN8’
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, J.J.; Wang, L.J.; Yan, D.H.; Lu, M.Z. Research and application of transgenic poplar in China. In Challenges and Opportunities forthe World’s Forests in the 21st Century; Springer: Dordrecht, The Netherlands, 2014; pp. 567–584. [Google Scholar] [CrossRef]
- Liu, B.; Yan, J.Y.; Wang, D.; Wang, Y.; Zhou, Y.T.; Chen, Y.F. Occurrence of major forest pests in China in 2023 and prediction for trend in 2024. For. Pest Dis. 2024, 43, 41–45. (In Chinese) [Google Scholar]
- Ning, J.; Lu, P.; Fan, J.; Ren, L.; Zhao, L. American fall webworm in China: A new case of global biological invasions. Innovation 2022, 3, 100201. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Fan, G.; Wang, R.; Yao, W.; Zhou, B.; Jiang, T. Multi-omics analysis of Populus simonii × P. nigra leaves under Hyphantria cunea stress. Front. Plant Sci. 2024, 15, 1392433. [Google Scholar] [CrossRef]
- Ding, Y.; Shen, J.; Li, H.; Sun, Y.; Jiang, T.; Kong, X.; Han, R.; Zhao, C.; Zhang, X.; Zhao, X. Physiological and molecular mechanism of Populus pseudo-cathayana × Populus deltoides response to Hyphantria cunea. Pestic. Biochem. Physiol. 2024, 202, 105969. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Q. Occurrence, Forest control status and prospect of fall-webworm (Hyphantria cunea Drury) in China. J. Shenyang Agric. Univ. 2022, 53, 630–640. (In Chinese) [Google Scholar] [CrossRef]
- Hanley, M.E.; Lamont, B.B.; Fairbanks, M.M.; Rafferty, C.M. Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol. Evol. Syst. 2007, 4, 157–178. [Google Scholar] [CrossRef]
- Hu, C.; Li, Y.T.; Liu, Y.X.; Hao, G.F.; Yang, X.Q. Molecular interaction network of plant-herbivorous insects. Adv. Agrochem 2024, 3, 74–82. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.T.; Hwang, S.Y. Chemical-based resistance of Brassica oleracea and Rorippa dubia in response to Spodoptera litura attack. J. Appl. Entomol. 2020, 144, 201–211. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Pichersky, E.; Lewinsohn, E. Convergent evolution in plant specialized metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef] [PubMed]
- Lattanzino, V.; Lattanzino, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochemistry 2006, 661, 23–67. [Google Scholar]
- Hirayama, J.; Cho, S.; Sassone-Corsi, P. Circadian control by the reduction/oxidation pathway: Catalase represses light-dependent clock gene expression in the zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 15747–15752. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, U.; Gershenzon, J. Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 2002, 5, 300–307. [Google Scholar] [CrossRef]
- Kessler, A. The information landscape of plant constitutive and induced secondary metabolite production. Curr. Opin. Insect Sci. 2015, 8, 47–53. [Google Scholar] [CrossRef]
- Inderjit; Wardle, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef]
- Brody, A.K.; Karban, R. Lack of a Tradeoff between constitutive and induced defenses among varieties of cotton. Oikos 1992, 65, 301–306. [Google Scholar] [CrossRef]
- Chen, M.S. Inducible direct plant defense against insect herbivores: A review. Insect Sci. 2008, 15, 101–114. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jaros, A.; Lee, G.; Mozola, C.; Weir, Q.; Salminen, J.P. Hydrolyzable tannins as “quantitative defenses”: Limited impact against Lymantria dispar caterpillars on hybrid poplar. J. Insect Physiol. 2009, 55, 297–304. [Google Scholar] [CrossRef]
- Barakat, A.; Bagniewska-Zadworna, A.; Frost, C.J.; Carlson, J.E. Phylogeny and expression profiling of CAD and CAD-like genes in hybrid Populus (P. deltoides × P. nigra): Evidence from herbivore damage for subfunctionalization and functional divergence. BMC Plant Biol. 2010, 10, 100. [Google Scholar] [CrossRef]
- Peters, D.J.; Constabel, C.P. Molecular analysis of herbivore-induced condensed tannin synthesis: Cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides). Plant J. 2002, 32, 701–712. [Google Scholar] [CrossRef]
- Young, B.; Wagner, D.; Doak, P.; Clausen, T. Induction of phenolic glycosides by quaking aspen (Populus tremuloides) leaves in relation to extrafloral nectaries and epidermal leaf mining. J. Chem. Ecol. 2010, 36, 369–377. [Google Scholar] [CrossRef]
- Wang, L.J.; Qu, L.J.; Zhang, L.; Hu, J.J.; Tang, F.; Lu, M.Z. Metabolic responses of poplar to Apripona germari (Hope) as revealed by metabolite profiling. Int. J. Mol. Sci. 2016, 17, 923. [Google Scholar] [CrossRef]
- Wang, L.J.; Qu, L.J.; Hu, J.J.; Zhang, L.W.; Tang, F.; Lu, M.Z. Metabolomics reveals constitutive metabolites that contribute resistance to fall webworm (Hyphantria cunea) in Populus deltoides. Environ. Exp. Bot. 2017, 136, 31–40. [Google Scholar] [CrossRef]
- Li, S.Y.; Yu, X.H.; Fan, B.Q.; Hao, D.J. A gut-isolated enterococcus strain (HcM7) triggers the expression of antimicrobial peptides that aid resistance to nucleopolyhedrovirus infection of Hyphantria cunea larvae. Pest Manag. Sci. 2023, 79, 3529–3537. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Zhou, X.; Wang, Y.; Liang, Y.J.; Luo, B.S.; Dai, Y.H.; Wei, Z.L.; Li, S.L.; He, R.; et al. Molecular mechanism of plant elicitor daphnetin-carboxymethyl chitosan nanoparticles against Ralstonia solanacearum by activating plant system resistance. Int. J. Biol. Macromol. 2023, 241, 124580. [Google Scholar] [CrossRef]
- Ge, X.L.; Zhang, L.; Du, J.J.; Wen, S.S.; Qu, G.Z.; Hu, J.J. Transcriptome analysis of Populus euphratica under salt treatment and PeERF1 gene enhances salt tolerance in transgenic Populus alba × Populus glandulosa. Int. J. Mol. Sci. 2022, 23, 3727. [Google Scholar] [CrossRef]
- Duan, L.X.; Chen, T.L.; Li, M.; Chen, M.; Zhou, Y.Q.; Cui, G.H.; Zhao, A.H.; Jia, W.; Huang, L.Q.; Qi, X. Use of the metabolomics approach to characterize Chinese medicinal material Huangqi. Mol. Plant 2012, 5, 376–386. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.G.; Wang, M.X.; Cui, L.; Han, B.Y. Research progress on the underlying mechanisms of plant defense enzymes in response to pest stress. Ying Yong Sheng Tai Xue Bao 2018, 29, 4248–4258. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Whitehill, J.G.; Opiyo, S.O.; Koch, J.L.; Herms, D.A.; Cipollini, D.F.; Bonello, P. Interspecific comparison of constitutive ash phloem phenolic chemistry reveals compounds unique to manchurian ash, a species resistant to emerald ash borer. J. Chem. Ecol. 2012, 38, 499–511. [Google Scholar] [CrossRef]
- Qazi, S.S.; Lombardo, D.A.; Abou-Zaid, M.M. A metabolomic and HPLC-MS/MS analysis of the foliar phenolics, flavonoids and coumarins of the Fraxinus species resistant and susceptible to emerald ash borer. Molecules 2018, 23, 2734. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jaros, A.; Lee, G.; Mozola, C.; Weir, Q.; Salminen, J.P. Tree resistance to Lymantria dispar caterpillars: Importance and limitations of foliar tannin composition. Oecologia 2009, 159, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Bertić, M.; Schroeder, H.; Kersten, B.; Fladung, M.; Orgel, F.; Buegger, F.; Schnitzler, J.P.; Ghirardo, A. European oak chemical diversity—From ecotypes to herbivore resistance. New Phytol. 2021, 232, 818–834. [Google Scholar] [CrossRef]
- Simmonds, M.S. Importance of flavonoids in insect-plant interactions: Feeding and oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef]
- Schnarr, L.; Segatto, M.L.; Olsson, O.; Zuin, V.G.; Kümmerer, K. Flavonoids as biopesticides—Systematic assessment of sources, structures, activities and environmental fate. Sci. Total Environ. 2022, 824, 153781. [Google Scholar] [CrossRef]
- Gould, K.S.; Lister, C. Flavonoid functions in plants. In Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2006; pp. 397–441. [Google Scholar]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Mirnezhad, M.; Romero-González, R.R.; Leiss, K.A.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G. Metabolomic analysis of host plant resistance to thrips in wild and cultivated tomatoes. Phytochem. Anal. 2010, 21, 110–117. [Google Scholar] [CrossRef]
- Leiss, K.A.; Cristofori, G.; van Steenis, R.; Verpoorte, R.; Klinkhamer, P.G. An eco-metabolomic study of host plant resistance to Western flower thrips in cultivated, biofortified and wild carrots. Phytochemistry 2013, 93, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Lin, S.B.; Xing, Y.X.; Zhang, X.; Ye, M.; Chang, Y.L.; Guo, H.W.; Sun, X.L. (+)-Catechin, epicatechin and epigallocatechin gallate are important inducible defensive compounds against Ectropis grisescens in tea plants. Plant Cell Environ. 2022, 45, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Li, T.; Haider, M.S.; Sadeghnezhad, E.; Pang, Q.; Han, J.; Zhang, P.; Su, L.; Jia, H.; Fang, J. Comparative transcriptomic and metabolomic profiling of Grapevine leaves (cv. Kyoho) upon infestation of grasshopper and Botrytis cinerea. Plant Mol. Biol. Report. 2022, 40, 539–555. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Qin, C.X.; Wang, X.; Ding, N.Z. Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Sakthi, A.R.; Selvi, C.; Poorniammal, R. Role of phytohormones in plant defence against insects: Signalling and crosstalk. In Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology; Springer: Singapore, 2021; pp. 215–231. [Google Scholar] [CrossRef]
- Han, Y.; Luthe, D. Key genes in the JAZ signaling pathway are up-regulated faster and more abundantly in caterpillar-resistant maize. J. Chem. Ecol. 2022, 48, 179–195. [Google Scholar] [CrossRef]
- Jing, T.; Du, W.; Qian, X.; Wang, K.; Luo, L.; Zhang, X.; Deng, Y.; Li, B.; Gao, T.; Zhang, M.; et al. UGT89AC1-mediated quercetin glucosylation is induced upon herbivore damage and enhances Camellia sinensis resistance to insect feeding. Plant Cell Environ. 2024, 47, 682–697. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ignacimuthu, S.; Sharma, H.C. Induced resistance to Helicoverpa armigera through exogenous application of jasmonic acid and salicylic acid in groundnut, Arachis hypogaea. Pest Manag. Sci. 2015, 71, 72–82. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Zu, H.; Zeng, X.; Baldwin, I.T.; Lou, Y.; Li, R. Molecular dissection of rice phytohormone signaling involved in resistance to a piercing-sucking herbivore. New Phytol. 2021, 230, 1639–1652. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Qu, L.; Fan, Z.; Hou, L.; Hu, J.; Wang, L. Dynamic Metabolic Responses of Resistant and Susceptible Poplar Clones Induced by Hyphantria cunea Feeding. Biology 2024, 13, 723. https://doi.org/10.3390/biology13090723
Wang Z, Qu L, Fan Z, Hou L, Hu J, Wang L. Dynamic Metabolic Responses of Resistant and Susceptible Poplar Clones Induced by Hyphantria cunea Feeding. Biology. 2024; 13(9):723. https://doi.org/10.3390/biology13090723
Chicago/Turabian StyleWang, Zheshu, Liangjian Qu, Zhibin Fan, Luxuan Hou, Jianjun Hu, and Lijuan Wang. 2024. "Dynamic Metabolic Responses of Resistant and Susceptible Poplar Clones Induced by Hyphantria cunea Feeding" Biology 13, no. 9: 723. https://doi.org/10.3390/biology13090723
APA StyleWang, Z., Qu, L., Fan, Z., Hou, L., Hu, J., & Wang, L. (2024). Dynamic Metabolic Responses of Resistant and Susceptible Poplar Clones Induced by Hyphantria cunea Feeding. Biology, 13(9), 723. https://doi.org/10.3390/biology13090723






