Evaluation of Cytotoxicity and Metabolic Profiling of Synechocystis sp. Extract Encapsulated in Nano-Liposomes and Nano-Niosomes Using LC-MS, Complemented by Molecular Docking Studies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Cultures
2.2. Extract Preparation
2.3. Metabolic Profiling and Peak Annotation in LC-MS
2.4. Nanoparticle Synthesis
2.4.1. Nano-Liposome and Nano-Niosome Preparation
2.4.2. Vesicle Characterization
Visual Observation and Physicochemical Evaluation
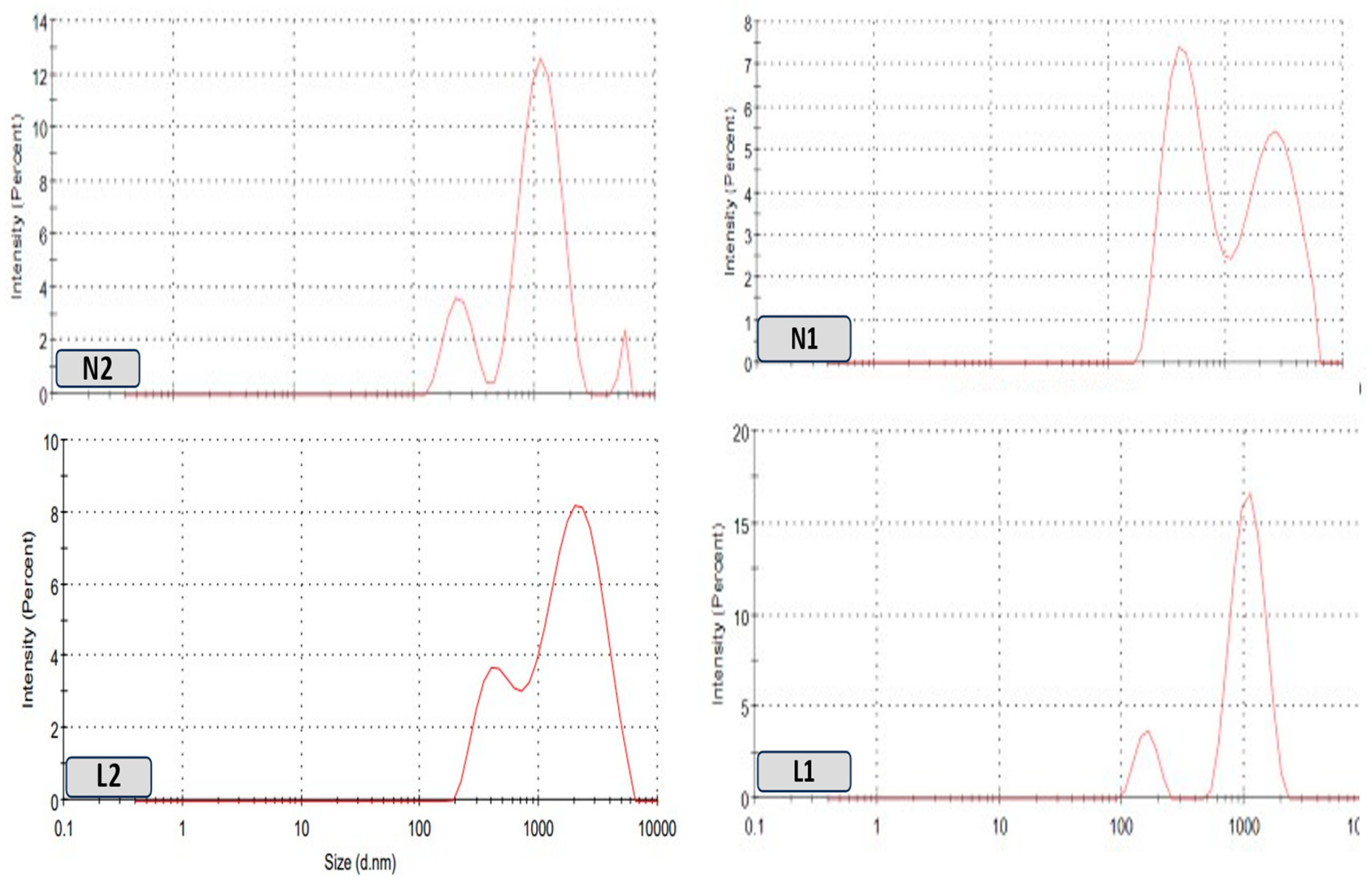
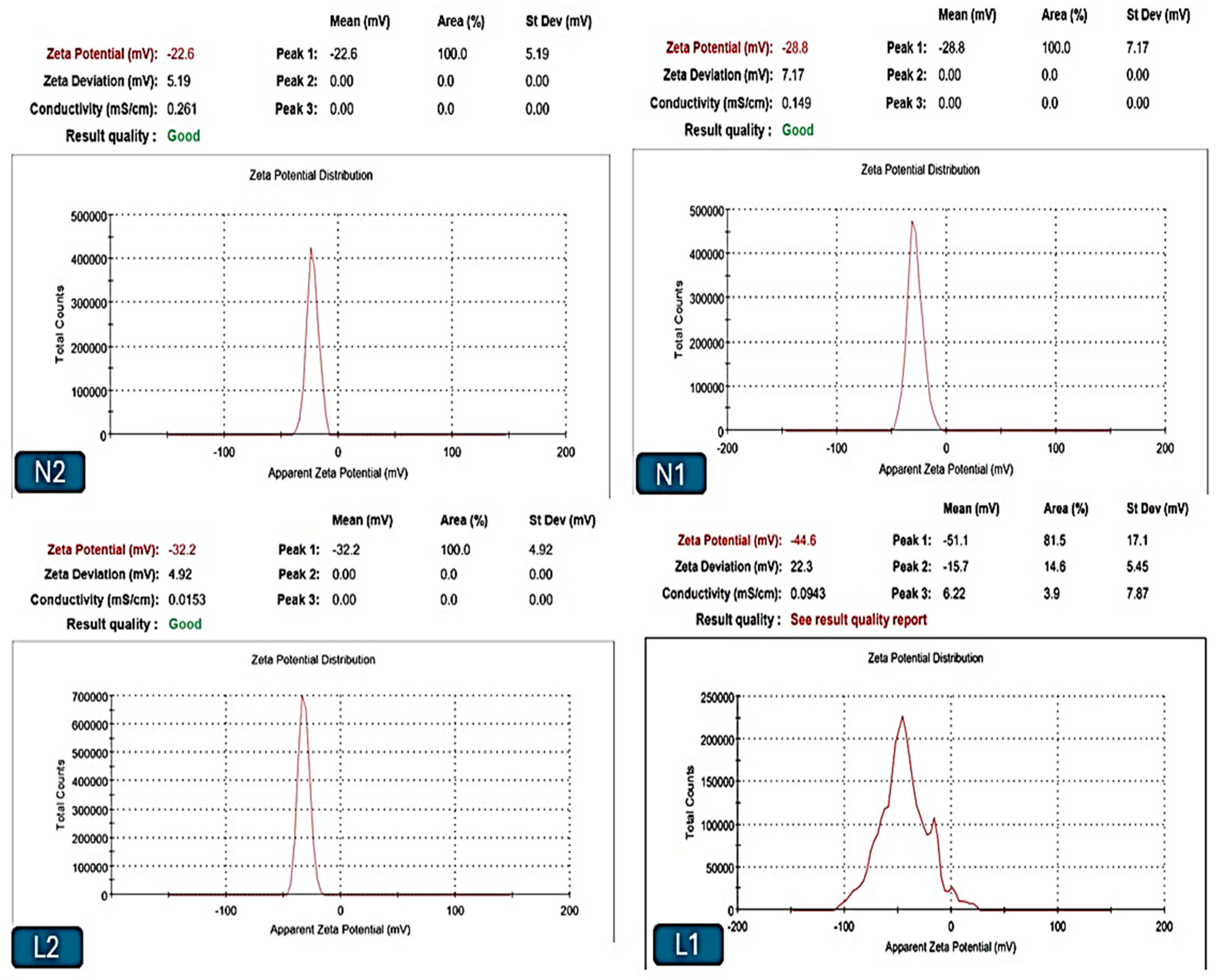
Vesicle Size Measurement and Zeta Potential Measurement
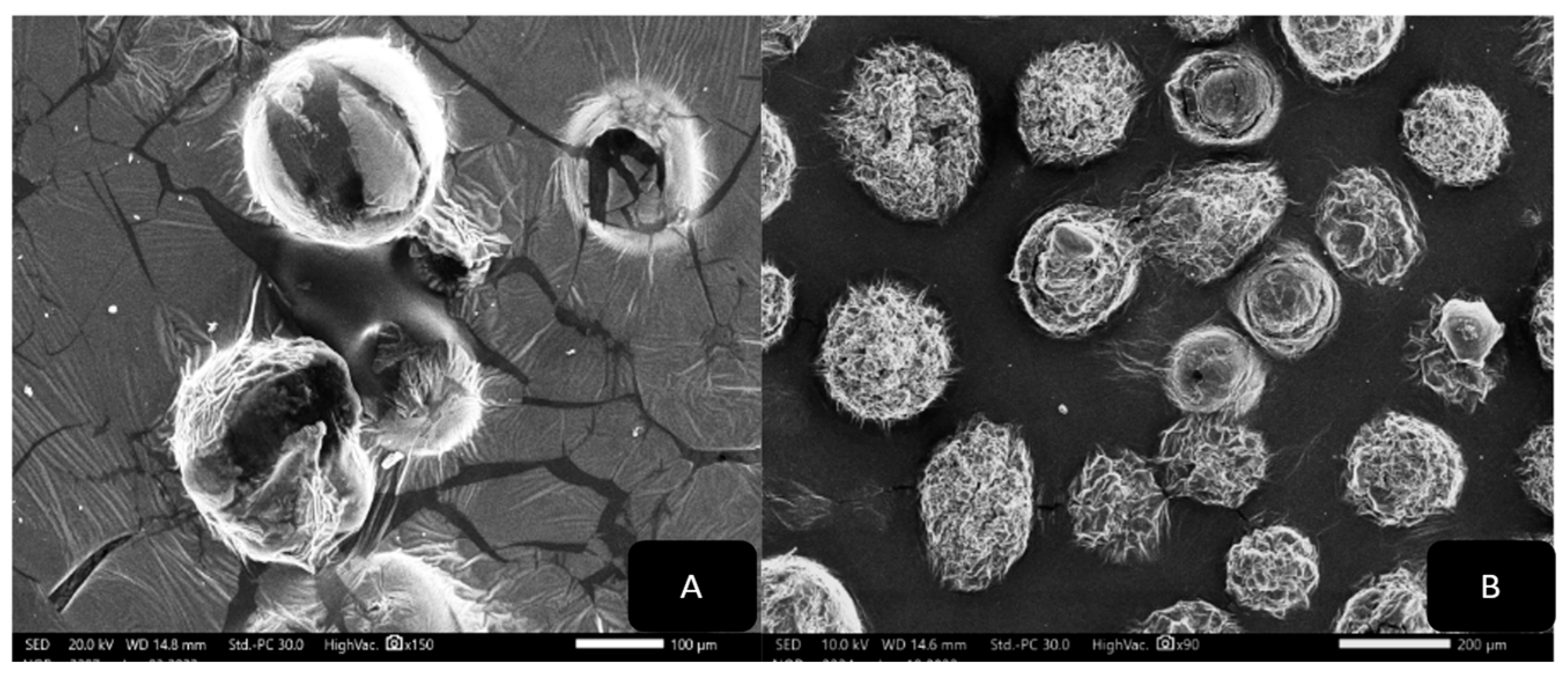
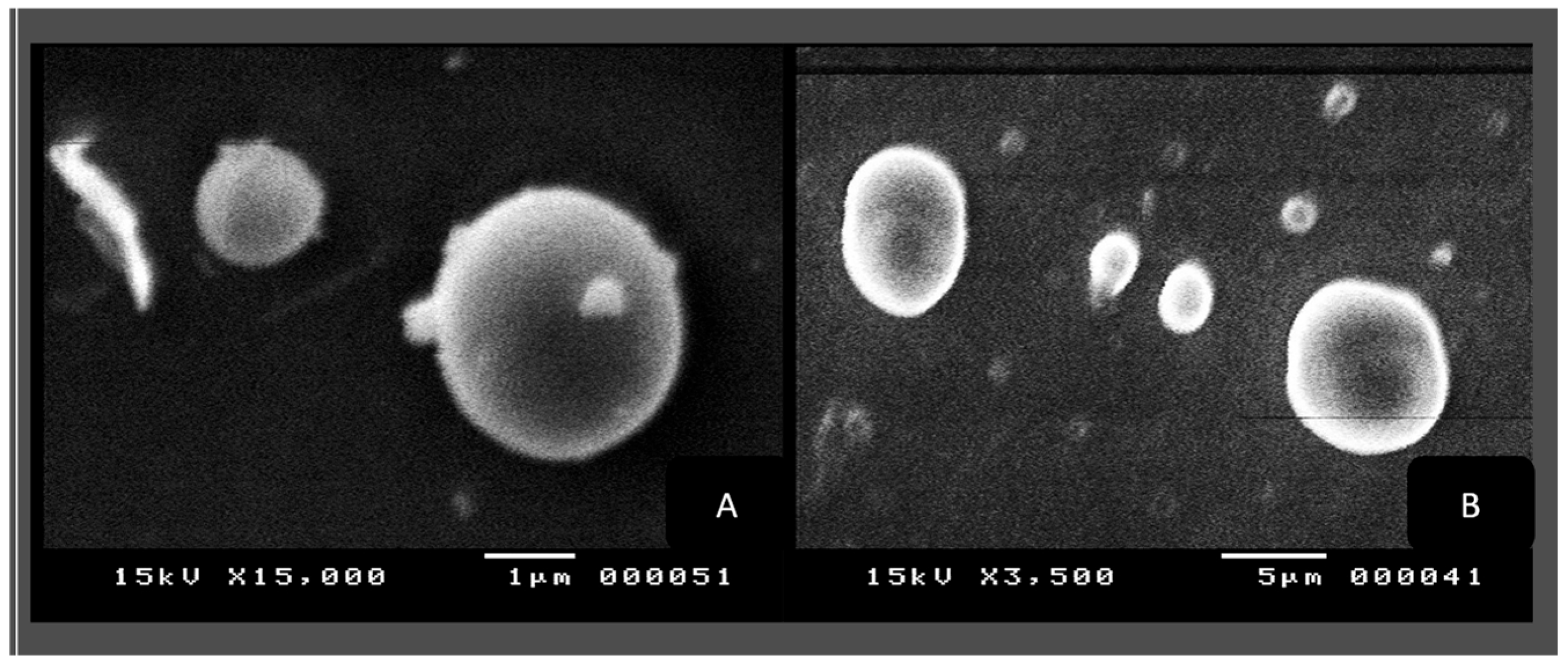
SEM Microscopic Analysis
2.5. Cytotoxicity Assay
2.5.1. Cell Culture Protocol
2.5.2. MTT–Cytotoxicity Assay Protocol
2.5.3. Statistical Analysis
2.6. Molecular Docking
3. Results
3.1. LC-MS and Metabolite Identification
3.2. Nanoparticle Vesicle Characterization
3.2.1. Visual Examination
3.2.2. Size and Zeta Analysis
3.2.3. SEM Morphological Analysis
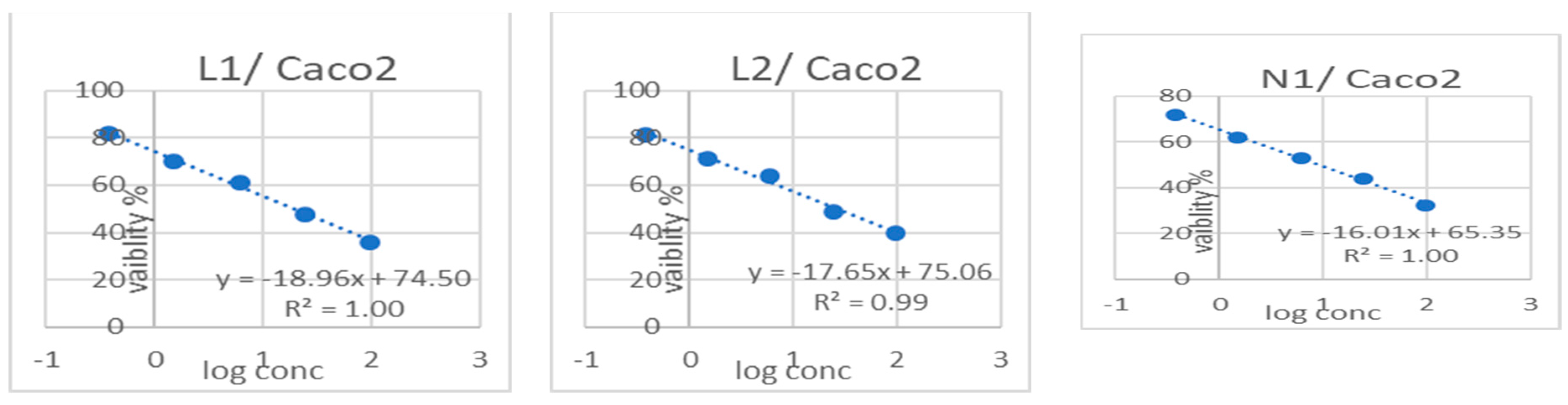
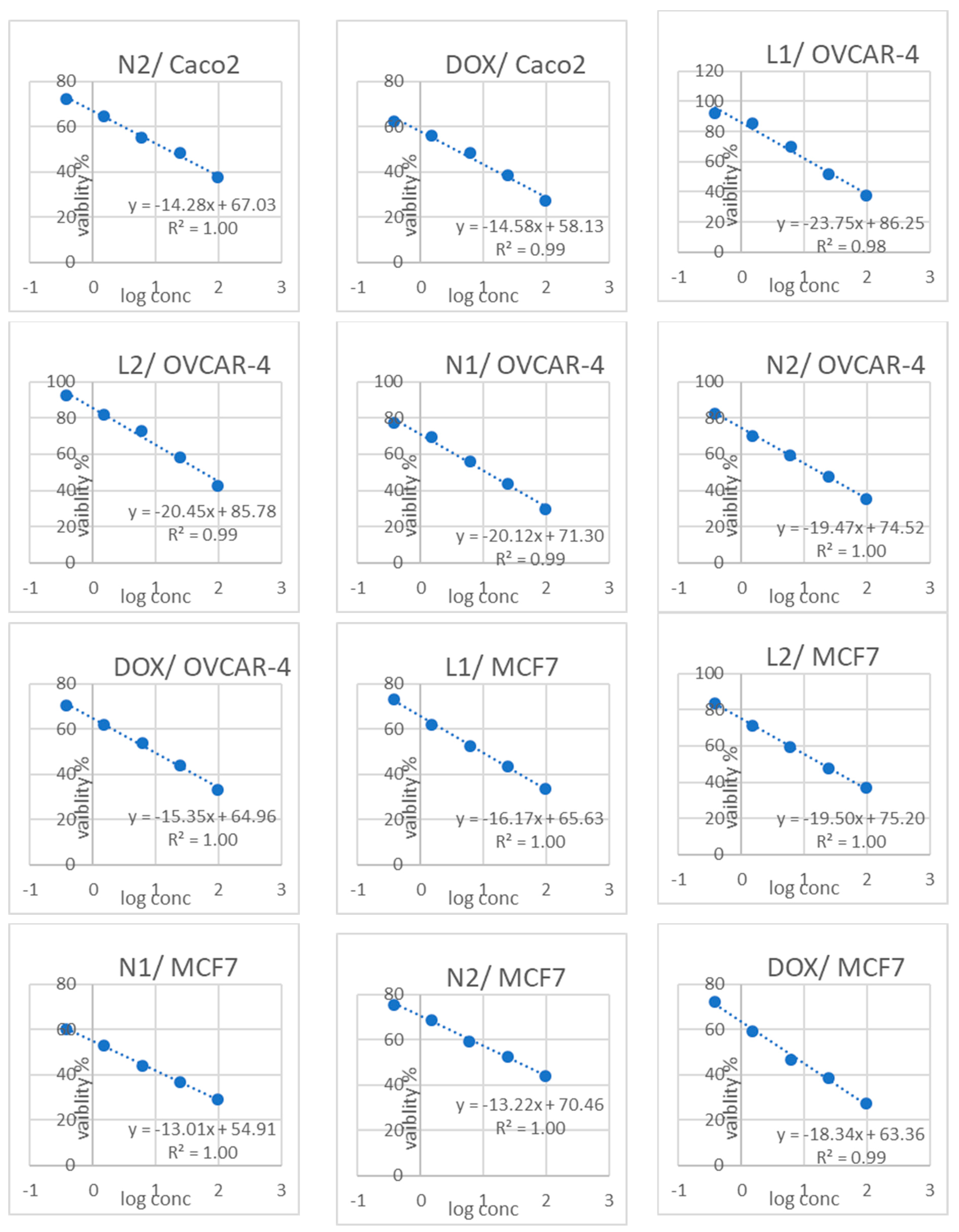
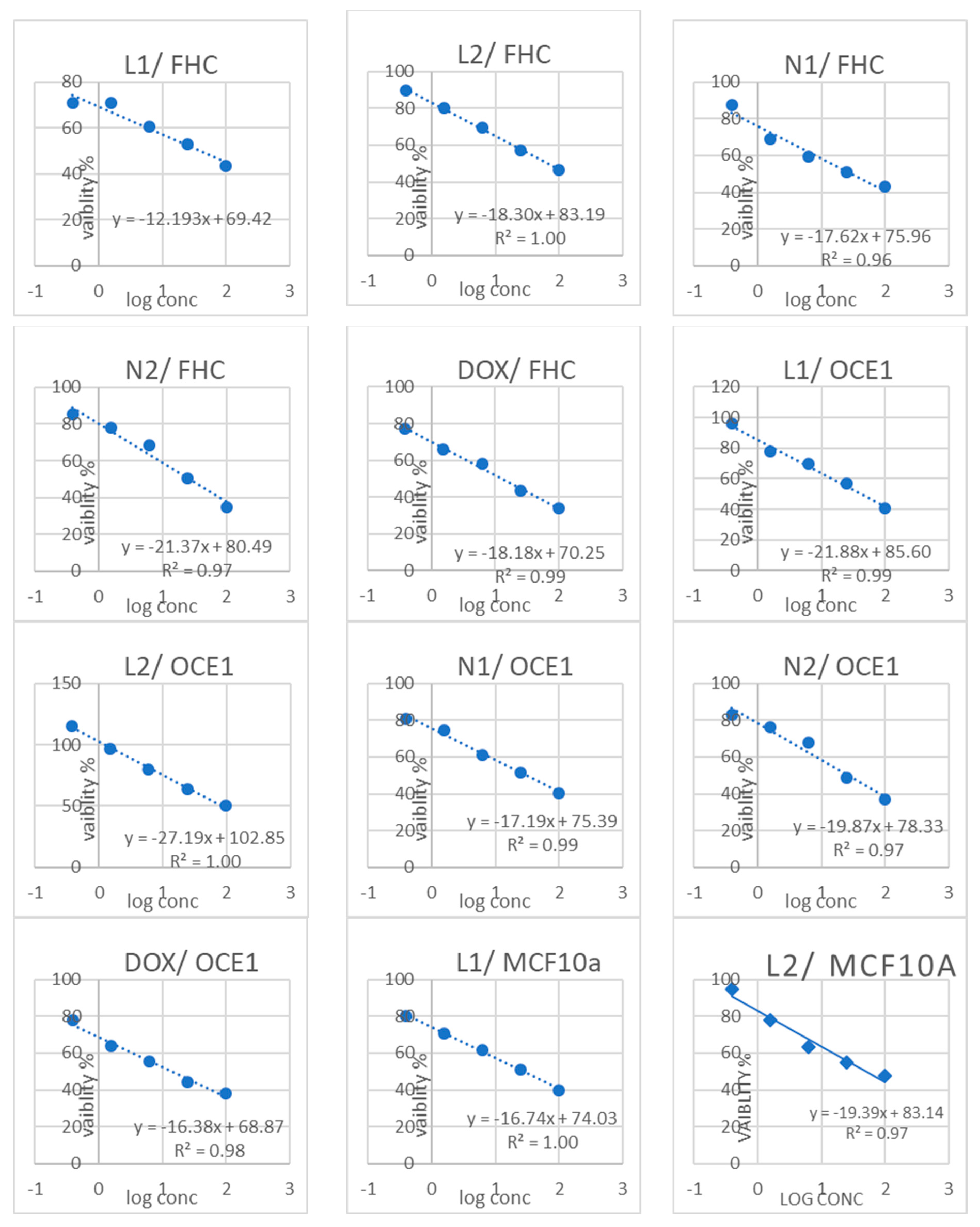
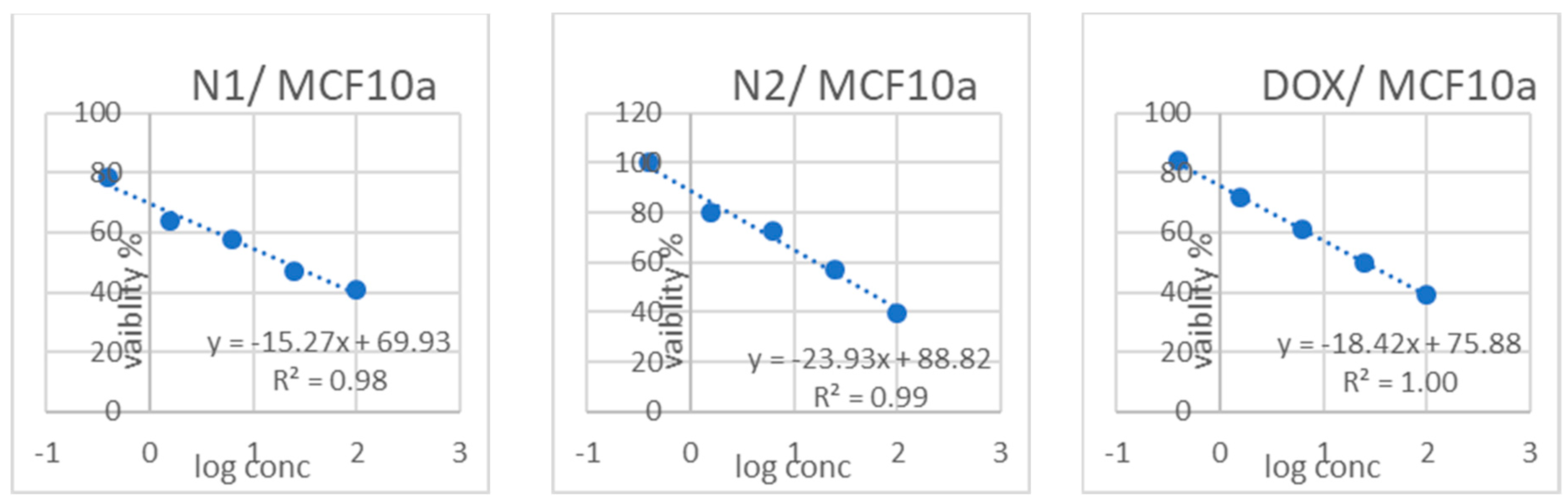
3.3. Cytotoxicity Results
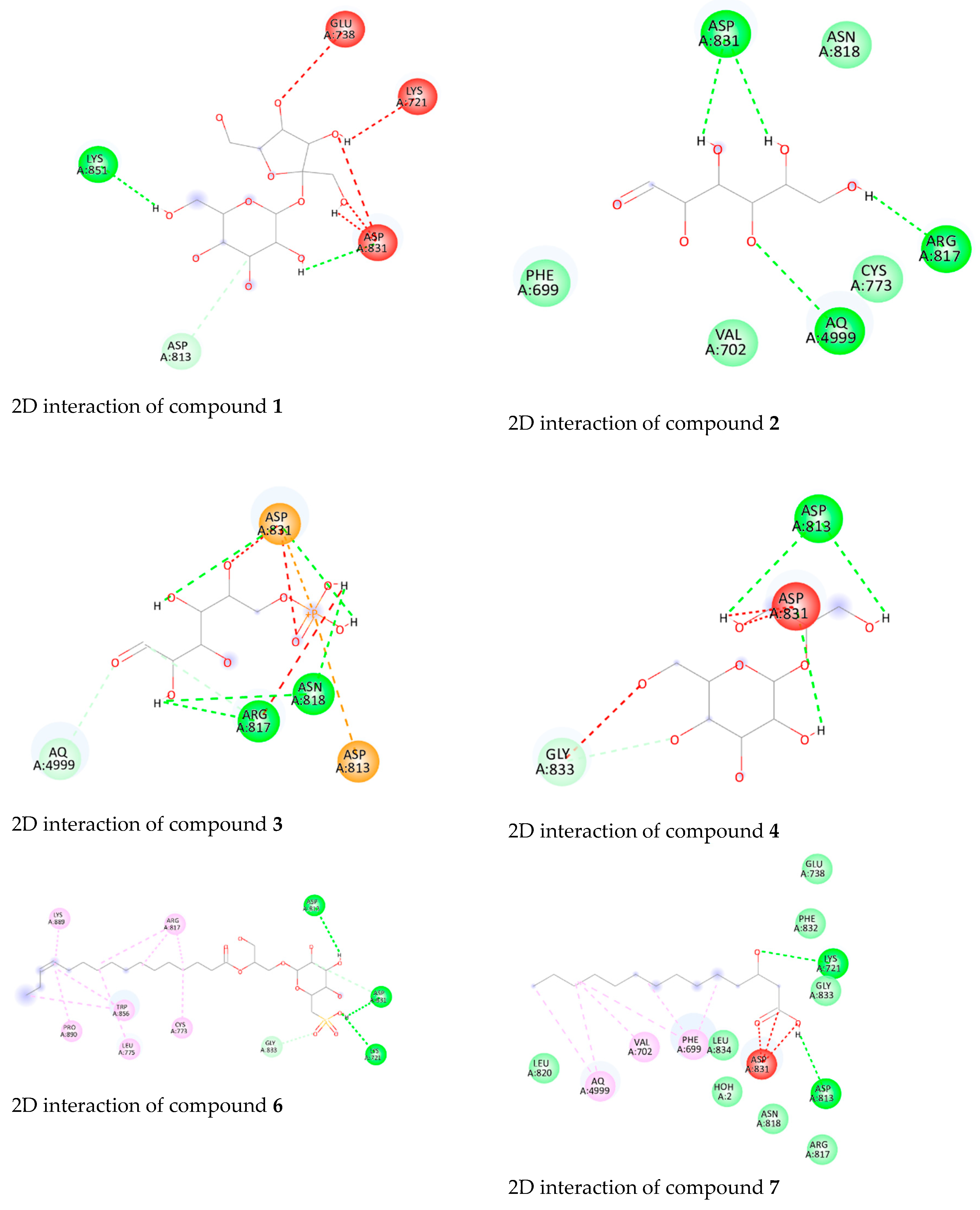
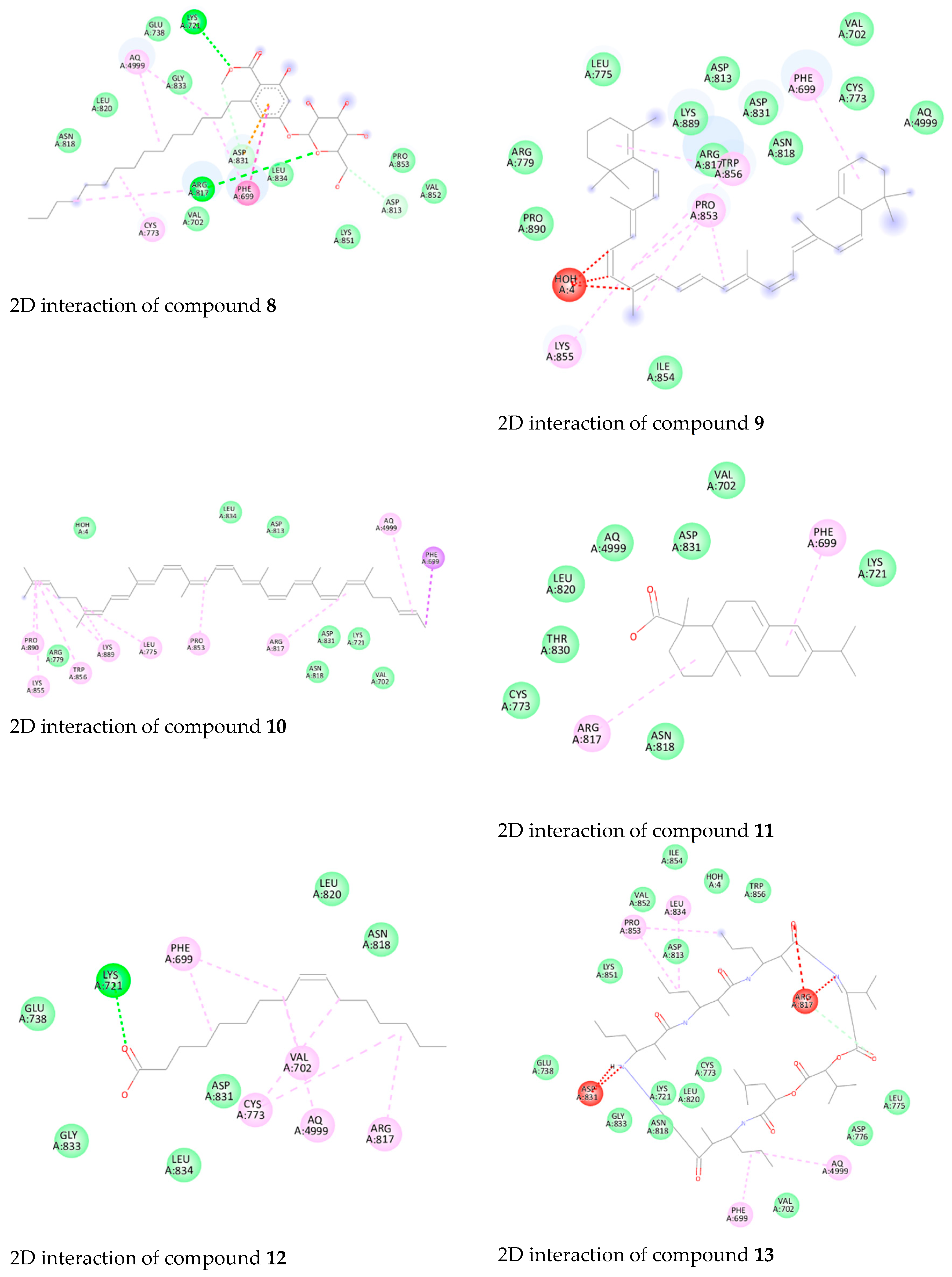
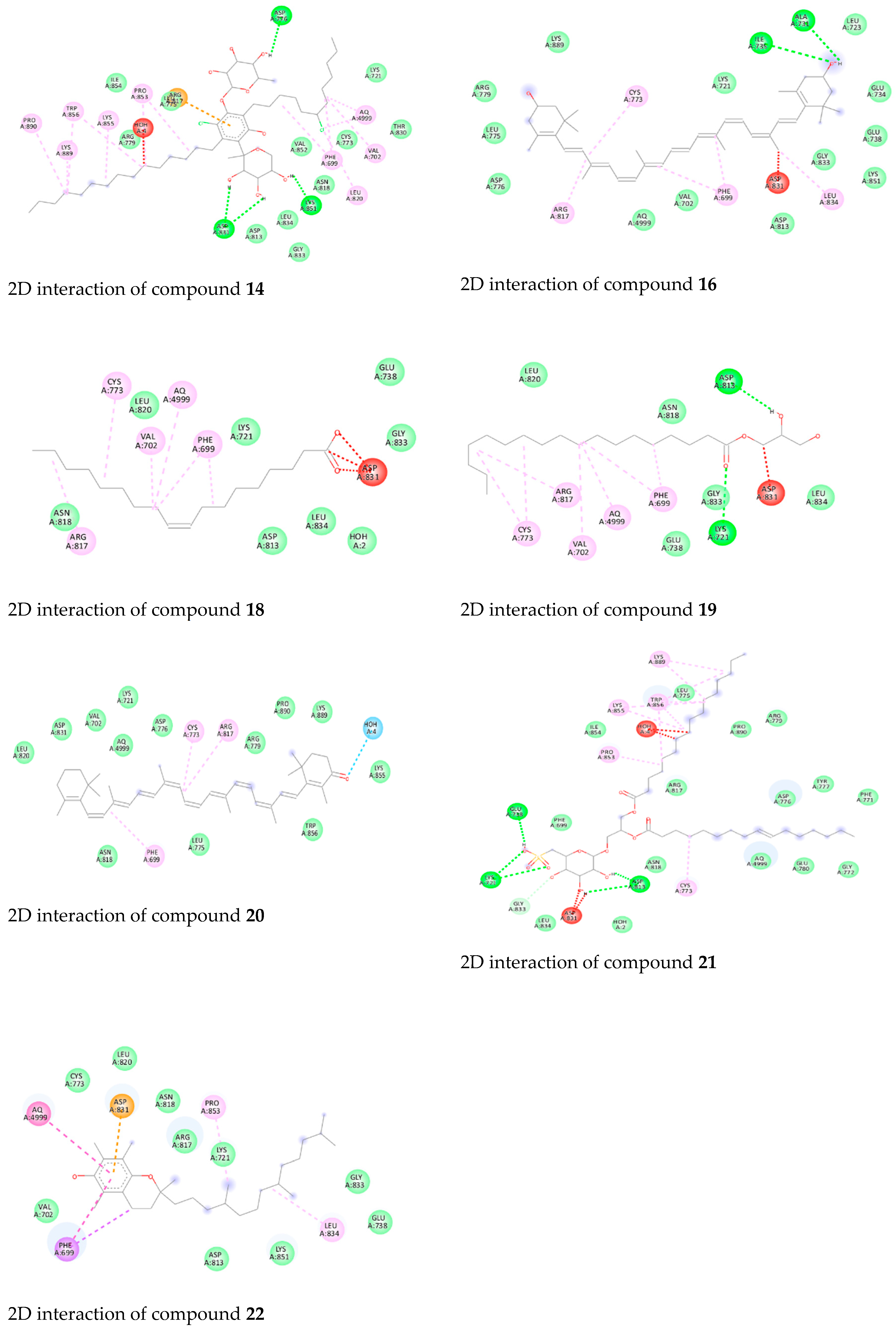
3.4. Molecular Docking Studies of Isolated Compounds toward EGFR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krylova, J.; Kurashov, E. Potential applications of the low-molecular-weight metabolome of Synechocystis aquatilis Sauvageau, 1892 (Cyanophyceae: Merismopediaceae). In Algal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 347–376. [Google Scholar]
- Abed, R.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh Allaf, M.; Peerhossaini, H. Cyanobacteria: Model microorganisms and beyond. Microorganisms 2022, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Jassim, Y.A.; Awadh, E.F.A.; Al-Amery, S.M.H. A review of general properties of blue-green algae (Cyanobacteria). Biomed. Chem. Sci. 2023, 2, 143–148. [Google Scholar] [CrossRef]
- Saad, M.H.; El-Fakharany, E.M.; Salem, M.S.; Sidkey, N.M. The use of cyanobacterial metabolites as natural medical and biotechnological tools. J. Biomol. Struct. Dyn. 2022, 40, 2828–2850. [Google Scholar] [CrossRef] [PubMed]
- Naveed, S.; Yu, Q.; Zhang, C.; Ge, Y. Extracellular polymeric substances alter cell surface properties, toxicity, and accumulation of arsenic in Synechocystis PCC6803. Environ. Pollut. 2020, 261, 114233. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.; Oliveira, R.; Dias, A.C. Microalgae and cyanobacteria as sources of bioactive compounds for cosmetic applications: A systematic review. Algal Res. 2023, 76, 103287. [Google Scholar] [CrossRef]
- Assunção, J.; Amaro, H.M.; Lopes, G.; Tavares, T.; Malcata, F.X.; Guedes, A.C. Synechocystis salina: Potential bioactivity and combined extraction of added-value metabolites. J. Appl. Phycol. 2021, 33, 3731–3746. [Google Scholar] [CrossRef]
- Martins, R.F.; Ramos, M.F.; Herfindal, L.; Sousa, J.A.; Skærven, K.; Vasconcelos, V.M. Antimicrobial and cytotoxic assessment of marine cyanobacteria-Synechocystis and Synechococcus. Mar. Drugs 2008, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Bakrim, S.; Chamkhi, I.; Taha, D.; El Omari, N.; El Mneyiy, N.; El Hachlafi, N.; El-Shazly, M.; Khalid, A.; Abdalla, A.N. Bioactive substances of cyanobacteria and microalgae: Sources, metabolism, and anticancer mechanism insights. Biomed. Pharmacother. 2024, 170, 115989. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawe, R.A.D.; Yusof, Z.N.B. Cyanobacteria as a Source of Bioactive Compounds with Anticancer, Antibacterial, Antifungal, and Antiviral Activities: A Review. Microb. Bioact. 2023, 6, 1–16. [Google Scholar]
- Abedin, M.R.; Barua, S. Isolation and purification of glycoglycerolipids to induce apoptosis in breast cancer cells. Sci. Rep. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Pandey, S.; Rai, L.C.; Dubey, S.K. Cyanobacteria: Miniature factories for green synthesis of metallic nanomaterials: A review. Biometals 2022, 35, 653–674. [Google Scholar] [CrossRef]
- El Semary, N.A.; Abd El Naby, M. Characterization of a Synechocystis sp. from Egypt with the potential of bioactive compounds production. World J. Microbiol. Biotechnol. 2010, 26, 1125–1133. [Google Scholar] [CrossRef]
- Yucetepe, A. Strategies for Nanoencapsulation of Algal Proteins, Protein Hydrolysates and Bioactive Peptides: The Effect of Encapsulation Techniques on Bioactive Properties. In Bioprospecting Algae for Nanosized Materials; Springer: Cham, Switzerland, 2022; pp. 211–227. [Google Scholar]
- Hamida, R.S.; Abdelmeguid, N.E.; Ali, M.A.; Bin-Meferij, M.M.; Khalil, M.I. Synthesis of silver nanoparticles using a novel cyanobacteria Desertifilum sp. extract: Their antibacterial and cytotoxicity effects. Int. J. Nanomed. 2020, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a unique position in medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Sinani, G.; Durgun, M.E.; Cevher, E.; Özsoy, Y. Polymeric-Micelle-Based delivery systems for nucleic acids. Pharmaceutics 2023, 15, 2021. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.B.; Kim, H.J.; Kang, S.-W.; Yoo, T.-H. Exosome-based drug delivery: Translation from bench to clinic. Pharmaceutics 2023, 15, 2042. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, O.H.; Ali, T.F.S.; Fahim, J.R.; Desoukey, S.Y.; Ahmed, S.; Behery, F.A.; Kamel, M.S.; Gulder, T.A.; Abdelmohsen, U.R. Anti-inflammatory potential of green synthesized silver nanoparticles of the soft coral Nephthea sp. supported by metabolomics analysis and docking studies. Int. J. Nanomed. 2020, 15, 5345–5360. [Google Scholar] [CrossRef]
- Mohammed, M.H.H.; Hamed, A.N.E.; Elhabal, S.F.; Mokhtar, F.A.; Abdelmohsen, U.R.; Fouad, M.A.; Kamel, M.S. Metabolic profiling and cytotoxic activities of ethanol extract of Dypsis leptocheilos aerial parts and its green synthesized silver nanoparticles supported by network pharmacology analysis. S. Afr. J. Bot. 2023, 161, 648–665. [Google Scholar] [CrossRef]
- El-Hawwary, S.S.; Abd Almaksoud, H.M.; Saber, F.R.; Elimam, H.; Sayed, A.M.; El Raey, M.A.; Abdelmohsen, U.R. Green-synthesized zinc oxide nanoparticles, anti-Alzheimer potential and the metabolic profiling of Sabal blackburniana grown in Egypt supported by molecular modelling. RSC Adv. 2021, 11, 18009–18025. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Mohamad, S.A.; Yahia, R.; Badawi, A.M.; Sayed, A.M.; Abdelmohsen, U.R. Anti-otomycotic potential of nanoparticles of Moringa oleifera leaf extract: An integrated in vitro, in silico and phase 0 clinical study. Food Funct. 2022, 13, 11083–11096. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, B.H.; Lashin, M.M.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and inorganic nanomaterials: Fabrication, properties and applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Lal, S. Synthesis of organic nanoparticles and their applications in drug delivery and food nanotechnology: A review. J. Nanomater. Mol. Nanotechnol. 2014, 3, 1–11. [Google Scholar]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, E09394. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Bach, H.; Lorenzo-Leal, A.C. Use of niosomes for the treatment of intracellular pathogens infecting the lungs. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1891. [Google Scholar] [CrossRef] [PubMed]
- Yasamineh, S.; Yasamineh, P.; Kalajahi, H.G.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Kheirkhah, A.H.; Taghizadeh, M.; Yazdani, Y. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Hajinezhad, M.R.; Zargari, F.; Shahraki, S.; Davodabadi, F.; Mirinejad, S.; Sargazi, S.; Rahdar, A.; Díez-Pascual, A.M. Preparation, characterization, cytotoxicity and pharmacokinetics of niosomes containing gemcitabine: In vitro, in vivo, and simulation studies. J. Drug Deliv. Sci. Technol. 2023, 84, 104505. [Google Scholar] [CrossRef]
- Minamisakamoto, T.; Nishiguchi, S.; Hashimoto, K.; Ogawara, K.-I.; Maruyama, M.; Higaki, K. Sequential administration of PEG-Span 80 niosome enhances anti-tumor effect of doxorubicin-containing PEG liposome. Eur. J. Pharm. Biopharm. 2021, 169, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Marazioti, A.; Mourtas, S.; Muzzalupo, R.; Antimisiaris, S.G. Liposomes Coated with Novel Synthetic Bifunctional Chitosan Derivatives as Potential Carriers of Anticancer Drugs. Pharmaceutics 2024, 16, 319. [Google Scholar] [CrossRef] [PubMed]
- Rommasi, F.; Esfandiari, N. Liposomal nanomedicine: Applications for drug delivery in cancer therapy. Nanoscale Res. Lett. 2021, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Allahou, L.W.; Madani, S.Y.; Seifalian, A. Investigating the application of liposomes as drug delivery systems for the diagnosis and treatment of cancer. Int. J. Biomater. 2021, 2021, 3041969. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Liu, B. Formulation strategies for folate-targeted liposomes and their biomedical applications. Pharmaceutics 2019, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhai, B.-T.; Fan, Y.; Sun, J.; Shi, Y.-J.; Zhang, X.-F.; Zou, J.-B.; Wang, J.-W.; Guo, D.-Y. Targeted drug delivery systems for curcumin in breast cancer therapy. Int. J. Nanomed. 2023, 18, 4275–4311. [Google Scholar] [CrossRef] [PubMed]
- Sahab-Negah, S.; Ariakia, F.; Jalili-Nik, M.; Afshari, A.R.; Salehi, S.; Samini, F.; Rajabzadeh, G.; Gorji, A. Curcumin loaded in niosomal nanoparticles improved the anti-tumor effects of free curcumin on glioblastoma stem-like cells: An in vitro study. Mol. Neurobiol. 2020, 57, 3391–3411. [Google Scholar] [CrossRef] [PubMed]
- Abdin, N.; Sahu, B.P.; Rahman, S.S. A Review on Formulation and Evaluation of Nanoniosomal Topical gel of Paclitaxel for skin cancer. Res. J. Pharm. Technol. 2022, 15, 2849–2854. [Google Scholar] [CrossRef]
- Sharma, S.; Garg, A.; Agrawal, R.; Chopra, H.; Pathak, D. A Comprehensive Review on Niosomes as a Tool for Advanced Drug Delivery. Pharm. Nanotechnol. 2024, 12, 206–228. [Google Scholar] [CrossRef] [PubMed]
- Abdin, N.; Bordoloi, B.; Rabha, T.; Dutta, J.J. A Review on Nanoniosomes. World J. Pharm. Res. 2021, 10, 443–450. [Google Scholar]
- Machado, A.; Assis, L.; Costa, J.; Badiale-Furlong, E.; Motta, A.; Micheletto, Y.; Souza-Soares, L. Application of sonication and mixing for nanoencapsulation of the cyanobacterium Spirulina platensis in liposomes. Int. Food Res. J. 2014, 21, 2201. [Google Scholar]
- Rigi, M.; Ojagh, S.M.; Alishahi, A.; Hasani, S. Characterizing and developing the antioxidant and antimicrobial properties of the nano-encapsulated bioactive compounds of spirulina platensis in liposome. J. Aquat. Food Prod. Technol. 2022, 31, 591–598. [Google Scholar] [CrossRef]
- Karnakis, T.; Souza, P.M.R.d.; Kanaji, A.L.; Chinaglia, L.; Bezerra, M.R.; Almeida, O.L.S. The role of geriatric oncology in the care of older people with cancer: Some evidence from Brazil and the world. Rev. Assoc. Méd. Bras. 2024, 70, e2024S2118. [Google Scholar] [CrossRef] [PubMed]
- Brotzman, L.E.; Zikmund-Fisher, B.J. Perceived barriers among clinicians and older adults aged 65 and older regarding use of life expectancy to inform cancer screening: A narrative review and comparison. Med. Care Res. Rev. 2023, 80, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Sachdeo, R.A.; Charde, M.S.; Chakole, R.D. Colorectal cancer: An overview. Asian J. Res. Pharm. Sci. 2020, 10, 211–223. [Google Scholar] [CrossRef]
- Parhizgar, P.; Bahadori Monfared, A.; Mohseny, M.; Keramatinia, A.; Hashemi Nazari, S.S.; Rahman, S.A.; Al Marzouqi, A.; Al-Yateem, N.; Mosavi Jarrahi, A. Risk of second primary cancer among breast cancer patients: A systematic review and meta-analysis. Front. Oncol. 2023, 12, 1094136. [Google Scholar] [CrossRef] [PubMed]
- Atallah, G.A.; Kampan, N.C.; Chew, K.T.; Mohd Mokhtar, N.; Md Zin, R.R.; Shafiee, M.N.b.; Abd. Aziz, N.H.b. Predicting prognosis and platinum resistance in ovarian cancer: Role of immunohistochemistry biomarkers. Int. J. Mol. Sci. 2023, 24, 1973. [Google Scholar] [CrossRef]
- Guardiola, S.; Varese, M.; Sanchez-Navarro, M.; Giralt, E. A third shot at EGFR: New opportunities in cancer therapy. Trends Pharmacol. Sci. 2019, 40, 941–955. [Google Scholar] [CrossRef]
- Citri, A.; Yarden, Y. EGF–ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Depuydt, S.; Choi, S.; Kim, G.; Kim, Y.; Pandey, L.K.; Häder, D.-P.; Han, T.; Park, J. Potential use of nuisance cyanobacteria as a source of anticancer agents. In Natural Bioactive Compounds; Academic Press: Cambridge, MA, USA, 2021; pp. 203–231. [Google Scholar]
- Saritha, S.; Nair, S.M. Isolation and Characterization of Glycolipids from Synechocystis sp. and Its Cytotoxic Potential against Colon Cancer. Ph.D. Thesis, Cochin University of Science and Technology, Cochin, India, 2017. [Google Scholar]
- Piawah, S.; Venook, A.P. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019, 125, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Solmi, R.; Lauriola, M.; Francesconi, M.; Martini, D.; Voltattorni, M.; Ceccarelli, C.; Ugolini, G.; Rosati, G.; Zanotti, S.; Montroni, I. Displayed correlation between gene expression profiles and submicroscopic alterations in response to cetuximab, gefitinib and EGF in human colon cancer cell lines. BMC Cancer 2008, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; El-Ashry, D.; Cheville, A.; Liu, Y.; McLeskey, S.; Kern, F. Emergence of MCF-7 cells over-expressing a transfected epidermal growth factor receptor (EGFR) under estrogen-depleted conditions: Evidence for a role of EGFR in breast cancer growth and progression. Cell Growth Differ. 1994, 5, 1263–1274. [Google Scholar]
- Takabatake, D.; Fujita, T.; Shien, T.; Kawasaki, K.; Taira, N.; Yoshitomi, S.; Takahashi, H.; Ishibe, Y.; Ogasawara, Y.; Doihara, H. Tumor inhibitory effect of gefitinib (ZD1839, Iressa) and taxane combination therapy in EGFR-overexpressing breast cancer cell lines (MCF7/ADR, MDA-MB-231). Int. J. Cancer 2007, 120, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fang, Y.; Shi, X.; Zhang, M.; Wang, X.; Tan, Y. Effect of bisphenol A on the EGFR-STAT3 pathway in MCF-7 breast cancer cells. Mol. Med. Rep. 2012, 5, 41–47. [Google Scholar] [PubMed]
- Kaufman, N.E.; Dhingra, S.; Jois, S.D.; Vicente, M.d.G.H. Molecular targeting of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR). Molecules 2021, 26, 1076. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Ray, S.; Banerji, A. Epidermal growth factor receptor-mediated regulation of matrix metalloproteinase-2 and matrix metalloproteinase-9 in MCF-7 breast cancer cells. Mol. Cell. Biochem. 2019, 452, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Fathy, W.; Elsayed, K.; Essawy, E.; Tawfik, E.; Zaki, A.; Abdelhameed, M.S.; Hammouda, O. Biosynthesis of silver nanoparticles from Synechocystis sp to be used as a flocculant agent with different microalgae strains. Curr. Nanomater. 2020, 5, 175–187. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E.; El Semary, N.A. Green synthesis of silver nanoparticles by the Cyanobacteria synechocystis sp.: Characterization, antimicrobial and diabetic wound-healing actions. Mar. Drugs 2022, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Tang, L.; Zhu, H. Titanium dioxide nanoparticles enhance photocurrent generation of cyanobacteria. Biochem. Biophys. Res. Commun. 2023, 672, 113–119. [Google Scholar] [CrossRef] [PubMed]
- ElFar, O.A.; Billa, N.; Lim, H.R.; Chew, K.W.; Cheah, W.Y.; Munawaroh, H.S.H.; Balakrishnan, D.; Show, P.L. Advances in delivery methods of Arthrospira platensis (spirulina) for enhanced therapeutic outcomes. Bioengineered 2022, 13, 14681–14718. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Shukla, S.; Kang, S.-M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.-K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, K. Production of Biofuels from Microalgae; IRC-Library, Information Resource Center der Jacobs University Bremen: Bremen, Germany, 2017. [Google Scholar]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and challenges of large-scale cultivation of photosynthetic microalgae and cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef] [PubMed]
- Aljabri, H.; Cherif, M.; Siddiqui, S.A.; Bounnit, T.; Saadaoui, I. Evidence of the drying technique’s impact on the biomass quality of Tetraselmis subcordiformis (Chlorophyceae). Biotechnol. Biofuels Bioprod. 2023, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Owis, A.I.; El-Hawary, M.S.; El Amir, D.; Aly, O.M.; Abdelmohsen, U.R.; Kamel, M.S. Molecular docking reveals the potential of Salvadora persica flavonoids to inhibit COVID-19 virus main protease. RSC Adv. 2020, 10, 19570–19575. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, M.; Steinbeck, C. Review on natural products databases: Where to find data in 2020. J. Cheminformatics 2020, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Yeo, P.L.; Lim, C.L.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Niosomes: A review of their structure, properties, methods of preparation, and medical applications. Asian Biomed. 2017, 11, 301–314. [Google Scholar] [CrossRef]
- Šturm, L.; Poklar Ulrih, N. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols. Int. J. Mol. Sci. 2021, 22, 6547. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Rai, M.; Varma, R.S.; Hamidi, M.; Mozafari, M. Conventional and novel methods for the preparation of micro and nanoliposomes. Micro Nano Bio Asp. 2022, 1, 18–29. [Google Scholar]
- Mazlumoğlu, B.Ş. In Vitro Cytotoxicity Test Methods: MTT and NRU. Int. J. PharmATA 2023, 3, 50–53. [Google Scholar]
- Sun, M.; Abdelwahab, M.F.; Zhang, J.; Samy, M.N.; Mohamed, N.M.; Abdel-Rahman, I.M.; Alsenani, F.; Abdelmohsen, U.R.; Mahmoud, B.K. Cytotoxic metabolites from Sinularia levi supported by network pharmacology. PLoS ONE 2024, 19, e0294311. [Google Scholar] [CrossRef]
- Ekpenyong, M.; Asitok, A.; Antigha, R.; Ogarekpe, N.; Ekong, U.; Asuquo, M.; Essien, J.; Antai, S. Bioprocess optimization of nutritional parameters for enhanced anti-leukemic L-asparaginase production by Aspergillus candidus UCCM 00117: A sequential statistical approach. Int. J. Pept. Res. Ther. 2021, 27, 1501–1527. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Perez de Souza, L.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the context of plant natural products research: From sample preparation to metabolite analysis. Metabolites 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.; Lee, H.; Hong, S.-J.; Lee, H.; Cho, B.-K.; Lee, C.-G.; Choi, H.-K. Comparative Primary Metabolic and Lipidomic Profiling of Freshwater and Marine Synechocystis Strains Using by GC-MS and NanoESI-MS Analyses. Biotechnol. Bioprocess Eng. 2020, 25, 308–319. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Cyanobacterial sunscreen scytonemin: Role in photoprotection and biomedical research. Appl. Biochem. Biotechnol. 2015, 176, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pichel, F.; Castenholz, R.W. Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment 1. J. Phycol. 1991, 27, 395–409. [Google Scholar] [CrossRef]
- Stevenson, C.S.; Capper, E.A.; Roshak, A.K.; Marquez, B.; Eichman, C.; Jackson, J.R.; Mattern, M.; Gerwick, W.H.; Jacobs, R.S.; Marshall, L.A. The identification and characterization of the marine natural product scytonemin as a novel antiproliferative pharmacophore. J. Pharmacol. Exp. Ther. 2002, 303, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and red macroalgae as potential sources of antioxidants and UV radiation-absorbing compounds for cosmeceutical applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- Jala, R.C.R.; Vudhgiri, S.; Kumar, C.G. A comprehensive review on natural occurrence, synthesis and biological activities of glycolipids. Carbohydr. Res. 2022, 516, 108556. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Thapa, S.; Siddiqui, N.; Chakdar, H. Cyanobacterial secondary metabolites towards improved commercial significance through multiomics approaches. World J. Microbiol. Biotechnol. 2022, 38, 100. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarzyk, D.; Fulda, M. Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol. 2010, 152, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Wahidullah, S.; Khan, S.; Devi, P. ESI-MS/MS Characterization of Glycerolipids and Seasonal Alteration in their Composition in Sesuvium portulacastrum (Linnaeus)—A Salt Marsh Halophyte. J. Glycobiol. 2021, 10, 180. [Google Scholar]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Zabetakis, I.; Murray, P.; Saha, S.K. Anti-inflammatory and antithrombotic properties of polar lipid extracts, rich in unsaturated fatty acids, from the Irish marine cyanobacterium Spirulina subsalsa. J. Funct. Foods 2022, 94, 105124. [Google Scholar] [CrossRef]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Biosurfactants as anticancer agents: Glycolipids affect skin cells in a differential manner dependent on chemical structure. Pharmaceutics 2022, 14, 360. [Google Scholar] [CrossRef]
- Levert, A.; Alvariño, R.; Bornancin, L.; Abou Mansour, E.; Burja, A.M.; Genevière, A.-M.; Bonnard, I.; Alonso, E.; Botana, L.; Banaigs, B. Structures and activities of tiahuramides A–C, cyclic depsipeptides from a Tahitian collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2018, 81, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Assunção, J.; Pagels, F.; Tavares, T.; Malcata, F.X.; Guedes, A. Light Modulation for Bioactive Pigment Production in Synechocystis salina. Bioengineering 2022, 9, 331. [Google Scholar] [CrossRef]
- Costa, M.S.; Rego, A.; Ramos, V.; Afonso, T.B.; Freitas, S.; Preto, M.; Lopes, V.; Vasconcelos, V.; Magalhães, C.; Leão, P.N. The conifer biomarkers dehydroabietic and abietic acids are widespread in Cyanobacteria. Sci. Rep. 2016, 6, 23436. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Tian, C.; Tang, J.-X.; Dumbuya, J.S.; Li, W.; Lu, J. Anticancer activities of natural abietic acid. Front. Pharmacol. 2024, 15, 1392203. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Ryu, J.; Lee, H.; Choi, H.-K. Enhanced growth, chlorophyll a and phycobiliprotein content, and modulation of bioactive metabolite profiles in Synechocystis sp. PCC 6803 culture by (+)-usnic acid. J. Appl. Phycol. 2023, 35, 1047–1059. [Google Scholar] [CrossRef]
- Bermúdez, M.A.; Pereira, L.; Fraile, C.; Valerio, L.; Balboa, M.A.; Balsinde, J. Roles of palmitoleic acid and its positional isomers, hypogeic and sapienic acids, in inflammation, metabolic diseases and cancer. Cells 2022, 11, 2146. [Google Scholar] [CrossRef] [PubMed]
- Fenner, A.M.; Engene, N.; Spadafora, C.; Gerwick, W.H.; Balunas, M.J. Medusamide A, a Panamanian Cyanobacterial Depsipeptide with Multiple β-Amino Acids. Org. Lett. 2016, 18, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.Z.; Parvez, S.; Tabassum, H. Cyanobacterial peptides with respect to anticancer activity: Structural and functional perspective. Stud. Nat. Prod. Chem. 2020, 67, 345–388. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Multiple roles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites 2013, 3, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Lanfer-Marquez, U.M.; Barros, R.M.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Terlikowska, K.M.; Dobrzycka, B.; Kinalski, M.; Terlikowski, S.J. Serum Concentrations of Carotenoids and Fat-Soluble Vitamins in Relation to Nutritional Status of Patients with Ovarian Cancer. Nutr. Cancer 2021, 73, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.; Kim, Y. Effects of β-carotene on expression of selected microRNAs, histone acetylation, and DNA methylation in colon cancer stem cells. J. Cancer Prev. 2019, 24, 224. [Google Scholar] [CrossRef] [PubMed]
- Janik-Hazuka, M.; Szafraniec-Szczęsny, J.; Kamiński, K.; Odrobińska, J.; Zapotoczny, S. Uptake and in vitro anticancer activity of oleic acid delivered in nanocapsules stabilized by amphiphilic derivatives of hyaluronic acid and chitosan. Int. J. Biol. Macromol. 2020, 164, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Dailey, O.D.; Wang, X.; Chen, F.; Huang, G. Anticancer activity of branched-chain derivatives of oleic acid. Anticancer Res. 2011, 31, 3165–3169. [Google Scholar] [PubMed]
- Su, W.; Polyakov, N.E.; Xu, W.; Su, W. Preparation of astaxanthin micelles self-assembled by a mechanochemical method from hydroxypropyl β-cyclodextrin and glyceryl monostearate with enhanced antioxidant activity. Int. J. Pharm. 2021, 605, 120799. [Google Scholar] [CrossRef] [PubMed]
- Assunção, J.; Amaro, H.M.; Tavares, T.; Malcata, F.X.; Guedes, A.C. Effects of temperature, pH, and NaCl concentration on biomass and bioactive compound production by Synechocystis salina. Life 2023, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Y.-H.; Chang, G.-P.; Yang, Y.-F.; Du, X.; Jiang, Z.-D.; Ni, H.; Li, Q.-B. Understanding the mechanism of action of echinenone on Alzheimer’s disease from the perspective of acetylcholinesterase and oxidative stress. Food Sci. 2022, 43, 105–112. [Google Scholar]
- Aoki, M.; Tsuzuki, M.; Sato, N. Involvement of sulfoquinovosyl diacylglycerol in DNA synthesis in Synechocystis sp. PCC 6803. BMC Res. Notes 2012, 5, 98. [Google Scholar] [CrossRef]
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Zimetti, F.; Marchi, C.; Bernini, F.; Bettini, R.; Elviri, L. Biocompatible 3d printed chitosan-based scaffolds containing α-tocopherol showing antioxidant and antimicrobial activity. Appl. Sci. 2021, 11, 7253. [Google Scholar] [CrossRef]
- Alrbyawi, H.; Poudel, I.; Annaji, M.; Boddu, S.H.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. pH-sensitive liposomes for enhanced cellular uptake and cytotoxicity of daunorubicin in melanoma (B16-BL6) cell lines. Pharmaceutics 2022, 14, 1128. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Krzywik, J.; Mozga, W.; Aminpour, M.; Janczak, J.; Maj, E.; Wietrzyk, J.; Huczyński, A. Synthesis, Antiproliferative Activity and Molecular Docking Studies of Novel Doubly Modified Colchicine Amides and Sulfonamides as Anticancer Agents. Molecules 2020, 25, 1789. [Google Scholar] [CrossRef] [PubMed]
- Kigondu, E.V.M.; Rukunga, G.M.; Gathirwa, J.W.; Irungu, B.N.; Mwikwabe, N.M.; Amalemba, G.M.; Omar, S.A.; Kirira, P.G. Antiplasmodial and cytotoxicity activities of some selected plants used by the Maasai community, Kenya. S. Afr. J. Bot. 2011, 77, 725–729. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Zhang, R.; Ni, H. Eriodictyol exerts potent anticancer activity against A549 human lung cancer cell line by inducing mitochondrial-mediated apoptosis, G2/M cell cycle arrest and inhibition of m-TOR/PI3K/Akt signalling pathway. Arch. Med. Sci. AMS 2020, 16, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, D. In-Silico Molecular Binding Prediction for Human Drug Targets Using Deep Neural Multi-Task Learning. Genes 2019, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.F.; Alcorn, J. Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharmaceuticals 2019, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Harari, P.M. Epidermal growth factor receptor inhibition strategies in oncology. Endocr. Relat. Cancer 2004, 11, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Koklesova, L.; Samec, M.; Varghese, E.; Abotaleb, M.; Samuel, S.M.; Smejkal, K.; Biringer, K.; Petras, M.; Blahutova, D.; et al. Implications of flavonoids as potential modulators of cancer neovascularity. J. Cancer Res. Clin. Oncol. 2020, 146, 3079–3096. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Kandaswami, C.; Lee, L.T.; Lee, P.P.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909. [Google Scholar] [PubMed]
- Zhou, K.; Raffoul, J.J. Potential Anticancer Properties of Grape Antioxidants. J. Oncol. 2012, 2012, 803294. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, H.; Ishitani, R.; Nureki, O.; Fukai, S.; Yamanaka, M.; Kim, J.H.; Saito, K.; Sakamoto, A.; Inoue, M.; Shirouzu, M.; et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell 2002, 110, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fernandez, M.L.; Clarke, D.T.; Roberts, S.K.; Zanetti-Domingues, L.C.; Gervasio, F.L. Structure and Dynamics of the EGF Receptor as Revealed by Experiments and Simulations and Its Relevance to Non-Small Cell Lung Cancer. Cells 2019, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Schlessinger, J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 2002, 110, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xie, L.; Bourne, P.E. Structural Insights into Characterizing Binding Sites in Epidermal Growth Factor Receptor Kinase Mutants. J. Chem. Inf. Model. 2019, 59, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Nowroozi, F.; Almasi, A.; Javidi, J.; Haeri, A.; Dadashzadeh, S. Effect of surfactant type, cholesterol content and various downsizing methods on the particle size of niosomes. Iran. J. Pharm. Res. IJPR 2018, 17, 1. [Google Scholar] [PubMed]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; Ebert, S.; Hinrichs, J.; Weiss, J. Effect of precipitation, lyophilization, and organic solvent extraction on preparation of protein-rich powders from the microalgae Chlorella protothecoides. Algal Res. 2018, 29, 266–276. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and nonconventional extraction techniques for optimal extraction processes of rosmarinic acid from six Lamiaceae plants as determined by HPLC-DAD measurement. J. Pharm. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef] [PubMed]
- Deepak, P.; Amutha, V.; Kamaraj, C.; Balasubramani, G.; Aiswarya, D.; Perumal, P. Chemical and green synthesis of nanoparticles and their efficacy on cancer cells. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–387. [Google Scholar]
- Dou, L.; Zhang, X.; Zangeneh, M.M.; Zhang, Y. Efficient biogenesis of Cu2O nanoparticles using extract of Camellia sinensis leaf: Evaluation of catalytic, cytotoxicity, antioxidant, and anti-human ovarian cancer properties. Bioorganic Chem. 2021, 106, 104468. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Jiang, B.; Xu, X.; Wan, Y.; Wang, L.; Chen, J. Anti-human ovarian cancer and cytotoxicity effects of nickel nanoparticles green-synthesized by Alhagi maurorum leaf aqueous extract. J. Exp. Nanosci. 2022, 17, 113–125. [Google Scholar] [CrossRef]
- Li, J.; Feizipour, S.; Delirezh, N.; Sheikhzadeh, S.; Hobbenaghi, R.; Amraii, S.A.; Khorasani, F.; Hemmati, S.; Joshani, Z.; Kamangar, S.A. Green synthesis of gold nanoparticles using potato starch as a phytochemical template, green reductant and stabilizing agent and investigating its cytotoxicity, antioxidant and anti-ovarian cancer effects. Inorg. Chem. Commun. 2023, 155, 111002. [Google Scholar] [CrossRef]
- Santhosh, S.; Chandrasekar, M.; Kaviarasan, L.; Deepak, P.; Silambarasan, T.; Gayathri, B.; Natarajan, D. Chemical composition, antibacterial, anti-oxidant and cytotoxic properties of green synthesized silver nanoparticles from Annona muricata L. (Annonaceae). Res. J. Pharm. Technol. 2020, 13, 33–39. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C. Marine cyanobacteria and microalgae metabolites—A rich source of potential anticancer drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef] [PubMed]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on anti-inflammatory molecular mechanisms induced by oleic acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-inflammatory and anticancer effects of microalgal carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef] [PubMed]
- Scanferlato, R.; Bortolotti, M.; Sansone, A.; Chatgilialoglu, C.; Polito, L.; De Spirito, M.; Maulucci, G.; Bolognesi, A.; Ferreri, C. Hexadecenoic fatty acid positional isomers and de novo PUFA synthesis in colon cancer cells. Int. J. Mol. Sci. 2019, 20, 832. [Google Scholar] [CrossRef]
- Sheng, Y.-N.; Luo, Y.-H.; Liu, S.-B.; Xu, W.-T.; Zhang, Y.; Zhang, T.; Xue, H.; Zuo, W.-B.; Li, Y.-N.; Wang, C.-Y. Zeaxanthin induces apoptosis via ROS-regulated MAPK and AKT signaling pathway in human gastric cancer cells. OncoTargets Ther. 2020, 13, 10995–11006. [Google Scholar] [CrossRef] [PubMed]
- Musa, A.; Elmaidomy, A.H.; Sayed, A.M.; Alzarea, S.I.; Al-Sanea, M.M.; Mostafa, E.M.; Hendawy, O.M.; Abdelgawad, M.A.; Youssif, K.A.; Refaat, H. Cytotoxic potential, metabolic profiling, and liposomes of Coscinoderma sp. crude extract supported by in silico analysis. Int. J. Nanomed. 2021, 16, 3861–3874. [Google Scholar] [CrossRef] [PubMed]

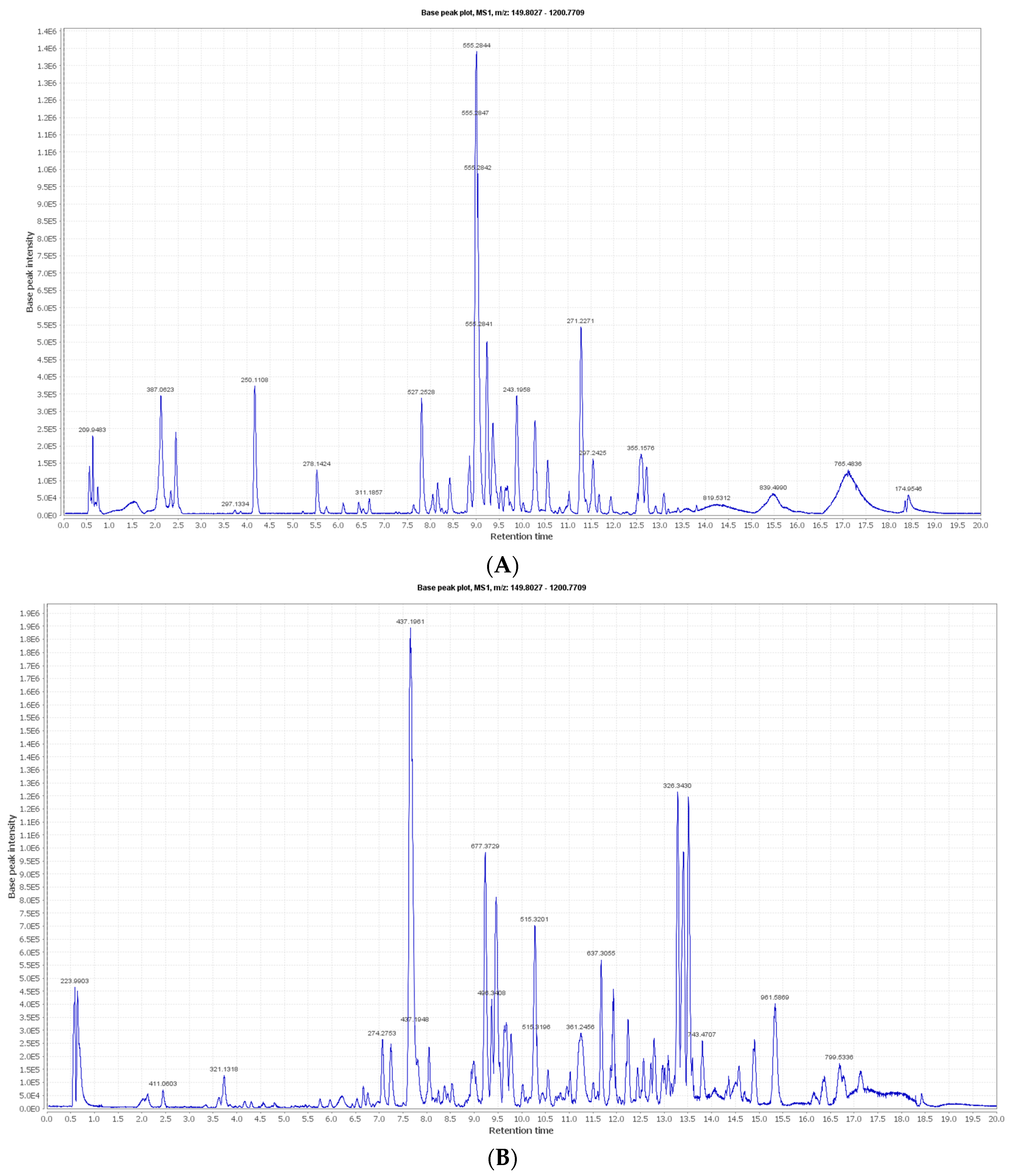

| Identified Compound | Molecular Formula | Phytochemical Class | m/z | RT (min) | M wt. | LM1.Mzxml Peak Area | Blank_Neg.Mzxml Peak Area | Exact Mass Difference | Potential Bioactivities | Source | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sucrose | C12H22O11 | Sugars | 341.1076 | 0.652017 | 342.1149 | 0 | 0 | 1 | Not reported | Synechocystis 7338 and Synechocystis 6803 | [77] |
| Glucose | C6H12O6 | Sugars | 179.0545 | 2.326483 | 180.0618 | 0 | 0 | 1 | Not reported | Synechocystis sp. | [77] |
| Glucose-6-phosphate | C6H13O9P | Sugars | 259.0235 | 3.106233 | 260.0308 | 0 | 0 | 1 | Not reported | Synechocystis sp. | [77] |
| Glucosyl glycerol | C9H18O8 | Sugars | 253.1047 | 3.679633 | 254.112 | 0 | 0 | 10 | Not reported | Synechocystis sp. | [77] |
| Scytonemin | C36H20N2O4 | Phenolic pigments | 543.1698 | 3.708917 | 544.1771 | 0 | 0 | 10 | Photoprotective; anticancer agent | Synechocystis sp. | [78,79,80,81] |
| Sulfoquinovosyl monoacylgycerol | C25H46O11S | Sulfolipids | 553.2688 | 8.159594 | 554.2761 | 383,504 | 0 | 0 | Antiviral effect | Synechocystis sp. | [82,83] |
| 3-Hydroxymyristic acid | C14H28O3 | Hydroxy fatty acid | 243.1959 | 8.872878 | 244.2031 | 1,436,656 | 2610.386 | 0 | Not reported | Synechocystis sp. | [84] |
| Glycolipid PF2 | C29H48O9 | Glycolipids | 541.3353 | 10.54202 | 540.328 | 434,467.4 | 0 | 0 | Anti-inflammatory, anticancer | Synechocystis sp. PCC | [85,86,87] |
| β-Carotene | C40H56 | Carotenoids | 537.4475 | 10.73032 | 536.4402 | 0 | 0 | 5 | Neuroprotective, antioxidant, anticancer | All Cyanobacterial species | [76,78,88,89,90] |
| Lycopene | C40H56 | Carotenoids | 537.4475 | 10.73032 | 536.4402 | 0 | 0 | 2 | Anticarcinogenic, antiatherogenic | Synechocystis salina | [90,91] |
| Abietic acid | C20H30O2 | Diterpenoids | 303.232 | 11.02424 | 302.2247 | 0 | 411,183.5 | 0.2 | Anticancer | Synechocystis sp. | [92,93] |
| Palmitoleic acid | C16H30O2 | Fatty acids | 253.2162 | 11.61243 | 254.2235 | 4613.705 | 0 | 1 | Anticancer | Synechocystis 6803 | [94,95] |
| Medusamide A | C44H79N5O9 | Depsipeptides | 822.5954 | 11.67082 | 821.5881 | 1798.30 | 0 | 4 | Anticancer | Synechocystis sp. | [96,97] |
| Bartoloside D | C44H75Cl3O10 | Alkylresorcinols | 869.4769 | 11.73558 | 870.4842 | 13,325.94 | 0 | 0 | Not reported | Synechocystis salina | [98] |
| Chlorophyll b | C55H70MgN4O6 | Chlorophyll | 905.513 | 12.0794 | 906.5203 | 0 | 0 | 5 | Not reported | All Cyanobacrerial sp. | [99,100] |
| Zeaxanthin | C40H56O2 | Xanthophylls | 569.4195 | 12.31082 | 568.4122 | 0 | 0 | 10 | Anticancer | Synechocystis salina | [94,101,102] |
| Chlorophyll a | C55H72O5N4Mg | Chlorophyll | 891.5305 | 12.34362 | 892.5377 | 0 | 0 | 2 | Neuroprotective | All Cyanobacterium sp. | [88,99,100] |
| Oleic acid | C18H34O2 | Fatty acids | 281.2473 | 12.69699 | 282.2546 | 411,919.7 | 0 | 1 | Anticancer | Synechocystis 6803 | [94,103,104] |
| Glyceryl monostearate | C21H42O4 | Glycerides | 357.2998 | 12.90373 | 358.3071 | 93,861.73 | 0 | 1 | Antioxidant | Synechocystis 7338/Synechocystis 6803 | [77,105] |
| Echinenone | C40H54O | Carotenoids | 551.4238 | 13.09513 | 550.4165 | 0 | 0 | 0.9 | Anti-Alzheimer | Synechocystis salina | [106,107] |
| 1-hexadecanoyl-2-(9Z-hexadecenoyl)-3-(6′-sulfo-alpha-D-quinovosyl)-sn-glycerol | C41H76O12S | Sulfolipids | 791.4996 | 17.22822 | 792.5069 | 3,203,772 | 0 | 0 | Not reported | Synechocystis sp. PCC 6803 | [108] |
| α-Tocopherol | C29H50O2 | Vitamins | 431.3854 | 17.65833 | 430.3782 | 0 | 0 | 2 | Antioxidant, antimicrobial | Synechocystis 6803 | [94,109] |
| Formulation Codes | Surfactant Ratio Ch:Lec * | Sample Type | Amount (mL) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| L1 * | 0:1 | Emulsion | 0.5 | 419 ± 114 | 0.27 ± 0.32 | −43.7 ± 1.65 |
| N1 * | 1:1 | Emulsion | 541 ± 479.4 | 0.31 ± 0.1 | −28.7 ± 1.3 | |
| L2 * | 0:1 | Emulsion | 847 ± 173.2 | 0.24 ± 0.22 | −31.6 ± 1.29 | |
| N2 * | 1:1 | Emulsion | 1051 ± 479.4 | 0.35 ± 0.33 | −22.2 ± 1.8 |
| Sample Code | Cytotoxicity IC50 (µg/mL) | (SI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FHC | Caco2 | OCE1 | OVCAR4 | MCF10a | MCF7 | FHC/Caco2 | OCE1/OVCAR4 | MCF10a/MCF7 | |
| L1 | 39.029 ± 1.36 b | 19.56 ± 0.72 b | 42.228 ± 2.5 b | 33.52 ± 1.55 b | 27.177 ± 1.12 c | 9.24 ± 0.41 c | 1.99 | 1.25 | 2.94 |
| L2 | 65.135 ± 2.27 a | 26.27 ± 0.94 a | 87.903 ± 5.21 a | 56.23 ± 2.38 a | 51.169 ± 2.11 a | 19.61 ± 0.78 b | 2.47 | 1.5 | 2.60 |
| N1 | 29.637 ± 1.04 c | 9.09 ± 0.33 d | 29.911 ± 1.77 c | 11.42 ± 0.72 d | 20.135 ± 0.83 d | 2.38 ± 0.13 e | 3.26 | 2.61 | 8.46 |
| N2 | 26.724 ± 0.93 c | 15.57 ± 0.81 c | 26.651 ± 1.58 c | 18.17 ± 0.61 c | 41.865 ± 1.72 b | 35.31 ± 1.29 a | 1.7 | 1.46 | 1.18 |
| Doxorubicin | 12.982 ± 0.45 d | 3.61 ± 0.26 e | 14.157 ± 0.84 d | 9.41 ± 0.39 d | 25.36 ± 1.04 c | 5.35 ± 0.22 d | 3.59 | 1.50 | 4.74 |
| Compound | Binding Energy (kcal/mol) | Key Interactions |
|---|---|---|
| 1 | −8.7 | Asp831 (H-bond) |
| 2 | −8.5 | Asp831 (H-bond) |
| 3 | −8.9 | Asp831 (H-bond), |
| Arg817 (H-bond) | ||
| 6 | −9.2 | Lys721 (H-bond), |
| Asp831 (H-bond) | ||
| 7 | −9.2 | Lys721 (H-bond), |
| Asp831 (H-bond) | ||
| 8 | −9.2 | Lys721 (H-bond), |
| Glu738 (H-bond) | ||
| 12 | −9.2 | Lys721 (H-bond) |
| 14 | −8.8 | Asp831 (H-bond) |
| 19 | −9.2 | Lys721 (H-bond), |
| Glu738 (H-bond) | ||
| 21 | −9.5 | Lys721 (H-bond), |
| Glu738 (H-bond) | ||
| 22 | −8.8 | Asp831 (H-bond) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azmy, L.; Ibraheem, I.B.M.; Alsalamah, S.A.; Alghonaim, M.I.; Zayed, A.; Abd El-Aleam, R.H.; Mohamad, S.A.; Abdelmohsen, U.R.; Elsayed, K.N.M. Evaluation of Cytotoxicity and Metabolic Profiling of Synechocystis sp. Extract Encapsulated in Nano-Liposomes and Nano-Niosomes Using LC-MS, Complemented by Molecular Docking Studies. Biology 2024, 13, 581. https://doi.org/10.3390/biology13080581
Azmy L, Ibraheem IBM, Alsalamah SA, Alghonaim MI, Zayed A, Abd El-Aleam RH, Mohamad SA, Abdelmohsen UR, Elsayed KNM. Evaluation of Cytotoxicity and Metabolic Profiling of Synechocystis sp. Extract Encapsulated in Nano-Liposomes and Nano-Niosomes Using LC-MS, Complemented by Molecular Docking Studies. Biology. 2024; 13(8):581. https://doi.org/10.3390/biology13080581
Chicago/Turabian StyleAzmy, Lamya, Ibraheem B. M. Ibraheem, Sulaiman A. Alsalamah, Mohammed Ibrahim Alghonaim, Ahmed Zayed, Rehab H. Abd El-Aleam, Soad A. Mohamad, Usama Ramadan Abdelmohsen, and Khaled N. M. Elsayed. 2024. "Evaluation of Cytotoxicity and Metabolic Profiling of Synechocystis sp. Extract Encapsulated in Nano-Liposomes and Nano-Niosomes Using LC-MS, Complemented by Molecular Docking Studies" Biology 13, no. 8: 581. https://doi.org/10.3390/biology13080581
APA StyleAzmy, L., Ibraheem, I. B. M., Alsalamah, S. A., Alghonaim, M. I., Zayed, A., Abd El-Aleam, R. H., Mohamad, S. A., Abdelmohsen, U. R., & Elsayed, K. N. M. (2024). Evaluation of Cytotoxicity and Metabolic Profiling of Synechocystis sp. Extract Encapsulated in Nano-Liposomes and Nano-Niosomes Using LC-MS, Complemented by Molecular Docking Studies. Biology, 13(8), 581. https://doi.org/10.3390/biology13080581








