Statistical Learning of Incidental Perceptual Regularities Induces Sensory Conditioned Cortical Responses
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.3. EEG Data Acquisition and Preprocessing
2.4. EEG Data Analysis
2.5. Hierarchical Gaussian Filter Modelling
3. Results
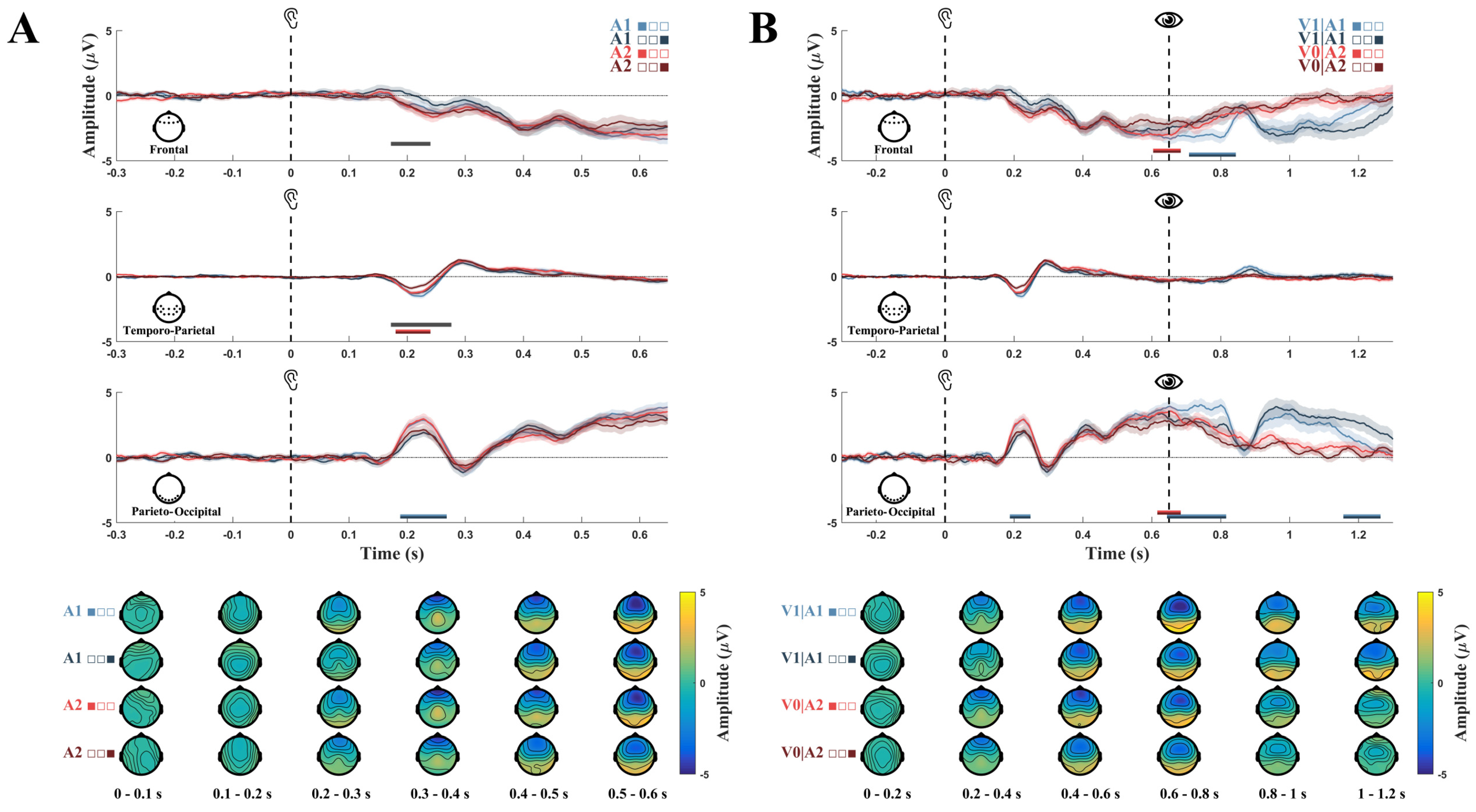
3.1. Event-Related Potentials
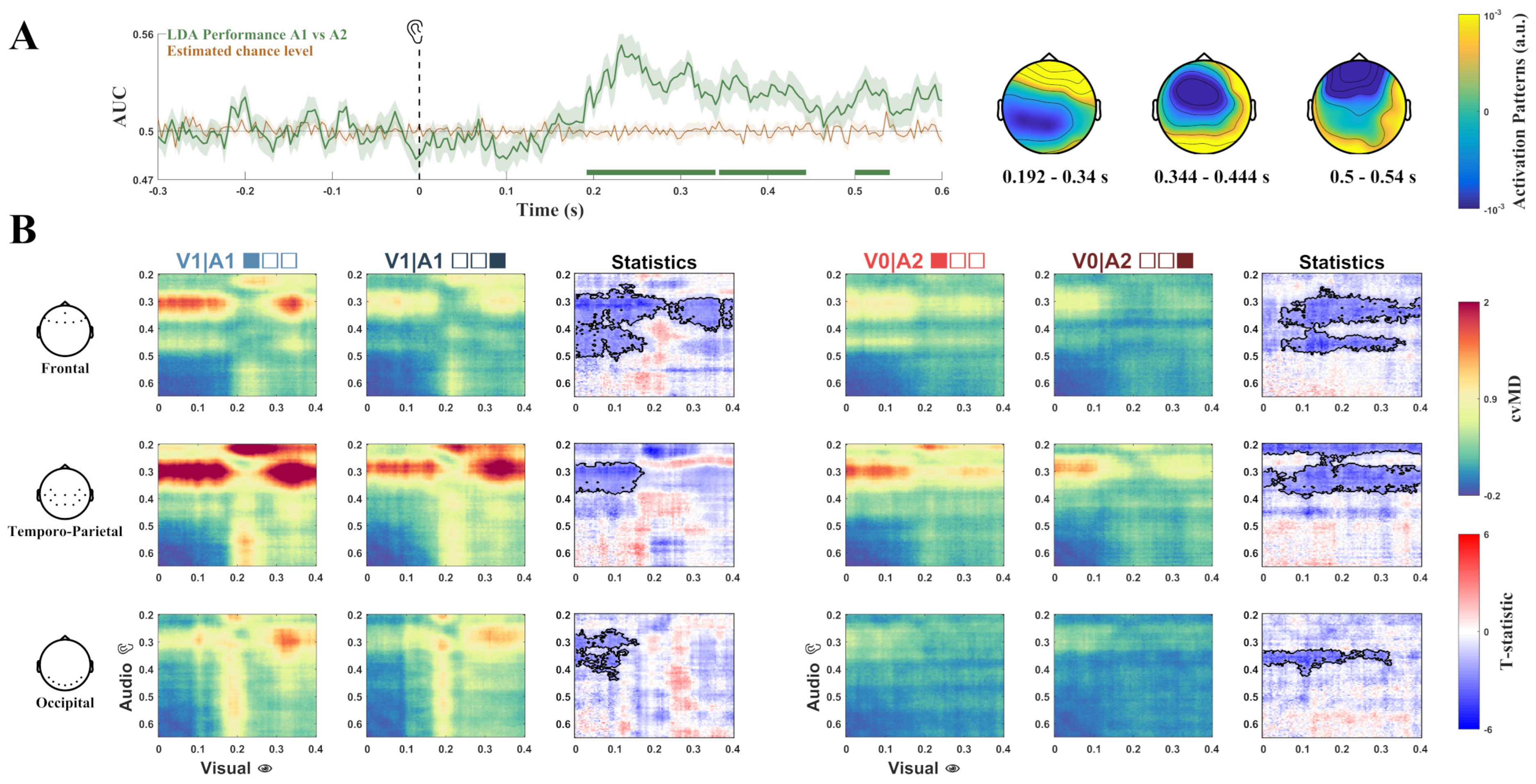
3.2. Multivariate Classification
3.3. Pattern Similarity Analysis
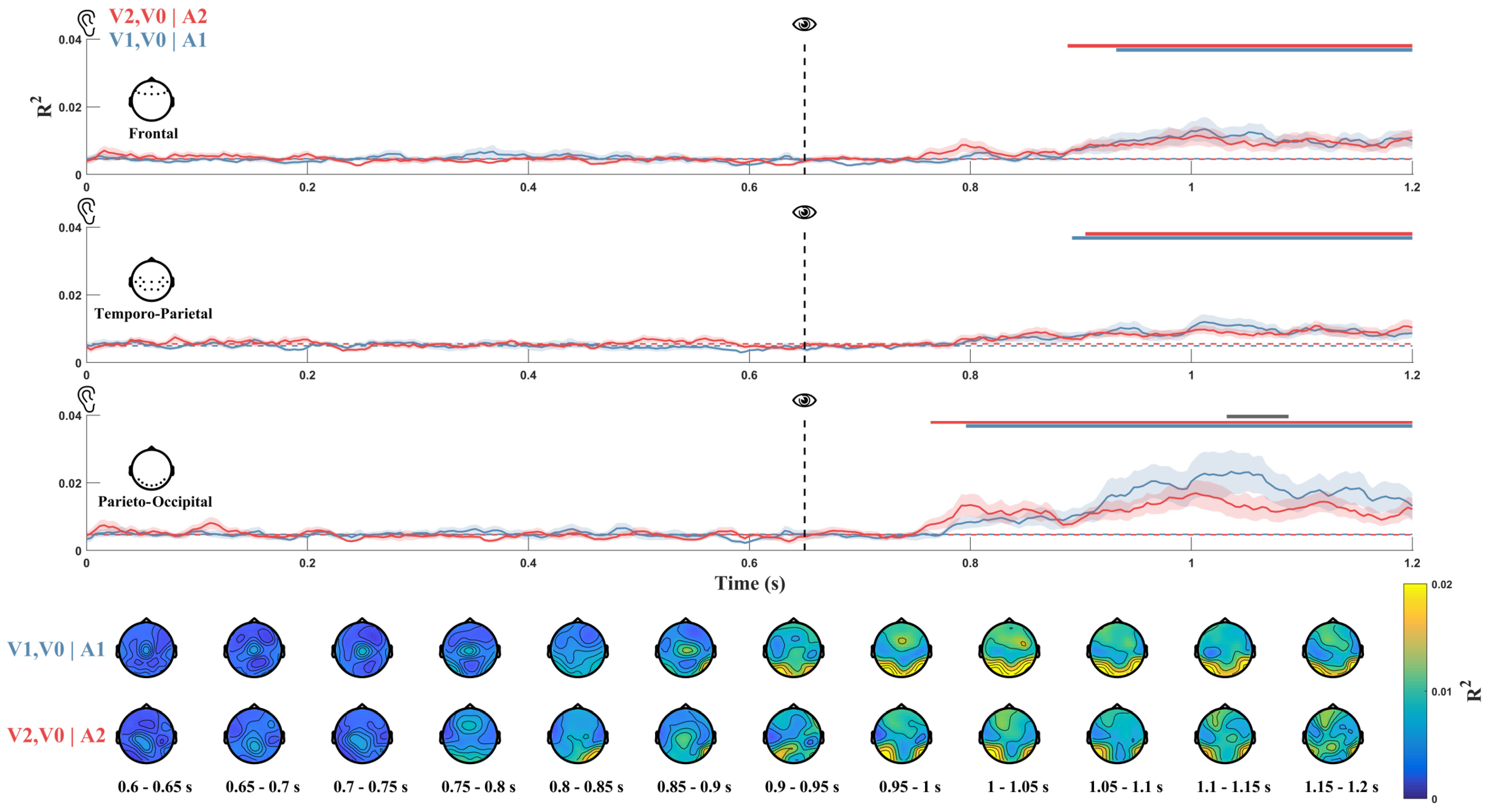
3.4. HGF Modelling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnal, L.H.; Giraud, A.L. Cortical oscillations and sensory predictions. Trends Cogn. Sci. 2012, 16, 390–398. [Google Scholar] [CrossRef] [PubMed]
- de Lange, F.P.; Heilbron, M.; Kok, P. How Do Expectations Shape Perception? Trends Cogn. Sci. 2018, 22, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: A rough guide to the brain? Trends Cogn. Sci. 2009, 13, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Press, C.; Kok, P.; Yon, D. The Perceptual Prediction Paradox. Trends Cogn. Sci. 2020, 24, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Wacongne, C.; Changeux, J.P.; Dehaene, S. A neuronal model of predictive coding accounting for the mismatch negativity. J. Neurosci. 2012, 32, 3665–3678. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.M.; Lundqvist, M.; Waite, A.S.; Kopell, N.; Miller, E.K. Layer and rhythm specificity for predictive routing. Proc. Natl. Acad. Sci. USA 2020, 117, 31459–31469. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Stephan, K.E. Free-energy and the brain. Synthese 2007, 159, 417–458. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.; Jehee, J.F.; de Lange, F.P. Less is more: Expectation sharpens representations in the primary visual cortex. Neuron 2012, 75, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.; Failing, M.F.; de Lange, F.P. Prior expectations evoke stimulus templates in the primary visual cortex. J. Cogn. Neurosci. 2014, 26, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Malekshahi, R.; Seth, A.; Papanikolaou, A.; Mathews, Z.; Birbaumer, N.; Verschure, P.F.; Caria, A. Differential neural mechanisms for early and late prediction error detection. Sci. Rep. 2016, 6, 24350. [Google Scholar] [CrossRef] [PubMed]
- Melloni, L.; Schwiedrzik, C.M.; Muller, N.; Rodriguez, E.; Singer, W. Expectations change the signatures and timing of electrophysiological correlates of perceptual awareness. J. Neurosci. 2011, 31, 1386–1396. [Google Scholar] [CrossRef]
- Powers, A.R.; Mathys, C.; Corlett, P.R. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 2017, 357, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Wyart, V.; Nobre, A.C.; Summerfield, C. Dissociable prior influences of signal probability and relevance on visual contrast sensitivity. Proc. Natl. Acad. Sci. USA 2012, 109, 3593–3598. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.E.; Graves, K.N.; Turk-Browne, N.B. The prevalence and importance of statistical learning in human cognition and behavior. Curr. Opin. Behav. Sci. 2020, 32, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bar, M. The proactive brain: Using analogies and associations to generate predictions. Trends Cogn. Sci. 2007, 11, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Clark, A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 2013, 36, 181–204. [Google Scholar] [CrossRef]
- Enns, J.T.; Lleras, A. What’s next? New evidence for prediction in human vision. Trends Cogn. Sci. 2008, 12, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.P.; Ballard, D.H. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 1999, 2, 79–87. [Google Scholar] [CrossRef]
- Alink, A.; Schwiedrzik, C.M.; Kohler, A.; Singer, W.; Muckli, L. Stimulus predictability reduces responses in primary visual cortex. J. Neurosci. 2010, 30, 2960–2966. [Google Scholar] [CrossRef] [PubMed]
- den Ouden, H.E.; Friston, K.J.; Daw, N.D.; McIntosh, A.R.; Stephan, K.E. A dual role for prediction error in associative learning. Cereb. Cortex 2009, 19, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, C.; Trittschuh, E.H.; Monti, J.M.; Mesulam, M.M.; Egner, T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat. Neurosci. 2008, 11, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, C.; Wyart, V.; Johnen, V.M.; de Gardelle, V. Human Scalp Electroencephalography Reveals that Repetition Suppression Varies with Expectation. Front. Hum. Neurosci. 2011, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Feuerriegel, D.; Vogels, R.; Kovacs, G. Evaluating the evidence for expectation suppression in the visual system. Neurosci. Biobehav. Rev. 2021, 126, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.S.; McGovern, D.P.; Clark, A.; O’Connell, R.G. Evaluating the neurophysiological evidence for predictive processing as a model of perception. Ann. N. Y. Acad. Sci. 2020, 1464, 242–268. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.I.; Rowe, E.G.; Halasz, V.; Mattingley, J.B. Bayesian Mapping Reveals That Attention Boosts Neural Responses to Predicted and Unpredicted Stimuli. Cereb. Cortex 2018, 28, 1771–1782. [Google Scholar] [CrossRef]
- Wacongne, C.; Labyt, E.; van Wassenhove, V.; Bekinschtein, T.; Naccache, L.; Dehaene, S. Evidence for a hierarchy of predictions and prediction errors in human cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 20754–20759. [Google Scholar] [CrossRef] [PubMed]
- Aitken, F.; Menelaou, G.; Warrington, O.; Koolschijn, R.S.; Corbin, N.; Callaghan, M.F.; Kok, P. Prior expectations evoke stimulus-specific activity in the deep layers of the primary visual cortex. PLoS Biol. 2020, 18, e3001023. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.R.; Cabeza, R.E.; Lobaugh, N.J. Analysis of neural interactions explains the activation of occipital cortex by an auditory stimulus. J. Neurophysiol. 1998, 80, 2790–2796. [Google Scholar] [CrossRef]
- Meyer, T.; Olson, C.R. Statistical learning of visual transitions in monkey inferotemporal cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 19401–19406. [Google Scholar] [CrossRef]
- Sakai, K.; Miyashita, Y. Neural organization for the long-term memory of paired associates. Nature 1991, 354, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.; Mostert, P.; de Lange, F.P. Prior expectations induce prestimulus sensory templates. Proc. Natl. Acad. Sci. USA 2017, 114, 10473–10478. [Google Scholar] [CrossRef] [PubMed]
- SanMiguel, I.; Widmann, A.; Bendixen, A.; Trujillo-Barreto, N.; Schroger, E. Hearing silences: Human auditory processing relies on preactivation of sound-specific brain activity patterns. J. Neurosci. 2013, 33, 8633–8639. [Google Scholar] [CrossRef] [PubMed]
- Blom, T.; Feuerriegel, D.; Johnson, P.; Bode, S.; Hogendoorn, H. Predictions drive neural representations of visual events ahead of incoming sensory information. Proc. Natl. Acad. Sci. USA 2020, 117, 7510–7515. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, S.E.P.; Stokes, M.G.; Nobre, A.C.; van Ede, F. One Thing Leads to Another: Anticipating Visual Object Identity Based on Associative-Memory Templates. J. Neurosci. 2020, 40, 4010–4020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Perez-Bellido, A.; Haegens, S.; de Lange, F.P. Perceptual Expectations Modulate Low-Frequency Activity: A Statistical Learning Magnetoencephalography Study. J. Cogn. Neurosci. 2020, 32, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Manahova, M.E.; Mostert, P.; Kok, P.; Schoffelen, J.M.; de Lange, F.P. Stimulus Familiarity and Expectation Jointly Modulate Neural Activity in the Visual Ventral Stream. J. Cogn. Neurosci. 2018, 30, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Rungratsameetaweemana, N.; Itthipuripat, S.; Salazar, A.; Serences, J.T. Expectations Do Not Alter Early Sensory Processing during Perceptual Decision-Making. J. Neurosci. 2018, 38, 5632–5648. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.S.; Tang, H.; Sussman, E.; Kohn, A. Limited Evidence for Sensory Prediction Error Responses in Visual Cortex of Macaques and Humans. Cereb. Cortex 2021, 31, 3136–3152. [Google Scholar] [CrossRef]
- Hall, M.G.; Mattingley, J.B.; Dux, P.E. Electrophysiological correlates of incidentally learned expectations in human vision. J. Neurophysiol. 2018, 119, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- den Ouden, C.; Zhou, A.; Mepani, V.; Kovacs, G.; Vogels, R.; Feuerriegel, D. Stimulus expectations do not modulate visual event-related potentials in probabilistic cueing designs. Neuroimage 2023, 280, 120347. [Google Scholar] [CrossRef] [PubMed]
- den Ouden, H.E.; Daunizeau, J.; Roiser, J.; Friston, K.J.; Stephan, K.E. Striatal prediction error modulates cortical coupling. J. Neurosci. 2010, 30, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Egner, T.; Monti, J.M.; Summerfield, C. Expectation and surprise determine neural population responses in the ventral visual stream. J. Neurosci. 2010, 30, 16601–16608. [Google Scholar] [CrossRef]
- Richter, D.; Ekman, M.; de Lange, F.P. Suppressed Sensory Response to Predictable Object Stimuli throughout the Ventral Visual Stream. J. Neurosci. 2018, 38, 7452–7461. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; de Lange, F.P. Statistical learning attenuates visual activity only for attended stimuli. eLife 2019, 8, e47869. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, C.; de Lange, F.P. Expectation in perceptual decision making: Neural and computational mechanisms. Nat. Rev. Neurosci. 2014, 15, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.; Theeuwes, J. Statistical learning in the absence of explicit top-down attention. Cortex 2020, 131, 54–65. [Google Scholar] [CrossRef] [PubMed]
- St John-Saaltink, E.; Utzerath, C.; Kok, P.; Lau, H.C.; de Lange, F.P. Expectation Suppression in Early Visual Cortex Depends on Task Set. PLoS ONE 2015, 10, e0131172. [Google Scholar] [CrossRef] [PubMed]
- Auksztulewicz, R.; Schwiedrzik, C.M.; Thesen, T.; Doyle, W.; Devinsky, O.; Nobre, A.C.; Schroeder, C.E.; Friston, K.J.; Melloni, L. Not All Predictions Are Equal: “What” and “When” Predictions Modulate Activity in Auditory Cortex through Different Mechanisms. J. Neurosci. 2018, 38, 8680–8693. [Google Scholar] [CrossRef]
- Moskowitz, H.S.; Sussman, E.S. Sound category habituation requires task-relevant attention. Front. Neurosci. 2023, 17, 1228506. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.G.; Myers, N.E.; Turnbull, J.; Nobre, A.C. Preferential encoding of behaviorally relevant predictions revealed by EEG. Front. Hum. Neurosci. 2014, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Brogden, W.J. Sensory pre-conditioning of human subjects. J. Exp. Psychol. 1947, 37, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Chernikoff, R.; Brogden, W.J. The effect of instructions upon sensory preconditioning of human subjects. J. Exp. Psychol. 1949, 39, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Headley, D.B.; Weinberger, N.M. Relational associative learning induces cross-modal plasticity in early visual cortex. Cereb. Cortex 2015, 25, 1306–1318. [Google Scholar] [CrossRef]
- Hoffeld, D.R.; Kendall, S.B.; Thompson, R.F.; Brogden, W.J. Effect of amount of preconditioning training upon the magnitude of sensory preconditioning. J. Exp. Psychol. 1960, 59, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Etzel, J.A.; Courtney, Y.; Carey, C.E.; Gehred, M.Z.; Agrawal, A.; Braver, T.S. Pattern Similarity Analyses of FrontoParietal Task Coding: Individual Variation and Genetic Influences. Cereb. Cortex 2020, 30, 3167–3183. [Google Scholar] [CrossRef] [PubMed]
- Sommer, V.R.; Mount, L.; Weigelt, S.; Werkle-Bergner, M.; Sander, M.C. Spectral pattern similarity analysis: Tutorial and application in developmental cognitive neuroscience. Dev. Cogn. Neurosci. 2022, 54, 101071. [Google Scholar] [CrossRef]
- Walther, A.; Nili, H.; Ejaz, N.; Alink, A.; Kriegeskorte, N.; Diedrichsen, J. Reliability of dissimilarity measures for multi-voxel pattern analysis. Neuroimage 2016, 137, 188–200. [Google Scholar] [CrossRef]
- Lieder, F.; Daunizeau, J.; Garrido, M.I.; Friston, K.J.; Stephan, K.E. Modelling trial-by-trial changes in the mismatch negativity. PLoS Comput. Biol. 2013, 9, e1002911. [Google Scholar] [CrossRef] [PubMed]
- Lieder, F.; Stephan, K.E.; Daunizeau, J.; Garrido, M.I.; Friston, K.J. A neurocomputational model of the mismatch negativity. PLoS Comput. Biol. 2013, 9, e1003288. [Google Scholar] [CrossRef]
- Mathys, C.D.; Lomakina, E.I.; Daunizeau, J.; Iglesias, S.; Brodersen, K.H.; Friston, K.J.; Stephan, K.E. Uncertainty in perception and the Hierarchical Gaussian Filter. Front. Hum. Neurosci. 2014, 8, 825. [Google Scholar] [CrossRef] [PubMed]
- Mathot, S.; Schreij, D.; Theeuwes, J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behav. Res. Methods 2012, 44, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Gabard-Durnam, L.J.; Mendez Leal, A.S.; Wilkinson, C.L.; Levin, A.R. The Harvard Automated Processing Pipeline for Electroencephalography (HAPPE): Standardized Processing Software for Developmental and High-Artifact Data. Front. Neurosci. 2018, 12, 97. [Google Scholar] [CrossRef]
- Lopez, K.L.; Monachino, A.D.; Morales, S.; Leach, S.C.; Bowers, M.E.; Gabard-Durnam, L.J. HAPPILEE: HAPPE In Low Electrode Electroencephalography, a standardized pre-processing software for lower density recordings. Neuroimage 2022, 260, 119390. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.J.; Sejnowski, T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995, 7, 1129–1159. [Google Scholar] [CrossRef] [PubMed]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. Neuroimage 2019, 198, 181–197. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.E.; Stokes, M.G.; Walther, L.; Nobre, A.C. Oscillatory brain state predicts variability in working memory. J. Neurosci. 2014, 34, 7735–7743. [Google Scholar] [CrossRef] [PubMed]
- Treder, M.S. MVPA-Light: A Classification and Regression Toolbox for Multi-Dimensional Data. Front. Neurosci. 2020, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Pernet, C.R.; Latinus, M.; Nichols, T.E.; Rousselet, G.A. Cluster-based computational methods for mass univariate analyses of event-related brain potentials/fields: A simulation study. J. Neurosci. Methods 2015, 250, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Meinecke, F.; Gorgen, K.; Dahne, S.; Haynes, J.D.; Blankertz, B.; Biessmann, F. On the interpretation of weight vectors of linear models in multivariate neuroimaging. Neuroimage 2014, 87, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Mathys, C.; Daunizeau, J.; Friston, K.J.; Stephan, K.E. A bayesian foundation for individual learning under uncertainty. Front. Hum. Neurosci. 2011, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.U.; Iannaccone, R.; Ball, J.; Mathys, C.; Brandeis, D.; Walitza, S.; Brem, S. Role of the medial prefrontal cortex in impaired decision making in juvenile attention-deficit/hyperactivity disorder. JAMA Psychiatry 2014, 71, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Stefanics, G.; Heinzle, J.; Horvath, A.A.; Stephan, K.E. Visual Mismatch and Predictive Coding: A Computational Single-Trial ERP Study. J. Neurosci. 2018, 38, 4020–4030. [Google Scholar] [CrossRef] [PubMed]
- Rescorla, R.A.; Wagner, A. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non reinforcement. In Classical Conditioning II: Current Research and Theory; Black, A.H., Prokasy, W.F., Eds.; Appleton-Century-Crofts: New York, NY, USA, 1972; pp. 64–99. [Google Scholar]
- Bueti, D.; Macaluso, E. Auditory temporal expectations modulate activity in visual cortex. Neuroimage 2010, 51, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.J.; Stormer, V.S.; Martinez, A.; Feng, W.; Hillyard, S.A. Salient sounds activate human visual cortex automatically. J. Neurosci. 2013, 33, 9194–9201. [Google Scholar] [CrossRef] [PubMed]
- Garner, A.R.; Keller, G.B. A cortical circuit for audio-visual predictions. Nat. Neurosci. 2022, 25, 98–105. [Google Scholar] [CrossRef]
- Fiser, J.; Lengyel, G. A common probabilistic framework for perceptual and statistical learning. Curr. Opin. Neurobiol. 2019, 58, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Sasaki, Y. Perceptual learning: Toward a comprehensive theory. Annu. Rev. Psychol. 2015, 66, 197–221. [Google Scholar] [CrossRef] [PubMed]
- Rahnev, D.; Lau, H.; de Lange, F.P. Prior expectation modulates the interaction between sensory and prefrontal regions in the human brain. J. Neurosci. 2011, 31, 10741–10748. [Google Scholar] [CrossRef] [PubMed]
- Giustino, T.F.; Maren, S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front. Behav. Neurosci. 2015, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ghim, J.W.; Lee, J.H.; Jung, M.W. Neural correlates of interval timing in rodent prefrontal cortex. J. Neurosci. 2013, 33, 13834–13847. [Google Scholar] [CrossRef] [PubMed]
- Sznabel, D.; Land, R.; Kopp, B.; Kral, A. The relation between implicit statistical learning and proactivity as revealed by EEG. Sci. Rep. 2023, 13, 15787. [Google Scholar] [CrossRef] [PubMed]
- Donchin, E.; Heffley, E.; Hillyard, S.A.; Loveless, N.; Maltzman, I.; Ohman, A.; Rosler, F.; Ruchkin, D.; Siddle, D. Cognition and event-related potentials. II. The orienting reflex and P300. Ann. N. Y. Acad. Sci. 1984, 425, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Stefanics, G.; Kremlacek, J.; Czigler, I. Visual mismatch negativity: A predictive coding view. Front. Hum. Neurosci. 2014, 8, 666. [Google Scholar] [CrossRef]
- Arnal, L.H.; Wyart, V.; Giraud, A.L. Transitions in neural oscillations reflect prediction errors generated in audiovisual speech. Nat. Neurosci. 2011, 14, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, N.E.; Xiao, D.; Baker, C.I.; Oram, M.W.; Perrett, D.I. Integration of visual and auditory information by superior temporal sulcus neurons responsive to the sight of actions. J. Cogn. Neurosci. 2005, 17, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Donchin, E.; Coles, M.G.H. Is the P300 Component a Manifestation of Context Updating. Behav. Brain Sci. 1988, 11, 357–374. [Google Scholar] [CrossRef]
- Verleger, R. Event-Related Potentials and Memory—A Critique of the Context Updating Hypothesis and an Alternative Interpretation of P3. Behav. Brain Sci. 1988, 11, 343–3568. [Google Scholar] [CrossRef]
- Schwartenbeck, P.; Passecker, J.; Hauser, T.U.; FitzGerald, T.H.; Kronbichler, M.; Friston, K.J. Computational mechanisms of curiosity and goal-directed exploration. eLife 2019, 8, e41703. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Gershman, S.J. The algorithmic architecture of exploration in the human brain. Curr. Opin. Neurobiol. 2019, 55, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; Wiley: New York, NY, USA, 1949; p. xix. 335p. [Google Scholar]
- Rescorla, R.A. Pavlovian Conditioning—Its Not what You Think It Is. Am. Psychol. 1988, 43, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Rescorla, R.A. Behavioral-Studies of Pavlovian Conditioning. Annu. Rev. Neurosci. 1988, 11, 329–352. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, A.; D’Alessandro, M.; Gallitto, G.; Rastelli, C.; Braun, C.; Caria, A. Statistical Learning of Incidental Perceptual Regularities Induces Sensory Conditioned Cortical Responses. Biology 2024, 13, 576. https://doi.org/10.3390/biology13080576
Greco A, D’Alessandro M, Gallitto G, Rastelli C, Braun C, Caria A. Statistical Learning of Incidental Perceptual Regularities Induces Sensory Conditioned Cortical Responses. Biology. 2024; 13(8):576. https://doi.org/10.3390/biology13080576
Chicago/Turabian StyleGreco, Antonino, Marco D’Alessandro, Giuseppe Gallitto, Clara Rastelli, Christoph Braun, and Andrea Caria. 2024. "Statistical Learning of Incidental Perceptual Regularities Induces Sensory Conditioned Cortical Responses" Biology 13, no. 8: 576. https://doi.org/10.3390/biology13080576
APA StyleGreco, A., D’Alessandro, M., Gallitto, G., Rastelli, C., Braun, C., & Caria, A. (2024). Statistical Learning of Incidental Perceptual Regularities Induces Sensory Conditioned Cortical Responses. Biology, 13(8), 576. https://doi.org/10.3390/biology13080576




