Early Peri-Implant Bone Healing on Laser-Modified Surfaces with and without Hydroxyapatite Coating: An In Vivo Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Surfaces
2.1.1. Laser Beam
2.1.2. Laser Beam Followed by Incorporation of Hydroxyapatite Biomimetic Method
2.2. Topographic Characterization of the Implants
2.2.1. Scanning Electron Microscopy Analysis Coupled to the X-ray Dispersive-Energy Spectrometry System—SEM-EDX
2.2.2. Contact Angle Measurements
2.3. Animals and Ethics Committee
2.4. Application of Fluorochromes
2.5. Euthanasia and Material Collection
2.6. Biomechanical Analysis
2.7. Fluorochrome Analysis (Active Mineralized Surface)
2.8. Histometric Analysis
2.9. Statistical Analysis
3. Results
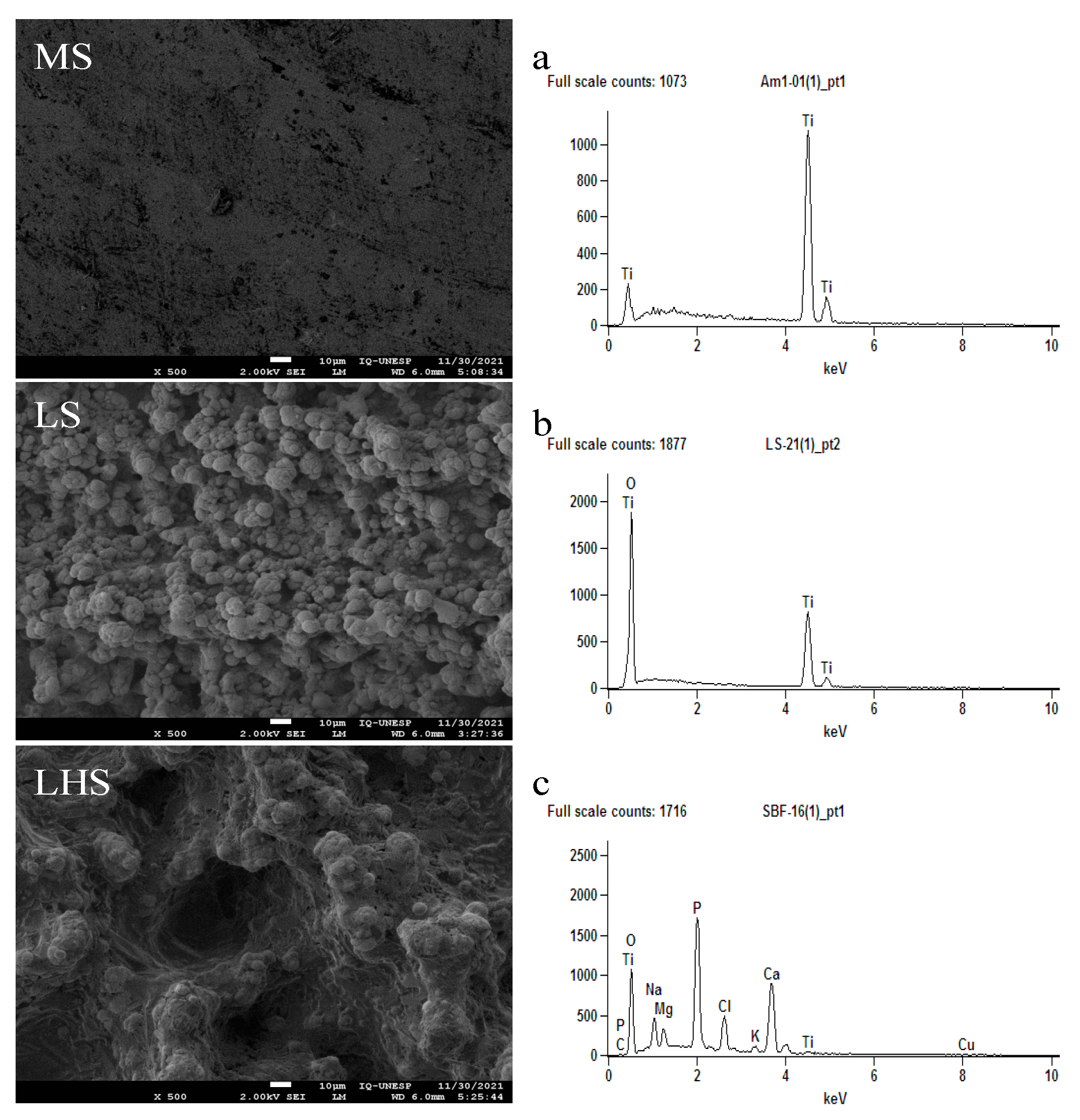
3.1. SEM/EDX
3.2. Contact Angle Measurements
3.3. SEM/EDX of the Removed Implants
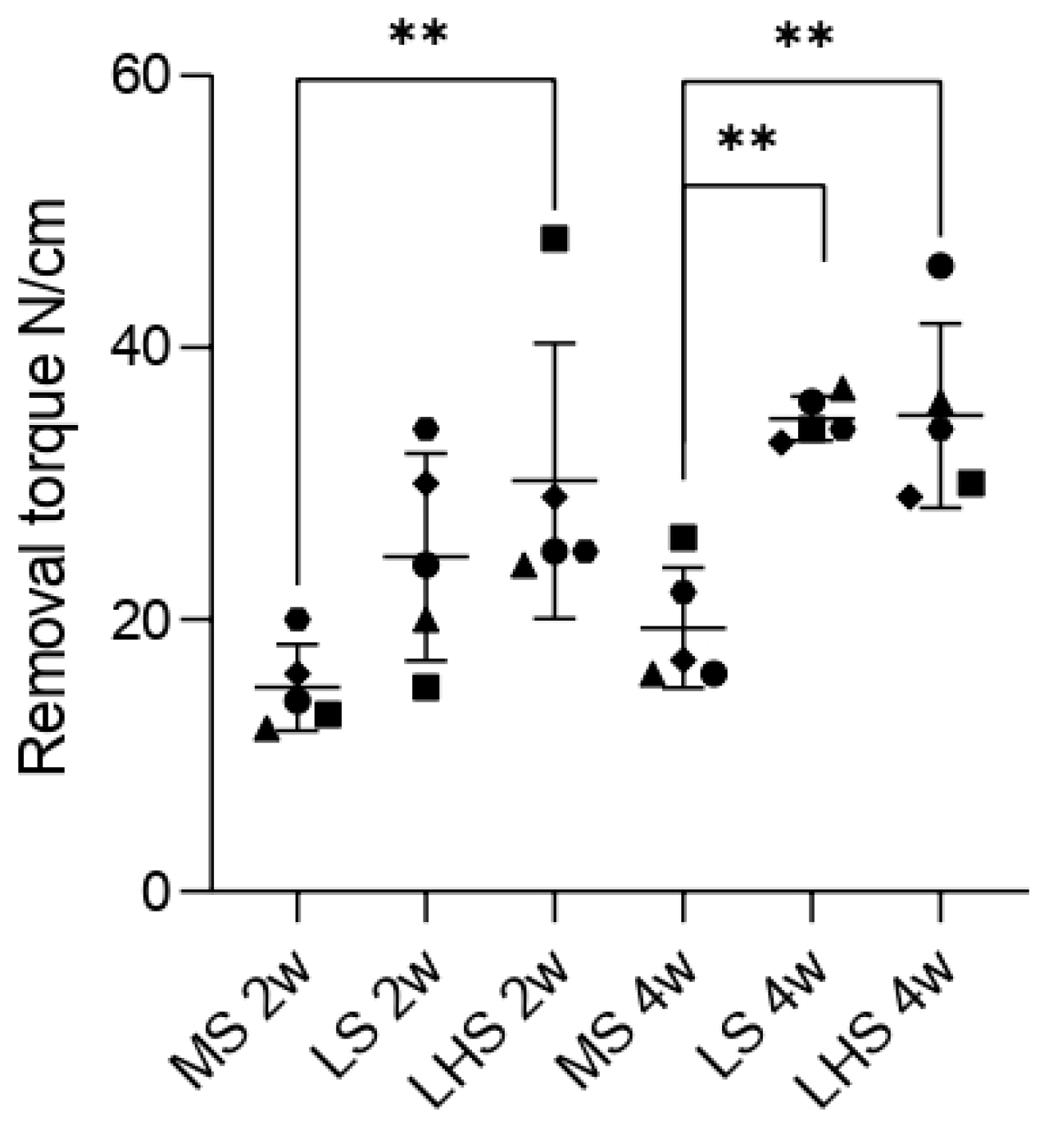
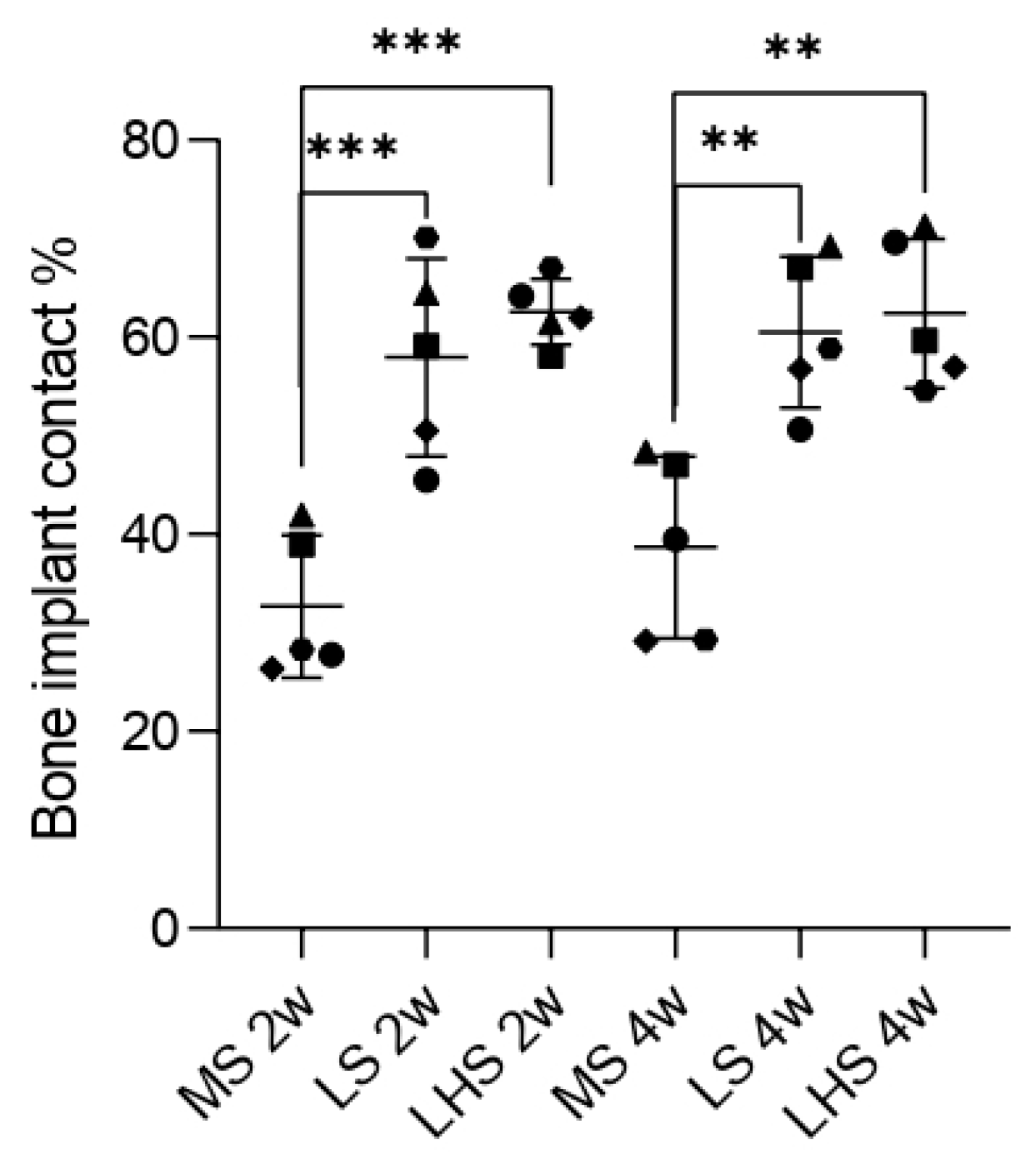
3.4. Biomechanical Analysis
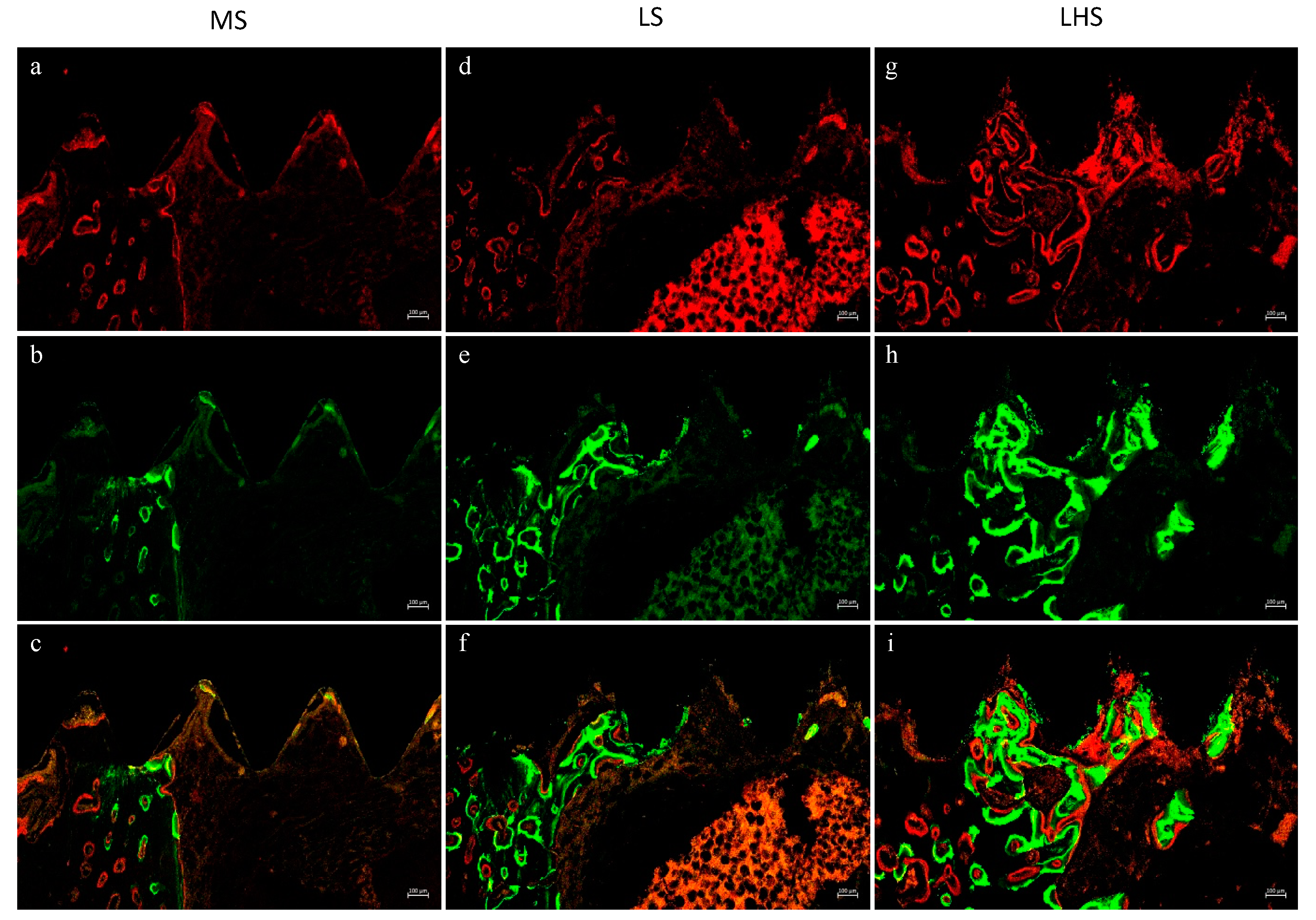
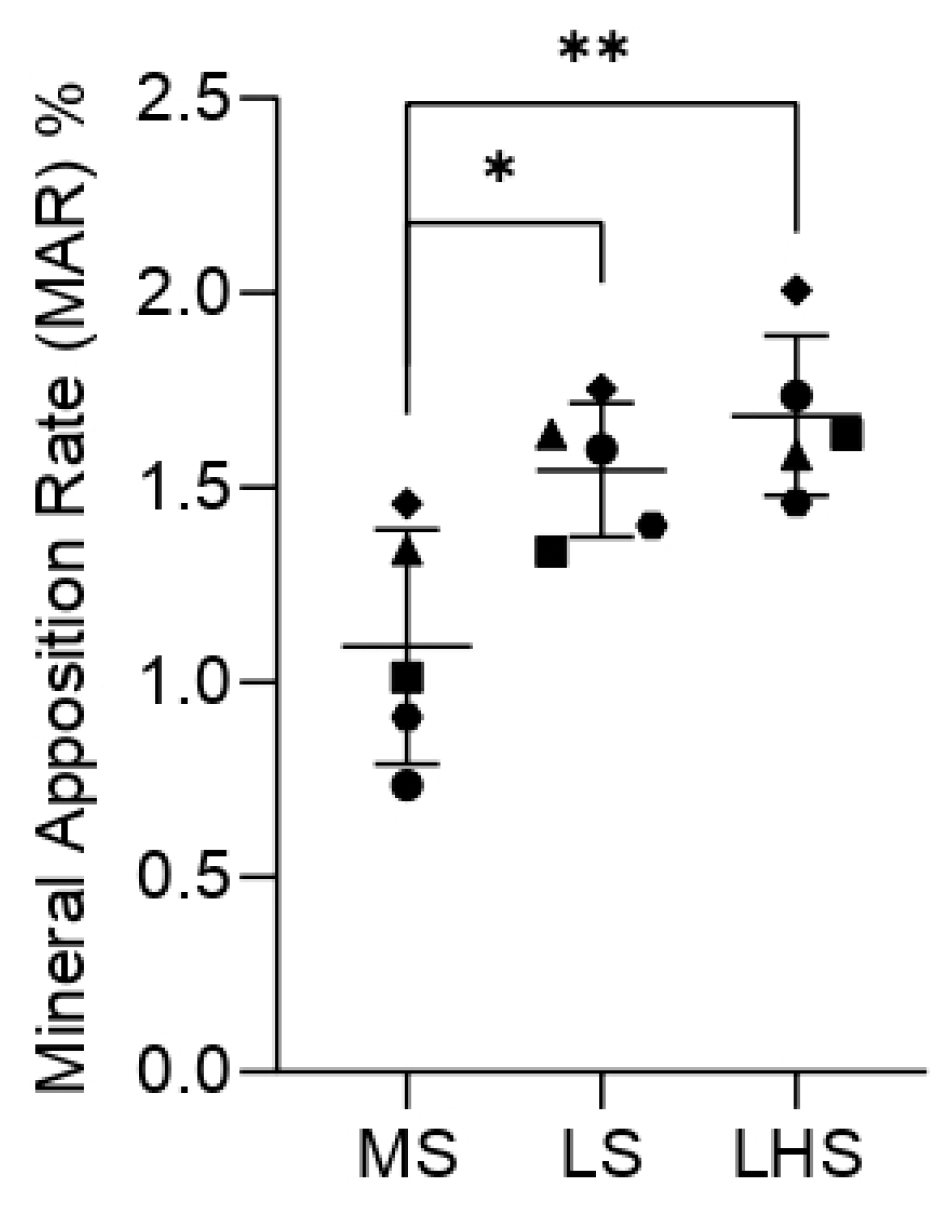
3.5. Fluorochrome Analysis
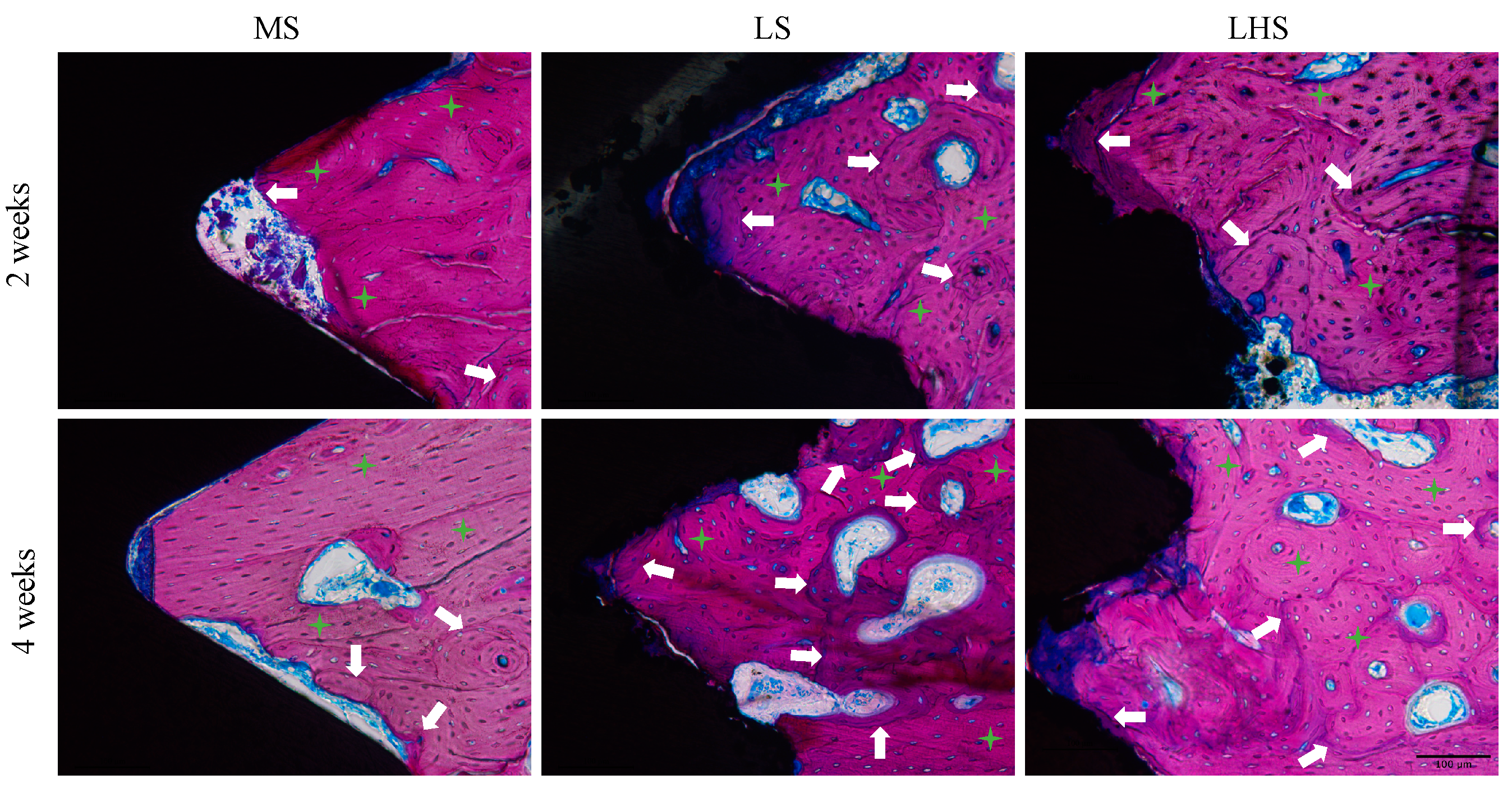
3.6. Qualitative Histological Analysis
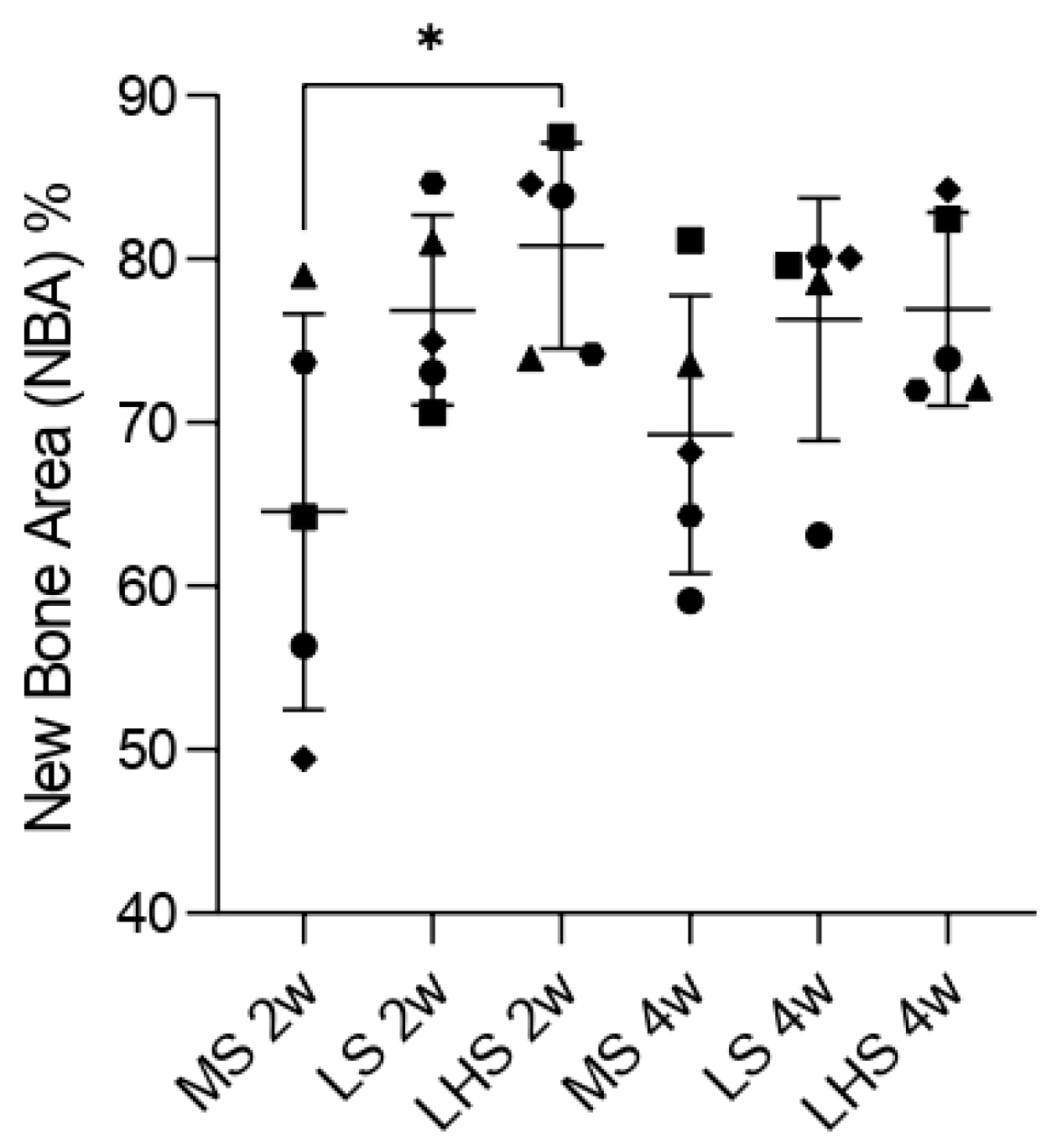
3.7. Histometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abu Alfaraj, T.; Al-Madani, S.; Alqahtani, N.S.; Almohammadi, A.A.; Alqahtani, A.M.; AlQabbani, H.S.; Bajunaid, M.K.; Alharthy, B.A.; Aljalfan, N. Optimizing Osseointegration in Dental Implantology: A Cross-Disciplinary Review of Current and Emerging Strategies. Cureus 2023, 15, e47943. [Google Scholar] [CrossRef] [PubMed]
- Matos, G.R.M. Surface Roughness of Dental Implant and Osseointegration. J. Maxillofac. Oral. Surg. 2021, 20, e47943. [Google Scholar] [CrossRef]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel Surfaces and Osseointegration in Implant Dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lundkvist, S.; Rockler, B. Osseointegrated Titanium Fixtures in the Treatment of Edentulousness. Biomaterials 1983, 4, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-Osseous Anchorage of Dental Prostheses. I. Experimental Studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated Titanium Implants. Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-Year Study of Osseointegrated Implants in the Treatment of the Edentulous Jaw. Int. J. Oral. Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological Factors Contributing to Failures of Osseointegrated Oral Implants. (II). Etiopathogenesis. Eur. J. Oral. Sci. 1998, 106, 721–764. [Google Scholar] [CrossRef]
- Faeda, R.S.; Tavares, H.S.; Sartori, R.; Guastaldi, A.C.; Marcantonio, E. Biological Performance of Chemical Hydroxyapatite Coating Associated with Implant Surface Modification by Laser Beam: Biomechanical Study in Rabbit Tibias. J. Oral. Maxillofac. Surg. 2009, 67, 1706–1715. [Google Scholar] [CrossRef]
- Queiroz, T.P.; Souza, F.Á.; Guastaldi, A.C.; Margonar, R.; Garcia-Júnior, I.R.; Hochuli-Vieira, E. Commercially Pure Titanium Implants with Surfaces Modified by Laser Beam with and without Chemical Deposition of Apatite. Biomechanical and Topographical Analysis in Rabbits. Clin. Oral. Implant. Res. 2013, 24, 896–903. [Google Scholar] [CrossRef]
- Souza, F.A.; Queiroz, T.P.; Guastaldi, A.C.; Garcia-Júnior, I.R.; Magro-Filho, O.; Nishioka, R.S.; Sisti, K.E.; Sonoda, C.K. Comparative in Vivo Study of Commercially Pure Ti Implants with Surfaces Modified by Laser with and without Silicate Deposition: Biomechanical and Scanning Electron Microscopy Analysis. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.Á.; Queiroz, T.P.; Sonoda, C.K.; Okamoto, R.; Margonar, R.; Guastaldi, A.C.; Nishioka, R.S.; Garcia Júnior, I.R. Histometric Analysis and Topographic Characterization of Cp Ti Implants with Surfaces Modified by Laser with and without Silica Deposition. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, T.P.; de Molon, R.S.; Souza, F.Á.; Margonar, R.; Thomazini, A.H.A.; Guastaldi, A.C.; Hochuli-Vieira, E. In Vivo Evaluation of Cp Ti Implants with Modified Surfaces by Laser Beam with and without Hydroxyapatite Chemical Deposition and without and with Thermal Treatment: Topographic Characterization and Histomorphometric Analysis in Rabbits. Clin. Oral. Investig. 2017, 21, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-G.; Jeong, Y.-S.; Huh, Y.-H.; Park, C.-J.; Cho, L.-R. Impact of Surface Chemistry Modifications on Speed and Strength of Osseointegration. Int. J. Oral. Maxillofac. Implant. 2018, 33, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Kessler-Liechti, G.; Zix, J.; Mericske-Stern, R. Stability Measurements of 1-Stage Implants in the Edentulous Mandible by Means of Resonance Frequency Analysis. Int. J. Oral. Maxillofac. Implant. 2008, 23, 353–358. [Google Scholar]
- Carlsson, L.; Röstlund, T.; Albrektsson, B.; Albrektsson, T. Removal Torques for Polished and Rough Titanium Implants. Int. J. Oral. Maxillofac. Implant. 1988, 3, 21–24. [Google Scholar]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. Biomed. Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone Tissue Responses to Surface-Modified Zirconia Implants: A Histomorphometric and Removal Torque Study in the Rabbit. Clin. Implant. Dent. Relat. Res. 2005, 7 (Suppl. S1), S13–S20. [Google Scholar] [CrossRef]
- Chauhan, P.; Srivastava, A.; Bhati, P.; Chaturvedi, M.; Patil, V.; Kunnoth, S.; Kumari, N.; Arya, V.; Pandya, M.; Agarwal, M.; et al. Enhanced Osseointegration of Drug Eluting Nanotubular Dental Implants: An in Vitro and in Vivo Study. Bioact. Mater. 2023, 28, 432–447. [Google Scholar] [CrossRef]
- Qahash, M.; Hardwick, W.R.; Rohrer, M.D.; Wozney, J.M.; Wikesjö, U.M.E. Surface-Etching Enhances Titanium Implant Osseointegration in Newly Formed (RhBMP-2-Induced) and Native Bone. Int. J. Oral. Maxillofac. Implant. 2007, 22, 472–477. [Google Scholar]
- Xavier, S.P.; Carvalho, P.S.P.; Beloti, M.M.; Rosa, A.L. Response of Rat Bone Marrow Cells to Commercially Pure Titanium Submitted to Different Surface Treatments. J. Dent. 2003, 31, 173–180. [Google Scholar] [CrossRef]
- Thomas, K.A.; Cook, S.D. Relationship between Surface Characteristics and the Degree of Bone-Implant Integration. J. Biomed. Mater. Res. 1992, 26, 831–833. [Google Scholar] [CrossRef]
- Bressel, T.A.B.; de Queiroz, J.D.F.; Gomes Moreira, S.M.; da Fonseca, J.T.; Filho, E.A.; Guastaldi, A.C.; Batistuzzo de Medeiros, S.R. Laser-Modified Titanium Surfaces Enhance the Osteogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cell Res. Ther. 2017, 8, 269. [Google Scholar] [CrossRef]
- Mariscal-Muñoz, E.; Costa, C.A.S.; Tavares, H.S.; Bianchi, J.; Hebling, J.; Machado, J.P.B.; Lerner, U.H.; Souza, P.P.C. Osteoblast Differentiation Is Enhanced by a Nano-to-Micro Hybrid Titanium Surface Created by Yb:YAG Laser Irradiation. Clin. Oral Investig. 2016, 20, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.F.L.; de Souza, J.A.C.; Magalhães, F.A.C.; de Oliveira, G.J.P.L.; de Santis, J.B.; de Souza Costa, C.A.; de Souza, P.P.C. Laser-Modified Ti Surface Improves Paracrine Osteogenesis by Modulating the Expression of DKK1 in Osteoblasts. J. Funct. Biomater. 2023, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Faeda, R.S.; Tavares, H.S.; Sartori, R.; Guastaldi, A.C.; Marcantonio, E. Evaluation of Titanium Implants with Surface Modification by Laser Beam. Biomechanical Study in Rabbit Tibias. Braz. Oral Res. 2009, 23, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Laoui, T.; Santos, E.; Osakada, K.; Shiomi, M.; Morita, M.; Shaik, S.K.; Tolochko, N.K.; Abe, F.; Takahashi, M. Properties of Titanium Dental Implant Models Made by Laser Processing. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2006, 220, 857–863. [Google Scholar] [CrossRef]
- Braga, F.J.; Marques, R.F.; de AFilho, E.; Guastaldi, A.C. Surface Modification of Ti Dental Implants by Nd:YVO4 Laser Irradiation. Appl. Surf. Sci. 2007, 253, 9203–9208. [Google Scholar] [CrossRef]
- Hallgren, C.; Reimers, H.; Chakarov, D.; Gold, J.; Wennerberg, A. An in Vivo Study of Bone Response to Implants Topographically Modified by Laser Micromachining. Biomaterials 2003, 24, 701–710. [Google Scholar] [CrossRef]
- Marticorena, M.; Corti, G.; Olmedo, D.; Guglielmotti, M.B.; Duhalde, S. Laser Surface Modification of Ti Implants to Improve Osseointegration. J. Phys. Conf. Ser. 2007, 59, 662–665. [Google Scholar] [CrossRef]
- Filiberto, M.; Daniele, B.; Franco, B.; Antonio, S.; Adriano, P.; Giovanna, I.; Raimondo, Q. Histological and Histomorphometric Comparison of Innovative Dental Implants Laser Obtained: Animal Pilot Study. Materials 2021, 14, 1830. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.-S.; Li, L.-J.; Cho, S.-A. Comparison of Removal Torques between Laser-Treated and SLA-Treated Implant Surfaces in Rabbit Tibiae. J. Adv. Prosthodont. 2014, 6, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Pálka, V.; Postrková, E.; Koerten, H.K. Some Characteristics of Hydroxylapatite Powder Particles after Plasma Spraying. Biomaterials 1998, 19, 1763–1772. [Google Scholar] [CrossRef]
- Guo, L.; Li, H. Fabrication and Characterization of Thin Nano-Hydroxyapatite Coatings on Titanium. Surf. Coat. Technol. 2004, 185, 268–274. [Google Scholar] [CrossRef]
- Nazir, M.; Pei Ting, O.; See Yee, T.; Pushparajan, S.; Swaminathan, D.; Kutty, M.G. Biomimetic Coating of Modified Titanium Surfaces with Hydroxyapatite Using Simulated Body Fluid. Adv. Mater. Sci. Eng. 2015, 2015, 407379. [Google Scholar] [CrossRef]
- Baino, F.; Yamaguchi, S. The Use of Simulated Body Fluid (SBF) for Assessing Materials Bioactivity in the Context of Tissue Engineering: Review and Challenges. Biomimetics 2020, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Aparecida, A.H.; Fook, M.V.L.; Guastaldi, A.C. Biomimetic Apatite Formation on Ultra-High Molecular Weight Polyethylene (UHMWPE) Using Modified Biomimetic Solution. J. Mater. Sci. Mater. Med. 2009, 20, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Florian, F.; Guastaldi, F.P.S.; Cominotte, M.A.; Pires, L.C.; Guastaldi, A.C.; Cirelli, J.A. Behavior of Rat Bone Marrow Stem Cells on Titanium Surfaces Modified by Laser-Beam and Deposition of Calcium Phosphate. J. Mater. Sci. Mater. Med. 2021, 32, 57. [Google Scholar] [CrossRef]
- Sisti, K.E.; Piattelli, A.; Guastaldi, A.C.; Queiroz, T.P.; de Rossi, R. Nondecalcified Histologic Study of Bone Response to Titanium Implants Topographically Modified by Laser with and without Hydroxyapatite Coating. Int. J. Periodontics Restor. Dent. 2013, 33, 689–696. [Google Scholar] [CrossRef]
- Sisti, K.E.; de Andrés, M.C.; Johnston, D.; Almeida-Filho, E.; Guastaldi, A.C.; Oreffo, R.O.C. Skeletal Stem Cell and Bone Implant Interactions Are Enhanced by LASER Titanium Modification. Biochem. Biophys. Res. Commun. 2016, 473, 719–725. [Google Scholar] [CrossRef] [PubMed]
- NC3Rs Reporting Guidelines Working Group. Animal Research: Reporting in Vivo Experiments: The ARRIVE Guidelines. J. Physiol. 2010, 588, 2519–2521. [Google Scholar] [CrossRef] [PubMed]
- Luvizuto, E.R.; Dias, S.S.M.D.; Okamoto, T.; Dornelles, R.C.M.; Okamoto, R. Raloxifene Therapy Inhibits Osteoclastogenesis during the Alveolar Healing Process in Rats. Arch. Oral Biol. 2011, 56, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry: A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Klokkevold, P.R.; Nishimura, R.D.; Adachi, M.; Caputo, A. Osseointegration Enhanced by Chemical Etching of the Titanium Surface. A Torque Removal Study in the Rabbit. Clin. Oral Implant. Res. 1997, 8, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of Surface Characteristics on Bone Integration of Titanium Implants. A Histomorphometric Study in Miniature Pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A Review on the Wettability of Dental Implant Surfaces I: Theoretical and Experimental Aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between Surface Properties (Roughness, Wettability and Morphology) of Titanium and Dental Implant Removal Torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.; Arvidsson, A.; Andersson, M.; Kjellin, P.; Albrektsson, T.; Wennerberg, A. Nano Hydroxyapatite Structures Influence Early Bone Formation. J. Biomed. Mater. Res. A 2008, 87, 299–307. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of Titanium Surface Topography on Bone Integration: A Systematic Review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Jung, U.-W.; Hwang, J.-W.; Choi, D.-Y.; Hu, K.-S.; Kwon, M.-K.; Choi, S.-H.; Kim, H.-J. Surface Characteristics of a Novel Hydroxyapatite-Coated Dental Implant. J. Periodontal Implant. Sci. 2012, 42, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Watzak, G.; Zechner, W.; Ulm, C.; Tangl, S.; Tepper, G.; Watzek, G. Histologic and Histomorphometric Analysis of Three Types of Dental Implants Following 18 Months of Occlusal Loading: A Preliminary Study in Baboons. Clin. Oral Implant. Res. 2005, 16, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Lotz, E.M.; Schwartz, Z. * Roughness and Hydrophilicity as Osteogenic Biomimetic Surface Properties. Tissue Eng. Part A 2017, 23, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Hyzy, S.L.; Cheng, A.; Cohen, D.J.; Yatzkaier, G.; Whitehead, A.J.; Clohessy, R.M.; Gittens, R.A.; Boyan, B.D.; Schwartz, Z. Novel Hydrophilic Nanostructured Microtexture on Direct Metal Laser Sintered Ti-6Al-4V Surfaces Enhances Osteoblast Response in Vitro and Osseointegration in a Rabbit Model. J. Biomed. Mater. Res. A 2016, 104, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Sartoretto, S.C.; Alves, A.T.N.N.; Resende, R.F.B.; Calasans-Maia, J.; Granjeiro, J.M.; Calasans-Maia, M.D. Early Osseointegration Driven by the Surface Chemistry and Wettability of Dental Implants. J. Appl. Oral Sci. 2015, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Gotfredsen, K.; Wennerberg, A.; Johansson, C.; Skovgaard, L.T.; Hjørting-Hansen, E. Anchorage of TiO2-Blasted, HA-Coated, and Machined Implants: An Experimental Study with Rabbits. J. Biomed. Mater. Res. 1995, 29, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.T.; Nanci, A. Nanotexturing of Titanium-Based Surfaces Upregulates Expression of Bone Sialoprotein and Osteopontin by Cultured Osteogenic Cells. Biomaterials 2004, 25, 403–413. [Google Scholar] [CrossRef]
- Messias, A.; Nicolau, P.; Guerra, F. Titanium Dental Implants with Different Collar Design and Surface Modifications: A Systematic Review on Survival Rates and Marginal Bone Levels. Clin. Oral Implant. Res. 2019, 30, 20–48. [Google Scholar] [CrossRef]
- Meijer, H.J.A.; Raghoebar, G.M.; Van’t Hof, M.A.; Visser, A. A Controlled Clinical Trial of Implant-Retained Mandibular Overdentures: 10 Years’ Results of Clinical Aspects and Aftercare of IMZ Implants and Brånemark Implants. Clin. Oral Implant. Res. 2004, 15, 421–427. [Google Scholar] [CrossRef]
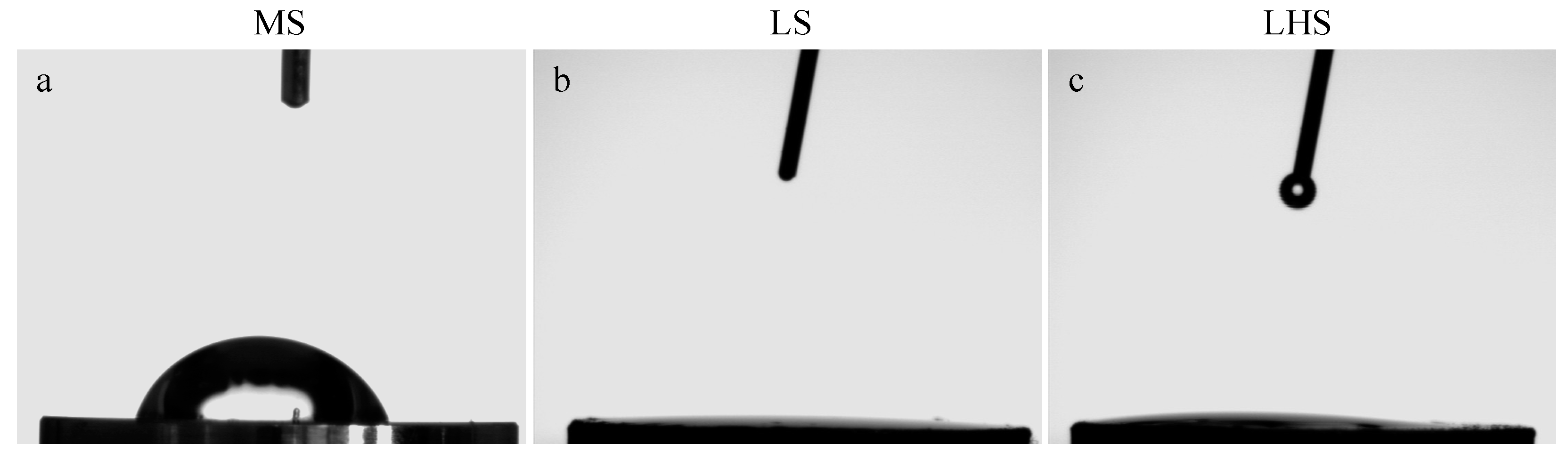

| Surface/Angle | MS | LS | LHS |
|---|---|---|---|
| First review | 52.9° ± 9.58 | 0° | 0° |
| Second review | 57.1° ± 9.58 | 0° | 0° |
| Third review | 71.2° ± 9.58 | 0° | 0° |
| Average | 60.4° ± 5.53 | 0° | 0° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.F.P.; da Silva, R.C.; Hadad, H.; de Jesus, L.K.; Pereira-Silva, M.; Nímia, H.H.; Oliveira, S.H.P.; Guastaldi, A.C.; Queiroz, T.P.; Poli, P.P.; et al. Early Peri-Implant Bone Healing on Laser-Modified Surfaces with and without Hydroxyapatite Coating: An In Vivo Study. Biology 2024, 13, 533. https://doi.org/10.3390/biology13070533
Santos AFP, da Silva RC, Hadad H, de Jesus LK, Pereira-Silva M, Nímia HH, Oliveira SHP, Guastaldi AC, Queiroz TP, Poli PP, et al. Early Peri-Implant Bone Healing on Laser-Modified Surfaces with and without Hydroxyapatite Coating: An In Vivo Study. Biology. 2024; 13(7):533. https://doi.org/10.3390/biology13070533
Chicago/Turabian StyleSantos, Ana Flávia Piquera, Rodrigo Capalbo da Silva, Henrique Hadad, Laís Kawamata de Jesus, Maísa Pereira-Silva, Heloisa Helena Nímia, Sandra Helena Penha Oliveira, Antônio Carlos Guastaldi, Thallita Pereira Queiroz, Pier Paolo Poli, and et al. 2024. "Early Peri-Implant Bone Healing on Laser-Modified Surfaces with and without Hydroxyapatite Coating: An In Vivo Study" Biology 13, no. 7: 533. https://doi.org/10.3390/biology13070533
APA StyleSantos, A. F. P., da Silva, R. C., Hadad, H., de Jesus, L. K., Pereira-Silva, M., Nímia, H. H., Oliveira, S. H. P., Guastaldi, A. C., Queiroz, T. P., Poli, P. P., Barbosa, D. d. B., da Silva Fabris, A. L., Garcia Júnior, I. R., Gruber, R., & Souza, F. Á. (2024). Early Peri-Implant Bone Healing on Laser-Modified Surfaces with and without Hydroxyapatite Coating: An In Vivo Study. Biology, 13(7), 533. https://doi.org/10.3390/biology13070533








