Jaboticaba Peel Extract Attenuates Ovariectomy-Induced Bone Loss by Preserving Osteoblast Activity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ovariectomy and JPE Treatment
2.2. Effect of JPE Treatment on Bone Tissue
2.2.1. µCT analysis
2.2.2. Counting of Osteocytes and Adipocytes
2.2.3. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
2.3. Effect of JPE Treatment on Osteoblast and Adipocyte Differentiation of MSCs
2.3.1. MSC Isolation and Osteoblast and Adipocyte Differentiation
2.3.2. Gene Expression of Osteogenic and Adipogenic Markers
2.3.3. Protein Expression of Osteogenic Markers
2.3.4. Extracellular Matrix Mineralization
2.3.5. Lipidic Accumulation
2.4. Statistical Analyses
3. Results
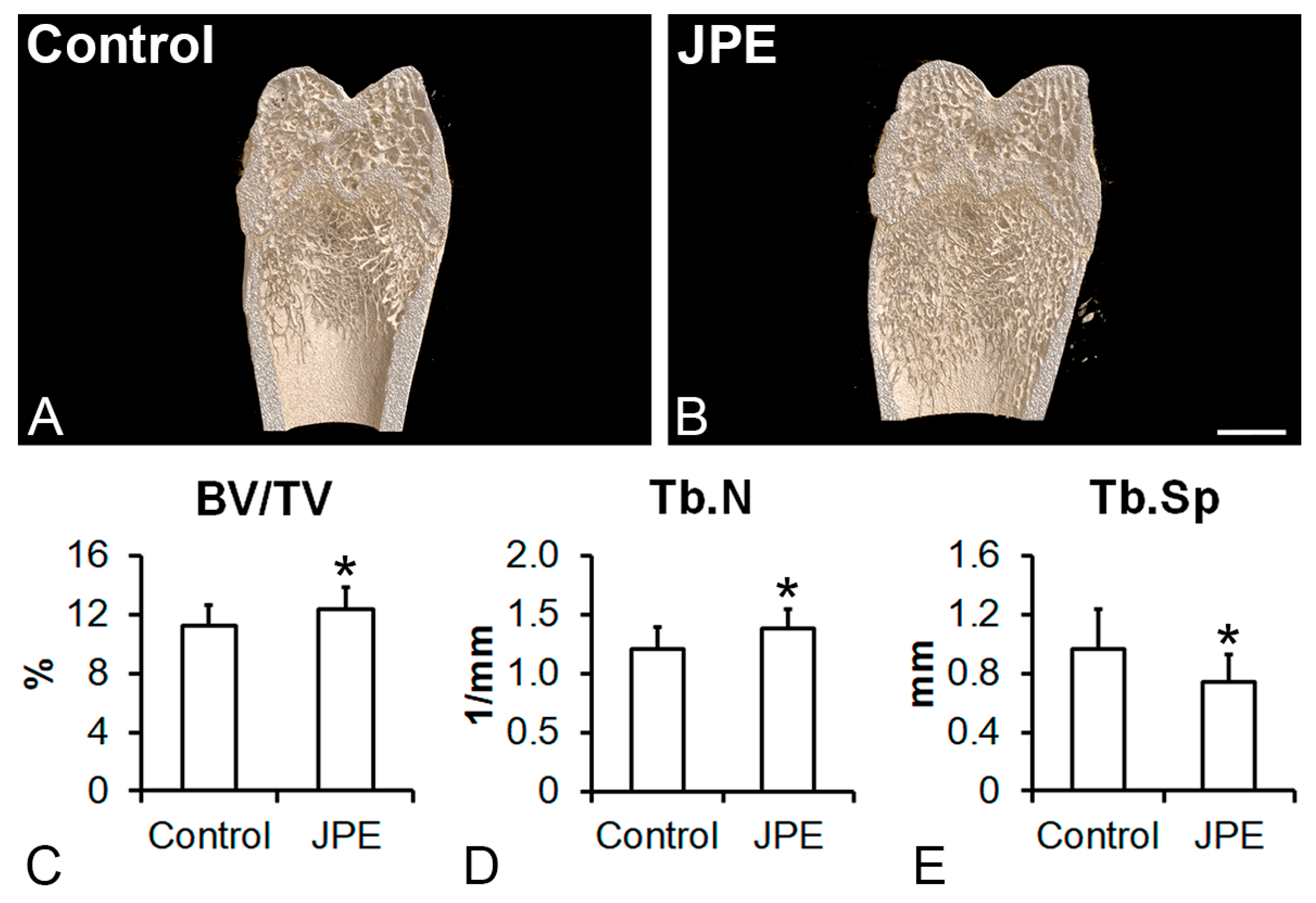
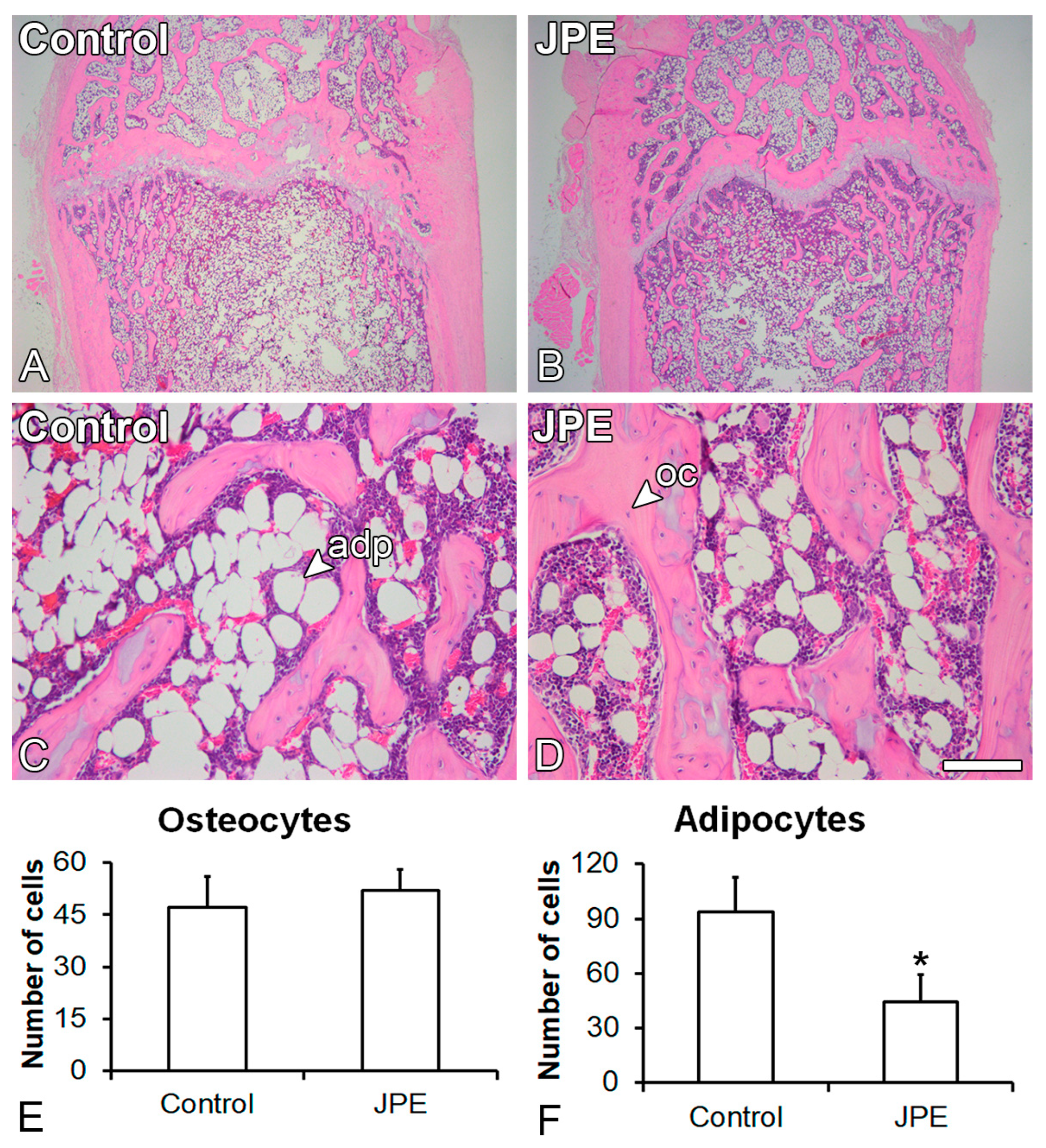
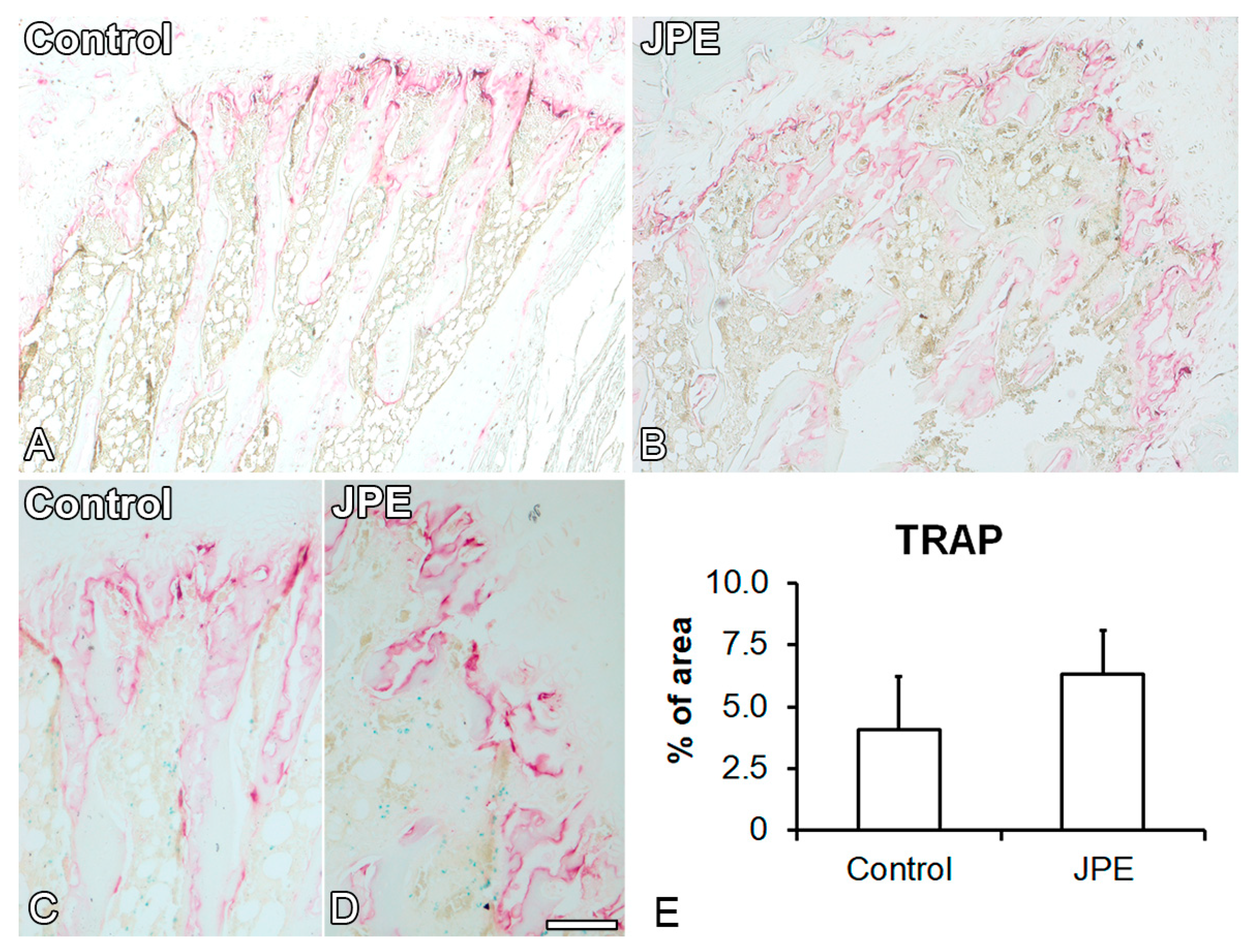
3.1. Effect of JPE Treatment on Bone Tissue
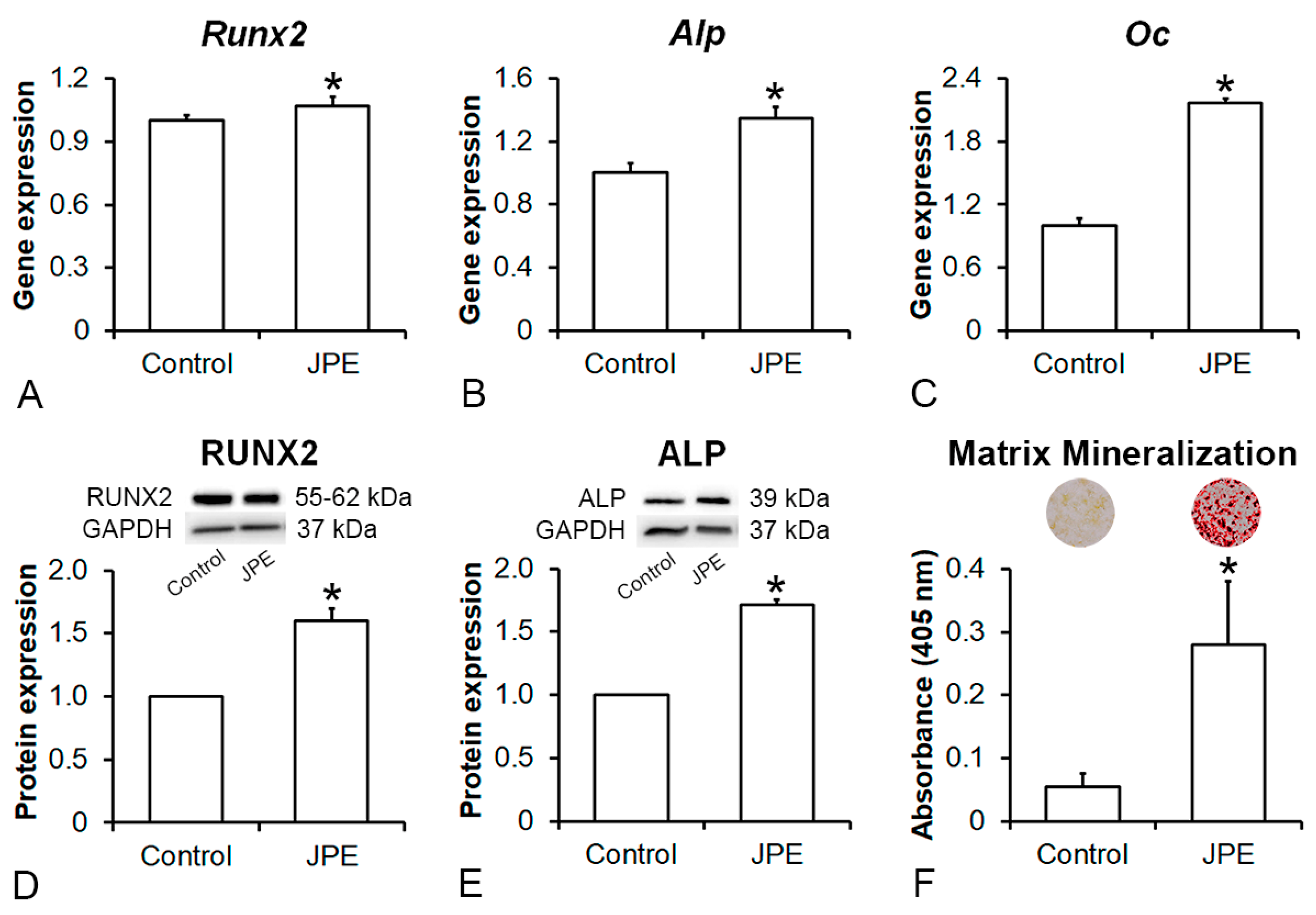
3.2. Effect of JPE Treatment on Osteoblast Differentiation of MSCs
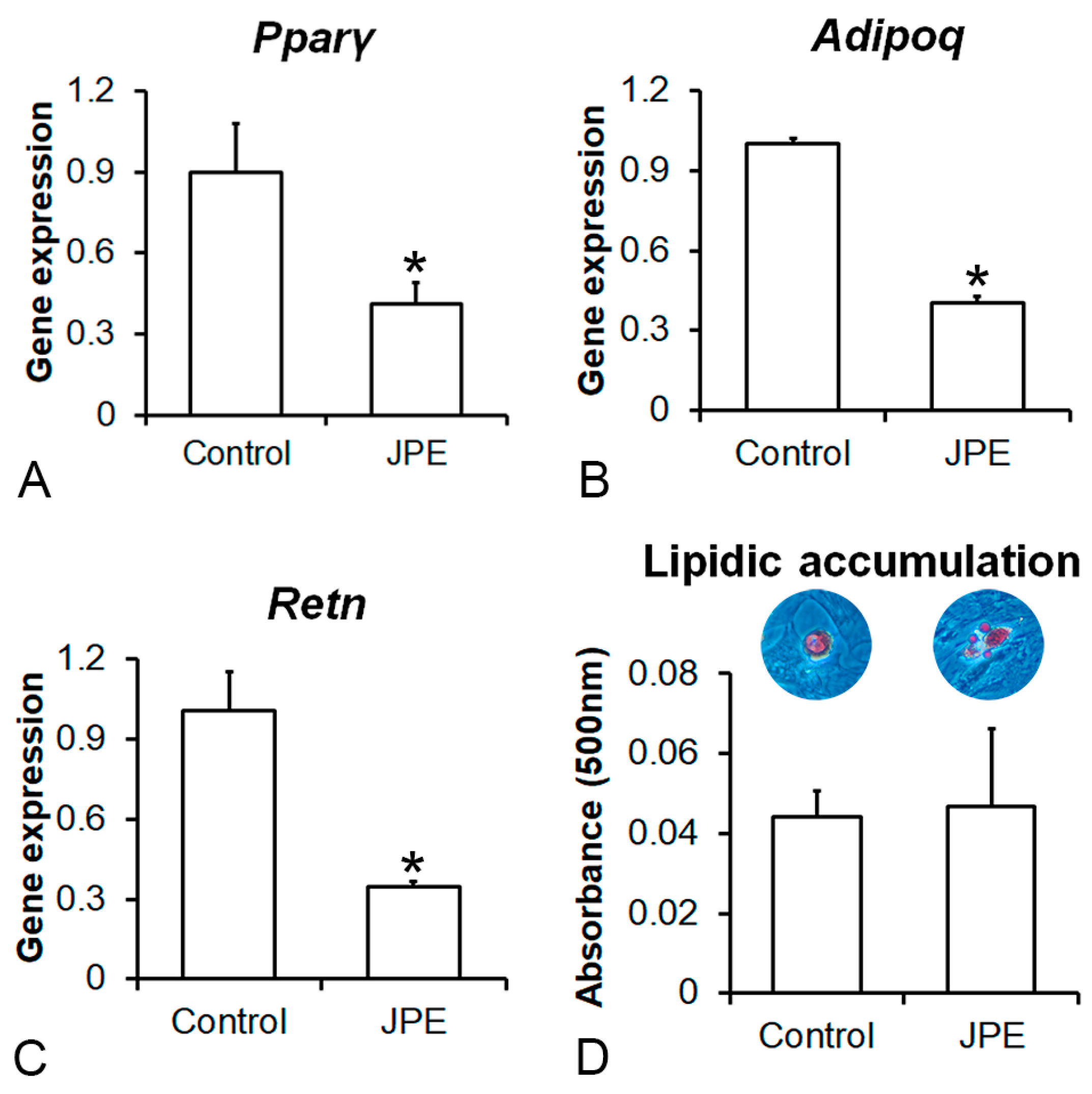
3.3. Effect of JPE Treatment on Adipocyte Differentiation of MSCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Hu, Y.; Guo, Y.; Liu, D. Modulation of bone remodeling by the gut microbiota: A new therapy for osteoporosis. Bone Res. 2023, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Hart, D.M.; Aitken, J.M.; MacDonald, E.B.; Anderson, J.B.; Clarke, A.C. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet 1976, 1, 1038–1041. [Google Scholar] [CrossRef]
- Vilaca, T.; Eastell, R.; Schini, M. Osteoporosis in men. Lancet Diabetes Endocrinol. 2022, 10, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.X.; Majumdar, S.R.; Dick, D.A.; Moreau, M.; Raso, J.; Otto, D.D.; Johnston, D.W. Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J. Bone Miner. Res. 2005, 20, 494–500. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, molecular target, and current status on drug development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Liu, S.; Looker, A.C.; Wallace, T.C.; Wang, D.D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016, 27, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.F.; Marangon, C.A.; Massimino, L.C.; Klingbeil, M.F.G.; Martins, V.C.A.; Plepis, A.M.G. Development of collagen/nanohydroxyapatite scaffolds containing plant extract intended for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111955. [Google Scholar] [CrossRef]
- Vargas-Sanchez, P.K.; Pitol, D.L.; de Sousa, L.G.; Beloti, M.M.; Rosa, A.L.; Rossi, A.C.; Siéssere, S.; Bombonato-Prado, K.F. Green tea extract rich in epigallocatechin gallate impairs alveolar bone loss in ovariectomized rats with experimental periodontal disease. Int. J. Exp. Pathol. 2020, 101, 277–288. [Google Scholar] [CrossRef]
- de Andrade Neves, N.; César Stringheta, P.; Ferreira da Silva, I.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Identification and quantification of phenolic composition from different species of Jabuticaba (Plinia spp.) by HPLC-DAD-ESI/MSn. Food Chem. 2021, 355, 129605. [Google Scholar] [CrossRef]
- Gadioli Tarone, A.; Keven Silva, E.; Dias de Freitas Queiroz Barros, H.; Baú Betim Cazarin, C.; Roberto Marostica Junior, M. High-intensity ultrasound-assisted recovery of anthocyanins from jabuticaba by-products using green solvents: Effects of ultrasound intensity and solvent composition on the extraction of phenolic compounds. Food Res. Int. 2021, 140, 110048. [Google Scholar] [CrossRef] [PubMed]
- Dragano, N.R.V.; Marques, A.C.; Cintra, D.E.C.; Solon, C.; Morari, J.; Leite-Legatti, A.V.; Velloso, L.A.; Maróstica-Júnior, M.R. Freeze-dried jaboticaba peel powder improves insulin sensitivity in high-fat-fed mice. Br. J. Nutr. 2013, 110, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lenquiste, S.A.; de Almeida Lamas, C.; da Silva Marineli, R.; Moraes, É.A.; Borck, P.C.; Camargo, R.L.; Quitete, V.H.A.C.; Carneiro, E.M.; Junior, M.R.M. Jaboticaba peel powder and jaboticaba peel aqueous extract reduces obesity, insulin resistance and hepatic fat accumulation in rats. Food Res. Int. 2019, 120, 880–887. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Maia, J.K.; Batista, A.G.; Cazarin, C.B.B.; Soares, E.S.; Bogusz Junior, S.; Leal, R.F.; da Cruz-Höfling, M.A.; Maróstica Junior, M.R. Aqueous extract of brazilian berry (Myrciaria jaboticaba) peel improves inflammatory parameters and modulates Lactobacillus and Bifidobacterium in rats with induced-colitis. Nutrients 2019, 11, 2776. [Google Scholar] [CrossRef]
- Nogueira-Lima, E.; Lamas, C.A.; Baseggio, A.M.; do Vale, J.S.F.; Maróstica Junior, M.R.; Cagnon, V.H.A. High-fat diet effects on the prostatic adenocarcinoma model and jaboticaba peel extract intake: Protective response in metabolic disorders and liver histopathology. Nutr. Cancer 2020, 72, 1366–1377. [Google Scholar] [CrossRef]
- Luz, R.F.; Ferreira, R.D.R.; Silva, C.N.S.; Miranda, B.M.; Piccoli, R.H.; Silva, M.S.; Paula, L.C.; Leles, M.I.G.; Fernandes, K.F.; Cruz, M.V.; et al. Development of a halochromic, antimicrobial, and antioxidant starch-based film containing phenolic extract from jaboticaba peel. Foods 2023, 12, 653. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Y.; Dou, C.; Dong, S. Microenvironment in subchondral bone: Predominant regulator for the treatment of osteoarthritis. Ann. Rheum. Dis. 2021, 80, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Gu, D.R.; So, H.S.; Kim, K.J.; Lee, S.H. Dual role of cyanidin-3-glucoside on the differentiation of bone cells. J. Dent. Res. 2015, 94, 1676–1683. [Google Scholar] [CrossRef]
- Souza, A.T.P.; Freitas, G.P.; Lopes, H.B.; Totoli, G.G.C.; Tarone, A.G.; Marostica-Junior, M.R.; Rosa, A.L.; Beloti, M.M. Jabuticaba peel extract modulates adipocyte and osteoblast differentiation of MSCs from healthy and osteoporotic rats. J. Bone Miner. Metab. 2021, 39, 163–173. [Google Scholar] [CrossRef]
- Kalu, D.N. Evaluation of the pathogenesis of skeletal changes in ovariectomized rats. Endocrinology 1984, 115, 507–512. [Google Scholar] [CrossRef]
- Freitas, G.P.; Souza, A.T.P.; Lopes, H.B.; Trevisan, R.L.B.; Oliveira, F.S.; Fernandes, R.R.; Ferreira, F.U.; Ros, F.A.; Beloti, M.M.; Rosa, A.L. Mesenchymal stromal cells derived from bone marrow and adipose tissue: Isolation, culture, characterization and differentiation. Bio-Protocol 2020, 10, e3534. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.J.; Chih-Hsing Wu, P.; Bergin, D. Risk assessment tools for osteoporosis and fractures in 2022. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101775. [Google Scholar] [CrossRef] [PubMed]
- Tarone, A.G.; Goupy, P.; Ginies, C.; Marostica Junior, M.R.; Dufour, C. Advanced characterization of polyphenols from Myrciaria jaboticaba peel and lipid protection in in vitro gastrointestinal digestion. Food Chem. 2021, 359, 129959. [Google Scholar] [CrossRef] [PubMed]
- Cladis, D.P.; Swallow, E.A.; Allen, M.R.; Hill Gallant, K.M.; Weaver, C.M. Blueberry polyphenols do not improve bone mineral density or mechanical properties in ovariectomized rats. Calcif. Tissue Int. 2022, 110, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Vashishth, D. Advanced glycation and glycoxidation end products in bone. Bone 2023, 176, 116880. [Google Scholar] [CrossRef] [PubMed]
- LLabre, J.E.; Gil, C.; Amatya, N.; Lagalwar, S.; Possidente, B.; Vashishth, D. Degradation of bone quality in a transgenic mouse model of Alzheimer’s disease. J. Bone Miner. Res. 2022, 37, 2548–2565. [Google Scholar] [CrossRef] [PubMed]
- Kaume, L.; Gilbert, W.; Smith, B.J.; Devareddy, L. Cyanidin 3-O-β-D-glucoside improves bone indices. J. Med. Food 2015, 18, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Constanze, B.; Popper, B.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-β suppresses osteoblast differentiation of mesenchymal stem cells and resveratrol reverses it through modulation of NF-κB, Sirt1 and Runx2. Cell Tissue Res. 2020, 381, 83–98. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Falsetti, I.; Bilia, A.R.; Vincenzini, M.T.; Brandi, M.L.; Iantomasi, T. Blueberry juice antioxidants protect osteogenic activity against oxidative stress and improve long-term activation of the mineralization process in human osteoblast-like SaOS-2 cells: Involvement of SIRT1. Antioxidants 2020, 9, 125. [Google Scholar] [CrossRef]
- He, N.; Zhu, X.; He, W.; Zhao, S.; Zhao, W.; Zhu, C. Resveratrol inhibits the hydrogen dioxide-induced apoptosis via Sirt 1 activation in osteoblast cells. Biosci. Biotechnol. Biochem. 2015, 79, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Zhang, L.; Yang, X. Ferulic acid, a natural polyphenol, protects against osteoporosis by activating SIRT1 and NF-κB in neonatal rats with glucocorticoid-induced osteoporosis. Biomed. Pharmacother. 2019, 120, 109205. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.L.; Sharma, A.K.; Roberts, R.L.; Yu, K.; Irsik, D.L.; Choudhary, V.; Dorn, J.S.; Bensreti, H.; Benson, R.D., Jr.; Kaiser, H.; et al. The glucocorticoid receptor in osterix-expressing cells regulates bone mass, bone marrow adipose tissue, and systemic metabolism in female mice during aging. J. Bone Miner. Res. 2022, 37, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, C.S.; Johncola, A.J.; Batzdorf, A.S.; Jones, B.C.; Al Mukaddam, M.; Sexton, K.; Shults, J.; Leonard, M.B.; Snyder, P.J.; Wehrli, F.W. Effect of low-intensity vibration on bone strength, microstructure, and adiposity in pre-osteoporotic postmenopausal women: A randomized placebo-controlled trial. J. Bone Miner. Res. 2021, 36, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Trindade, P.L.; Soares, E.D.R.; Inada, K.O.P.; Martins, F.F.; Rudnicki, M.; Perrone, D.; Monteiro, M.; Souza-Mello, V.; Daleprane, J.B. Consumption of phenolic-rich jabuticaba (Myrciaria jaboticaba) powder ameliorates obesity-related disorders in mice. Br. J. Nutr. 2022, 127, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Puga, C.; Torre-Villalvazo, I.; Noriega, L.G.; Rodríguez-López, L.A.; Alemán, G.; Torre-Anaya, E.A.; Cariño-Cervantes, Y.Y.; Palacios-Gonzalez, B.; Furuzawa-Carballeda, J.; Tovar, A.R.; et al. Pecans and its polyphenols prevent obesity, hepatic steatosis and diabetes by reducing dysbiosis, inflammation, and increasing energy expenditure in mice fed a high-fat diet. Nutrients 2023, 15, 2591. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tao, K.T.; Lin, J.; Liu, P.; Guan, Z.; Deng, J.; Wang, D.; Zeng, H. The role of m6A in osteoporosis and the differentiation of mesenchymal stem cells into osteoblasts and adipocytes. Curr. Stem Cell Res. Ther. 2023, 18, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Suo, J.; Zou, S.; Wang, J.; Han, Y.; Zhang, L.; Lv, C.; Jiang, B.; Ren, Q.; Chen, L.; Yang, L.; et al. The RNA-binding protein Musashi2 governs osteoblast-adipocyte lineage commitment by suppressing PPARγ signaling. Bone Res. 2022, 10, 31. [Google Scholar] [CrossRef]
- Abuna, R.P.F.; Almeida, L.O.; Souza, A.T.P.; Fernandes, R.R.; Sverzut, T.F.V.; Rosa, A.L.; Beloti, M.M. Osteoporosis and osteoblasts cocultured with adipocytes inhibit osteoblast differentiation by downregulating histone acetylation. J. Cell. Physiol. 2021, 236, 3906–3917. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adolpho, L.F.; Gomes, M.P.O.; Freitas, G.P.; Bighetti-Trevisan, R.L.; Ramos, J.I.R.; Campeoti, G.H.; Zatta, G.C.; Almeida, A.L.G.; Tarone, A.G.; Marostica-Junior, M.R.; et al. Jaboticaba Peel Extract Attenuates Ovariectomy-Induced Bone Loss by Preserving Osteoblast Activity. Biology 2024, 13, 526. https://doi.org/10.3390/biology13070526
Adolpho LF, Gomes MPO, Freitas GP, Bighetti-Trevisan RL, Ramos JIR, Campeoti GH, Zatta GC, Almeida ALG, Tarone AG, Marostica-Junior MR, et al. Jaboticaba Peel Extract Attenuates Ovariectomy-Induced Bone Loss by Preserving Osteoblast Activity. Biology. 2024; 13(7):526. https://doi.org/10.3390/biology13070526
Chicago/Turabian StyleAdolpho, Letícia Faustino, Maria Paula Oliveira Gomes, Gileade Pereira Freitas, Rayana Longo Bighetti-Trevisan, Jaqueline Isadora Reis Ramos, Gabriela Hernandes Campeoti, Guilherme Crepi Zatta, Adriana Luisa Gonçalves Almeida, Adriana Gadioli Tarone, Mario Roberto Marostica-Junior, and et al. 2024. "Jaboticaba Peel Extract Attenuates Ovariectomy-Induced Bone Loss by Preserving Osteoblast Activity" Biology 13, no. 7: 526. https://doi.org/10.3390/biology13070526
APA StyleAdolpho, L. F., Gomes, M. P. O., Freitas, G. P., Bighetti-Trevisan, R. L., Ramos, J. I. R., Campeoti, G. H., Zatta, G. C., Almeida, A. L. G., Tarone, A. G., Marostica-Junior, M. R., Rosa, A. L., & Beloti, M. M. (2024). Jaboticaba Peel Extract Attenuates Ovariectomy-Induced Bone Loss by Preserving Osteoblast Activity. Biology, 13(7), 526. https://doi.org/10.3390/biology13070526









