Biosynthesis and Pharmacological Activities of the Bioactive Compounds of White Mulberry (Morus alba): Current Paradigms and Future Challenges
Abstract
Simple Summary
Abstract
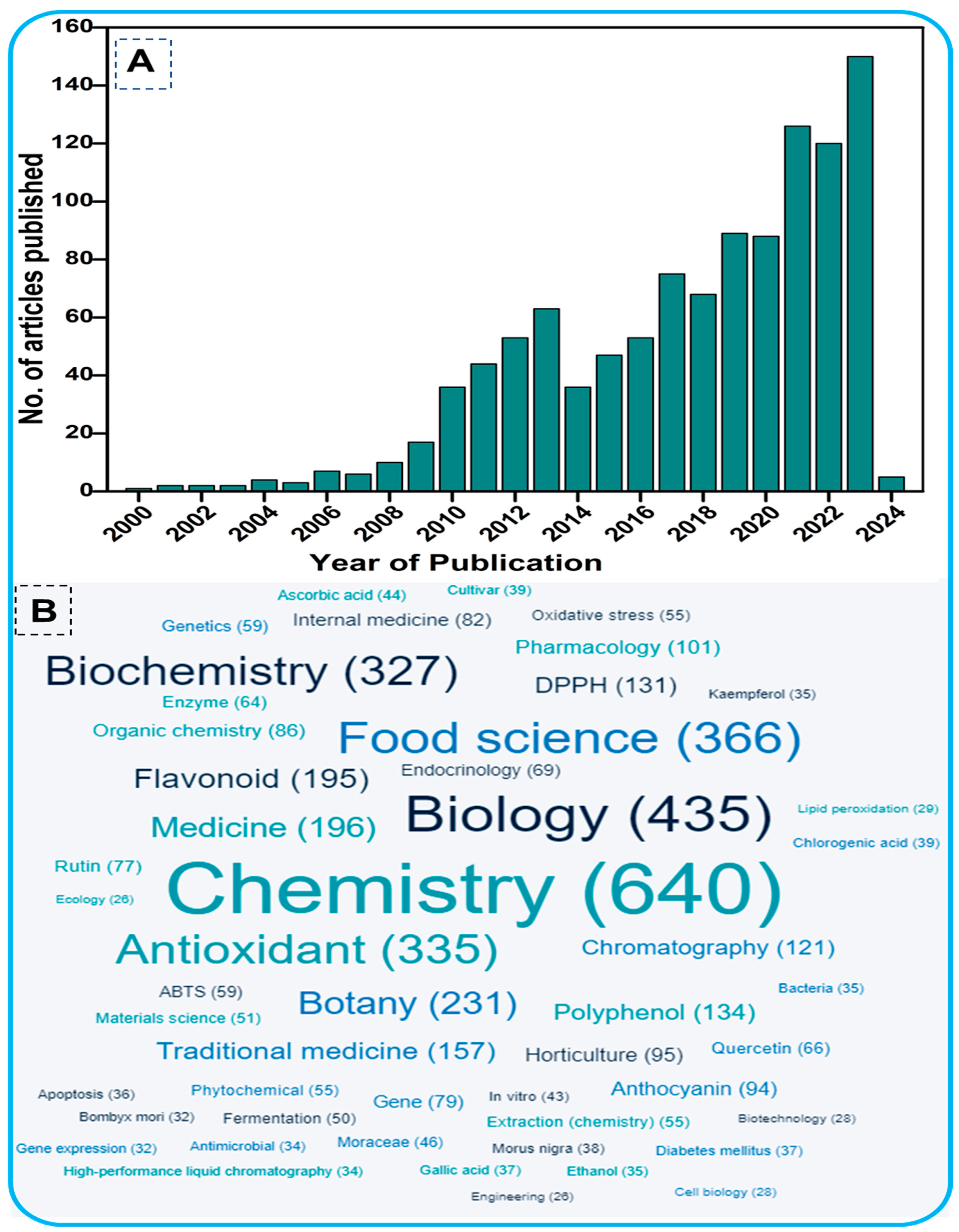
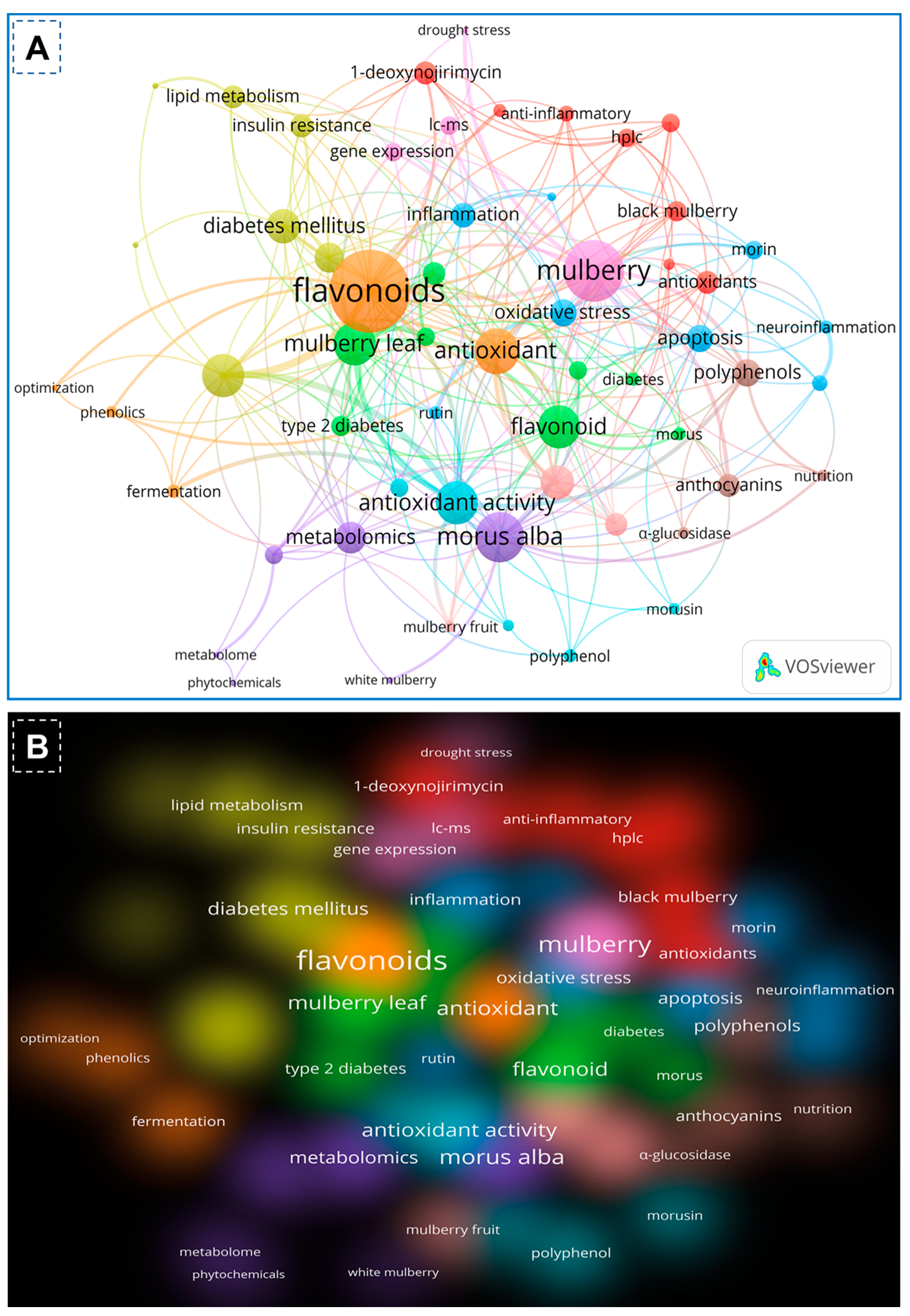
1. Introduction
2. Methodology
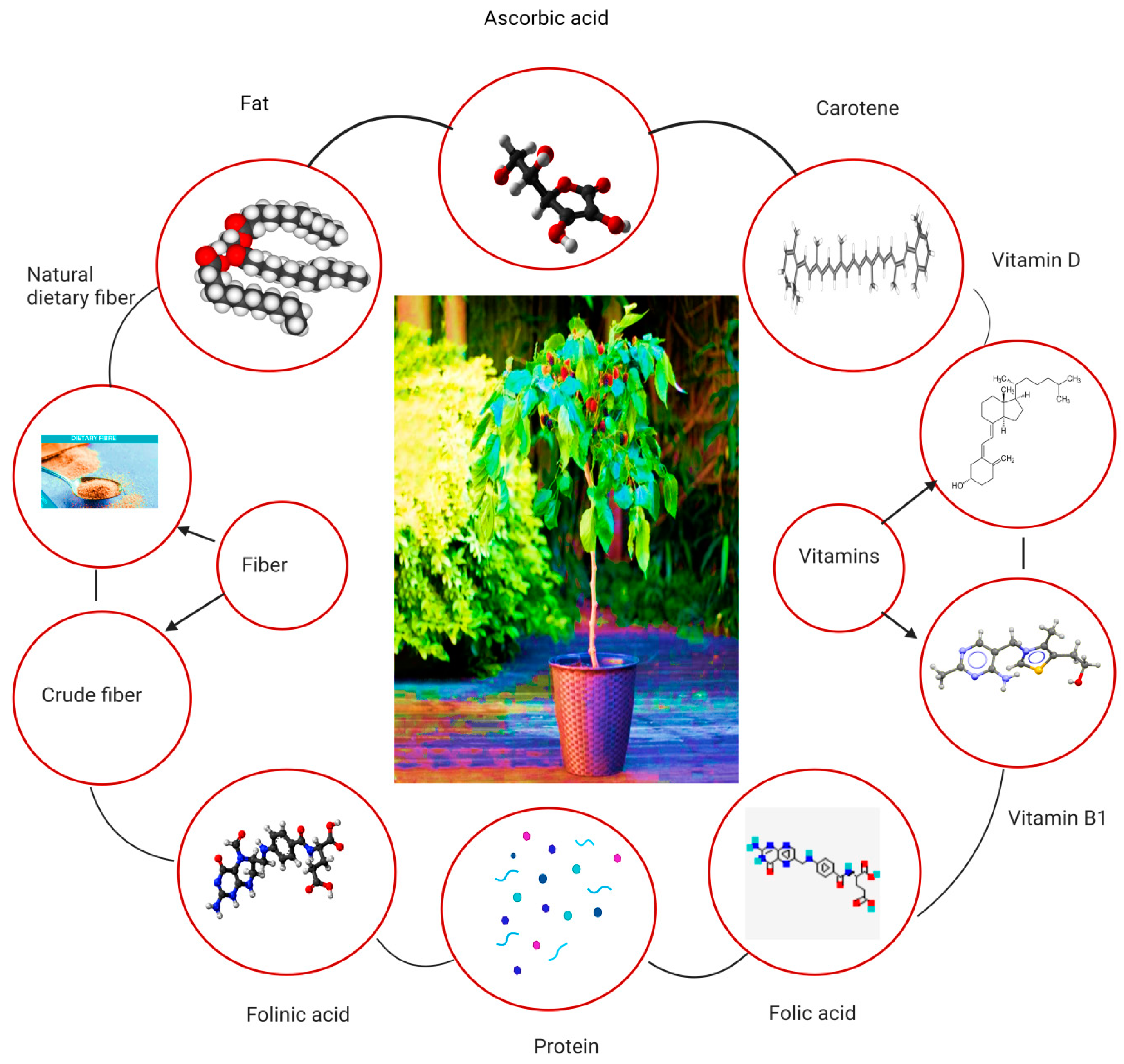
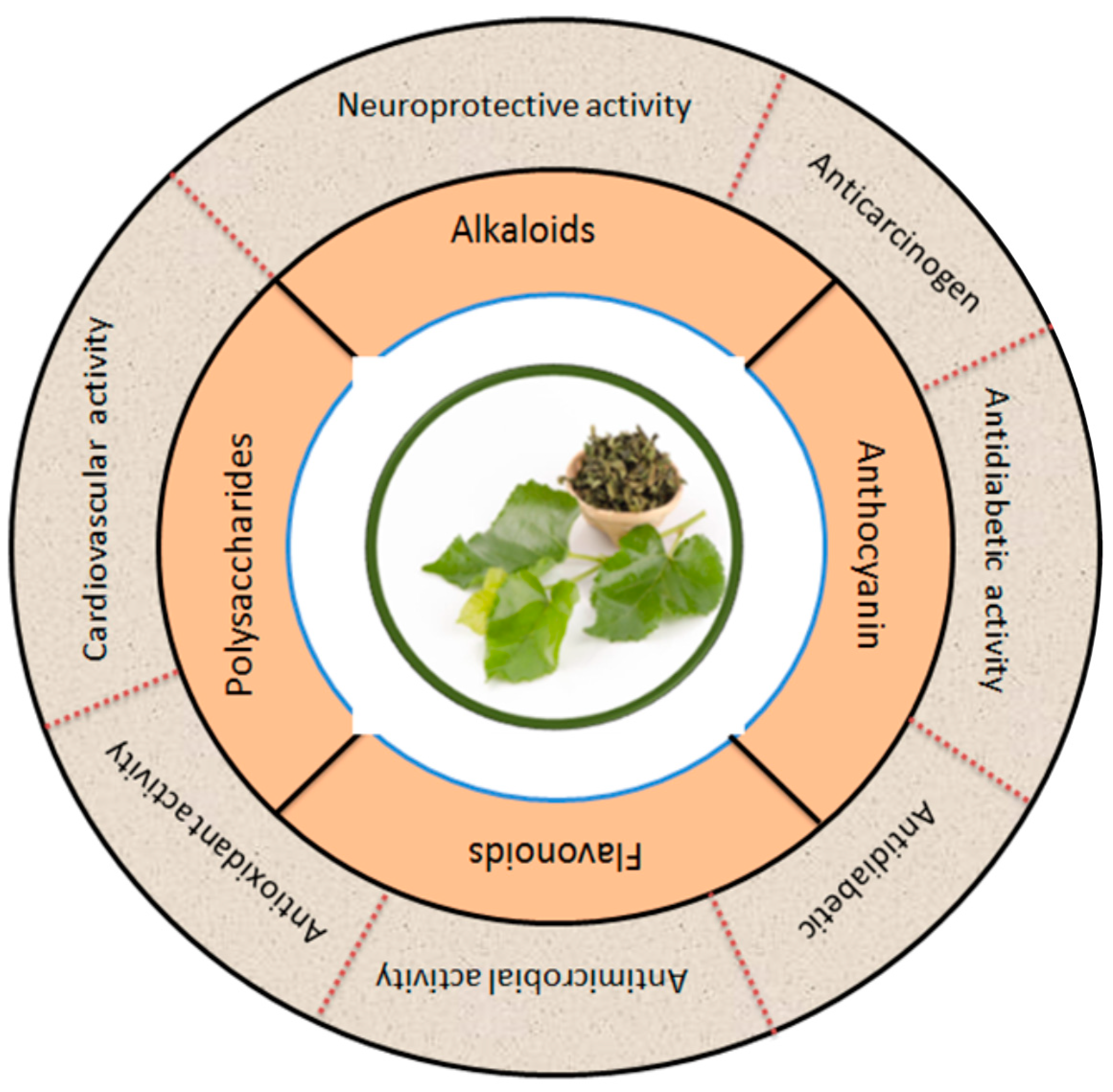
3. Phytochemistry and Nutritional Values of Mulberry Leaves
3.1. Flavonoids
3.2. Alkaloids
3.3. Anthocyanins
3.4. Polysaccharides
3.5. Amino Acids
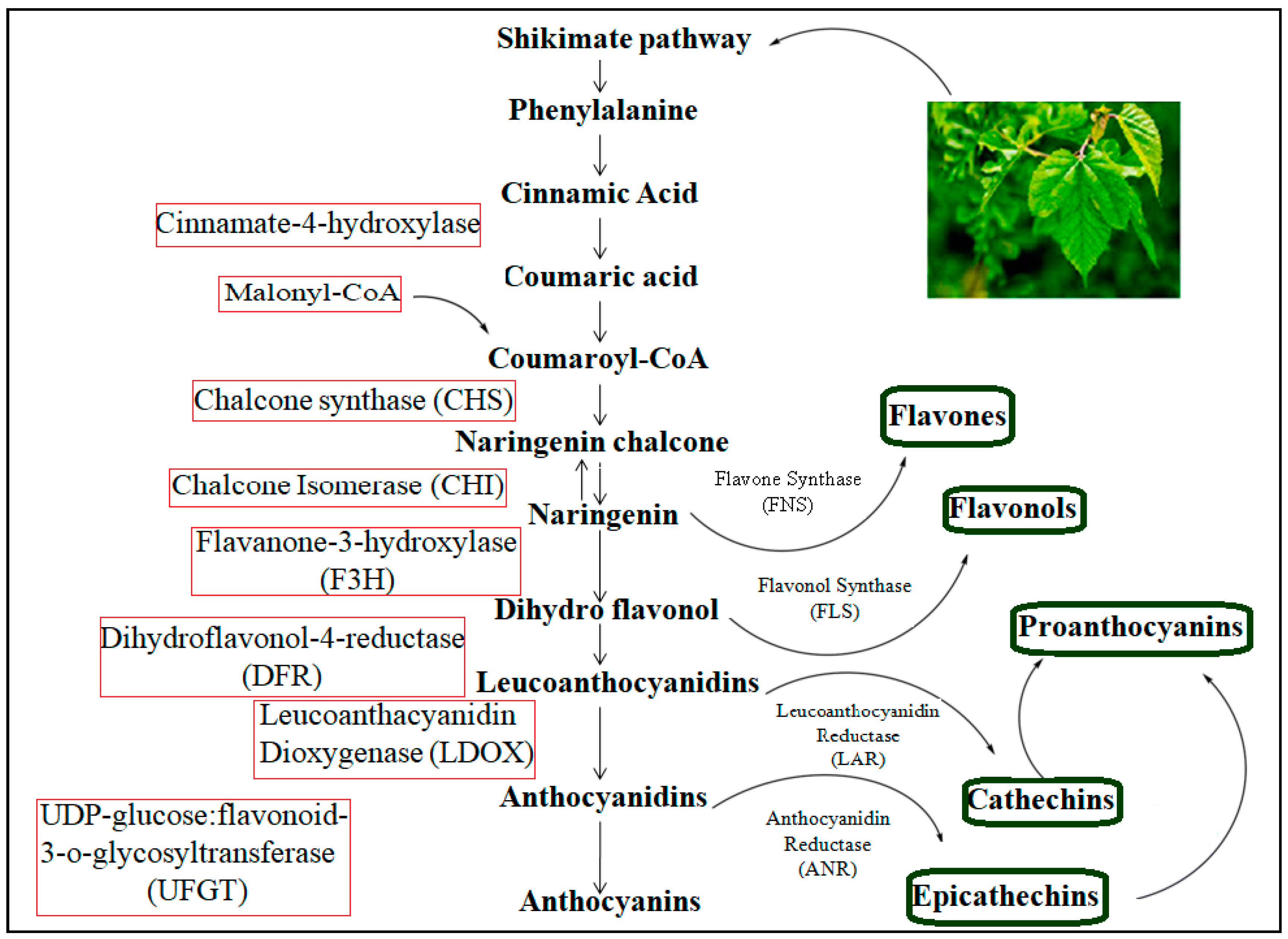
4. Biosynthetic Pathway for Flavonoids
4.1. Related Functional Genes in the Biosynthetic Pathway of Flavonoids in Mulberry
4.2. Factors Effecting the Biosynthesis of Flavonoids in Mulberry
4.2.1. Effect of Temperature on Flavonoid Biosynthesis
4.2.2. Effect of Light
4.2.3. Effect of Water Availability
5. Pharmacological Activities of Mulberry Leaves
5.1. Antioxidant Effects of Mulberry Leaves
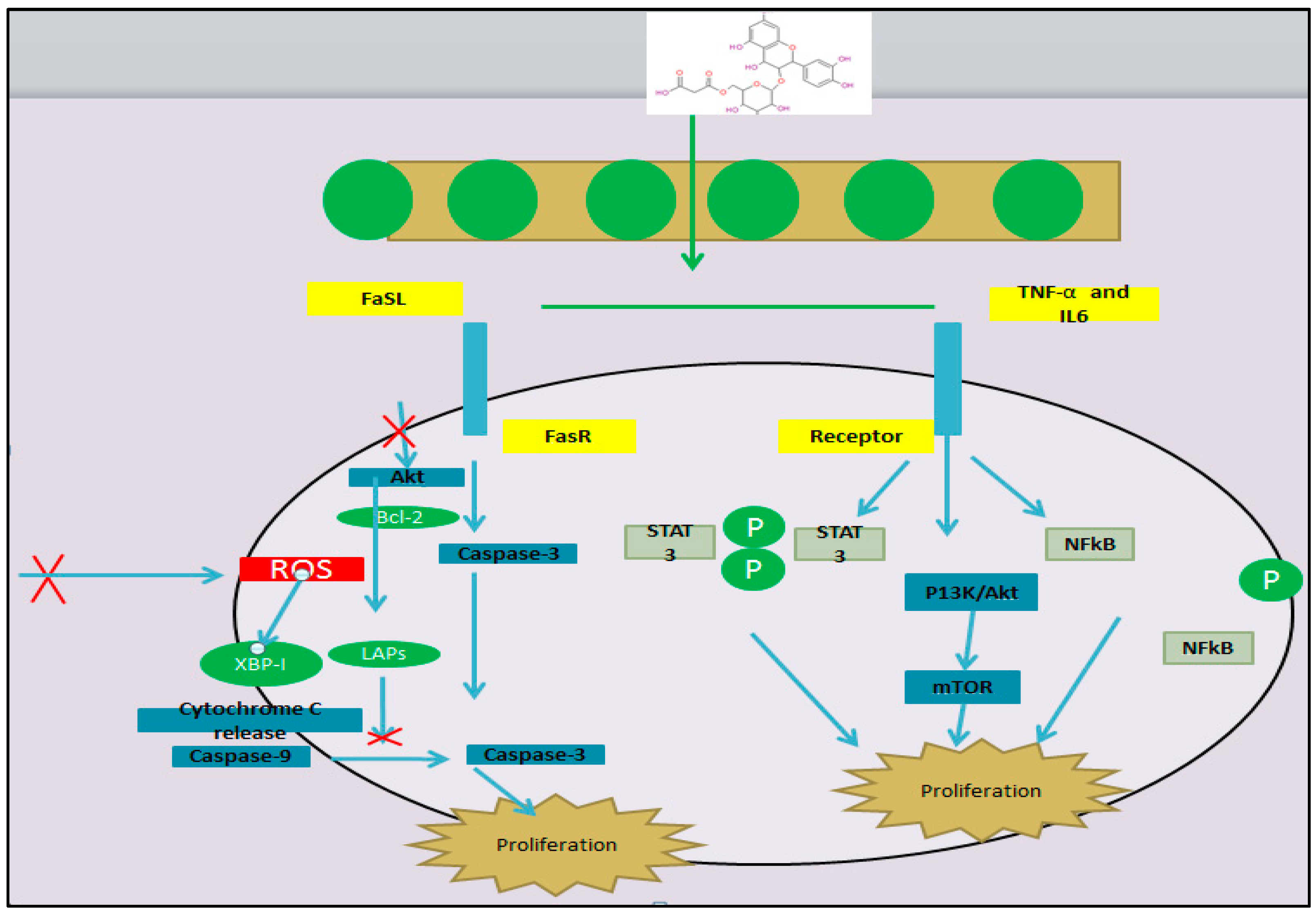
5.2. Anti-Carcinogenesis by Mulberry Leaf Extracts
5.3. Antidiabetic Activity
5.4. Antimicrobial Activities of Mulberry Leaves
5.5. Cardiovascular Activity
5.6. Neuroprotective Activity
| Type of Activity | Method of Extraction | Activity Unit | Model Cell/Animal Used | Bioactive Compound | Reference(s) |
|---|---|---|---|---|---|
| Antioxidant activity | Ethyl acetate | DPPF radical scavenging and reducing activity | Ferric reducing power | Maclurin, rutin, isoquercitin, resveratrol and morin | [128] |
| Antihyperlipidemic activity | Ethanol | ED50 | Rats | Mulberroside A (MUL) | [129,130] |
| Antimicrobial activity | Ethanol | MIC Viable Cell Count | Pathogenic bacteria: P.aeruginosa, E. coli, B. subtilis, S. mutans, S. sanguis, S. sobrinus | Kunwanon G | [131] |
| Neuroprotective activity | Ethanol | MTT assay | Foot shock induced aggression Water maze test | Isobavachalcone, morachalcone B, moracin N and morachalcone A | [132,133] |
| Anticancer activity | Ethyl acetate | IC50 | Hepatocellular carcinoma cells, hepatoma cells | Morushalunin, chalcomoracin guangsangon E, and kuwanon J | [88,100,134] |
| Antidiabetic activity | Ethanol | HbA1c levels | Rats | sanggenon C, morin, Kuwanon G, morusin, kaempferol, rutin, quercetin, isoquercitrin, 1-deoxynojirimycin | [135] |
| Anti-obesity activity | Water | Melanin-concentrating hormone receptor subtype 1 (MCH1) | Mice | Chlorogenic acid and Quercitrin | [136] |
| Tyrosinase inhibitory activity/skin whitening activity | Methanol | SOD | Melanin formation in melan A cells | Mulberroside F | [137] |
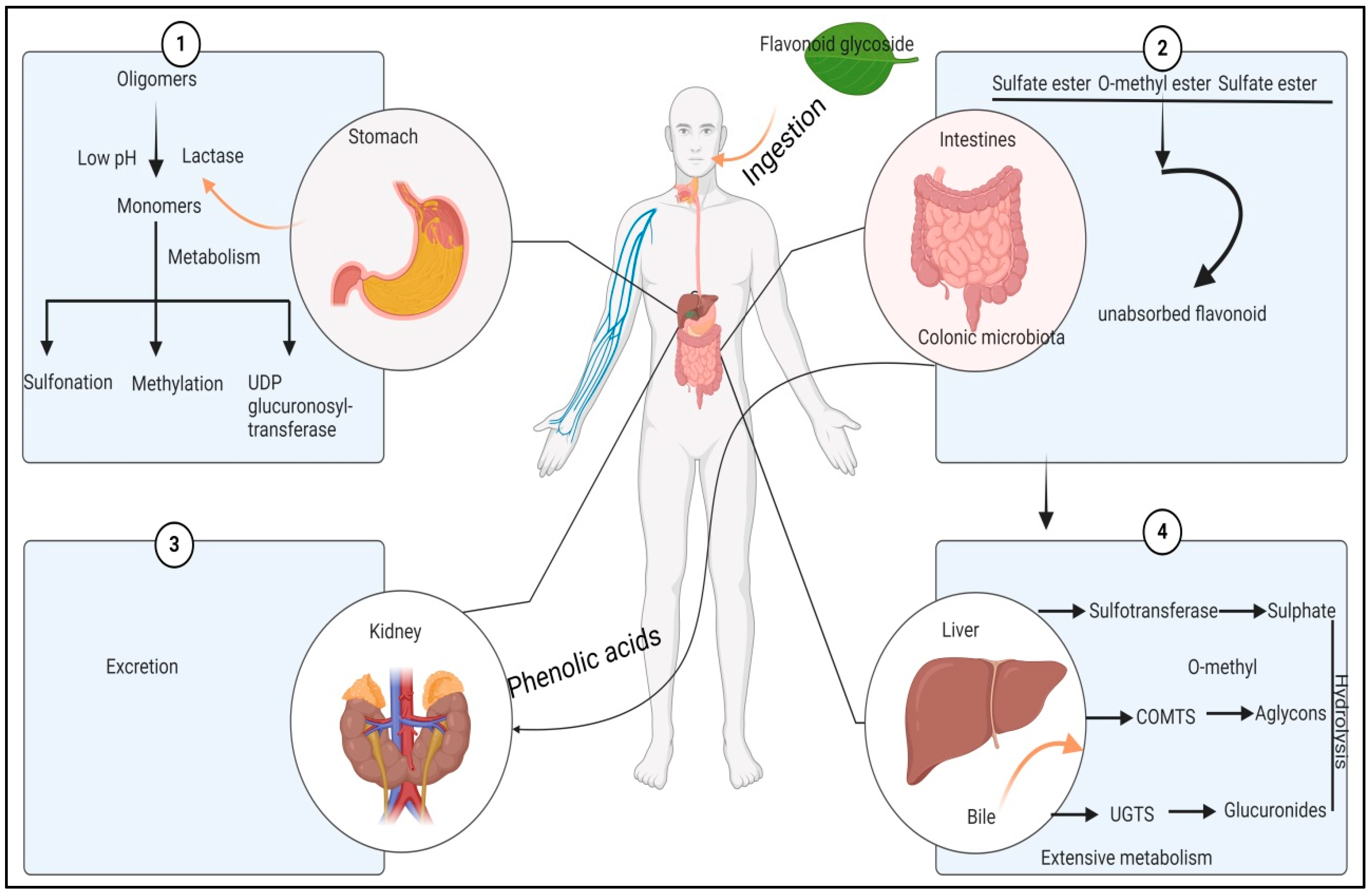
6. Methods for Extraction of Bioactive Compounds from Mulberry Leaves
7. Toxicological Impacts
8. Conclusions
9. Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Dadhwal, R.; Banerjee, R. Ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological profile of Morus alba L.: A comprehensive review. S. Afr. J. Bot. 2023, 158, 98–117. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Al-Snafi, A.E.; Thuwaini, M.M.; Teibo, J.O.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; et al. Morus alba: A comprehensive phytochemical and pharmacological review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ruan, J.; Huang, P.; Sun, F.; Zheng, D.; Zhang, Y.; Wang, T. The structure–activity relationship review of the main bioactive constituents of Morus genus plants. J. Nat. Med. 2020, 74, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Imawaka, N.; Kawano, Y.; Yamazaki, Y.; Shiwaku, K.; Yamane, Y. Antioxidant flavonol glycosides in mulberry (Morus alba L.) leaves isolated based on LDL antioxidant activity. Food Chem. 2006, 97, 25–31. [Google Scholar] [CrossRef]
- Chen, J.J.; Li, X.R. Hypolipidemic effect of flavonoids from mulberry leaves in triton WR-1339 induced hyperlipidemic mice. Asia Pac. J. Clin. Nutr. 2007, 16, 290–294. [Google Scholar] [PubMed]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.-L.; Aligiannis, N. Anti-Melanogenic Properties of Greek Plants. A Novel Depigmenting Agent from Morus alba Wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Masood, S.; Choudhary, M.I. Genus Morus: A source of universal therapeutic agents. Trop. J. Pharm. Res. 2017, 16, 963–971. [Google Scholar]
- Kaur, R.; Bhatia, R.; Pandey, A.; Jain, R. Mulberry (Morus spp.): A synopsis. J. Saudi Soc. Agric. Sci. 2019, 18, 207–217. [Google Scholar]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Zheng, Z.; Guo, Y.; Fu, Y.; Luo, F.; Zeng, B.; Li, T. Comparative analysis of the chemical constituents of white, red, and black mulberry. Int. J. Food Prop. 2016, 19, 1317–1327. [Google Scholar]
- Srivastava, S.; Kapoor, R.; Thathola, A.; Srivastava, R.P. Mulberry (Morus alba) leaves as human food: A new dimension of sericulture. Int. J. Food Sci. Nutr. 2003, 54, 411–416. [Google Scholar] [CrossRef]
- Kimura, T.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.C.; Ju, W.T.; Kim, H.B.; Sung, G.B.; Kim, Y.S. UPLC-DADQTOF/MS analysis of flavonoids from 12 varieties of Korean mulberry fruit. J. Food Qual. 2019. [Google Scholar] [CrossRef]
- Sugiyama, M.; Katsube, T.; Koyama, A.; Itamura, H. Seasonal Changes in Functional Component Contents in Mulberry (Morus alba L.) Leaves. Hortic. J. 2017, 86, 534–542. [Google Scholar] [CrossRef]
- Marchetti, L.; Saviane, A.; Montà, A.d.; Paglia, G.; Pellati, F.; Benvenuti, S.; Bertelli, D.; Cappellozza, S. Determination of 1-Deoxynojirimycin (1-DNJ) in Leaves of Italian or Italy-Adapted Cultivars of Mulberry (Morus sp.pl.) by HPLC-MS. Plants 2021, 10, 1553. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, F.; Tang, C.; Xiao, G.; Li, Z.; Luo, G. Quantitative determination of 1-deoxynojirimycin in 146 varieties of mulberry fruit. Int. J. Food Prop. 2021, 24, 1214–1221. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Sledzinski, T.; Goyke, E.; Wesolowski, M.; Viapiana, A. A comparative study on the phenolic composition and biological activities of Morus alba L. commercial samples. Molecules 2019, 24, 3082. [Google Scholar] [CrossRef]
- Chen, Z.; Du, X.; Yang, Y.; Cui, X.; Zhang, Z.; Li, Y. Comparative study of chemical composition and active components against α-glucosidase of various medicinal parts of Morus alba L. Biomed. Chromatogr. 2018, 32, e4328. [Google Scholar] [CrossRef]
- Memete, A.R.; Timar, A.V.; Vuscan, A.N.; Miere, F.; Venter, A.C.; Vicas, S.I. Phytochemical Composition of Different Botanical Parts of Morus Species, Health Benefits and Application in Food Industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Eruygur, N.; Dural, E. Determination of 1-deoxynojirimycin by a developed and validated HPLC-FLD method and assessment of in-vitro antioxidant, α-amylase and α-glucosidase inhibitory activity in mulberry varieties from Turkey. Phytomedicine 2019, 53, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Flaczyk, E.; Kobus-Cisowska, J.; Przeor, M.; Korczak, J.; Remiszewski, M.; Korbas, E.; Buchowski, M. Chemical characterization and antioxidative properties of Polish variety of Morus alba L. leaf aqueous extracts from the laboratory and pilot-scale processes. Agric. Sci. 2013, 4, 141–147. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Sirajuddin Chan, K.W.; Sarfraz, R.A.; Uddin, K. Proximate composition and antioxidant potential of leaves from three varieties of mulberry (Morus sp.): A comparative study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Chang, B.Y.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Pyrrole alkaloids from the fruits of Morus alba. Bioorgan. Med. Chem. Lett. 2014, 24, 5656–5659. [Google Scholar] [CrossRef]
- Sanchez-Salcedo, E.M.; Mena, P.; Garcia-Viguera, C.; Martinez, J.J.; Hernandez, F. Phytochemical evaluation of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits: A starting point for the assessment of their beneficial properties. J. Funct. Foods 2015, 12, 399–408. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Xu, B.; Zeng, G.; Tan, J.; He, X.; Hu, C.; Zhou, Y. Chemical constituents of Morus alba L. and their inhibitory effect on 3T3-L1 preadipocyte proliferation and differentiation. Fitoterapia 2014, 98, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Łochyńska, M.; Oleszak, G. Multi-use of the white mulberry (Morus alba L.). Ecol. Quest. 2011, 15, 91–95. [Google Scholar] [CrossRef][Green Version]
- Hunyadi, A.; Herke, I.; Veres, K.; Erdei, A.; Simon, A.; Tothb, G. Volatile glycosides from the leaves of Morus alba with a potential contribution to the complex anti-diabetic activity. Nat. Prod. Commun. 2014, 9, 145–147. [Google Scholar] [CrossRef]
- Sanchez-Salcedo, E.M.; Tassotti, M.; Rio, D.D.; Hernandez, F.; Martinez, J.J.; Mena, P. (Poly)phenolic fingerprint and chemometric analysis of white (Morus alba L.) and black (Morus nigra L.) mulberry leaves by using a non-targeted UHPLC-MS approach. Food Chem. 2016, 212, 250–255. [Google Scholar] [CrossRef]
- Hong, H.C.; Li, S.L.; Zhang, X.Q.; Ye, W.-C.; Zhang, Q.-W. Flavonoids with α-glucosidase inhibitory activities and their contents in the leaves of Morus atropurpurea. Chin. Med. 2013, 81, 19. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; EI-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-H.; Tsai, M.-C.; Wang, C.-C.; Wu, S.-W.; Chang, Y.-J.; Wu, C.-H.; Wang, C.-J. Mulberry Leaf Polyphenol Extract and Rutin Induces Autophagy Regulated by p53 in Human Hepatoma HepG2 Cells. Pharmaceuticals 2021, 14, 1310. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gan, T.; Huang, Y.; Bao, L.; Liu, S.; Cui, X.; Wang, H.; Jiao, F.; Zhang, M.; Su, C.; et al. Anti-Inflammatory Activity of Mulberry Leaf Flavonoids In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 7694. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lei, C.; Shu, T.; Zhang, Y.; Jin, J.; Li, S.; Liu, W. Effects of low temperature stress on secondary metabolism in mosses exposed to simulated N deposition. Plant Ecol. Div. 2015, 8, 415–426. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, X.; Wang, T.; Wang, G.; Cao, F. Regulation of flavonoid metabolism in ginkgo leaves in response to different day night temperature combination. Plant Physiol. Biochem. 2020, 147, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, C.; Guo, X.; Chen, D.; Zhou, W.; Chen, X.; Zhang, Q. Flavonoid levels and Antioxidant capacity of mulberry leaves: Effect of growth period and drying methods. Front. Plant Sci. 2021, 12, 684974. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Huang, C.N.; Chan, K.C.; Yang, Y.S.; Peng, C.H.; Wang, C.J. Mulberry leaf polyphenols possess antiatherogenesis effect via inhibiting LDL oxidation and foam cell formation. J. Agric. Food Chem. 2011, 59, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Liu, Q.; Kim, S.B.; Jo, Y.H.; Mo, E.J.; Yang, H.H.; Song, D.H.; Hwang, B.Y.; Lee, M.K. Characterization of Melanogenesis Inhibitory Constituents of Morus alba Leaves and Optimization of Extraction Conditions Using Response Surface Methodology. Molecules 2015, 20, 8730–8741. [Google Scholar] [CrossRef]
- Doi, K.; Kojima, T.; Makino, M.; Kimura, Y.; Fujimoto, Y. Studies on the constituents of the leaves of Morus alba L. Chem. Pharm. Bull. 2001, 49, 151–153. [Google Scholar] [CrossRef]
- Dugo, P.; Donato, P.; Cacciola, F.; Germanò, M.P.; Rapisarda, A.; Mondello, L. Characterization of the polyphenolic fraction of Morus alba leaves extracts by HPLC coupled to a hybrid IT-TOF MS system. J. Sep. Sci. 2009, 32, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Hernandez, F.; Martinez, J.J. (Poly)phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: Their potential for new products rich in phytochemicals. J. Funct. Foods 2015, 18, 1039–1046. [Google Scholar] [CrossRef]
- Lee, W.J.; Sang, W.C. Quantitative changes of polyphenolic compounds in mulberry (Morus alba L.) leaves in relation to varieties, harvest period, and heat processing. Prev. Nutr. Food Sci. 2012, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ouyang, Z.; Zhao, M.; Wei, Y.; Shao, Y.; Wang, Z.; Wang, Q. Analysis of expression differences of secondary metabolites in mulberry leaves before and after frost exposure. Food Sci. 2015, 36, 109–114. [Google Scholar]
- Yuanyuan, Y.; Deguang, W. LC-MS method verification of the rationality of “Jingshuang is the best” of mulberry leaves produced in Sichuan. Shizhen Tradit. Chin. Med. 2011, 22, 2596–2598. [Google Scholar]
- Wang, X.; Wang, Y.; Qiu, J. Research on the chemical components of mulberry leaves. Chin. Pat. Med. 2007, 30, 1–3. [Google Scholar]
- Thabti, I.; Elfalleh, W.; Hannachi, H.; Ferchichi, A.; Campos, M.D.A. Identification and quantification of phenolic acids and flavonol glycosides in Tunisian Morus species by HPLC-DAD and HPLC–MS. J. Funct. Foods 2012, 4, 367–374. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, C.; Yang, S.; Zhang, S. Study on the chemical composition of white mulberry leaves. Chin. Pat. Med. 2012, 34, 1126–1131. [Google Scholar]
- Ji, T.; Li, J.; Su, S.L.; Zhu, Z.H.; Guo, S.; Qian, D.W.; Duan, J.A. Identification and Determination of the Polyhydroxylated Alkaloids Compounds with α-Glucosidase Inhibitor Activity in Mulberry Leaves of Different Origins. Molecules 2016, 21, 206. [Google Scholar] [CrossRef]
- Hansawasdi, C.; Kawabata, J. α-Glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia 2006, 77, 568–573. [Google Scholar] [CrossRef]
- Kim, I.; Lee, J. Variations in Anthocyanin Profiles and Antioxidant Activity of 12 Genotypes of Mulberry (Morus spp.) Fruits and Their Changes during Processing. Antioxidants 2020, 9, 242. [Google Scholar] [CrossRef]
- Zhao, S.; Park, C.H.; Yang, J.; Yeo, H.J.; Kim, T.J.; Kim, J.K.; Park, S.U. Molecular characterization of anthocyanin and betulinic acid biosynthesis in red and white mulberry fruits using highthroughput sequencing. Food Chem. 2018, 279, 364–372. [Google Scholar] [CrossRef]
- Cui, X.Q.; Wang, L.; Yan, R.Y.; Tan, Y.X.; Chen, R.Y.; Yu, D.Q. A new Diels-Alder type adducts and two new flavones from the stem bark of Morus yunanensis koidz. J. Asian Nat. Prod. Res. 2008, 10, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Serce, S.; Kaya, C. Phytochemical and antioxidant properties of anthocyanin rich Morus nigra and Morus rubra fruits. Sci. Hortic. 2009, 119, 275–279. [Google Scholar] [CrossRef]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Lv, Z.Q.; Lin, T.B.; Chen, S.; Hu, G.Y. Hybrid of 1-deoxynojirimycin and polysaccharide from mulberry leaves treat diabetes mellitus by activating PDX-1/insulin-1 signaling pathway and regulating the expression of glucokinase, phosphoenolpyruvate carboxykinase and glucose-6-phosphate in alloxan-induced diabetic mice. J. Ethnopharmacol. 2011, 134, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Katyama, H.; Takano, R.; Sugimura, Y. Localization of mucilaginous polysaccharides in mulberry leaves. Protoplasma 2008, 233, 157–163. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N.; Zhao, Z.; Chang, Y.; Ning, N.; Guo, H.; et al. Structure, bioactivities and future prospective of polysaccharides from Morus alba (white mulberry): A review. Food Chem. 2018, 245, 899–910. [Google Scholar] [CrossRef]
- Suryanarayanan, N.; Murthy, T.C.S. Differences in amino acid contents in leaf blades of mulberry (Morus spp.) varieties. Adv. Plant Sci. 2002, 15, 475–481. [Google Scholar]
- do Nascimento, R.P.; Dos Santos, B.L.; Amparo, J.A.O.; Soares, J.R.P.; da Silva, K.C.; Santana, M.R.; Almeida, Á.M.A.N.; da Silva, V.D.A.; Costa, M.F.D.; Ulrich, H.; et al. Neuroimmunomodulatory Properties of Flavonoids and Derivates: A Potential Action as Adjuvants for the Treatment of Glioblastoma. Pharmaceutics 2022, 14, 116. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavnoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Tariq, H.; Asif, S.; Andleeb, A.; Hano, C.; Abbasi, B.H. Flavonoid Production: Current trends in plant metabolic engineering and de novo microbial production. Metabolites 2023, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2020, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Wohl, J.; Petersen, M. Functional expression and characterization of cinnamic acid 4-hydroxylase from the hornwort Anthoceros agrestis in Physcomitrella patens. Plant Cell Rep. 2020, 39, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska-Borek, M.; Chadzinikolau, T.; Kozlowska, M. Effect of urban pollution on 4-coumarate: CoA ligase and flavonoid accumulation in Berberis thunbergii. Dendrobiology 2010, 64, 79–85. [Google Scholar]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.A.; Ashraf, N. Functional characterization of flavonoid 3-hydroxylase, CsF3H, from Crocus sativus L.: Insights into substrate specificity and role in abiotic stress. Arch. Biochem. Biophys. 2019, 667, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, J.; Yao, Y.Y.; Zhang, J.; Song, T.T.; Li, K.T.; Yao, Y.C. Identification of leucoanthocyanidin reductase and anthocyanidin reductase genes in proanthocyanidin biosynthesis in Malus crab apple plants. Plant Physiol. Biochem. 2019, 139, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Hu, R.; Ma, J.; Fan, J.; Wu, F.; Wang, Y.; Huang, L.; Feng, G.; Li, D.; Nie, G.; et al. Integrative analysis of the metabolome and transcriptome provides insights into the mechanisms of anthocyanins and proanthocyanidins biosynthesis in Trifolium repens. Ind. Crop. Prod. 2022, 187, 115529. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Haq, I.U.; Patel, S.; Pan, X.; Naz, S.; Sanches, S.A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Tao, R.; Yin, L.; Gao, L.; Strid, Å.; Qian, M.; Li, J.; Li, Y.; Shen, J.; et al. Ethylene mediates the branching of the jasmonate-induces flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits. Plant Biotechnol. J. 2020, 18, 1223–1240. [Google Scholar] [CrossRef]
- Kim, M.J.; Paramanantham, A.; Lee, W.S.; Yun, J.W.; Chang, S.H.; Kim, D.C.; Park, H.S.; Choi, Y.H.; Kim, G.S.; Ryu, C.H.; et al. Anthocyanins derived from Vitis coignetiae Pulliat contributes Anti-cancer effects by suppressings NF-κB Pathways in Hep3B human hepatocellular carcinoma cells and In Vivo. Molecules 2020, 25, 5445. [Google Scholar] [CrossRef]
- Huang, M. Cloning of Mulberry C4H, 4CL and CHS Genes and Their Expression Differences among Different Mulberry Germplasms. Master’s Thesis, Jiangsu University of Science and Technology, Zhenjiang, China, 2014. [Google Scholar]
- Zhao, S.; Park, C.H.; Li, X.; Kim, Y.B.; Yang, J.; Sung, G.B.; Park, N.I.; Kim, S.; Park, S.U. Accumulation of Rutin and Betulinic Acid and Expression of Phenylpropanoid and Triterpenoid Biosynthetic Genes in Mulberry (Morus alba L.). J. Agric. Food Chem. 2015, 63, 8622–8630. [Google Scholar] [CrossRef]
- Liu, W. Study on the Dynamic Changes of Chemical Components of Mulberry Leaves and the Expression Levels of PAL and F3H Genes. Ph.D. Thesis, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2012. [Google Scholar]
- Liu, W.; Wang, A.; Wan, D.; Pei, J. PCR cloning and analysis of mulberry leaf f3h gene. Shizhen Chin. Med. 2017, 3, 756–757. [Google Scholar]
- Xing, A.; Wang, X.; Nazir, M.F.; Zhang, X.; Wang, X.; Yang, R.; Chen, B.; Fu, G.; Wang, J.; Ge, H.; et al. Transcriptomic and metabolomic profiling of flavonoid biosynthesis provides novel insights into petals coloration in Asian cotton (Gossypium arboreum L.). BMC Plant Biol. 2022, 22, 416. [Google Scholar] [CrossRef]
- Dwivedi, M.K.; Sonter, S.; Mishra, S.; Patel, D.K.; Sinhg, P.K. Antioxidant, antibacterial activity and phytochemical characterization of Carica papaya flowers. J. Basic Appl. Sci. 2020, 9, 23. [Google Scholar] [CrossRef]
- Caldwell, C.R.; Britz, S.J.; Mirecki, R.M. Effect of temperature, elevated carbon dioxide, and drought during seed development on the isoflavone content of dwarf soybean [Glycine max (L.) Merrill] grown in controlled environments. J. Agric. Food Chem. 2005, 53, 1125–1129. [Google Scholar] [CrossRef]
- Boo, H.O.; Heo, B.G.; Gorinstein, S.; Chon, S.U. Positive effects of temperature and growth conditions on enzymatic and antioxidant status in lettuce plants. Plant Sci. 2011, 181, 479–484. [Google Scholar] [CrossRef]
- Crifò, T.; Petrone, G.; Cicero, L.; Lo Piero, A.R. Short cold storage enhances the anthocyanin contents and level of transcripts related to their biosynthesis in blood oranges. J. Agric. Food Chem. 2012, 60, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.D.; Tarara, J.M.; Gambetta, G.A.; Matthews, M.A.; Kennedy, J.A. Impact of diurnal temperature variation on grape berry development, proanthocyanidin accumulation, and the expression of flavonoid pathway genes. J. Exp. Bot. 2012, 63, 2655–2665. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. The effect of bunch shading on berry development and flavonoid accumulation in Shiraz grapes. Aust. J. Grape Wine Res. 2004, 10, 55–73. [Google Scholar] [CrossRef]
- Cortell, J.M.; Kennedy, J.A. Effect of shading on accumulation of flavonoid compounds in (Vitis vinifera L.) pinot noir fruit and extraction in a model system. J. Agric. Food Chem. 2006, 54, 8510–8520. [Google Scholar] [CrossRef] [PubMed]
- Pastore, C.; Zenoni, S.; Fasoli, M.; Pezzotti, M.; Tornielli, G.B.; Filippetti, I. Selective defoliation affects plant growth, fruit transcriptional ripening program and flavonoid metabolism in grapevine. BMC Plant Biol. 2013, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light quality affects flavonoid biosynthesis in young berries of cabernet sauvignon grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in Agriculture: Chemistry and Roles in, Biotic and Abiotic Stress Responses, and Microbial Associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Grimplet, J.; Deluc, L.G.; Tillett, R.L.; Wheatley, M.D.; Schlauch, K.A.; Cramer, G.R.; Cushman, J.C. Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genom. 2007, 8, 187. [Google Scholar] [CrossRef]
- Braidot, E.; Petrussa, E.; Bertolini, A.; Peresson, C.; Ermacora, P.; Loi, N.; Terdoslavich, M.; Passamonti, S.; Macri, F.; Vianello, A. Evidence for a putative flavonoid translocator similar to mammalian bilitranslocase in grape berries (Vitis vinifera L.) during ripening. Planta 2008, 228, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; DI Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis inripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.; Koo, B.S.; Kim, S.Y. Pharmacological Activities for Morus alba L., Focusing on the Immunostimulatory Property from the Fruit Aqueous Extract. Foods 2021, 10, 1966. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Lye, P.-Y.; Wong, S.-K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed. Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, M.; Rajnarayana, K. Role of oxygen free radicals in human diseases. Indian Drugs 2001, 38, 545–553. [Google Scholar]
- Singhania, N.; Puri, D.; Madhu, S.V.; Sharma, S.B. Assessment of oxidative stress and endothelial dysfunction in Asian Indians with type 2 diabetes mellitus with and without macroangiopathy. QJM Int. J. Med. 2008, 101, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Tangah, J.; Inoue, T.; Chan, H.T. Phenolic constituents and anticancer properties of Morus alba (white mulberry) leaves. J. Integr. Med. 2020, 18, 189–195. [Google Scholar] [CrossRef]
- Deepa, M.; Priya, S. Purification and characterization of a novel anti-proliferative lectin from Morus alba L. Leaves. Protein Pept. Lett. 2012, 19, 839–845. [Google Scholar] [CrossRef]
- Naowaratwattana, W.; De-Eknamkul, W.; De Mejia, E.G. Phenolic containing organic extract of Mulbeery (Morus alba L.) leaves inhibit HepG2 hepatoma cells through G2/M phase arrest, induction of apoptosis and inhibition of topoisomerase IIa activity. J. Med. Food 2010, 13, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivators of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Tan, Y.X.; Liu, C.; Chen, R. Phenolic constituents from stem bark of Morus wittiorum and their anti-inflammation and cytotoxicity. Zhongguo Zhong Yao Za Zhi 2010, 35, 2700–2703. [Google Scholar] [PubMed]
- Asano, N.; Yamashita, T.; Yasuda, K.; Ikeda, K.; Kizu, H.; Kameda, Y.; Kato, A.; Nash, R.J.; Lee, H.S.; Ryu, K.S. Polyhydroxylates alkaloids isolated from mulnerry trees (Morus alba L.) and silkworms (Bombyx mori L.). J. Agric. Food Chem. 2001, 49, 4208–4213. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Shi, G.; Chen, C.; Sun, J.; Chen, X. Studies on inhibitor effects and activator of α-glucosidase in mulberry leaves. Shipin Kexue 2006, 27, 108–111. [Google Scholar]
- Hao, J.; Zhang, Y.; Wu, T.; Liu, R.; Sui, W.; Zhu, J.; Fang, S.; Geng, J.; Zhang, M. The antidiabetic effect of Bifidobacterium longum subsp. longum BL21 through regulating gut microbiota structure in type 2 diabetic mice. Food Funct. 2022, 13, 9947–9958. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.Y.; Son, K.H.; Kwon, C.S.; Kwon, G.S.; Kang, S.S. Antimicrobial and cytotoxicity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Muhammad, F.; Javed, I.; Akhtar, M.; Khaliq, T.; Aslam, B.; Waheed, A.; Yasmin, R.; Zafar, H. White Mulberry (Morus alba): A brief phytochemical and pharmacological evaluations Account. Int. J. Agric. Biol. 2013, 15, 612–620. [Google Scholar]
- Paiva, P.M.G.; Gomes, F.S.; Napoleao, T.H.; Sá, R.A.; Correia, M.T.S.; Coelho, L.C.B. Antmicrobial activity of secondary metabolites and lectins from plants. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 396–406. [Google Scholar]
- Utomo, R.Y.; Ikawati, M.; Meiyanto, E. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection. Preprints 2020. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral properties of polyphenols from plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Timalsina, D.; Pokhrel, K.P.; Bhusal, D. Pharmacologic activities of plant-derived natural products on respiratory diseases and inflammations. BioMed Res. Int. 2021, 2021, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Kojima, T.; Fujimoto, Y. Mulberry leaf extract inhibits the oxidative modification of rabbit and human low density lipoprotein. Biol. Pharm. Bull. 2010, 23, 1066–1071. [Google Scholar] [CrossRef]
- Kadam, R.A.; Dhumal, N.D.; Khyade, V. The mulberry Morus alba (L.): The medicinal herbal source for human health. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 270–274. [Google Scholar] [CrossRef]
- Naowaboot, J.; Pannangpetch, P.; Kukongviriyapan, V.; Kukongviriyapan, U.; Nakmareong, S.; Itharat, A. Mulberry leaf extract restores arterial pressure in streptozotocin-induced chronic diabetic rats. Nutr. Res. 2009, 29, 602–660. [Google Scholar] [CrossRef]
- Yang, S.J.; Park, N.Y.; Lim, Y. Anti-adipogenic effect of mulberry leaf ethanol extract in 3T3-L1 adipocytes. Nutr. Res. Pract. 2014, 8, 613–617. [Google Scholar] [CrossRef]
- Bao, T.; Xu, Y.; Gowd, V.; Zhao, J.; Xie, J.; Liang, W.; Chen, W. Systematic study on phyto-chemicals and antioxidant activity of some new and commonmulberry cultivars in China. J. Funct. Foods 2016, 25, 537–547. [Google Scholar]
- Kirisattayakul, W.; Wattanathorn, J.; Iamsaard, S.; Jittiwat, J.; Suriharn, B.; Lertrat, K. Neuroprotective and Memory-Enhancing Effect of the Combined Extract of Purple Waxy Corn Cob and Pandan in Ovariectomized Rats. Oxidative Med. Cell. Longev. 2017, 2017, 5187102. [Google Scholar] [CrossRef]
- Kawvised, S.; Wattanathorn, J.; Thukham-Mee, W. Neuroprotective and Cognitive-Enhancing Effects of Microencapsulation of Mulberry Fruit Extract in Animal Model of Menopausal Women with Metabolic Syndrome. Oxidative Med. Cell. Longev. 2017, 2017, 2962316. [Google Scholar] [CrossRef]
- Kim, J.; Yun, E.Y.; Quan, F.S.; Park, S.W.; Goo, T.W. Central administration of 1-deoxynojirimycin attenuates hypothalamic endoplasmic reticulum stress and regulates food intake and body weight in mice with high-fat diet-induced obesity. Evid. -Based Complement. Altern. Med. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Fu, F.; Gen, M.; Jiang, Y.; Yang, J.; Jiang, W.; Wang, C.; Liu, K. Neuroprotective effect of 20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci. Lett. 2005, 374, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Takahashi, K.; Goto, Y.; Goh, S.; Tanaka, N.; Kamei, K.; Ichida, M.; Hara, S.; Akaike, A.; Kihara, T.; et al. Mulberry leaf extract prevents amyloid beta-peptide fibril formation and neurotoxicity. Neuroreport 2007, 18, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Shahana, S.; Nikalje, A.P.G. Development and evaluation of antidiabetic formulation of Trichosanthes dioica fruit extract. J. Pharmacogn. Phytochem. 2019, 8, 610–613. [Google Scholar]
- Kaewkaen, P.; Tong-un, T.; Wattanathorn, J.; Muchimapura, S.; Kaewrueng, W.; Wongcharoenwanakit, S. Mulberry Fruit Extract Protects against Memory Impairment and Hippocampal Damage in Animal Model of Vascular Dementia. Evid. Based Complement. Alternat. Med. 2012, 2012, 263520. [Google Scholar] [CrossRef] [PubMed]
- Kaewkaen, P.; Tong-un, T.; Wattanathorn, J.; Muchimapura, S.; Kaewrueng, W.; Wongcharoenwanakit, S. Effect of mulberry fruit powder in animal model of stroke. Am. J. Agric. Biol. Sci. 2012, 7, 322–329. [Google Scholar] [CrossRef]
- Sriraksa, N.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-enhancing effect of quercetin in a rat model of Parkinson’s disease induced by 6-hydroxydopamine. Evid. Based Complement. Alternat. Med. 2012, 2012, 823206. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Juang, L.J.; Wang, B.S.; Wang, M.Y.; Tai, H.M.; Hung, W.J.; Chen, Y.J.; Huang, M.H. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food Chem. Toxicol. 2011, 49, 785–790. [Google Scholar] [CrossRef]
- Zeni, A.L.B.; Dall’Molin, M. Hypotriglyceridemic efect of Morus alba L., Moraceae, leaves in hyperlipidemic rats. Braz. J. Pharmacogn. 2010, 20, 130–133. [Google Scholar] [CrossRef]
- Jo, S.P.; Kim, J.K.; Lim, Y.H. Antihyperlipidemic efects of stilbenoids isolated from Morus alba in rats fed a high-cholesterol diet. Food Chem. Toxicol. 2014, 65, 213–218. [Google Scholar] [CrossRef]
- Park, K.M.; You, J.S.; Lee, H.Y.; Baek, N.I.; Hwang, J.K. Kuwanon G: An antibacterial agent from the root bark of Morus alba against oral pathogens. J. Ethnopharmacol. 2003, 84, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.V.; Nade, V.S. Anti-dopaminergic efect of the methanolic extract of Morus alba L. leaves. Indian J. Pharmacol. 2008, 40, 221–226. [Google Scholar] [PubMed]
- Dat, N.T.; Binh, P.T.; le Quynh, T.P.; Van Minh, C.; Huong, H.T.; Lee, J.J. Cytotoxic prenylated favonoids from Morus alba. Fitoterapia 2010, 81, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, J.; Naik, P.R. The histopathologic efects of Morus alba leaf extract on the pancreas of diabetic rats. Turk. J. Biol. 2012, 36, 211–216. [Google Scholar]
- Oh, K.S.; Ryu, S.Y.; Lee, S.; Seo, H.W.; Oh, B.K.; Kim, Y.S.; Lee, B.H. Melanin- concentrating hormone-1 receptor antagonism and anti-obesity efects of ethanolic extract from Morus alba leaves in diet-induced obese mice. J. Ethnopharmacol. 2009, 122, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, S.Y.; Kim, H.; Hwang, J.S.; Lee, B.G.; Gao, J.J.; Kim, S.Y. Mulberroside F isolated from the leaves of Morus alba inhibits melanin biosynthesis. Biol. Pharm. Bull. 2002, 25, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Xie, Y.; Wang, W.; Yan, Y.; Ye, H.; Jabbar, S.; Zeng, X. Extraction optimization, characterization and antioxidant activity in vitro of polysaccharides from mulberry (Morus alba L.) leaves. Carbohydr. Polym. 2015, 128, 52–62. [Google Scholar] [CrossRef]
- Li, W.; Li, T.; Tang, K. Flavonoids from mulberry leaves by microwave assisted extract and anti-fatigue activity. Afr. J. Agric. Res. 2009, 4, 898–902. [Google Scholar]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Prasad, P.; Reddy, M. Nutritive value of mulberry (Morus alba) leaves in goats and sheep. Indian. J. Anim. Nutr. 1991, 8, 295–296. [Google Scholar]
- Park, S.W.; Shin, K.C.; Yoou, S.K.; Park, H.J.; Eun, S.H.; Bae, Y.M.; Lee, H.M.; Chae, H.J.; Chae, S.W.; Choi, B.H. Effects of an ethanolic extract of mulberry fruit on blood pressure and vascular remodeling in spontaneous hypertensive rats. Clin. Exp. Hypertens. 2019, 41, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Choi, D.H.; Kim, E.J.; Kim, H.Y.; Kwon, T.O.; Kang, D.G.; Lee, H.S. Hypotensive, hypolipidemic, and vascular protective effects of Morus alba L. in rats fed an atherogenic diet. Am. J. Chin. Med. 2011, 39, 39–52. [Google Scholar] [CrossRef] [PubMed]


| Sr. No. | Compound | Method of Extraction | Chemical Structure | Bioactive Potential | Reference(s) |
|---|---|---|---|---|---|
| 1 | Rutin | Methanol extraction | C27H30O16 | Inhibition of peroxidation and acts as an antioxidant, reverts β-amyloid toxicity | [33] |
| 2 | Isorhamnetin | Ethanol extraction | C16H12O7 | Antioxidant (IC50-14.3 µM), anti-inflammatory (IC50-3.9 µM), Neuroprotective, Anticancer | [34,35] |
| 3 | Myricetin | Methanol extraction | C15H10O8 | Antioxidant (IC50-3.1 µM), anti-inflammatory (IC50-5.7 µM), anti-cardiovascular | [32,36,37] |
| 4 | Quercetin | Solvent Extraction | C15H10O7 | Antioxidant | [38,39] |
| 5 | Kaempferol | Solvent Extraction | C15H10O6 | Ameliorate hyperglycemia, antioxidant effect | [40] |
| 6 | Quercetin-3,7-di-O-β-D-glucopyranoside | Solvent Extraction | C27H30O17 | Antioxidant (IC50-12.8 µM), anti-inflammatory (IC50-9.3 µM) | [41,42] |
| 7 | Kaempferol-3,7-di-O-β-glucopyranoside | Solvent Extraction | C27H30O16 | Antioxidant (IC50-16.4 µM), anti-inflammatory (IC50-12.1 µM) | [41,42] |
| 8 | Quercetin-3-O-β-D-glucopyranoside (isoquercitrin) | Solvent Extraction | C21H20O12 | Reduce oxidative stress | [43,44] |
| 9 | Kaempferol-3-O-β-D-glucopyranoside (milk vetch glycoside) | Solvent Extraction | C21H19O11 | Antioxidant (IC50-20.3 µM), anti-inflammatory (IC50-14.2 µM) | [43,44] |
| 10 | Quercetin-3-O-α-L-rhamnosyl-(1-6)-β-glucopyranose (rutin) | Solvent Extraction | C27H30O16 | Antioxidant (IC50-9.7 µM), anti-inflammatory (IC50-7.4 µM), Neuroprotective activity | [44,45] |
| 11 | Kaempferol-7-O-β-D-glucopyranoside | Solvent Extraction and Chromatography technique | C21H20O11 | Antioxidant (IC50-17.9 µM), anti-inflammatory (IC50-11.6 µM) | [46] |
| 12 | Quercetin-3-O-(6″-O-acetyl)-β-D-glucopyranoside | LC-MS | C23H22O13 | Antioxidant (IC50-14.2 µM), anti-inflammatory (IC50-9.8 µM) | [26] |
| 13 | Kaempferol-3-O-α-L-rhamnosyl-(1-6)-β-glucopyranoside | HPLC | C33H40O20 | Antioxidant (IC50-16.7 µM), anti-inflammatory (IC50-10.9 µM) | [41] |
| 14 | Quercetin-3-O-β-D-glucosyl-(1-6)-β-glucopyranoside | HPLC | C21H20O12 | Antioxidant (IC50-11.4 µM), anti-inflammatory (IC50-8.2 µM) | [41,42] |
| 15 | Kaempferol-3-O-(6″-O-acetyl)-β-D-glucopyranoside | HPLC | C23H22O12 | Antioxidant (IC50-15.6 µM), anti-inflammatory (IC50-10.3 µM) | [41,42] |
| 16 | Quercetin-3-O-(6″-O-malonyl)-β-D-glucopyranoside | HPLC | C24H22O15 | Antioxidant (IC50-12.9 µM), anti-inflammatory (IC50-9.1 µM) | [41,42] |
| 17 | Kaempferol-3-O-(6″-O-malonyl)-β-D-glucopyranoside | HPLC | C24H22O14 | Antioxidant (IC50-14.8 µM), anti-inflammatory (IC50-10.6 µM) | [41,42] |
| 18 | Kaempferol-3-O-β-D-glucosyl-(1-6)-β-glucopyranoside | Chromatography technique | C21H20O11 | Antioxidant (IC50-17.2 µM), anti-inflammatory (IC50-11.4 µM) | [46] |
| 19 | Quercetin-3-O-α-L-rhamnopyranoside | LC-MS | C21H20O11 | Antioxidant (IC50-10.2 µM), anti-inflammatory (IC50-7.8 µM) | [45] |
| Kaempferol-3- O-α-L-rhamnoside | Liquid Chromatography technique | C21H20O10 | Antioxidant (IC50-14.3 µM), anti-inflammatory (IC50-9.7 µM) | [47] | |
| Quercetin-3-O-rhamnose-7-O-glucoside | Chromatography technique | C27H30O16 | Antioxidant (IC50-12.0 µM), anti-inflammator (IC50-8.6 µM) | [48] | |
| Quercetin-3-O-glucose-7-O-rhamnoside | Chromatography technique | C27H30O16 | Antioxidant (IC50-11.8 µM), anti-inflammatory (IC50-8.4 µM) | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, M.; Dar, M.A.; Dhanavade, M.J.; Abbas, S.Z.; Bukhari, M.N.; Arsalan, A.; Liao, Y.; Wan, J.; Shah Syed Bukhari, J.; Ouyang, Z. Biosynthesis and Pharmacological Activities of the Bioactive Compounds of White Mulberry (Morus alba): Current Paradigms and Future Challenges. Biology 2024, 13, 506. https://doi.org/10.3390/biology13070506
Fatima M, Dar MA, Dhanavade MJ, Abbas SZ, Bukhari MN, Arsalan A, Liao Y, Wan J, Shah Syed Bukhari J, Ouyang Z. Biosynthesis and Pharmacological Activities of the Bioactive Compounds of White Mulberry (Morus alba): Current Paradigms and Future Challenges. Biology. 2024; 13(7):506. https://doi.org/10.3390/biology13070506
Chicago/Turabian StyleFatima, Maryam, Mudasir A. Dar, Maruti J. Dhanavade, Syed Zaghum Abbas, Mohd Nadeem Bukhari, Abdullah Arsalan, Yangzhen Liao, Jingqiong Wan, Jehangir Shah Syed Bukhari, and Zhen Ouyang. 2024. "Biosynthesis and Pharmacological Activities of the Bioactive Compounds of White Mulberry (Morus alba): Current Paradigms and Future Challenges" Biology 13, no. 7: 506. https://doi.org/10.3390/biology13070506
APA StyleFatima, M., Dar, M. A., Dhanavade, M. J., Abbas, S. Z., Bukhari, M. N., Arsalan, A., Liao, Y., Wan, J., Shah Syed Bukhari, J., & Ouyang, Z. (2024). Biosynthesis and Pharmacological Activities of the Bioactive Compounds of White Mulberry (Morus alba): Current Paradigms and Future Challenges. Biology, 13(7), 506. https://doi.org/10.3390/biology13070506








