Simple Summary
Chagas disease is endemic to the state of Texas in the United States but does not have consistent surveillance or reporting. We utilized multiple sampling sites and different species of triatomine to gain data on the blood meal sources found by DNA testing for the host and vector species identities. From domestic, peridomestic, and rural sites, we found a breadth of blood meal origins including mammals, chickens, and reptiles. Unique non-native taxa utilized for blood meals enabled us to also report on extensive foraging distances for the vectors. Understanding the diversity of blood meal sources and the distances the vectors travel between meals and daytime refuges are both important aspects for understanding the spread of this disease.
Abstract
The prevalence of Trypanosoma cruzi was assessed in 117 triatomine insects from central Texas. The qPCR-based results revealed T. cruzi in 59% of the insects (62 adults and eight nymphs), with overall prevalences of T. cruzi of 0% (0/9), 64% (11/17), 58% (10/17), 73% (30/41), and 57% (19/33) for the Bastrop, Caldwell, Gonzales, Guadalupe, and Hays counties, respectively. Analyses of 18S rRNA fragments confirmed T. cuzi in 81% of these samples. Vectors were identified as Triatoma gerstaeckeri (35% of which 65% were positive for T. cruzi), T. sanguisuga (21%, 43% positive), and Paratriatoma leticularia (0.3%, 100% positive). Food sources were recovered from 29% of the insects. Raccoons were 53% of the blood meals (83% positive for T. cruzi), while the remainder came from a variety of sources, including humans (33% positive), house geckos, Eastern woodrats, plain-bellied water snakes (50% positive), hispid cotton rats (0% positive), chickens (100% positive); Asian forest turtles, bison, and pigs (0% positive). The serendipitous detection of blood meal sources at known minimum distances from the collection of the vector insect enabled us to provide several instances where the insect foraging distance was greater than 400 m. These vector foraging distances are novel information that can assist in our understanding of the landscape dynamics for the spread of the pathogen.
1. Introduction
Chagas disease is one of many neglected tropical diseases shared across North, Central, and South America [1,2]. The causative agent of Chagas disease is Trypanosoma cruzi, a parasitic protozoan that has been detected in many mammalian hosts including domestic and wild animals such as rodents, opossums, raccoons, armadillos, bats, dogs, cats, goats, and pigs [2,3,4,5,6]. The parasite is naturally transmitted to mammals through infected triatomine fecal material introduced into a wound but also by oral ingestion or congenital routes [7].
In central Texas, a region with established populations of infected triatomine vectors [8,9,10,11], 70% of raccoons were found to be positive for T. cruzi, while other animals such as bobcats, ocelots, coyotes, and foxes revealed much a lower prevalence of 14% each [3,12]. T. cruzi infections were also reported from feral hogs (Sus scrofa), even though the prevalence was relatively low at 6% [13]. The Triatomine vector species in Texas have mainly been identified as Triatoma gerstaeckeri Stål 1859, though occasionally Triatoma sanguisuga Leconte 1855, Paratriatoma lecticularia Stål 1859, Triatoma rubida Uhler 1894, or Triatoma protracta woodi Uhler 1894 were observed as well [8,9,10,14]. Up to 50% and more of the insects have been reported to be infected with T. cruzi [8,10,14], with adult insects generally 5 to 10 times more likely to be infected than nymphs [10,15].
The diversity of potential blood meal source prey may be poorly characterized in Texas compared to the potential breadth of prey available. Similarly, while previous work has characterized the prevalence of T. cruzi detection in triatomines, concurrent tests for blood meal sources for the vectors are less frequently reported [8,14]. We sought to address some of these knowledge gaps for central Texas, USA. The goal of this study was to assess the prevalence of T. cruzi in triatomines in South Central Texas from a variety of sampling locations, characterize triatomine vectors, and subsequently determine their blood meal sources.
2. Materials and Methods
Triatomine insect specimen collection and preparation. Triatomine insects were iteratively collected opportunistically within five central Texas municipal counties (Bastrop, Caldwell, Gonzales, Guadalupe, and Hays), generally from peridomestic sites surrounding buildings and woodpiles of residential structures, except for two insects in Hays County and four insects in Caldwell County that were captured inside residences. We characterized these sites by the density of human habitation, alongside the captures (n = 6) within a home or outside it: domestic (>100 homes/km2), peridomestic (>10 homes/km2), and rural (<1/km2). Active search efforts were not made in non-disturbed or sylvatic environments. Additional triatomines were collected from peridomestic sites using intentionally placed wooden lumber debris piles outside a barn in Guadalupe County, Texas, or as bycatch in a sylvatic herpetofaunal study using pitfall arrays with associated bucket traps on the rural Griffith League Ranch in Bastrop County, Texas. Three adults of Arilus cristatus (wheel bug) and two nymphs of Pyrrhocoris apterus (fire bug) were used as negative controls for DNA-based analyses of T. cruzi and triatomine identification. Insect specimens were stored at −20 °C until extractions were performed on all available tissue recovered from the abdominal contents (about 25 mg) using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD, USA), following the instructions for tissue samples. DNA was resuspended in a final volume of 200 µL [8].
Detection of T. cruzi. The presence of T. cruzi in triatomine samples was assessed by qPCR targeting a 166 bp fragment of satellite DNA with primers Cruzi 1 and Cruzi 2 (Table 1) [16]. Standards used for qPCR reactions were PCR amplified products from T. cruzi ITRI/MX/99/Cari-006 originally isolated from Triatoma picturata and representing lineage TcI, obtained from Universidad Autonoma del Estado de Morelos, Mexico [17]. All DNA samples were diluted ten-fold for amplification and analyzed in triplicate. qPCRs were performed on an Eco Real-Time PCR System (Illumina, San Diego, CA, USA) in a 10 µL volume reaction with 5 µL SYBR® Green (SsoAdvanced Universal SYBR® Green Supermix), 0.2 µL of 10 µM Cruzi primer 1, 0.2 µL of 10 µM Cruzi 2 primer, 3.6 µL H2O, and 1 µL of DNA solution (Bio-Rad, Hercules, CA, USA). The qPCR parameters consisted of initial denaturation at 95 °C for 5 min, followed by 40 cycles at 95 °C for 15 s and 58 °C for 60 s (Table 1) [8].

Table 1.
Description of PCR utilized in detection of Trypanosoma cruzi, Triatomine species identity, and blood meal source organism.
Identification of T. cruzi. Samples with positive qPCR detection of T. cruzi using primers Cruzi 1 and Cruzi 2 were used to amplify a 667 bp fragment of the 18S rRNA gene of T. cruzi in a nested PCR, initially with primers of SSU4F and 18Sq1R (followed by using the amplicons as a template for primers SSU561F/SSU561R [19]. The initial amplicons were generated on a PTC-200 DNA Thermocycler (MJ Research, Watertown, MA, USA) of 25 µL with 1 µL of specimen sample, 12.5 µL of Green Taq polymerase, 0.8 µL of 10 µM SSU4F, 0.8 µL of 10 µM 18Sq1R, and 9.9 µL of nuclease-free H2O. PCR reaction parameters consisted of initial denaturation at 95 °C for 5 min, followed by 35 cycles at 95 °C for 30 s, 50 °C for 45 s, and 72 °C for 60 s (Table 1). Amplicons were cleaned using a PCR clean-up kit following standard protocols (Promega, Madison, WI, USA). The nested PCR reaction was performed with the same conditions as those listed above, with the exception of an increase in DNA template to 2 µL and a corresponding decrease in water to 8.9 µL.
Amplicons of the nested PCR were visualized on a 2% agarose gel, cleaned using Shrimp Alkaline Phosphate and Exonuclease I (Affymetrix, Santa Clara, CA, USA) following the manufacturer’s protocols, and then sequenced bidirectionally using BigDye Terminator v3.1 (Applied Biosystems, Foster City, CA, USA), with the same primers used for PCR. Sequences were analyzed on a 3500 Genetic Analyzer (Life Technologies, Carlsbad, CA, USA) and deposited at GenBank/EMBL under accession numbers MT548855-MT548904.
Identification of Triatomines. Triatomine insects were identified by comparative sequence analyses of partial cytochrome B or COI amplicons of mitochondrial DNA [8,27] using DNA extracts from insect intestinal samples. All COI results were obtained using identical primers and methods in our previous work [8] and utilized the same specimens. The cytochrome B amplicons reactions were performed on a PTC-200 DNA Thermocycler of 25 µL in volume with 1 µL of DNA template, 14.25 µL of nuclease-free H2O, 4.125 µL of 15 mg mL−1 BSA (Thermo Fisher Scientific, Waltham, MA, USA), 2.5 µL of 10× Taq Buffer, 0.5 µL of 10 µM each of 7432F forward primer and 7433R reverse primer (Table 1), 0.5 µL of 10 mM dNTP stock, 1.5 µL of 50 mM MgCl2, and 0.125 µL of 5 U µL−1 Taq polymerase [27]. Thermocycler parameters were 95 °C for 5 min followed by 34 cycles of 95 °C for 30 s, 45 °C for 45 s, 72 °C for 60 s, and, finally, 72 °C for 10 min [28] (Table 1). Amplicons were cleaned and sequenced bidirectionally using BigDye Terminator v3.1 and sequences were analyzed on a 3500 Genetic Analyzer as described above.
Identification of food sources. Blood meals of triatomines were analyzed from insect intestine samples by comparative sequence analyses of partial cytochrome B amplicons of mitochondrial DNA, with three different primer sets targeting vertebrates in general, mammals, or birds, respectively (Table 1) [22]. DNA extracted from tissue samples of bison, chicken, swallow, turkey, alligator, rat, mouse, turtle, and pig tissues were used as positive controls for these primer sets. While the primer set for mammals amplified all controls, the vertebrate primer set failed to detect turtles, and the avian primer set detected swallow, turkey, alligator, mouse, and rat but did not amplify chicken. Reactions were performed on a PTC-200 DNA Thermocycler of 25 µL with 3 µL of DNA template, 12.175 µL of nuclease-free H2O, 4.125 µL of 15 mg mL−1 BSA, 2.5 µL of 10× Taq Buffer, 0.5 µL of 10 µM each of forward primer and reverse primer (Table 1), 0.5 µL of 10 mM dNTP stock, 1.5 µL of 50 mM MgCl2, and 0.25 µL of 5 U µL−1 Taq polymerase. Amplicons were cleaned and sequenced bidirectionally using BigDye Terminator v3.1 and sequences were analyzed on a 3500 Genetic Analyzer as described above.
Phylogenetic Analyses. Sequences were assembled in Geneious 8.1.8 (Biomatters Ltd., Auckland, New Zealand) and checked in the GenBank/EMBL databases using the BLAST algorithm [29]. The identities and relationships among the sequences amplified were compared to available GenBank sequences using Neighbor-Joining (NJ) [30] and maximum likelihood (ML) [31] analyses, including bootstrap consensus for 1000 replicate analyses [32,33]. NJ trees used the HKY substitution method and 1000 bootstrap replicates. All trees included the percent consensus support from ML analysis where appropriate. Trees were generated to identify the strain identity of the T. cruzi sequences as well as phylogenetic results from the mitochondrial DNA sequences that enabled species identification of the insect vector.
3. Results
A total of 117 insects were classified as triatomines by sight identification, with 90 individuals characterized as adults and 27 as nymphs (Table 2). Further determination of instar stage was not performed. Morphological keys could not enable species-level determination because the frozen, individually stored specimens were in flexible plastic bags and experienced considerable damage to appendages and color changes attendant to such storage. Table 2 also includes arthropods we previously identified [8]. Those samples had been previously identified as species using the same methods as in this project but had not had blood meal assessments [8]. Those 30 insects were included in the blood meal assessments here. In 59% (70/117) of these insects (62 adults and eight nymphs), qPCR-based analyses indicated the presence of T. cruzi (Table 2). The prevalence of T. cruzi detected in these insects was 59% (19/33), 73% (30/41), 64% (11/17), 58% (10/17), and 0% (0/9), respectively, for the Hays, Guadalupe, Caldwell, Gonzales, and Bastrop counties (Table 2). Two of the five non-triatomines, i.e., one wheel bug and one fire bug, used as negative controls showed weak amplification, with Ct values close to the detection limit, while the remaining two wheel bugs and one fire bug did not show any amplification.

Table 2.
Detection of Trypanosoma cruzi in adults and nymphs of triatomine insects collected in five counties in Central Texas, USA.
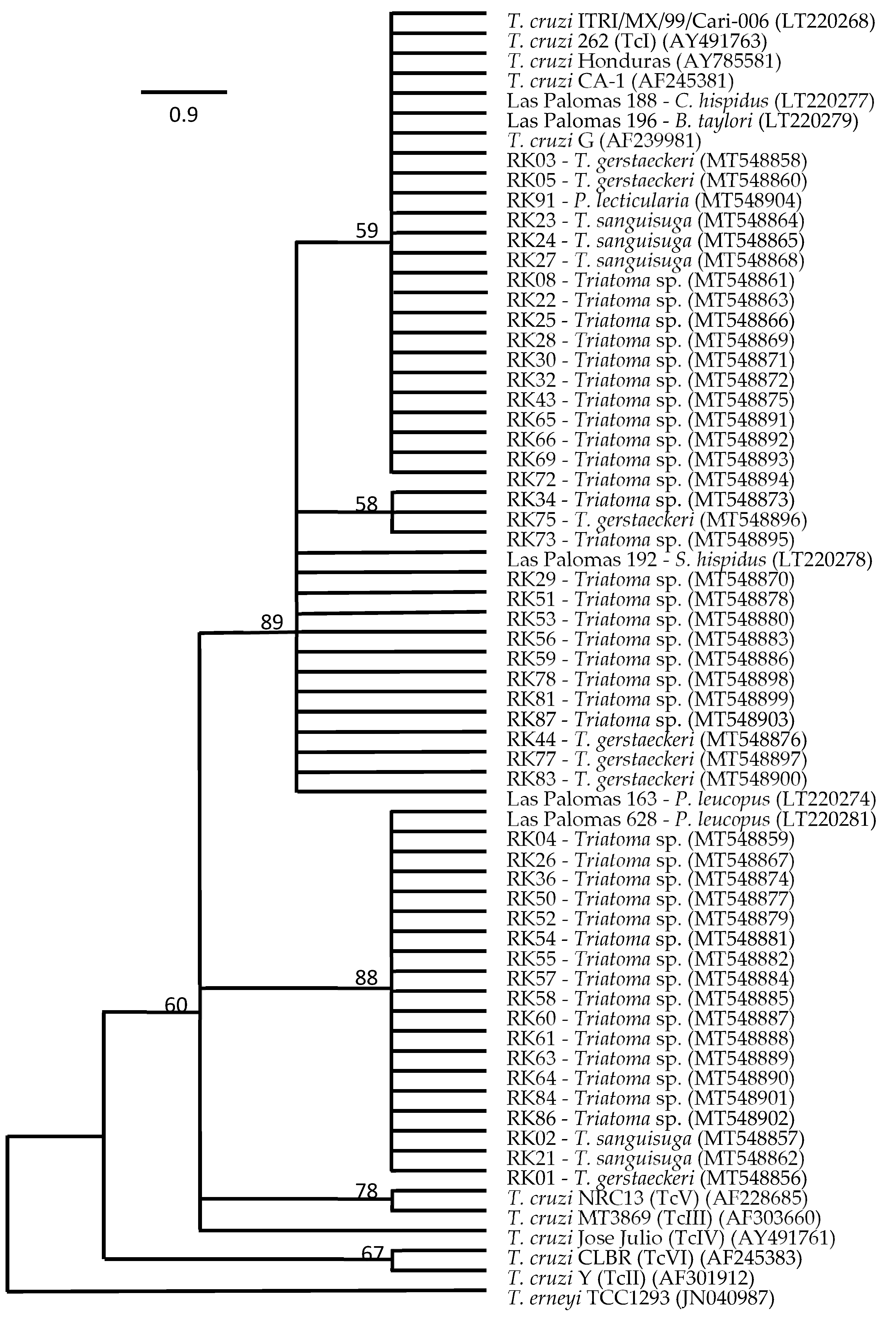
Comparative sequence analyses of a 667 bp fragment of the 18S rRNA gene targeting T. cruzi in a nested PCR allowed us to generate sequences from T. cruzi in 49 of the 70 samples, with T. cruzi from 31 triatomine insects from Hays, Guadalupe, and Caldwell counties clustering with representatives of Tc1 and the remaining related to Tc1, but distinct to TcII to TcVI (Figure 1). The presence of T. cruzi in our non-triatomine insects, i.e., the wheel bug and the fire bug, was not confirmed as T. cruzi by sequence data and thus was characterized as false-positive qPCR detections. Comparative sequence analyses of the 18S rRNA gene fragment targeting T. cruzi revealed a 98.9% pairwise identity match to Trypanomastidae sp. isolate PNG85 (MK929454) for the wheel bug sample and a 99.8% pairwise identity match to Blastocrithidia papi isolate Pa3 (KX641340) for the fire bug sample. Another sample, RK35, with a strong qPCR signal indicating the presence of T. cruzi, was also identified as a false-positive detection, with a 99.4% pairwise identity match to the same Blastocrithidia papi isolate Pa3 (KX641340). Table 3 provides the results for triatomine species, T. cruzi status, and Texas county of origin. Twenty-one of the triatomine insects positive for T. cruzi were identified as Triatoma gerstaeckeri by comparative CytB sequence analyses, with a 99.7 to 100% pairwise identity match to the sequence of specimen San Marcos I-13 (LT630441), 100% pairwise identity match to the sequence of I-JAM02 (LT630443), or a 98.2% pairwise identity match to the sequence of Parker 026 (KY305701). Twenty-five other individuals, negative for T. cruzi, were also identified as T. gerstaeckeri. Eight insects carrying T. cruzi and another five insects without T. cruzi were identified as Triatoma sanguisuga, with a 99.1 to 99.7% pairwise identity to the sequence of specimen PS099 (KY305708). Two adult insects, both positive for T. cruzi, were identified as Paratriatoma leticularia, with a 98.6 to 99.1% pairwise identity match to the sequence of specimen PS100 (KY305709). The remaining 39 insects positive for T. cruzi could not be identified at the species level and were further classified as Triatoma species (Figure 1).
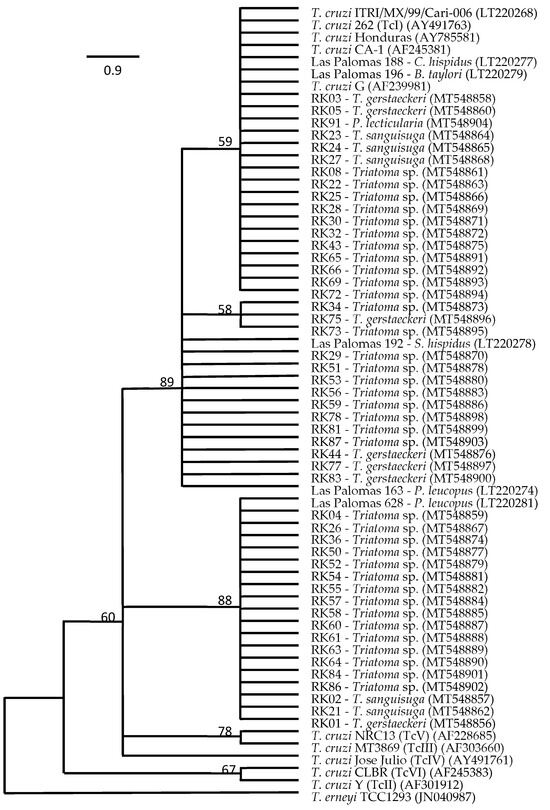
Figure 1.
Bootstrap consensus tree of the maximum likelihood (ML) final topology (100 replicates) showing the phylogenetic relationship of Trypanosoma cruzi detected in intestine samples from Triatoma gerstaeckeri, Triatoma sanguisuga, Paratriatoma lecticularia, and unidentified Triatoma sp. collected in Hays, Guadalupe, and Caldwell counties, TX, USA, based on comparative sequence analysis of 18S rRNA gene fragments with those of reference isolates.

Table 3.
Triatomine species identification of Triatoma gerstacekeri, Triatoma sanguisuga, and Paratriatoma lecticularia determined by amplification and comparative analysis of mitochondrial COI or CytB gene fragments as well as Trypanosoma cruzi infection status and life stage of triatomines collected in Bastrop, Caldwell, Gonzales, Guadalupe, and Hays County from 2016 to 2019. Infection status was determined by the detection of pathogen DNA through qPCR amplification of triplicate samples. The asterisks present in the table indicate that no insect of that species and life stage was identified by molecular methods.
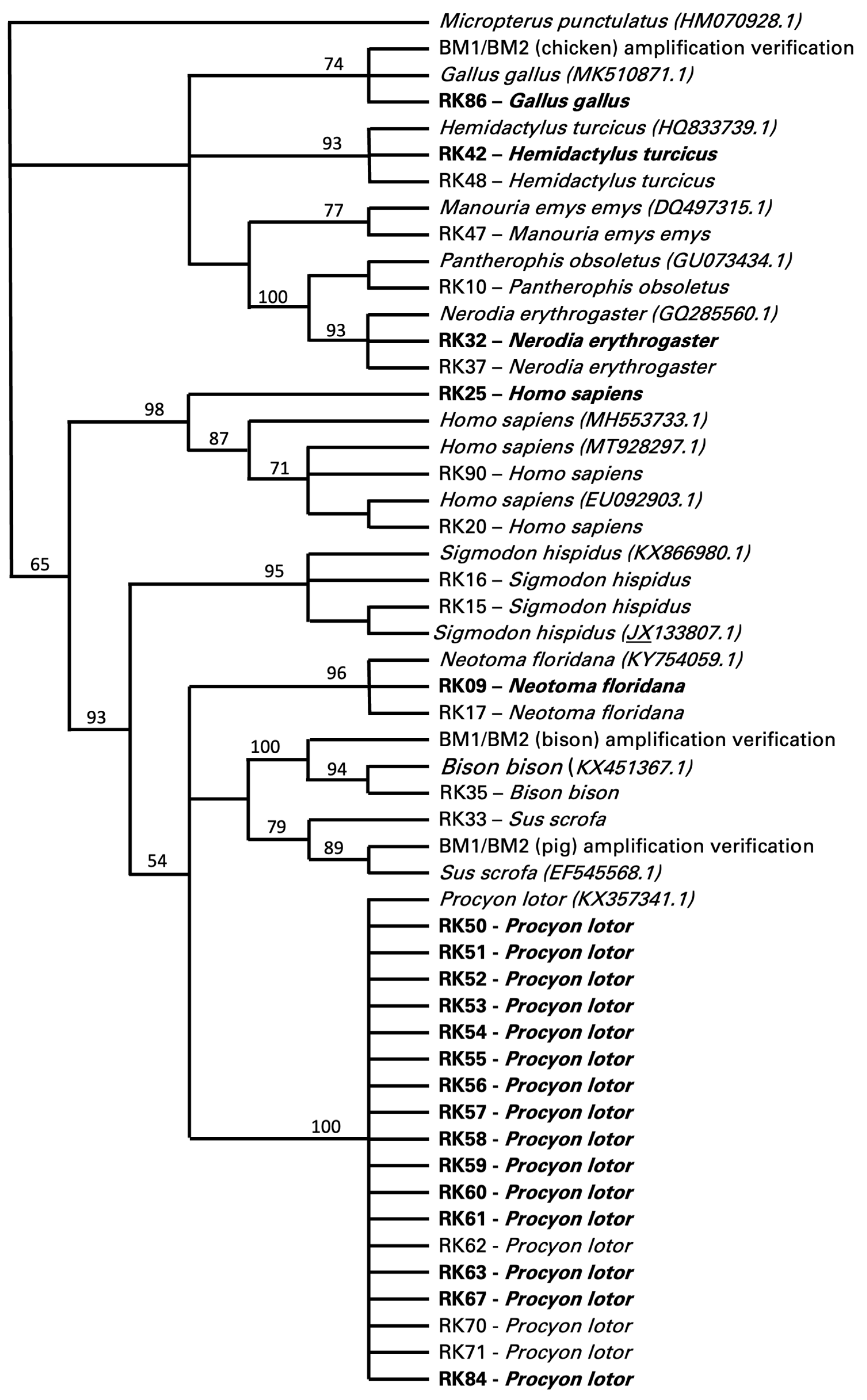
Food sources could only be determined in 34 of the 117 specimens, 20 of which were positive for T. cruzi and 14 were negative for T. cruzi (Figure 2). These data were all retrieved with the cytB primer set targeting vertebrates, with a few also confirmed by the cytB primer set targeting mammals. The cytB primer set designed to target only avian samples did not produce any results. The cytB primer set targeting vertebrates also retrieved non-target sequences from twenty-six insects (i.e., 23 sequences identifying Triatoma sp. and 3 sequences identifying Gryllus sp.). Of the food sources identified as vertebrates, 53% (18/34) were identified as Procyon lotor (raccoon), with 15 individuals positive and 3 negative for T. cruzi (Figure 2). Other food sources included Homo sapiens (human, 9%, 3/34), with one insect positive and two insects negative for T. cruzi; Hemidactylus turcicus (house gecko, 6%, 2/34), Neotoma floridana (Eastern woodrat, 6%, 2/34), and Nerodia erythrogaster (plain-bellied water snake, 6%, 2/34), all with one insect positive for T. cruzi and one negative; and Sigmodon hispidus (hispid cotton rat, 6%, 2/34), with two insects negative for T. cruzi (Figure 2). One insect feeding on Gallus gallus (chicken, 3%, 1/34) was positive for T. cruzi, while insects feeding on Manouria emys emys (Asian forest tortoise, 3%, 1/34), Bison bison (bison, 3%, 1/34), and Sus scrofa (pig, 3%, 1/34) were negative for T. cruzi (Figure 2).
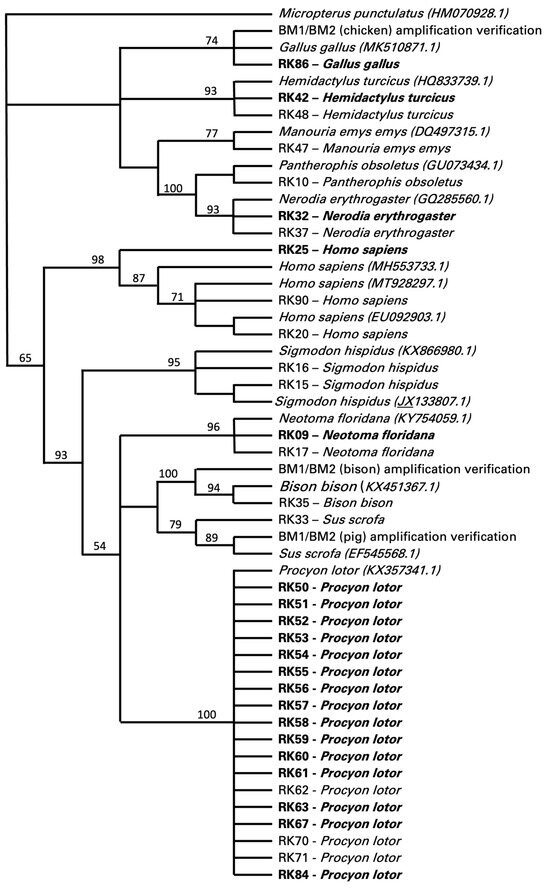
Figure 2.
Bootstrap consensus tree of the maximum likelihood (ML) final topology identifying vertebrate blood meals of triatomines collected from Central Texas. The presence of Trypanosoma cruzi in triatomine samples is highlighted in bold. Sequences were obtained from PCR analysis with CytB fragments. NJ analysis with HKY substitution and 1000 bootstrap replicates was used for the final topology, with ML consensus support values included.
Several of these blood meal taxa provided serendipitous information for the foraging distances of these vector insects. Specifically, the detection of these blood meals was novel among reported hosts for the vector insects, and detecting them enabled the calculation of the minimum distances between the blood meal source and the arthropod collection site using straight-line measurement. First, the Asian forest tortoise (Manouria emys) was captively held in an indoor/outdoor paddock. The minimum measured distance between the corner of that paddock and the insect vector collection site was 40 m. However, the tortoise could move as much as 110 m from the site of the vector. Next, a blood meal was detected from bison (Bison bison). These animals were held in a large, fenced facility that was a 600 m minimum distance from the insect vector location in the corner of that facility, proximal to the collection site. Importantly, this was the minimum distance, and this facility enabled the bison to travel as far as 1210 m from the site of insect collection. Finally, we also detected three instances of humans as the blood meal; one of these vector insects was also positive for T. cruzi. There were no human-occupied buildings, campsites, or other such occupancies near the vector collection site. The nearest potential occupied location was 430 m from the collection site, but other alternative locations were found at distances of 621–698 m (n = 5 sites). These all represent neighboring tracts of land adjacent to the insect collection location.
4. Discussion
Trypanosoma cruzi was detected in 59% of the 117 triatomine insects (76% adults and 23% nymphs), with 68% of the adults and 29% of the nymphs being positive. These results are in agreement with those of previous studies in Central Texas in which 50% or more of the triatomine insects analyzed were infected with T. cruzi [8,10,11,14,33]. A higher prevalence in adult insects compared to nymphs has also been reported [10,15], even though one study reported a much lower overall prevalence of 23% in adults and 4% in nymphs compared to our results [15]. High prevalence in triatomines was also found in other Texas areas [6], and the prevalence in triatomines collected in Texas was generally higher than in other states, including Oklahoma [34], Nebraska [35], or specific areas in Arizona, while other areas in Arizona were comparable to Texas values [36].
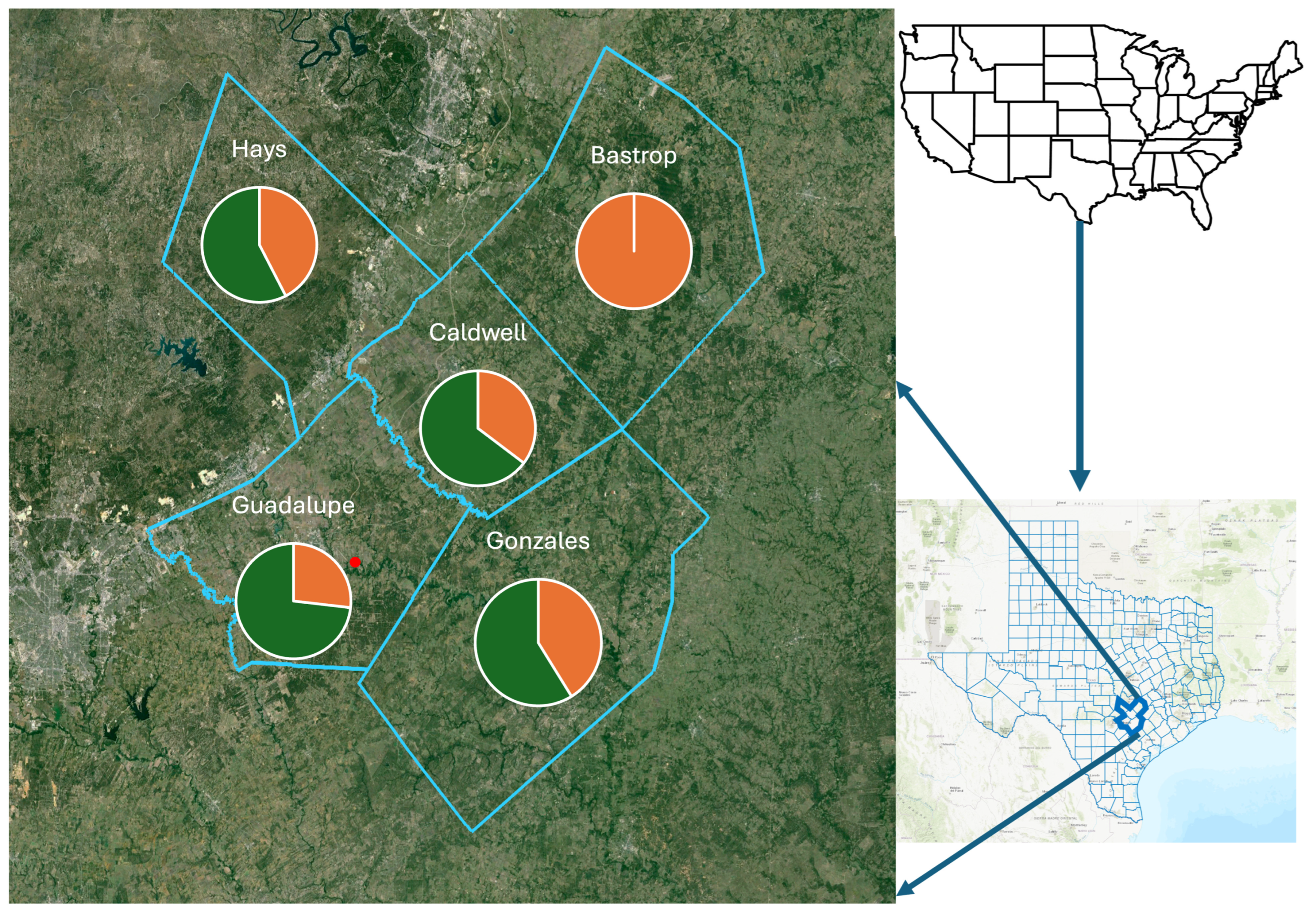
Triatomines were predominantly collected from peridomestic habitats in five counties in South Central Texas (Figure 3), with similar prevalence values for T. cruzi in four counties (Hays, Guadalupe, Caldwell, Gonzales), with 57% (19/33), 73% (30/41), 64% (11/17), and 58% (10/17), respectively, while T. cruzi was not detected in samples from Bastrop County (0/9). While prevalence values for Hays, Guadalupe, Caldwell, and Gonzales counties represented values generally found in Texas, the lack of any detection in neighboring Bastrop County was unexpected, and most likely a consequence of the small sample size. A small sample size might also have been the cause of the low prevalence detection in triatomes from Nebraska [35], but this was not considered to explain the samples from Arizona [36]. Here, the sample size was comparable, but the sites differed with respect to the most frequently encountered triatomine species, Triatoma rubida and Triatoma recurva, respectively [36].
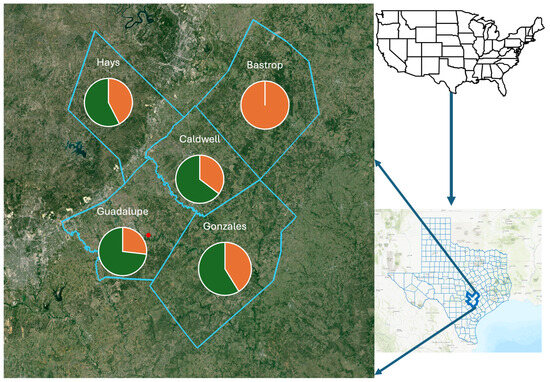
Figure 3.
The prevalence of Trypanosoma cruzi detected in five counties of South Central Texas, USA. Positive detection of the pathogen (green) in vector insects varied by county, with only Bastrop County providing only negative detections (orange). The red point provides the location enabling the determination of long-distance dispersal by the vector insects.
The presence of T. cruzi detected by qPCR-based analyses was confirmed by comparative sequence analyses of 18S rRNA gene fragments in 87% of the samples, with most of the sequences clustering with those of representatives of discrete typing unit (DTU) TcI [37] and the remaining related to TcI, but distinct from DTUs TcII to TcVI. DTU TcI is widely distributed in the Americas and found in Texas in Triatoma sp. from domestic and sylvatic areas [2,10,15]. Our phylogenetic analyses clearly identified members of the TcI lineage but could not explicitly characterize the remaining T. cruzi lineage with bootstrap support, even though underlying comparative raw sequence analyses showed a high similarity to TcIV for these isolates. Thus, while our analyses largely corroborate data on TcI lineage abundance in triatomes in Texas, the potential occurrence of TcIV in triatomines necessitates additional informative DNA sites that can enable a better-supported resolution of T. cruzi lineages found in our study.
Vectors mainly belonged to Triatoma gerstaeckeri (35% of which 65% were positive for T. cruzi), Triatoma sanguisuga (21%, 43% positive), and Paratriatoma leticularia (0.3%, 100% positive); however, 56% of the insects could not be identified at the species level using molecular tools. Of those identified, T. gerstaeckeri was confirmed as the most prominently encountered triatomine insect in Central Texas in our study, with T. sanguisuga and P. lecticularia detected much less frequently, as described in other studies [8,9,10,14,38]. Studies on triatomines in other southern states retrieved T. rubida and T. recurva (Arizona) [36], T. sanguisuga (Oklahoma, Nebraska) [34,35], or T. rubida (New Mexico, West Texas) [6] as the most commonly encountered triatomine insects, though studies often dealt with domestic rather than peridomestic or rural habitats.
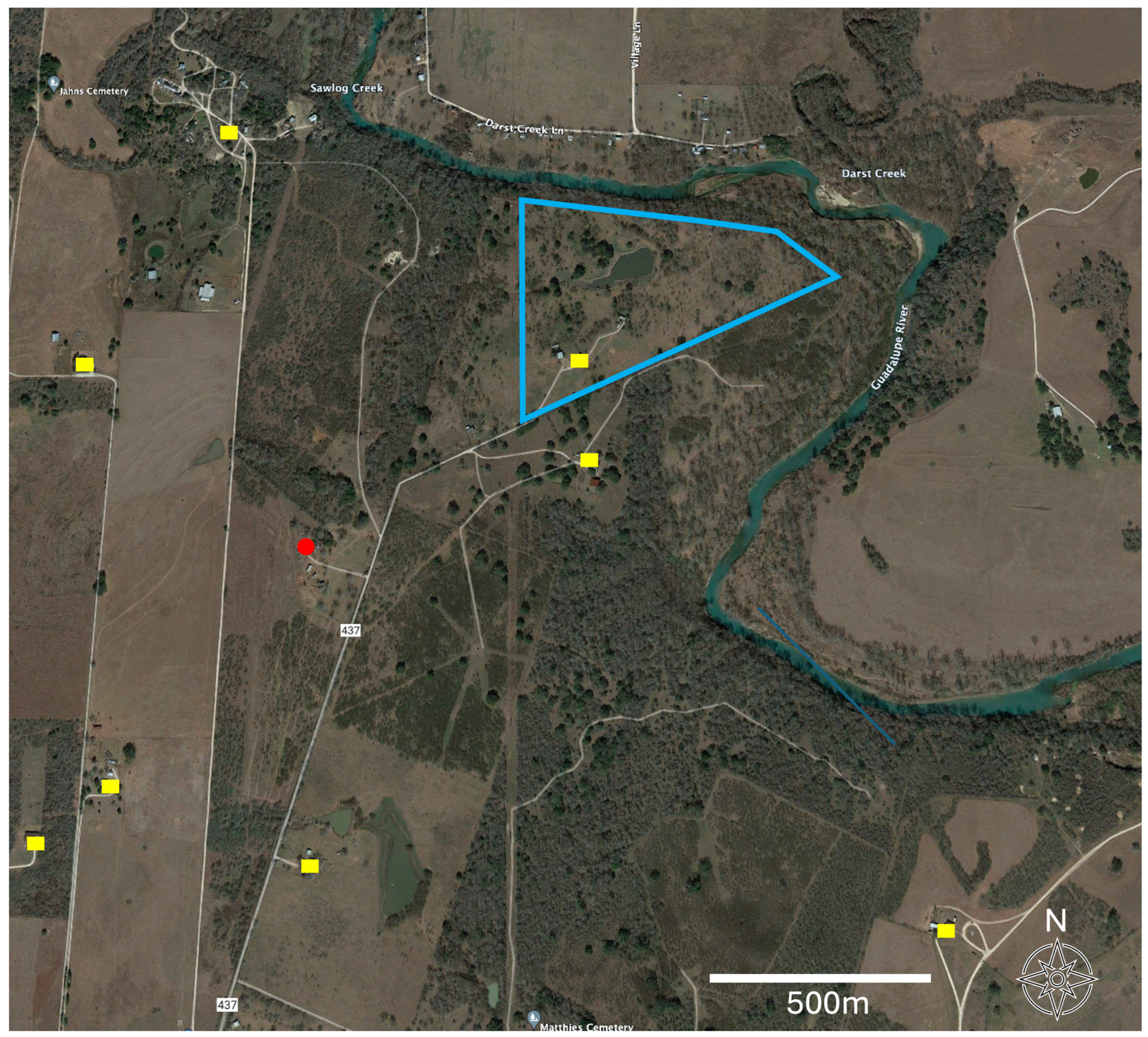
Food sources were determined in 29% (34/117) of the insects. This was a lower blood meal recovery rate than two other studies from Texas where the recovery was 92% [9] and 63% [22]. The rate of blood meal recovery success has been shown to decline with the satiation state of the vector insect [22] and when dead arthropods as opposed to living individuals are dissected [22]. It is possible that our generally rural sites provided fewer opportunities for regular meals by the insects, decreasing their satiation and our recovery rate. All of our arthropods were frozen and not living prior to dissection. For the blood meals recovered in this study, the majority of hosts were raccoons (P. lotor), which were identified in 42% (15/34) of these blood meals. With 83% (15/18) of those samples being positive for T. cruzi, the results were higher for raccoons than those reported from other states, with 34% and 43% [39,40]. These results are closer to those obtained for Texas in other studies with prevalence values of 62% and 70% [3,41]. In Texas, DTU TcIV has been identified as the sole T. cruzi lineage in raccoons [3,41], with one TcI/TcIV mixed infection as an exception [3]. Comparative raw sequence analyses in our study supported these results since T. cruzi from raccoons showed a high similarity to sequences identifying the TcIV lineage. Blood meal sources included humans, though expectedly represented a much lower prevalence among blood meals at 9% (33% positive) in our peridomestic habitats than was detected in domestic environments, providing up to 67% [9,15,42]. We detected a blood meal from an Asian forest tortoise (Manouria) without T. cruzi infection that was held at the location but contained in an enclosure 40–110 m from the arthropod capture site. Further, we noted that for one of the peridomestic samples, while the blood meal was human, the nearest domicile occupied by people at the time of the sampling was over 400 m away (Figure 4). Finally, we also detected bison as a blood meal source though without T. cruzi infection. Bison were held in paddocks spanning 600–1200 m from the sampling site, indicating the high mobility of triatomine insects (Figure 4). These last two serendipitous blood meal detections uniquely enabled us to report very large distances between the source of the blood meal to the subsequent Triatoma sp. capture sites. These foraging distances for the insect vectors indicate that a better understanding of the insect vector movement ecology is critical to understanding the potential for exposure and the modeling of this emerging disease.
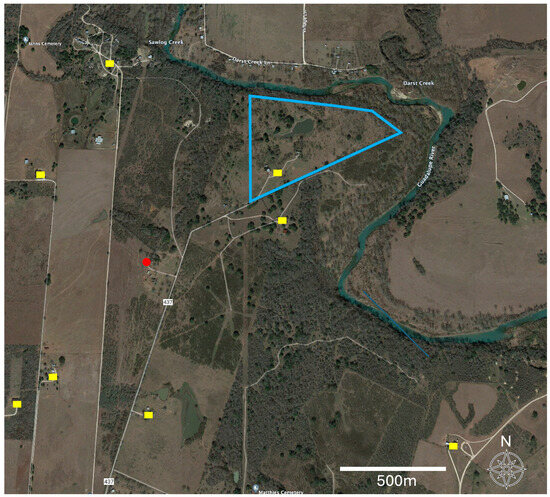
Figure 4.
Aerial image for a portion of Guadalupe County, Texas, USA. The site context can be examined in Figure 3. The image depicts a detailed landscape context for the sampling site for Triatomine insects (red point), human-occupied domiciles (yellow), and the boundaries (blue) of an adjacent American bison paddock. The unique detection of bison among the blood meals enabled a specific minimum dispersal distance to be calculated from the collection site to the bison enclosure (600–1200 m). Similarly, the human sites also provided a set of minimum options for the distance between the arthropod detection and its nearest potential human blood meal source (>400 m).
5. Conclusions
Our main goals for this project were to collect and identify both the triatomines potentially acting as vectors for Trypanosoma cruzi and further contribute to characterizing the blood sources that these insects use for meals in Texas. We were successful in triatomine sampling, with detections in all five counties in Central Texas. Trypanosoma cruzi was routinely detected among the potential triatomine vectors. Two species of Triatoma and one species of Paratriatoma were identified as positive for T. cruzi. The majority of blood meals were identified to have come from raccoons (Procyon lotor), but a wide array of vertebrate blood meals were confirmed including three samples of Homo sapiens. One of the human blood meals was also from a vector positive for T. cruzi. We were not able to successfully identify 44% (51/117) of the vector insects at the species level; simply storing individual specimens within freezer vials until laboratory processing would likely have prevented this difficulty.
The predominant sampling sites varied from peridomestic to rural, and the samples’ unique, non-native sources for the blood meals enabled us to infer foraging distances for the triatomine vectors. In one case this confirmed a 600 m minimum movement distance for the Triatomine from the blood meal to the daytime retreat where the insect was captured. We considered the variety of blood meal sources and the uniquely enabled forage distance measurements for the Triatoma sp. to be particularly relevant for modeling or assessments of the vector side of T. cruzi evaluations. However, these were a limited set of forage distances; moreover, they are unlikely to be the largest distances that occur. In Texas, but not only Texas, future research into these distances will be enabled because of the wide array of captive, enclosed exotic mammals and reptiles held privately across the landscape of the state. The occurrence of these non-native species could be leveraged by future research to reveal additional Triatomine dispersal/foraging data by conducting experiments collecting the vectors at known distances from unique exotic species enclosures.
Author Contributions
Conceptualization, R.J.K., M.R.J.F. and D.H.; methodology, R.J.K., T.G., H.B., A.V.G., M.R.J.F. and D.H.; validation, T.G., A.V.G., M.R.J.F. and D.H.; formal analysis, R.J.K., M.R.J.F. and D.H.; investigation, R.J.K. and H.B.; resources, M.R.J.F. and D.H.; data curation, T.G. and A.V.G.; writing—original draft preparation, R.J.K. and D.H.; writing—review and editing, M.R.J.F. and D.H.; visualization, R.J.K. and D.H.; supervision, M.R.J.F. and D.H.; project administration, D.H.; funding acquisition, M.R.J.F. and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Texas State University (protocol code 201648186 (06/01/2016); renewed #7994 06/15/2019), with Federal USFWS TE039544-1 and State TPWD permit SPR-0102-191 for studies involving animals.
Informed Consent Statement
Not Applicable.
Data Availability Statement
All sequences are available on GENBank/EMBL, as reported in the results.
Acknowledgments
We thank I. Castro-Arellano, M. Milholland, and S. Sirsi for their assistance in this project. We gratefully acknowledge the anonymous reviews and input from the editors, which improved the quality of this manuscript. The Alexander-Stone endowment at Texas State University provided partial funding for the project.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bern, C.; Messenger, L.A.; Whitman, J.D.; Maguire, J.H. Chagas disease in the United States: A public health approach. Clin. Microbiol. Rev. 2019, 33, e00023-19. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Kjos, S.; Yabsley, M.J.; Montgomery, S.P. Trypanosoma cruzi and Chagas’ disease in the United States. Clin. Microbiol. Rev. 2011, 24, 655–681. [Google Scholar] [CrossRef]
- Curtis-Robles, R.; Lewis, B.C.; Hamer, S.A. High Trypanosoma cruzi infection prevalence associated with minimal cardiac pathology among wild carnivores in central Texas. Int. J. Parasitol. Parasites Wildl. 2016, 5, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Munoz-San Martin, C.; Campo Verde Arbocco, F.; Saavedra, M.; Actis, E.A.; Rios, T.A.; Abba, A.M.; Morales, M.E.; Cattan, P.E.; Jahn, G.A.; Superina, M. High rates of Trypanosoma cruzi infection in goats from Mendoza province, Argentina: Parasite loads in blood and seasonal variation. Acta Trop. 2020, 208, 105493. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.; D’Andrea, P.S.; Xavier, S.C.; Mangia, R.H.; Fernandes, O.; Jansen, A.M. Trypanosoma cruzi infection in wild mammals of the National Park ‘Serra da Capivara’ and its surroundings (Piaui, Brazil), an area endemic for Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 379–388. [Google Scholar] [CrossRef]
- Rodriguez, F.; Luna, B.S.; Calderon, O.; Manriquez-Roman, C.; Amezcua-Winter, K.; Cedillo, J.; Garcia-Vazquez, R.; Tejeda, I.A.; Romero, A.; Waldrup, K.; et al. Surveillance of Trypanosoma cruzi infection in triatomine vectors, feral dogs and cats, and wild animals in and around El Paso County, Texas, and New Mexico. PLoS Negl. Trop. Dis. 2021, 15, e0009147. [Google Scholar] [CrossRef]
- Coura, J.R.; Dias, J.C. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem. Inst. Oswaldo Cruz. 2009, 104 (Suppl. 1), 31–40. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Guerra, T.; Maikis, T.J.; Milholland, M.T.; Castro-Arellano, I.; Forstner, M.R.J.; Hahn, D. The prevalence of Trypanosoma cruzi, the causal agent of Chagas disease, in Texas rodent populations. Ecohealth 2017, 14, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Gorchakov, R.; Trosclair, L.P.; Wozniak, E.J.; Feria, P.T.; Garcia, M.N.; Gunter, S.M.; Murray, K.O. Trypanosoma cruzi infection prevalence and bloodmeal analysis in triatomine vectors of Chagas disease from rural peridomestic locations in Texas, 2013–2014. J. Med. Entomol. 2016, 53, 911–918. [Google Scholar] [CrossRef]
- Curtis-Robles, R.; Auckland, L.D.; Snowden, K.F.; Hamer, G.L.; Hamer, S.A. Analysis of over 1500 triatomine vectors from across the US, predominantly Texas, for Trypanosoma cruzi infection and discrete typing units. Infect. Genet. Evol. 2018, 58, 171–180. [Google Scholar] [CrossRef]
- Curtis-Robles, R.; Wozniak, E.J.; Auckland, L.D.; Hamer, G.L.; Hamer, S.A. Combining public health education and disease ecology research: Using citizen science to assess Chagas disease entomological risk in Texas. PLoS Neglect. Trop. D 2015, 9, e0004235. [Google Scholar] [CrossRef] [PubMed]
- Hudson, F.P.; Homer, N.; Epstein, A.; Mondy, K. Acute Chagas disease manifesting as orbital cellulitis, Texas, USA. Emerg Infect. Dis. 2021, 27, 2937–2939. [Google Scholar] [CrossRef] [PubMed]
- Comeaux, J.M.; Curtis-Robles, R.; Lewis, B.C.; Cummings, K.J.; Mesenbrink, B.T.; Leland, B.R.; Bodenchuk, M.J.; Hamer, S.A. Survey of feral swine (Sus scrofa) infection with the agent of Chagas disease (Trypanosoma cruzi) in Texas, 2013–2014. J. Wildl. Dis. 2016, 52, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Kjos, S.A.; Snowden, K.F.; Olson, J.K. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector-Borne Zoonot. 2009, 9, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Curtis-Robles, R.; Meyers, A.C.; Auckland, L.D.; Zecca, I.B.; Skiles, R.; Hamer, S.A. Parasitic interactions among Trypanosoma cruzi, triatomine vectors, domestic animals, and wildlife in Big Bend National Park along the Texas-Mexico border. Acta Trop. 2018, 188, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Piron, M.; Fisa, R.; Casamitjana, N.; Lopez-Chejade, P.; Puig, L.; Verges, M.; Gascon, J.; Prat, J.G.I.; Portus, M.; Sauleda, S. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop. 2007, 103, 195–200. [Google Scholar] [PubMed]
- Bosseno, M.F.; Barnabe, C.; Gastelum, E.M.; Kasten, F.L.; Ramsey, J.; Espinoza, B.; Breniere, S.F. Predominance of Trypanosoma cruzi lineage I in Mexico. J. Clin. Microbiol. 2002, 40, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Moreira, O.C.; Ramirez, J.D.; Velazquez, E.; Melo, M.F.A.D.; Lima-Ferreira, C.; Guhl, F.; Sosa-Estani, S.; Marin-Neto, J.A.; Morillo, C.A.; Britto, C. Towards the establishment of a consensus real-time qPCR to monitor Trypanosoma cruzi parasitemia in patients with chronic Chagas disease cardiomyopathy: A substudy from the BENEFIT trial. Acta Trop. 2013, 125, 23–31. [Google Scholar]
- Pinto, C.M.; Ocana-Mayorga, S.; Tapia, E.E.; Lobos, S.E.; Zurita, A.P.; Aguirre-Villacís, F.; MacDonald, A.; Villacís, A.G.; Lima, L.; Teixeira, M.M.G.; et al. Bats, trypanosomes, and triatomines in Ecuador: New insights into the diversity, transmission, and origins of Trypanosoma cruzi and Chagas disease. PLoS ONE 2015, 10, e0139999. [Google Scholar]
- Noyes, H.A.; Stevens, J.R.; Teixeira, M.; Phelan, J.; Holz, P. A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. Int. J. Parasitol. 1999, 29, 331–339. [Google Scholar] [CrossRef]
- Murphy, W.J.; O’Brien, S.J. Designing and optimizing comparative anchor primers for comparative gene mapping and phylogenetic inference. Nat. Protoc. 2007, 2, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Kjos, S.A.; Marcet, P.L.; Yabsley, M.J.; Kitron, U.; Snowden, K.F.; Logan, K.S.; Barnes, J.C.; Dotson, E.M. Identification of bloodmeal sources and Trypanosoma cruzi infection in triatomine bugs (Hemiptera: Reduviidae) from residential settings in Texas, the United States. J. Med. Entomol. 2013, 50, 1126–1139. [Google Scholar] [CrossRef]
- Monteiro, F.A.; Barrett, T.V.; Fitzpatrick, S.; Cordon-Rosales, C.; Feliciangeli, D.; Beard, C.B. Molecular phylogeography of the Amazonian Chagas disease vectors Rhodnius prolixus and R. robustus. Mol. Ecol. 2003, 12, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Boakye, D.A.; Tang, J.; Truc, P.; Merriweather, A.; Unnasch, T.R. Identification of bloodmeals in haematophagous Diptera by Cytochrome B heteroduplex analysis. Med. Vet Entomol. 1999, 13, 282–287. [Google Scholar] [CrossRef]
- Molaei, G.; Oliver, J.; Andreadis, T.G.; Armstrong, P.M.; Howard, J.J. Molecular identification of blood-meal sources in Culiseta melanura and Culiseta morsitans from an endemic focus of eastern equine encephalitis virus in New York. Am. J. Trop. Med. Hyg. 2006, 75, 1140–1147. [Google Scholar] [CrossRef]
- Cicero, C.; Johnson, N.K. Higher-level phylogeny of new world vireos (Aves: Vireonidae) based on sequences of multiple mitochondrial DNA genes. Mol. Phylogenet. Evol. 2001, 20, 27–40. [Google Scholar] [CrossRef]
- Pfeiler, E.; Bitler, B.G.; Ramsey, J.M.; Palacios-Cardiel, C.; Markow, T.A. Genetic variation, population structure, and phylogenetic relationships of Triatoma rubida and T. recurva (Hemiptera: Reduviidae: Triatominae) from the Sonoran Desert, insect vectors of the Chagas’ disease parasite Trypanosoma cruzi. Mol. Phylogenet. Evol. 2006, 41, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Justi, S.A.; Russo, C.A.M.; Mallet, J.R.D.; Obara, M.T.; Galvao, C. Molecular phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae). Parasite Vector 2014, 7, 149. [Google Scholar] [CrossRef]
- Pearson, W.R.; Lipman, D.J. Improved tools for biological sequence comparison. Proc. Nat. Acad. Sci. USA 1988, 85, 2444–2448. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-joining Method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits of phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.E.; Lineberry, M.W. Detection of Trypansoma cruzi in kissing bugs (Hemiptera: Reduviidae: Triatominae) collected across Oklahoma. J. Med. Entomol. 2022, 59, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.H.; Koch, K.; Roachell, W.; Delgado, B.; Bast, J. First record of an established population of Triatoma sanguisuga (Hemiptera: Reduviidae) in Richardson County, Nebraska. J. Med. Entomol. 2021, 58, 2519–2523. [Google Scholar] [CrossRef]
- Behrens-Bradley, N.; Smith, S.; Beatty, N.L.; Love, M.; Ahmad, N.; Dorn, P.L.; Schmidt, J.O.; Klotz, S.A. Kissing bugs harboring Trypanosoma cruzi, frequently bite residents of the US Southwest but do not cause Chagas disease. Am. J. Med. 2020, 133, 108–114.e13. [Google Scholar] [CrossRef]
- Zingales, B.; Andrade, S.G.; Briones, M.R.S.; Campbell, D.A.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.M.; Machado, C.R.; et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. I Oswaldo Cruz. 2009, 104, 1051–1054. [Google Scholar] [CrossRef]
- Wozniak, E.J.; Lawrence, G.; Gorchakov, R.; Alamgir, H.; Dotson, E.; Sissel, B.; Sarkar, S.; Murray, K.O. The biology of the triatomine bugs native to South Central Texas and assessment of the risk they pose for autochthonous Chagas disease exposure. J. Parasitol. 2015, 101, 520–528. [Google Scholar] [CrossRef]
- Majeau, A.; Pronovost, H.; Sanford, A.; Cloherty, E.; Anderson, A.N.; Balsamo, G.; Gee, L.; Straif-Bourgeois, S.C.; Herrera, C. Raccoons as an important reservoir for Trypanosoma cruzi: A prevalence study from two metropolitan areas in Louisiana. Vector-Borne Zoonot. 2020, 20, 535–540. [Google Scholar] [CrossRef]
- Pietrzak, S.M.; Pung, O.J. Trypanosomiasis in raccoons from Georgia. J. Wildl. Dis. 1998, 34, 132–136. [Google Scholar] [CrossRef]
- Hodo, C.L.; Banuelos, R.M.; Edwards, E.E.; Wozniak, E.J.; Hamer, S.A. Pathology and discrete typing unit associations of Trypanosoma cruzi infection in coyotes (Canis latrans) and raccoons (Procyon lotor) of Texas, USA. J. Wildl. Dis. 2020, 56, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ordonez-Krasnowski, P.C.; Lanati, L.A.; Gaspe, M.S.; Cardinal, M.V.; Ceballos, L.A.; Gurtler, R.E. Domestic host availability modifies human-triatomine contact and host shifts of the Chagas disease vector Triatoma infestans in the humid Argentine Chaco. Med. Vet Entomol. 2020, 34, 459–469. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).