Simple Summary
Fatty liver injury is common in farmed fish, but its molecular mechanisms are not well understood. An eight-week feeding trial was conducted to investigate the effects of dietary chitosan on high-fat diet (HFD)-induced liver damage in Nile tilapia. Six diets with varying fat and chitosan levels were tested. Fish on the HFD showed increased growth, fat accumulation, elevated liver injury markers, and higher levels of pro-apoptotic and inflammatory markers. Chitosan supplementation mitigated these effects and reduced intestinal injury by improving antioxidant defense and reducing inflammation and apoptosis through the nrf2 and cox2 pathways.
Abstract
Fatty liver injury is a prevalent condition in most farmed fish, yet the molecular mechanisms underpinning this pathology remain largely elusive. A comprehensive feeding trial spanning eight weeks was conducted to discern the potential of dietary chitosan in mitigating the deleterious effects of a high-fat diet (HFD) while concurrently exploring the underlying mechanism. Growth performance, haemato-biochemical capacity, antioxidant capacity, apoptotic/anti-apoptotic gene expression, inflammatory gene expression, and histopathological changes in the liver, kidney, and intestine were meticulously assessed in Nile tilapia. Six experimental diets were formulated with varying concentrations of chitosan. The first three groups were administered a diet comprising 6% fat with chitosan concentrations of 0%, 5%, and 10% and were designated as F6Ch0, F6Ch5, and F6Ch10, respectively. Conversely, the fourth, fifth, and sixth groups were fed a diet containing 12% fat with chitosan concentrations of 0%, 5%, and 10%, respectively, for 60 days and were termed F12Ch0, F12Ch5, and F12Ch10. The results showed that fish fed an HFD demonstrated enhanced growth rates and a significant accumulation of fat in the perivisceral tissue, accompanied by markedly elevated serum hepatic injury biomarkers and serum lipid levels, along with upregulation of pro-apoptotic and inflammatory markers. In stark contrast, the expression levels of nrf2, sod, gpx, and bcl-2 were notably decreased when compared with the control normal fat group. These observations were accompanied by marked diffuse hepatic steatosis, diffuse tubular damage, and shortened intestinal villi. Intriguingly, chitosan supplementation effectively mitigated the aforementioned findings and alleviated intestinal injury by upregulating the expression of tight junction-related genes. It could be concluded that dietary chitosan alleviates the adverse impacts of an HFD on the liver, kidney, and intestine by modulating the impaired antioxidant defense system, inflammation, and apoptosis through the variation in nrf2 and cox2 signaling pathways.
1. Introduction
The aquaculture sector has grown and prospered over the past few decades [1]. Despite its advancement, the aquaculture sector faces significant challenges, such as the limited availability of high-performing fry strains, the elevated cost of aquafeeds, and limited disease resistance [2].
In fact, short-term feeding of fish benefits from boosting dietary lipid levels within specified bounds [3]. However, as the concentration of dietary lipids increases, a series of adverse effects begins to emerge [4].
The primary organ in charge of regulating lipid uptake, synthesis, redistribution, catabolism, and storage is the liver. Steatosis is largely caused by fatty acid and triacylglycerol imbalances [5,6].
A high-fat diet is posited as the instigator of hepatic lipidosis in farmed fish [3,7,8,9]. This can subsequently compromise fish health and reduce production [10]. Fat accumulation has been shown in several studies utilizing animal models to trigger inflammation [11,12,13,14,15] and contribute to liver damage, hypoimmunity, and decreased appetite [8]. Furthermore, fat buildup can accelerate lipid peroxidation, inducing oxidative stress [16], and disrupt lipid metabolism, thereby inhibiting growth [3,8,10,17].
nrf2 serves as a pivotal regulator of the adaptive response to oxidative stress. Upon exposure to oxidative stress, nrf2 is activated through its dissociation from the kelch-like ECH-associated protein-1 (Keap1), subsequently translocating to the nucleus [18], where it activates a battery of antioxidant genes [19]. However, severe oxidative stress suppresses the nrf2 pathway [20,21,22]. To counteract hepatic steatosis, nrf2 activation inhibits lipogenesis and promotes beta-oxidation of fatty acids [23], while nrf2 pathway inactivation could potentially exacerbate liver damage induced by hepatotoxicants [24,25]. Genetically regulated cell death is called apoptosis, and it may be brought on by a stream of physiological events. It is widely accepted in the scientific community that mitochondrial malfunction is a crucial indicator of apoptosis [26]. cyt-c is released by dysfunctional mitochondria, which then activates the downstream effector cas-3, resulting in the execution of apoptotic changes [27].
Occludin, a trans-membrane protein, members of the claudin family, junction adhesion molecules, and linker proteins such as zo-1 are all components of tight junctions [28]. Increased intestinal permeability results from decreased intestinal tight junction protein expression, which can seriously jeopardize intestinal barrier integrity [29]. Impaired intestinal integrity is accompanied by intestinal permeability changes and histological changes [30,31,32]. The intestine predominantly responds to various stressors, including oxidative stress and inflammation [33,34]. Hence, oxidative stress and inflammation in the intestines are crucial for maintaining intestinal function. Consequently, to maintain intestinal function in the face of oxidative stress, an optimal candidate with free radical scavenging activity as well as antioxidant and anti-inflammatory capacity is urgently needed.
Natural materials have recently shown distinctive, affordable, and secure antibacterial properties. Recently, a remarkable number of organic bioactive substances have been investigated for potential use as methods of reducing the detrimental effects of an HFD in farmed fish [35,36,37].
Chitosan (β-(1-4)-N-acetyl-D-glucosamine) has proven to be among the most intelligent and safe natural cationic biopolymers. It is a member of a class of glycosaminoglycan-like polysaccharides having structural characteristics [38]. Following cellulose, chitosan is the second-most abundant polymer [39]. It offers exceptional qualities such as a non-toxic nature, biocompatibility, biodegradability, and increased solubility, along with immunological restoration attributes [40]. Chitosan is derived from the de-acetylation process of chitin and is recognized as a crucial constituent found in the exoskeletons of aquatic crustaceans, such as crab, crayfish, and shrimp, as well as in certain terrestrial organisms and the cell walls of some microorganisms [41]. Chitosan is receiving considerable interest and has already begun to contribute significantly to aquaculture’s sustainability. It satisfies environmental standards since it is an eco-friendly substance that also promotes the effective use of reagents and minimizes potential waste [42]. There are several uses for chitosan in the biomedical and pharmaceutical industries [15]. Chitosan is an active growth promoter that can be considered a crucial element for the growth of aquatic animals. The major effect of chitosan is improving the morphological structure of the small intestine, which may potentially augment nutrient absorption and subsequently improve growth performance [43]. Even at low concentrations, dietary chitosan was found to enhance nitrogen utilization and amino acid digestibility [44].
Nile tilapia (Oreochromis niloticus) is highly favored among cultured fish, ranking as the third most prominent group of cultured fish globally, surpassed by carp and salmonids. It is extensively cultured in about 100 countries in both tropical and subtropical regions [45,46]. The success of Nile tilapia in aquaculture is ascribed to its superior survival rate in poorly oxygenated environments, comparatively high resistance to diseases, and adaptability to consume a diverse range of foods [47]. Moreover, in cultured fish, there is a paucity of evidence supporting the potential pathogenesis of fatty liver injury. Consequently, a prevailing challenge in aquaculture is the development of strategies to mitigate the oxidative stress and inflammation induced by high-fat diets. Chitosan’s antioxidant activity has garnered significant attention. Research indicates that chitosan exhibits hepatoprotective effects due to its antioxidant properties [48]. Similarly, AlKandari et al. [49] stated that supplementing with natural antioxidants like propolis, chitosan, or their combination during ibuprofen use enhanced the reduction in toxic effects and improved the antioxidant system and anti-inflammatory response.
Our study constituted novel insights on hepatotoxicity and intestinal injury induced by an HFD and evaluated the ameliorative effects of dietary chitosan supplementation on intestinal integrity, oxidative status, and apoptosis/anti-apoptosis.
2. Materials and Methods
2.1. Ethical Approval
Following the normal operating procedures approved by the Institutional Animal Care and Animal Ethics Committee, Faculty Aquatic and Fisheries Sciences, Kafrelsheikh University, Egypt, the current experiment was conducted on Nile tilapia (Oreochromis niloticus) (IAACUC-KSU-028-2022).
2.2. Experimental Design
The experiment was carried out at the Fish Processing and Biotechnology department of the Faculty of Aquatic and Fisheries Sciences at Kafrelsheikh University in Egypt. A total of 216 juvenile Nile tilapia with an initial mean weight of 17.38 g ± 0.22 g (mean ± SD) were procured from a private farm located in Kafrelsheikh, Egypt. The fish were then acclimated to the experimental system for two weeks in glass aquariums and fed on the basal diet (Table 1) twice daily. Next, they were equally distributed among eighteen glass aquariums (80 × 45 × 35 cm, 12 fish/tank) with three replicates per treatment (six treatments, as shown in Table 2). Each aquarium was equipped with a mechanical filter (JAD, China) to eliminate waste from the water and an air stone for oxygen supply. Throughout the 60-day trial, water quality parameters, including temperature, pH, and dissolved oxygen, were monitored daily and recorded with an average of 26.19 ± 4 °C, 5.9 ± 0.8 mg/L, and 7.50 ± 0.1, respectively. The total ammonia level was checked once a week. All fish groups were fed twice daily with 4–6% of their body weight divided into two meals at 8:00 am and 3:00 pm.

Table 1.
Ingredient and proximate compositions (g/kg, as-fed) of the normal and high-fat diets supplemented with low and high doses of chitosan at both levels of dietary fat.

Table 2.
Experimental groups.
2.3. Experimental Diet Designs
Six iso-nitrogenous (32% crude protein) test diets were designed as displayed in Table 1 and Table 2. The experimental diets were divided into two categories: normal fat diets (F6, 6% fat) and high-fat diets (F12, 12% fat), then each category was divided into three groups: chitosan-free diet (devoid of chitosan), low chitosan diet (5 g/kg diet), and high chitosan diet (10 g/kg diet) [50,51]. Diets were then extruded through a 2.33-mm-diameter die in a meat grinder, air-dried at room temperature, and stored in the freezer at −20 °C until used. A sample of each diet was collected and analyzed for proximate composition (g 100 g−1 as is) following AOAC (1995) procedures. Crude protein was determined based on the AOAC 990.03 Kjeldahl method by using the Kjeldahl apparatus (Infitek Co., Ltd. Shandong, China). Also, the Pet Ether Soxhlet Extraction Method (AOAC 945.16) was followed for the analysis of crude fat. Ash was measured using a muffle furnace at 600 °C for 5 to 6 h (AOAC 942.05). The digestible energy (DE), crude fiber (CF), calcium (Ca), and phosphorus (P) content of each diet were calculated using the DE, Ca, and P values stated in the feed ingredient composition table reported in NRC (1993).
2.4. Sample Collection
Following a fasting period of 24 h, all fish in each group were counted and weighed. From each aquarium tank, six fish (18 fish/group) were randomly chosen and anesthetized with FA-100 anesthetic (diluted 1:5000, DS Pharma Animal Health Company, Osaka, Japan). Of these, three fish per tank were designated for blood aspiration and tissue sampling (including liver, kidney, and intestine). Importantly, tissue samples were subsequently divided into two parts: the first part was kept in 10% formalin for histopathological analysis, while the other part was immediately submerged in liquid nitrogen and then stored at −80 °C for subsequent gene expression analysis. Furthermore, three fish from each tank (totaling nine fish per group) were randomly chosen for liver weighting and measurement of liver fat, hepato-somatic index (HSI), and intra-peritoneal fat index (IPF), and then the whole fish were stored at −20 °C to determine their chemical body composition.
2.5. Growth Performance and Morphometric Indices
The fish in each tank were weighed and counted at the beginning of the experiment, then biweekly, and at the end of the experiment. Growth and feed utilization parameters, including the initial body weight (IBW), final body weight (FBW), body weight gain (BWG), specific growth rate (SGR), feed conversion ratio (FCR), and protein efficiency rate (PER), were calculated. At the end of the experiment, 3 fish per tank were randomly chosen, euthanized with tricaine methane sulphonate, weighed, and dissected to obtain liver and visceral weights for further calculation of the hepato-somatic index (HIS) and viscerosomatic index (VSI). Also, from each group, 9 fish were analyzed (3 fish per tank) to determine the crude protein, crude fat, and ash content according to AOAC [52] guidelines. Liver samples were collected and analyzed to measure liver fat content according to the instructions of the Folch method [53].
The growth parameters were determined as follows:
Body weight gain (BWG, g) = FBW − IBW
Specific growth rate (SGR) (% body weight gain/day) = ((Ln FW − Ln IW)/t) × 100
Feed conversion rate (FCR) = Feed intake (g)/Weight gain (g)
Protein efficiency ratio (PER) = Live weight gain (g)/Dry protein intake (g)
Hepato-somatic index (HSI) = (Liver weight/Whole body weight) × 100
Intra-peritoneal fat (IPF) = (Visceral fat weight/Whole body weight) × 100
2.6. Hematological and Serum Biochemical Analysis
At the end of the experiment, two different blood samples were collected from the caudal vein using a 3 mL disposable syringe. The first blood sample was collected in tubes containing EDTA (Ethylenediaminetetraacetic acid) as an anticoagulant to quantify erythrocytes, hemoglobin (Hb), packed cell volume (PCV), total leukocyte counts, and differential leucocyte counts (monocytes, lymphocytes, heterophils, and eosinophils) according to [54]. The second blood sample was collected without anticoagulant and then centrifuged at 3000 rpm for 10 min at 4 °C using a benchtop refrigerated centrifuge (Heraeus Megafuge 8R, Thermo Fisher Scientific, Germany) to separate the serum, which has been stored at −20 °C until biochemical analyses. Commercial kits (BIODIGNOSTIC, Giza, Egypt) were used to test plasma for total proteins, albumin, cholesterol, triglycerides, high-density lipoprotein-C (HDL-C), low-density lipoprotein (LDL), urea, creatinine, glucose, globulins, aspartate aminotransferase (Ast), alanine aminotransferase (Alt), and lactate dehydrogenase (LDH). The globulin concentration (Glob) and consequently the albumin-to-globulin ratio (A/G) were calculated. VLDL-C concentrations were calculated using the standard Friedewald equation [55].
2.7. Histopathology Study
Liver specimens were immersed in 10% phosphate-buffered neutral formalin (dehydrated and cleared in xylene), and then all specimens were processed into paraffin blocks and cut off at a thickness of 5 μm. Sections were stained by standard histology techniques using hematoxylin and eosin, along with Masson trichrome for collagen fiber staining. Liver sections were oxidized with 1% periodic acid for 5 min, washed, and incubated in Schiff’s reagent for 10 min at room temperature. The sections were then washed and counterstained with Mayer’s hematoxylin for 30 s, washed, dehydrated, and mounted in DPX for histological analysis. Images were then acquired using an inverted light microscope at the Institute of Nanoscience and Nanotechnology, Kafrelsheikh University, Egypt (Leica Microsystems-Fluorescence Model, DMi8 manual, Wetzlar, Germany).
2.8. Total RNA Extraction, cDNA Synthesis, and RT-qPCR Assay
Total RNA was extracted from 50 mg of the livers and intestines of Oreochromis niloticus using GENEzolTM Reagent (Geneaid, UK) following the manufacturer’s instructions. The integrity of the extracted RNA was confirmed by electrophoresis using an ethidium bromide-stained 2% agarose gel. The concentration and purity of RNA were determined using a Nanodrop BioDrop spectrophotometer (Biochrom Ltd., Cambridge CB23 6DW, Cambridgeshire, UK) based on the A260/A280 nm ratio. Five μg of RNA samples were reverse transcribed using the TOP script TM RT Dry Mix (dt18/dN6 plus) kit (enzynomics, Daejeon, Republic of Korea). Gene expression profiling was performed in Rotor Gene-Q (Qiagen, 40724 Hilden, Germany) using gene-specific primer sequences for the amplification of immune-related genes such as tumor necrosis factor alpha (tnfa), interleukin-1β (il1b), interleukin-10 (il10), and cyclooxygenase 2 (cox2); antioxidant genes such as nuclear factor-E2-related factor 2 (nrf2), kelch-like ECH-associated protein 1 (keap1), glutathione peroxidase (gpx), and superoxide dismutase (sod2); and apoptosis genes such as baxa, B-cell lymphoma 2 (bcl2a), cytochrome c (cyc1), cysteine-aspartic acid protease 9 (casp9), and cysteine-aspartic acid protease 3 (casp3). Tight junction genes, including claudin 3 (cldn3c), claudin 7 (cldn7), and tight junction protein 1a (tjp1a), encode for the Zonula occludens-1 protein, and tight junction protein 1a (tjp2a) encodes for the Zonula occludens-2 protein, as shown in Table 3 [56,57,58,59,60,61,62,63,64,65]. The amplification reaction was performed using the TOPrealTM qPCR 2X PreMIX (SYBR Green with Low Rox) kit (enzynomics, Daejeon, Republic of Korea). The reaction volume was adjusted to 20 μL consisting of 10 µL SYBR Green, 0.6 µL of forward and reverse specific primer, 1 µL of cDNA template, and nuclease-free water to make the final volume 20 µL. The PCR program was carried out with the following conditions: activation at 95 °C for 15 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at the primer-specific temperature for 15 s, and extension at 72 °C for 25 s. This was followed by a melt curve analysis to assess the specificity of amplification at 72 °C to 95 °C. The efficiency of amplification was estimated depending on the qPCR slope by using the formula: E = 10−1/slope. Normalization of qPCR data was performed using the geometric averaging of two internal reference genes: 18S ribosomal RNA (18s rRNA) and Ubiquitin C (ubce) to calculate fold change. All genes were tested in triplicate. CT values for each sample were determined and incorporated in an efficiency-corrected fold change (2−ΔΔCT) calculation based on [66], and mRNA expressions for each sample were normalized against the average of 18s ribosomal rRNA (18srRNA) and Ubiquitin C (ubce) as internal reference genes.

Table 3.
Primers used for qRT-PCR analysis.
2.9. Statistical Analysis
Data are demonstrated as the mean ± standard error of the mean (M ± SEM). Preliminary, Shapiro–Wilk’s test was employed to assess data normality, while Levene’s test was used to evaluate variance homogeneity, both conducted with a significance level set at p < 0.05. Percentage data were subjected to arcsine transformation prior to analysis of variances. To investigate the differential effects of high fat and chitosan and their interaction, body indices, growth performances, hematological parameters, biochemical analyses, histo-morphometric measurements, and relative gene expression were analyzed using a two-way ANOVA, followed by Tukey’s multiple comparison test (p < 0.05). All statistical analyses were conducted using GraphPad Prism (version 9.5, GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Effects on Growth, Feed Utilization, Morphometric Parameters, and Body Nutritional Composition
As presented in Table 4, no significant differences in final body weight (FBW), body weight gain (BWG), or specific growth rate (SGR) were detected among the experimental groups. Nile tilapia exposed to an HFD without chitosan supplementation (F12Ch0) showed a non-statistical decrease in BWG and SGR with the highest FCR, HSI, IPF, liver fat %, and body fat %, and the lowest PER and body protein % after 8 weeks of dietary intervention compared with the control untreated group and the other treatment groups. However, dietary supplementation with chitosan significantly reverses the aforementioned findings in a dose–response manner. Moreover, the ash content was significantly higher in F12Ch5 and F12Ch10 compared to the HFD-fed group (F12Ch0) and the other groups.

Table 4.
Growth, feed utilization, morphometric indices, and proximate analysis of Nile tilapia (Oreochromis niloticus) fed on normal and high-fat diets supplemented with low and high doses of chitosan at both levels of dietary fat for 8 weeks.
3.2. Hematological Parameters
As seen in Table 5, the erythrogram parameters showed significantly higher RBCs, Hb, and PCV in the F6Ch10 group compared to the control normal fat diet group and the other treatment groups. However, the lowest value for these parameters was detected in the HFD-fed group F12Ch0, while dietary chitosan supplementation significantly increased the RBC count in the F12Ch5 and F12Ch10 groups compared with the HFD-fed group F12C0. No significant differences were observed in mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) among all the groups. The leukogram parameter showed that the highest white blood cell (WBC) count, heterophil, lymphocytes, monocytes, and eosinophil counts were detected in the HFD-fed group (F12Ch0) compared with the control normal fat diet group and the other treatment groups. Dietary chitosan given to HFD-fed fish led to a significant decrease in the number of WBCs, lymphocytes, monocytes, and eosinophils, with a non-significant increase in heterophil count compared with the HFD-fed group (F12Ch0).

Table 5.
Hematological parameters of Nile tilapia (Oreochromis niloticus) fed on normal and high-fat diets supplemented with low and high doses of chitosan at both levels of dietary fat for 8 weeks.
3.3. Serum Biochemical Parameters
As explained in Table 6, the results of serum biochemical findings exhibited significantly higher AST, ALT, and LDH enzyme activities and TG, TC, LDL-C, VLDL-C, creatinine, and glucose in the HFD-fed group (F12Ch0) compared with the control and the other treatment groups. There was no significant difference among the groups in blood urea nitrogen. There was a significant decrease in total proteins, albumin, globulins, and high-density lipoprotein (HDL) in the F12Ch0 and F12Ch5 groups compared to the control group and other treated groups. Moreover, chitosan supplementation either with or without an HFD succeeded in normalizing these parameters compared to the HFD-fed (F12Ch0) group in a dose–response manner.

Table 6.
Biochemical parameters of Nile tilapia (Oreochromis niloticus) reared for 8 weeks and fed on normal and high-fat diets supplemented with low and high doses of chitosan at both levels of dietary fat.
3.4. Histopathological Examination
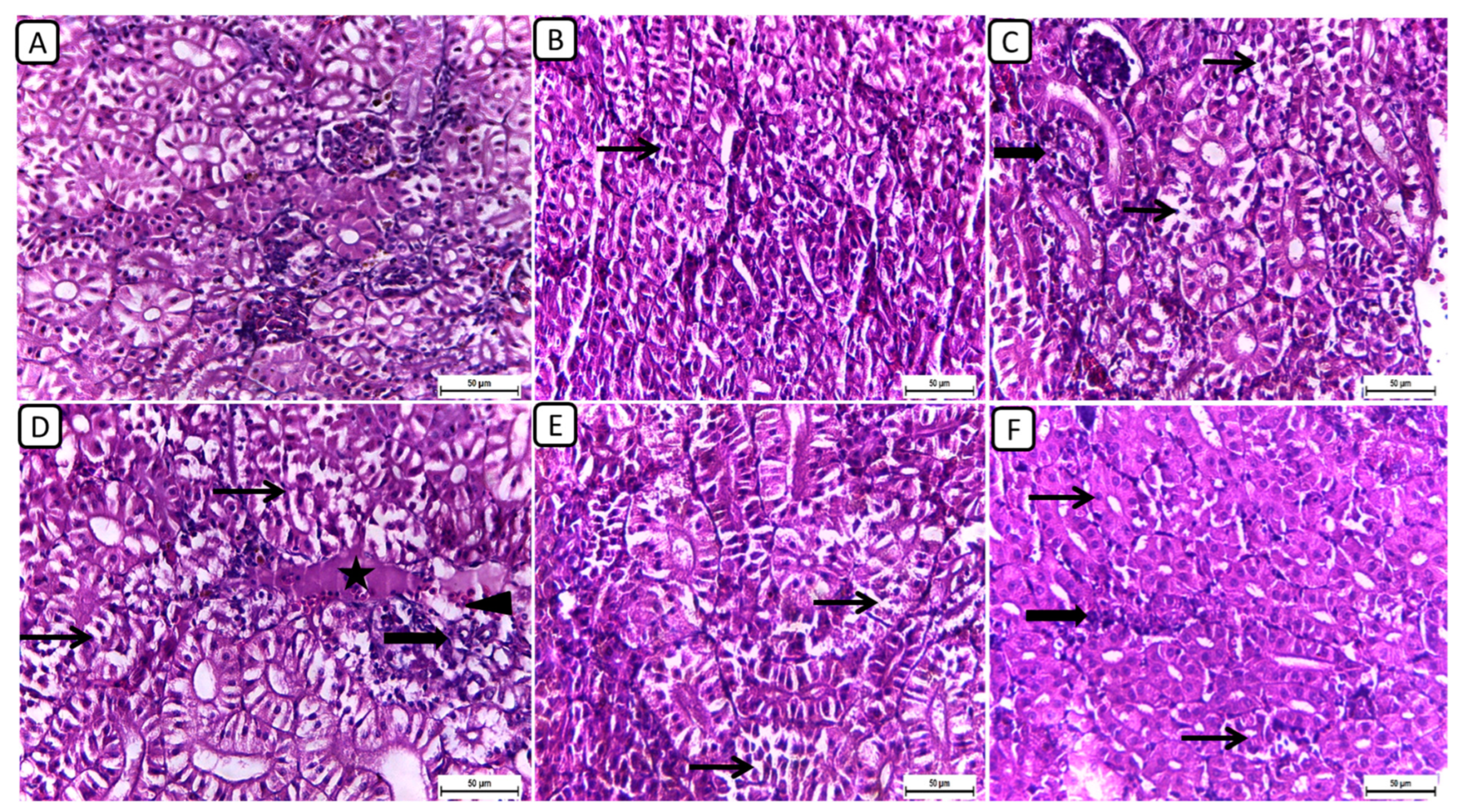
In Figure 1, the histopathological examination of kidney sections from different treatment groups revealed notable variations in renal architecture and cellular morphology. In the F6Ch0 group, the kidney displayed a histologically normal tubular and glomerular structure. The renal cells exhibited moderately vacuolar cytoplasm and were accompanied by adequate interstitial cells. In the F6Ch5 group, the histological structure was nearly normal, characterized by less vacuolated cytoplasm and only slight interstitial cell infiltration. However, the kidney sections from the F6Ch10 group showed focal tubular degeneration and interstitial inflammatory aggregations. Conversely, the F12Ch0 group exhibited severe renal damage, with diffuse tubular injury represented by severe necrotic tubular epithelial cells, intraluminal eosinophilic hyaline degeneration (renal casts), and interstitial fibrosis. These changes were accompanied by an infiltration of inflammatory cells and numerous RBCs, illustrating extensive renal damage in the high-fat diet group. In the F12Ch5 group, the kidney demonstrated mild tubular necrosis. Additionally, in the F12Ch10 group, the kidney sections showed mostly normal tubular endothelial lining with reduced cytoplasmic vacuolation and few interstitial inflammatory cell infiltrations.
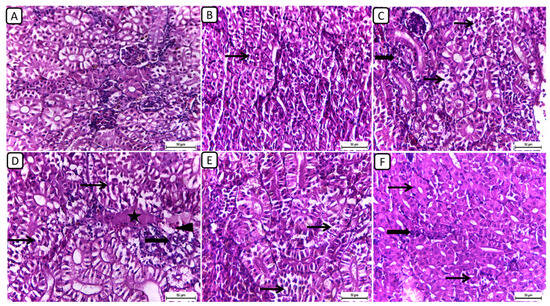
Figure 1.
Representative photomicrographs of kidneys from different treatment groups. (A) F6Ch0 shows normal tubular and glomerular structure with moderately vacuolar cytoplasm in addition to adequate interstitial cells. (B) F6Ch5 shows nearly normal histological structure with less vacuolated cytoplasm in addition to slight interstitial cell infiltration (thin arrow). (C) F6Ch10 shows focal tubular degeneration (thin arrow) with interstitial inflammatory aggregations (thick arrow). (D) F12Ch0 shows diffuse tubular damage represented by severe necrotic tubular epithelial cells (thin arrows), intraluminal esinophilic cellular cast (star), and interstitial fibrosis (thick arrow) admixed with lymphocytes and numerous RBCs (arrowhead). (E) F12Ch5 shows mild tubular necrosis (thin arrows). (F) F12Ch10 shows mostly normal tubular endothelial lining with less vacuolation (thin arrows) and little interstitial inflammatory cell infiltration (thick arrow). Scale bar = 50 μm.
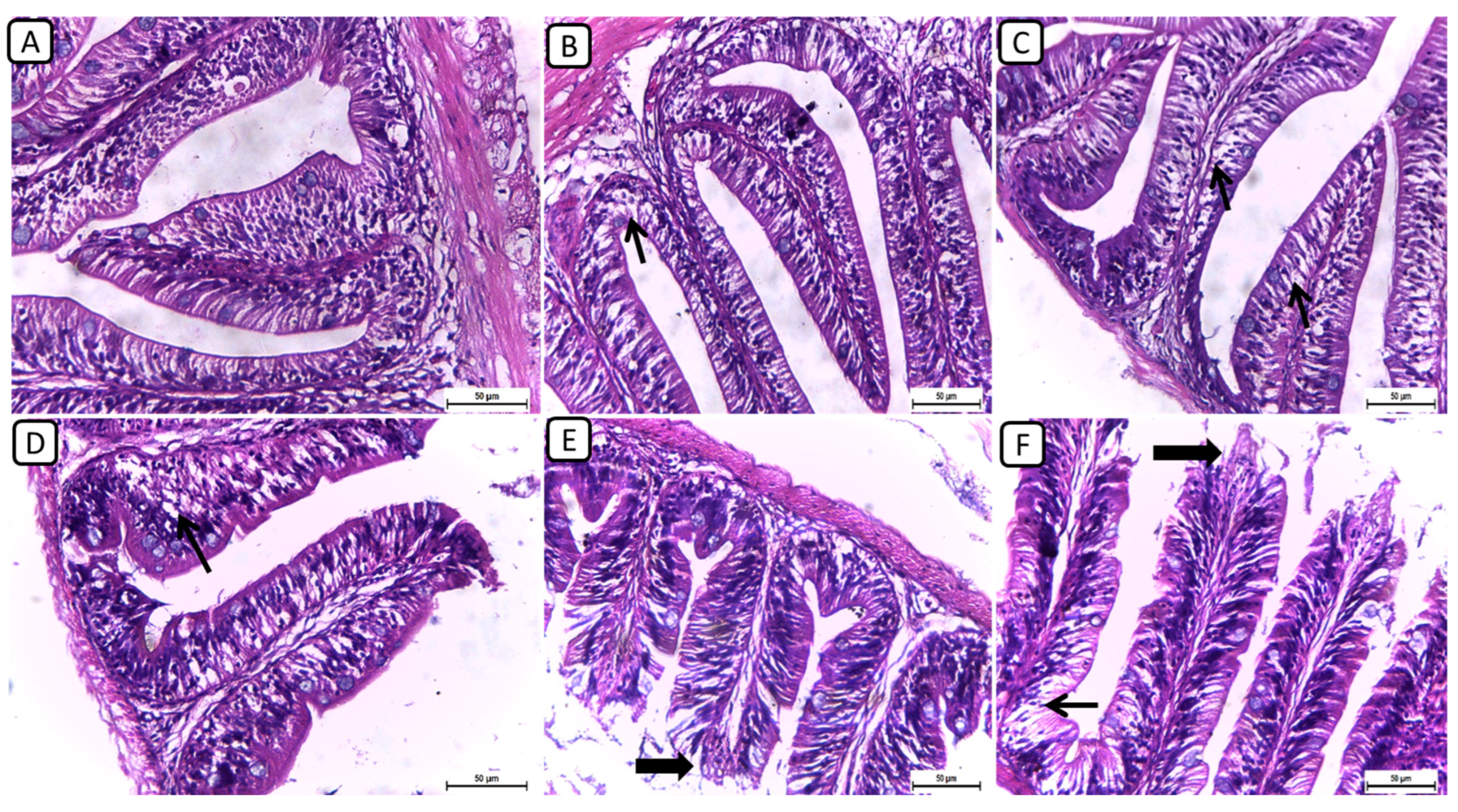
On the other hand, the histopathological examination of intestinal sections from various treatment groups revealed distinctive findings in the intestinal architecture and mucosal integrity (Figure 2); however, no severe degenerative or necrotic changes were detected. In F6Ch0, the intestine exhibited a normal histological structure, characterized by well-preserved intestinal villi with an intact mucous membrane. In both the F6Ch5 and F6Ch10 chitosan groups, the intestinal mucosa remained mostly normal, with few intracellular vacuoles. Conversely, the F12Ch0 group displayed shortened intestinal villi with numerous vacuoles. In the F12Ch5 group, there was minimal apical mucosal loss, and the intestinal mucosa appeared expanded, reflecting partial improvement compared to the F12Ch0 group. Additionally, the F12Ch10 group showed elongated intestinal villi, slight apical mucosal loss, and a few intestinal vacuoles.
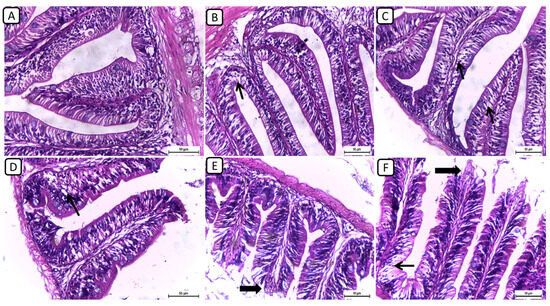
Figure 2.
Representative photomicrographs of intestines from different treatment groups. (A) F6Ch0 shows a normal structure of intestinal villi with an intact mucous membrane. (B) F6Ch5 shows normal intestinal mucosa except for a few intestinal vacuoles (thin arrow). (C) F6Ch10 shows mild intestinal vacuolation (thin arrows). (D) F12Ch0 shows shortened intestinal villi with many vacuoles (thin arrow). (E) F123Ch5 shows minimal apical loss (thick arrow) and expanded intestinal mucosa. (F) F12Ch10 shows elongation of the intestinal villi, slight apical mucosal loss (thick arrow), and little intestinal vacuolation (thin arrow). Scale bar = 50 μm.
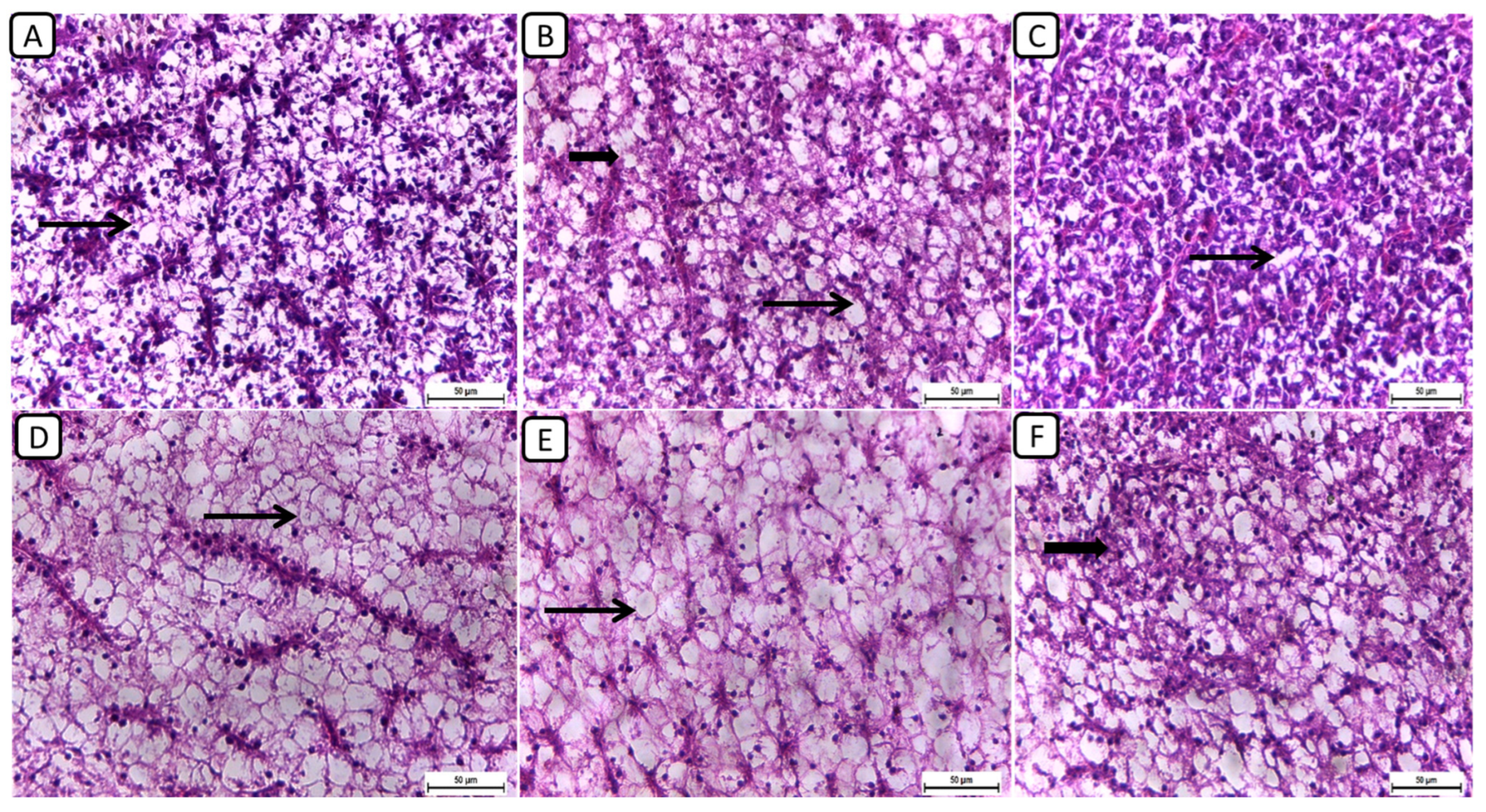
Regarding the histopathological examination of liver sections from different groups (Figure 3), the F6Ch0 group demonstrated a generally normal appearance, characterized by normal lobular architecture and normal hepatocytes with normal vacuoles of lipid and glycogen. In the F6Ch5 chitosan group, hepatocytes appeared normal, displaying normal lipid vacuoles with slight vascular dilatation. For the F6Ch10 chitosan group, signs of hydropic degeneration and slight blood vessel dilatation appeared, suggesting mild hepatocellular changes in response to chitosan supplementation. In contrast, the F12Ch0 group exhibited disarrangement of the lobular hepatic structure and marked hepatocellular necrosis, indicating severe liver damage; the F12Ch5 group showed a relatively normal hepatic structure with slight vascular dilatation; and the liver sections from the F12Ch10 group exhibited dilatation of the hepatic blood vessels with little inflammatory cell aggregation, hydropic degeneration of some hepatocytes, and increased eosinophilia of the cytoplasm, along with pyknosis of the nuclei.
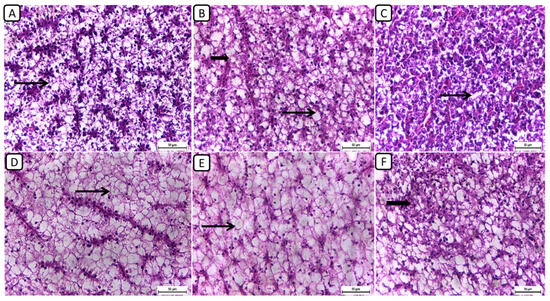
Figure 3.
Representative photomicrographs of livers from different treatment groups. (A) F6Ch0 shows normal hepatic architecture with a normal vacuolation appearance. (B) F6Ch5 shows mostly normal hepatocytes and normal lipid vacuoles (thin arrow) with a few slight degenerative changes (thick arrow). (C) F6Ch10 shows hydropic degeneration (thin arrow) with slight blood vessel dilatation. (D) F12Ch0 shows marked hepatocellular necrosis with a loss of most nuclei (thin arrow). (E) F12Ch5 shows relatively vacuolated cytoplasm with intact nuclei. (F) F12Ch10 shows normal hepatocytes with slight degenerative changes in some cells (thick arrow). Scale bar = 50 μm.
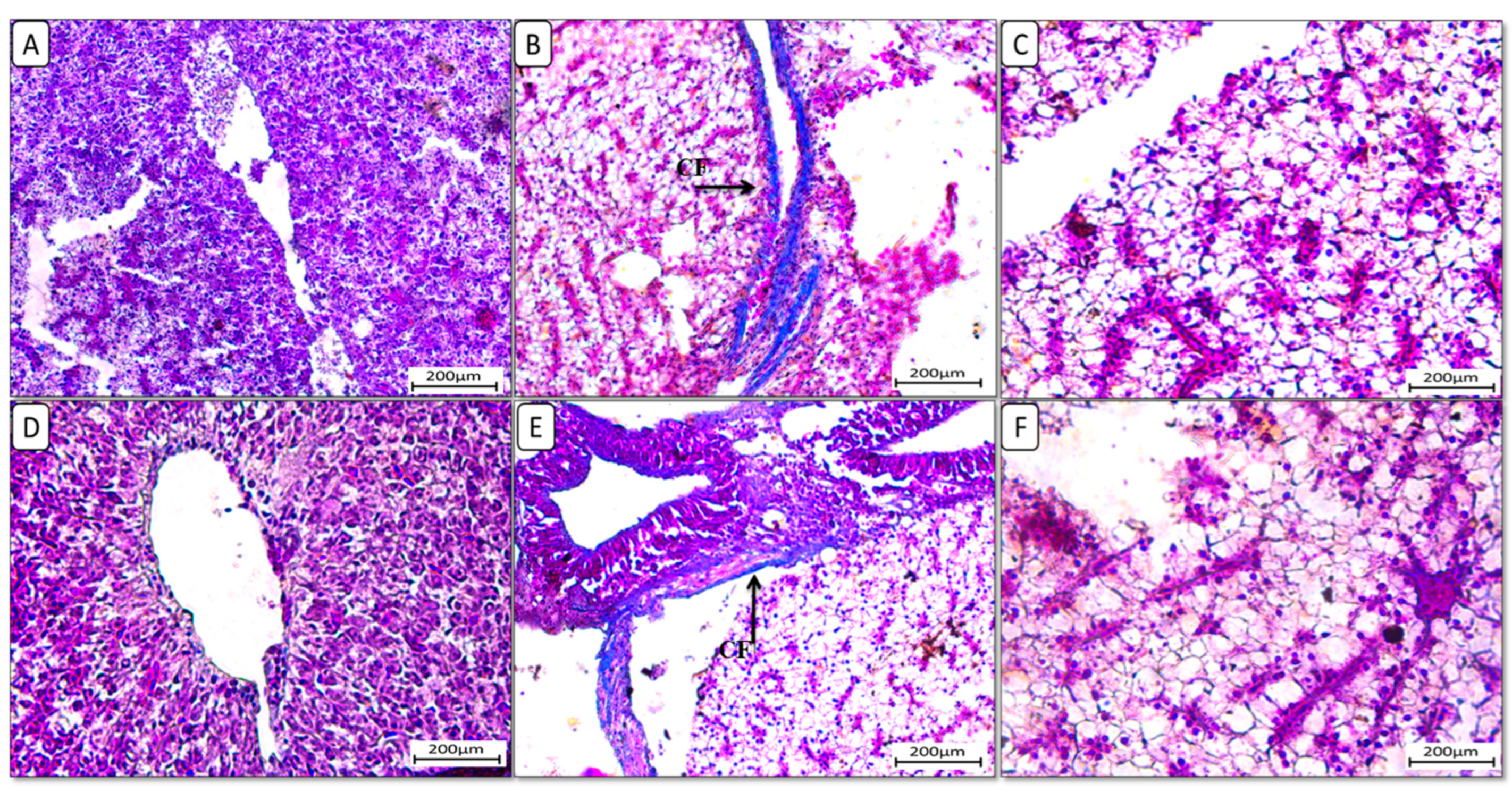
Additionally, liver sections stained with Masson trichrome stain (Figure 4) showed the accumulation of collagen fibers in the HFD-fed group F12Ch0, while the control normal fat diet group showed an absence of collagen fibers. Groups F6Ch5 and F6Ch10, fed low and high chitosan doses for 8 weeks, respectively, displayed no collagen fibers. Nile tilapia fed with F12Ch5 had slight collagen fibers accumulated around blood vessels, while collagen fibers were completely reduced in those fed with F12Ch10.
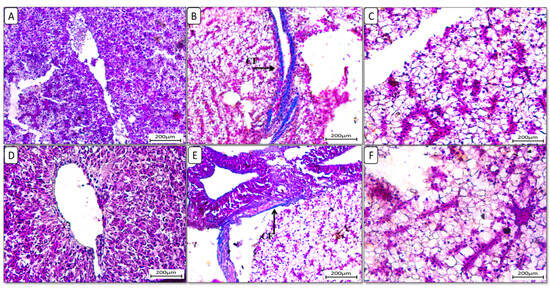
Figure 4.
Liver histological morphology in Nile tilapia fed with different experimental diets for 8 weeks. Liver tissue sections were stained with Masson trichrome stain. (A): F6Ch0; (B): F12Ch0 (accumulation of collagen fibers (CFs)); (C): F6Ch5; (D): F6Ch10; (E): F12Ch5 (mild accumulation of collagen fibers (CFs)); (F): F12Ch10. Scale bar = 200 μm.
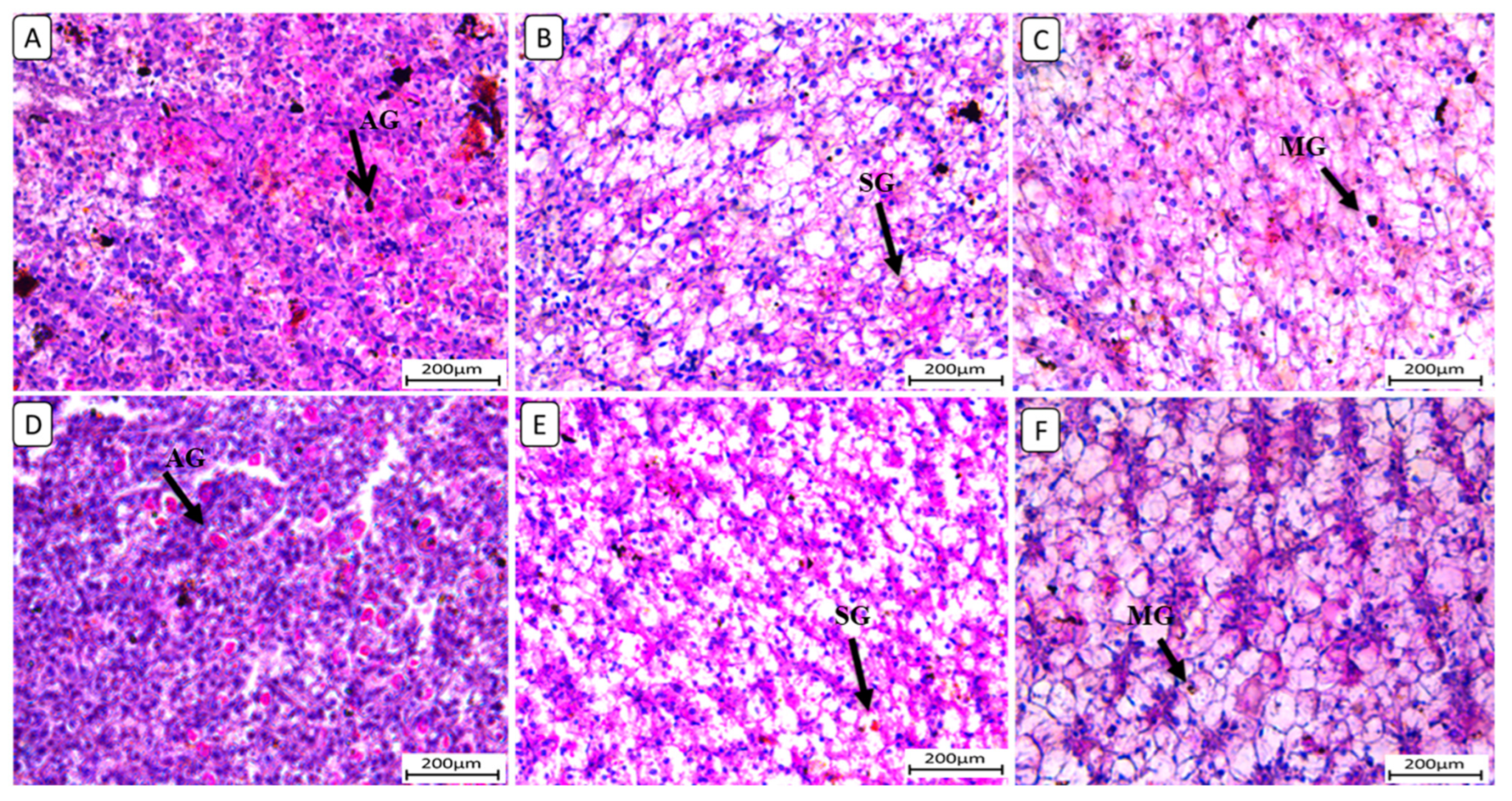
Also, liver sections stained with periodic acid Schiff reagent (PAS) (Figure 5) in the control group revealed that glycogen granules occupied most of the cytoplasm of hepatocytes in F6Ch0. The HFD-fed group (F12Ch0) showed a reduction in PAS reaction compared to the control group. Glycogen granules appeared in groups of Nile tilapia fed with F6Ch5 and F6Ch10, respectively. Glycogen granules disappeared in Nile tilapia fed with F12Ch5, but initially appeared in those fed with F12Ch10.
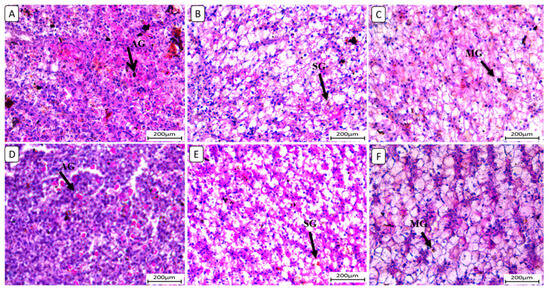
Figure 5.
Histological liver sections of Nile tilapia fed with different experimental diets for 8 weeks. Tissue sections were stained with a periodic acid Schiff reagent (PAS) stain. (A): F6Ch0 (accumulated glycogen (AG)); (B): F12Ch0 (slight glycogen (SG)); (C): F6Ch5 (mild glycogen (MG)); (D): F6Ch10 (accumulated glycogen (AG)); (E): F12Ch5 (slight glycogen (SG)); (F): F12Ch10 (mild glycogen (MG)). Scale bar = 200 μm.
At the same time, histomorphometric analysis of muscle tissue samples from all experimental groups showed the effect of adding chitosan to HFD-fed Nile tilapia (Table 7), where there was a significant improvement in muscle fiber count, total area, average size of muscle fiber, and area percent by adding chitosan to an HFD.

Table 7.
Histomorphometric analysis of the muscle fiber of Nile tilapia (Oreochromis niloticus) reared for 8 weeks and fed on normal and high-fat fat diets supplemented with low and high doses of chitosan at both levels of dietary fat.
3.5. Differential Gene Expression Analysis
3.5.1. Antioxidative Function-Related Gene Expression
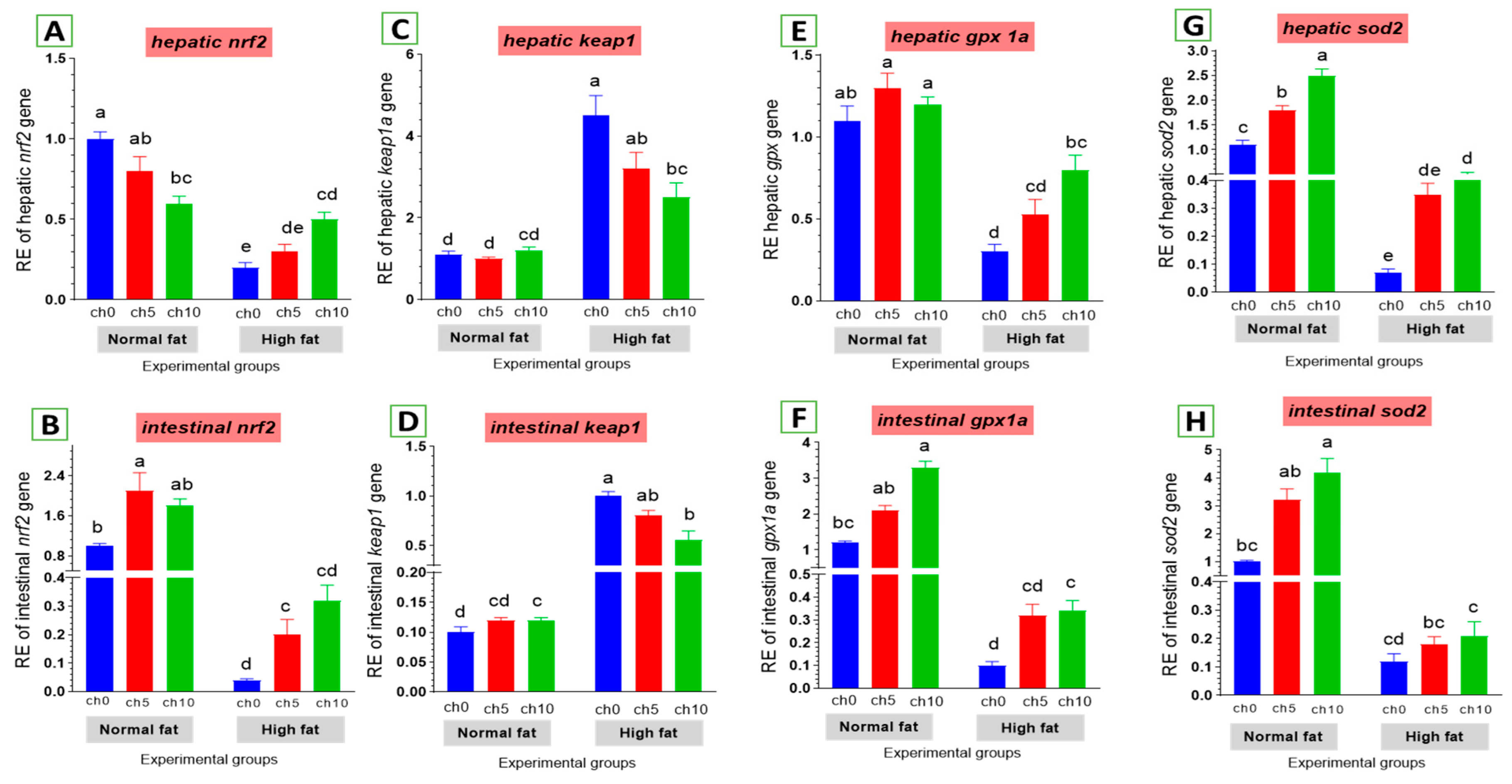
Oxidative stress is considered a crucial factor in liver and intestinal injuries. We also examined the expression of critical genes involved in the antioxidant signaling pathway in the liver and intestine of tilapia after 60 days of HFD administration, as shown in Figure 6. The hepatic and intestinal nrf2 expression levels exhibited a decreasing tendency (Figure 6A,B), but kaep1 expression in the liver and intestine showed an upward tendency (Figure 6C,D) in HFD-treated tilapia (F12C0) compared with the control, and the other treated groups suggested that excess deposition of fat impaired the nrf2 pathway and attenuated antioxidant defense. Moreover, reduced hepatic and intestinal expression levels of gpx (Figure 6E,F) and sod (Figure 6G,H) were also detected in HFD-treated tilapia (F12Ch0) compared with the control normal fat group and the other treated groups. However, dietary chitosan, either with or without an HFD, significantly reverses these findings in a dose-dependent manner.
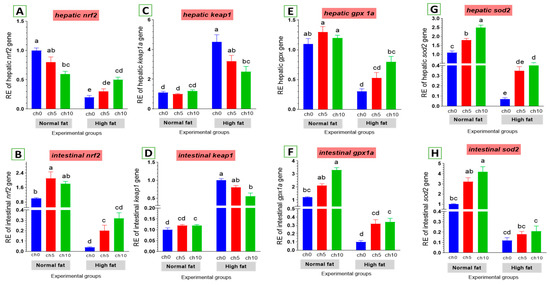
Figure 6.
Differential expression of different antioxidant genes in the liver and intestine of Nile tilapia groups fed on normal and high-fat diets with chitosan. (A,B) Nrf2: Nuclear factor erythroid 2-related factor 2, (C,D) Kaep1: Kelch-like ECH-associated protein 1, (E,F) GPx: Glutathione peroxidase, (G,H) SOD: Superoxide dismutase. Columns with different superscript letters in the same figure are significantly different (p ≤ 0.05).
3.5.2. Apoptosis-Related Gene Expression
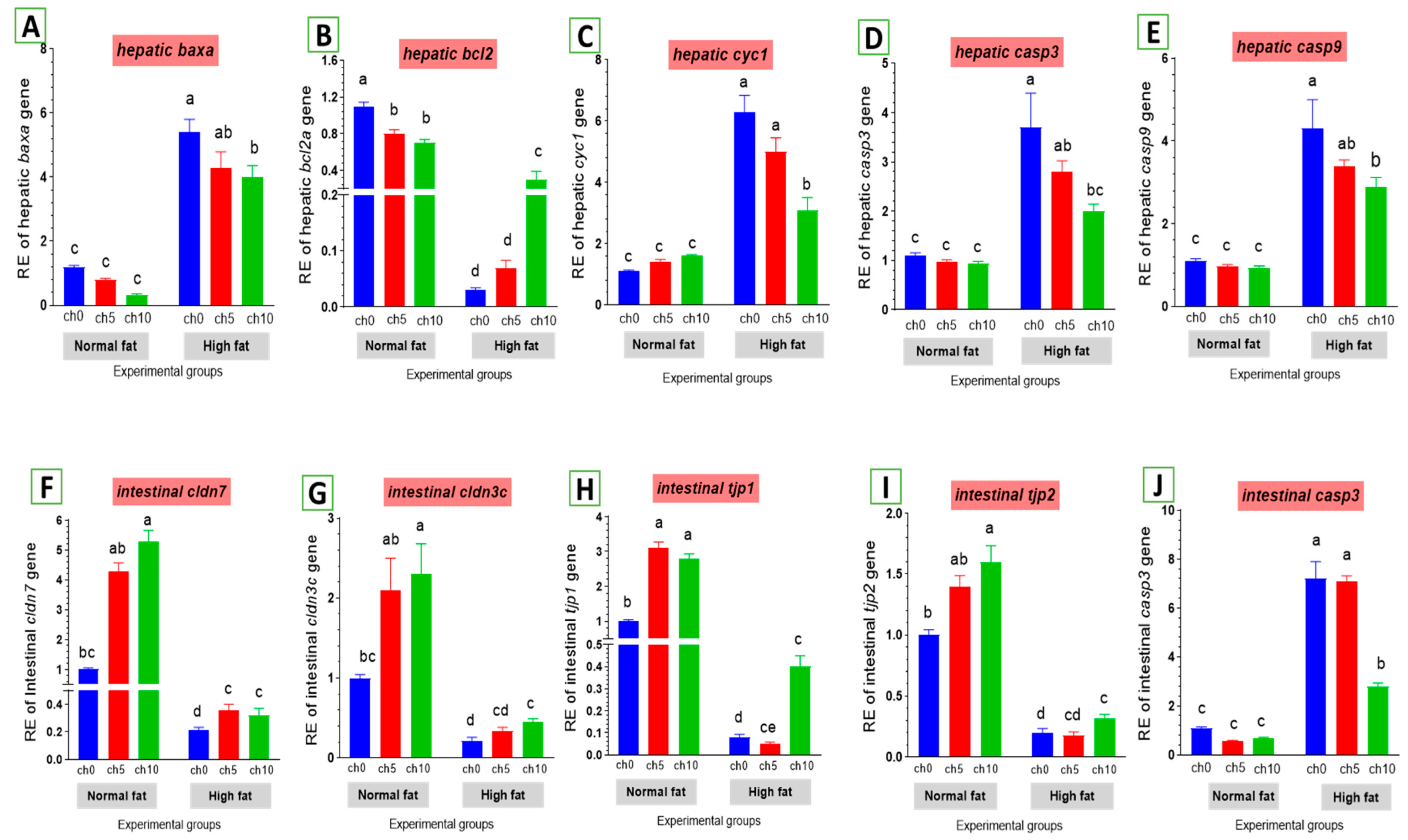
The expression levels of apoptosis-related genes are listed in Figure 7. The mRNA levels of baxa (Figure 7A), cytochrome c (cytc) (Figure 7C), caspase-3 (cas-3) (Figure 7D), and caspase-9 (Figure 7E) were greatly elevated. Conversely, the bcl-2 inhibitor of apoptosis was signally downregulated in the liver and intestine of HFD-fed tilapia compared to the control and the other treated groups. However, dietary chitosan inclusion markedly downregulated the apoptotic genes while enhancing the anti-apoptotic gene (bcl-2) (Figure 7B).
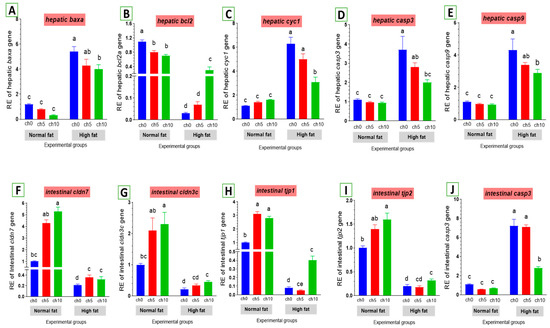
Figure 7.
Differential expression of apoptosis-related genes in the liver and tight junction-related genes in the intestine of Nile tilapia groups fed on normal and high-fat diets with chitosan. (A) Bax: Bcl-2 associated X-protein, (B) Bcl2: B-cell lymphoma 2, (C) Cyc1: Cytochrome c, (D) Casp3: Cysteine-aspartic acid protease3 in the liver, (E) Casp9: Cysteine-aspartic acid protease9, (F) cldn7: Claudin7, (G) cldn3c: Claudin3c, (H) tjp1: Zonula occludens-1, (I) tjp2: Zonula occludens-2, (J) casp3: Cysteine-aspartic acid protease3 in the intestine. Columns with different superscript letters in the same figure are significantly different (p ≤ 0.05).
3.5.3. Tight Junction Gene Expression in the Intestine
As shown in Figure 7, an HFD significantly reduced the expression of tight junction protein genes in the intestine, including claudin-3 (Figure 7G), claudin-7 (Figure 7F), tjp1 (Figure 7H), and tjp2 (Figure 7I). Moreover, dietary chitosan significantly increased the levels of mRNA expression of claudin-3, claudin-7, tjp1, and tjp2 compared with an HFD.
3.5.4. Inflammatory-Related Gene Expression
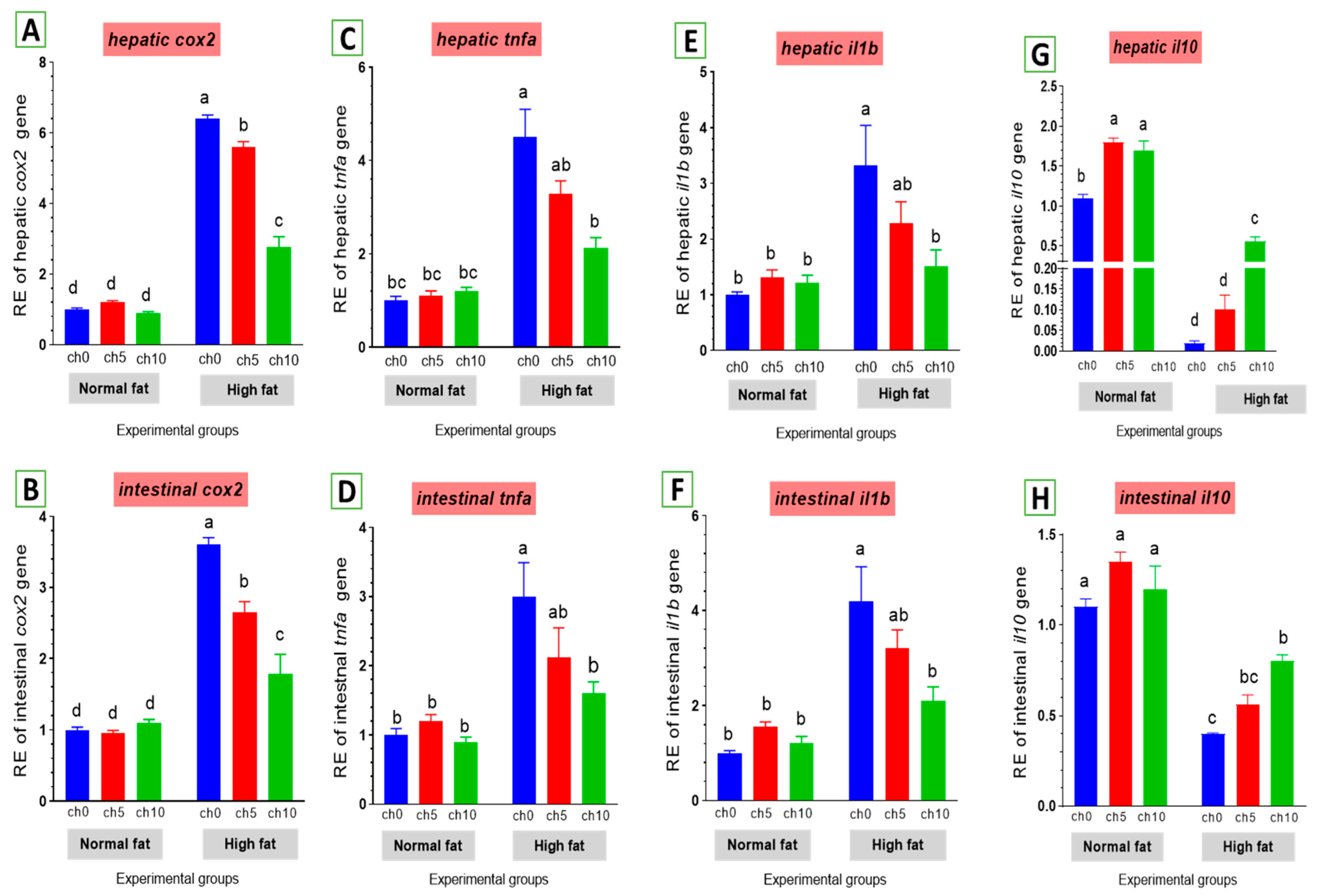
Chronic inflammation was evaluated in fatty liver injury via measuring changes in cox2 and other inflammatory factors. The mRNA levels of cox2 and pro-inflammatory factors, including tnf-α and il-1β, were elevated by an HFD after 60 days of feeding (Figure 8). tnf-α (Figure 8C,D) and il-1β (Figure 8E,F) were markedly upregulated in the liver and intestine of the high-fat diet-fed group F12Ch0 in Nile tilapia after 60 days compared with F6Ch0 and the other treated groups. Moreover, hepatic and intestinal il-10 expression levels were markedly inhibited compared with the control and the other treatment groups (Figure 8G,H). However, chitosan supplementation markedly reverses these observations compared with the HFD-fed group, F12Ch0.
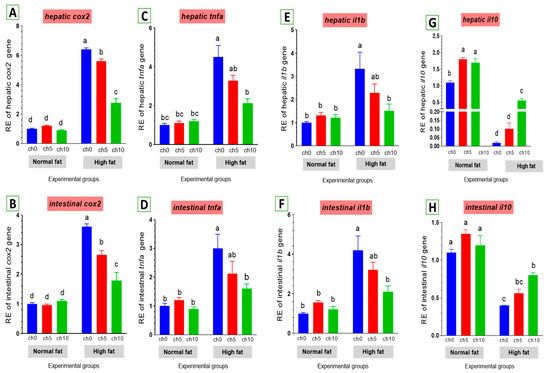
Figure 8.
Differential expression of immune-related genes in the liver and intestine of Nile tilapia groups fed on normal and high-fat diets with chitosan. (A,B) cox2: Cyclooxygenase 2 in the liver and intestine, (C,D) tnf-a: Tumor necrosis factor alpha, (E,F) il-1β: Interleukin-1beta, (G,H) il-10: Interleukin-10. Columns with different superscript letters in the same figure are significantly different (p ≤ 0.05).
4. Discussion
Lipids are a dietary energy resource that plays a consequential role in fish growth. However, excessive amounts of lipids in the diet will have adverse impacts on fish growth and health, including poor feed intake, slow growth, low immunity, and oxidative stress [67,68]. The formation of a fatty liver is mostly a result of the high content of fat in feed, which renders the body unable to consume too much fat itself. The deposition of fat will consequently cause an increase in the fat content in the fish body, which is principally deposited in liver cells and can result in a fatty liver [69].
In mammals, lipotoxicity is the primary contributor to several diseases associated with excess fat accumulation, such as a fatty liver, obesity, and diabetes [70,71]. Excessive deposition of triglycerides in the liver is considered an important biomarker of a fatty liver. Moreover, excessive dietary fat might also cause adverse effects on fish [72].
The negative impacts caused by an HFD in fish have been investigated in different species. It has been noticed in grass carp (Ctenopharyngodon idella) and tilapia (Oreochromis niloticus) that diets high in fat (15 or 16%) decreased feed intake and growth performance, lowered immunological function, and changed lipid metabolism [67,68,73]. Moreover, it has been reported in turbot (Scophthalmus maximus) and black seabream (Acanthopagrus schlegelii) that a high-fat diet (17–19.5%) induces lipid peroxidation and oxidative stress [9,74]. An HFD (11 or 12% fat) exacerbated intestinal and liver health in tilapia [17] and triggered endoplasmic reticulum (ER) stress to modulate hepatic lipid secretion in blunt snout bream (Megalobrama amblycephala) [3]. Excessive lipid consumption beyond the body’s capacity for utilization is the main risk factor for hepatic steatosis in most cultured fish. Hepatic steatosis is a typical form of abnormal lipid metabolism in the liver that is characterized by excessive deposition of triacylglycerol in hepatic tissue [3,72]. Growth, feed utilization rate, immunity, and stress tolerance are negatively impacted by steatosis [8,69,75]. It was explained by Tanaka et al. [76] that an HFD induces hepatic steatosis by inhibiting hepatic autophagic activity, which triggers fat accumulation. Similarly, in mammals, lipotoxicity is the main contributor to various diseases, such as a fatty liver, obesity, and diabetes [70,71]. The present study showed that Nile tilapia exposed to a high-fat diet without chitosan supplementation (F12Ch0) had the lowest values for FBW, BWG, SGR, PER, and body protein content and the highest values for FCR, HSI, IPF, liver, and body fat content after 8 weeks of a feeding trial. Tilapia groups offered trial diets fortified with 5 and 10 g/kg chitosan (100%) exhibited the opposite trend. Previous research studied the negative impacts of an HFD on tilapia and indicated that an HFD reduces feed intake, impairs growth performance, damages fish’s liver and intestinal tissue, and causes oxidative damage [75,77,78]. Also, Dai et al. [8] stated that an HFD-related inflammation response reduces the appetite of blunt snout bream and is closely related to lipid utilization abnormality and exacerbation of growth performance. The lowered growth performance and poor body indices obtained in Nile tilapia fed with an HFD (F12Ch0) could be due to the reduced feed intake, efficiency, digestion, absorption, and metabolism that resulted from the oxidative damage and inflammatory effect of an HFD on the liver and intestine, which are considered the main organs involved in nutrient digestion, absorption, and metabolism, as explained previously by Ding et al. [79]. This evidence is confirmed by the results of serum antioxidant enzymes and liver and intestine histopathology (Table 6 and Figure 2 and Figure 3). Subsequently, the nutrient deposition in the whole fish body, such as protein, decreased in this group, as seen in Table 4, as did the distorted tight junction proteins (Figure 8). Furthermore, excessive lipid intake causes over-deposition of fat in hepatic tissue and viscera, causing an increase in relative liver size (HSI), viscerosomal index (VSI), metabolic disturbances, and a reduction in liver activity [72,79,80,81]. Similar findings were observed in grass carp (Ctenopharyngodon idella) [13], turbot (Scophthalmus maximus) [74], black seabream (Acanthopagrus schlegelii) [9], largemouth bass (Micropterus salmoides), and orgiant croaker (Nibea japonica) fed with a high-fat diet [72,81].
In this feeding trial, Nile tilapia fed the high-fat diet (F12) supplemented with chitosan demonstrated better growth, feed utilization, body indices, body protein content, and lower fat accumulation in the body and liver. It indicates that adding chitosan mitigated the deleterious impacts induced by the high-fat diet (12% fat) without chitosan. The beneficial effects of chitosan supplementation on fish diets have been studied in different species. Chitosan plays various biological roles, including hypolipidemic, immunomodulatory, and antioxidant properties [82,83,84]. It has been observed that diets containing chitosan enhance the growth of shrimp (Penaeus monodon), loaches (Misgurnus anguillicadatus), and caspian kutum (Rutilus frisii kutum Kamenskii) fingerlings [85,86,87,88]. Similarly, Wu [50] noticed that diets containing 4 g kg−1 chitosan increased tilapia body weight gain, feed conversion rate, specific growth rate, body protein content, decreased lipid in the whole body, and hepatopancreas. It is linked to the modulatory action of chitosan on a variety of receptors, including the calcium-sensing receptor (CaSR), olfactory receptor, epidermal growth factor receptor, Tolllike receptor 4, TLR4/MD-2 receptor, scavenger receptor BI, and CYP7A1 [89,90]. Thus, certain receptors mediated the effects of chitosan, which in turn triggered the production of proteins. Furthermore, a large dose of chitosan probably displayed hypolipidemic action, restricting the tilapia from synthesizing lipids [83].
Hematological response is an essential index of fish health, which varies according to the type of stressor [91]. In the present study, it was notable that dietary chitosan positively impacts the hematological parameters in Nile tilapia by enhancing the RBC, PCV, and hemoglobin concentration. This may be attributed to the ability of chitosan to improve the absorption of macronutrients and micronutrients [92] that lead to good health. The count of WBCs is used to evaluate both fish innate immunity and health [93]. Moreover, chitosan-treated fish might receive a sufficient amount of oxygen for respiration and high metabolic activity [94,95]. Mubarak Ali et al. [96] stated that dietary chitosan and chitosan nanoparticles recorded better non-specific defenses due to the immunostimulatory effect and antimicrobial properties of chitosan nanoparticles. Similarly, Meshkini et al. [97] indicated that dietary chitosan at concentrations of 0.25% showed improving effects on the hematological indices, a result that agreed with the biochemical findings of the present study regarding enhanced total serum proteins in fish fed chitosan, which suggest better innate immunity of the fish. Comparable outcomes have been reported in many fish species when fed chitosan [98] and chitosan nanoparticles [99].
Fish biochemical parameters are regularly used as reliable diagnostic tools in biomonitoring, allowing for detection of the pathophysiological changes attributable to nutrition [100]. In this study, we found that fish fed an HFD, F12Ch0, showed significantly higher serum activities of AST, ALT, and LDH compared to the F6Ch0 group (Table 6). Their abnormal elevations implied the occurrence of both liver injury and hepatotoxicity, which are firmly associated with both hyperlipidemia and hepatic steatosis [101]. This is consistent with the work of Li et al. [102] and Chen et al. [103] in M. amblycephala and in blunt snout bream (Megalobrama amblycephala) fed an HFD. However, in the present study, AST and ALT activities were reduced by increasing dietary chitosan in HFD-fed fish, implying that dietary chitosan incorporation could mitigate the HFD-induced damage in Nile tilapia, which may be attributed to the antioxidative capability of chitosan that protects liver cells from damage. Current results coincided with Mehrpak et al. [104] and El-Naby et al. [92], who reported declining AST and ALT activities in fish fed a chitosan-supplemented diet. Elevated ALT and AST with raised creatinine levels may also affect other organs, such as the gills and kidney [105].
The concentrations of energetic metabolites triglycerides and cholesterol, as well as VLDL-C and LDL-C, were significantly increased in fish fed an HFD (F12Ch0). According to Mensinger et al. [106], cholesterol levels can result in disorders of lipid and lipoprotein metabolism, particularly liver dysfunction. Higher levels of lipid in fish feeds also make hepatocytes work harder, probably stressing them and contributing to liver damage [3]. Moreover, chitosan supplementation in an HFD-fed fish exhibited a beneficial effect on the lipid profile, as seen in a previous study on Nile tilapia (Oreochromis niloticus) [107,108]. Kang et al. [109] stated that chitosan oligosaccharide (COS) can inhibit the activity of pancreatic lipase and reduce the absorption of intestinal fat in combination with bile acids, as well as increase the excretion of fecal fat [110]. Accumulated shreds of evidence have demonstrated that these results were analogous to data in B. bidyanus [111], C. carpio [112], and Ctenopharyngodon Idella [113], and Xu et al. [114] indicated that adding 5% chitosan can promote liver LDL receptor (LDLR) mRNA expression, improve the clearance of LDL into the liver, and diminish the plasma cholesterol level. Furthermore, the current reduction in glucose levels may indicate the ability of chitosan to reduce liver gluconeogenesis and increase glucose consumption in the skeletal muscles [115].
Oxidative stress is a major cause of the progression of liver disease, which is induced by an HFD and leads to mitochondrial dysfunction, apoptosis, ER stress, and an inflammatory response [5,116]. Fish fed an HFD have a notable hepatic lipid accumulation and peroxidation [81,117]. As a result of lipid deposition and peroxidation, excessive production of ROS will deteriorate the integrity of organelles and cause severe damage to cells and tissues [81,118]. In this study, we found that the liver and intestine of HFD-fed Nile tilapia (F12Ch0) signally downregulated the mRNA levels of antioxidant enzymes (SOD and GPX), as well as nrf2, while elevating kaep1, which reflected the occurrence of severe oxidative stress and redox imbalance. SOD, GSH-Px, and CAT were considered the main antioxidant enzymes for free radical scavenging [119]. In this work, dietary chitosan could alleviate intestinal mucosal oxidative stress by upregulating the gene expression of the antioxidative enzyme activities of SOD and GSH-PX. These findings are consistent with Lan et al. [120], who indicated COS could increase SOD, CAT, GSH-Px, and T-AOC activity and decrease the MDA level after H2O2 challenge. Li et al. [121] also indicated COS enhanced SOD activity in the duodenum’s mucosa and decreased the MDA level in the jejunum and ileum’s mucosa in broilers. Furthermore, Li et al. [122] indicated that dietary COS supplementation elevated the inhibition of hydroxy radical capacity while decreasing MDA content in the ileum mucosa of broilers. On the same line, Assar et al. [123] stated that chitosan dietary inclusion in HFD-fed Zaraibi goat bucks caused a notable enhancement in antioxidant enzyme activities and suppressed the elevated MDA levels.
Many studies concluded that the accumulation of fat intensified vulnerability to oxidative stress and debilitated the antioxidant defense system in HFD-induced liver injury [116,124,125]. However, dietary chitosan enhanced nrf2 mRNA expression in the liver and intestine, similar to other similar studies on doxorubicin-challenged rats [126] and mice fed a high-fat diet [127].
Some studies have concluded that adding chitosan induces nuclear factor erythroid-derived2-like2 (nrf2) activation, which plays a vital role in cellular protection in opposition to free radical damage and reduces the incidence of severe oxidative stress as well as redox imbalance and chronic inflammation in the liver [3,8].
In this study, we explore the mechanism by which an HFD promotes hepatic and intestinal injury by focusing on mitochondrial dysfunction, which has been identified as a crucial driver for both cell injury and cell death. Apoptosis is a genetically programmed type of cell death that can be triggered by a variety of physiological changes. It is commonly accepted that mitochondrial dysfunction is a crucial indicator of apoptosis, which is mediated by the bcl-2 family of proteins, including both bcl-2 and bax [26]. Mitochondrial dysfunction causes the release of cyt c, which activates the downstream effector cas-3 and finally executes the apoptotic changes [27]. In the current investigation, apoptosis of hepatocytes was also associated with intrinsic mitochondrial pathways characterized by mitochondrial damage with the release of cytochrome c and activation of caspase-9 and, subsequently, caspase 3 as well as baxa in the HFD-fed group (F12Ch0). This is in line with Lu et al. [128], who detected that cytochrome c was released from mitochondria into the cytosol after feeding fish an HFD, causing damage to mitochondrial permeability. Cytochrome c release from mitochondria is considered the principal event for apoptosis. Therefore, we believe that the anti-apoptotic activity of chitosan stems from its ability to protect mitochondrial integrity. Among proapoptotic members, bax is perhaps the best-studied protein and is essential for mitochondrion-mediated apoptosis [129]. Functional analysis showed that bax could promote the release of cytochrome c from mitochondria [129,130]. Dietary chitosan supplementation significantly downregulated the expressions of cytochrome c, caspase-3, and caspase-9 compared to the HFD-fed group (F12Ch0). Conversely, bcl-2 plays an anti-apoptosis role by ameliorating cytochrome c release, and a decrease in the bax/bcl-2 ratio alleviates the amount of apoptosis [131,132]. In this study, dietary chitosan inclusion downregulated the bax expression level while upregulating the bcl-2 expression level compared with the HFD-fed fish group, F12Ch0. An HFD induces hepatocyte apoptosis in the blunt snout bream [133]. In line with earlier findings, apoptosis was also enhanced through upregulating pro-apoptotic genes cytc, bax, cas-3, cas-8, and p53 and downregulating anti-apoptotic genes bcl-2 and xiap in HFD-fed tilapia [134]. Similarly, Dai et al. [8] observed an increase in hepatocyte gene expression of caspase-3, caspase-9, and CD68. These genes are known for their functions in promoting intrinsic apoptosis, regulating physiological cell death, and regulating pathological tissue degeneration in M. amblycephala fish.
High-fat diets are well recognized to induce metabolic inflammation throughout the tissues. High-fat diets elevate the amounts of endotoxins, circulating free fatty acids, and inflammatory mediators, resulting in low-grade systemic inflammation and disturbed homeostasis in many tissues [135]. Li et al. [122] indicated that long-term HFD feeding induced an increase in inflammatory markers in zebrafish (Danio rerio). Inflammatory cytokines are intimately associated with the development of metabolic disorders [131,132,136]. Many studies demonstrated that an HFD could impair lipid homeostasis and the rate of fat peroxidation and induce oxidative stress, thus inducing inflammatory responses in various marine fish species, notably black seabream (Acanthopagrus schlegelii), blunt snout bream (Megalobrama amblycephala), and large yellow croaker (Larimichthys crocea) [8,9,128,137,138]. We relate these adverse inflammatory responses to oxidative stress induced by an HFD diet (Figure 8). Previous investigations have revealed that oxidative stress precedes metabolic disorders induced by a high-fat diet in mice [139] and rats [140] and mitochondrial damage and apoptosis in rats [141]. The Nile tilapia, when under sustained oxidative stress, modulates the mRNA of nrf2/kaep1 signaling as a signal for cell damage. Our results were consistent with earlier research demonstrating that inflammation was a key factor for liver injury after feeding an HFD to tilapia [142]. Meanwhile, dietary chitosan supplementation could attenuate inflammation by modulating cox2 signaling molecules, increasing expression of anti-inflammatory markers il-10, and decreasing tnf-α and il-1b levels [124,143,144]. Bai et al. [145] reported that chitosan oligosaccharide supplementation resulted in downregulation of mRNA levels of pro-inflammatory cytokines (il-6, tnf-α, and monocyte chemo-attractant protein 1(MCP-1) in the liver of mice fed an HFD.
A healthy intestine morphology improves the capacity to absorb different nutrients and acts as an immune barrier [36]. However, consumption of an HFD causes an increase in intestinal permeability, impairs its mucosal defenses, and promotes intestinal inflammation [146]. A high-fat diet (15%) fed to Nile tilapia for a period of 8 weeks significantly reduced the length of intestinal villi, decreased the number of goblet cells, downregulated the mRNA expressions of tight junction proteins, i.e., occludin and claudin, and prompted the expression of the intestinal inflammatory factor il-1b [146]. The accumulating literature illustrates that inflammation is an important marker in intestinal dysfunction [147,148]. Chen et al. [147] demonstrated that overproduction of inflammatory cytokines could alter the intestinal permeability and tight junction structure by modulating tight junction-related gene expression in weaning piglets. At the same time, the overproduction of il-1β, il-6, and tnf-α directly causes intestinal mucosal injury [149]. Therefore, suppressing the overproduction of intestinal mucosal il-1β, il-6, and tnf-α proved to be effective in maintaining intestinal function. Previous research suggested that stressors could disturb the balance between anti-inflammatory and pro-inflammatory responses by boosting the production of pro-inflammatory cytokines [147,150]. In this study, intestinal il-1b and tnf-α levels were higher, whereas intestinal il-10 levels were lower in the HFD-fed group compared to the F6Ch0 group, indicating that oxidative stress resulted in inflammation in the intestine. Dietary chitosan supplementation decreased the intestinal il-1b and tnf-α levels while enhancing the intestinal il-10 levels, all of which was inconsistent with the results of Hu et al. [151], who reported that COS reduced il-1β and tnf-α mRNA expression levels in the jejunum mucosa of weaning pigs. Also, COS reduced the il-6 and tnf-α mRNA expression levels in the liver of mice after being fed a high-fat diet [123]. These findings suggest that COS may mitigate intestinal inflammation by suppressing the levels of il-1β, il-6, and tnf-α [152,153].
Normal intestinal permeability is maintained by tight junctions, which are multi-protein complexes made up of various proteins, namely Zonula-Occludens, Occludin, and Claudin [154]. Paola et al. [155] suggested that intestinal hyperpermeability is a causative agent of both liver fibrosis and hepatic inflammation. A high-fat diet can significantly decrease the expression of tight junction proteins and thus increase intestinal permeability by weakening the intestinal barrier. Previous studies have demonstrated that mice fed with an HFD showed increased intestinal damage when compared to mice fed a low-fat diet [156].
In this study, HFD-induced oxidative stress downregulated the protein expression of claudin3, claudin7, tjp1, and tjp2, which reflected intestinal barrier dysfunction and was consistent with the results reported by Song et al. [157] and Cao et al. [158]. Moreover, dietary chitosan upregulated the expression of tight junction proteins, similar to other studies on mice fed high-fat diets [159], dexamethasone-challenged broilers [160], and weaning pigs [151,161]. These results indicated that chitosan could modulate oxidative-induced intestinal barrier function relatively well by maintaining the intestinal structure, intestinal permeability, and tight junction functionality.
In this investigation, we observed histological alterations in the liver, kidney, and intestine that are linked to a high-fat diet. These alterations were mainly in the form of renal tubular damage, hepatic necrosis, and shortening of intestinal villi. However, these changes showed less severe patterns emerging in the groups that received chitosan supplements.
Surprisingly, the F12Ch10 group had the best results, providing features of healing in the form of enlarged intestinal villi with a minor loss of apical mucosa, normal renal tubular endothelial lining, and restricted hepatic inflammatory cell infiltration, suggesting that increased chitosan supplementation may have a protective effect against fat droplet accumulation. Similar results were previously reported on common carp [162].
High fat storage in hepatocytes disrupts the normal regulation of glycogen metabolism in the liver. It decreases glycogen synthesis, increases gluconeogenesis, and impairs the response to insulin [163,164]. These changes, in turn, result in reduced glycogen storage in the liver and explain the low glycogen level in hepatocytes in the F12Ch0 group. Also, such results suggest the effect of chitosan treatment in alleviating fat accumulation and maintaining the normal metabolism of glucose, as observed by the higher storage of glycogen in hepatocytes from the F12Ch10 group.
Collectively, the histopathological findings suggest that chitosan supplementation, particularly at higher levels, may have a protective role in mitigating the severe damage induced by a high-fat diet.
In summary, the present study demonstrated that an HFD could promote liver and intestinal injury that might be closely related to oxidative damage, inflammation, and apoptosis. An impaired nrf2 pathway may depress the antioxidant defense system, leading to oxidative damage. Concurrently, apoptosis was induced by activating the mitochondria pathway after feeding an HFD, which triggered cox2, led to the release of pro-inflammatory factors, and exaggerated liver and intestinal injury.
5. Conclusions
This study suggests that dietary chitosan supplementation may be an effective nutritional strategy to mitigate the deleterious effects of HFD-induced oxidative stress via modulating the nrf2/kaep1 signaling pathway and anti-apoptotic action by increasing the expression of bcl-2. Therefore, it attenuates intestinal barrier damage by improving intestinal morphology and tight junction protein expression, which may be involved in suppressing intestinal inflammation and increasing antioxidant capacity in HFD-fed Nile tilapia.
Author Contributions
A.G.R.: Conceptualization, Methodology, Software. D.H.A.: Data curation, Methodology, Writing—Original draft preparation. A.S.S.: Visualization, Investigation, Statistical analysis, Methodology. X.L.: Resources, Writing—Reviewing and editing. I.I.A.-H.: Supervision, reviewing-editing final manuscript. M.H.A.-A.: Resources, Writing—Reviewing and editing. S.M.R.S.: Methodology, Feed formulation, Analysis, Reviewing. K.K., A.A. and N.A.N.H.: Pathological analysis, Validation. L.S.: Supervision, Resources, Writing—Reviewing and editing. Z.I.E.: Methodology, Molecular analysis, Writing—Reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Graduate Studies and Scientific Research at the University of Bisha, Saudi Arabia, through the Promising Program under grant number (UB-Promising-34-1445).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Faculty of Aquatic and Fisheries Sciences Ethics Committee, Kafrelsheikh University, Egypt (IAACUC-KSU-028-2022). All precautions were followed to diminish animal suffering during the experiment.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
The authors would like to acknowledge the support of the Biotechnology Lab, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Egypt, where the laboratory investigations were carried out. The authors extend their appreciation to the Deanship of Graduate Studies and Scientific Research at the University of Bisha for funding this research through the Promising Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Agricultural Organization (FAO). The state of world fisheries and aquaculture. In Towards Blue Transformation; Food and Agricultural Organization (FAO): Rome, Italy, 2022; 266p. [Google Scholar] [CrossRef]
- Khalil, M.T. Achieving Sustainability in the Fishery Sector. Road Map for Green Economy in Egypt; Workshop on 25/6/2014; Academy of Science and Technologies: Cairo, Egypt, 2014. [Google Scholar]
- Cao, X.-F.; Dai, Y.-J.; Liu, M.-Y.; Yuan, X.-Y.; Wang, C.-C.; Huang, Y.-Y.; Liu, W.-B.; Jiang, G.-Z. High-fat diet induces aberrant hepatic lipid secretion in blunt snout bream by activating endoplasmic-reticulum stress-associated IRE1/XBP1 pathway. Biochim. Et. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 213–223. [Google Scholar] [CrossRef]
- Lu, K.; Xu, W.; Li, J.; Li, X.; Huang, G.; Liu, W. Alterations of liver histology and blood biochemistry in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish. Sci. 2013, 79, 661–671. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F.W. Steatohepatitis: A tale of two ’Hits’? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Bass, N.M.; Merriman, R.B. Fatty Acid Metabolism and Lipotoxicity in the Pathogenesis of NAFLD/NASH. In Fatty Liver Disease; Farrell, G.C., George, J., Hall, P.D.L.M., McCullough, A.J., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2005; pp. 109–122. [Google Scholar]
- Du, Z. Causes of fatty liver in farmed fish: A review and new perspectives. J. Fish. China 2014, 38, 1628–1638. [Google Scholar]
- Dai, Y.-J.; Cao, X.-F.; Zhang, D.-D.; Li, X.-F.; Liu, W.-B.; Jiang, G.-Z. Chronic inflammation is a key to inducing liver injury in blunt snout bream (Megalobrama amblycephala) fed with high-fat diet. Dev. Comp. Immunol. 2019, 97, 28–37. [Google Scholar] [CrossRef]
- Jin, M.; Pan, T.; Cheng, X.; Zhu, T.T.; Sun, P.; Zhou, F.; Ding, X.; Zhou, Q. Effects of supplemental dietary L-carnitine and bile acids on growth performance, antioxidant and immune ability, histopathological changes and inflammatory response in juveNile black seabream (Acanthopagrus schlegelii) fed high-fat diet. Aquaculture 2019, 504, 199–209. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, Z.; Zhou, Y.; Guo, D.; Wang, H.; Chen, X. Hepatic lipid metabolism and oxidative stress responses of grass carp (Ctenopharyngodon idella) fed diets of two different lipid levels against Aeromonas hydrophila infection. Aquaculture 2019, 509, 149–158. [Google Scholar] [CrossRef]
- Oka, T.; Nishimura, Y.; Zang, L.; Hirano, M.; Tanaka, T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010, 10, 21. [Google Scholar] [CrossRef]
- Chen, F.; Chen, D.; Zhao, X.; Yang, S.; Li, Z.; Sanchis, D.; Jin, L.; Qiang, X.; Wang, K.; Xu, Y.; et al. Interleukin-6 deficiency facilitates myocardial dysfunction during high fat diet-induced obesity by promoting lipotoxicity and inflammation. Biochim. Biophys. Acta 2017, 1863, 3128. [Google Scholar] [CrossRef]
- Jabri, M.A.; Sakly, M.; Marzouki, L.; Sebai, H. Chamomile (Matricaria recutita L.) decoction extract inhibits intestinal glucose absorption and attenuates high fat diet-induced lipotoxicity and oxidative stress. Biomed. Pharmacother. 2017, 87, 153–159. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Charradi, K.; Limam, F.; Aouani, E. Grape seed and skin extract as an adjunct to xenical therapy reduces obesity, brain lipotoxicity and oxidative stress in high fat diet fed rats. Obes. Res. Clin. Pract. 2018, 12 (Suppl. 2), 115–126. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of CS and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Negm, S.S.; Ghazanfar, S.; Shukry, M.; Abdelnour, S.A. The risk assessment of high-fat diet in farmed fish and its mitigationapproaches: A review. J. Anim. Physiol. Anim. Nutr. 2023, 107, 948–969. [Google Scholar] [CrossRef]
- Limbu, S.M.; Ma, Q.; Zhang, M.-L.; Du, Z.-Y. High fat diet worsens the adverse effects of antibiotic on intestinal health in juveNile Nile tilapia (Oreochromis niloticus). Sci. Total Environ. 2019, 680, 169–180. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef]
- Narasimhan, M.; Riar, A.K.; Rathinam, M.L.; Vedpathak, D.; Henderson, G.; Mahimainathan, L. Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicol. Lett. 2014, 228, 179–191. [Google Scholar] [CrossRef]
- Assar, D.H.; Abou Asa, S.; El Abasy, M.A.; Elbialy, Z.I.; Shukry, M.; Abd El Latif, A.; BinMowyna, M.N.; Althobaiti, N.A.; El Magd, M.A. Aspergillus awamori attenuates ochratoxin A induced renal and cardiac injuries in rabbits by activating the Nrf2/HO-1 signaling pathway and downregulating IL1β, TNFα, and iNOS gene expressions. Environ. Sci. Pollut. Res. 2022, 29, 69798–69817. [Google Scholar] [CrossRef]
- Assar, D.H.; Elbialy, Z.I.; Al Wakeel, R.; Gomaa, N.; El-Maghraby, M.M.; Nagy, W.M.; El-Badawy, A.A.E.A.; Abdel-Khalek, A.-K.E.-S. Dietary Chitosan Supplementation Modulates Hematology, Lipid Profile, Rumen Function, Antioxidant Status, and Thyroxin in Zaraibi Goat Bucks Fed on High-Fat Diets. Adv. Appl. Physiol. 2023, 8, 8–16. [Google Scholar] [CrossRef]
- Chambel, S.S.; Santos-Goncalves, A.; Duarte, T.L. The dual role of Nrf2 in nonalcoholic fatty liver disease: Regulation of antioxidant defenses and hepatic lipid metabolism. BioMed Res. Int. 2015, 2015, 597134. [Google Scholar] [CrossRef]
- Ahmed-Farid, O.A.; Rizk, H.A.; Shehata, A.M. Hydrogen peroxide modulates redox status, energy metabolism, and gene expression in a dose- and time-dependent manner in rat liver. J. Biochem. Mol. Toxicol. 2018, 32, e22199. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Li, Y.; Johnson, O.; Gu, Z.; Jeney, Z.; Xu, P.; Yin, G. Antioxidative, inflammatory and immune responses in hydrogen peroxide-induced liver injury of tilapia (GIFT, Oreochromis niloticus). Fish. Shellfish. Immunol. 2019, 84, 894–905. [Google Scholar] [CrossRef]
- Alkhouri, N.; Carter-Kent, C.; Feldstein, A.E. Apoptosis in nonalcoholic fatty liver disease: Diagnostic and therapeutic implications. Expet Rev. Gastroenterol. Hepatol. 2011, 5, 201–212. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692–701. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health & disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar]
- Wu, Q.; Liu, N.; Wu, X.; Wang, G.; Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018, 97, 2675–2683. [Google Scholar] [CrossRef]
- Liu, W.; Lu, Z.; Jiang, M.; Wu, F.; Tian, J.; Yu, L.; Wen, H. Effects of dietary niacin on liver health in genetically improved farmed tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 16, 100243. [Google Scholar] [CrossRef]
- Wu, S.; Pan, L.; Liao, H.; Yao, W.; Shen, N.; Chen, C.; Liu, D.; Ge, M. High-fat diet increased NADPH-oxidase-related oxidative stress and aggravated LPS-induced intestine injury. Life Sci. 2020, 253, 117539. [Google Scholar] [CrossRef]
- Yun, S.H.; Moon, Y.S.; SoHn, S.H.; Jang, I.S. Effects of cyclic heat stress or vitamin C supplementation during cyclic heat stress on HSP70, inflammatory cytokines, and the antioxidant defense system in Sprague Dawley rats. Exp. Anim. 2012, 61, 543–553. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 16530. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Kim, S.K. Marine invertebrate natural products for anti-inflammatory and chronic diseases. Evid. Based Complement. Altern. Med. 2013, 2013, 572859. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Zaineldin, A.I.; Van Doan, H.; Moustafa, E.M.; Abdel-Daim, M.M.; Angeles Esteban, M.; Hassaan, M.S. Dietary supplementation of selenium nanoparticles modulated systemic and mucosal immune status and stress resistance of red sea bream (Pagrus major). Fish. Physiol. Biochem. 2019, 45, 219–230. [Google Scholar] [CrossRef]
- Sheiha, A.M.; Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Metwally, K.A.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; El- Saadony, M.T. Effects of dietary biological or chemical- synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals 2020, 10, 430. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Baldassarre, V.; Conti, F.; Ferrara, P.; Biagini, G.; Gazzanelli, G.; Vasi, V. Biological activity of chitosan: Ultrastructural study. Biomaterials 1988, 9, 247–252. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, G.G.; Heras, A. Funcional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Shard, P.; Bhatia, A.; Sharma, D. Optimization and physico-chemical parameters on synthesis of chitosan nanoparticles by ionic gelation technique. Int. J. Drug Deliv. 2014, 6, 58–63. [Google Scholar]
- Xu, Y.; Gallert, C.; Winter, J. Chitin purification from shrimp wastes by microbial deproteination and decalcification. Appl. Microbiol. Biotechnol. 2008, 79, 687–697. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.; Salaah, S.; El-Shabaka, H.; Abd El-Rahman, F.; Khalil, M.; Suloma, A. Efficacy of dietary chitosan and chitosan nanoparticles supplementation on health status of Nile tilapia, Oreochromis niloticus (L.). Aquac. Rep. 2021, 19, 100628. [Google Scholar] [CrossRef]
- Zaki, M.; Salem, M.; Gaber, M.; Nour, A. Effect of Chitosan Supplemented Diet on Survival, Growth, Feed Utilization, Body Composition & Histology of Sea Bass (Dicentrarchus labrax). World J. Eng. Technol. 2015, 3, 38–47. [Google Scholar] [CrossRef]
- Shi, B.L.; Li, D.F.; Piao, X.S.; Yan, S.M. Effects of CS on growth performance and energy and protein utilization in broiler chickens. Br. Poult. Sci. 2005, 46, 516–519. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.F.M. Tilapia Culture; CABI Pub.: Wallingford, GB, USA, 2006; 256p. [Google Scholar]
- General Authority for Fisheries Resources Development (GAFRD). Yearly Book for Fish Production in Egypt; Agriculture Ministry: Washington, DC, USA, 2022. [Google Scholar]
- Prabu, E.; Rajagopalsamy, C.; Ahilan, B.; Jeevagan, I.J.M.A.; Renuhadevi, M. Tilapia—An excellent candidate species for world aquaculture: A Review. Ann. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Ban, K.; Peng, Z.; Kozar, R.A. Inhibition of ERK1/2 worsen intestinal ischemia/reperfusion injury. PLoS ONE 2013, 8, e76790. [Google Scholar] [CrossRef]
- AlKandari, F.M.; Mohamed, H.S.; Ahmed, S.A.; Mahmoud, B.; Mahmoud, A.M. Protective Effects of Propolis and Chitosan Nanoparticles against Ibuprofen-Induced Hepatotoxicity in Albino Rats. Diseases 2024, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Wu, S. The growth performance, body composition and nonspecific immunity of Tilapia (Oreochromis niloticus) affected by chitosan. Int. J. Biol. Macromol. 2020, 145, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Fadl, S.E.; El-Gammal, G.E.; Abdo, W.S.; Barakat, M.; Sakr, O.A.; Nassef, E.; Gad, D.M.; El-Sheshtawy, H.S. Evaluation of dietary chitosan effects on growth performance, immunity, body composition and histopathology of Nile tilapia (Oreochromis niloticus) as well as the resistance to Streptococcus agalactiae infection. Aquac. Res. 2019, 51, 1120–1132. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497. [Google Scholar] [CrossRef]
- Stoskopf, M.K. Fish Medicine; W.B. Sannders: Philadelphia, PA, USA, 1993. [Google Scholar]
- Friedwald, W.T.; Levy, R.I.; Fredriekson, D.S. Estimation of concentration of low density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Salah, A.S.; Elsheshtawy, A.; Elkatatny, N.M.; Fouad, A.M.; Abo-Al-Ela, H.G. Differential tissue regulation of nrf2/keap1 crosstalk in response to Aeromonas infection in Nile tilapia: A comparative study. Aquacult Int. 2023, 32, 545–562. [Google Scholar] [CrossRef]
- Qiang, J.; He, J.; Yang, H.; Xu, P.; Michael Habte-Tsion, H.; Ma, X.Y.; Zhu, Z.X. The changes in cortisol and expression of immune genes of GIFT tilapia Oreochromis niloticus (L.) at different rearing densities under Streptococcus iniae infection. Aquacult Int. 2016, 24, 1365–1378. [Google Scholar] [CrossRef]
- Standen, B.T.; Peggs, D.L.; Rawling, M.D.; Foey, A.; Davies, S.J.; Santos, G.A.; Merrifield, D.L. Dietary administration of a commercial mixed-species probiotic improves growth performance and modulates the intestinal immunity of tilapia, Oreochromis niloticus. Fish. Shellfish. Immunol. 2016, 49, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.L.; Pan, B.S. Anti-Stress Effects of Glycine tomentella Hayata in Tilapia: Inhibiting COX-2 Expression and Enhancing EPA Synthesis in Erythrocyte Membrane and Fish Growth. J. Agric. Food Chem. 2011, 59, 9532–9541. [Google Scholar] [CrossRef]
- Jia, R.; Li, Y.; Cao, L.; Du, J.; Zheng, T.; Qian, H.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part. C 2019, 215, 56–66. [Google Scholar] [CrossRef]
- Han, C.Y.; Zheng, Q.M.; Sun, Z.T. Gene Expression and Activities of Antioxidant Enzymes in Liver of Hybrid Tilapia, Oreochromis niloticus × Oreochromis aureus, Under Acute pH Stress. J. World Aquac. Soc. 2016, 47, 260–267. [Google Scholar] [CrossRef]
- Mu, L.L.; Yin, X.; Yang, Y.; Wu, L.; Wu, H.; Li, B.; Guo, Z.; Ye, J. Functional characterization of a mannose-binding lectin (MBL) from Nile tilapia (Oreochromis niloticus) in non-specific cell immunity and apoptosis in monocytes/macrophages. Fish. Shellfish. Immunol. 2019, 87, 265–274. [Google Scholar] [CrossRef]
- Zheng, Y.; Qiu, L.; Fan, L.; Song, C.; Meng, S.; Chen, J. Effect of polychlorinated biphenyls on osmoregulatory response and apoptosis in GIFT tilapia, Oreochromis niloticus. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Jiang, Z.Y.; Han, S.L.; Li, L.Y.; Qiao, F.; Zhang, M.L.; Du, Z.Y. Inhibition of intestinal lipases alleviates the adverse effects caused by high-fat diet in Nile tilapia. Fish. Physiol. Biochem. 2020, 46, 111–123. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, H.; Ma, T.; Yan, Z.; Zhang, Y.; Geng, Y.; Zhu, Y.; Shi, Y. Effect of chronic cyclic heat stress on the intestinal morphology, oxidative status and cecal bacterial communities in broilers. J. Therm. Biol. 2020, 91, 102619. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xu, J.H.; Qin, J.; Yan, B.-L.; Zhu, M.; Luo, G. Effects of dietary lipid levels on growth performance, feed utilization and fatty acid composition of juveNile Japanese seabass (Lateolabrax japonicus) reared in seawater. Aquac. Int. 2011, 19, 79–89. [Google Scholar] [CrossRef]
- Meng, Y.; Qian, K.; Ma, R.; Liu, X.; Han, B.; Wu, J.; Zhang, L.; Zhan, T.; Hu, X.; Tian, H. Effects of dietary lipid levels on sub-adult triploid rainbow trout (Oncorhynchus mykiss): 1. Growth performance, digestive ability, health status and expression of growth-related genes. Aquaculture 2019, 513, 394–404. [Google Scholar] [CrossRef]
- De Zeng, M.; Li, Y.M.; Chen, C.W.; Lu, L.G.; Fan, J.G.; Wang, B.Y.; Mao, Y.M. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J. Dig. Dis. 2008, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Brookheart, R.T.; Michel, C.I.; Schaffer, J.E. As a Matter of Fat. Cell Metab. 2009, 10, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.A.; Summers, S.A. Lipid oversupply, selective insulin resistance, and lipotoxicity: Molecular mechanisms. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2010, 1801, 252–265. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Zhuang, Z.; He, X.; Tang, X.; Tian, L.; Liu, Y.; Niu, J. Dietary supplementation of bile acid attenuate adverse effects of high-fat diet on growth performance, antioxidant ability, lipid accumulation and intestinal health in juveNile largemouth bass (Micropterus salmoides). Aquaculture 2021, 531, 735864. [Google Scholar] [CrossRef]
- Tang, T.; Hu, Y.; Peng, M.; Chu, W.Y.; Hu, Y.J.; Zhong, L. Effects of high-fat diet on growth performance, lipid accumulation and lipid metabolismrelated MicroRNA/gene expression in the liver of grass carp (Ctenopharyngodon idella). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 234, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Jing, Q.; Niu, H.; Huang, B. Ameliorative effect of vitamin E on hepatic oxidative stress and hypoimmunity induced by high-fat diet in turbot (Scophthalmus maximus). Fish. Shellfish. Immunol. 2017, 67, 634–642. [Google Scholar] [CrossRef]
- Xu, F.; Xu, C.; Xiao, S.; Lu, M.; Limbu, S.M.; Wang, X.; Du, Z.; Qin, J.G.; Chen, L. Effects of a- lipoic acid on growth performance, body composition, antioxidant profile and lipid metabolism of the GIFT tilapia (Oreochromis niloticus) fed high-fat diets. Aquac. Nutr. 2019, 25, 585–596. [Google Scholar] [CrossRef]
- Tanaka, S.; Hikita, H.; Tatsumi, T.; Sakamori, R.; Nozaki, Y.; Sakane, S.; Shiode, Y.; Nakabori, T.; Saito, Y.; Hiramatsu, N.; et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 2016, 64, 1994–2014. [Google Scholar] [CrossRef]
- Lin, J.-J.; Liu, Y.-C.; Chang, C.-J.; Pan, M.-H.; Lee, M.-F.; Pan, B.S. Hepatoprotective mechanism of freshwater clam extract alleviates nonalcoholic fatty liver disease: Elucidated in vitro and in vivo models. Food Funct. 2018, 9, 6315–6325. [Google Scholar] [CrossRef]
- Qiang, J.; Tao, Y.F.; Bao, J.W.; Chen, D.J.; Li, H.X.; He, J.; Xu, P. High Fat Diet-Induced miR-122 regulates lipid metabolism and fat deposition in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) Liver. Front. Physiol. 2018, 9, 1422. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Xu, N.; Liu, Y.; Du, J.; Xiang, X.; Xu, D.; Liu, Q.; Yin, Z.; Li, J.; Mai, K.; et al. Effect of Dietary Bile Acid (BA) on the Growth Performance, Body Composition, Antioxidant Responses and Expression of Lipid Metabolism-Related Genes of JuveNile Large Yellow Croaker (Larimichthys crocea) Fed High-Fat Diets. Aquaculture 2020, 518, 734768. [Google Scholar] [CrossRef]
- Wang, J.T.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Du, Z.Y.; Wang, Y.; Yang, H.-J. Effect of Dietary Lipid Level on Growth Performance, Lipid Deposition, Hepatic Lipogenesis in JuveNile Cobia (Rachycentron canadum). Aquaculture 2005, 249, 439–447. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Guo, J.L.; Tang, R.J.; Ma, H.J.; Chen, Y.J.; Lin, S.M. High Dietary Lipid Level Alters the Growth, Hepatic Metabolism Enzyme, and Anti-oxidative Capacity in JuveNile Largemouth Bass Micropterus salmoides. Fish. Physiol. Biochem. 2020, 46, 125–134. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, J.S.; Balasundaram, C.; Heo, M.S. Immunomodulatory effects of chitin and chitosan enriched diets in Epinephelus bruneus against Vibrio alginolyticus infection. Aquaculture 2012, 326–329, 46–52. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Jiang, Q.; Xia, W. A comparative study on hypolipidemic activities of high and low molecular weight chitosan in rats. Int. J. Biol. Macromol. 2012, 51, 504–508. [Google Scholar] [CrossRef]
- Chang, S.H.; Wu, C.H.; Tsai, G.J. Effects of chitosan molecular weight on its antioxidant and antimutagenic properties. Carbohydr. Polym. 2018, 181, 1026–1032. [Google Scholar] [CrossRef]
- Niu, J.; Li, C.H.; Tian, L.X.; Liu, Y.J.; Chen, X.; Wu, K.C.; Jun, W.; Huang, Z.; Wang, Y.; Lin, H.Z. Suitable dietary chitosan improves the growth performance, survival and immune function of tiger shrimp, Penaeus monodon. Aquac. Res. 2015, 46, 1668–1678. [Google Scholar] [CrossRef]
- Kamali Najafabad, M.; Imanpoor, M.R.; Taghizadeh, V.; Alishahi, A. Effect of dietary chitosan on growth performance, hematological parameters, intestinal histology and stress resistance of Caspian kutum (Rutilus frisii kutum Kamenskii, 1901) fingerlings. Fish Physiol. Biochem. 2016, 42, 1063–1071. [Google Scholar] [CrossRef]
- Yan, J.; Guo, C.; Dawood, M.A.O.; Gao, J. Effects of dietary chitosan on growth, lipidmetabolism, immune response and antioxidant-related gene expression in misgurnus anguillicaudatus. Benef. Microbes. 2017, 8, 439–449. [Google Scholar] [CrossRef]
- Jing, C.; Li, L. Effects of chitosan-supplemented diets on the growth performance, nonspecific immunity and health of loach fish (Misgurnus anguillicadatus). Carbohydr. Polym. 2019, 225, 115227. [Google Scholar]
- Li, S.T.; Young, T.H.; Wang, C.T.; Huang, T.W. Chitosan films promote formation of olfactory neurospheres and differentiation of olfactory receptor neurons. Rhinology 2018, 56, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Xiao, D.F.; Tan, B.; Xiao, H.; Wang, J.; Yin, J.; Duan, G.L.; Huang, R.K.; Yang, C.B.; Yin, Y.L. Chitosan oligosaccharide reduces intestinal inflammation that involves calcium-sensing receptor (CaSR) activation in lipopolysaccharide (LPS)-challenged piglets. J. Agr. Food Chem. 2016, 64, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.L.; Mouriño, J.L.; Amaral, G.V.; Vieira, F.N.; Dotta, G.; Jatobá, A.M.; Pedrotti, F.S.; Jerônimo, G.T.; Buglione-Neto, C.C.; Pereira, G., Jr. Haematological changes in Nile tilapia experimentally infected with Enterococcus sp. Braz. J. Biol. 2008, 68, 657–661. [Google Scholar] [CrossRef] [PubMed]
- El-Naby, F.S.; MA Naiel, A.A. Al-Sagheer, S.S. Negm. Dietary CS nanoparticles enhance the growth, production performance, and immunity in Oreochromis niloticus. Aquaculture 2018, 501, 82–89. [Google Scholar] [CrossRef]
- Duncan, P.L.; Klesius, P.H. Effects of Feeding Spirulina on Specific and Nonspecific Immune Responses of Channel Catfish. J. Aquat. Anim. Health 1996, 8, 308–313. [Google Scholar] [CrossRef]
- Lenfant, C.; Johansen, K. Gas exchange in gill, skin, and lung breathing. Respir. Physiol. 1972, 14, 211–218. [Google Scholar] [CrossRef]
- Mohapatra, S.; Chakraborty, T.; Prusty, A.K.; PaniPrasad, K.; Mohanta, K.N. Beneficial effects of dietary probiotics mixture on hemato-immunology and cell apoptosis of Labeo rohita fingerlings reared at higher water temperatures. PLoS ONE 2014, 9, e100929. [Google Scholar] [CrossRef] [PubMed]
- MubarakAli, D.; LewisOscar, F.; Gopinath, V.; Alharbi, N.S.; Alharbi, S.A.; Thajuddin, N. An inhibitory action of CS nanoparticles against pathogenic bacteria and fungi and their potential applications as biocompatible antioxidants. Microb. Pathog. 2018, 114, 323–327. [Google Scholar] [CrossRef]
- Meshkini, S.; Tafy, A.A.; Tukmechi, A.; Farhang-Pajuh, F. Effects of chitosan on hematological parameters and stress resistance in rainbow trout (Oncorhynchus mykiss). Vet. Res. Forum 2012, 3, 49. [Google Scholar] [PubMed]
- Sheikhzadeh, N.; Kouchaki, M.; Mehregan, M.; Tayefi-Nasrabadi, H.; Divband, B.; Khataminan, M.; Khani Oushani, A.; Shabanzadeh, S. Influence of nanoCS/zeolite composite on growth performance, digestive enzymes and serum biochemical parameters in rainbow trout (Oncorhynchus mykiss). J. Aquac. Res. Dev. 2017, 48, 5955–5964. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.K.; Tripathi, M. Melatonin induced changes in specific growth rate, gonadal maturity, lipid and protein production in Nile tilapia Oreochromis niloticus (Linnaeus 1758) Asian-Australas. J. Anim. Sci. 2012, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Folmar, L.C.; Moody, T.; Bonomelli, S.; Gibson, J. Annual of blood chemistry parameters in striped mullet (Mugil cephalus L.) and pinfish (Lagodon rhomboides L.) from the Gulf of Mexico. J. Fish. Biol. 1992, 41, 999–1011. [Google Scholar] [CrossRef]
- Chang, H.C.; Huang, C.N.; Yeh, D.M.; Wang, S.J.; Peng, C.H.; Wang, C.J. Oat prevents obesity and abdominal fat distribution, and improves liver function in humans. Plant Foods Hum. Nutr. 2013, 68, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, D.; Xu, W.; Jiang, G.-Z.; Zhang, C.-N.; Li, X.-F.; Liu, W.-B. Effects of dietary choline supplementation on growth performance and hepatic lipid transport in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Aquaculture 2014, 434, 340–347. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Ma, L.; Ni, Y.; Zhao, H. Nrf2 plays a pivotal role in protection against burn trauma-induced intestinal injury and death. Oncotarget 2016, 7, 19272. [Google Scholar] [CrossRef]
- Mehrpak, M.; Banaee, M.; Nematdoost Hagh, B.; Noori, A. Protective effects of vitamin C and CS against cadmium-induced oxidative stress in the liver of common carp (Cyprinus carpio). Iran. J. Toxicol. 2015, 9, 1360–1367. [Google Scholar]
- Shahsavani, D.; Mohri, M.; Gholipour Kanani, H. Determination of normal values of some blood serum enzymes in Acipenser stellatus Pallas. Fish. Physiol. Biochem. 2010, 36, 39–43. [Google Scholar] [CrossRef]
- Mensinger, A.F.; Walsh, P.J.; Halon, R.T. Blood biochemistry of the oyster toadfish. J. Aquat. Anim. Health 2005, 17, 170–176. [Google Scholar] [CrossRef]
- He, A.Y.; Ning, L.J.; Chen, L.Q.; Chen, Y.-L.; Xing, Q.; Li, J.-M.; Qiao, F.; Li, D.-L.; Zhang, M.-L.; Du, Z.-Y. Systemic adaptation of lipid metabolism in response to low- and high-fat diet in Nile tilapia (Oreochromis niloticus). Physiol. Rep. 2015, 3, e12485. [Google Scholar] [CrossRef]
- Pan, H.; Yang, Q.; Huang, G.; Ding, C.; Cao, P.; Huang, L.; Xiao, T.; Guo, J.; Su, Z. Hypolipidemic effects of chitosan and its derivatives in hyperlipidemic rats induced by a high-fat diet. Food Nutr. Res. 2016, 60, 31137. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.H.; Lee, W.K.; Yi, B.R.; Park, M.A.; Lee, H.R.; Park, S.K.; Hwang, K.-A.; Park, H.K.; Choi, K.-C. Modulation of lipid metabolism by mixtures of protamine and chitooligosaccharide through pancreatic lipase inhibitory activity in a rat model. Lab. Anim. Res. 2012, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, M.; Kimura, Y. Low molecular weight chitosan inhibits obesity induced by feeding a high-fat diet long-term in mice. J. Pharm. Pharmacol. 2010, 58, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.D.; Liu, F.G.; Liou, C.H. Effects of Dietary L-Carnitine, Plant Proteins and Lipid Levels on Growth Performance, Body Composition, Blood Traits and Muscular Carnitine Status in JuveNile Silver Perch (Bidyanus Bidyanus). Aquaculture 2012, 342–343, 48–55. [Google Scholar] [CrossRef]
- Sabzi, E.; Mohammadiazarm, H.; Salati, A.P. Effect of Dietary LCarnitine and Lipid Levels on Growth Performance, Blood Biochemical Parameters and Antioxidant Status in JuveNile Common Carp (Cyprinus Carpio). Aquaculture 2017, 480, 89–93. [Google Scholar] [CrossRef]
- Du, Z.U.; Clouet, P.; Zheng, W.H.; Degrace, P.; Tian, L.X.; Liu, Y.J. Biochemical Hepatic Alterations and Body Lipid Composition in the Herbivorous Grass Carp (Ctenopharyngodon Idella) Fed High-Fat Diets. Br. J. Nutr. 2006, 95, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Huang, X.; Qiu, L.; Wu, J.; Hu, Y. Mechanism study of chitosan on lipid metabolism in hyperlipidemic rats. Asia Pac. J. Clin. Nutr. 2007, 16, 313–317. [Google Scholar] [PubMed]
- Shiau, S.Y.; Yu, Y.P. Dietary supplementation of chitin and CS depresses growth in tilapia, Oreochromis niloticus× O. Aureus. Aquaculture 1999, 179, 439–446. [Google Scholar] [CrossRef]
- Spahis, S.; Delvin, E.; Borys, J.M.; Levy, E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid. Redox Signal. 2017, 26, 519–541. [Google Scholar] [CrossRef]
- Fang, H.H.; Xie, J.J.; Zhao, W.; Liu, Z.L.; Liu, Y.J.; Tian, L.X.; Niu, J. Study Supplementation of Astaxanthin in High-Fat Diet on Growth Performance, Antioxidant Ability, Anti-Inflammation, Non-Specific Immunity and Intestinal Structure of JuveNile Trachinotus Ovatus. Aquac. Nutr. 2021, 27, 2575–2586. [Google Scholar] [CrossRef]
- Hou, J.; Jeon, B.; Baek, J.; Yun, Y.; Kim, D.; Chang, B.; Kim, S.; Kim, S. High fat diet-induced brain damaging effects through autophagy-mediated senescence, inflammation and apoptosis mitigated by ginsenoside F1-enhanced mixture. J. Ginseng Res. 2022, 46, 79–90. [Google Scholar] [CrossRef]
- Lan, R.; Chang, Q.; Wei, L.; Zhao, Z. The Protect Effects of Chitosan Oligosaccharides on Intestinal Integrity by Regulating Oxidative Status and Inflammation under Oxidative Stress. Mar. Drugs 2021, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Chang, Q.; An, L.; Zhao, Z. Dietary supplementation with chitosan oligosaccharides alleviates oxidative stress in rats challenged with hydrogen peroxide. Animals 2020, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, Y.; Chen, Y.; Qu, H.; Zhao, Y.; Wen, C.; Zhou, Y. Dietary chito-oligosaccharide inclusion as an alternative to antibiotics improves intestinal morphology, barrier function, antioxidant capacity, and immunity of broilers at early age. Animals 2019, 9, 493. [Google Scholar] [CrossRef]
- Li, X.; Ding, X.; Peng, X.; Chi, X.; Cui, H.; Zuo, Z.; Fang, J. Effect of chitosan oligosaccharides on antioxidant function, lymphocyte cycle and apoptosis in ileum mucosa of broiler. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 571–577. [Google Scholar]
- Assar, D.H.; Ragab, A.E.; Abdelsatar, E.; Salah, A.S.; Salem, S.M.R.; Hendam, B.M.; Al Jaouni, S.; Al Wakeel, R.A.A.; AbdEl-Kader, M.F.; Elbialy, Z.I. Dietary Olive Leaf Extract Differentially Modulates Antioxidant Defense of Normal and Aeromonas hydrophila-Infected Common Carp (Cyprinus carpio) via Keap1/Nrf2 Pathway Signaling: A Phytochemical and Biological Link. Animals 2023, 13, 2229. [Google Scholar] [CrossRef] [PubMed]
- Adjoumani, J.-J.Y.; Wang, K.; Zhou, M.; Liu, W.; Zhang, D. Effect of dietary betaine on growth performance, antioxidant capacity and lipid metabolism in blunt snout bream fed a high-fat diet. Fish Physiol. Biochem. 2017, 43, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Li, Y.; Hu, D.; Xie, L.; Ke, H.; Zheng, X.; Chen, W. Procyanidin B2 ameliorates free fatty acids-induced hepatic steatosis through regulating TFEB-mediated lysosomal pathway and redox state. Free Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ahmad, K.A.; Khan, F.U.; Yan, S.; Ihsan, A.U.; Ding, Q. Chitosan oligosaccharides prevent doxorubicin-induced oxidative stress and cardiac apoptosis through activating p38 and JNK MAPK mediated Nrf2/ARE pathway. Chem. Biol. Interact. 2019, 305, 54–65. [Google Scholar] [CrossRef]
- Tao, W.; Sun, W.; Liu, L.; Wang, G.; Xiao, Z.; Pei, X.; Wang, M. Chitosan oligosaccharide attenuates nonalcoholic fatty liver disease induced by high fat diet through reducing lipid accumulation, inflammation and oxidative stress in C57BL/6 mice. Mar. Drugs 2019, 17, 645. [Google Scholar] [CrossRef]
- Lu, K.L.; Xu, W.N.; Liu, W.B.; Wang, L.N.; Zhang, C.N.; Li, X.F. Association of Mitochondrial Dysfunction with Oxidative Stress and Immune Suppression in Blunt Snout Bream Megalobrama amblycephala Fed a High-Fat Diet. J. Aquat. Anim. Health 2014, 26, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.M.; Xue, L.Y.; Usuda, J.; Azizuddin, K.; Oleinick, N.L. Bax is essential for mitochondrion-mediated apoptosis but not for cell death caused by photodynamic therapy. Brit. J. Cancer 2003, 89, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Mcarthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; San, C.H.; et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 2018, 359, eaao6047. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, L.; Lu, T.; Zhang, Y.; Sui, X.; Li, Y.; Huang, X.; He, L.; Cai, J.; Zhou, C.; et al. MSCs ameliorate hepatocellular apoptosis mediated by PINK1-dependent mitophagy in liver ischemia/reperfusion injury through AMPKα activation. Cell Death Dis. 2020, 11, 256. [Google Scholar] [CrossRef]
- Xie, S.; Lin, Y.; Wu, T.; Tian, L.; Liang, J.; Tan, B. Dietary lipid levels affected growth performance, lipid accumulation, inflammatory response and apoptosis of japanese seabass (Lateolabrax japonicus). Aquacult. Nutr. 2021, 27, 807–816. [Google Scholar] [CrossRef]
- Lu, K.; Wang, L.; Zhang, D.; Liu, W.; Xu, W. Berberine attenuates oxidative stress and hepatocytes apoptosis via protecting mitochondria in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish. Physiol. Biochem. 2017, 43, 65–76. [Google Scholar] [CrossRef]
- Jia, R.; Cao, L.-P.; Du, J.-L.; He, Q.; Gu, Z.-Y.; Jeney, G.; Xu, P.; Yin, G.-J. Effects of high-fat diet on antioxidative status, apoptosis and inflammation in liver of tilapia (Oreochromis niloticus) via Nrf2, TLRs and JNK pathways. Fish. Shellfish. Immunol. 2020, 104, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory links between high fat diets and diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Ahonen, T.M.; Saltevo, J.T.; Kautiainen, H.J.; Kumpusalo, E.A.; Vanhala, M.J. The association of adiponectin and low-grade inflammation with the course of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 285–291. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Hou, C.; Gao, Y.; Wang, Y. Physiological and molecular changes in large yellow croaker (Pseudosciaena crocea R.) with high-fat diet-induced fatty liver disease. Aquac. Res. 2015, 46, 272–282. [Google Scholar] [CrossRef]
- Aloui, F.; Charradi, K.; Hichami, A.; Subramaniam, S.; Khan, N.A.; Limam, F.; Aouani, E. Grape seed and skin extract reduces pancreas lipotoxicity, oxidative stress and inflammation in high fat diet fed rats. Biomed. Pharmacother. 2016, 84, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa-Nagata, N.; Takamura, T.; Ando, H.; Nakamura, S.; Kurita, S.; Misu, H.; Ota, T.; Yokoyama, M.; Honda, M.; Miyamoto, K.I.; et al. Increased oxidative stress precedes the onset of high-fat diet–induced insulin resistance and obesity. Metabolism 2008, 57, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Yang, Y.; Hu, Y.; Sun, Y.; Zhang, S.; Peng, W.; Zhong, Y.; Huang, X.; Kong, W. A long-term high-fat diet increases oxidative stress, mitochondrial damage and apoptosis in the inner ear of d-galactose-induced aging rats. Hear. Res. 2012, 287, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jiang, W.D.; Jiang, J.; Zhao, J.; Liu, Y.; Zhang, Y.A.; Zhou, X.Q.; Feng, L. Dietary choline deficiency and excess induced intestinal inflammation and alteration of intestinal tight junction protein transcription potentially by modulating NF-κB, STAT and p38 MAPK signaling molecules in juveNile Jian carp. Fish. Shellfish. Immunol. 2016, 58, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Saleh, H.; El-Shorbagy, H.M. Chitosan protects liver against ischemia-reperfusion injury via regulating Bcl-2/Bax, TNF-α and TGF-β expression. Int. J. Biol. Macromol. 2020, 164, 1565–1574. [Google Scholar] [CrossRef]
- Bai, Y.; Zheng, J.; Yuan, X.; Jiao, S.; Feng, C.; Du, Y.; Liu, H.; Zheng, L. Chitosan Oligosaccharides Improve Glucolipid Metabolism Disorder in Liver by Suppression of Obesity-Related Inflammation and Restoration of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ). Mar. Drugs 2018, 16, 455. [Google Scholar] [CrossRef]
- Ma, Q.; Li, L.Y.; Le, J.Y.; Lu, D.L.; Qiao, F.; Zhang, M.L.; Du, Z.Y.; Li, D.L. Dietary microencapsulated oil improves immune function and intestinal health in Nile tilapia fed with high-fat diet. Aquaculture 2018, 496, 19–29. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef]
- Cheng, K.; Song, Z.; Li, S.; Yan, E.; Zhang, H.; Zhang, L.; Wang, C.; Wang, T. Effects of resveratrol on intestinal oxidative status and inflammation in heat-stressed rats. J. Therm. Biol. 2019, 85, 102415. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, K.; Luan, Z.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Liu, S.; Zhou, Y.; Mi, S.; Liu, G.; Wu, X.; Yao, K.; Assaad, H.; Deng, Z.; Hou, Y. Chlorogenic acid decreases intestinal permeability and increases expression of intestinal tight junction proteins in weaned rats challenged with LPS. PLoS ONE 2014, 9, e97815. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Wen, X.; Wang, L.; Jiang, Z.; Zheng, C. Effects of low-molecular-weight chitosan on the growth performance, intestinal morphology, barrier function, cytokine expression and antioxidant system of weaned piglets. BMC Vet. Res. 2018, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Huang, P.; Ma, P.; Liu, Q.S.; Yu, C.; Du, Y.G. Chitosan oligosaccharides suppress LPS-induced IL-8 expression in human umbilical vein endothelial cells through blockade of p38 and Akt protein kinases. Acta Pharmacol. Sin. 2011, 32, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hyung, J.H.; Ahn, C.B.; Kim, B.I.; Kim, K.; Je, J.Y. Involvement of Nrf2-mediated heme oxygenase-1 expression in anti-inflammatory action of chitosan oligosaccharides through MAPK activation in murine macrophages. Eur. J. Pharmacol. 2016, 793, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Vermette, D.; Hu, P.; Canarie, M.F.; Funaro, M.; Glover, J.; Pierce, R.W. Tight junction structure, function, and assessment in the critically ill: A systematic review. Intensive Care Med. Exp. 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Paola, B.; Ignazio, C.; Vincenza, D.L.; Andrea, B.; Massimo, P.; Giorgio, P.; Diego, M. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar]
- Sambolin-Escobales, L.; Nazario, M.; Torres-Aguiar, R.; Cruz, M.L.; Yamamura, Y.; Appleyard, C.B.; Chompre, G. High fat diet induces decreased levels of colonic tight junctions and inflammatory cytokine expression in sprague dawley rats. FASEB J. 2016, 30, lb698. [Google Scholar] [CrossRef]
- Song, D.; Cheng, Y.; Li, X.; Wang, F.; Lu, Z.; Xiao, X.; Wang, Y. Biogenic nanoselenium particles effectively attenuate oxidative stress-induced intestinal epithelial barrier injury by activating the Nrf2 antioxidant pathway. ACS Appl. Mater. Interfaces 2017, 9, 14724–14740. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Bio. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef]
- He, N.; Wang, S.; Lv, Z.; Zhao, W.; Li, S. Low molecular weight chitosan oligosaccharides (LMW-COSs) prevent obesity-related metabolic abnormalities in association with the modification of gut microbiota in high-fat diet (HFD)-fed mice. Food Funct. 2020, 11, 9947–9959. [Google Scholar] [CrossRef]
- Osho, S.; Adeola, O. Chitosan oligosaccharide supplementation alleviates stress stimulated by in-feed dexamethasone in broiler chickens. Poult. Sci. 2020, 99, 2061–2067. [Google Scholar] [CrossRef]
- Wan, J.; Jiang, F.; Xu, Q.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Yu, J.; He, J. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Adv. 2017, 7, 9669–9679. [Google Scholar] [CrossRef]
- Stanek, M.; Mazurkiewicz, J.; Rawski, M.; Bogucka, J.; Ziółkowska, E.; Dankowiakowska, A.; Kierończyk, B. Effect of chitosan on common carp (Cyprinus carpio) fry growth performance, feed utilization and nutriphysiological status. Aquac. Rep. 2023, 30, 101622. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).