Simple Summary
To reveal the effects of waterborne copper stress on gene expression changes, molecular pathways, and physiological functions in Coilia nasus, two libraries were constructed from a copper treatment group (Cu) and a control group (C) and sequenced using Illumina sequencing technology. The present study indicates the main view: copper induces the aberrant expression of immune and metabolic aspects of genes, suggesting that copper causes metabolic disorders and insufficient energy supply in the body, and it induces oxidative stress, which results in reduced immune functions.
Abstract
To reveal the effects of waterborne copper stress on gene expression changes, molecular pathways, and physiological functions in Coilia nasus, juvenile fish were equally divided into two experimental groups, and the copper levels were 1.61 ± 0.03 mg/L (copper-exposed group) and 0 mg/L (control group), respectively. After 4 h, gill tissue samples were collected for transcript sequencing analysis, and two libraries were constructed from the copper treatment group (Cu) and the control group (C) and sequenced using Illumina sequencing technology. The results showed that approximately 40.2–46.0 M clean reads were obtained from each library, and the percentage of uniquely mapped transcripts ranged from 80.57 to 84.93%. A total of 3915 differentially expressed genes (DEGs) were identified under waterborne copper stress, among which 1300 genes were up-regulated, and 2615 genes were down-regulated. Twelve DEGs were randomly selected for quantitative RT-PCR (qRT-PCR) analysis, and the results confirmed that the transcriptome analysis was reliable. Furthermore, the DEGs were subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis, and the results showed that most of the DEGs were involved in metabolic pathways, including steroid biosynthesis, glutathione metabolism, and peroxisome proliferator-activated receptor (PPAR) signaling pathways. Furthermore, due to the waterborne copper levels, gsk-3β was significantly up-regulated, while other metabolism-related genes (tor, pi3k, lpl, aqp7, fabp3) were significantly down-regulated. In addition, the copper-exposed group significantly reduced the expression of some immunity genes (ifn-γ, stat1, cxcl10, and tgf-β), and enhanced the expression of il-1β and tnf-α. In summary, these results indicated that copper causes metabolic disorders and insufficient energy supply in the body, and induces oxidative stress, which results in reduced immune functions.
1. Introduction
In recent years, with the rapid development of industry and agriculture, copper (Cu) is widely used as a raw material in the chemical industry, and is discharged in the industrial production process [1]. Copper is an effective component in algaecides and fungicides that are widely used in agricultural production activities. Excessive copper ions in water will cause serious pollution to aquaculture water, and will have strong toxic effects on fish through biological enrichment [2], causing abnormal behavior of fish, physiological function disorders, tissue lesions, and even death [3,4]. On the other hand, after the aquatic products with high copper content enter the food chain, the copper ions in the body will be enriched in the food chain through the enrichment effect, and eventually endanger human health [5]. Therefore, the toxicity of waterborne copper to aquatic animals should be widely concerning.
Gills have many physiological functions such as immune regulation, respiration, osmotic pressure regulation and excretion, and acid–base balance, and are also one of the organs directly in contact with the external environment in aquatic animals [6]. A change in the water environment will directly affect the physiological state of gills, and even aquatic animals. In previous studies, it has been confirmed that gills are directly exposed to heavy metals, so heavy metals accumulate in gill tissue [7]. Common lesions in the gill tissue of the Senegalese sole (Solea senegalensis) were observed under copper exposure [8], such as the lamellar fusion and proliferation of epithelial cells, the rupture of capillaries, and the release of red blood cells. Ortiz et al. [9] observed that copper increased gill mucous secretions, hypertrophy of chlorine cells, and blood exosmosis in mummichogs (Fundulus heteroclitus). Thus, gills are very sensitive to changes in the physical and chemical properties of water environments; they are not only the main sensing organs of pollutants in water environments, but also provide an important model to evaluate environmental risks in ecotoxicological studies [10].
With the continuous development of omics technology and tools, to deeply understand the molecular regulatory mechanisms of aquatic animals in response to changes in water environments, most researchers adopt omics technology to explore the mechanisms of gill adaptations to environmental stressors [11,12,13] and find effective ways to improve aquatic animals’ responses to adverse environments. So far, more and more studies have been conducted on the transcriptome sequencing analysis of aquatic animal gills under different environmental factors (heavy metal pollutants, hypoxia, salinity, etc.); the expression of Cu/Zn-sod, cat, idh1, phyh, and decr2 in the gill peroxisome pathway is significantly up-regulated by copper stress in red swamp crayfish (Procambarus cruzii), suggesting that the regulatory mechanism is related to the function of oxidative stress [14]. Transcriptome analysis has become a powerful tool for studying the regulatory mechanism of branchial responses to changes in water environments, due to the fact that the results of transcriptome sequencing vary with physiological states and external environments.
Coilia nasus is an important migratory fish in the Yangtze River and enjoys the reputation of being one of the “three delicacies of the Yangtze River” due to its nutritional richness. With the breakthrough of artificial breeding and pond breeding technology, C. nasus has gradually become a breeding variety with higher economic value [15,16]. Studies have shown that the concentration coefficient of heavy metal copper in Yangtze River sediments is high [17]; although an appropriate amount of copper ions is conducive to the stability of aquatic animals’ internal environments, excessive copper ions will lead to abnormal structures and physiological functions of aquatic animals, and various diseases will occur [18]. So far, no studies have been reported on the immune, metabolic, and oxidative stress responses of C. nasus under copper stress. Therefore, this study was aimed at revealing the effects of waterborne copper stress on the gene expression changes, molecular pathways, and physiological functions related to C. nasus gills through transcriptional sequencing analysis, so as to provide new thoughts and directions for the healthy culturing of C. nasus.
2. Materials and Methods
2.1. Experimental Materials and Fish
Healthy juvenile Coilia nasus (5.0 ± 0.2 g) were collected from the Yangzhong Base of the Freshwater Fisheries Research Center (Zhenjiang, China), and the feeding was stopped 2 days before the formal experiment.
The copper sulfate (CuSO4-5H2O) used was analytically pure, and the Cu2+ in the water was diluted to the intended concentration (1.68 mg/L) according to the results of previous experiments [19]. The water used in the experiment was water purified after 3 days of aeration, with the following water quality indexes: pH = 7.2, dissolved oxygen >7.0 mg/L, water temperature 25.0 ± 0.5 °C, copper level 1.61 ± 0.03 mg/L (copper-exposed group), 0 mg/L (control group), respectively.
2.2. Management Methods
At the beginning of the experiment, the juvenile fish were equally divided into six tanks and divided into two experimental groups (copper-exposed and control), with three tanks of 20 fish in each experimental group. All the juvenile fish were placed in six tanks at the same time, and the behavioral status and poisoning symptoms of the fish were observed immediately during the experiment. After 4 h, when the fish in the copper-treated group showed a near-death state, gill tissue samples were collected from Coilia nasus in each experimental group for transcript sequencing analysis. To prevent feeding effects, no bait was fed during the experiment.
2.3. RNA Extraction, Library Construction, and Sequencing
This experiment was performed according to the instructions of the Trizol kit (Invitrogen, Carlsbad, CA, USA) for total RNA extraction. RNA quality was determined on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). After extraction of total RNA, eukaryotic mRNA was enriched with Oligo(dT) beads. After that, the enriched mRNA was fragmented into short fragments using a fragmentation buffer, and sequencing libraries were prepared after quality control of the RNA samples. The resulting cDNA library was amplified by polymerase chain reaction (PCR) and sequenced by Genetic Variation Biotechnology (Guangzhou, China) using Illumina Novaseq6000 (DynaMax Biotech. Co., Ltd., Shanghai, China).
2.4. Bioinformatics Analysis
First, fastp [20] was used to perform quality control; low-quality data were filtered and clean reads were obtained. After data filtering, the data quality was visualized. Next, HISAT2 (v2.1.0) [21] software was utilized to carry out reference genome-based comparison analysis.
Then, according to the comparison results, Stringtie was used to reconstruct the transcripts [22], and then RSEM was performed to calculate the gene expression [23]. After that, to exclude outlier samples, the expression results of each sample were analyzed using PCA, and Pearson correlation coefficients were calculated between samples as a method to understand the reproducibility of the samples.
2.5. Differentially Expressed Genes (DEGs)
RNAs were analyzed for differential expression between the two groups using DESeq2 (v1.20.0) [24] and edgeR (v3.32.1) [25] software. Differentially expressed genes/transcripts were defined as having a false discovery rate (FDR) parameter below 0.05 and an absolute fold change of ≥2.
2.6. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
First, differential genes were mapped to each term in the GO database (http://www.geneontology.org/, accessed on 5 June 2023) and the number of differential genes per term was calculated to count the number of differential genes with some GO function. Pathway significance enrichment analyses were performed in terms of KEGG pathways, and hypergeometric tests were applied to identify GO terms and KEGG pathways that were significantly enriched in differential genes compared to the whole background.
2.7. Real-Time PCR Validation Analysis for Differentially Expressed Genes
Twelve DEGs were selected for RT-qPCR, and the expression levels of the target genes were normalized to those of β-actin (forward primer: AACGGATCCGGTATGTGCAAAGC, reverse primer: GGGTCAGGATACCTCTCTTGCTCTG) [26] to validate the results of the RNA-Seq analysis. Total RNA was extracted from the gill tissues of swordfish from control and copper-exposed groups. The extracted RNA was used as a template for quantitative analysis of the genes on a CFX96 fluorescent PCR instrument (BioRad, Shanghai, China) according to the instructions of the HiScript ®II One Step qRT–PCR SYBR Green Kit (Vazyme Biotech Co., Ltd., Nanjing, China). The relative expression of each sample was calculated by the 2−ΔΔCt method [27]. Details of the primers are shown in Table 1.

Table 1.
Primers used in the experiment.
3. Results
3.1. Sequence Comparison Reference Statistics
Table 2 shows the sequencing results of the C. nasus gill tissue RNA library; approximately 40.2–46.0 M clean reads of each library were obtained. The percentage of uniquely mapped transcripts ranged from 80.57 to 84.93%, while the percentage of transcripts showing multiple mapping results ranged from 2.63 to 3.79%.

Table 2.
Sequence comparison reference statistics.
3.2. Sample Relationship Analysis
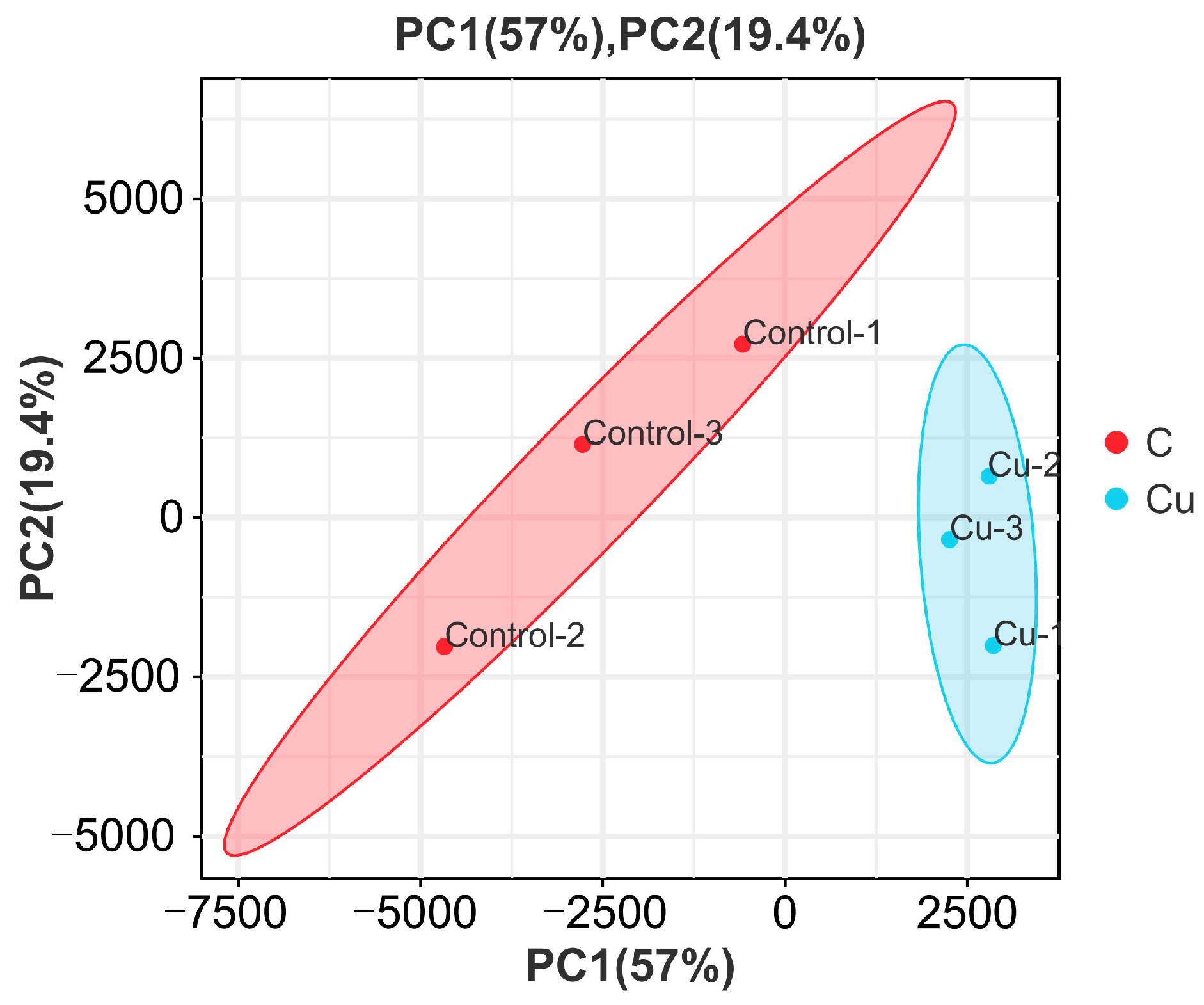
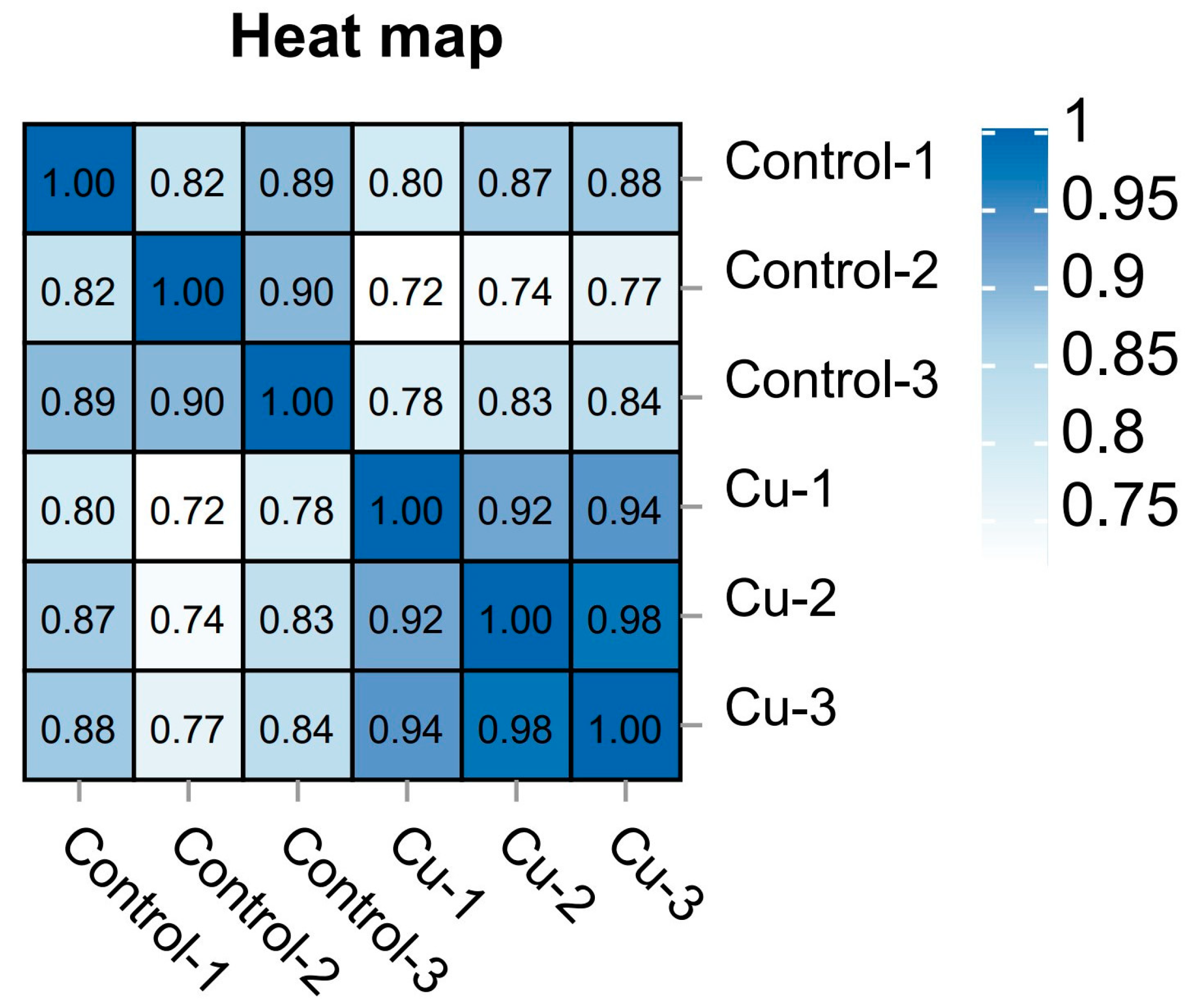
Based on the expression volume information, we carried out Principal Component Analysis (PCA). In the analyzed results of the PCA plots, the closer reflected distances represent the more similar sample compositions, and the samples from different effective treatments tend to show their own clustered distribution (Figure 1); it can be seen that the control group is clearly separated from the Cu group in the PCA plot. In addition, the Pearson correlation coefficient visualizes the correlation between any two samples in the form of a heat map, which in our study showed that the repeatability between duplicate samples within groups was good (Figure 2). In the correlation heat map, the correlation coefficients between the three replicates of the control group are all above 0.8, as well as the Cu group. Furthermore, there is a strong positive correlation between samples that belong to different treatment groups, and the lowest correlation coefficient (Cu-1/Control-2) was also greater than 0.72.
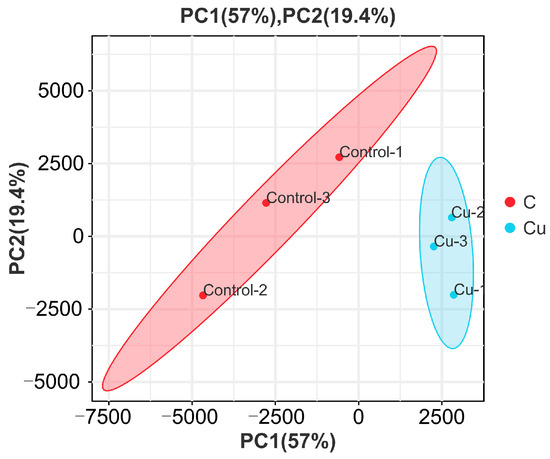
Figure 1.
Principal Component Analysis of samples.
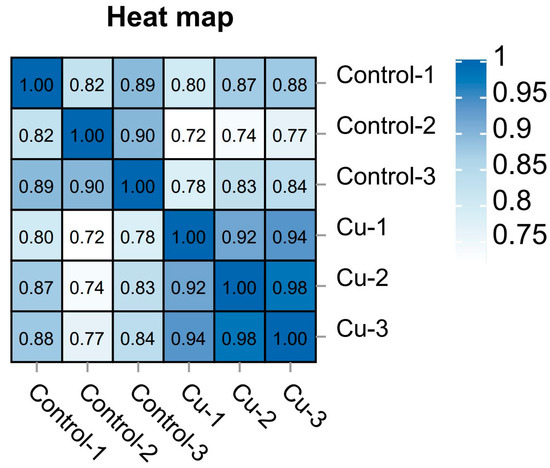
Figure 2.
Sample correlation heat map.
3.3. Differentially Expressed Genes (DEGs)
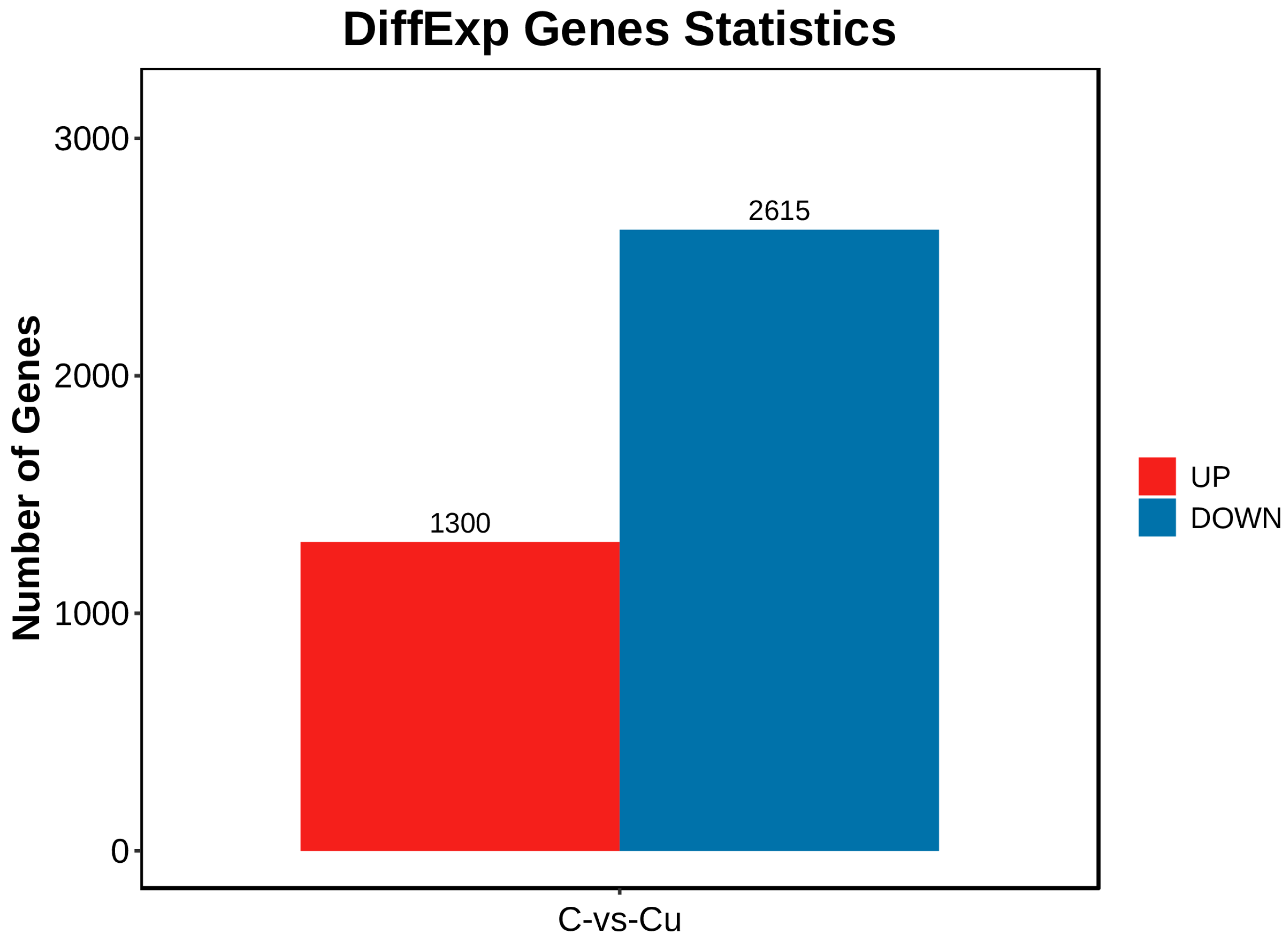
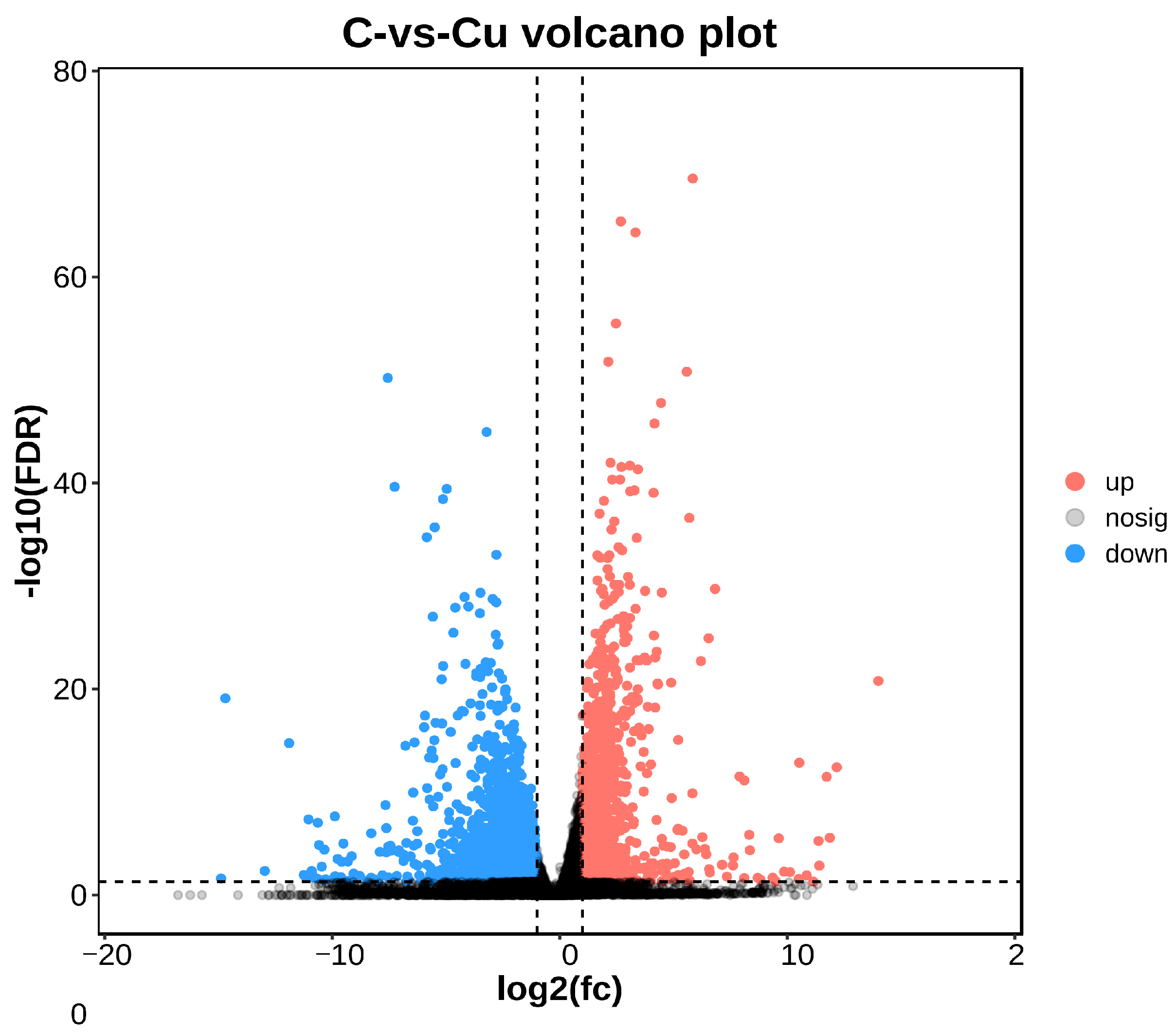
DEGs between the copper-exposed and control groups (Cu vs. C) were identified; 3915 differential genes (DEGs) were identified in the Cu group, of which 1300 were up-regulated and 2615 were down-regulated (Figure 3). Volcano plots were constructed with DEGs to visualize genes that differed between the two groups, with genes closer to the ends of the plot representing a greater degree of difference (Figure 4).
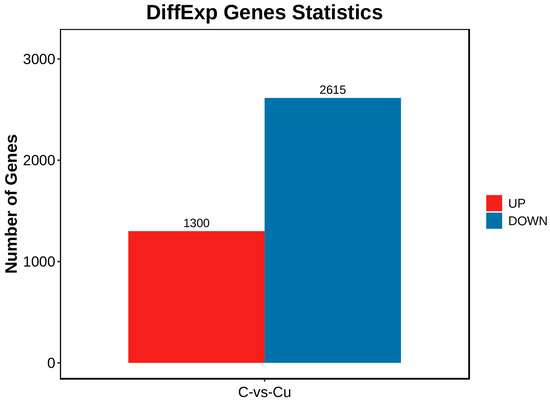
Figure 3.
Differential genes statistics chart.
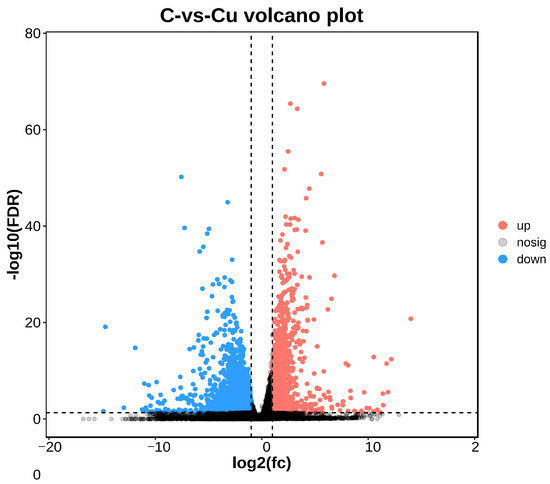
Figure 4.
Comparison between C and Cu groups volcano plot.
3.4. GO and KEGG Pathway Enrichment Analysis
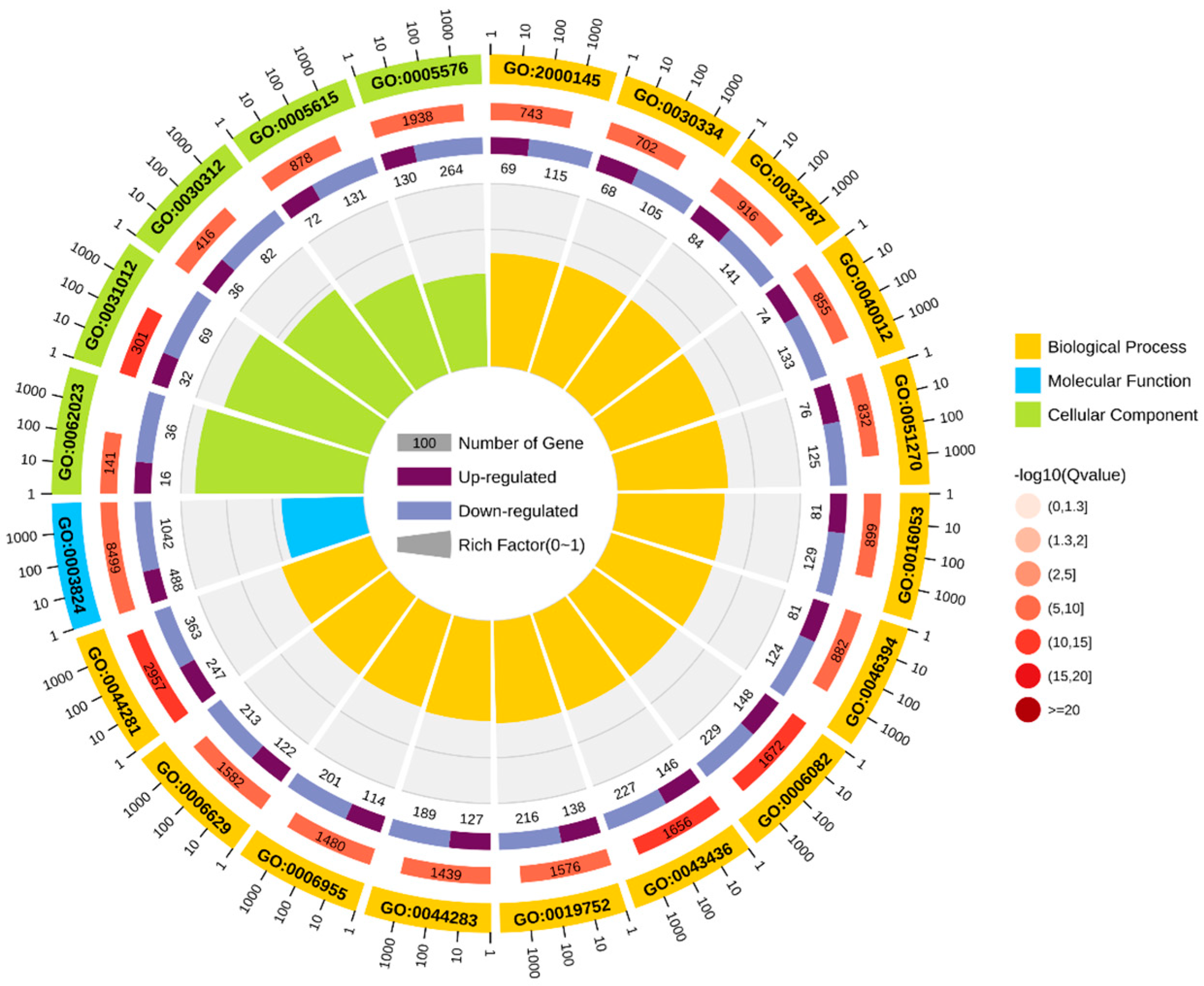
GO enrichment analysis was performed based on DEGs, and the results were categorized into three categories: cellular components (CCs), molecular functions (MFs), and biological processes (BPs) (Figure 5). Among cellular components, the main categories were the extracellular matrix (GO: 0031012) and external encapsulated structures (GO: 0030312). For molecular functions, the main categories were catalytic activity (GO: 0003824) and small molecule binding (GO: 0036094). For biological processes, the main categories represented are small molecule metabolic processes (GO: 0044281) and organic acid metabolic processes (GO: 0006082).
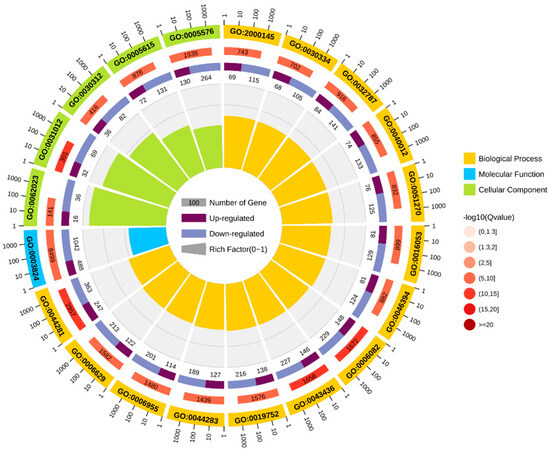
Figure 5.
GO enrichment circle diagram.
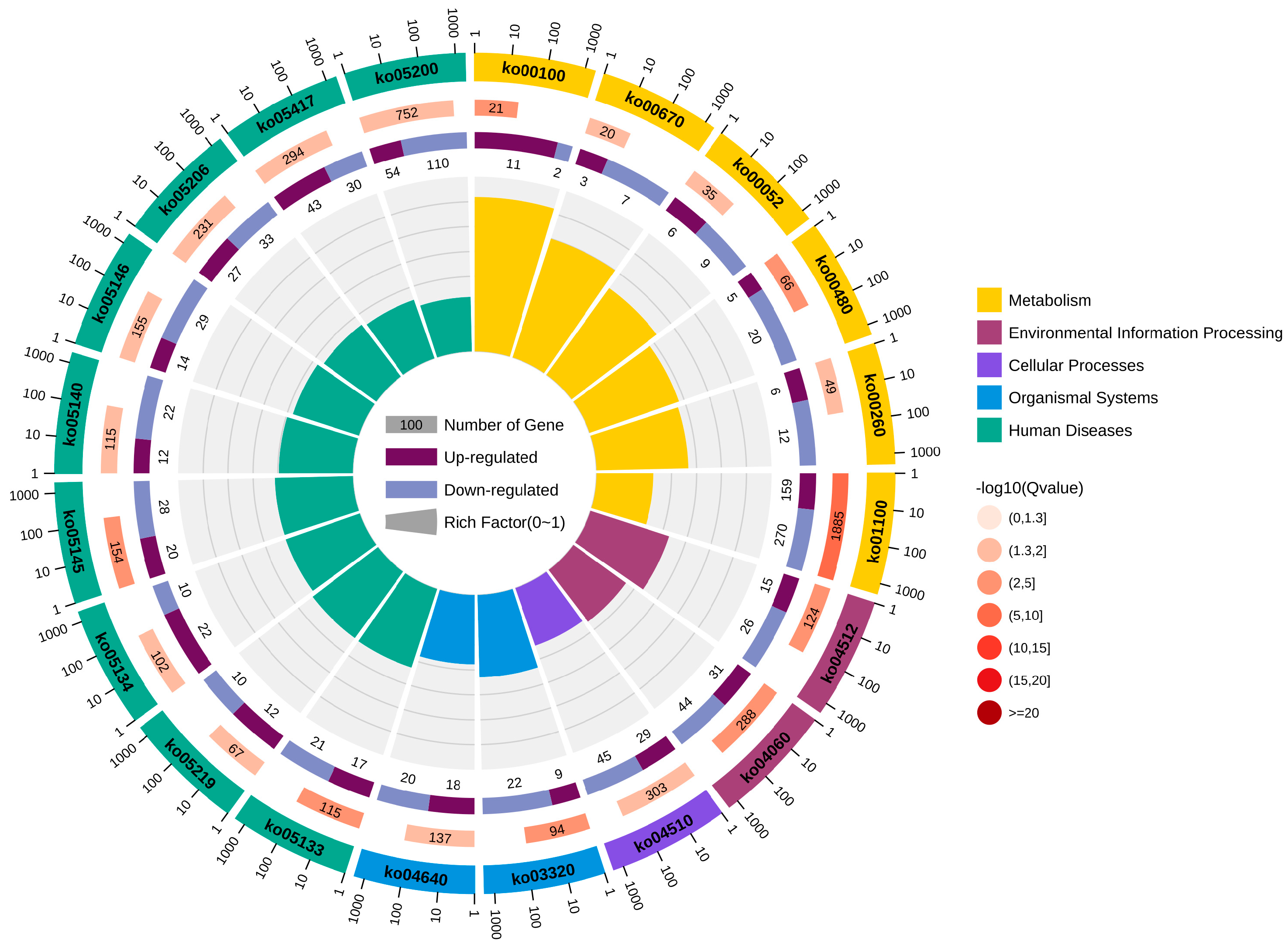
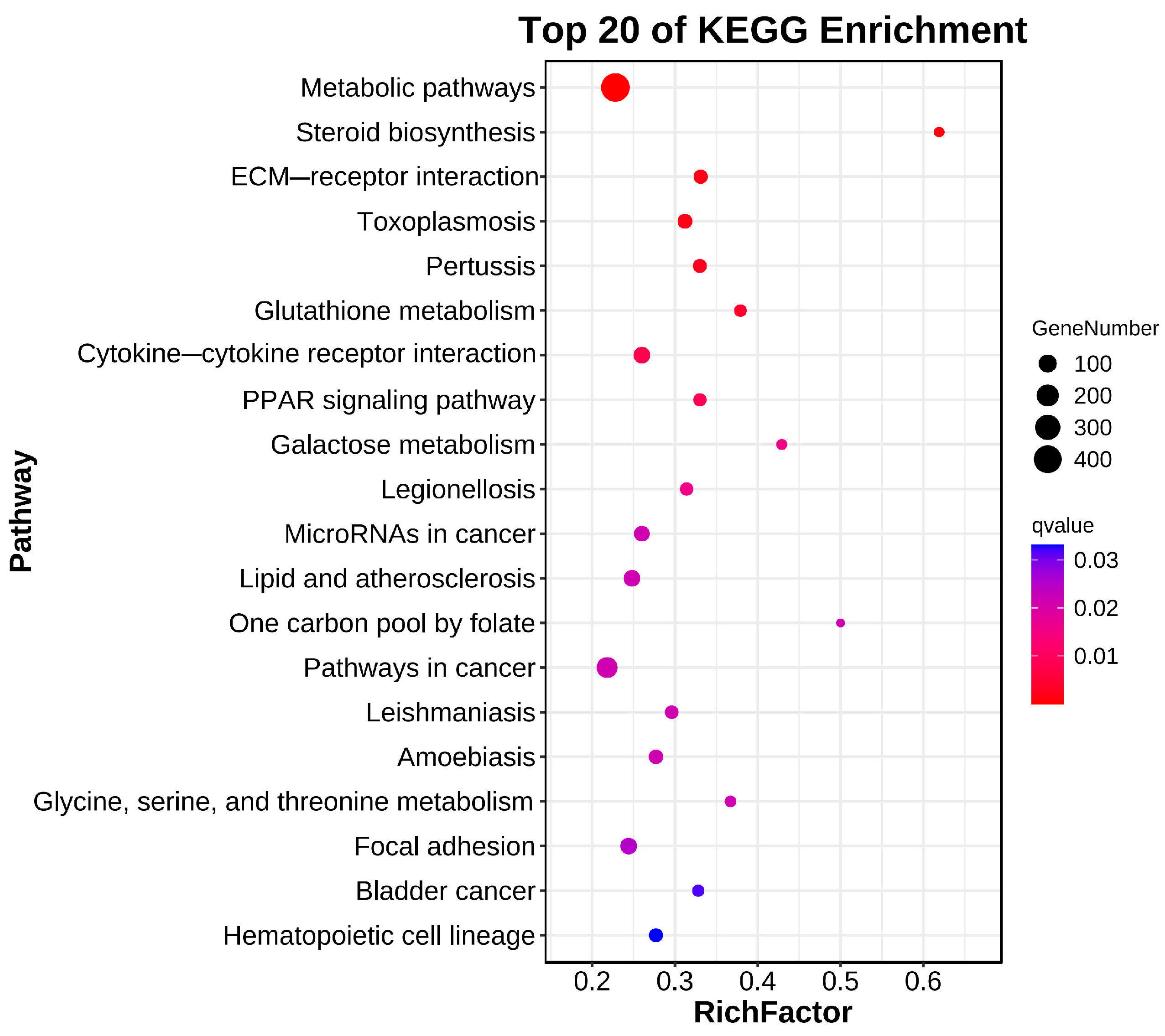
In total, 20 significantly enriched pathways were identified based on KEGG functional annotation (p-value < 0.05) (Figure 6). In the Cu and control group comparison, the identified differentially expressed genes were mainly enriched in metabolic pathways (ko01100), steroid biosynthesis (ko00100), ECM receptor interactions (ko04512), glutathione metabolism (ko00480), and the PPAR signaling pathway (ko03320) (Figure 7).
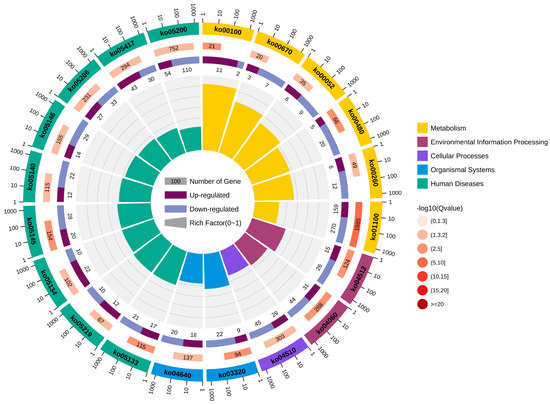
Figure 6.
KEGG enrichment circle diagram.
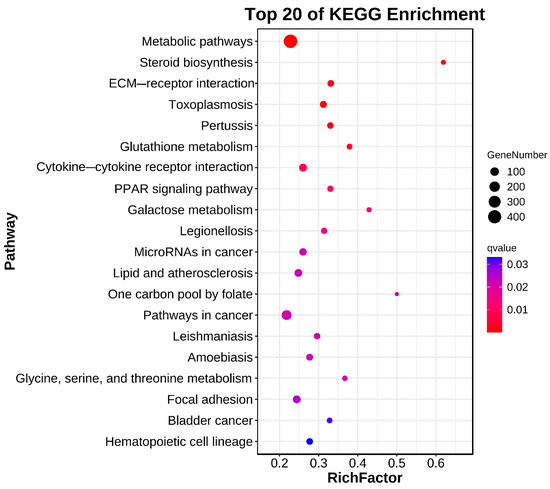
Figure 7.
Bubble diagram of KEGG enrichment.
3.5. Validation of RNA-Seq Results with qRT-RCR
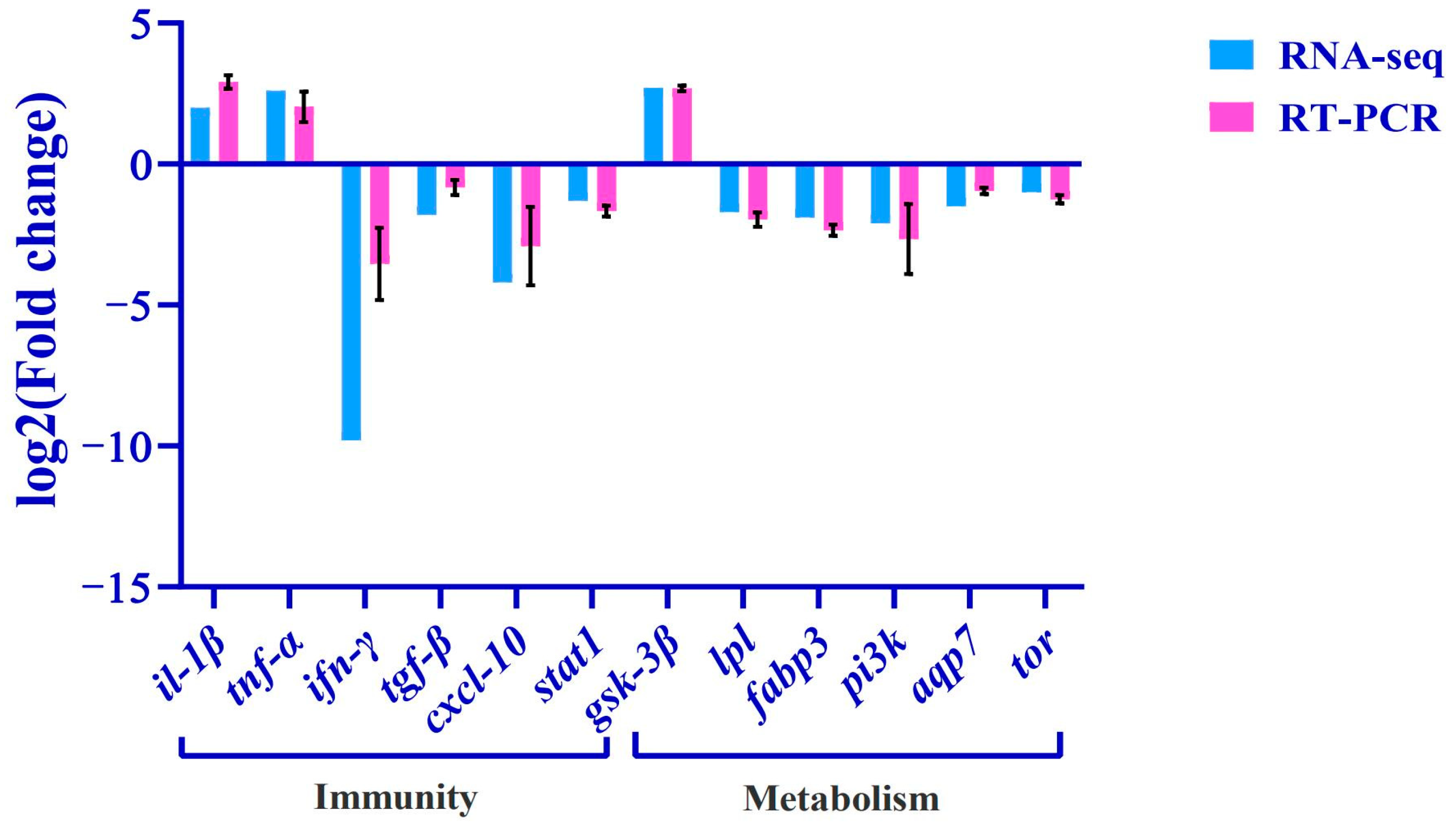
Twelve metabolism- or immunity-related differential genes (il-1β, tnf-α, gsk-3β, ifn-γ, pi3k, tgf-β, cxcl10, tor, fabp3, aqp7, lpl, stat1) were selected from the transcriptome database for qRT-PCR validation, and the relative expression levels (expressed as mean ± standard error) of the differentially expressed genes caused by copper stress were quantitatively analyzed using the 2−ΔΔCt method (Figure 8), and the expression trends of the differentially expressed genes were found to be consistent with the quantitative results of the RNA-Seq, which indicated that the results of transcriptome sequencing are reliable.
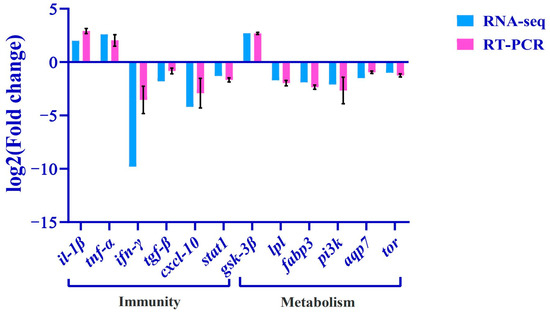
Figure 8.
Validation of RNA-Seq results using RT-PCR. The transcript expression levels of the selected genes were normalized to that of the β-actin.
4. Discussion
The irrational discharge of industrial wastewater and the misuse of agricultural pesticides can have a serious impact on the ecosystems of water bodies and threaten the survival of fish [17]. As an indispensable trace element for fish, a high concentration of waterborne copper affects the healthy culture of C. nasus [2]. There is an urgent need for transcriptional sequencing technology to reveal the mechanisms of physiological functional changes in C. nasus in response to waterborne copper stress. In this experiment, based on PCA and heat map analysis, the Pearson correlation coefficient can reach 0.8 between the three biological replicates within the same group, and the vast majority of gene expression proved that between the three biological replicates in the control and Cu groups, the reproducibility was still relatively good, with no obvious outlier samples, which indicated that the transcriptome results were reliable. In this experiment, the copper-exposed group showed a total of 3915 DEGs compared to the control group, of which 1300 were up-regulated genes and 2615 down-regulated genes. Furthermore, in terms of GO and KEGG enrichment analyses, most differential genes cluster in metabolic pathways including steroid biosynthesis, glutathione metabolism, and PPAR signaling pathway, etc. As reported, after a certain period of copper exposure, it can cause damage to the tissue structure and inhibit the activity of relevant enzymes in the tissues [28,29]. In addition to this, there are also immune pathways (cytokine–cytokine receptor interaction, focal adhesion) involved where some of the inflammatory factors are located. Ma et al. [30] found that copper exposure induces oxidative stress and triggers an inflammatory response in Fugu obscurus (Takifugu fasciatus), and Bu et al. [31] reported that copper induces oxidative stress, hepatopancreatic injury, apoptosis, and inflammatory responses in Chinese mitten crabs (Eriocheir sinensis). Therefore, it is worth exploring the effects of waterborne copper stress on the molecular pathways and physiological functions of C. nasus through transcriptional sequencing analysis, to provide new insights into copper exposure-induced dysfunctions in C. nasus.
In recent years, it has been reported that waterborne copper significantly affects lipid metabolism in fish. Studies based on different concentrations of Cu exposure showed that high concentrations of Cu exposure reduced hepatic lipid synthase activity and gene expression in catfish (Pelteobagrus fulvidraco), and the lipid contents in the liver and abdominal adipose tissue gradually decreased with the gradual increase in Cu exposure concentration [29]. In this experiment, some DEGs were also enriched in lipid metabolism-related pathways, which in turn resulted in significant changes in the expression of some key genes. Cardiac fatty acid-binding protein 3 (FABP3), whose main function is to bind to intracellular long-chain fatty acids, promotes intracellular long-chain fatty acid transport from the plasma membrane of the cell to the mitochondria, then takes part in β-oxidation in the mitochondria, and ultimately generates ATP, which provides energy for the cell. When C. nasus were stressed by copper, the expression of fabp3 decreased; this implies that avoiding fabp3 overexpression by decreasing fabp3 expression will lead to a significant decrease in ATP levels, thus providing a protective effect on the organism [32]. In addition, lipoprotein lipase (LPL) is closely related to adipocyte differentiation and lipid deposition [33], and aquaporin 7 (AQP7) also mediates lipid metabolism in adipocytes [34]. In this experiment, the expression of both lpl and aqp7 was suppressed remarkably; this indicates a reduction in fatty acid uptake and the hydrolysis of triglycerides. Guo et al. [35] reported regarding grass carp (Ctenopharyngodon idellus) that the mRNA expression and enzyme activity of lipolytic LPL were significantly decreased after bacterial stress. The increased expression of AQP7 promotes glycerol excretion to the outside of adipocytes and reduces the burden on hypertrophic adipocytes [36]. These results show that lipid has a diminished ability to be oxidized for energy and does not provide more energy to the body.
In addition to lipid metabolism, protein and glucose metabolism were also significantly affected by copper stress. The expression of pi3k, which is involved in protein and glucose metabolism, was significantly suppressed. The PI3K/TOR pathway takes part in the regulation of protein synthesis [37]. Phosphatidylinositol 3-kinase (PI3K) can deregulate the inhibitory effect of target of rapamycin (TOR), and activated TOR can phosphorylate S6K, which in turn phosphorylates ribosomal protein S6 kinase, thereby initiating protein synthesis [38]. In this study, copper stress inhibited the pi3k and tor mRNA expression levels, which in turn inhibited protein synthesis in the organism, resulting in reduced protein deposition. Similar results were also found in swimming crabs (Portunus trituberculatus) [39] and the juvenile cobia (Rachycentron canadum) [40]. Furthermore, recent studies have revealed that glycogen synthase kinase-3β (GSK-3β) can phosphorylate a variety of endogenous substrates, including multiple proteins and transcription factors involved in metabolism, and, thus, it promotes cell growth and development, and the regulation of glucose homeostasis [41]. Inhibition of GSK-3β activity promotes the dephosphorylation of glycogen synthase, so its activation converts glucose to glycogen, and blood glucose decreases. Conversely, if GSK-3β activity is increased, glycogen synthase activity is inhibited, causing disorders of glucose metabolism [42]. In this experiment, copper stress activated the expression of gsk-3β mRNA, and reduced glycogen synthesis, which may lead to insufficient energy reserves in the body. However, further experiments are required to investigate the specific mechanism by which copper stress induces the disruption of glucose metabolism by increasing GSK3β activity.
Interferon γ (IFN-γ) plays important biological functions in immunomodulation, antiviral activities, and the mediation of inflammatory responses [43]. Moreover, signal transducer and activator of the transcription l (STAT1) gene is also involved in the regulation of the type I IFNs signaling pathway and can be induced to be expressed by IFN-γ [44]. In this study, the ifn-γ and stat1 mRNAs were significantly inhibited, which means that cellular immunity and resistance to viral infections began to weaken. As studies have confirmed, similarly to in mammals, IFN-γ in fish has an enhanced ability to clear bacteria [45] and a strong antiviral activity [46]. Furthermore, the downstream signaling molecule stat1 is also inhibited by copper stress, and dysregulation of this signaling pathway indicates multiple immune system disorders in the body. In addition, interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α) play important roles in the immune response and are important pro-inflammatory factors [47,48]. In this experiment, il-1β and tnf-α were clearly activated, which indicated that the body exhibited a pronounced inflammatory response. Further, the up-regulation of transforming growth factor-β (TGF-β) is induced by an increase in inflammatory mediators to suppress the inflammatory response [49], and chemokine 10 (CXCL10) is also associated with inflammatory diseases, autoimmune diseases, an tumors, and has antibacterial immunity when activated [50], but in this experiment, tgf-β and cxcl10 were both decreased significantly by copper stress. This implies that copper stress poses a serious threat to the C. nasus organism, with reduced immune functions.
5. Conclusions
In summary, transcriptional sequencing indicates that copper stress has profound effects on C. nasus. Copper induces the aberrant expression of immune and metabolic aspects of genes, suggesting that copper causes metabolic disorders and insufficient energy supply in the body, and it induces oxidative stress, which results in reduced immune functions. The enrichment of copper in water bodies has a great toxic effect on C. nasus, which seriously affects its normal growth and development and physiological functions and may even lead to death; at the same time, the enrichment of copper in water bodies directly reduces the safety of consumption, and indirectly threatens the health of human beings.
Author Contributions
Conceptualization, D.H. and H.L.; methodology, M.R.; software, D.H.; validation, D.H.; investigation, T.T.; resources, L.Z., H.M.; data curation, D.H.; writing—original draft preparation, D.H.; writing—review and editing, H.L., M.R.; supervision, T.T., L.Z., H.M.; project administration, M.R.; funding acquisition, D.H., M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFD2400904); the Central Public-interest Scientific Institution Basal Research Fund, Freshwater Fisheries Research Center, CAFS (NO. 2024JBFR01).
Institutional Review Board Statement
This study was conducted according to the Management Rule of Laboratory Animals (Chinese Order No. 676 of the State Council, revised 1 March 2017). The study was approved by the Laboratory Animal Ethics Committee of the Freshwater Fisheries Research Center (LAECFFRC-2023-03-13).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We are grateful to/thank Guangzhou Genedenovo Biotechnology Co., Ltd. for assisting in sequencing and/or bioinformatics analysis.
Conflicts of Interest
Authors Tao Teng, Lu Zhang, and Haifeng Mi are employed by Tongwei Agricultural Development Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Pierri, B.D.; Silva, A.D.; Cadorin, D.I.; Ferreira, T.H.; Mourino, J.L.P.; Filer, K.; Pettigrew, J.E.; Fracalossi, D.M. Different levels of organic trace minerals in diets for Nile tilapia juveniles alter gut characteristics and body composition, but not growth. Aquacult. Nutr. 2021, 27, 176–186. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, Y.P.; Jing, J.J.; Jiang, Q.; Xu, T.; Tu, Z.Y.; Li, W.M. Effect of copper exposure on metabolism behavior of juvenile grass carp (Ctenopharyngodon idella). J. Agro-Environ. Sci. 2016, 35, 261–265. [Google Scholar]
- Luo, J.; Zhu, T.; Wang, X.; Cheng, X.; Yuan, Y.; Jin, M.; Zhou, Q. Toxicological mechanism of excessive copper supplementation: Effects on coloration, copper bioaccumulation and oxidation resistance in mud crab Scylla paramamosain. J. Hazard. Mater. 2020, 395, 122600. [Google Scholar] [CrossRef] [PubMed]
- Zebral, Y.D.; Anni, I.S.A.; Afonso, S.B.; Abril, S.I.M.; Klein, R.D.; Bianchini, A. Effects of life-time exposure to waterborne copper on the somatotropic axis of the viviparous fish Poecilia vivipara. Chemosphere 2018, 203, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Dornelles Zebral, Y.; Roza, M.; da Silva Fonseca, J.; Gomes Costa, P.; Stürmer de Oliveira, C.; Gubert Zocke, T.; Bianchini, A. Waterborne copper is more toxic to the killifish Poecilia vivipara in elevated temperatures: Linking oxidative stress in the liver with reduced organismal thermal performance. Aquat. Toxicol. 2019, 209, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Mallatt, J. Fish gill structural changes induced by toxicants and other irritants: A statistical review. Can. J. Fish. Aquat. Sci. 1985, 42, 630–648. [Google Scholar] [CrossRef]
- Arellano, J.M.; Storch, V.; Sarasquete, C. Histological changes and copper accumulation in liver and gills of the Senegales sole, Solea senegalensi. Ecotoxicol. Environ. Saf. 1999, 44, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.B.; González De Canales, M.L.; Sarasquete, C. Quantification and histopathological alterations produced by sublethal concentrations of copper in Fundulus heteroclitus. Cienc. Mar. 1999, 25, 119–143. [Google Scholar] [CrossRef][Green Version]
- Nunes, B.; Antunes, S.C.; Gomes, R.; Campos, J.; Braga, M.R.; Ramos, A.S.; Correia, A.T. Acute effects of tetracycline exposure in the freshwater fish Gambusia holbrooki: Antioxidant effects, neurotoxicity and histological alterations. Arch. Environ. Contam. Toxicol. 2015, 68, 371–381. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.K.F.; Sun, J.; Zhang, H.M.; Lai, K.P.; Gu, J.; Qiu, J.W.; Kong, C.; Wong, C. iTRAQ-base quantitative proteomic analysis reveals acute hypoosmotic responsive proteins in the gills of the Japanese eel (Anguilla japonica). J. Proteom. 2014, 105, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.J.; Chen, J.; Huang, Z.A.; Shi, Y.H.; Wang, F. Proteomic analysis on the alteration of protein expression in gills of ayu (Plecoglossus altivelis) associated with salinity change. Comp. Biochem. Physiol.—Part D Genom. Proteom. 2010, 5, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Shi, X.L.; Guo, H.Y.; Bai, Y.Z.; Shen, C.C.; Zhang, Y.P.; Wang, Z.F. Comparative transcriptome analysis of the gills of Procambarus clarkii provides novel insights into the immune-related mechanism of copper stress tolerance. Fish Shellfish. Immunol. 2020, 96, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.C.; Tang, X.; Zhang, C.X.; Gu, R.B.; Zheng, J.L.; Xu, P.; Le, G.W. First studies of embryonic and larval development of Coilia nasus (Engraulidae) under controlled conditions. Aquac. Res. 2011, 42, 593–601. [Google Scholar] [CrossRef]
- Xu, G.C.; Xu, P.; Gu, R.B.; Zhang, C.X.; Zheng, J.L. Feeding habits and growth characteristics of pond-cultured Coilia nasus fingerlings. Chin. J. Ecol. 2011, 30, 2014–2018. [Google Scholar] [CrossRef]
- Shan, L.L.; Yuan, X.Y.; Mao, C.P.; Ji, J.F. Characteristics of heavy metals in sediments from different sources and their ecological risks in the lower reaches of the Yangtze River. Environ. Sci. 2008, 29, 2399–2404. [Google Scholar]
- Herkovits, J.; Alejandra Helguero, L. Copper toxicity and copper-zinc interactions in amphibian embryos. Sci. Total Environ. 1998, 221, 1–10. [Google Scholar] [CrossRef]
- Nie, Z.J.; Xu, G.C.; Zhang, S.L.; Xu, P.; Gu, R.B. Acute effects of copper on survival of fingerlings, antioxidant enzyme activities in liver and structure of gill and liver of Coilia nasus. J. Fish. Sci. China 2014, 21, 161–168. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Ben Langmead, C.A.; Steven, L.S. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq read. Nat. Biotechnol. 2015, 33, 290. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Du, F.K.; Xu, G.C.; Gao, J.W.; Nie, Z.J.; Xu, P.; Gu, R.B. Transport-induced changes in hypothalamic–pituitary–interrenal axis gene expression and oxidative stress responses in Coilia nasus. Aquac. Res. 2016, 47, 3599–3607. [Google Scholar] [CrossRef]
- Pfafff, M.W. A new mathematical model for relative quantification in real-time RTPCR. Nucleic. Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
- Matsuo, A.Y.O.; Playle, R.C.; Val, A.L.; Wood, C.M. Physiological action of dissolved organic matter in rainbow trout in the presence and absence of copper: Sodium uptake kinetics and unidirectional flux rates in hard and soft water. Aquat. Toxicol. 2004, 70, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Luo, Z.; Liu, X.; Song, Y.F.; Zhao, Y.H. Effects of Waterborne Chronic Copper Exposure on Hepatic Lipid Metabolism and Metal-Element Composition in Synechogobius hasta. Arch. Environ. Contam. Toxicol. 2012, 64, 301–315. [Google Scholar] [CrossRef]
- Ma, S.S.; Liu, Y.X.; Zhao, C.; Chu, P.; Yin, S.W.; Wang, T. Copper induced intestinal inflammation response through oxidative stress induced endoplasmic reticulum stress in Takifugu fasciatus. Aquat. Toxicol. 2023, 261, 106634. [Google Scholar] [CrossRef]
- Bu, X.Y.; Song, Y.; Pan, J.Y.; Wang, X.D.; Qin, C.J.; Jia, Y.Y.; Du, Z.Y.; Qin, J.G.; Chen, L.Q. Toxicity of chronic copper exposure on Chinese mitten crab (Eriocheir sinensis) and mitigation of its adverse impact by myo-inositol. Aquaculture 2022, 547, 737511. [Google Scholar] [CrossRef]
- Dubey, P.K.; Goyal, S.; Mishra, S.K.; Arora, R.; Mukesh, M.; Niranjan, S.K.; Kathiravan, P.; Kataria, R.S. Identification of polymorphism in fatty acid binding protein 3 (fabp3) gene and its association with milk fat traits in riverine buffalo (bubalus bubalis). Trop. Anim. Health Prod. 2016, 48, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, P.P.; Zhang, D.W.; Zheng, X.L.; Cayabyab, F.S.; Yin, W.D.; Tang, C.K. Lipoprotein lipase: From gene to atherosclerosis. Atherosclerosis 2014, 237, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Gimenez, L.; Becerril, S.; Moncada, R.; Valenti, V.; Ramirez, B.; Lancha, A.; Gurbindo, J.; Balaguer, I.; Cienfuegos, J.A.; Catalan, V.; et al. Sleeve gastrectomy reduces hepatic steatosis by improving the coordinated regulation of aquaglyceroporins in adipose tissue and liver in obese rats. Obes. Surg. 2015, 25, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zheng, H.O.; Liu, X.; Chi, S.Y.; Xu, Z.; Wang, Q.C. Nutrient sensing signaling functions as the sensor and regulator of immunometabolic changes in grass carp during Flavobacterium columnare infection. Fish Shellfish. Immunol. 2019, 93, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Sjoholm, K.; Palming, J.; Olofsson, L.E.; Gummesson, A.; Svensson, P.A.; Lystig, T.C.; Jennische, E.; Brandberg, J.; Torgerson, J.S.; Carlsson, B.; et al. A microarray search for genes predominantly expressed in human omental adipocytes: Adipose tissue as a major production site of serum amyloid A. J. Clin. Endocrinol. Metab. 2005, 90, 2233–2239. [Google Scholar] [CrossRef]
- Mammana, S.; Bramanti, P.; Mazzon, E.; Cavalli, E.; Basile, M.S.; Fagone, P.; Petralia, M.C.; Mccubrey, J.A.; Nicoletti, F.; Mangano, K. Preclinical evaluation of the pi3k/akt/mtor pathway in animal models of multiple sclerosis. Impact J. 2015, 9, 8263. [Google Scholar] [CrossRef] [PubMed]
- Miron, M.; Lasko, P.; Sonenberg, N. Signaling from akt to frap/tor targets both 4e-bp and s6k in drosophila melanogaster. Mol. Cell. Biol. 2003, 23, 9117. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.C.; Zhou, Q.C.; Zhang, X.S.; Zhu, T.T.; Guo, C.; Yang, Z.; Luo, J.X.; Yuan, Y.; Hu, X.Y.; Jiao, L.F.; et al. Effect of dietary replacement of fish meal with low-gossypol cottonseed protein concentrate on growth performance and expressions of genes related to protein metabolism for swimming crab (Portunus trituberculatus). Aquaculture 2022, 549, 737820. [Google Scholar] [CrossRef]
- He, Y.F.; Chi, S.Y.; Tan, B.P.; Dong, X.H.; Yang, Q.H.; Liu, H.Y.; Zhang, S.; Han, F.L.; Liu, D. dl-Methionine supplementation in a low-fishmeal diet affects the TOR/S6K pathway by stimulating ASCT2 amino acid transporter and insulin-like growth factor I in the dorsal muscle of juvenile cobia (Rachycentron canadum). Br. J. Nutr. 2019, 122, 734–744. [Google Scholar] [CrossRef]
- Lochhead, P.A.; Coghlan, M.; Rice, S.Q.; Sutherland, C. Inhibition of GSK-3 selectively reduces glucose-6-phosphatase and phosphatase and phosphoenolypyruvate carboxykinase gene expression. Diabetes 2001, 50, 937–946. [Google Scholar] [CrossRef]
- Patel, S.; Doble, B.W.; Macaulay, K.; Sinclair, E.M.; Drucker, D.J.; Woodgett, J.R. Tissue-specific role of glycogen synthase kinase 3β in glucose homeostasis and insulin action. Mol. Cell. Biol. 2008, 28, 6314–6328. [Google Scholar] [CrossRef]
- Fathy, S.A.; Mohamed, M.R.; Ali, M.A.M.; El-Helaly, A.E.; Alattar, A.T. Influence of il-6, il-10, ifn-γ and tnf-α genetic variants on susceptibility to diabetic kidney disease in type 2 diabetes mellitus patients. Biomarkers 2019, 24, 43–55. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.Y.; Lu, X.Y.; Xiao, J.; Feng, P.H.; Feng, H. STAT1a and STAT1b of black carp play important roles in the innate immune defense against GCRV. Fish Shellfish. Immunol. 2019, 87, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Lv, M.; Qiu, X.; Wang, X.; Zhou, H. Ifn-γ manipulates nod1-mediated interaction of autophagy and Edwardsiella piscicida to augment intracellular clearance in fish. J. Immunol. 2021, 207, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, S.N.; Laghari, Z.A.; Huo, H.J.; Hou, J.; Huang, L.; Li, N.; Nie, P. Myxovirus resistance (Mx) gene and its differential expression regulated by three type I and two type II IFNs in mandarin fish, Siniperca chuatsi. Dev. Comp. Immunol. 2020, 105, 103604. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Huang, C.H.; Kato-Maeda, M.; Hopewell, P.C.; Daley, C.L.; Krensky, A.M.; Clayberger, C. Messenger RNA expression of IL-8, FOXP3, and IL-12β differentiates latent tuberculosis infection from disease. J. Immunol. 2007, 178, 3688–3694. [Google Scholar] [CrossRef]
- Caminero, A.; Comabella, M.; Montalban, X. Tumor necrosis factor alpha (tnf-α), anti-tnf-α and demyelination revisited: An ongoing story. J. Neuroimmunol. 2011, 234, 1–6. [Google Scholar] [CrossRef]
- Faliex, E.; Dasilva, C.; Simon, G.; Sasal, P. Dynamic expression of immune response genes in the sea bass, Dicentrarchus labrax, experimentally infected with the monogenean Diplectanum aequans. Fish Shellfish. Immunol. 2008, 24, 759–767. [Google Scholar] [CrossRef]
- Gasper, N.A.; Petty, C.C.; Schrum, L.W. Bacterium-induced CXCL10 secretion by osteoblasts can be mediated in part through toll-like receptor 4. Infect. Immun. 2002, 70, 4075–4082. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).