Exosomes in the Diagnosis of Neuropsychiatric Diseases: A Review
Abstract
Simple Summary
Abstract
1. Introduction
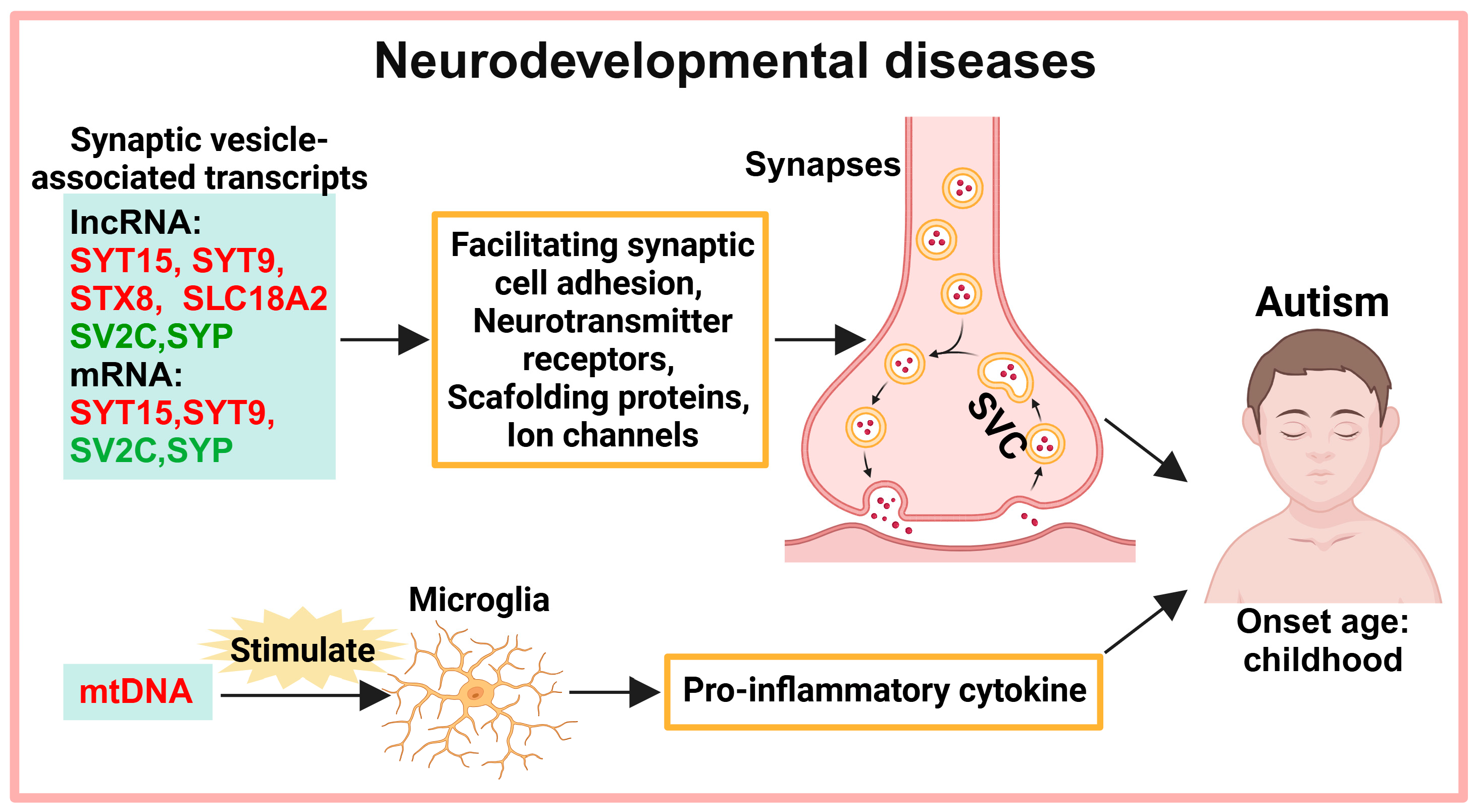
2. Exosomes in the Diagnosis of Neurodevelopmental Diseases
2.1. Neurotransmitter Transmission
2.2. MtDNA-Mediated Neuroinflammation
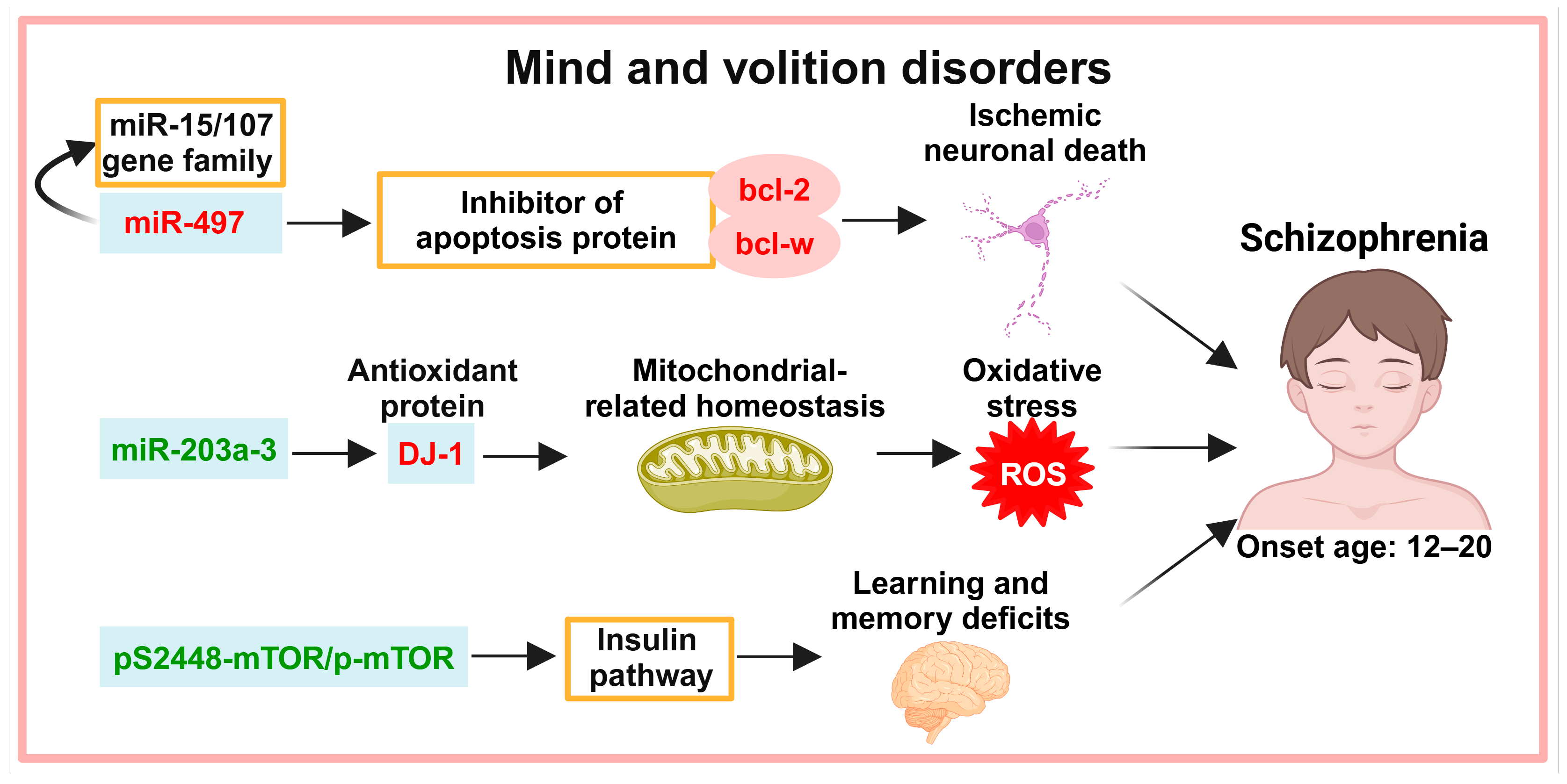
3. Exosomes in the Diagnosis of Mind and Volition Disorders
3.1. Neuronal Apoptosis
3.2. Oxidative Stress
3.3. Insulin Pathways
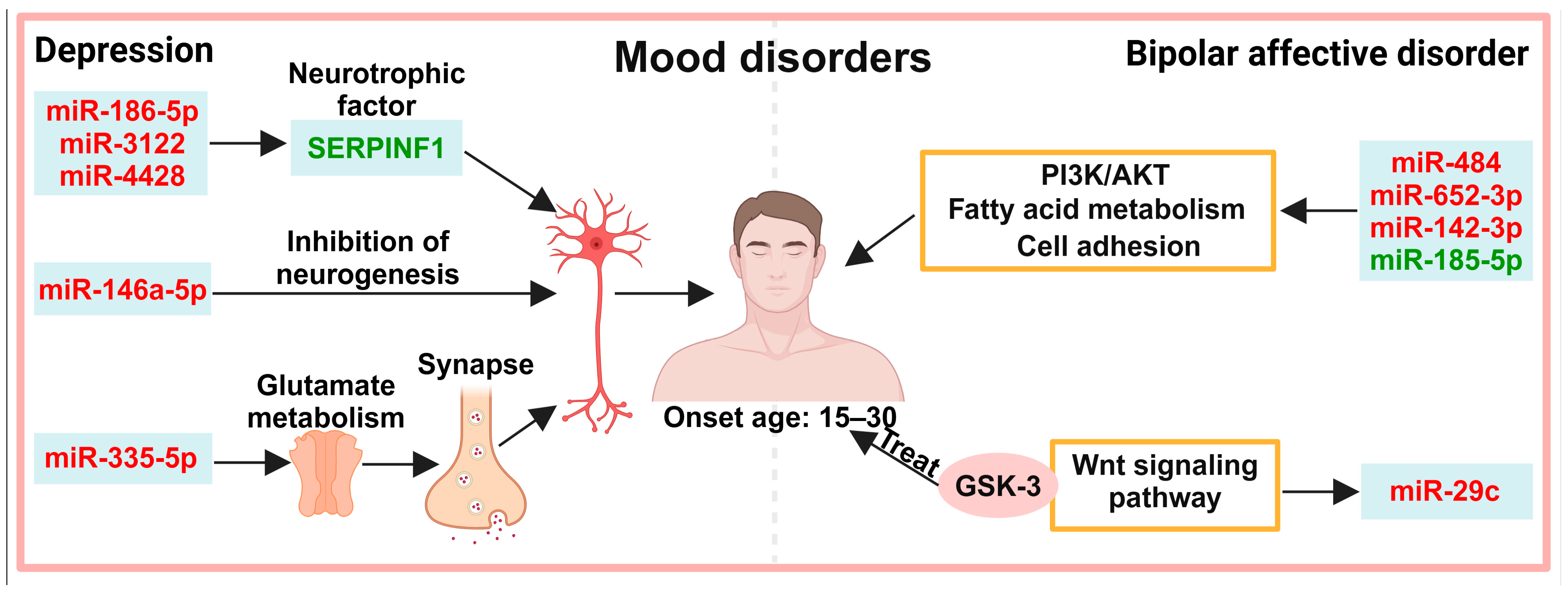
4. Exosomes in the Diagnosis of Mood Disorders
4.1. Neuronal Activity
4.2. Other Pathways
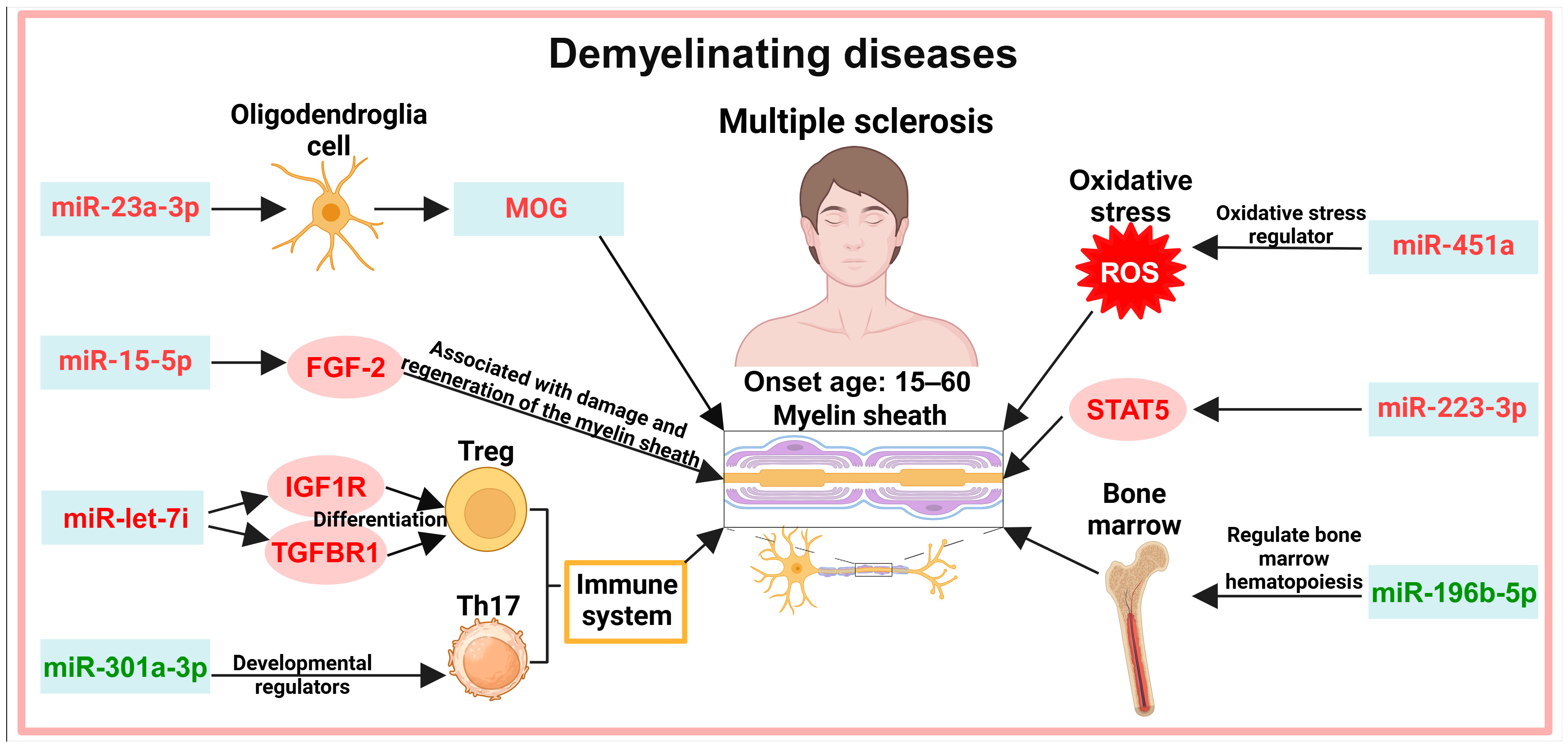
5. Exosomes in the Diagnosis of Demyelinating Diseases
5.1. Myelin Demyelination
5.2. Immune System
5.3. Other Pathways
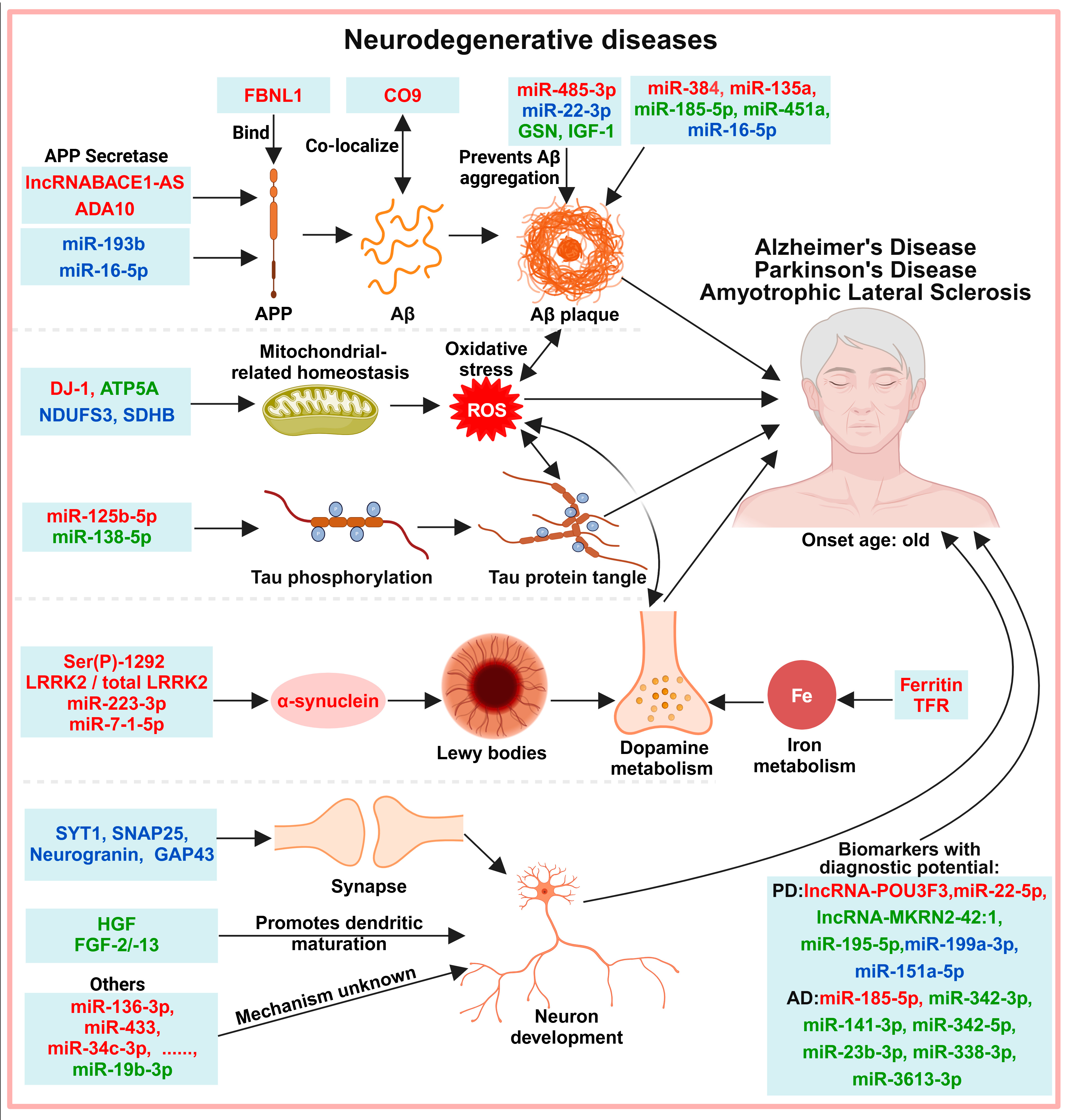
6. Exosomes in the Diagnosis of Neurodegenerative Diseases
6.1. Protein-Misfolding-Induced Neurotoxicity
6.2. Neuronal Development and Function
6.3. Other Molecules
7. Discussion
7.1. Isolation, Analysis, and Validation of Exosome Biomarkers
7.2. Exosome Biomarkers Classification
7.3. Ethical Concerns
8. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J. Eextracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Stanca, S.; Rossetti, M.; Bokulic Panichi, L.; Bongioanni, P. The cellular dysfunction of the brain-blood barrier from endothelial cells to astrocytes: The pathway towards neurotransmitter impairment in Schizophrenia. Int. J. Mol. Sci. 2024, 25, 1250. [Google Scholar] [CrossRef]
- Roghani, A.K.; Garcia, R.I.; Roghani, A.; Reddy, A.; Khemka, S.; Reddy, R.P.; Pattoor, V.; Jacob, M.; Reddy, P.H.; Sehar, U. Treating Alzheimer’s disease using nanoparticle-mediated drug delivery strategies/systems. Ageing Res. Rev. 2024, 97, 102291. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Li, J.W.; Zhu, X.; Jiang, H.S.; Zhao, H.; Zhang, L.M. MiR-184 mediated the expression of znf865 in exosome to promote procession in the PD model. Mol. Neurobiol. 2024, 61, 3397–3408. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Hornung, S.; Kruayatidee, A.; Maina, K.N.; Del Rosario, I.; Paul, K.C.; Wong, D.Y.; Duarte Folle, A.; Markovic, D.; Palma, J.A.; et al. α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson’s disease from multiple system atrophy. Acta Neuropathol. 2021, 142, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Agliardi, C.; Guerini, F.R.; Zanzottera, M.; Bolognesi, E.; Picciolini, S.; Marano, M.; Magliozzi, A.; Di Fonzo, A.; Arighi, A.; et al. Oligomeric α-synuclein and tau aggregates in NDEVs differentiate Parkinson’s disease from atypical parkinsonisms. Neurobiol. Dis. 2023, 176, 105947. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.; Mukherjee, R.; Singh, E.; Kumar, S.; Sharma, V.; Vishwakarma, P.; Bharti, P.S.; Nikolajeff, F.; Dinda, A.K.; Goyal, V.; et al. Neuronal exosomes in saliva of Parkinson’s disease patients: A pilot study. Park. Relat. Disord. 2019, 67, 21–23. [Google Scholar] [CrossRef]
- Rusconi, F.; Battaglioli, E.; Venturin, M. Psychiatric disorders and lncRNAs: α-synaptic match. Int. J. Mol. Sci. 2020, 21, 3030. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, R.; Lu, G.; Zhou, Y.; Li, J.; Jiang, X.; Gu, S.; Liang, H.; Wang, J. Brain-derived exosomal circrnas in plasma serve as diagnostic biomarkers for acute ischemic stroke. J. Neuroimmune Pharmacol. 2024, 19, 15. [Google Scholar] [CrossRef]
- Lei, X.; Xie, X.N.; Yang, J.X.; Li, Y.M. The emerging role of extracellular vesicles in the diagnosis and treatment of autism spectrum disorders. Psychiatry Res. 2024, 337, 115954. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wan, C.; Wen, Y.; Wu, Z.; Pan, J.; Zhong, M.; Zhong, N. Autism-associated synaptic vesicle transcripts are differentially expressed in maternal plasma exosomes of physiopathologic pregnancies. J. Transl. Med. 2021, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M.L.; Dunn, A.R.; Bradner, J.M.; Egerton, K.S.; Burkett, J.P.; Johnson, M.A.; Miller, G.W. Synaptic vesicle glycoprotein 2C enhances vesicular storage of dopamine and counters dopaminergic toxicity. Eur. J. Neurosci. 2024, 59, 2483–2501. [Google Scholar] [CrossRef] [PubMed]
- Bera, M.; Radhakrishnan, A.; Coleman, J.; RV, K.S.; Ramakrishnan, S.; Pincet, F.; Rothman, J.E. Synaptophysin chaperones the assembly of 12 snarepins under each ready-release vesicle. Proc. Natl. Acad. Sci. USA 2023, 120, e2311484120. [Google Scholar] [CrossRef] [PubMed]
- Seibert, M.J.; Evans, C.S.; Stanley, K.S.; Wu, Z.; Chapman, E.R. Synaptotagmin 9 modulates spontaneous neurotransmitter release in striatal neurons by regulating Substance P secretion. J. Neurosci. 2023, 43, 1475–1491. [Google Scholar] [CrossRef] [PubMed]
- Hansel, C. Deregulation of synaptic plasticity in autism. Neurosci. Lett. 2019, 688, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Baronio, D.; Chen, Y.C.; Decker, A.R.; Enckell, L.; Fernandez-Lopez, B.; Semenova, S.; Puttonen, H.A.J.; Cornell, R.A.; Panula, P. Vesicular monoamine transporter 2 (SLC18A2) regulates monoamine turnover and brain development in zebrafish. Acta Physiol. 2022, 234, e13725. [Google Scholar] [CrossRef]
- Zhang, B.; Angelidou, A.; Alysandratos, K.D.; Vasiadi, M.; Francis, K.; Asadi, S.; Theoharides, A.; Sideri, K.; Lykouras, L.; Kalogeromitros, D.; et al. Mitochondrial DNA and anti-mitochondrial antibodies in serum of autistic children. J. Neuroinflamm. 2010, 7, 80. [Google Scholar] [CrossRef]
- Tsilioni, I.; Theoharides, T.C. Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β. J. Neuroinflamm. 2018, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; McAllister, A.K. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat. Rev. Neurosci. 2015, 16, 469–486. [Google Scholar] [CrossRef] [PubMed]
- González-Madrid, E.; Rangel-Ramírez, M.A.; Opazo, M.C.; Méndez, L.; Bohmwald, K.; Bueno, S.M.; González, P.A.; Kalergis, A.M.; Riedel, C.A. Gestational hypothyroxinemia induces ASD-like phenotypes in behavior, proinflammatory markers, and glutamatergic protein expression in mouse offspring of both sexes. Front. Endocrinol. 2024, 15, 1381180. [Google Scholar] [CrossRef] [PubMed]
- Ferencova, N.; Visnovcova, Z.; Ondrejka, I.; Hrtanek, I.; Bujnakova, I.; Kovacova, V.; Macejova, A.; Tonhajzerova, I. Peripheral inflammatory markers in autism spectrum disorder and attention deficit/hyperactivity disorder at adolescent age. Int. J. Mol. Sci. 2023, 24, 11710. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Ma, X.; Chen, Z.; Zhao, Y.; Yao, Q.; Zhou, C.; Zhao, X.; Meng, X. The mtDNA fragments within exosomes might be novel diagnostic biomarkers of non-small cell lung cancer. Pathol. Res. Pract. 2023, 249, 154718. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Johnson, A. Exosome DNA: Critical regulator of tumor immunity and a diagnostic biomarker. J. Cell Physiol. 2020, 235, 1921–1932. [Google Scholar] [CrossRef]
- Tang, T.Z.; Zhao, Y.; Agarwal, D.; Tharzeen, A.; Patrikeev, I.; Zhang, Y.; DeJesus, J.; Bossmann, S.H.; Natarajan, B.; Motamedi, M.; et al. Serum amyloid a and mitochondrial DNA in extracellular vesicles are novel markers for detecting traumatic brain injury in a mouse model. iScience 2024, 27, 108932. [Google Scholar] [CrossRef] [PubMed]
- Konaka, H.; Kato, Y.; Hirano, T.; Tsujimoto, K.; Park, J.; Koba, T.; Aoki, W.; Matsuzaki, Y.; Taki, M.; Koyama, S.; et al. Secretion of mitochondrial DNA via exosomes promotes inflammation in Behcet’s syndrome. EMBO J. 2023, 42, e112573. [Google Scholar] [CrossRef]
- Banigan, M.G.; Kao, P.F.; Kozubek, J.A.; Winslow, A.R.; Medina, J.; Costa, J.; Schmitt, A.; Schneider, A.; Cabral, H.; Cagsal-Getkin, O.; et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS ONE 2013, 8, e48814. [Google Scholar] [CrossRef]
- Spreafico, M.; Grillo, B.; Rusconi, F.; Battaglioli, E.; Venturin, M. Multiple layers of CDK5R1 regulation in Alzheimer’s Disease implicate long non-coding RNAs. Int. J. Mol. Sci. 2018, 19, 2022. [Google Scholar] [CrossRef]
- Turco, C.; Donzelli, S.; Fontemaggi, G. miR-15/107 microRNA gene group: Characteristics and functional implications in cancer. Front. Cell Dev. Biol. 2020, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, Q.; Zhang, Y.; Guan, X.; Xiu, M.; Zhang, X. Superoxide dismutase, BDNF, and cognitive improvement in drug-naive first-episode patients with schizophrenia: A 12-week longitudinal study. Int. J. Neuropsychopharmacol. 2022, 25, 128–135. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, Y.; Zhao, G.; Zhang, Y.; Sun, Y.; Liao, Y.; Kang, Z.; Feng, X.; Sun, J.; Yue, W. The association of redox regulatory drug target genes with psychiatric disorders: A mendelian randomization study. Antioxidants 2024, 13, 398. [Google Scholar] [CrossRef]
- Zhao, P.; Shi, W.; Ye, Y.; Xu, K.; Hu, J.; Chao, H.; Tao, Z.; Xu, L.; Gu, W.; Zhang, L.; et al. Atox1 protects hippocampal neurons after traumatic brain injury via DJ-1 mediated anti-oxidative stress and mitophagy. Redox Biol. 2024, 72, 103156. [Google Scholar] [CrossRef]
- Nam, Y.; Na, J.; Ma, S.X.; Park, H.; Park, H.; Lee, E.; Kim, H.; Jang, S.M.; Ko, H.S.; Kim, S. DJ-1 protects cell death from a mitochondrial oxidative stress due to GBA1 deficiency. Genes Genom. 2024, 46, 519–529. [Google Scholar] [CrossRef]
- Tsoporis, J.N.; Ektesabi, A.M.; Gupta, S.; Izhar, S.; Salpeas, V.; Rizos, I.K.; Kympouropoulos, S.P.; Dos Santos, C.C.; Parker, T.G.; Rizos, E. A longitudinal study of alterations of circulating DJ-1 and miR-203a-3p in association to olanzapine medication in a sample of first episode patients with schizophrenia. J. Psychiatr. Res. 2022, 146, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Wijtenburg, S.A.; Kapogiannis, D.; Korenic, S.A.; Mullins, R.J.; Tran, J.; Gaston, F.E.; Chen, S.; Mustapic, M.; Hong, L.E.; Rowland, L.M. Brain insulin resistance and altered brain glucose are related to memory impairments in schizophrenia. Schizophr. Res. 2019, 208, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ksiezak-Reding, H.; Riggio, S.; Haroutunian, V.; Pasinetti, G.M. Insulin receptor deficits in schizophrenia and in cellular and animal models of insulin receptor dysfunction. Schizophr. Res. 2006, 84, 1–14. [Google Scholar] [CrossRef]
- Emamian, E.S.; Hall, D.; Birnbaum, M.J.; Karayiorgou, M.; Gogos, J.A. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat. Genet. 2004, 36, 131–137. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Dobrowolny, H.; Tran, J.; Mustapic, M.; Frodl, T.; Meyer-Lotz, G.; Schiltz, K.; Schanze, D.; Rietschel, M.; Bernstein, H.G.; et al. Insulin-signaling abnormalities in drug-naive first-episode schizophrenia: Transction protein analyses in extracellular vesicles of putative neuronal origin. Eur. Psychiatry 2019, 62, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Prigerson, H.G.; Boelen, P.A.; Xu, J.; Smith, K.V.; Maciejewski, P.K. Validation of the new DSM-5-TR criteria for prolonged grief disorder and the PG-13-Revised (PG-13-R) scale. World Psychiatry 2021, 20, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Gu, Y.-f.; Cai, J.-f.; Wang, A.; He, Y.; Feng, Y.-l. miR-186-5p dysregulation leads to depression-like behavior by de-repressing SERPINF1 in hippocampus. Neuroscience 2021, 479, 48–59. [Google Scholar] [CrossRef]
- Deng, Y.; Gong, P.; Han, S.; Zhang, J.; Zhang, S.; Zhang, B.; Lin, Y.; Xu, K.; Wen, G.; Liu, K. Reduced cerebral cortex thickness is related to overexpression of exosomal miR-146a-5p in medication-free patients with major depressive disorder. Psychol. Med. 2022, 53, 6253–6260. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, Y.; Lan, T.; Wang, W.; Long, Y.; Yu, S.Y. Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Mol. Ther. 2022, 30, 1300–1314. [Google Scholar] [CrossRef] [PubMed]
- Iacona, J.R.; Lutz, C.S. miR-146a-5p: Expression, regulation, and functions in cancer. Wiley Interdiscip. Rev. RNA 2019, 10, e1533. [Google Scholar] [CrossRef] [PubMed]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef]
- Li, L.D.; Naveed, M.; Du, Z.W.; Ding, H.; Gu, K.; Wei, L.L.; Zhou, Y.P.; Meng, F.; Wang, C.; Han, F.; et al. Abnormal expression profile of plasma-derived exosomal microRNAs in patients with treatment-resistant depression. Hum. Genom. 2021, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Baniahmad, A.; Taheri, M.; Samadian, M. A review on the role of DANCR in the carcinogenesis. Cancer Cell Int. 2022, 22, 194. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Y.; Chen, W.; Liang, T. Epigenetic deregulation of MLF1 drives intrahepatic cholangiocarcinoma progression through EGFR/AKT and Wnt/β-catenin signaling. Hepatol. Commun. 2023, 7, e0204. [Google Scholar] [CrossRef]
- Liu, M.; Liu, C.; Li, X.; Li, S. RP11-79H23.3 Inhibits the proliferation and metastasis of non-small-cell lung cancer through promoting miR-29c. Biochem. Genet. 2023, 61, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, D.; Tufekci, K.U.; Keskinoglu, P.; Genc, S.; Ozerdem, A. Circulating exosomal microRNAs in bipolar disorder. J. Affect. Disord. 2020, 262, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis-a review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yu, W.; Sun, M.; Li, X.; Zhang, W.; Wang, M. Dabrafenib mitigates the neuroinflammation caused by ferroptosis in experimental autoimmune encephalomyelitis by up regulating Axl receptor. Eur. J. Pharmacol. 2024, 973, 176600. [Google Scholar] [CrossRef]
- Hasaniani, N.; Nouri, S.; Shirzad, M.; Rostami-Mansoor, S. Potential therapeutic and diagnostic approaches of exosomes in multiple sclerosis pathophysiology. Life Sci. 2024, 347, 122668. [Google Scholar] [CrossRef]
- Katz Sand, I. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr. Opin. Neurol. 2015, 28, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Chico-Garcia, J.L.; Sainz-Amo, R.; Monreal, E.; Rodriguez-Jorge, F.; Sainz de la Maza, S.; Masjuan, J.; Villar, L.M.; Costa-Frossard França, L. Passive assessment of tapping speed through smartphone is useful for monitoring multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 86, 105595. [Google Scholar] [CrossRef] [PubMed]
- Lugosi, K.; Engh, M.A.; Huszár, Z.; Hegyi, P.; Mátrai, P.; Csukly, G.; Molnár, Z.; Horváth, K.; Mátis, D.; Mezei, Z. Domain-specific cognitive impairment in multiple sclerosis: A systematic review and meta-analysis. Ann. Clin. Transl. Neurol. 2024, 11, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Androutsou, M.E.; Tapeinou, A.; Vlamis-Gardikas, A.; Tselios, T. Myelin oligodendrocyte glycoprotein and multiple sclerosis. Med. Chem. 2018, 14, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Mehan, S.; Gupta, G.D.; Narula, A.S. Immune System Dysregulation in the Progression of Multiple Sclerosis: Molecular Insights and Therapeutic Implications. Neuroscience 2024, 548, 9–26. [Google Scholar] [CrossRef]
- Galazka, G.; Mycko, M.P.; Selmaj, I.; Raine, C.S.; Selmaj, K.W. Multiple sclerosis: Serum-derived exosomes express myelin proteins. Mult. Scler. 2018, 24, 449–458. [Google Scholar]
- Sarchielli, P.; Di Filippo, M.; Ercolani, M.V.; Chiasserini, D.; Mattioni, A.; Bonucci, M.; Tenaglia, S.; Eusebi, P.; Calabresi, P. Fibroblast growth factor-2 levels are elevated in the cerebrospinal fluid of multiple sclerosis patients. Neurosci. Lett. 2008, 435, 223–228. [Google Scholar] [CrossRef]
- Rajendran, R.; Böttiger, G.; Stadelmann, C.; Karnati, S.; Berghoff, M. FGF/FGFR pathways in multiple sclerosis and in its disease models. Cells 2021, 10, 884. [Google Scholar] [CrossRef]
- Fenoglio, C.; Ridolfi, E.; Galimberti, D.; Scarpini, E. MicroRNAs as active players in the pathogenesis of multiple sclerosis. Int. J. Mol. Sci. 2012, 13, 13227–13239. [Google Scholar] [CrossRef]
- Schelch, K.; Kirschner, M.B.; Williams, M.; Cheng, Y.Y.; van Zandwijk, N.; Grusch, M.; Reid, G. A link between the fibroblast growth factor axis and the miR-16 family reveals potential new treatment combinations in mesothelioma. Mol. Oncol. 2018, 12, 58–73. [Google Scholar] [CrossRef]
- Ebrahimkhani, S.; Vafaee, F.; Young, P.E.; Hur, S.S.J.; Hawke, S.; Devenney, E.; Beadnall, H.; Barnett, M.H.; Suter, C.M.; Buckland, M.E. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci. Rep. 2017, 7, 14293. [Google Scholar] [CrossRef]
- Qin, D.; Wang, C.; Li, D.; Guo, S. Exosomal miR-23a-3p derived from human umbilical cord mesenchymal stem cells promotes remyelination in central nervous system demyelinating diseases by targeting Tbr1/Wnt pathway. J. Biol. Chem. 2024, 300, 105487. [Google Scholar] [CrossRef]
- Junker, A.; Krumbholz, M.; Eisele, S.; Mohan, H.; Augstein, F.; Bittner, R.; Lassmann, H.; Wekerle, H.; Hohlfeld, R.; Meinl, E. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 2009, 132 Pt 12, 3342–3352. [Google Scholar] [CrossRef]
- Astier, A.L.; Kofler, D.M. Editorial: Dysregulation of Th17 and Treg cells in autoimmune diseases. Front. Immunol. 2023, 14, 1151836. [Google Scholar]
- Kimura, K.; Hohjoh, H.; Fukuoka, M.; Sato, W.; Oki, S.; Tomi, C.; Yamaguchi, H.; Kondo, T.; Takahashi, R.; Yamamura, T. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat. Commun. 2018, 9, 17. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Xiao, L.; Wang, Y.; Liu, G.; Li, J.; Liang, F. Identification of differentially expressed mirnas in the response of spleen CD4(+) T Cells to electroacupuncture in senescence-accelerated mice. Cell Biochem. Biophys. 2020, 78, 89–100. [Google Scholar] [CrossRef]
- Selmaj, I.; Cichalewska, M.; Namiecinska, M.; Galazka, G.; Horzelski, W.; Selmaj, K.W.; Mycko, M.P. Global exosome transcriptome profiling reveals biomarkers for multiple sclerosis. Ann. Neurol. 2017, 81, 703–717. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, C.; Martincuks, A.; Herrmann, A.; Yu, H. STAT proteins in cancer: Orchestration of metabolism. Nat. Rev. Cancer 2023, 23, 115–134. [Google Scholar] [CrossRef]
- Aziz, F. The emerging role of miR-223 as novel potential diagnostic and therapeutic target for inflammatory disorders. Cell Immunol. 2016, 303, 1–6. [Google Scholar] [CrossRef]
- Tothova, Z.; Tomc, J.; Debeljak, N.; Solár, P. STAT5 as a key protein of erythropoietin signalization. Int. J. Mol. Sci. 2021, 22, 7109. [Google Scholar] [CrossRef]
- Zhang, M.W.; Shen, Y.J.; Shi, J.; Yu, J.G. MiR-223-3p in cardiovascular diseases: A biomarker and potential therapeutic target. Front. Cardiovasc. Med. 2020, 7, 610561. [Google Scholar] [CrossRef]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Naude, J.; Wang, M.; Leon, R.; Smith, E.; Ismail, Z. Tau-PET in early cortical Alzheimer brain regions in relation to mild behavioral impairment in older adults with either normal cognition or mild cognitive impairment. Neurobiol. Aging 2024, 138, 19–27. [Google Scholar] [CrossRef]
- Haghdel, A.; Smith, N.; Glodzik, L.; Li, Y.; Wang, X.; Crowder, T.; Zhu, Y.S.; Butler, T.; Blennow, K.; McIntire, L.B.; et al. Evidence of pericyte damage in a cognitively normal cohort: Association with CSF and PET biomarkers of Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 2024. [Google Scholar] [CrossRef]
- Koga, S.; Sekiya, H.; Kondru, N.; Ross, O.A.; Dickson, D.W. Neuropathology and molecular diagnosis of synucleinopathies. Mol. Neurodegener. 2021, 16, 83. [Google Scholar] [CrossRef]
- Li, B.; Xiao, X.; Bi, M.; Jiao, Q.; Chen, X.; Yan, C.; Du, X.; Jiang, H. Modulating α-synuclein propagation and decomposition: Implications in Parkinson’s disease therapy. Ageing Res. Rev. 2024, 98, 102319. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.H.; Yang, W.; Chen, L.; Xue, Y.; Chen, X.Y. GLP-1 modulated the firing activity of nigral dopaminergic neurons in both normal and parkinsonian mice. Neuropharmacology 2024, 252, 109946. [Google Scholar] [CrossRef]
- Do, Q.B.; Noor, H.; Marquez-Gomez, R.; Cramb, K.M.L.; Ng, B.; Abbey, A.; Ibarra-Aizpurua, N.; Caiazza, M.C.; Sharifi, P.; Lang, C.; et al. Early deficits in an in vitro striatal microcircuit model carrying the Parkinson’s GBA-N370S mutation. NPJ Park. Dis. 2024, 10, 82. [Google Scholar] [CrossRef]
- Jerusalem, F.; Pohl, C.; Karitzky, J.; Ries, F. ALS. Neurology 1996, 47 (Suppl. 4), S218–S220. [Google Scholar] [CrossRef]
- Gendron, T.F.; Petrucelli, L. Immunological drivers of amyotrophic lateral sclerosis. Sci. Transl. Med. 2023, 15, eadj9332. [Google Scholar] [CrossRef]
- Zhao, A.; Li, Y.; Yan, Y.; Qiu, Y.; Li, B.; Xu, W.; Wang, Y.; Liu, J.; Deng, Y. Increased prediction value of biomarker combinations for the conversion of mild cognitive impairment to Alzheimer’s dementia. Transl. Neurodegener. 2020, 9, 30. [Google Scholar] [CrossRef]
- Rani, K.; Rastogi, S.; Vishwakarma, P.; Bharti, P.S.; Sharma, V.; Renu, K.; Modi, G.P.; Vishnu, V.Y.; Chatterjee, P.; Dey, A.B.; et al. A novel approach to correlate the salivary exosomes and their protein cargo in the progression of cognitive impairment into Alzheimer’s disease. J. Neurosci. Methods 2021, 347, 108980. [Google Scholar] [CrossRef]
- Sun, R.; Wang, H.; Shi, Y.; Sun, Z.; Jiang, H.; Zhang, J. Changes in the morphology, number, and pathological protein levels of plasma exosomes may help diagnose Alzheimer’s disease. J. Alzheimers Dis. 2020, 73, 909–917. [Google Scholar] [CrossRef]
- Jia, L.; Qiu, Q.; Zhang, H.; Chu, L.; Du, Y.; Zhang, J.; Zhou, C.; Liang, F.; Shi, S.; Wang, S.; et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019, 15, 1071–1080. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Wang, J.; Xu, L.; Yu, S.; Fu, J.; Yan, X.; Su, J. Aβ and Tau regulate microglia metabolism via exosomes in Alzheimer’s disease. Biomedicines 2022, 10, 1800. [Google Scholar] [CrossRef]
- Pacheco-Quinto, J.; Clausen, D.; Perez-Gonzalez, R.; Peng, H.; Meszaros, A.; Eckman, C.B.; Levy, E.; Eckman, E.A. Intracellular metalloprotease activity controls intraneuronal Aβ aggregation and limits secretion of Aβ via exosomes. FASEB J. 2019, 33, 3758–3771. [Google Scholar] [CrossRef]
- Jiang, Y.; Wan, M.; Xiao, X.; Lin, Z.; Liu, X.; Zhou, Y.; Liao, X.; Lin, J.; Zhou, H.; Zhou, L.; et al. GSN gene frameshift mutations in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2023, 94, 436–447. [Google Scholar] [CrossRef]
- Mantik, K.E.K.; Kim, S.; Gu, B.; Moon, S.; Kwak, H.B.; Park, D.H.; Kang, J.H. Repositioning of anti-diabetic drugs against dementia: Insight from molecular perspectives to clinical trials. Int. J. Mol. Sci. 2023, 24, 11450. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Liu, L.; Xie, A. The Role of Insulin/IGF-1/PI3K/Akt/GSK3β signaling in Parkinson’s disease dementia. Front. Neurosci. 2018, 12, 73. [Google Scholar] [CrossRef]
- Soares Martins, T.; Marcalo, R.; Ferreira, M.; Vaz, M.; Silva, R.M.; Martins Rosa, I.; Vogelgsang, J.; Wiltfang, J.; Cruz e Silva, O.A.B.; Henriques, A.G. Exosomal Aβ-binding proteins identified by “in silico” analysis represent putative blood-derived biomarker candidates for Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 3933. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Nogueras-Ortiz, C.; Mustapic, M.; Mullins, R.J.; Abner, E.L.; Schwartz, J.B.; Kapogiannis, D. Deficient neurotrophic factors of CSPG4-type neural cell exosomes in Alzheimer disease. FASEB J. 2018, 33, 231–238. [Google Scholar] [CrossRef]
- Barger, S.W.; Mattson, M.P. Isoform-specific modulation by apolipoprotein of the activities of secreted beta-amyloid precursor protein. J. Neurochem. 1997, 69, 60–67. [Google Scholar] [CrossRef]
- Shen, L.; Liao, L.; Chen, C.; Guo, Y.; Song, D.; Wang, Y.; Chen, Y.; Zhang, K.; Ying, M.; Li, S.; et al. Proteomics analysis of blood serums from Alzheimer’s disease patients using iTRAQ labeling technology. J. Alzheimers Dis. 2017, 56, 361–378. [Google Scholar] [CrossRef]
- Ohsawa, I.; Takamura, C.; Kohsaka, S. Fibulin-1 binds the amino-terminal head of beta-amyloid precursor protein and modulates its physiological function. J. Neurochem. 2001, 76, 1411–1420. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Chen, B.; Song, L.; Yang, J.; Zhou, W.-Y.; Cheng, Y.-Y. Proteomics of serum exosomes identified fibulin-1 as a novel biomarker for mild cognitive impairment. Neural Regen. Res. 2023, 18, 587–593. [Google Scholar] [CrossRef]
- Tenner, A.J. Complement-mediated events in Alzheimer’s disease: Mechanisms and potential therapeutic targets. J. Immunol. 2020, 204, 306–315. [Google Scholar] [CrossRef]
- Cai, H.; Pang, Y.; Wang, Q.; Qin, W.; Wei, C.; Li, Y.; Li, T.; Li, F.; Wang, Q.; Li, Y.; et al. Proteomic profiling of circulating plasma exosomes reveals novel biomarkers of Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 181. [Google Scholar] [CrossRef]
- Ryu, I.S.; Kim, D.H.; Ro, J.-Y.; Park, B.-G.; Kim, S.H.; Im, J.-Y.; Lee, J.-Y.; Yoon, S.J.; Kang, H.; Iwatsubo, T.; et al. The microRNA-485-3p concentration in salivary exosome-enriched extracellular vesicles is related to amyloid β deposition in the brain of patients with Alzheimer’s disease. Clin. Biochem. 2023, 118, 110603. [Google Scholar] [CrossRef]
- Koh, H.; Lee, S.; Lee, H.; Min, J.-W.; Iwatsubo, T.; Teunissen, C.; Cho, H.-J.; Ryu, J.-H. Targeting microRNA-485-3p blocks Alzheimer’s disease progression. Int. J. Mol. Sci. 2021, 22, 13136. [Google Scholar] [CrossRef]
- Dong, Z.; Gu, H.; Guo, Q.; Liang, S.; Xue, J.; Yao, F.; Liu, X.; Li, F.; Liu, H.; Sun, L.; et al. Profiling of serum exosome miRNA reveals the potential of a miRNA panel as diagnostic biomarker for Alzheimer’s disease. Mol. Neurobiol. 2021, 58, 3084–3094. [Google Scholar] [CrossRef]
- Gamez-Valero, A.; Campdelacreu, J.; Vilas, D.; Ispierto, L.; Reñé, R.; Álvarez, R.; Armengol, M.P.; Borras, F.E.; Beyer, K. Exploratory study on microRNA profiles from plasma-derived extracellular vesicles in Alzheimer’s disease and dementia with Lewy bodies. Transl. Neurodegener. 2019, 3, 31. [Google Scholar] [CrossRef]
- McKeever, P.M.; Schneider, R.; Taghdiri, F.; Weichert, A.; Multani, N.; Brown, R.A.; Boxer, A.L.; Karydas, A.; Miller, B.; Robertson, J.; et al. MicroRNA expression levels are altered in the cerebrospinal fluid of patients with young-onset Alzheimer’s disease. Mol. Neurobiol. 2018, 55, 8826–8841. [Google Scholar] [CrossRef]
- Li, Y.; Meng, S.; Di, W.; Xia, M.; Dong, L.; Zhao, Y.; Ling, S.; He, J.; Xue, X.; Chen, X.; et al. Amyloid-β protein and microRNA-384 in NCAM-Labeled exosomes from peripheral blood are potential diagnostic markers for Alzheimer’s disease. CNS Neurosci. Ther. 2022, 28, 1093–1107. [Google Scholar] [CrossRef]
- Ho, D.H.; Yi, S.; Seo, H.; Son, I.; Seol, W. Increased DJ-1 in urine exosome of Korean males with Parkinson’s disease. Biomed Res. Int. 2014, 2014, 704678. [Google Scholar] [CrossRef]
- Liu, C.-G.; Zhao, Y.; Lu, Y.; Wang, P.-C.; Cao, D.-Y. ABCA1-labeled exosomes in serum contain higher microRNA-193b levels in Alzheimer’s disease. Biomed Res. Int. 2021, 2021, 5450397. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Marini, F.; Biancolillo, A.; Landi, G.; Beli, R.; Landi, F.; Bernabei, R.; Bentivoglio, A.R.; et al. Mitochondrial signatures in circulating extracellular vesicles of older adults with Parkinson’s disease: Results from the exosomes in Parkinson’s disease study. J. Clin. Med. 2020, 9, 504. [Google Scholar] [CrossRef]
- Wang, D.; Wang, P.; Bian, X.; Xu, S.; Zhou, Q.; Zhang, Y.; Ding, M.; Han, M.; Huang, L.; Bi, J.; et al. Elevated plasma levels of exosomal BACE1-AS combined with the volume and thickness of the right entorhinal cortex may serve as a biomarker for the detection of Alzheimer’s disease. Mol. Med. Rep. 2020, 22, 227–238. [Google Scholar] [CrossRef]
- Cortini, F.; Roma, F.; Villa, C. Emerging roles of long non-coding RNAs in the pathogenesis of Alzheimer’s disease. Ageing Res. Rev. 2019, 50, 19–26. [Google Scholar] [CrossRef]
- Luo, Q.; Chen, Y. Long noncoding RNAs and Alzheimer’s disease. Clin. Interv. Aging 2016, 11, 867–872. [Google Scholar] [CrossRef]
- Wegmann, S.; Biernat, J.; Mandelkow, E. A current view on Tau protein phosphorylation in Alzheimer’s disease. Curr. Opin. Neurobiol. 2021, 69, 131–138. [Google Scholar] [CrossRef]
- Jia, L.; Zhu, M.; Yang, J.; Pang, Y.; Wang, Q.; Li, T.; Li, F.; Wang, Q.; Li, Y.; Wei, Y. Exosomal microRNA-based predictive model for preclinical Alzheimer’s disease: A multicenter study. Biol. Psychiatry 2022, 92, 44–53. [Google Scholar] [CrossRef]
- Volta, M. Roles of neuronal lysosomes in the etiology of Parkinson’s disease. Neural Regen. Res. 2024, 19, 1981–1983. [Google Scholar] [CrossRef]
- Leyns, C.E.G.; Prigent, A.; Beezhold, B.; Yao, L.; Hatcher, N.G.; Tao, P.; Kang, J.; Suh, E.; Van Deerlin, V.M.; Trojanowski, J.Q.; et al. Glucocerebrosidase activity and lipid levels are related to protein pathologies in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 74. [Google Scholar] [CrossRef]
- Yao, Y.F.; Qu, M.W.; Li, G.C.; Zhang, F.B.; Rui, H.C. Circulating exosomal miRNAs as diagnostic biomarkers in Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5278–5283. [Google Scholar]
- Citterio, L.A.; Mancuso, R.; Agostini, S.; Meloni, M.; Clerici, M. Serum and Exosomal miR-7-1-5p and miR-223-3p as possible biomarkers for Parkinson’s disease. Biomolecules 2023, 13, 865. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, M.; Pan, S.; Zhou, R.; Yao, J.; Fu, R.; Yu, H.; Lu, Z. MicroRNA-23a-3p is upregulated in plasma exosomes of bulbar-onset ALS patients and targets ERBB4. Neuroscience 2023, 524, 65–78. [Google Scholar] [CrossRef]
- Parsi, S.; Smith, P.Y.; Goupil, C.; Dorval, V.; Hebert, S.S. Preclinical evaluation of miR-15/107 family members as multifactorial drug targets for Alzheimer’s disease. Mol. Ther. Nucleic Acids 2015, 4, e256. [Google Scholar] [CrossRef]
- Reddy, P.H.; Mani, G.; Park, B.S.; Jacques, J.; Murdoch, G.; Whetsell, W., Jr.; Kaye, J.; Manczak, M. Differential loss of synaptic proteins in Alzheimer’s disease: Implications for synaptic dysfunction. J. Alzheimers Dis. 2005, 7, 103–117; discussion 173–180. [Google Scholar] [CrossRef]
- He, L.; Chen, Y.; Lin, S.; Shen, R.; Pan, H.; Zhou, Y.; Wang, Y.; Chen, S.; Ding, J. Regulation of Hsa-miR-4639-5p expression and its potential role in the pathogenesis of Parkinson’s disease. Aging Cell 2023, 22, e13840. [Google Scholar] [CrossRef]
- Matsumoto, K.; Funakoshi, H.; Takahashi, H.; Sakai, K. HGF-met pathway in regeneration and drug discovery. Biomedicines 2014, 2, 275–300. [Google Scholar] [CrossRef]
- Baldassarro, V.A.; Perut, F.; Cescatti, M.; Pinto, V.; Fazio, N.; Alastra, G.; Parziale, V.; Bassotti, A.; Fernandez, M.; Giardino, L.; et al. Intra-individual variability in the neuroprotective and promyelinating properties of conditioned culture medium obtained from human adipose mesenchymal stromal cells. Stem Cell Res. Ther. 2023, 14, 128. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tufekci, K.U.; Olcum, M.; Durur, D.Y.; Akarlar, B.A.; Ozlu, N.; Bagriyanik, H.A.; Keskinoglu, P.; Yener, G.; Genc, S. Proteome profiling of neuron-derived exosomes in Alzheimer’s disease reveals hemoglobin as a potential biomarker. Neurosci. Lett. 2021, 755, 135914. [Google Scholar] [CrossRef]
- Chi, H.; Yao, R.; Sun, C.; Leng, B.; Shen, T.; Wang, T.; Zhang, S.; Li, M.; Yang, Y.; Sun, H.; et al. Blood neuroexosomal mitochondrial proteins predict alzheimer disease in diabetes. Diabetes 2022, 71, 1313–1323. [Google Scholar] [CrossRef]
- Jain, G.; Stuendl, A.; Rao, P.; Berulava, T.; Pena Centeno, T.; Kaurani, L.; Burkhardt, S.; Delalle, I.; Kornhuber, J.; Hüll, M.; et al. A combined miRNA–piRNA signature to detect Alzheimer’s disease. Transl. Psychiatry 2019, 9, 250. [Google Scholar] [CrossRef]
- Sun, W.; Samimi, H.; Gamez, M.; Zare, H.; Frost, B. Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat. Neurosci. 2018, 21, 1038–1048. [Google Scholar] [CrossRef]
- Carles, A.; Freyssin, A.; Perin-Dureau, F.; Rubinstenn, G.; Maurice, T. Targeting n-methyl-d-aspartate receptors in neurodegenerative diseases. Int. J. Mol. Sci. 2024, 25, 3733. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Colella, M.; De Carluccio, M.; Lelli, D.; Paffi, A.; Carducci, F.; Apollonio, F.; Palacios, D.; Viscomi, M.T.; Liberti, M.; et al. Astrocyte responses influence local effects of whole brain magnetic stimulation in Parkinsonian rats. Mov. Disord. 2023, 38, 2173–2184. [Google Scholar] [CrossRef] [PubMed]
- Buoso, C.; Seifert, M.; Lang, M.; Griffith, C.M.; Talavera Andújar, B.; Castelo Rueda, M.P.; Fischer, C.; Doerrier, C.; Talasz, H.; Zanon, A.; et al. Dopamine-iron homeostasis interaction rescues mitochondrial fitness in Parkinson’s disease. Neurobiol. Dis. 2024, 196, 106506. [Google Scholar] [CrossRef]
- Dorszewska, J.; Kowalska, M.; Prendecki, M.; Piekut, T.; Kozłowska, J.; Kozubski, W. Oxidative stress factors in Parkinson’s disease. Neural Regen. Res. 2021, 16, 1383–1391. [Google Scholar] [CrossRef]
- Chen, Z.-t.; Pan, C.-z.; Ruan, X.-l.; Lei, L.-p.; Lin, S.-m.; Wang, Y.-z.; Zhao, Z.-H. Evaluation of ferritin and TfR level in plasma neural-derived exosomes as potential markers of Parkinson’s disease. Front. Aging Neurosci. 2023, 15, 1216905. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Biswas, A.; Biswas, S.C. Brain-enriched miR-128: Reduced in exosomes from Parkinson’s patient plasma, improves synaptic integrity, and prevents 6-OHDA mediated neuronal apoptosis. Front. Cell Neurosci. 2023, 16, 1037903. [Google Scholar] [CrossRef]
- Shim, K.H.; Go, H.G.; Bae, H.; Jeong, D.-E.; Kim, D.; Youn, Y.C.; Kim, S.; An, S.S.A.; Kang, M.J. Decreased exosomal acetylcholinesterase activity in the plasma of patients with Parkinson’s disease. Front. Aging Neurosci. 2021, 28, 665400. [Google Scholar] [CrossRef]
- Jeong, S.; Shim, K.H.; Kim, D.; Bae, H.; Jeong, D.E.; Kang, M.J.; An, S.S.A. Assessment of acetylcholinesterase activity in CD9-positive exosomes from patients with Parkinson’s disease. Front. Aging Neurosci. 2024, 16, 1332455. [Google Scholar] [CrossRef] [PubMed]
- Raver-Shapira, N.; Marciano, E.; Meiri, E.; Spector, Y.; Rosenfeld, N.; Moskovits, N.; Bentwich, Z.; Oren, M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell 2007, 26, 731–743. [Google Scholar] [CrossRef]
- Bellver-Sanchis, A.; Avila-Lopez, P.A.; Tic, I.; Valle-Garcia, D.; Ribalta-Vilella, M.; Labrador, L.; Banerjee, D.R.; Guerrero, A.; Casadesus, G.; Poulard, C.; et al. Neuroprotective effects of G9a inhibition through modulation of peroxisome-proliferator activator receptor gamma-dependent pathways by miR-128. Neural Regen. Res. 2024, 19, 2532–2542. [Google Scholar] [CrossRef]
- Zou, J.; Guo, Y.; Wei, L.; Yu, F.; Yu, B.; Xu, A. Long noncoding RNA POU3F3 and α-synuclein in plasma L1CAM exosomes combined with β-glucocerebrosidase activity: Potential predictors of Parkinson’s disease. Neurotherapeutics 2020, 17, 1104–1119. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Hu, J.; Zheng, P.; Lv, Y.; Liu, H.; Zhang, G.; Jiang, H. LncRNA PANTR1 is associated with poor prognostic and suppresses apoptosis in glioma. J. Oncol. 2023, 2023, 8537036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Li, J.; Sun, C. LncRNA NEAT1 promotes MPP+ induced injury of PC12 cells and accelerates the progression of Parkinson’s disease in mice through FUS mediated inhibition of PI3K/AKT/mTOR signalling pathway. Exp. Gerontol. 2024, 191, 112436. [Google Scholar] [CrossRef]
- Wang, Q.; Han, C.L.; Wang, K.L.; Sui, Y.P.; Li, Z.B.; Chen, N.; Fan, S.Y.; Shimabukuro, M.; Wang, F.; Meng, F.G. Integrated analysis of exosomal lncRNA and mRNA expression profiles reveals the involvement of lnc-MKRN2-42:1 in the pathogenesis of Parkinson’s disease. CNS Neurosci. Ther. 2020, 26, 527–537. [Google Scholar] [CrossRef]
- Zhang, B.; Lugli, G.; Cohen, A.M.; Bennett, D.A.; Shah, R.C.; Fields, C.J.; Hernandez, A.G.; Smalheiser, N.R. Plasma exosomal miRNAs in persons with and without Alzheimer disease: Altered expression and prospects for biomarkers. PLoS ONE 2015, 10, e0139233. [Google Scholar]
- He, S.; Huang, L.; Shao, C.; Nie, T.; Xia, L.; Cui, B.; Lu, F.; Zhu, L.; Chen, B.; Yang, Q. Several miRNAs derived from serum extracellular vesicles are potential biomarkers for early diagnosis and progression of Parkinson’s disease. Transl. Neurodegener. 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Wang, J.; Ni, S.; Lu, Z.; Guo, Y.; Yobas, L. High-performance gel-free and label-free size fractionation of extracellular vesicles with two-dimensional electrophoresis in a microfluidic artificial sieve. Anal. Chem. 2024, 96, 3508–3516. [Google Scholar] [CrossRef] [PubMed]
- Shirejini, S.Z.; Inci, F. The Yin and Yang of exosome isolation methods: Conventional practice, microfluidics, and commercial kits. Biotechnol. Adv. 2022, 54, 107814. [Google Scholar] [CrossRef]
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef]
- Mao, Z.; Wu, Y.; Kong, L.; Zhou, L.; Zhang, X.; Geng, A.; Cai, J.; Yang, H.; Peili, H. Changes in cargoes of platelet derived extracellular vesicles heterogeneous subpopulations induced by PM(0.1)—Undisclosed cardiovascular injury communication mechanism. Environ. Pollut. 2024, 348, 123845. [Google Scholar] [CrossRef]
- Taylor, M.L.; Giacalone, A.G.; Amrhein, K.D.; Wilson, R.E., Jr.; Wang, Y.; Huang, X. Nanomaterials for molecular detection and analysis of extracellular vesicles. Nanomaterials 2023, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lei, C.; Fan, W.; Sun, Y.; Liu, C. Ultrasensitive protein and exosome analysis based on a rolling circle amplification assisted-CRISPR/Cas12a strategy. Talanta 2024, 273, 125906. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, Q.; Zhao, Y.; Zhou, H.; Yan, Y.; Kong, R.M.; Tan, Q.; Kong, W.; Qu, F. Dual-aptamer recognition of DNA logic gate sensor-based specific exosomal proteins for ovarian cancer diagnosis. ACS Sens. 2024. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Shen, C.; Li, C.; Xu, X.; Li, Q.; Wu, Z.; Ma, H.; Chen, F.; Mao, H. Rapid automated extracellular vesicle isolation and miRNA preparation on a cost-effective digital microfluidic platform. Anal. Chim. Acta 2024, 1296, 342337. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yu, Y.; Hu, Y.; Li, X.W.; Wei, Z.X.; Pan, R.Y.; Li, X.S.; Zheng, G.-E.; Qin, X.Y.; Liu, Q.-S.; et al. Genome-wide, integrative analysis implicates exosome-derived microRNA dysregulation in schizophrenia. Schizophr. Bull. 2019, 45, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Q.; Liao, H.R.; Xu, C.X.; Li, X.L.; Wei, Z.X.; Xie, G.J.; Cheng, Y. Serum exosome-derived miR-139-5p as a potential biomarker for major depressive disorder. Neuropsychiatr. Dis. Treat. 2020, 16, 2689–2693. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Sun, H.; Li, M.; Zhao, J.; Liu, X.; Yao, R.; Shen, T.; Li, Z.; Zhang, J. Blood neuro exosomal excitatory amino acid transporter-2 is associated with cognitive decline in Parkinson’s disease with RBD. Front. Aging Neurosci. 2022, 14, 952368. [Google Scholar] [CrossRef]
- Squadrito, M.L.; Baer, C.; Burdet, F.; Maderna, C.; Gilfillan, G.D.; Lyle, R.; Ibberson, M.; De Palma, M. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep. 2014, 8, 1432–1446. [Google Scholar] [CrossRef]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. miR-212 and miR-132 are downregulated in neurally derived plasma exosomes of Alzheimer’s patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Xu, Y.; Xu, W.; Zhou, Q.; Chen, Q.; Yang, M.; Feng, F.; Liu, Y.; Zhu, X.; Yu, M.; et al. Serum exosomal miR-223 serves as a potential diagnostic and prognostic biomarker for dementia. Neuroscience 2018, 379, 167–176. [Google Scholar] [CrossRef]
- Tan, Y.J.; Wong BY, X.; Vaidyanathan, R.; Sreejith, S.; Chia, S.Y.; Kandiah, N.; Ng AS, L.; Zeng, L. Altered cerebrospinal fluid exosomal microRNA levels in young-onset Alzheimer’s disease and frontotemporal dementia. J. Alzheimer’s Dis. Rep. 2021, 5, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, L.; Tu, Y.; Wei, J.; Zhang, J.; Jiang, G.; Shi, Q.; Ying, H. Circulating exo-miR-154-5p regulates vascular dementia through endothelial progenitor cell-mediated angiogenesis. Front. Cell Neurosci. 2022, 16, 881175. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Kluge, A.; Bunk, J.; Schaeffer, E.; Drobny, A.; Xiang, W.; Knacke, H.; Bub, S.; Lückstädt, W.; Arnold, P.; Lucius, R.; et al. Detection of neuron-derived pathological α-synuclein in blood. Brain 2022, 145, 3058–3071. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.D.; Xu, J.H.; Yu, T.; Li, J.Y.; Zhao, L.N.; Ouyang, H.; Luo, S.; Lu, X.J.; Huang, C.Z.; Lan, Q.S.; et al. Knockdown of linc-POU3F3 suppresses the proliferation, apoptosis, and migration resistance of colorectal cancer. Oncotarget 2016, 7, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L. Circulating Exosomal miRNA as Diagnostic Biomarkers of Neurodegenerative Diseases. Front. Mol. Neurosci. 2020, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Zhang, P.; Hu, W.; Zhang, K.; Chen, X.; Aasly, J. Diagnostic test to identify Parkinson’s disease from the blood sera of chinese population: A cross-sectional study. Parkinson’s Dis. 2022, 2022, 8683877. [Google Scholar] [CrossRef]
- Barbagallo, C.; Mostile, G.; Baglieri, G.; Giunta, F.; Luca, A.; Raciti, L.; Zappia, M.; Purrello, M.; Ragusa, M.; Nicoletti, A. Specific signatures of serum miRNAs as potential biomarkers to discriminate clinically similar neurodegenerative and vascular-related diseases. Cell. Mol. Neurobiol. 2019, 40, 531–546. [Google Scholar] [CrossRef]
| Symbol (Name) | Also Known as | Functions |
|---|---|---|
| SYT15 (Synaptotagmin 15) | SytXV, CHR10SYT, SYT15B, Sytbeta | Membrane transport proteins of the synaptotagmin family |
| SYT9 (Synaptotagmin 9) | SytIX, syt9a, syt9b, Syt1 | Calcium ion binding activity; phospholipid binding activity; and syntaxin binding activity |
| STX8 (Syntaxin-8) | CARB, Hsap | Neurotransmitter release; neuronal membrane maturation |
| SLC18A2 (Solute carrier protein 18 A2) | PKDYS2, SVMT, VAT2, VMAT2, SVAT | Monoaminergic system |
| SV2C (Synaptic vesicle glycoprotein 2C) | SLC22B3, KIAA1054 | Transmembrane transporter activity; protein binding |
| SYP (Synaptophysin) | MRX96, MRXSYP, XLID96 | Synapsin, a phosphoprotein associated with synaptic vesicles |
| DJ-1 (Parkinsonism-associated deglycase) | DJ-1, DJ1, PARK7, GATD2, HEL-S-67p | Antioxidant protein |
| SERPINF1 (Serpin family F member 1) | EPC-1, OI12, OI6, PEDF, PIG35 | Serine-type endopeptidase inhibitor activity; neurotrophic factors |
| GRM4 (Glutamate metabotropic receptor 4) | GPRC1D, MGLUR4, mGlu4 | Adenylate cyclase inhibiting G protein-coupled glutamate receptor activity; G protein-coupled receptor activity |
| AKT (Serine/threonine kinase) | RAC, PKB, PRKBA | Protein serine/threonine/tyrosine kinase activity |
| GSK-3β (Glycogen synthase kinase-3β) | CiGSK; Gsk3b | p53 binding; protein serine/threonine kinase activity |
| MOG (Myelin oligodendrocyte glycoprotein) | BTN6, BTNL11, MOGIG2, NRCLP7 | CNS-related functions, signaling receptor binding; virus receptor activity |
| FGF-2 (Fibroblast growth factor-2) | BFGF, FGF2, FGFB, HBGF-2 | Fibroblast growth factor receptor binding; cytokine activity; integrin binding |
| IGF1R (Insulin-like growth factor 1 receptor) | CD221, IGFIR, IGFR, JTK13 | G-protein alpha-subunit binding; protein tyrosine kinase activity |
| TGFBR1 (Transforming growth factor β receptor 1) | AAT5, ACVRLK4, ALK-5, ALK5, ESS1, LDS1, LDS1A, LDS2A, MSSE, SKR4, TBR-i, TBRI, TGFR-1, tbetaR-I | Protein serine/threonine kinase activity; protein serine/threonine kinase activity |
| STAT5 (Signal transducer and activator of transcription 5) | MGF; STAT5A, Stat92E | DNA-binding transcription factor activity, RNA polymerase II-specific |
| APP (Amyloid precursor protein) | AAA, ABETA, ABPP, AD1, APPI, CVAP, PN-II, PN2, alpha-sAPP, preA4 | RNA polymerase II cis-regulatory region sequence-specific DNA binding; serine-type endopeptidase inhibitor activity |
| ADAM10 (A disintegrin and metalloproteinase) | ADA10, MADM, HsT18717, CD156C, | Endopeptidase activity; metalloendopeptidase activity; signaling receptor binding |
| IGF-1 (Insulin-like growth factor 1) | IGF, IGF-I, IGFI, MGF, IBP1 | Insulin-like growth factor receptor binding; hormone activity |
| GSN (Gelsolin) | ADF, AGEL | Phosphatidylinositol 3-kinase catalytic subunit binding; calcium ion binding |
| FBLN1 (Fibulin-1) | FBLN, FIBL1 | Extracellular matrix structural constituent; fibronectin binding; calcium ion binding |
| CO9 (Complement C9) | C9, ARMD15, C9D | Protein binding |
| BACE1 (β-secretase 1) | ASP2, BACE, HSPC104 | Amyloid-beta binding; endopeptidase activity; aspartic-type endopeptidase activity |
| LRRK2 (Leucine-rich repeat kinase 2) | AURA17, DARDARIN, PARK8, RIPK7, ROCO2 | Magnesium ion binding; actin binding |
| α-synuclein | SNCA, NACP, PARK1, PARK4, PD1, | Magnesium ion binding; transcription cis-regulatory region binding; protein kinase inhibitor activity |
| SYT1 (Synaptotagmin 1) | BAGOS, P65, SVP65, SYT, Syt1 | Phosphatidylserine binding; calcium ion binding; calmodulin binding |
| SNAP25 (Synaptosome-associated protein 25) | CMS18, RIC-4, RIC4, SEC9, SNAP, SNAP-25, SUP, bA416n4.2, dJ1068f16.2 | Voltage-gated potassium channel activity; lipid binding |
| GAP43 (Growth-associated protein 43) | B-50, GAP-43, PP46 | Phosphatidylserine binding; calmodulin binding; lysophosphatidic acid binding |
| HGF (Hepatocyte growth factor) | DFNB39, F-TCF, HGFB, HPTA, SF, Tequila | Endopeptidase activity; serine-type endopeptidase activity; signaling receptor binding |
| FGF-13 (Fibroblast growth factor 13) | FGF2; FHF2; DEE90; FHF-2; FGF-13; XLID110; LINC00889 | Microtubule binding; growth factor activity; sodium channel regulator activity; transmembrane transporter binding |
| RSU1 (Ras suppressor protein 1) | RSP-1, FLJ31034, Rsu-1 | Protein binding |
| NDUFS3 (Nicotinamide adenine dinucleotide (NADH) ubiquinone oxidoreductase core subunit S3) | CI-30, MC1DN8 | NADH dehydrogenase activity; oxidoreductase activity |
| SDHB (Succinate dehydrogenase complex subunit B) | CWS2, IP, MC2DN4, PGL4, PPGL4, SDH, SDH1, SDH2, SDHIP | Succinate dehydrogenase (quinone) activity; electron transfer activity; oxidoreductase activity |
| GP1BB (Glycoprotein Ib Platelet subunit β) | BDPLT1, BS, GPIBB, CD42C, GPI-b Beta | Transmembrane signaling receptor activity; identical protein binding |
| TFR (Total ferritin receptor) | T9; TR; TFRC; p90; CD71; TFR1; TRFR; IMD46 | Virus receptor activity; double-stranded RNA binding; transferrin receptor activity |
| ATP5A (ATP Synthase F1 Subunit Alpha) | OMR; ORM; ATPM; MOM2; ATP5A1; hATP1; ATP5A1; MC5DN4; ATP5AL2; COXPD22; MC5DN4A; MC5DN4B; HEL-S-123m | Protease binding; protein binding; ATP binding |
| VGLUT-1 (Vesicular glutamate transporter-1) | SLC17A7, BNPI | Chloride channel activity; inorganic phosphate transmembrane transporter activity; L-glutamate transmembrane transporter activity; neurotransmitter transmembrane transporter activity; sodium:phosphate symporter activity |
| EAAT-2 (Excitatory amino acid transporter-2) | SLC1A2, GLT1; HBGT; DEE41; EAAT2; GLT-1; EIEE41 | L-glutamate transmembrane transporter activity; monoatomic anion transmembrane transporter activity; neutral L-amino acid transmembrane transporter activity |
| AChE (Acetylcholinesterase) | ACEE, ARACHE, N-ACHE, YT | Amyloid-beta binding; acetylcholinesterase activity; cholinesterase activity; collagen binding |
| SOD1 (Superoxide dismutase 1) | ALS, ALS1, HEL-S-44, IPOA, SOD, STAHP, hSod1, homodimer | Superoxide dismutase activity; copper ion binding; zinc ion binding; antioxidant activity |
| POU3F3 (POU Class 3 Homeobox 3) | BRN1, OTF8, SNIBFIS, brain-1, oct-8 | RNA polymerase II cis-regulatory region sequence-specific DNA binding; DNA-binding transcription factor activity |
| MKRN2 (Makorin ring finger protein 2) | HSPC070, RNF62 | Metal ion binding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Shang, X.; Guo, M.; Su, L.; Wang, J. Exosomes in the Diagnosis of Neuropsychiatric Diseases: A Review. Biology 2024, 13, 387. https://doi.org/10.3390/biology13060387
Wu S, Shang X, Guo M, Su L, Wang J. Exosomes in the Diagnosis of Neuropsychiatric Diseases: A Review. Biology. 2024; 13(6):387. https://doi.org/10.3390/biology13060387
Chicago/Turabian StyleWu, Song, Xinmiao Shang, Meng Guo, Lei Su, and Jun Wang. 2024. "Exosomes in the Diagnosis of Neuropsychiatric Diseases: A Review" Biology 13, no. 6: 387. https://doi.org/10.3390/biology13060387
APA StyleWu, S., Shang, X., Guo, M., Su, L., & Wang, J. (2024). Exosomes in the Diagnosis of Neuropsychiatric Diseases: A Review. Biology, 13(6), 387. https://doi.org/10.3390/biology13060387







