Disrupted Endoplasmic Reticulum Ca2+ Handling: A Harβinger of β-Cell Failure
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Ca2+ER in β-Cell Survival and Health
1.2. Ca2+ER Control of Insulin Secretion
2. Ca2+ER Handling Proteins and Their β-Cell Function(s)
2.1. Sarco/Endoplasmic Reticulum Calcium ATPase (SERCA)
2.2. Ryanodine Receptor (RyR)
2.3. Inositol Trisphosphate Receptor (IP3R)
2.4. Ca2+ER Leak Channels
2.5. Two-Pore Domain K+ Channel (K2P)
2.6. Store-Operated Ca2+ Entry (SOCE)
3. Mutations That Affect Ca2+ER Function and Ca2+ Handling
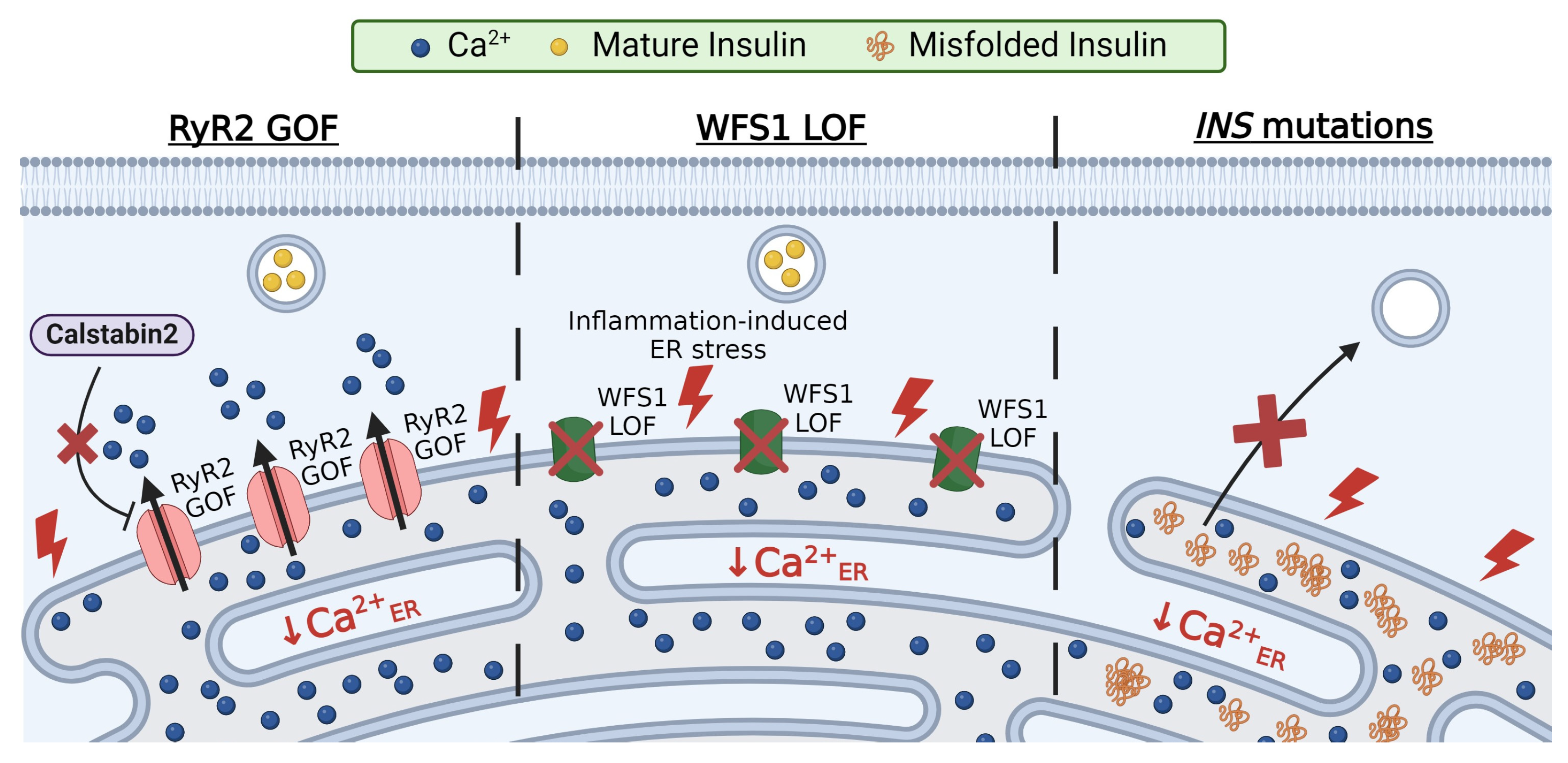
3.1. RyR2 Mutations
3.2. Wolfram Syndrome 1 (WFS1) Mutations
3.3. Insulin Mutations
4. Ca2+ER in the Context of Cellular Function
4.1. Chaperone-Mediated Ca2+ Binding
4.2. Protein Folding
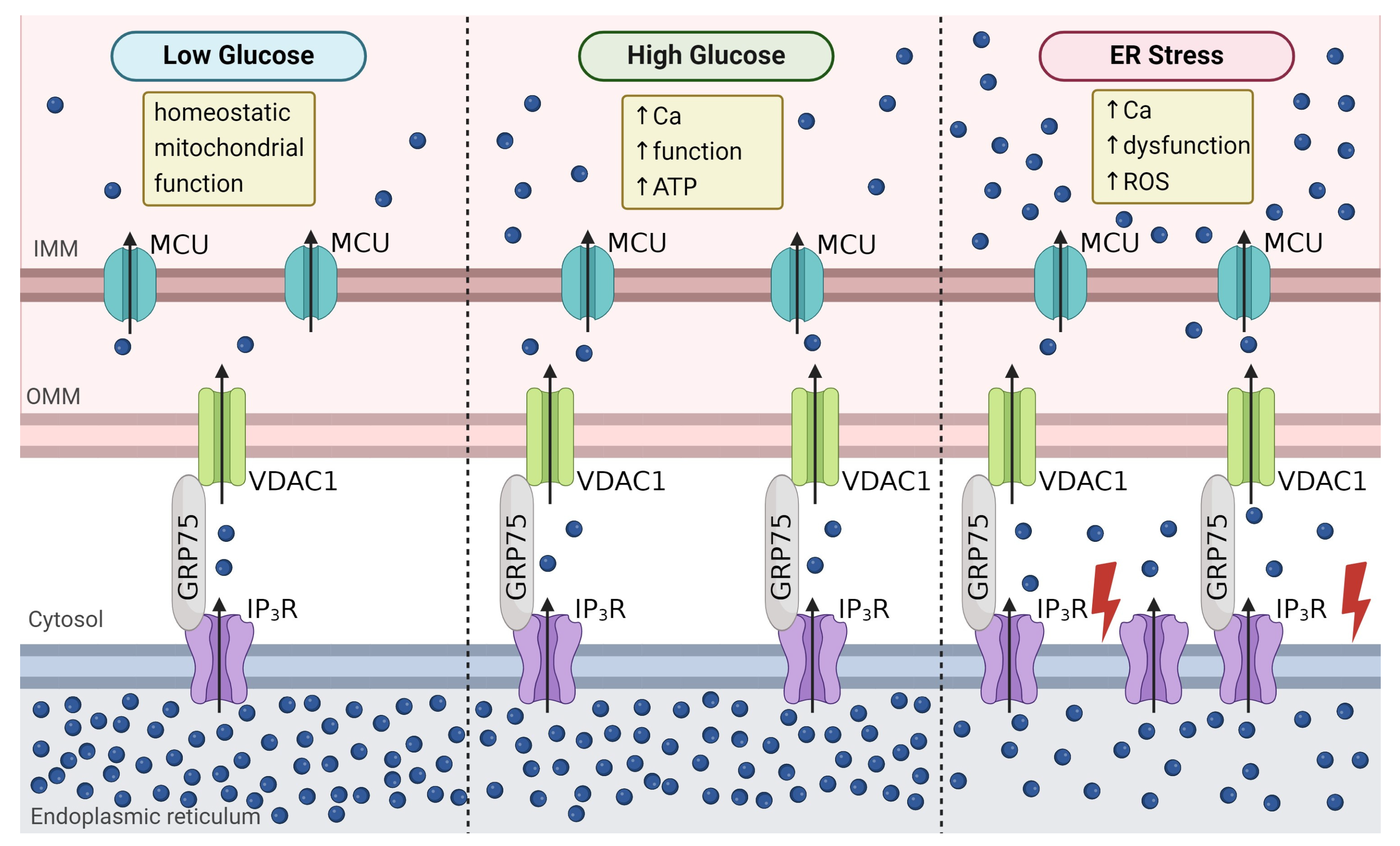
4.3. Mitochondrial Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rutter, G.A.; Hodson, D.J.; Chabosseau, P.; Haythorne, E.; Pullen, T.J.; Leclerc, I. Local and regional control of calcium dynamics in the pancreatic islet. Diabetes Obes. Metab. 2017, 19, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.; Gromada, J.; Urano, F. Endoplasmic Reticulum Stress and Pancreatic Beta-Cell Death. Trends Endocrinol. Metab. 2011, 22, 266–274. [Google Scholar]
- Gilon, P.; Chae, H.Y.; Rutter, G.A.; Ravier, M.A. Calcium Signaling in Pancreatic Beta-Cells in Health and in Type 2 Diabetes. Cell Calcium 2014, 56, 340–361. [Google Scholar] [CrossRef]
- Shrestha, N.; De Franco, E.; Arvan, P.; Cnop, M. Pathological Beta-Cell Endoplasmic Reticulum Stress in Type 2 Diabetes: Current Evidence. Front. Endocrinol. 2021, 12, 650158. [Google Scholar] [CrossRef]
- Scheuner, D.; Kaufman, R.J. The Unfolded Protein Response: A Pathway That Links Insulin Demand with Beta-Cell Failure and Diabetes. Endocr. Rev. 2008, 29, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Aguayo-Mazzucato, C.; Thompson, P.J. Pancreatic Beta-Cell Senescence in Diabetes: Mechanisms, Markers and Therapies. Front. Endocrinol. 2023, 14, 1212716. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Haataja, L.; Wright, J.; Wickramasinghe, N.P.; Hua, Q.-X.; Phillips, N.F.; Barbetti, F.; Weiss, M.A.; Arvan, P. Mutant Ins-Gene Induced Diabetes of Youth: Proinsulin Cysteine Residues Impose Dominant-Negative Inhibition on Wild-Type Proinsulin Transport. PLoS ONE 2010, 5, e13333. [Google Scholar] [CrossRef]
- Hodish, I.; Liu, M.; Rajpal, G.; Larkin, D.; Holz, R.W.; Adams, A.; Liu, L. Arvan Misfolded Proinsulin Affects Bystander Proinsulin in Neonatal Diabetes. J. Biol. Chem. 2010, 285, 685–694. [Google Scholar] [CrossRef]
- Kang, S.; Dahl, R.; Hsieh, W.; Shin, A.; Zsebo, K.M.; Buettner, C.; Hajjar, R.J.; Lebeche, D. Small Molecular Allosteric Activator of the Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) Attenuates Diabetes and Metabolic Disorders. J. Biol. Chem. 2016, 291, 5185–5198. [Google Scholar] [CrossRef]
- Tong, X.T.; Kono, E.K.; Anderson-Baucum, W.; Yamamoto, P.; Gilon, D.; Lebeche, R.N.; Day, R.; Shull, G.E.; Evans-Molina, C. Serca2 Deficiency Impairs Pancreatic Beta-Cell Function in Response to Diet-Induced Obesity. Diabetes 2016, 65, 3039–3052. [Google Scholar] [CrossRef]
- Cardozo, A.K.F.; Ortis, J.; Storling, Y.M.; Feng, J.; Rasschaert, M.; Van Eylen, F.T.; Mandrup-Poulsen, T.; Herchuelz, A.; Eizirik, D.L. Cytokines Downregulate the Sarcoendoplasmic Reticulum Pump Ca2+ Atpase 2b and Deplete Endoplasmic Reticulum Ca2+, Leading to Induction of Endoplasmic Reticulum Stress in Pancreatic Beta-Cells. Diabetes 2005, 54, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Ly, L.D.; Xu, S.; Choi, S.-K.; Ha, C.-M.; Thoudam, T.; Cha, S.-K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.-K.; Park, K.-S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef]
- Zhang, I.X.; Raghavan, M.; Satin, L.S. The Endoplasmic Reticulum and Calcium Homeostasis in Pancreatic Beta Cells. Endocrinology 2020, 161, bqz028. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Gutierrez, G.D.; Okamoto, H.; Kim, J.; Lee, A.H.; Adler, C.; Ni, M.; Yancopoulos, G.D.; Murphy, A.J.; Gromada, J. Pseudotime Ordering of Single Human Beta-Cells Reveals States of Insulin Production and Unfolded Protein Response. Diabetes 2018, 67, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Gilon, P.; Arredouani, A.; Gailly, P.; Gromada, J.; Henquin, J.-C. Uptake and Release of Ca2+ by the Endoplasmic Reticulum Contribute to the Oscillations of the Cytosolic Ca2+ Concentration Triggered by Ca2+ Influx in the Electrically Excitable Pancreatic B-cell. J. Biol. Chem. 1999, 274, 20197–20205. [Google Scholar] [CrossRef] [PubMed]
- Llanos, P.; Contreras-Ferrat, A.; Barrientos, G.; Valencia, M.; Mears, D.; Hidalgo, C. Glucose-Dependent Insulin Secretion in Pancreatic Beta-Cell Islets from Male Rats Requires Ca2+ Release Via Ros-Stimulated Ryanodine Receptors. PLoS ONE 2015, 10, e0129238. [Google Scholar]
- Usui, R.; Yabe, D.; Fauzi, M.; Goto, H.; Botagarova, A.; Tokumoto, S.; Tatsuoka, H.; Tahara, Y.; Kobayashi, S.; Manabe, T.; et al. Gpr40 Activation Initiates Store-Operated Ca2+ Entry and Potentiates Insulin Secretion Via the Ip3r1/Stim1/Orai1 Pathway in Pancreatic Beta-Cells. Sci. Rep. 2019, 9, 15562. [Google Scholar] [CrossRef] [PubMed]
- Arredouani, A.; Guiot, Y.; Jonas, J.C.; Liu, L.H.; Nenquin, M.; Pertusa, J.A.; Rahier, J.; Rolland, J.F.; Shull, G.E.; Stevens, M.; et al. Serca3 Ablation Does Not Impair Insulin Secretion but Suggests Distinct Roles of Different Sarcoendoplasmic Reticulum Ca2+ Pumps for Ca2+ Homeostasis in Pancreatic Beta-Cells. Diabetes 2002, 51, 3245–3253. [Google Scholar] [CrossRef] [PubMed]
- Roe, M.W.; Philipson, L.H.; Frangakis, C.J.; Kuznetsov, A.; Mertz, R.J.; Lancaster, M.E.; Spencer, B.; Worley, J.F., 3rd; Dukes, I.D. Defective Glucose-Dependent Endoplasmic Reticulum Ca2+ Sequestration in Diabetic Mouse Islets of Langerhans. J. Biol. Chem. 1994, 269, 18279–18282. [Google Scholar] [CrossRef]
- Roe, M.W.; Mertz, R.J.; Lancaster, M.E., 3rd; Worley, J.F.; Dukes, I.D. Thapsigargin Inhibits the Glucose-Induced Decrease of Intracellular Ca2+ in Mouse Islets of Langerhans. Am. J. Physiol. Endocrinol. Metab. 1994, 266, E852–E862. [Google Scholar] [CrossRef]
- Váradi, A.; Molnár, E.; Östenson, C.-G.; Ashcroft, S.J.H. Isoforms of endoplasmic reticulum Ca2+-ATPase are differentially expressed in normal and diabetic islets of Langerhans. Biochem. J. 1996, 319, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Ahn, G.; Moss, D.R.; Gann, L.; Zarain-Herzberg, A.; Nishiki, Y.; Fueger, P.T.; Ogihara, T.; Evans-Molina, C. Ppar-Gamma Activation Restores Pancreatic Islet Serca2 Levels and Prevents Beta-Cell Dysfunction under Conditions of Hyperglycemic and Cytokine Stress. Mol. Endocrinol. 2012, 26, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Zarain-Herzberg, A.; García-Rivas, G.; Estrada-Avilés, R. Regulation of SERCA pumps expression in diabetes. Cell Calcium 2014, 56, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Beauvois, M.C.; Merezak, C.; Jonas, J.C.; Ravier, M.A.; Henquin, J.C.; Gilon, P. Glucose-Induced Mixed [Ca2+]C Oscillations in Mouse Beta-Cells Are Controlled by the Membrane Potential and the Serca3 Ca2+-Atpase of the Endoplasmic Reticulum. Am. J. Physiol. Cell Physiol. 2006, 290, C1503–C1511. [Google Scholar] [CrossRef]
- Varadi, A.; Rutter, G.A. Dynamic Imaging of Endoplasmic Reticulum Ca2+ Concentration in Insulin-Secreting Min6 Cells Using Recombinant Targeted Cameleons: Roles of Sarco(Endo)Plasmic Reticulum Ca2+-Atpase (Serca)-2 and Ryanodine Receptors. Diabetes 2002, 51, S190–S201. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.Y.; Borge, P.D., Jr.; Jegier, P.A.; Young, R.A.; Wolf, B.A. Insulin Regulation of Beta-Cell Function Involves a Feedback Loop on Serca Gene Expression, Ca2+ Homeostasis, and Insulin Expression and Secretion. Biochemistry 2000, 39, 14912–14919. [Google Scholar]
- Gao, Z.Y.; Borge, P.D., Jr.; Wolf, B.A. Insulin Receptor Substrate 1-Induced Inhibition of Endoplasmic Reticulum Ca2+ Uptake in Beta-Cells. Autocrine Regulation of Intracellular Ca2+ Homeostasis and Insulin Secretion. J. Biol. Chem. 1999, 274, 18067–18074. [Google Scholar]
- Borge, P.D.; Wolf, B.A., Jr. Insulin Receptor Substrate 1 Regulation of Sarco-Endoplasmic Reticulum Calcium Atpase 3 in Insulin-Secreting Beta-Cells. J. Biol. Chem. 2003, 278, 11359–11368. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.N.; Roper, M.G.; Dahlgren, G.; Shih, D.Q.; Kauri, L.M.; Peters, J.L.; Stoffel, M.; Kennedy, R.T. Islet Secretory Defect in Insulin Receptor Substrate 1 Null Mice Is Linked With Reduced Calcium Signaling and Expression of Sarco(endo)plasmic Reticulum Ca2+-ATPase (SERCA)-2b and -3. Diabetes 2004, 53, 1517–1525. [Google Scholar] [CrossRef]
- Takatani, T.; Shirakawa, J.; Roe, M.W.; Leech, C.A.; Maier, B.F.; Mirmira, R.G.; Kulkarni, R.N. Irs1 Deficiency Protects Beta-Cells against Er Stress-Induced Apoptosis by Modulating Sxbp-1 Stability and Protein Translation. Sci. Rep. 2016, 6, 28177. [Google Scholar] [CrossRef]
- Borge, P.D.; Moibi, J.; Greene, S.R.; Trucco, M.; Young, R.A.; Gao, Z.; Wolf, B.A. Insulin Receptor Signaling and Sarco/Endoplasmic Reticulum Calcium Atpase in Beta-Cells. Diabetes 2002, 51 (Suppl. 3), S427–S433. [Google Scholar] [CrossRef] [PubMed]
- Withers, D.J.; Gutierrez, J.S.; Towery, H.; Burks, D.J.; Ren, J.-M.; Previs, S.; Zhang, Y.; Bernal, D.; Pons, S.; Shulman, G.I.; et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 1998, 391, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Tamemoto, H.; Kadowaki, T.; Tobe, K.; Yagi, T.; Sakura, H.; Hayakawa, T.; Terauchi, Y.; Ueki, K.; Kaburagi, Y.; Satoh, S.; et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 1994, 372, 182–186. [Google Scholar] [CrossRef]
- Lockridge, A.; Jo, S.; Gustafson, E.; Damberg, N.; Mohan, R.; Olson, M.; Abrahante, J.E.; Alejandro, E.U. Islet O-Glcnacylation Is Required for Lipid Potentiation of Insulin Secretion through Serca2. Cell Rep. 2020, 31, 107609. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, E.U.; Bozadjieva, N.; Kumusoglu, D.; Abdulhamid, S.; Levine, H.; Haataja, L.; Vadrevu, S.; Satin, L.S.; Arvan, P.; Bernal-Mizrachi, E. Disruption of O-Linked N-Acetylglucosamine Signaling Induces Er Stress and Beta Cell Failure. Cell Rep. 2015, 13, 2527–2538. [Google Scholar] [CrossRef]
- Sánchez-Gómez, F.J.; Espinosa-Díez, C.; Dubey, M.; Dikshit, M.; Lamas, S. S-glutathionylation: Relevance in diabetes and potential role as a biomarker. Biol. Chem. 2013, 394, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Weisbrod, R.M.; Pimentel, D.R.; Ying, J.; Sharov, V.S.; Schöneich, C.; Cohen, R.A. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004, 10, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Fu, A.; Robson-Doucette, C.; Allister, E.M.; Wheeler, M.B.; Screaton, R.; Harper, M.-E. Glutathionylation State of Uncoupling Protein-2 and the Control of Glucose-stimulated Insulin Secretion. J. Biol. Chem. 2012, 287, 39673–39685. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.; Grimm, A.; Lutz, S.; Wagner-Teschner, D.; Handel, M.; Hagenloh, I. Islet Glutathione and Insulin Release. Diabetes 1980, 29, 830–834. [Google Scholar] [CrossRef]
- Rebelato, E.; Abdulkader, F.; Curi, R.; Carpinelli, A.R. Control of the Intracellular Redox State by Glucose Participates in the Insulin Secretion Mechanism. PLoS ONE 2011, 6, e24507. [Google Scholar] [CrossRef]
- Liang, K.; Du, W.; Zhu, W.; Liu, S.; Cui, Y.; Sun, H.; Luo, B.; Xue, Y.; Yang, L.; Chen, L.; et al. Contribution of Different Mechanisms to Pancreatic Beta-cell Hyper-secretion in Non-obese Diabetic (NOD) Mice during Pre-diabetes. J. Biol. Chem. 2011, 286, 39537–39545. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, M.T.; Bogart, A.M.; Altman, M.K.; Milian, S.C.; Jordan, K.L.; Dadi, P.K.; Jacobson, D.A. Cytokine-Mediated Changes in K+ Channel Activity Promotes an Adaptive Ca2+ Response That Sustains Beta-Cell Insulin Secretion During Inflammation. Sci. Rep. 2018, 8, 1158. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Kono, T.; Lee, C.-C.; Krishnan, P.; Arvin, M.C.; Weaver, S.A.; Jarvela, T.S.; Branco, R.C.S.; McLaughlin, M.R.; Bone, R.N.; et al. SERCA2 regulates proinsulin processing and processing enzyme maturation in pancreatic beta cells. Diabetologia 2023, 66, 2042–2061. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kagami, K.; Sato, A.; Osaki, A.; Ito, K.; Horii, S.; Toya, T.; Masaki, N.; Yasuda, R.; Nagatomo, Y.; et al. Sarco/Endoplasmic Reticulum Ca2+ ATPase 2 Activator Ameliorates Endothelial Dysfunction; Insulin Resistance in Diabetic Mice. Cells 2022, 11, 1488. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Polo, C.N.; Wiederkehr, A.; Wollheim, C.B.; Park, K.S. Cdn1163, an Activator of Sarco/Endoplasmic Reticulum Ca2+ Atpase, up-Regulates Mitochondrial Functions and Protects against Lipotoxicity in Pancreatic Beta-Cells. Br. J. Pharmacol. 2023, 180, 2762–2776. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Van Remmen, H. The Sarcoendoplasmic Reticulum Calcium Atpase (Serca) Pump: A Potential Target for Intervention in Aging and Skeletal Muscle Pathologies. Skelet. Muscle 2021, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Gwiazda, K.S.; Yang, T.L.; Lin, Y.; Johnson, J.D. Effects of Palmitate on Er and Cytosolic Ca2+ Homeostasis in Beta-Cells. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E690–E701. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.A.; Hekerman, P.; Ladriere, L.; Bazarra-Castro, A.; Ortis, F.; Wakeham, M.C.; Moore, F.; Rasschaert, J.; Cardozo, A.K.; Bellomo, E.; et al. Initiation and Execution of Lipotoxic Er Stress in Pancreatic Beta-Cells. J. Cell Sci. 2008, 121, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, Y.; Li, B.; Xu, H.; Wang, C.; Liu, Y.; Li, Y.; Li, C.; Gao, N.; Li, L. Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca2+ homeostasis in human adenoid cystic carcinoma cells. Cell Biosci. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W.; Lin, X.; Watkins, S.M.; Ivanov, A.R.; Hotamisligil, G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011, 473, 528–531. [Google Scholar] [CrossRef]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine Receptors: Structure, Expression, Molecular Details, and Function in Calcium Release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef] [PubMed]
- Meissner, G.; Rios, E.; Tripathy, A.; Pasek, D.A. Regulation of Skeletal Muscle Ca2+ Release Channel (Ryanodine Receptor) by Ca2+ and Monovalent Cations and Anions. J. Biol. Chem. 1997, 272, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. The Ryanodine Receptor Calcium Channel of Beta-Cells: Molecular Regulation and Physiological Significance. Diabetes 2002, 51, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C. Cyclic ADP-ribose and Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) as Messengers for Calcium Mobilization. J. Biol. Chem. 2012, 287, 31633–31640. [Google Scholar] [CrossRef] [PubMed]
- Mu-U-Min, R.B.A.; Diane, A.; Allouch, A.; Al-Siddiqi, H.H. Ca2+-Mediated Signaling Pathways: A Promising Target for the Successful Generation of Mature and Functional Stem Cell-Derived Pancreatic Beta Cells In Vitro. Biomedicines 2023, 11, 1577. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, W.R.; Bone, R.N.; Sohn, P.; Syed, F.; Reissaus, C.A.; Mosley, A.L.; Wijeratne, A.B.; True, J.D.; Tong, X.; Kono, T.; et al. Endoplasmic Reticulum Stress Alters Ryanodine Receptor Function in the Murine Pancreatic Beta Cell. J. Biol. Chem. 2019, 294, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Pagano, G.; Sardu, C.; Xie, W.; Reiken, S.; D’Ascia, S.L.; Cannone, M.; Marziliano, N.; Trimarco, B.; Guise, T.A.; et al. Calcium Release Channel Ryr2 Regulates Insulin Release and Glucose Homeostasis. J. Clin. Investig. 2015, 125, 4316. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, S.; Akiyama, T.; Nata, K.; Kuroki, M.; Tohgo, A.; Noguchi, N.; Kobayashi, S.; Kato, I.; Katada, T.; Okamoto, H. Cyclic Adp-Ribose and Inositol 1,4,5-Trisphosphate as Alternate Second Messengers for Intracellular Ca2+ Mobilization in Normal and Diabetic Beta-Cells. J. Biol. Chem. 1998, 273, 2497–2500. [Google Scholar] [CrossRef] [PubMed]
- Postic, S.; Sarikas, S.; Pfabe, J.; Pohorec, V.; Bombek, L.K.; Sluga, N.; Klemen, M.S.; Dolensek, J.; Korosak, D.; Stozer, A.; et al. High-Resolution Analysis of the Cytosolic Ca2+ Events in Beta Cell Collectives in Situ. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E42–E55. [Google Scholar] [CrossRef]
- Dror, V.; Kalynyak, T.B.; Bychkivska, Y.; Frey, M.H.; Tee, M.; Jeffrey, K.D.; Nguyen, V.; Luciani, D.S.; Johnson, J.D. Glucose and Endoplasmic Reticulum Calcium Channels Regulate Hif-1beta Via Presenilin in Pancreatic Beta-Cells. J. Biol. Chem. 2018, 283, 9909–9916. [Google Scholar] [CrossRef]
- Zhang, I.X.; Herrmann, A.; Leon, J.; Jeyarajan, S.; Arunagiri, A.; Arvan, P.; Gilon, P.; Satin, L.S. ER stress increases expression of intracellular calcium channel RyR1 to modify Ca2+ homeostasis in pancreatic beta cells. J. Biol. Chem. 2023, 299, 105065. [Google Scholar] [CrossRef] [PubMed]
- Marmugi, A.; Parnis, J.; Chen, X.; Carmichael, L.; Hardy, J.; Mannan, N.; Marchetti, P.; Piemonti, L.; Bosco, D.; Johnson, P.; et al. Sorcin Links Pancreatic Beta-Cell Lipotoxicity to Er Ca2+ Stores. Diabetes 2016, 65, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Foskett, J.K.; Mak, D.O.D. Regulation of Ip(3)R Channel Gating by Ca2+ and Ca2+ Binding Proteins. Curr. Top. Membr. 2010, 66, 235–272. [Google Scholar] [PubMed]
- Stutzmann, G.E.; Mattson, M.P. Endoplasmic Reticulum Ca2+Handling in Excitable Cells in Health and Disease. Pharmacol. Rev. 2011, 63, 700–727. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.; Han, S.J.; Hamdan, F.F.; Jeon, J.; Li, B.; Li, J.H.; Cui, Y.; Mears, D.; Lu, H.; Deng, C.; et al. A Critical Role for Beta Cell M3 Muscarinic Acetylcholine Receptors in Regulating Insulin Release and Blood Glucose Homeostasis in Vivo. Cell Metab. 2006, 3, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Dyachok, O.; Gylfe, E. Ca2+-Induced Ca2+ Release Via Inositol 1,4,5-Trisphosphate Receptors Is Amplified by Protein Kinase a and Triggers Exocytosis in Pancreatic Beta-Cells. J. Biol. Chem. 2004, 279, 45455–45461. [Google Scholar] [CrossRef]
- Thore, S.; Dyachok, O.; Tengholm, A. Oscillations of Phospholipase C Activity Triggered by Depolarization and Ca2+ Influx in Insulin-secreting Cells. J. Biol. Chem. 2004, 279, 19396–19400. [Google Scholar] [CrossRef] [PubMed]
- Tamarina, N.A.; Kuznetsov, A.; Rhodes, C.J.; Bindokas, V.P.; Philipson, L.H. Inositol (1,4,5)-Trisphosphate Dynamics and Intracellular Calcium Oscillations in Pancreatic Beta-Cells. Diabetes 2005, 54, 3073–3081. [Google Scholar] [CrossRef]
- Hauge-Evans, A.C.; Reers, C.; Kerby, A.; Franklin, Z.; Amisten, S.; King, A.J.; Hassan, Z.; Vilches-Flores, A.; Tippu, Z.; Persaud, S.J.; et al. Effect of hyperglycaemia on muscarinic M3 receptor expression and secretory sensitivity to cholinergic receptor activation in islets. Diabetes, Obes. Metab. 2014, 16, 947–956. [Google Scholar] [CrossRef]
- Zhu, L.; Rossi, M.; Cohen, A.; Pham, J.; Zheng, H.; Dattaroy, D.; Mukaibo, T.; Melvin, J.E.; Langel, J.L.; Hattar, S.; et al. Allosteric Modulation of Beta-Cell M(3) Muscarinic Acetylcholine Receptors Greatly Improves Glucose Homeostasis in Lean and Obese Mice. Proc. Natl. Acad. Sci. USA 2019, 116, 18684–18690. [Google Scholar] [CrossRef] [PubMed]
- Oduori, O.S.; Murao, N.; Shimomura, K.; Takahashi, H.; Zhang, Q.; Dou, H.; Sakai, S.; Minami, K.; Chanclon, B.; Guida, C.; et al. Gs/Gq Signaling Switch in Beta Cells Defines Incretin Effectiveness in Diabetes. J. Clin. Investig. 2020, 130, 6639–6655. [Google Scholar] [CrossRef] [PubMed]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, E.A.; Almeida, D.C.; Roma, L.P.; Ortis, F.; Carpinelli, A.R. Lipotoxicity and Beta-Cell Failure in Type 2 Diabetes: Oxidative Stress Linked to Nadph Oxidase and Er Stress. Cells 2021, 10, 3328. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Gambardella, J.; Sorriento, D.; Santulli, G. Mechanistic Role of IP3R Calcium Release Channel in Pancreatic Beta-Cell Function. Diabetes 2018, 67, 313. [Google Scholar] [CrossRef]
- Cassel, R.; Ducreux, S.; Alam, M.R.; Dingreville, F.; Berlé, C.; Burda-Jacob, K.; Chauvin, M.A.; Chikh, K.; Païta, L.; Al-Mawla, R.; et al. Protection of Human Pancreatic Islets from Lipotoxicity by Modulation of the Translocon. PLoS ONE 2016, 11, e0148686. [Google Scholar] [CrossRef] [PubMed]
- Klec, C.; Madreiter-Sokolowski, C.T.; Stryeck, S.; Sachdev, V.; Duta-Mare, M.; Gottschalk, B.; Depaoli, M.R.; Rost, R.; Hay, J.; Waldeck-Weiermair, M.; et al. Glycogen Synthase Kinase 3 Beta Controls Presenilin-1-Mediated Endoplasmic Reticulum Ca2+ Leak Directed to Mitochondria in Pancreatic Islets and beta-Cells. Cell. Physiol. Biochem. 2019, 52, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Klec, C.; Madreiter-Sokolowski, C.T.; Ziomek, G.; Stryeck, S.; Sachdev, V.; Duta-Mare, M.; Gottschalk, B.; Depaoli, M.R.; Rost, R.; Hay, J.; et al. Presenilin-1 Established Er-Ca2+ Leak: A Follow up on Its Importance for the Initial Insulin Secretion in Pancreatic Islets and Beta-Cells Upon Elevated Glucose. Cell Physiol. Biochem. 2019, 53, 573–586. [Google Scholar] [PubMed]
- Parys, J.B.; Van Coppenolle, F. Sec61 complex/translocon: The role of an atypical ER Ca2+-leak channel in health and disease. Front. Physiol. 2022, 13, 991149. [Google Scholar] [CrossRef]
- Bhadra, P.; Dos Santos, S.; Gamayun, I.; Pick, T.; Neumann, C.; Ogbechi, J.; Hall, B.S.; Zimmermann, R.; Helms, V.; Simmonds, R.E.; et al. Mycolactone enhances the Ca2+ leak from endoplasmic reticulum by trapping Sec61 translocons in a Ca2+ permeable state. Biochem. J. 2021, 478, 4005–4024. [Google Scholar] [CrossRef]
- Schäuble, N.; Sven, L.; Martin, J.; Cappel, S.; Schorr, S.; Ulucan, Ö.; Linxweiler, J.; Dudek, J.; Blum, R.; Helms, V.; et al. Bip-Mediated Closing of the Sec61 Channel Limits Ca2+ Leakage from the Er. EMBO J. 2012, 31, 3282–3296. [Google Scholar] [CrossRef]
- Lemos, F.O.; Bultynck, G.; Parys, J.B. A comprehensive overview of the complex world of the endo- and sarcoplasmic reticulum Ca2+-leak channels. Biochim. Biophys. Acta BBA Mol. Cell Res. 2021, 1868, 119020. [Google Scholar] [CrossRef]
- Lloyd, D.J.; Wheeler, M.C.; Gekakis, N. A Point Mutation in Sec61α1 Leads to Diabetes and Hepatosteatosis in Mice. Diabetes 2009, 59, 460–470. [Google Scholar] [CrossRef]
- Erdmann, F.; Schäuble, N.; Lang, S.; Jung, M.; Honigmann, A.; Ahmad, M.; Dudek, J.; Benedix, J.; Harsman, A.; Kopp, A.; et al. Interaction of calmodulin with Sec61α limits Ca2+leakage from the endoplasmic reticulum. EMBO J. 2010, 30, 17–31. [Google Scholar] [CrossRef]
- Tu, H.; Nelson, O.; Bezprozvanny, A.; Wang, Z.; Lee, S.F.; Hao, Y.H.; Serneels, L.; De Strooper, B.; Yu, G.; Bezprozvanny, I. Presenilins Form Er Ca2+ Leak Channels, a Function Disrupted by Familial Alzheimer’s Disease-Linked Mutations. Cell 2006, 126, 981–993. [Google Scholar] [CrossRef]
- Shilling, D.; Mak, D.-O.D.; Kang, D.E.; Foskett, J.K. Lack of Evidence for Presenilins as Endoplasmic Reticulum Ca2+ Leak Channels. J. Biol. Chem. 2012, 287, 10933–10944. [Google Scholar] [CrossRef]
- Kasri, N.N.; Kocks, S.L.; Verbert, L.; Hébert, S.S.; Callewaert, G.; Parys, J.B.; Missiaen, L.; De Smedt, H. Up-regulation of inositol 1,4,5-trisphosphate receptor type 1 is responsible for a decreased endoplasmic-reticulum Ca2+ content in presenilin double knock-out cells. Cell Calcium 2006, 40, 41–51. [Google Scholar] [CrossRef]
- Vierra, N.C.; Dadi, P.K.; Milian, S.C.; Dickerson, M.T.; Jordan, K.L.; Gilon, P.; Jacobson, D.A. Talk-1 Channels Control Beta Cell Endoplasmic Reticulum Ca2+ Homeostasis. Sci. Signal 2017, 10, eaan2883. [Google Scholar] [CrossRef]
- Yazawa, M.; Ferrante, C.; Feng, J.; Mio, K.; Ogura, T.; Zhang, M.; Lin, P.-H.; Pan, Z.; Komazaki, S.; Kato, K.; et al. TRIC channels are essential for Ca2+ handling in intracellular stores. Nature 2007, 448, 78–82. [Google Scholar] [CrossRef]
- Vierra, N.C.; Dadi, P.K.; Jeong, I.; Dickerson, M.; Powell, D.R.; Jacobson, D.A. Type 2 Diabetes-Associated K+ Channel Talk-1 Modulates Beta-Cell Electrical Excitability, Second-Phase Insulin Secretion, and Glucose Homeostasis. Diabetes 2015, 64, 3818–3828. [Google Scholar] [CrossRef]
- Graff, S.M.; Johnson, S.R.; Leo, P.J.; Dadi, P.K.; Dickerson, M.T.; Nakhe, A.Y.; McInerney-Leo, A.M.; Marshall, M.; Zaborska, K.E.; Schaub, C.M.; et al. A KCNK16 mutation causing TALK-1 gain of function is associated with maturity-onset diabetes of the young. J. Clin. Investig. 2021, 6, e138057. [Google Scholar] [CrossRef]
- Nakhe, A.; Prasanna, Y.; Dadi, K.; Kim, J.; Shrestha, S.; Cartailler, J.-P.; Sampson, L.; Magnuson, M.A.; Jacobson, D.A. The Mody-Associated Talk-1 L114p Mutation Causes Islet A-Cell Overactivity and Β-Cell Inactivity Resulting in Transient Neonatal Diabetes and Glucose Dyshomeostasis in Adults; eLife Sciences Publications, Ltd.: Cambridge, UK, 2023; 114p. [Google Scholar]
- Varshney, A.; Scott, L.J.; Welch, R.P.; Erdos, M.R.; Chines, P.S.; Narisu, N.; Albanus, R.D.; Orchard, P.; Wolford, B.N.; Kursawe, R.; et al. Genetic regulatory signatures underlying islet gene expression and type 2 diabetes. Proc. Natl. Acad. Sci. USA 2017, 114, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Duprat, F.; Girard, C.; Jarretou, G.; Lazdunski, M. Pancreatic Two P Domain K+ Channels Talk-1 and Talk-2 Are Activated by Nitric Oxide and Reactive Oxygen Species. J. Physiol. 2005, 562, 235–244. [Google Scholar] [CrossRef]
- Riel, E.B.; Jürs, B.C.; Cordeiro, S.; Musinszki, M.; Schewe, M.; Baukrowitz, T. The versatile regulation of K2P channels by polyanionic lipids of the phosphoinositide and fatty acid metabolism. J. Gen. Physiol. 2021, 154, e202112989. [Google Scholar] [CrossRef]
- Khoubza, L.; Gilbert, N.; Kim, E.J.; Chatelain, F.C.; Feliciangeli, S.; Abelanet, S.; Kang, D.; Lesage, F.; Bichet, D. Alkaline-Sensitive Two-Pore Domain Potassium Channels Form Functional Heteromers in Pancreatic Beta-Cells. J. Biol. Chem. 2022, 298, 102447. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W., Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, J.; Allagnat, F. Store-operated Ca2+ entry: A key component of the insulin secretion machinery. J. Mol. Endocrinol. 2016, 57, F35–F39. [Google Scholar] [CrossRef]
- Klec, C.; Ziomek, G.; Pichler, M.; Malli, R.; Graier, W.F. Calcium Signaling in ß-cell Physiology and Pathology: A Revisit. Int. J. Mol. Sci. 2019, 20, 6110. [Google Scholar] [CrossRef]
- Sabourin, J.; Le Gal, L.; Saurwein, L.; Haefliger, J.A.; Raddatz, E.; Allagnat, F. Store-Operated Ca2+ Entry Mediated by Orai1 and Trpc1 Participates to Insulin Secretion in Rat Beta-Cells. J. Biol. Chem. 2015, 290, 30530–30539. [Google Scholar] [CrossRef]
- Kono, T.; Tong, X.; Taleb, S.; Bone, R.N.; Iida, H.; Lee, C.C.; Sohn, P.; Gilon, P.; Roe, M.W.; Evans-Molina, C. Impaired Store-Operated Calcium Entry and Stim1 Loss Lead to Reduced Insulin Secretion and Increased Endoplasmic Reticulum Stress in the Diabetic Beta-Cell. Diabetes 2018, 67, 2293–2304. [Google Scholar] [CrossRef]
- Zhang, I.X.; Ren, J.; Vadrevu, S.; Raghavan, M.; Satin, L.S. ER stress increases store-operated Ca2+ entry (SOCE) and augments basal insulin secretion in pancreatic beta cells. J. Biol. Chem. 2020, 295, 5685–5700. [Google Scholar] [CrossRef]
- Lehnart, S.E.; Wehrens, X.H.; Marks, A.R. Calstabin Deficiency, Ryanodine Receptors, and Sudden Cardiac Death. Biochem. Biophys. Res. Commun. 2004, 322, 1267–1279. [Google Scholar] [CrossRef]
- Dixit, S.S.; Wang, T.; Manzano, E.J.; Yoo, S.; Lee, J.; Chiang, D.Y.; Ryan, N.; Respress, J.L.; Yechoor, V.K.; Wehrens, X.H. Effects of Camkii-Mediated Phosphorylation of Ryanodine Receptor Type 2 on Islet Calcium Handling, Insulin Secretion, and Glucose Tolerance. PLoS ONE 2013, 8, e58655. [Google Scholar] [CrossRef]
- Kõks, S. Genomics of Wolfram Syndrome 1 (WFS1). Biomolecules 2023, 13, 1346. [Google Scholar] [CrossRef]
- Didmoad (Wolfram) Syndrome. Lancet 1986, 1, 1075–1076.
- Cremers, C.W.; Wijdeveld, P.G.; Pinckers, A.J. Juvenile Diabetes Mellitus, Optic Atrophy, Hearing Loss, Diabetes Insipidus, Atonia of the Urinary Tract and Bladder, and Other Abnormalities (Wolfram Syndrome). A Review of 88 Cases from the Literature with Personal Observations on 3 New Patients. Acta Paediatr. Scand. Suppl. 1977, 264, 1–16. [Google Scholar] [CrossRef]
- Gong, Y.; Xiong, L.; Li, X.; Su, L.; Xiao, H. A novel mutation of WFS1 gene leading to increase ER stress and cell apoptosis is associated an autosomal dominant form of Wolfram syndrome type 1. BMC Endocr. Disord. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Minton, J.A.; Hattersley, A.T.; Owen, K.; McCarthy, M.I.; Walker, M.; Latif, F.; Barrett, T.; Frayling, T.M. Association Studies of Genetic Variation in the Wfs1 Gene and Type 2 Diabetes in U.K. Populations. Diabetes 2002, 51, 1287–1290. [Google Scholar] [CrossRef]
- Ishihara, H.; Takeda, S.; Tamura, A.; Takahashi, R.; Yamaguchi, S.; Takei, D.; Yamada, T.; Inoue, H.; Soga, H.; Katagiri, H.; et al. Disruption of the Wfs1 Gene in Mice Causes Progressive Beta-Cell Loss and Impaired Stimulus-Secretion Coupling in Insulin Secretion. Hum. Mol. Genet. 2004, 13, 1159–1170. [Google Scholar] [CrossRef]
- Hara, T.; Mahadevan, J.; Kanekura, K.; Hara, M.; Lu, S.; Urano, F. Calcium Efflux from the Endoplasmic Reticulum Leads to Beta-Cell Death. Endocrinology 2014, 155, 758–768. [Google Scholar] [CrossRef]
- Morikawa, S.; Blacher, L.; Onwumere, C.; Urano, F. Loss of Function of WFS1 Causes ER Stress-Mediated Inflammation in Pancreatic Beta-Cells. Front. Endocrinol. 2022, 13, 849204. [Google Scholar] [CrossRef]
- Nguyen, L.D.; Fischer, T.T.; Abreu, D.; Arroyo, A.; Urano, F.; Ehrlich, B.E. Calpain Inhibitor and Ibudilast Rescue Beta Cell Functions in a Cellular Model of Wolfram Syndrome. Proc. Natl. Acad. Sci. USA 2020, 117, 17389–17398. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J.; Cui, J.; Chen, W.; Guo, H.; Barbetti, F.; Arvan, P. INS-gene mutations: From genetics and beta cell biology to clinical disease. Mol. Asp. Med. 2015, 42, 3–18. [Google Scholar] [CrossRef]
- Støy, J.; Edghill, E.L.; Flanagan, S.E.; Ye, H.; Paz, V.P.; Pluzhnikov, A.; Below, J.E.; Hayes, M.G.; Cox, N.J.; Lipkind, G.M.; et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 15040–15044. [Google Scholar] [CrossRef]
- Ataie-Ashtiani, S.; Forbes, B. A Review of the Biosynthesis and Structural Implications of Insulin Gene Mutations Linked to Human Disease. Cells 2023, 12, 1008. [Google Scholar] [CrossRef]
- Liu, M.; Hodish, I.; Haataja, L.; Lara-Lemus, R.; Rajpal, G.; Wright, J.; Arvan, P. Proinsulin misfolding and diabetes: Mutant INS gene-induced diabetes of youth. Trends Endocrinol. Metab. 2010, 21, 652–659. [Google Scholar] [CrossRef]
- Rajan, S.; Eames, S.C.; Park, S.-Y.; Labno, C.; Bell, G.I.; Prince, V.E.; Philipson, L.H. In vitro processing and secretion of mutant insulin proteins that cause permanent neonatal diabetes. Am. J. Physiol. Metab. 2010, 298, E403–E410. [Google Scholar] [CrossRef]
- Wang, J.; Takeuchi, T.; Tanaka, S.; Kubo, S.K.; Kayo, T.; Lu, D.; Takata, K.; Koizumi, A.; Izumi, T. A Mutation in the Insulin 2 Gene Induces Diabetes with Severe Pancreatic Beta-Cell Dysfunction in the Mody Mouse. J. Clin. Investig. 1999, 103, 27–37. [Google Scholar] [CrossRef]
- Liu, M.; Lara-Lemus, R.; Shan, S.-O.; Wright, J.; Haataja, L.; Barbetti, F.; Guo, H.; Larkin, D.; Arvan, P. Impaired Cleavage of Preproinsulin Signal Peptide Linked to Autosomal-Dominant Diabetes. Diabetes 2012, 61, 828–837. [Google Scholar] [CrossRef]
- Wang, W.-A.; Agellon, L.B.; Michalak, M. Organellar Calcium Handling in the Cellular Reticular Network. Cold Spring Harb. Perspect. Biol. 2019, 11, a038265. [Google Scholar] [CrossRef]
- Michalak, M. Calreticulin: Endoplasmic Reticulum Ca2+ Gatekeeper. J. Cell Mol. Med. 2023, 28, e17839. [Google Scholar] [CrossRef]
- Gelebart, P.; Opas, M.; Michalak, M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int. J. Biochem. Cell Biol. 2005, 37, 260–266. [Google Scholar] [CrossRef]
- Mery, L.; Mesaeli, N.; Michalak, M.; Opas, M.; Lew, D.P.; Krause, K.-H. Overexpression of Calreticulin Increases Intracellular Ca2+ Storage and Decreases Store-operated Ca2+ Influx. J. Biol. Chem. 1996, 271, 9332–9339. [Google Scholar] [CrossRef]
- Oyadomari, S.; Takeda, K.; Takiguchi, M.; Gotoh, T.; Matsumoto, M.; Wada, I.; Akira, S.; Araki, E.; Mori, M. Nitric Oxide-Induced Apoptosis in Pancreatic Beta Cells Is Mediated by the Endoplasmic Reticulum Stress Pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 10845–10850. [Google Scholar] [CrossRef]
- Gupta, D.; Jetton, T.L.; LaRock, K.; Monga, N.; Satish, B.; Lausier, J.; Peshavaria, M.; Leahy, J.L. Temporal Characterization of Beta Cell-Adaptive and -Maladaptive Mechanisms During Chronic High-Fat Feeding in C57bl/6ntac Mice. J. Biol. Chem. 2017, 292, 12449–12459. [Google Scholar] [CrossRef]
- Franklin, J.L.; Amsler, M.O.; Messina, J.L. Regulation of glucose responsive protein (GRP) gene expression by insulin. Cell Stress Chaperon 2022, 27, 27–35. [Google Scholar] [CrossRef]
- Lièvremont, J.-P.; Rizzuto, R.; Hendershot, L.; Meldolesi, J. BiP, a Major Chaperone Protein of the Endoplasmic Reticulum Lumen, Plays a Direct and Important Role in the Storage of the Rapidly Exchanging Pool of Ca2+. J. Biol. Chem. 1997, 272, 30873–30879. [Google Scholar] [CrossRef]
- Teodoro-Morrison, T.; Schuiki, I.; Zhang, L.; Belsham, D.D.; Volchuk, A. GRP78 overproduction in pancreatic beta cells protects against high-fat-diet-induced diabetes in mice. Diabetologia 2013, 56, 1057–1067. [Google Scholar] [CrossRef]
- Biswas, C.; Ostrovsky, O.; Makarewich, C.A.; Wanderling, S.; Gidalevitz, T.; Argon, Y. The peptide-binding activity of GRP94 is regulated by calcium. Biochem. J. 2007, 405, 233–241. [Google Scholar] [CrossRef]
- Poirier, S.; Mamarbachi, M.; Chen, W.-T.; Lee, A.S.; Mayer, G. GRP94 Regulates Circulating Cholesterol Levels through Blockade of PCSK9-Induced LDLR Degradation. Cell Rep. 2015, 13, 2064–2071. [Google Scholar] [CrossRef]
- Mekahli, D.; Bultynck, G.; Parys, J.B.; De Smedt, H.; Missiaen, L. Endoplasmic-Reticulum Calcium Depletion and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a004317. [Google Scholar] [CrossRef] [PubMed]
- Daverkausen-Fischer, L.; Prols, F. Regulation of Calcium Homeostasis and Flux between the Endoplasmic Reticulum and the Cytosol. J. Biol. Chem. 2022, 298, 102061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lai, E.; Teodoro, T.; Volchuk, A. Grp78, but Not Protein-Disulfide Isomerase, Partially Reverses Hyperglycemia-Induced Inhibition of Insulin Synthesis and Secretion in Pancreatic Beta-Cells. J. Biol. Chem. 2009, 284, 5289–5298. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, S.M.; Dahlby, T.; Andersen, C.H.; Haataja, L.; Petersen, S.; Omar-Hmeadi, M.; Yang, M.; Pihl, C.; Bresson, S.E.; Khilji, M.S.; et al. Endoplasmic Reticulum Chaperone Glucose-Regulated Protein 94 Is Essential for Proinsulin Handling. Diabetes 2019, 68, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Bugliani, M.; Lupi, R.; Marselli, L.; Masini, M.; Boggi, U.; Filipponi, F.; Weir, G.C.; Eizirik, D.L.; Cnop, M. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 2007, 50, 2486–2494. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, N.F.; Amend, A.L.; Bastidas-Ponce, A.; Sabrautzki, S.; Tarquis-Medina, M.; Sachs, S.; Rubey, M.; Lorenz-Depiereux, B.; Feuchtinger, A.; Bakhti, M.; et al. A Point Mutation in the Pdia6 Gene Results in Loss of Pancreatic Beta-Cell Identity Causing Overt Diabetes. Mol. Metab. 2021, 54, 101334. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, G.; Schuiki, I.; Liu, M.; Volchuk, A.; Arvan, P. Action of Protein Disulfide Isomerase on Proinsulin Exit from Endoplasmic Reticulum of Pancreatic Beta-Cells. J. Biol. Chem. 2012, 287, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Avezov, E.; Konno, T.; Zyryanova, A.; Chen, W.; Laine, R.; Crespillo-Casado, A.; Melo, E.P.; Ushioda, R.; Nagata, K.; Kaminski, C.F.; et al. Retarded PDI diffusion and a reductive shift in poise of the calcium depleted endoplasmic reticulum. BMC Biol. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Szabadkai, G.; Bianchi, K.; Várnai, P.; De Stefani, D.; Wieckowski, M.R.; Cavagna, D.; Nagy, A.I.; Balla, T.; Rizzuto, R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006, 175, 901–911. [Google Scholar] [CrossRef]
- Dingreville, F.; Panthu, B.; Thivolet, C.; Ducreux, S.; Gouriou, Y.; Pesenti, S.; Chauvin, M.A.; Chikh, K.; Errazuriz-Cerda, E.; Van Coppenolle, F.; et al. Differential Effect of Glucose on Er-Mitochondria Ca2+ Exchange Participates in Insulin Secretion and Glucotoxicity-Mediated Dysfunction of Beta-Cells. Diabetes 2019, 68, 1778–1794. [Google Scholar] [CrossRef]
- Thivolet, C.; Vial, G.; Cassel, R.; Rieusset, J.; Madec, A.-M. Reduction of endoplasmic reticulum- mitochondria interactions in beta cells from patients with type 2 diabetes. PLoS ONE 2017, 12, e0182027. [Google Scholar] [CrossRef] [PubMed]
- Graff, S.M.; Nakhe, A.Y.; Dadi, P.K.; Dickerson, M.T.; Dobson, J.R.; Zaborska, K.E.; Ibsen, C.E.; Butterworth, R.B.; Vierra, N.C.; Jacobson, D.A. Talk-1-Mediated Alterations of Beta-Cell Mitochondrial Function and Insulin Secretion Impair Glucose Homeostasis on a Diabetogenic Diet. Cell Rep. 2024, 43, 113673. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Erikson, G.; Lyon, J.; Spigelman, A.F.; Bautista, A.; Fox, J.E.M.; dos Santos, C.; Shokhirev, M.; Cartailler, J.-P.; Hetzer, M.W.; et al. Aging compromises human islet beta cell function and identity by decreasing transcription factor activity and inducing ER stress. Sci. Adv. 2022, 8, eabo3932. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobson, J.R.; Jacobson, D.A. Disrupted Endoplasmic Reticulum Ca2+ Handling: A Harβinger of β-Cell Failure. Biology 2024, 13, 379. https://doi.org/10.3390/biology13060379
Dobson JR, Jacobson DA. Disrupted Endoplasmic Reticulum Ca2+ Handling: A Harβinger of β-Cell Failure. Biology. 2024; 13(6):379. https://doi.org/10.3390/biology13060379
Chicago/Turabian StyleDobson, Jordyn R., and David A. Jacobson. 2024. "Disrupted Endoplasmic Reticulum Ca2+ Handling: A Harβinger of β-Cell Failure" Biology 13, no. 6: 379. https://doi.org/10.3390/biology13060379
APA StyleDobson, J. R., & Jacobson, D. A. (2024). Disrupted Endoplasmic Reticulum Ca2+ Handling: A Harβinger of β-Cell Failure. Biology, 13(6), 379. https://doi.org/10.3390/biology13060379





