Modulation of Glucose Consumption and Uptake in HepG2 Cells by Aqueous Extracts from the Coelomic Fluid of the Edible Holothuria tubulosa Sea Cucumber
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Analysis of the CFE
2.2. Cell Culture and Treatment
2.3. PAS Staining
2.4. Extracellular Glucose Determination
2.5. Flow Cytometric Assays for Glucose Uptake Determination and GLUT-2 and -4 Exposure on the Plasma Membrane
2.6. Conventional and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Western Blotting
2.8. Statistics
3. Results
3.1. Glycogen Synthesis by CFE-Treated HepG2 Cells
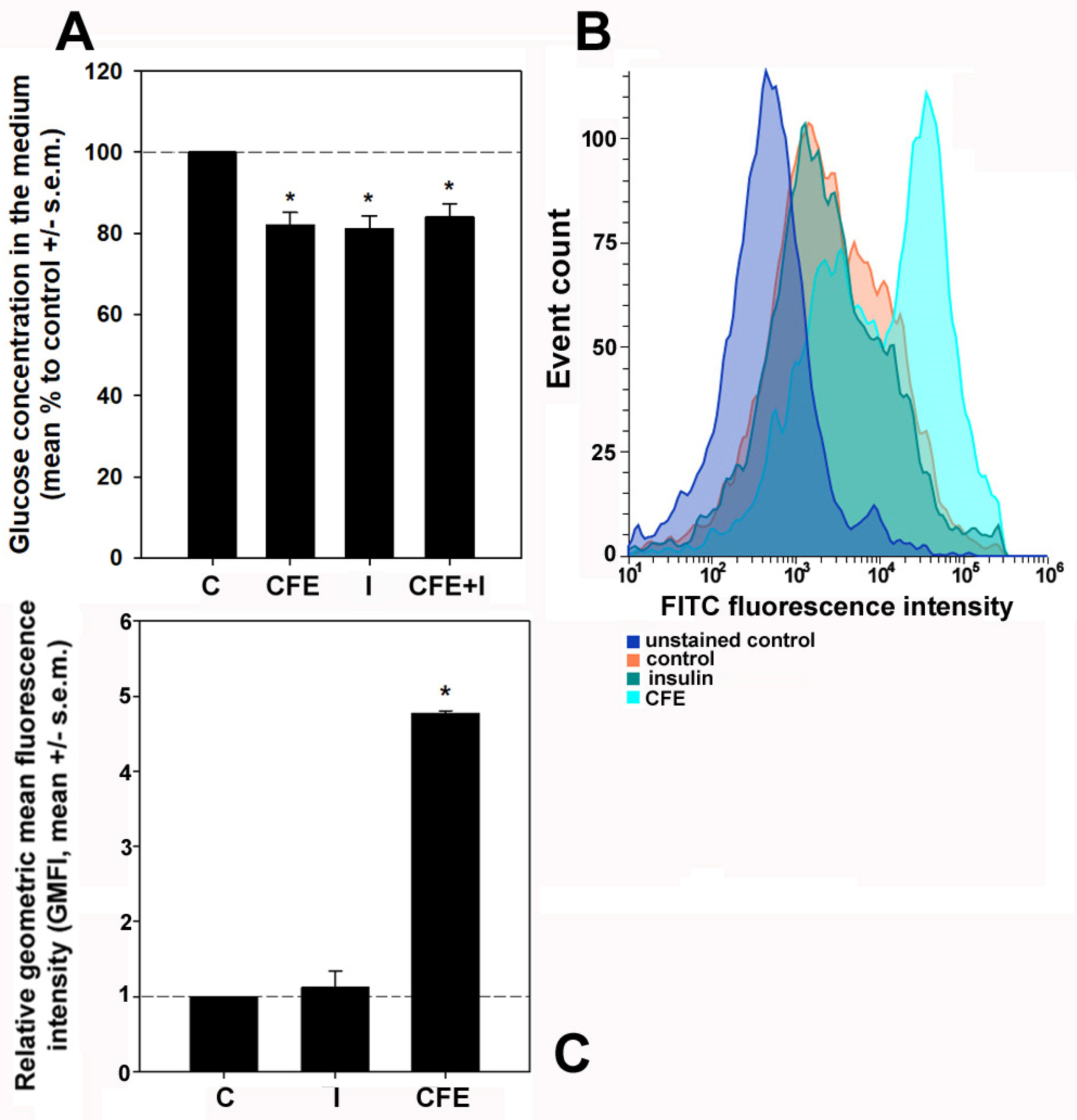
3.2. Glucose Consumption and Glucose Uptake by CFE-Treated HepG2 Cells
3.3. Expression of Glucose Transporters and Upstream Regulators in CFE-Treated HepG2 Cells
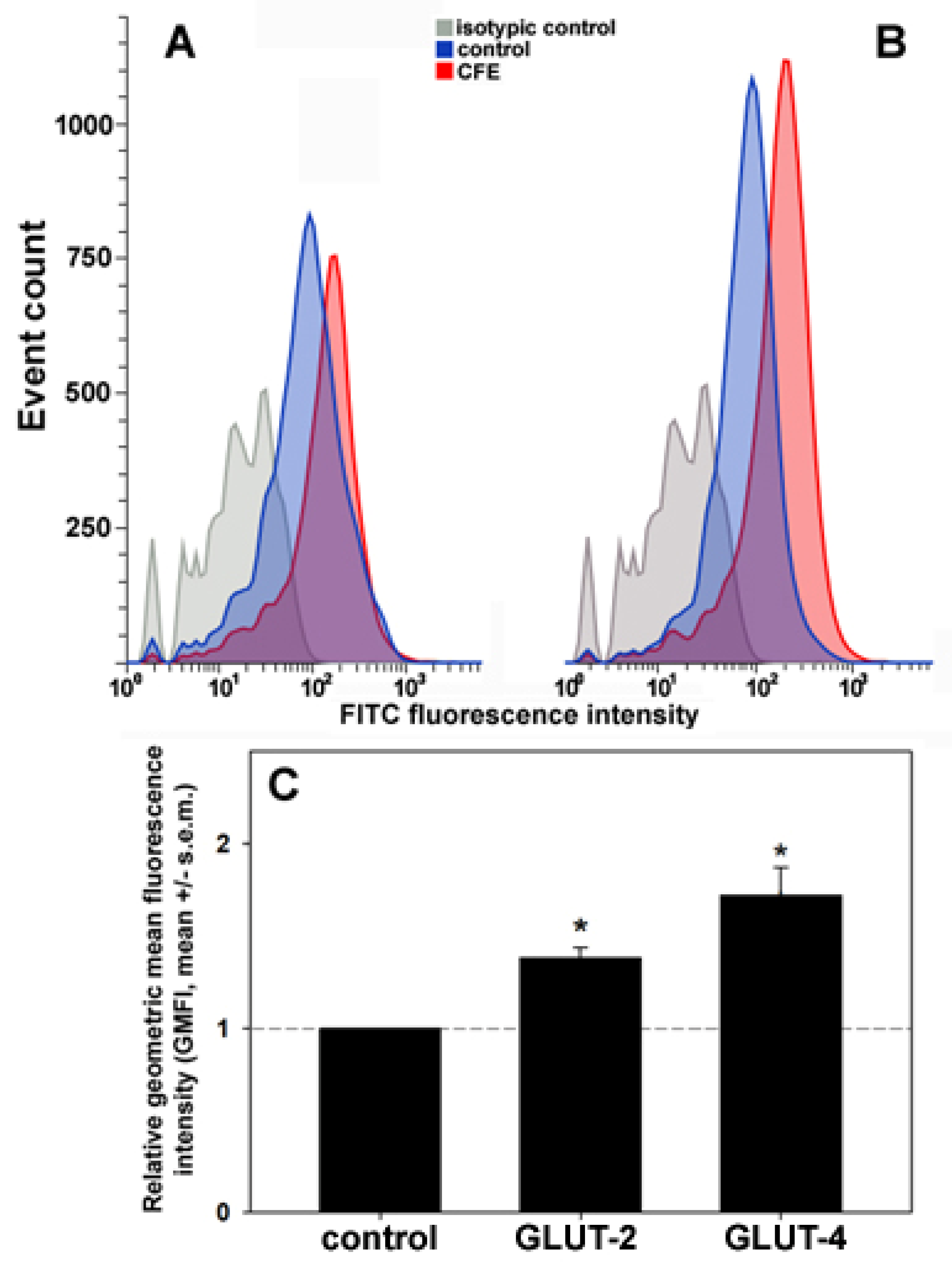
3.4. Exposure of the GLUTs on the Plasma Membrane in CFE-Treated HepG2 Cells
4. Discussion
- (i)
- No insulin or insulin analogs were found among the protein components of the CFE;
- (ii)
- As already reported [16], the analysis of the mixture showed the presence of typical proteins of the exosomes which are conceivably kept intact by the method of preparation of the CFE and therefore can stimulate the observed effects upon fusion with HepG2 cells and intracellular transfer of their cargo;
- (iii)
- Among the other protein signatures identified in the comprehensive analysis, three of them might be related to the increased recruitment and activation of GLUT-4. Their peptide sequences and the results of alignments selected on the basis of sorting by the best E value are reported in Table 2. In particular, they are the following:
- (1)
- Huntingtin-interacting protein 1 (HIP1), which is implicated in clathrin-mediated endocytosis and intracellular protein trafficking [51]. It is known that HIP1 interacts with the CHC22 clathrin isoform, expressed also in HepG2 cells [52], thereby maintaining its proper functioning which aims at the correct formation of the intracellular storage compartment for the GLUT-4 transporter [53]. The dysfunction of this mechanism has been linked to the onset of diabetes mellitus [54].
- (2)
- Small ubiquitin-like modifier (SUMO)/sentrin-specific protease 1 (SENP1), which is a cysteine protease that catalyzes the deSUMOylation of protein substrates, thereby controlling the intracellular localization and function of the targets [55]. Among them, the transcription factor HIF1α, which regulates the mobilization of GLUT-4-containing vesicles to the plasma membrane in skeletal muscle cells [56], is stabilized by deSUMOylation through SENP1 activity. Therefore, it might conceivably stimulate the increase in glucose uptake via the surface accumulation of GLUT-4 [57].
- (3)
- TBC1 domain family member 17 (TBC1D17), which is a Rab5 GTPase-activating protein. In myoblasts and skeletal muscle cells, the AMPK-induced phosphorylation of TBC1D17 leads to the activation of Rab5, which is known to recruit multiple molecules that intervene in GLUT-4 translocation [58].
| Peptide Sequence | Sequence ID (Range) | Expected | Identities (%) | Positives (%) | Protein Description (Organism) |
|---|---|---|---|---|---|
| GRSAPSQGPNNGR | PIK47307.1 (459–471) | 0.008 | 100 | 100 | Putative huntingtin-interacting protein 1 isoform X3 (Apostichopus japonicus) |
| MSVSILDSMDTGKG | PIK42614.1 (182–195) | 1 × 10−4 | 100 | 100 | Putative sentrin-specific protease 1-like (Apostichopus japonicus) |
| KQVLTQAEGLVRE | PIK61193.1 (430–442) | 0.002 | 100 | 100 | Putative TBC1 domain family member 17 (Apostichopus japonicus) |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, M.M.; Jennings, L.; Thomas, O.P. Marine Biodiscovery in a Changing World. Prog. Chem. Org. Nat. Prod. 2021, 116, 1–36. [Google Scholar] [PubMed]
- Lazzara, V.; Arizza, V.; Luparello, C.; Mauro, M.; Vazzana, M. Bright Spots in the Darkness of Cancer: A Review of Starfishes-Derived Compounds and Their Anti-Tumor Action. Mar. Drugs 2019, 17, 617. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Mauro, M.; Lazzara, V.; Vazzana, M. Collective Locomotion of Human Cells, Wound Healing and Their Control by Extracts and Isolated Compounds from Marine Invertebrates. Molecules 2020, 25, 2471. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Mauro, M.; Arizza, V.; Vazzana, M. Histone Deacetylase Inhibitors from Marine Invertebrates. Biology 2020, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Lazzara, V.; Punginelli, D.; Arizza, V.; Vazzana, M. Antitumoral compounds from vertebrate sister group: A review of Mediterranean ascidians. Dev. Comp. Immunol. 2020, 108, 103669. [Google Scholar] [CrossRef] [PubMed]
- Librizzi, M.; Martino, C.; Mauro, M.; Abruscato, G.; Arizza, V.; Vazzana, M.; Luparello, C. Natural Anticancer Peptides from Marine Animal Species: Evidence from In Vitro Cell Model Systems. Cancers 2024, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant Potential of Sea Cucumbers and Their Beneficial Effects on Human Health. Mar. Drugs 2022, 20, 521. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Feng, C.; Lu, Q.; Mao, X.; Huang, H.; Su, Z.; Guo, H.; Huang, Z. Research progress on the chemical components and biological activities of sea cucumber polypeptides. Front. Pharmacol. 2023, 14, 1290175. [Google Scholar] [CrossRef] [PubMed]
- Bay-Nouailhat, A. Description of Holothuria (Holothuria) tubulosa. Available online: https://www.european-marine-life.org/30/holothuria-(holothuria)-tubulosa.php (accessed on 2 April 2024).
- Zhu, Y.; Tian, Y.; Wang, N.; Chang, Y.; Xue, C.; Wang, J. Structure-function relationship analysis of fucoidan from sea cucumber (Holothuria tubulosa) on ameliorating metabolic inflammation. J. Food Biochem. 2021, 45, e13500. [Google Scholar] [CrossRef]
- Zmemlia, N.; Bejaoui, S.; Khemiri, I.; Bouriga, N.; Louiz, I.; El-Bok, S.; Ben-Attia, M.; Souli, A. Biochemical composition and antioxidant potential of the edible Mediterranean sea cucumber Holothuria tubulosa. Grasas Aceites 2020, 71, e364. [Google Scholar] [CrossRef]
- Alper, M.; Güneş, M. Evaluation of cytotoxic, apoptotic effects and phenolic compounds of sea cucumber Holothuria tubulosa (Gmelin, 1791) extracts. Turk. J. Vet. Anim. Sci. 2020, 44, 641–655. [Google Scholar] [CrossRef]
- Mecheta, A.; Hanachi, A.; Jeandel, C.; Arab-Tehrany, E.; Bianchi, A.; Velot, E.; Mezali, K.; Linder, M. Physicochemical Properties and Liposomal Formulations of Hydrolysate Fractions of Four Sea Cucumbers (Holothuroidea: Echinodermata) from the Northwestern Algerian Coast. Molecules 2020, 25, 2972. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, D.; Cusimano, M.G.; Cunsolo, V.; Saletti, R.; Russo, D.; Vazzana, M.; Vitale, M.; Arizza, V. Immune mediators of sea-cucumber Holothuria tubulosa (Echinodermata) as source of novel antimicrobial and anti-staphylococcal biofilm agents. AMB Express 2013, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Ragona, D.; Asaro, D.M.L.; Lazzara, V.; Affranchi, F.; Celi, M.; Arizza, V.; Vazzana, M. Cytotoxic Potential of the Coelomic Fluid Extracted from the Sea Cucumber Holothuria tubulosa against Triple-Negative MDA-MB231 Breast Cancer Cells. Biology 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Branni, R.; Abruscato, G.; Lazzara, V.; Drahos, L.; Arizza, V.; Mauro, M.; Di Stefano, V.; Vazzana, M. Cytotoxic capability and the associated proteomic profile of cell-free coelomic fluid extracts from the edible sea cucumber Holothuria tubulosa on HepG2 liver cancer cells. Excli. J. 2022, 21, 722–743. [Google Scholar] [PubMed]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, F.; Wang, M. Bioactive Substances of Animal Origin. In Handbook of Food Chemistry; Cheung, P., Mehta, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1009–1033. [Google Scholar]
- Abdel Raoof, G.F.; Mohamed, K.Y. Chapter 10—Natural Products for the Management of Diabetes. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 323–374. [Google Scholar]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Meth. Mol. Biol. 2015, 1250, 77–93. [Google Scholar]
- Karim, S.; Adams, D.H.; Lalor, P.F. Hepatic expression and cellular distribution of the glucose transporter family. World J. Gastroenterol. 2012, 18, 6771–6781. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Kang, Y.H.; Kim, K.K.; Kim, T.W.; Park, J.B.; Choe, M. Increased glucose metabolism and alpha-glucosidase inhibition in Cordyceps militaris water extract-treated HepG2 cells. Nutr. Res. Pract. 2017, 11, 180–189. [Google Scholar] [CrossRef]
- Ramachandran, V.; Saravanan, R. Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum. Exp. Toxicol. 2015, 34, 884–893. [Google Scholar] [CrossRef]
- Mokashi, P.; Khanna, A.; Pandita, N. Flavonoids from Enicostema littorale blume enhances glucose uptake of cells in insulin resistant human liver cancer (HepG2) cell line via IRS-1/PI3K/Akt pathway. Biomed. Pharmacother. 2017, 90, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, X.C.; Wang, Y.; Cao, F.F.; You, J.; Uzan, G.; Peng, B.; Zhang, D.H. Celastrol Reverses Palmitic Acid-Induced Insulin Resistance in HepG2 Cells via Restoring the miR-223 and GLUT4 Pathway. Can. J. Diabetes 2019, 43, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Bermúdez, A.; Laza-Briviesca, R.; Vicente-Blanco, R.J.; García-Grande, A.; Coronado, M.J.; Laine-Menéndez, S.; Palacios-Zambrano, S.; Moreno-Villa, M.R.; Ruiz-Valdepeñas, A.M.; Lendinez, C.; et al. Cisplatin resistance involves a metabolic reprogramming through ROS and PGC-1α in NSCLC which can be overcome by OXPHOS inhibition. Free Radic. Biol. Med. 2019, 135, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, R.; Temesfői, V.; Sali, N.; Poór, M.; Needs, P.W.; Kroon, P.A.; Kőszegi, T. A One-Step Extraction and Luminescence Assay for Quantifying Glucose and ATP Levels in Cultured HepG2 Cells. Int. J. Mol. Sci. 2018, 19, 2670. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.; Alizadeh, P.; Timchenko, L.T.; Beeton, C. Quantitative measurement of GLUT4 translocation to the plasma membrane by flow cytometry. J. Vis. Exp. 2010, 7, 2429. [Google Scholar]
- Bruzzone, S.; Ameri, P.; Briatore, L.; Mannino, E.; Basile, G.; Andraghetti, G.; Grozio, A.; Magnone, M.; Guida, L.; Scarfì, S.; et al. The plant hormone abscisic acid increases in human plasma after hyperglycemia and stimulates glucose consumption by adipocytes and myoblasts. FASEB J. 2012, 26, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Abruscato, G.; Chiarelli, R.; Lazzara, V.; Punginelli, D.; Sugár, S.; Mauro, M.; Librizzi, M.; Di Stefano, V.; Arizza, V.; Vizzini, A.; et al. In Vitro Cytotoxic Effect of Aqueous Extracts from Leaves and Rhizomes of the Seagrass Posidonia oceanica (L.) Delile on HepG2 Liver Cancer Cells: Focus on Autophagy and Apoptosis. Biology 2023, 12, 616. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Kim, D.J.; Kim, K.K.; Lee, S.M.; Choe, M. Study of the mechanisms underlying increased glucose absorption in Smilax china L. leaf extract-treated HepG2 cells. J. Nutr. Health 2014, 47, 167–175. [Google Scholar] [CrossRef]
- Párrizas, M.; Maestro, M.A.; Boj, S.F.; Paniagua, A.; Casamitjana, R.; Gomis, R.; Rivera, F.; Ferrer, J. Hepatic nuclear factor 1-alpha directs nucleosomal hyperacetylation to its tissue-specific transcriptional targets. Mol. Cell. Biol. 2001, 21, 3234–3243. [Google Scholar] [CrossRef]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT Pathway in Insulin-Mediated Glucose Uptake. In Blood Glucose Levels; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Hu, S.; Xu, L.; Shi, D.; Wang, J.; Wang, Y.; Lou, Q.; Xue, C. Eicosapentaenoic Acid-Enriched Phosphatidylcholine Isolated From Cucumaria frondosa Exhibits Anti-Hyperglycemic Effects Via Activating Phosphoinositide 3-Kinase/Protein Kinase B Signal Pathway. J. Biosci. Bioeng. 2014, 117, 457–463. [Google Scholar] [CrossRef] [PubMed]
- El Barky, A.R.; Hussein, S.A.; Alm-Eldeen, A.A.; Hafez, Y.A.; Mohamed, T.M. Anti-diabetic activity of Holothuria thomasi saponin. Biomed. Pharmacother. 2016, 84, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Yang, S.; Yu, M.; Jiang, T.; Lv, Z. Glycosaminoglycan from Apostichopus japonicus Improves Glucose Metabolism in the Liver of Insulin Resistant Mice. Mar. Drugs 2020, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, Q.; Cao, J.; Xu, Y.; Pei, Z.; Fan, H.; Yuan, Y.; Shen, X.; Li, C. A sea cucumber (Holothuria leucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food Chem. Toxicol. 2020, 135, 110886. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Lin, L.; Zhao, M. Sulfated fucan/fucosylated chondroitin sulfate-dominated polysaccharide fraction from low-edible-value sea cucumber ameliorates type 2 diabetes in rats: New prospects for sea cucumber polysaccharide based-hypoglycemic functional food. Int. J. Biol. Macromol. 2020, 159, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Purwanto, B.; Wiyasihati, S.I.; Masyitha, P.A.; Wigati, K.W.; Irwadi, I. Golden Sea cucumber extract revives glucose transporter-4 and interleukin-6 protein level in diabetic mouse muscle. Vet. World 2019, 12, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.L.; Trumbly, A.R.; Gibson, G.V. Insulin-mediated GLUT4 translocation is dependent on the microtubule network. J. Biol. Chem. 2001, 276, 10706–10714. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, S.; Huang, M.; Ma, X.; Yang, J.; Deng, S.; Huang, Y.; Wen, Y.; Yang, X. β-ecdysterone from Cyanotis arachnoidea exerts hypoglycemic effects through activating IRS-1/Akt/GLUT4 and IRS-1/Akt/GLUT2 signal pathways in KK-Ay mice. J. Funct. Foods 2017, 39, 123–132. [Google Scholar] [CrossRef]
- Xu, M.; Xue, H.; Kong, L.; Lin, L.; Zheng, G. Smilax china L. Polyphenols Improves Insulin Resistance and Obesity in High-fat Diet-induced Mice Through IRS/AKT-AMPK and NF-κB Signaling Pathways. Plant Foods Hum. Nutr. 2023, 78, 299–306. [Google Scholar] [CrossRef]
- Liu, T.Y.; Shi, C.X.; Gao, R.; Sun, H.J.; Xiong, X.Q.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef]
- Ban, N.; Yamada, Y.; Someya, Y.; Miyawaki, K.; Ihara, Y.; Hosokawa, M.; Toyokuni, S.; Tsuda, K.; Seino, Y. Hepatocyte nuclear factor-1alpha recruits the transcriptional co-activator p300 on the GLUT2 gene promoter. Diabetes 2002, 51, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yao, Y.; Zhao, J.; Cao, J.; Ma, H. Dehydroepiandrosterone protects against hepatic glycolipid metabolic disorder and insulin resistance induced by high fat via activation of AMPK-PGC-1α-NRF-1 and IRS1-AKT-GLUT2 signaling pathways. Int. J. Obes. 2020, 44, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Niu, W.; Wang, Z.; Wang, X.; Xia, G. The effect of gonadotropin on glucose transport and apoptosis in rat ovary. PLoS ONE 2012, 7, e42406. [Google Scholar] [CrossRef]
- Fu, H.; Vuononvirta, J.; Fanti, S.; Bonacina, F.; D’Amati, A.; Wang, G.; Poobalasingam, T.; Fankhaenel, M.; Lucchesi, D.; Coleby, R.; et al. The glucose transporter 2 regulates CD8+ T cell function via environment sensing. Nat. Metab. 2023, 5, 1969–1985. [Google Scholar] [CrossRef]
- Shabelnikov, S.V.; Bobkov, D.E.; Sharlaimova, N.S.; Petukhova, O.A. Injury affects coelomic fluid proteome of the common starfish, Asterias rubens. J. Exp. Biol. 2019, 222, jeb198556. [Google Scholar]
- Gomes, L.P.; Silva, C.G.E.; Gaillard, J.C.; Armengaud, J.; Coelho, A.V. Characterization of Soluble Cell-Free Coelomic Fluid Proteome from the Starfish Marthasterias glacialis. Methods Mol. Biol. 2022, 2450, 583–597. [Google Scholar]
- Hyun, T.S.; Ross, T.S. HIP1: Trafficking roles and regulation of tumorigenesis. Trends Mol. Med. 2004, 10, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Esk, C.; Chen, C.Y.; Johannes, L.; Brodsky, F.M. The clathrin heavy chain isoform CHC22 functions in a novel endosomal sorting step. J. Cell Biol. 2010, 188, 131–144. [Google Scholar] [CrossRef]
- Ybe, J.A. Novel clathrin activity: Developments in health and disease. Biomol. Concepts 2014, 5, 175–182. [Google Scholar] [CrossRef]
- Vassilopoulos, S.; Esk, C.; Hoshino, S.; Funke, B.H.; Chen, C.Y.; Plocik, A.M.; Wright, W.E.; Kucherlapati, R.; Brodsky, F.M. A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism. Science 2009, 324, 1192–1196. [Google Scholar] [CrossRef]
- Vertegaal, A.C.O. Signalling mechanisms and cellular functions of SUMO. Nat. Rev. Mol. Cell. Biol. 2022, 23, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Makino, Y.; Mizumoto, K.; Isoe, T.; Takeda, Y.; Watanabe, J.; Fujita, Y.; Takiyama, Y.; Abiko, A.; Haneda, M. Loss of HIF-1α impairs GLUT4 translocation and glucose uptake by the skeletal muscle cells. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1065–E1076. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Kang, X.; Zhang, S.; Yeh, E.T. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell 2007, 131, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.S.; Cong, X.X.; Gao, X.K.; Shi, Y.P.; Shi, L.J.; Wang, J.F.; Ni, C.Y.; He, M.J.; Xu, Y.; Yi, C.; et al. AMPK-mediated phosphorylation enhances the auto-inhibition of TBC1D17 to promote Rab5-dependent glucose uptake. Cell Death Differ. 2021, 28, 3214–3234. [Google Scholar] [CrossRef] [PubMed]


| Gene (Primer) | Sequence (5′ → 3′) | Reference |
|---|---|---|
| GLUT2 (sense) | GATGAACTGCCCACAATCTC | [31] |
| GLUT2 (antisense) | CTGATGAAAAGTGCCAAGTG | |
| GLUT4 (sense) | GTTAATCGGCATTCTGATCG | [31] |
| GLUT4 (antisense) | GTGAAGACTGTGTTGACCAC | |
| AKT2 (sense) | GCTAGGTGACAGCGTACCAC | [24] |
| AKT2 (antisense) | GGCCTCTCGGTCTTCATCAG | |
| IRS1 (sense) | TATCTGCATGGGTGGCAAGG | [24] |
| IRS1 (antisense) | GGGTAGGCAGGCATCATCTC | |
| HNF1A (sense) | GAATGCATCCAGAGAGGGGT | [31] |
| HNF1A (antisense) | GTGGACCTTACTGGGGGAGA | |
| ACTB (sense) | GGAAGGTGGACAGCGAGGCC | [30] |
| ACTB (antisense) | GTGACGTGGACATCCGCAAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abruscato, G.; Tarantino, R.; Mauro, M.; Chiarelli, R.; Vizzini, A.; Arizza, V.; Vazzana, M.; Luparello, C. Modulation of Glucose Consumption and Uptake in HepG2 Cells by Aqueous Extracts from the Coelomic Fluid of the Edible Holothuria tubulosa Sea Cucumber. Biology 2024, 13, 378. https://doi.org/10.3390/biology13060378
Abruscato G, Tarantino R, Mauro M, Chiarelli R, Vizzini A, Arizza V, Vazzana M, Luparello C. Modulation of Glucose Consumption and Uptake in HepG2 Cells by Aqueous Extracts from the Coelomic Fluid of the Edible Holothuria tubulosa Sea Cucumber. Biology. 2024; 13(6):378. https://doi.org/10.3390/biology13060378
Chicago/Turabian StyleAbruscato, Giulia, Roberta Tarantino, Manuela Mauro, Roberto Chiarelli, Aiti Vizzini, Vincenzo Arizza, Mirella Vazzana, and Claudio Luparello. 2024. "Modulation of Glucose Consumption and Uptake in HepG2 Cells by Aqueous Extracts from the Coelomic Fluid of the Edible Holothuria tubulosa Sea Cucumber" Biology 13, no. 6: 378. https://doi.org/10.3390/biology13060378
APA StyleAbruscato, G., Tarantino, R., Mauro, M., Chiarelli, R., Vizzini, A., Arizza, V., Vazzana, M., & Luparello, C. (2024). Modulation of Glucose Consumption and Uptake in HepG2 Cells by Aqueous Extracts from the Coelomic Fluid of the Edible Holothuria tubulosa Sea Cucumber. Biology, 13(6), 378. https://doi.org/10.3390/biology13060378








