Influence of Cluster-Situated Regulator PteF in Filipin Biosynthetic Cluster on Avermectin Biosynthesis in Streptomyces avermitilis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids and Culture Conditions
2.2. Construction of In-Frame Deletion Mutant Strains
2.3. Transcriptome Analysis
2.4. Extraction and High-Performance Liquid Chromatography (HPLC) Analysis of Avermectin B1a and Filipin III
2.5. Genomic Synteny Analysis
2.6. Statistical Analysis
3. Results
3.1. Analysis of Avermectin and Filipin Biosynthesis in S. avermitilis S0
3.2. Influence of PteF and PteR on Avermectin and Filipin Production
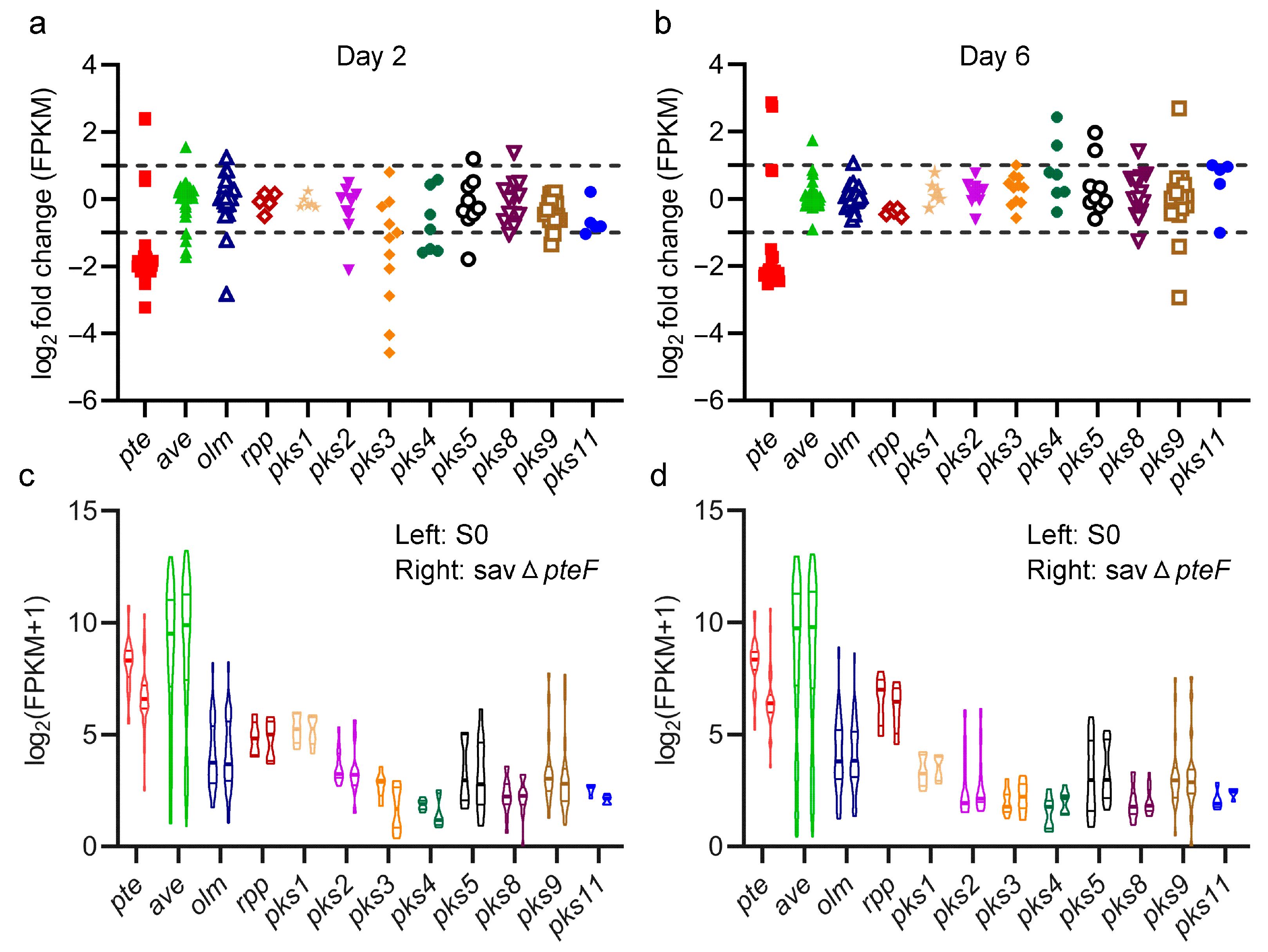
3.3. Influence of pteF Deletion on Transcription of Genes Involved in Polyketide Synthase (PKS) BGCs
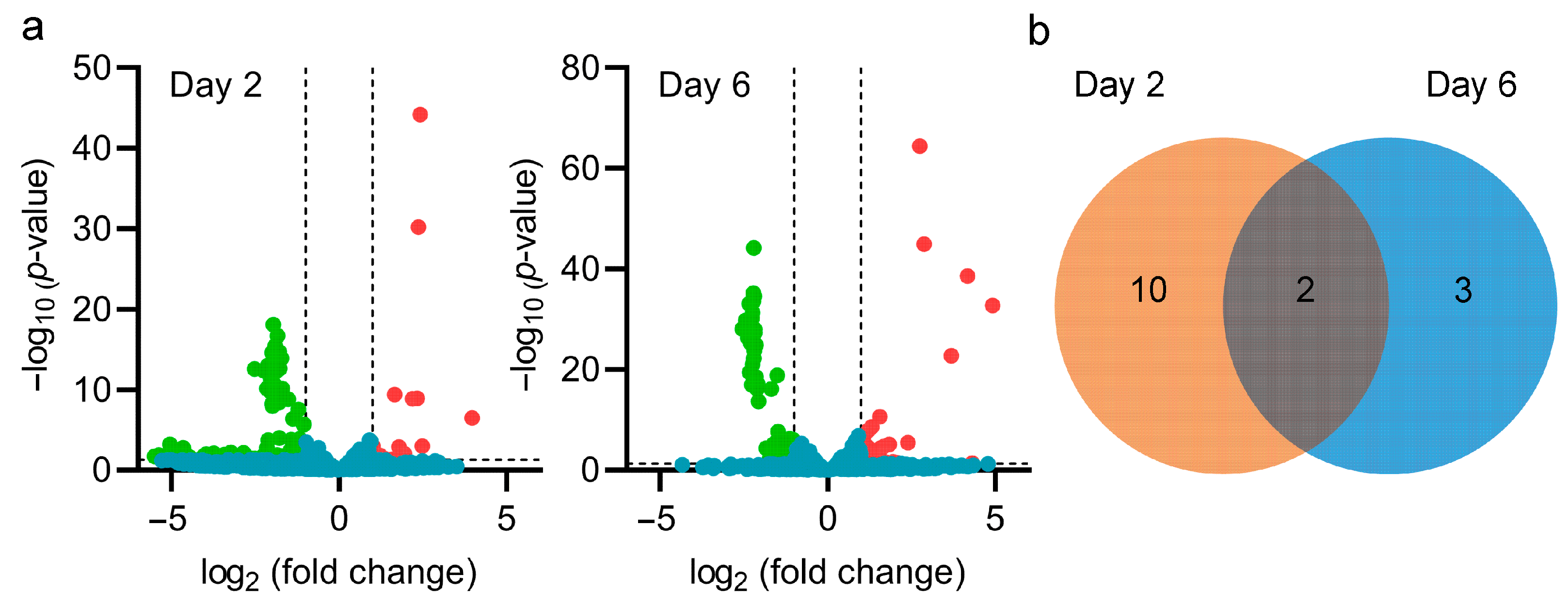
3.4. Influence of pteF Deletion on Global Regulatory Network
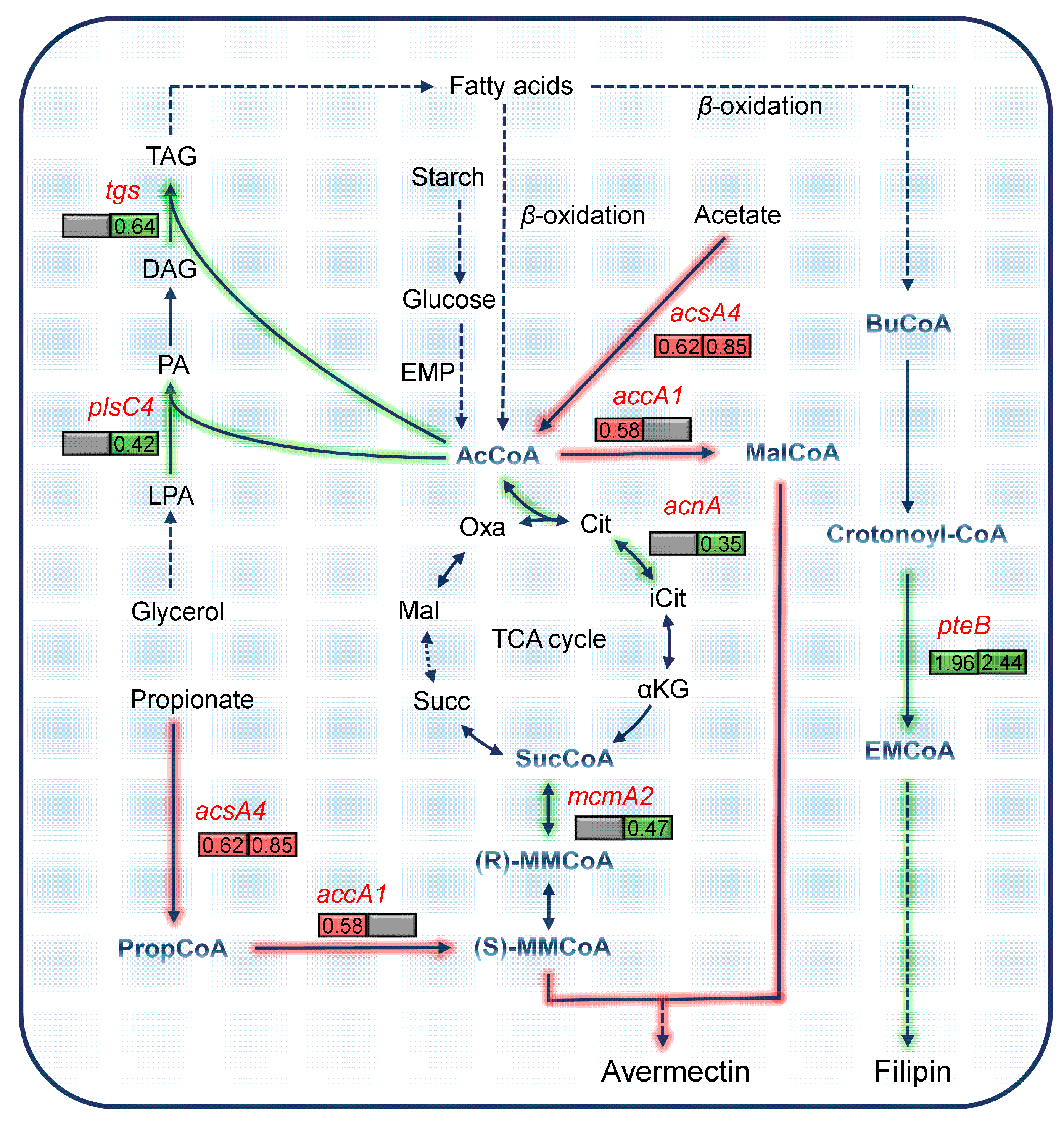
3.5. Influence of pteF Deletion on Cell Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Yang, B.; Tan, G.-Y.; Ouyang, L.-M.; Qiu, S.; Wang, W.; Xiang, W.; Zhang, L. Polyketide pesticides from actinomycetes. Curr. Opin. Biotechnol. 2021, 69, 299–307. [Google Scholar] [CrossRef]
- Ikeda, H.; Ishikawa, J.; Hanamoto, A.; Shinose, M.; Kikuchi, H.; Shiba, T.; Sakaki, Y.; Hattori, M.; Omura, S. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 2003, 21, 526–531. [Google Scholar] [CrossRef]
- Komatsua, M.; Uchiyama, T.; Omura, S.; Cane, D.E.; Ikeda, H. Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 2646–2651. [Google Scholar] [CrossRef]
- Siddique, S.; Syed, Q.; Adnan, A.; Nadeem, M.; Irfan, M.; Qureshi, F.A. Production of avermectin B1b from Streptomyces avermitilis 41445 by batch submerged fermentation. Jundishapur. J. Microbiol. 2013, 6, e7198. [Google Scholar] [CrossRef]
- He, F.; Liu, W.; Sun, D.; Luo, S.; Chen, Z.; Wen, Y.; Li, J. Engineering of the TetR family transcriptional regulator SAV151 and its target genes increases avermectin production in Streptomyces avermitilis. Appl. Microbiol. Biotechnol. 2014, 98, 399–409. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Guo, J.; Chen, Z.; Li, J.; Wen, Y. Increasing avermectin production in Streptomyces avermitilis by manipulating the expression of a novel TetR-Family regulator and its target gene product. Appl. Environ. Microbiol. 2015, 81, 5157–5173. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.; Lu, X.; Liu, W.; Chen, Z.; Li, J.; Deng, L.; Wen, Y. SAV4189, a MarR-Family regulator in Streptomyces avermitilis, activates avermectin biosynthesis. Front. Microbiol. 2018, 9, 1358. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhang, W.; Chen, D.; Gao, H.; Tao, J.; Liu, M.; Gou, Z.; Zhou, X.; Ye, B.C.; Zhang, Q.; et al. Reverse biological engineering of hrdB to enhance the production of avermectins in an industrial strain of Streptomyces avermitilis. Proc. Natl. Acad. Sci. USA 2010, 107, 11250–11254. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Zhang, X.; Song, Y.; Wen, Y.; Li, J. Enhancement of avermectin and ivermectin production by overexpression of the maltose ATP-binding cassette transporter in Streptomyces avermitilis. Bioresour. Technol. 2010, 101, 9228–9235. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Li, S.; Zhang, Y.; Chu, L.; He, H.; Dong, Z.; Xiang, W. Mining and fine-tuning sugar uptake system for titer improvement of milbemycins in Streptomyces bingchenggensis. Synth. Syst. Biotechnol. 2020, 5, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Li, L.; Du, G.; Zhang, Y.; Wang, X.; Li, S.; Xiang, W. A previously unidentified sugar transporter for engineering of high-yield Streptomyces. Appl. Microbiol. Biotechnol. 2024, 108, 72. [Google Scholar] [CrossRef]
- Qiu, J.; Zhuo, Y.; Zhu, D.; Zhou, X.; Zhang, L.; Bai, L.; Deng, Z. Overexpression of the ABC transporter AvtAB increases avermectin production in Streptomyces avermitilis. Appl. Microbiol. Biotechnol. 2011, 92, 337–345. [Google Scholar] [CrossRef]
- Chu, L.; Li, S.; Dong, Z.; Zhang, Y.; Jin, P.; Ye, L.; Wang, X.; Xiang, W. Mining and engineering exporters for titer improvement of macrolide biopesticides in Streptomyces. Microb. Biotechnol. 2022, 15, 1120–1132. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Li, Z.; Zhang, J.; Fan, K.; Tan, G.; Ai, G.; Lam, S.M.; Shui, G.; Yang, Z.; et al. Harnessing the intracellular triacylglycerols for titer improvement of polyketides in Streptomyces. Nat. Biotechnol. 2020, 38, 76–83. [Google Scholar] [CrossRef]
- Hao, Y.; You, Y.; Chen, Z.; Li, J.; Liu, G.; Wen, Y. Avermectin B1a production in Streptomyces avermitilis is enhanced by engineering aveC and precursor supply genes. Appl. Microbiol. Biotechnol. 2022, 106, 2191–2205. [Google Scholar] [CrossRef]
- Culp, E.J.; Yim, G.; Waglechner, N.; Wang, W.; Pawlowski, A.C.; Wright, G.D. Hidden antibiotics in actinomycetes can be identified by inactivation of gene clusters for common antibiotics. Nat. Biotechnol. 2019, 37, 1149–1154. [Google Scholar] [CrossRef]
- Niu, G.; Chater, K.F.; Tian, Y.; Zhang, J.; Tan, H. Specialised metabolites regulating antibiotic biosynthesis in Streptomyces spp. FEMS Microbiol. Rev. 2016, 40, 554–573. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, X.; Jiang, M.; Bai, L. Enhanced salinomycin production by adjusting the supply of polyketide extender units in Streptomyces albus. Metab. Eng. 2016, 35, 129–137. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Cheng, X.; Zhang, Y.; Li, S.; Wang, X.; Xiang, W. Titer improvement of milbemycins via coordinating metabolic competition and transcriptional co-activation controlled by Streptomyces antibiotic regulatory protein family regulator in Streptomyces bingchenggensis. Biotechnol. Bioeng. 2022, 119, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Rateb, M.E.; Wang, N.; Shen, B. Competition and co-regulation of spirotoamide and tautomycetin biosynthesis in Streptomyces griseochromogenes, and isolation and structural elucidation of spirotoamide C and D. J. Antibiot. 2017, 70, 710–714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omura, S.; Ikeda, H.; Ishikawa, J.; Hanamoto, A.; Takahashi, C.; Shinose, M. Genome sequence of an industrial microorganism Streptomyces avermitilis: Deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. USA 2001, 98, 12215–12220. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, J.; Li, L.; Chen, Z.; Wen, Y.; Li, J. The pathway-specific regulator AveR from Streptomyces avermitilis positively regulates avermectin production while it negatively affects oligomycin biosynthesis. Mol. Genet. and Genom. 2010, 283, 123–133. [Google Scholar] [CrossRef]
- Vicente, C.M.; Payero, T.D.; Santos-Aberturas, J.; Barreales, E.G.; de Pedro, A.; Aparicio, J.F. Pathway-specific regulation revisited: Cross-regulation of multiple disparate gene clusters by PAS-LuxR transcriptional regulators. Appl. Microbiol. Biotechnol. 2015, 99, 5123–5135. [Google Scholar] [CrossRef]
- Vicente, C.M.; Payero, T.D.; Rodriguez-Garcia, A.; Barreales, E.G.; de Pedro, A.; Santos-Beneit, F.; Aparicio, J.F. Modulation of multiple gene clusters’ expression by the PAS-LuxR transcriptional regulator PteF. Antibiotics 2022, 11, 994. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000; Volume 291. [Google Scholar]
- Gao, H.; Liu, M.; Liu, J.; Dai, H.; Zhou, X.; Liu, X.; Zhuo, Y.; Zhang, W.; Zhang, L. Medium optimization for the production of avermectin B1a by Streptomyces avermitilis 14-12A using response surface methodology. Bioresour. Technol. 2009, 100, 4012–4016. [Google Scholar] [CrossRef]
- Vicente, C.M.; Santos-Aberturas, J.; Payero, T.D.; Barreales, E.G.; de Pedro, A.; Aparicio, J.F. PAS-LuxR transcriptional control of filipin biosynthesis in S. avermitilis. Appl. Microbiol. Biotechnol. 2014, 98, 9311–9324. [Google Scholar] [CrossRef]
- Kurtz, S.; Phillippy, A.; Delcher, A.L.; Smoot, M.; Shumway, M.; Antonescu, C.; Salzberg, S.L. Versatile and open software for comparing large genomes. Genome. Biol. 2004, 5, R12. [Google Scholar] [CrossRef]
- Chiaromonte, F.; Yap, V.B.; Miller, W. Scoring pairwise genomic sequence alignments. Pac. Symp. Biocomput. 2002, 7, 115–126. [Google Scholar]
- Bergy, M.E.; Eble, T.E. The filipin complex. Biochem. 1968, 7, 653–659. [Google Scholar] [CrossRef] [PubMed]
- van der Heul, H.U.; Bilyk, B.L.; McDowall, K.J.; Seipke, R.F.; van Wezel, G.P. Regulation of antibiotic production in Actinobacteria: New perspectives from the post-genomic era. Nat. Prod. Rep. 2018, 35, 575–604. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Bai, L.; Zhou, X.; Deng, Z. Inactivation of the positive LuxR-type oligomycin biosynthesis regulators OlmRI and OlmRII increases avermectin production in Streptomyces avermitilis. Chin. Sci. Bull. 2011, 57, 869–876. [Google Scholar] [CrossRef]
- Sun, D.; Liu, C.; Zhu, J.; Liu, W. Connecting metabolic pathways: Sigma factors in Streptomyces spp. Front. Microbiol. 2017, 8, 2546. [Google Scholar] [CrossRef]
- Fujii, T.; Gramajo, H.C.; Takano, E.; Bibb, M.J. redD and actII-ORF4, pathway-specific regulatory genes for antibiotic production in Streptomyces coelicolor A3(2), are transcribed in vitro by an RNA polymerase holoenzyme containing σhrdD. J. Bacteriol. 1996, 178, 3402–3405. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Wang, P.; Wen, Y.; Song, Y.; Chen, Z.; Li, J. Inactivation of the extracytoplasmic function sigma factor Sig6 stimulates avermectin production in Streptomyces avermitilis. Biotechnol. Lett. 2011, 33, 1955–1961. [Google Scholar] [CrossRef]
- Luo, S.; Sun, D.; Zhu, J.Y.; Chen, Z.; Wen, Y.; Li, J.L. An extracytoplasmic function sigma factor, σ25, differentially regulates avermectin and oligomycin biosynthesis in Streptomyces avermitilis. Appl. Microbiol. Biot. 2014, 98, 7097–7112. [Google Scholar] [CrossRef]
- Sun, D.; Wang, Q.; Chen, Z.; Li, J.; Wen, Y. An alternative σ factor, σ8, controls avermectin production and multiple stress responses in Streptomyces avermitilis. Front. Microbiol. 2017, 8, 736. [Google Scholar] [CrossRef]
- Sivapragasam, S.; Grove, A. The link between purine metabolism and production of antibiotics in Streptomyces. Antibiotics 2019, 8, 76. [Google Scholar] [CrossRef]
- Bierman, M.; Logan, R.; O’Brien, K.; Seno, E.T.; Rao, R.N.; Schoner, B.E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 1992, 116, 43–49. [Google Scholar] [CrossRef]


| Category | Cluster | Length | Product |
|---|---|---|---|
| terpene | ams | 1008 bp | Avermitilol, avermitilone |
| polyketide | pks11 | 5227 bp | Polyketide 1 |
| other | mcj1 | 3368 bp | Microcin 1 |
| polyketide | pte | 80,344 bp | Filipin |
| polyketide | ave | 80,849 bp | Avermectin |
| terpene | crt | 8718 bp | Isorenieratene |
| Gene ID | Functional Description | log2 (Fold Change) | p-Value |
|---|---|---|---|
| GM007052 | putative RNA polymerase ECF subfamily sigma factor | −5.05 | 0.001 |
| GM006412 | putative IclR family transcriptional regulator | −2.29 | 0.020 |
| GM003939 | putative anti-sigma regulatory factor | 2.19 | 1.42 × 10−9 |
| GM000222 | putative TetR family transcriptional regulator | −1.68 | 0.022 |
| GM007217 | putative two-component system response regulator | 1.57 | 0.042 |
| GM003574 | putative anti-sigma regulatory factor | −1.51 | 1.46 × 10−9 |
| GM003618 | AsnC family transcriptional regulator | −1.47 | 0.044 |
| GM001189 | putative RNA polymerase ECF subfamily sigma factor | −1.30 | 0.037 |
| GM000932 | putative LacI family transcriptional regulator | −1.24 | 0.049 |
| GM005356 | putative two-component system sensor kinase | −1.06 | 0.001 |
| GM007707 | putative MerR family transcriptional regulator | 1.02 | 0.036 |
| GM001883 | putative ArsR family transcriptional regulator | −1.00 | 0.030 |
| Gene ID | Functional Description | log2 (Fold Change) | p-Value |
|---|---|---|---|
| GM003939 | putative anti-sigma regulatory factor | 4.17 | 2.51 × 10−39 |
| GM003307 | putative regulatory protein | 1.95 | 0.024 |
| GM008359 | LytR family transcriptional regulator | 1.23 | 0.037 |
| GM003574 | putative anti-sigma regulatory factor | −1.13 | 6.45 × 10−7 |
| GM002997 | TetR family transcriptional regulator | −1.01 | 3.05 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, G.; Yang, X.; Wu, Z.; Pan, M.; Dong, Z.; Zhang, Y.; Xiang, W.; Li, S. Influence of Cluster-Situated Regulator PteF in Filipin Biosynthetic Cluster on Avermectin Biosynthesis in Streptomyces avermitilis. Biology 2024, 13, 344. https://doi.org/10.3390/biology13050344
Du G, Yang X, Wu Z, Pan M, Dong Z, Zhang Y, Xiang W, Li S. Influence of Cluster-Situated Regulator PteF in Filipin Biosynthetic Cluster on Avermectin Biosynthesis in Streptomyces avermitilis. Biology. 2024; 13(5):344. https://doi.org/10.3390/biology13050344
Chicago/Turabian StyleDu, Guozhong, Xue Yang, Zhengxiong Wu, Minghui Pan, Zhuoxu Dong, Yanyan Zhang, Wensheng Xiang, and Shanshan Li. 2024. "Influence of Cluster-Situated Regulator PteF in Filipin Biosynthetic Cluster on Avermectin Biosynthesis in Streptomyces avermitilis" Biology 13, no. 5: 344. https://doi.org/10.3390/biology13050344
APA StyleDu, G., Yang, X., Wu, Z., Pan, M., Dong, Z., Zhang, Y., Xiang, W., & Li, S. (2024). Influence of Cluster-Situated Regulator PteF in Filipin Biosynthetic Cluster on Avermectin Biosynthesis in Streptomyces avermitilis. Biology, 13(5), 344. https://doi.org/10.3390/biology13050344






