Examining the Influence of Cognitive Load and Environmental Conditions on Autonomic Nervous System Response in Military Aircrew: A Hypoxia–Normoxia Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Subjects
2.3. O2 Exposure (Factor A)
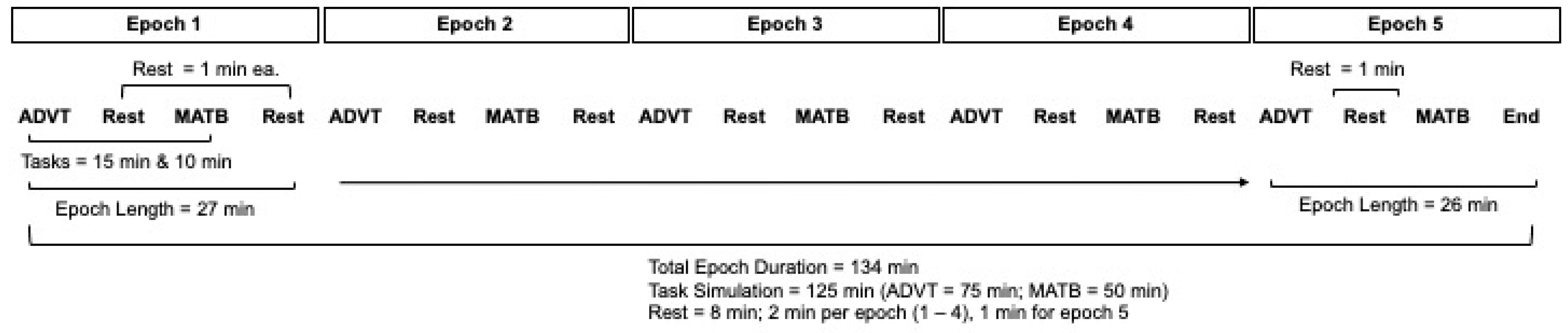
2.4. Cognitive Load: Simulated In-Flight Tasks (Factor B)
Automated Desktop Vision Tester and Enhanced Air Force Multi-Attribute Task Battery
2.5. Autonomic Nervous System Response (Outcome)
2.6. Statistical Analysis
3. Results
3.1. ANS Response to High and Low Cognitive Loads
3.1.1. Hypoxic Condition
3.1.2. Normoxic Condition
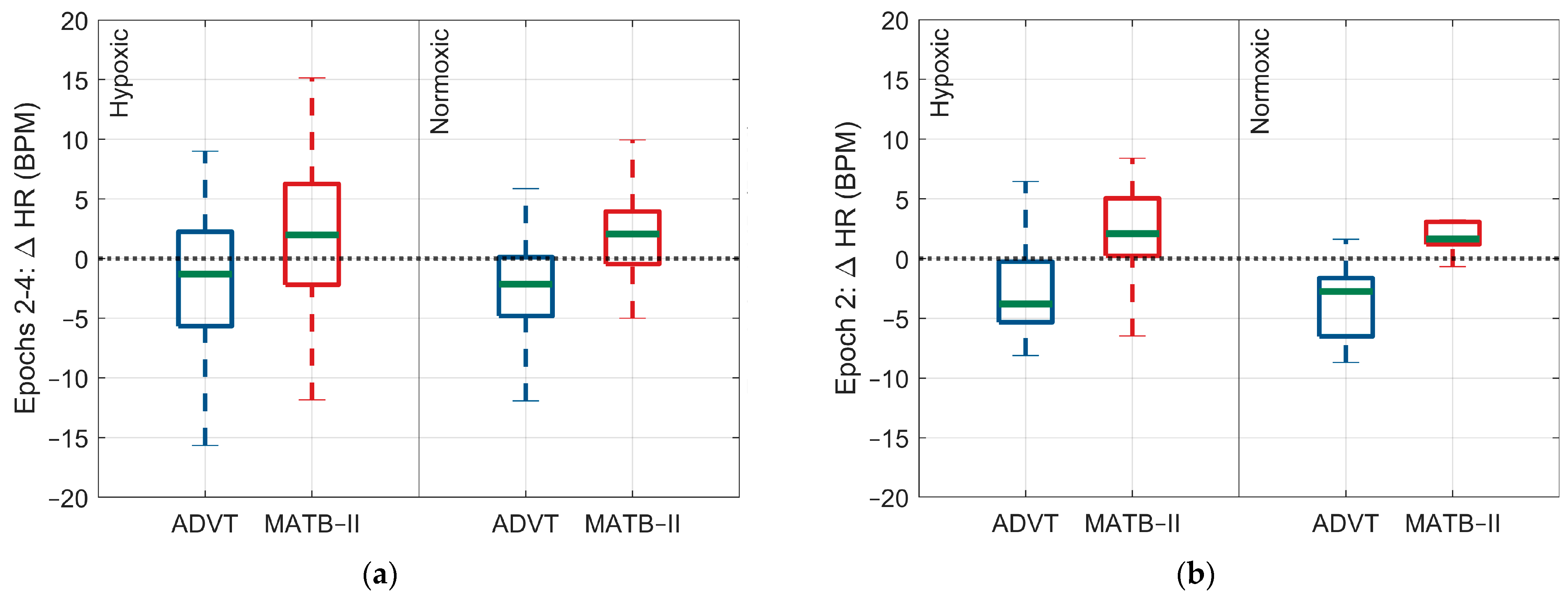
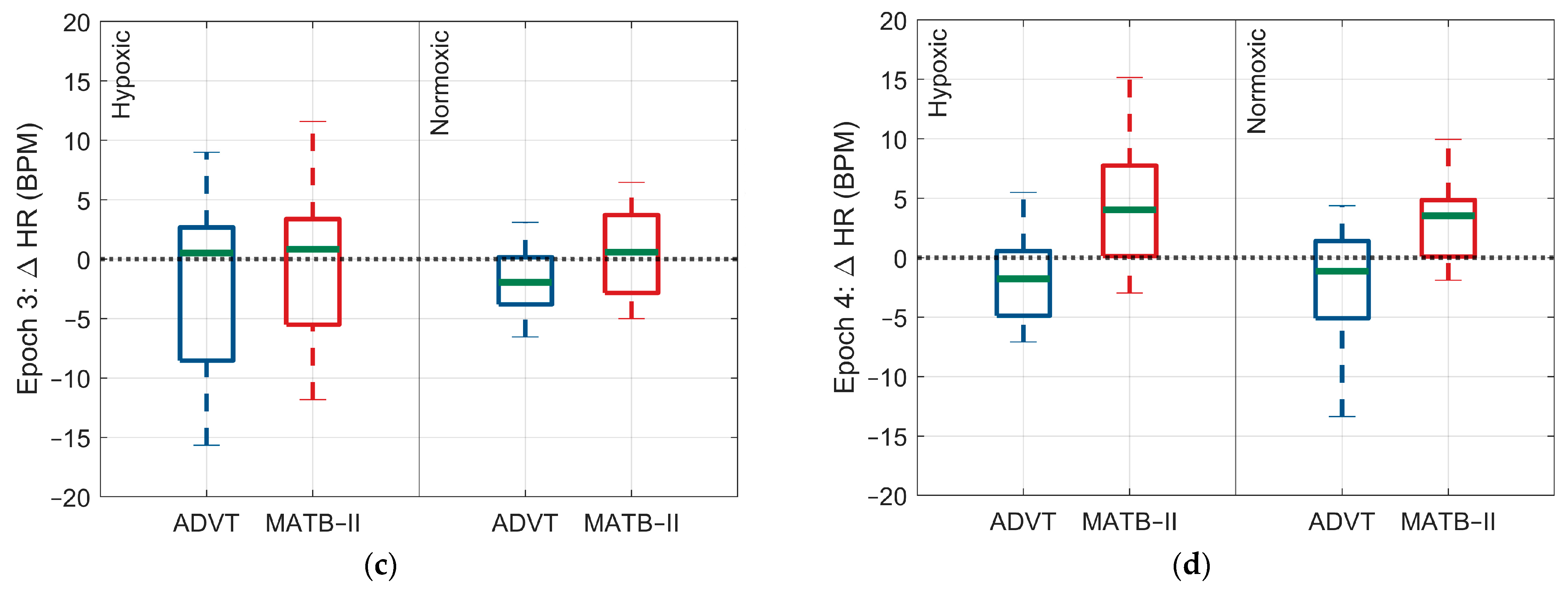
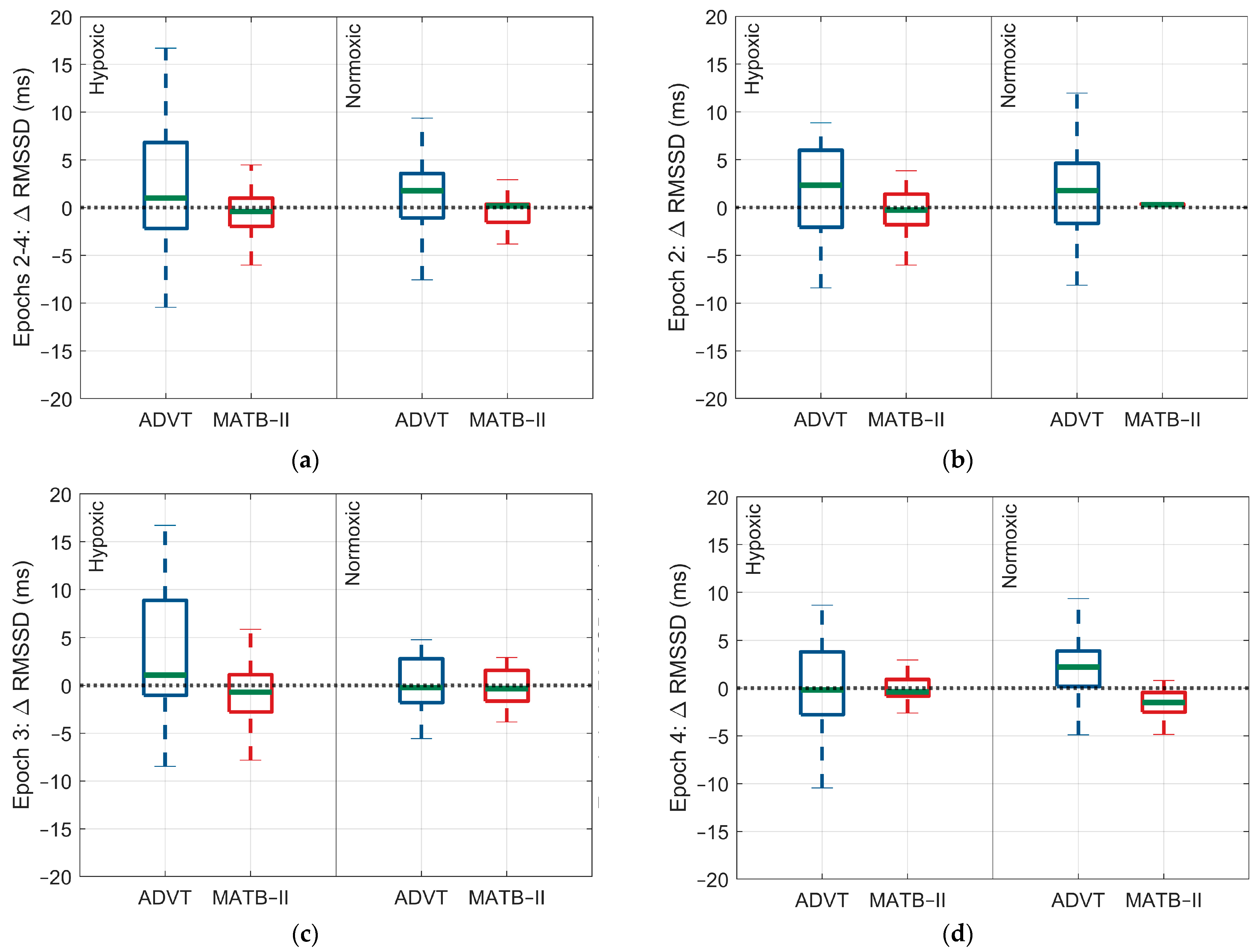
3.2. Differences in ANS Response between High and Low Cognitive Loads
3.2.1. Hypoxic Condition
3.2.2. Normoxic Condition
3.3. Differences in the ANS Response to Cognitive Load between Factor A Conditions
3.3.1. MATB-II
3.3.2. ADVT
4. Discussion
5. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Jiang, N.; Pan, T.; Si, H.; Li, Y.; Zou, W. Cognitive Load Identification of Pilots Based on Physiological-Psychological Characteristics in Complex Environments. J. Adv. Transp. 2020, 2020, 5640784. [Google Scholar] [CrossRef]
- Bowers, M.A.; Christensen, J.C.; Eggemeier, F.T. The Effects of Workload Transitions in a Multitasking Environment. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2014, 58, 220–224. [Google Scholar] [CrossRef]
- Grassmann, M.; Vlemincx, E.; Von Leupoldt, A.; Mittelstädt, J.M.; Van den Bergh, O. Respiratory Changes in Response to Cognitive Load: A Systematic Review. Neural Plast. 2016, 2016, 8146809. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; Benson, S.; Sela-Venter, S.; Mackus, M.; Moss, M.C. Oxygen Administration and Acute Human Cognitive Enhancement: Higher Cognitive Demand Leads to a More Rapid Decay of Transient Hyperoxia. J. Cogn. Enhanc. 2020, 4, 94–99. [Google Scholar] [CrossRef]
- Hoshi, Y.; Tamura, M. Detection of Dynamic Changes in Cerebral Oxygenation Coupled to Neuronal Function during Mental Work in Man. Neurosci. Lett. 1993, 150, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Physiology and Pathophysiology of the Autonomic Nervous System. Continuum 2020, 26, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Bouak, F.; Vartanian, O.; Hofer, K.; Cheung, B. Acute Mild Hypoxic Hypoxia Effects on Cognitive and Simulated Aircraft Pilot Performance. Aerosp. Med. Hum. Perform. 2018, 89, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.; Kennedy, K.; Napoli, N.; Demas, M.; Barnes, L.; Crook, B.; Williams, R.; Last, M.C.; Schutte, P. Effects on Task Performance and Psychophysiological Measures of Performance during Normobaric Hypoxia Exposure. In Proceedings of the 19th International Symposium on Aviation Psychology, Dayton, OH, USA, 8–11 May 2017; p. 202. [Google Scholar]
- Shaw, D.M.; Cabre, G.; Gant, N. Hypoxic Hypoxia and Brain Function in Military Aviation: Basic Physiology and Applied Perspectives. Front. Physiol. 2021, 12, 665821. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.; Harrell, J.W. Integrating Physiological Monitoring Systems in Military Aviation: A Brief Narrative Review of Its Importance, Opportunities, and Risks. Ergonomics 2023, 66, 2242–2254. [Google Scholar] [CrossRef]
- Temme, L.A.; Wittels, H.L.; Wishon, M.J.; St. Onge, P.; McDonald, S.M.; Hecocks, D.; Wittels, S.H. Continuous Physiological Monitoring of the Combined Exposure to Hypoxia and High Cognitive Load in Military Personnel. Biology 2023, 12, 1398. [Google Scholar] [CrossRef]
- Backs, R.W.; Ryan, A.M. Multimodal Measures of Mental Workload during Dual-Task Performance: Energetic Demands of Cognitive Processes. Proc. Hum. Factors Soc. Annu. Meet. 1992, 36, 1413–1417. [Google Scholar] [CrossRef]
- Kubisiak, C.; Katz, L.U.S. Army Aviator Job Analysis; United States Army Research Institute for the Behavioral and Social Sciences: Alexandria, VA, USA, 2006; p. 91. [Google Scholar]
- Wright, S.; Rousse, D.; Van Atta, A.; Gaska, J.; Winterbottom, M.; Hadley, S.; Lamothe, D. Automated Vision Test Development and Validation; Special Report: #2016-6017; United States Air Force School of Aerospace Medicine, Aerospace Medicine Department/FECO: Wright Patterson AFB, OH, USA, 2016; Available online: https://apps.dtic.mil/sti/pdfs/AD1021902.pdf (accessed on 2 May 2024).
- Cegarra, J.; Valéry, B.; Avril, E.; Calmettes, C.; Navarro, J. OpenMATB: A Multi-Attribute Task Battery Promoting Task Customization, Software Extensibility and Experiment Replicability. Behav. Res. Methods 2020, 52, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Hilla, Y.; Stefani, M.; Nitzschner, M.; Mack, W. Multi-Attribute Task Battery Performance Predicts Military Multitasking Better than Multitasking Costs. Predict. Mil. Multitask. 2023; preprint. [Google Scholar] [CrossRef]
- Bourdillon, N.; Yazdani, S.; Vesin, J.-M.; Schmitt, L.; Millet, G.P. RMSSD Is More Sensitive to Artifacts Than Frequency-Domain Parameters: Implication in Athletes’ Monitoring. J. Sports Sci. Med. 2022, 21, 260–266. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart Rate Variability. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse Oximetry: Understanding Its Basic Principles Facilitates Appreciation of Its Limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Peck, J.; Wishon, M.; Wittels, H.; Hasty, F.; Hendricks, S.; Lee, S.; Wittels, S. COVID-19-Induced Changes in Photoplethysmography. Mil. Med. 2023, 188, e2661–e2669. [Google Scholar] [CrossRef]
- Peck, J.; Wishon, M.J.; Wittels, H.; Lee, S.J.; Hendricks, S.; Davila, H.; Wittels, S.H. Single Limb Electrocardiogram Using Vector Mapping: Evaluation and Validation of a Novel Medical Device. J. Electrocardiol. 2021, 67, 136–141. [Google Scholar] [CrossRef]
- Mizuno, K.; Tanaka, M.; Yamaguti, K.; Kajimoto, O.; Kuratsune, H.; Watanabe, Y. Mental Fatigue Caused by Prolonged Cognitive Load Associated with Sympathetic Hyperactivity. Behav. Brain Funct. 2011, 7, 17. [Google Scholar] [CrossRef]
- Soga, C.; Miyake, S.; Wada, C. Recovery Patterns in the Physiological Responses of the Autonomic Nervous System Induced by Mental Workload. In Proceedings of the SICE Annual Conference 2007, Takamatsu, Japan, 17–20 September 2007; pp. 1366–1371. [Google Scholar] [CrossRef]
- Bouak, F.; Vartanian, O.; Hofer, K. Performance and Health Effects of Mild Hypoxic Hypoxia in Simulated 6-Hour Exposures between 2,438 and 3,048 Metres. J. Mil. Veteran Fam. Health 2019, 5, 40–49. [Google Scholar] [CrossRef]
- Smith, A.M. Acute Hypoxia and Related Symptoms on Mild Exertion at Simulated Altitudes below 3048 m. Aviat. Space Environ. Med. 2007, 78, 979–984. [Google Scholar] [CrossRef]
- Cohen-Koren, R.; Garbi, D.; Gordon, S.; Yavnai, N.; Erlich Shoham, Y.; Shelef, L. Predictors of Emotional Distress in Combat Military Flight Engineers. Mil. Med. 2023, 188, e301–e310. [Google Scholar] [CrossRef]
- Joseph, C. An Overview of Psychological Factors and Interventions in Air Combat Operations. Indian J. Aerosp. Med. 2007, 51, 1–16. [Google Scholar]
- Potter, A.; Baker, M.; Sanders, C.; Peterson, A. Combat Stress Reactions During Military Deployments: Evaluation of the Effectiveness of Combat Stress Control Treatment. J. Ment. Health Couns. 2009, 31, 137–148. [Google Scholar] [CrossRef]
- Sampson, J.B. Anxiety As a Factor in the Incidence of Combat Cold Injury: A Review. Mil. Med. 1984, 149, 89–91. [Google Scholar] [CrossRef]
- Ernst, G. Hidden Signals-The History and Methods of Heart Rate Variability. Front Public Health 2017, 5, 265. [Google Scholar] [CrossRef]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L. The Mechanism of Cardiac Sympathetic Activity Assessment Methods: Current Knowledge. Front. Cardiovasc. Med. 2022, 9, 931219. [Google Scholar] [CrossRef]
- McDougall, S.J.; Widdop, R.E.; Lawrence, A.J. Central Autonomic Integration of Psychological Stressors: Focus on Cardiovascular Modulation. Auton. Neurosci. 2005, 123, 1–11. [Google Scholar] [CrossRef]
- Delliaux, S.; Delaforge, A.; Deharo, J.-C.; Chaumet, G. Mental Workload Alters Heart Rate Variability, Lowering Non-Linear Dynamics. Front. Physiol. 2019, 10, 565. [Google Scholar] [CrossRef]
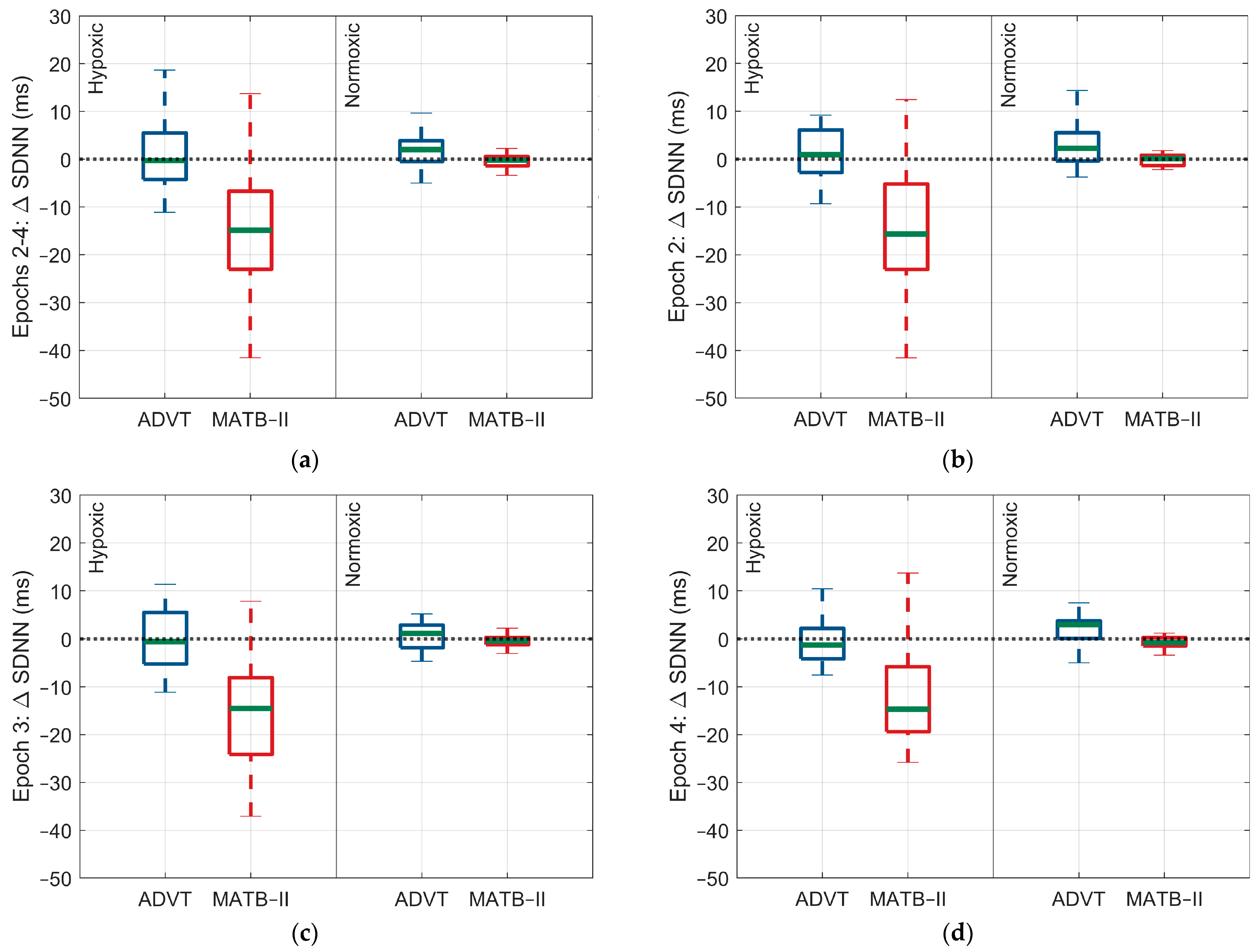


| Hypoxic | Normoxic | |||
|---|---|---|---|---|
| MATB-II | ADVT | MATB-II | ADVT | |
| Mean Change (SD) | Mean Change (SD) | Mean Change (SD) | Mean Change (SD) | |
| Epoch 2 | ||||
| Heart rate (bpm) | 1.8 (5.7) | −2.7 (5.0) | 2.6 (3.96) | −3.3 (3.8) |
| rMSSD (ms) | −0.4 (2.4) | 1.7 (4.9) | 0.3 (0.2) | 0.99 (5.2) |
| SDNN (ms) | −13.8 (15.7) | 0.97 (5.6) | 0.1 (1.6) | 2.7 (5.7) |
| SpO2 (%) | −0.2 (0.1) | 0.14 (0.01) | −0.2 (0.1) | 0.02 (0.01) |
| Epoch 3 | ||||
| Heart rate (bpm) | 1.04 (8.7) | −2.0 (7.04) | −0.6 (8.8) | −1.9 (2.7) |
| rMSSD (ms) | −0.9 (3.8) | 3.01 (6.7) | 0.3 (4.2) | 0.2 (2.9) |
| SDNN (ms) | −13.6 (15.1) | 0.9 (6.7) | −0.30 (1.5) | 0.7 (2.8) |
| SpO2 (%) | 0.2 (0.03) | 0.8 (0.2) | 0.2 (0.03) | 0.04 (0.02) |
| Epoch 4 | ||||
| Heart rate (bpm) | 4.4 (5.6) | −3.2 (7.0) | 3.3 (5.2) | −2.3 (5.2) |
| rMSSD (ms) | 0.03 (1.5) | 0.5 (6.5) | −1.5 (2.3) | 1.99 (4.2) |
| SDNN (ms) | −13.4 (14.1) | 0.3 (6.7) | −0.7 (1.2) | 1.8 (3.7) |
| SpO2 (%) | 0.2 (0.00) | 0.2 (0.02) | 0.2 (0.00) | 0.2 (0.1) |
| Epochs (2–4) | ||||
| Heart rate (bpm) | 2.4 (6.9) | −2.6 (6.3) | 1.8 (6.4) | −2.51 (3.9) |
| rMSSD (ms) | −0.4 (2.7) | 1.8 (6.04) | −0.3 (2.8) | 1.0 (4.2) |
| SDNN (ms) | −13.6 (14.7) | 0.7 (6.3) | −0.3 (1.4) | 1.7 (4.3) |
| SpO2 (%) | 0.1 (0.3) | 0.4 (0.3) | 0.1 (0.2) | 0.1 (0.10) |
| Hypoxia | Normoxia | |||
|---|---|---|---|---|
| MATB-II vs. ADVT | MATB-II vs. ADVT | |||
| Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | |
| Epoch 2 | ||||
| Heart rate (bpm) | 4.5 (−2.8, 11.8) | 0.74 | 5.9 (−1.9, 13.6) | 0.40 |
| rMSSD (ms) | −2.1 (−7.1, 2.9) | 0.99 | −0.7 (−6.7, 4.5) | 0.99 |
| SDNN (ms) | −14.7 (−24.4, −5.0) | <0.0001 | −2.6 (−12.6, 7.3) | 0.99 |
| SpO2 (%) | −0.5 (−1.2, 3.0) | 0.82 | −0.2 (−0.9, 0.6) | 0.99 |
| Epoch 3 | ||||
| Heart rate (bpm) | 3.0 (−4.3, 10.3) | 0.99 | 1.3 (−6.6, 1.9) | 0.99 |
| rMSSD (ms) | −3.9 (−8.9, 1.1) | 0.34 | 0.2 (−5.2, 5.6) | 0.99 |
| SDNN (ms) | −14.4 (−24.2, −4.6) | <0.0001 | −0.9 (−11.0, 9.2) | 0.99 |
| SpO2 (%) | −0.4 (−1.2, 0.4) | 0.92 | −0.1 (−0.6, 0.9) | 0.99 |
| Epoch 4 | ||||
| Heart rate (bpm) | 7.6 (0.1, 15.1) | 0.04 | 5.6 (−2.4, 13.6) | 0.55 |
| rMSSD (ms) | −0.5 (−5.7, 4.7) | 0.99 | 3.5 (−1.9, 8.9) | 0.69 |
| SDNN (ms) | −13.6 (−23.6, −3.7) | 0.0003 | 2.6 (−7.6, 12.8) | 0.99 |
| SpO2 (%) | −0.02 (−0.8, 0.8) | 0.99 | −0.02 (−0.8, 0.8) | 0.99 |
| Epoch 2–4 | ||||
| Heart rate (bpm) | 5.0 (2.1, 7.9) | <0.0001 | 4.3 (1.2, 7.4) | 0.002 |
| rMSSD (ms) | −2.2 (−4.2, −0.2) | 0.03 | −1.3 (−3.5, 0.8) | 0.39 |
| SDNN (ms) | −14.3 (−18.4, −10.1) | <0.0001 | −2.0 (−6.3, 2.2) | 0.61 |
| SpO2 (%) | −0.3 (−0.4, −0.2) | <0.0001 | −0.01 (−0.11, 0.01) | 0.99 |
| MATB-II | ADVT | |||
|---|---|---|---|---|
| Hypoxia vs. Normoxia | Hypoxia vs. Normoxia | |||
| Mean (95% CI) | p-Value | Mean (95% CI) | p-Value | |
| Epoch 2 | ||||
| Heart rate (bpm) | −0.8 (−8.3, 6.8) | 0.99 | 0.6 (−6.9, 8.1) | 0.99 |
| rMSSD (ms) | −0.6 (−5.8, 4.6) | 0.99 | 0.7 (−4.4, 5.9) | 0.99 |
| SDNN (ms) | −13.8 (−23.8, 3.9) | 0.0002 | −1.7 (−11.4, 7.9) | 0.99 |
| SpO2 (%) | −0.2 (−0.9, 0.6) | 0.99 | 0.1 (−0.7, 0.9) | 0.99 |
| Epoch 3 | ||||
| Heart rate (bpm) | 1.7 (−5.9, 9.3) | 0.99 | −0.1 (−7.6, 7.4) | 0.99 |
| rMSSD (ms) | −1.3 (−6.5, 3.9) | 0.99 | 2.9 (−2.3, 8.0) | 0.88 |
| SDNN (ms) | −13.3 (−23.5, −3.0) | 0.001 | 0.2 (−9.5, 9.9) | 0.99 |
| SpO2 (%) | 0.2 (−0.6, 0.9) | 0.99 | 0.7 (−0.03, 1.5) | 0.08 |
| Epoch 4 | ||||
| Heart rate (bpm) | 1.1 (−6.6, 8.9) | 0.99 | −0.9 (−8.6, 6.9) | 0.99 |
| rMSSD (ms) | 1.6 (−3.8, 6.9) | 0.99 | −1.5 (−6.8, 3.9) | 0.99 |
| SDNN (ms) | −12.6 (−22.9, −2.4) | 0.003 | −1.5 (−11.5, 8.4) | 0.99 |
| SpO2 (%) | −0.04 (−0.8, 0.7) | 0.99 | 0.03 (−0.8, 0.7) | 0.99 |
| Epoch 2–4 | ||||
| Heart rate (bpm) | 0.6 (−2.4, 3.7) | 0.95 | −0.1 (−3.1, 2.9) | 0.99 |
| rMSSD (ms) | −0.1 (−2.2, 1.9) | 0.99 | 0.7 (−1.3, 2.8) | 0.79 |
| SDNN (ms) | −13.3 (−17.5, −8.9) | <0.0001 | −1.0 (−5.1, 3.1) | 0.92 |
| SpO2 (%) | −0.01 (−0.1, 0.1) | 0.99 | 0.3 (0.2, 0.4) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittels, H.L.; Wittels, S.H.; Wishon, M.J.; Vogl, J.; St. Onge, P.; McDonald, S.M.; Temme, L.A. Examining the Influence of Cognitive Load and Environmental Conditions on Autonomic Nervous System Response in Military Aircrew: A Hypoxia–Normoxia Study. Biology 2024, 13, 343. https://doi.org/10.3390/biology13050343
Wittels HL, Wittels SH, Wishon MJ, Vogl J, St. Onge P, McDonald SM, Temme LA. Examining the Influence of Cognitive Load and Environmental Conditions on Autonomic Nervous System Response in Military Aircrew: A Hypoxia–Normoxia Study. Biology. 2024; 13(5):343. https://doi.org/10.3390/biology13050343
Chicago/Turabian StyleWittels, Harrison L., S. Howard Wittels, Michael J. Wishon, Jonathan Vogl, Paul St. Onge, Samantha M. McDonald, and Leonard A. Temme. 2024. "Examining the Influence of Cognitive Load and Environmental Conditions on Autonomic Nervous System Response in Military Aircrew: A Hypoxia–Normoxia Study" Biology 13, no. 5: 343. https://doi.org/10.3390/biology13050343
APA StyleWittels, H. L., Wittels, S. H., Wishon, M. J., Vogl, J., St. Onge, P., McDonald, S. M., & Temme, L. A. (2024). Examining the Influence of Cognitive Load and Environmental Conditions on Autonomic Nervous System Response in Military Aircrew: A Hypoxia–Normoxia Study. Biology, 13(5), 343. https://doi.org/10.3390/biology13050343





