Dietary Tryptophan Plays a Role as an Anti-Inflammatory Agent in European Seabass (Dicentrarchus labrax) Juveniles during Chronic Inflammation
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Dietary Treatments
2.2. Fish and Experimental Design
2.3. Haematological Profile
2.4. Differential Peritoneal Leucocyte Counts
2.5. Humoral Parameters
2.6. Gut Oxidative Stress
Tissue Samples Homogenisation and Protein Quantification
2.7. Gut Immune Parameters
2.8. Head Kindey Gene Expression
2.9. Statistical Analysis
3. Results
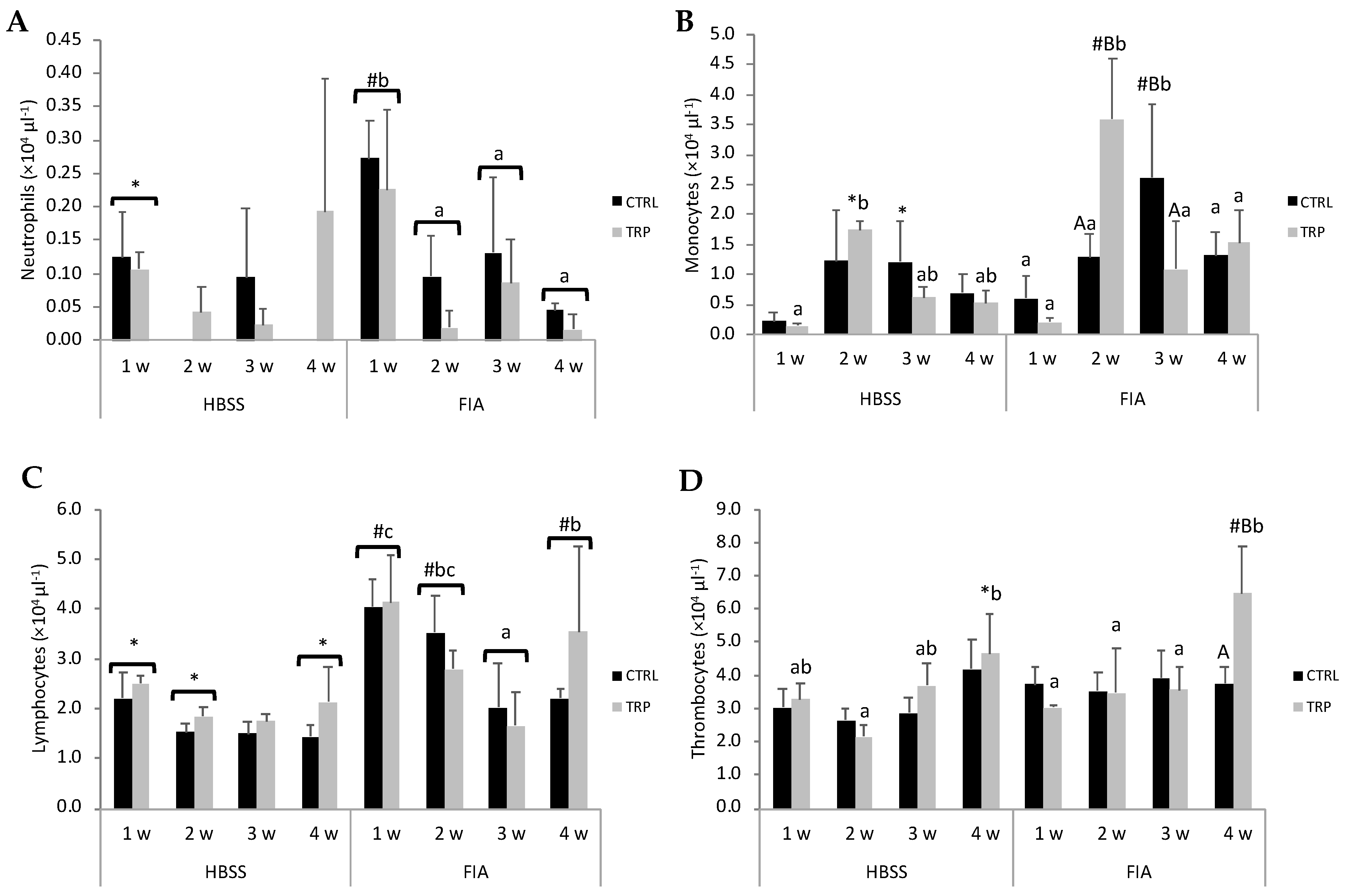
3.1. Haematological Profile
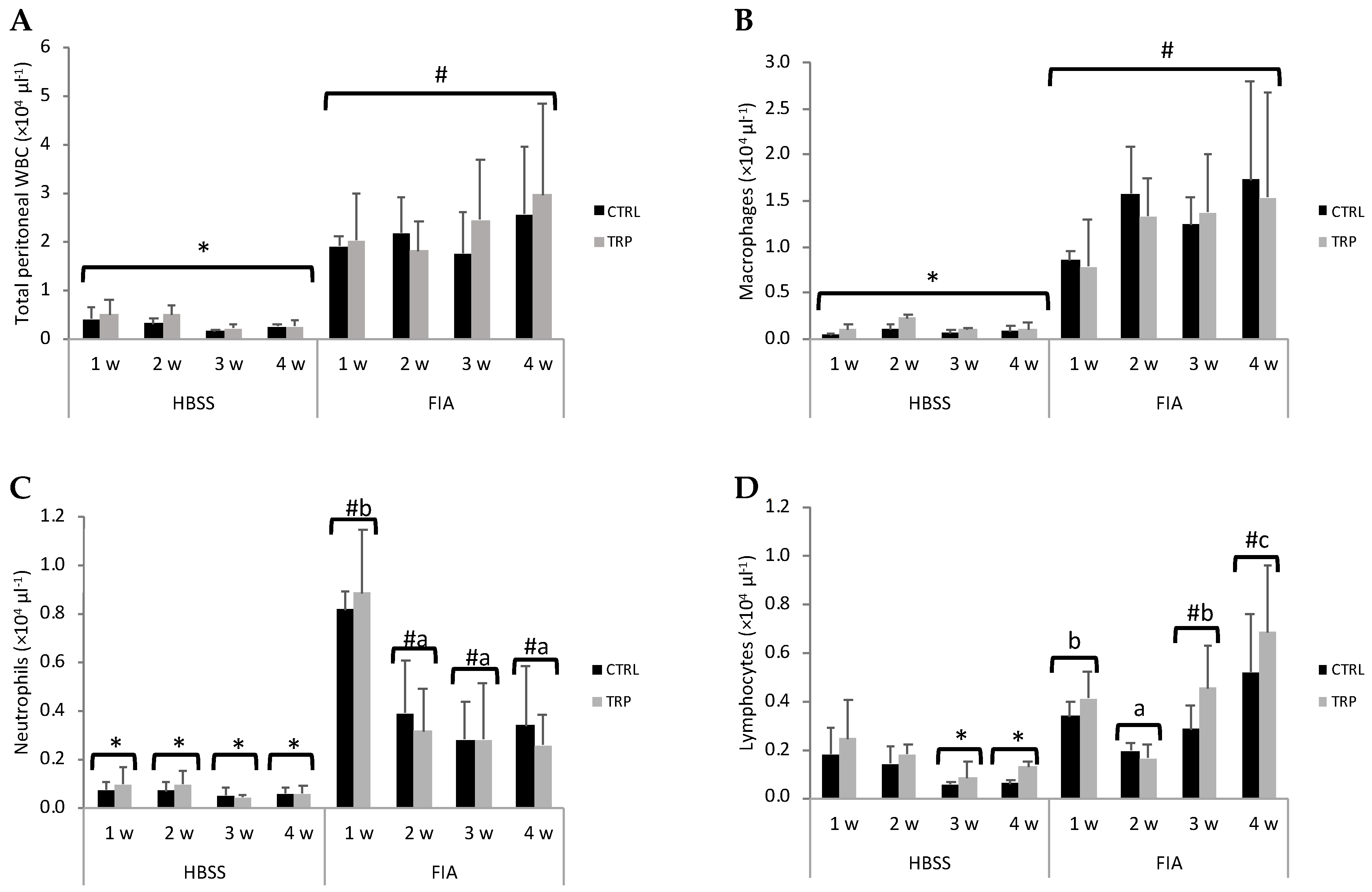
3.2. Peritoneal Leucocytes
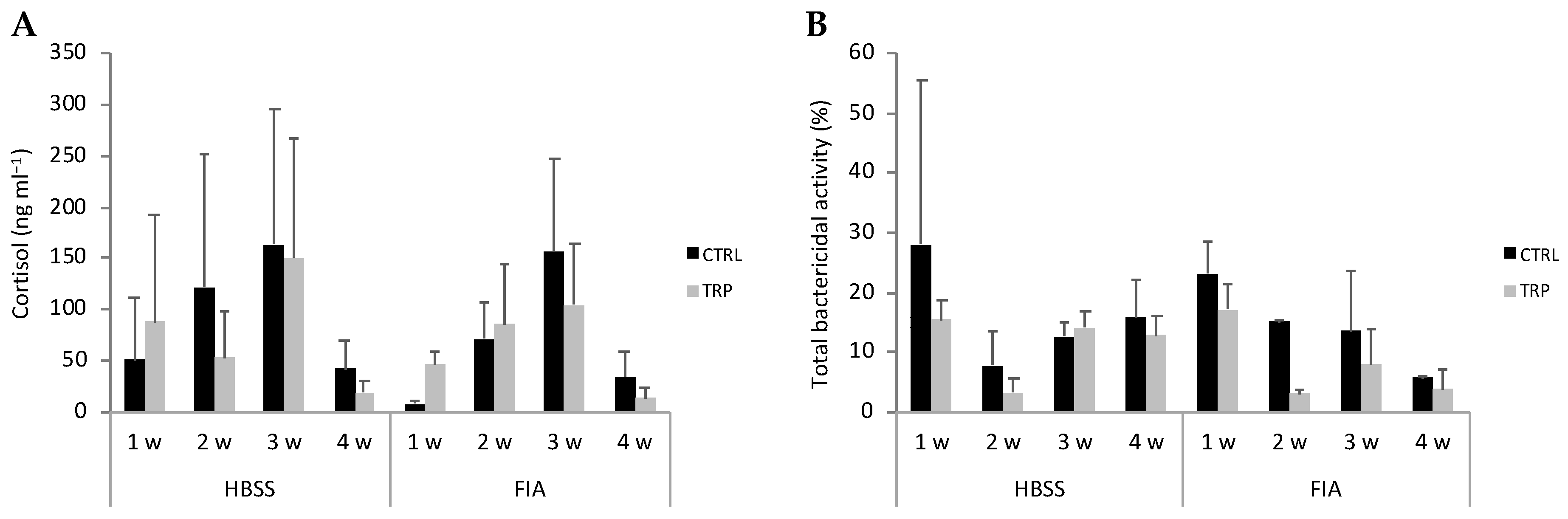
3.3. Plasma Cortisol and Immune Parameters
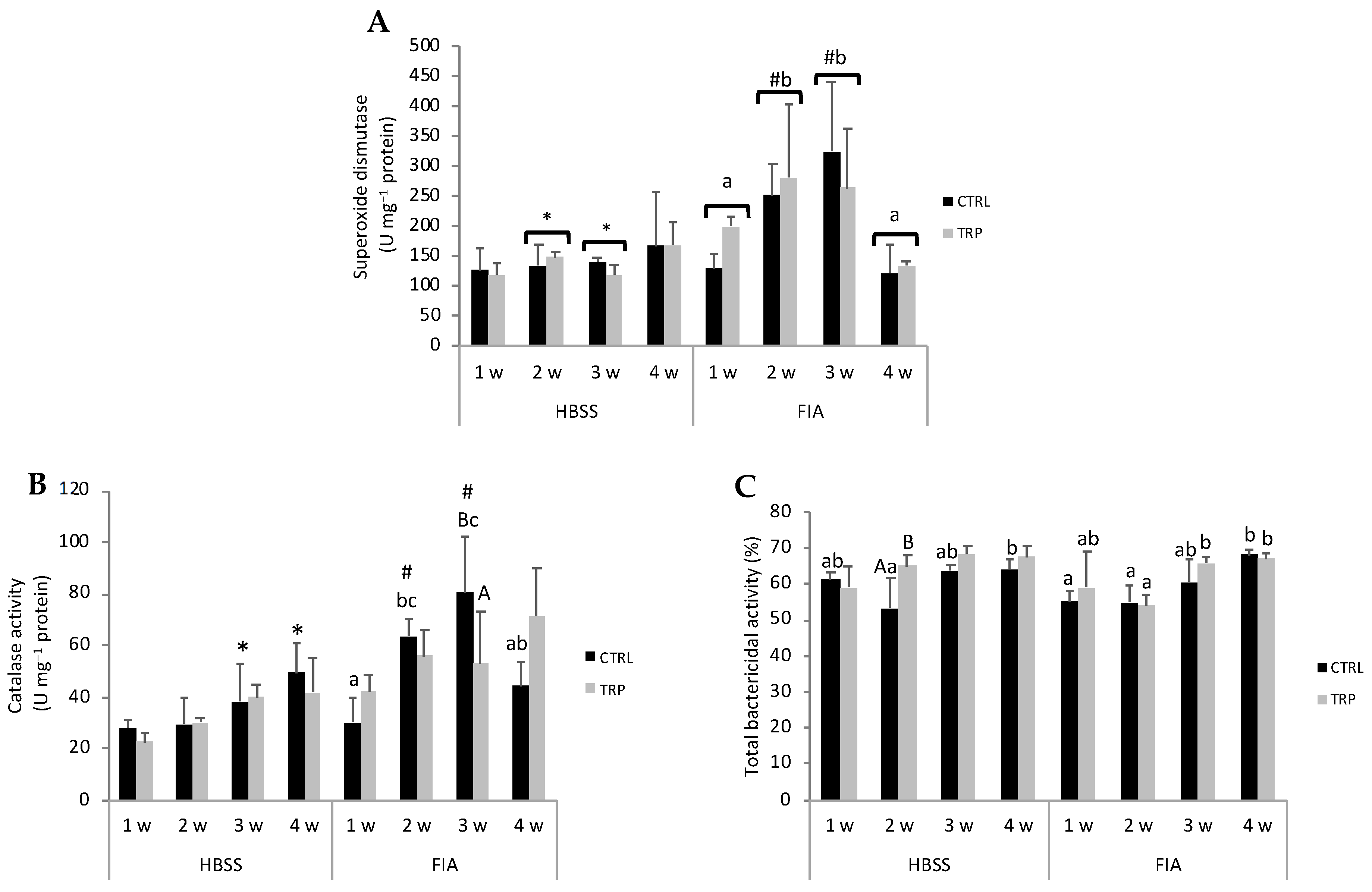
3.4. Gut Oxidative Stress and Immune Parameters
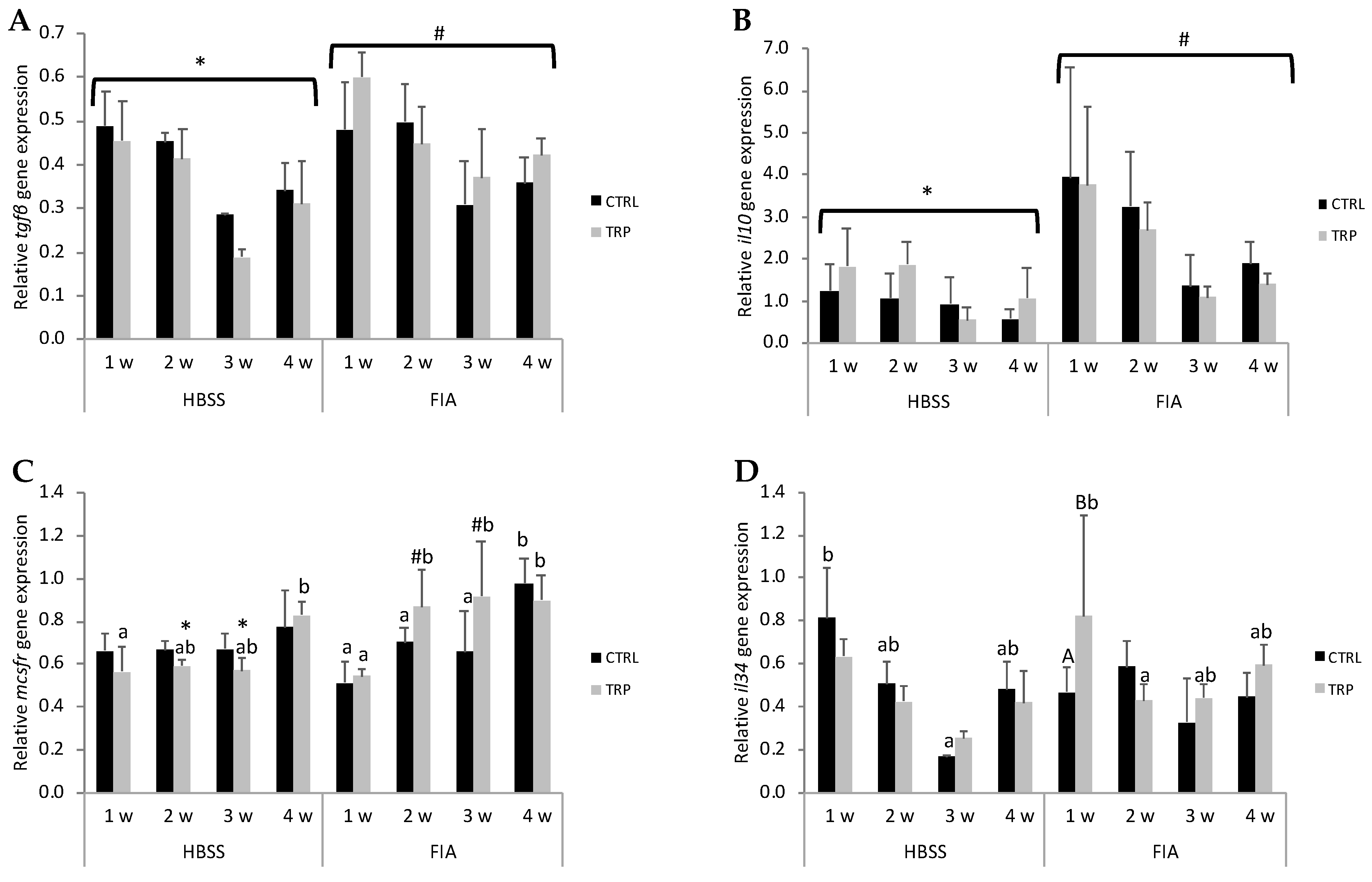
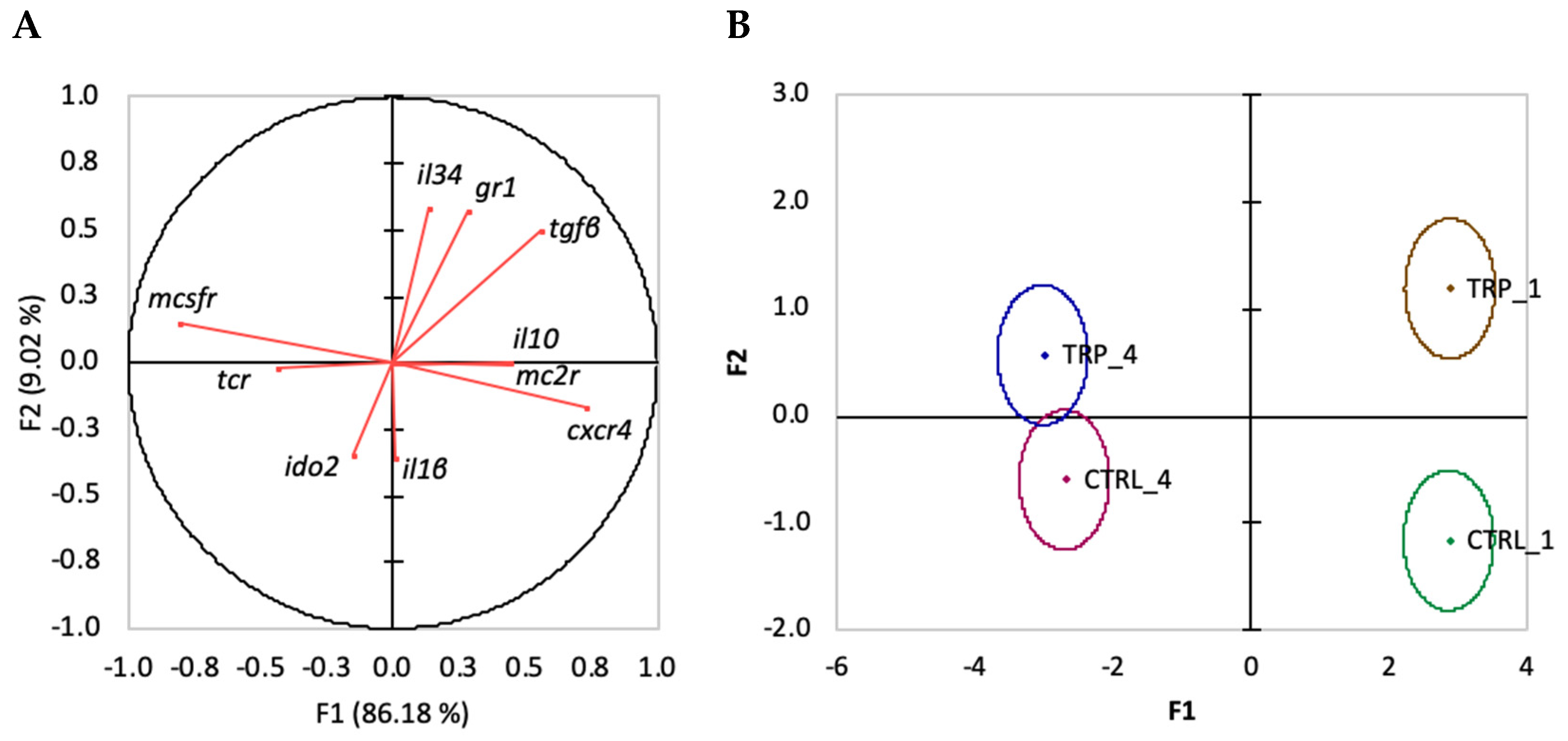
3.5. Gene Expression
3.5.1. Inflammation-Induced Changes
3.5.2. Dietary Treatment-Induced Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barreto, M.O.; Planellas, S.R.; Yang, Y.F.; Phillips, C.; Descovich, K. Emerging indicators of fish welfare in aquaculture. Rev. Aquac. 2022, 14, 343–361. [Google Scholar] [CrossRef]
- Bergqvist, J.; Gunnarsson, S. Finfish Aquaculture: Animal Welfare, the Environment, and Ethical Implications. J. Agric. Environ. Ethic 2013, 26, 75–99. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef]
- Costas, B.; Aragao, C.; Mancera, J.M.; Dinis, M.T.; Conceicao, L.E.C. High stocking density induces crowding stress and affects amino acid metabolism in Senegalese sole Solea senegalensis (Kaup 1858) juveniles. Aquac. Res. 2008, 39, 1–9. [Google Scholar] [CrossRef]
- Aragao, C.; Corte-Real, J.; Costas, B.; Dinis, M.T.; Conceicao, L.E.C. Stress response and changes in amino acid requirements in Senegalese sole (Solea senegalensis Kaup 1858). Amino Acids 2008, 34, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.M.; Waagbo, R.; Espe, M. Functional amino acids in fish nutrition, health and welfare. Front. Biosci. (Elite Ed.) 2016, 8, 143–169. [Google Scholar] [CrossRef]
- Azeredo, R.; Serra, C.R.; Oliva-Teles, A.; Costas, B. Amino acids as modulators of the European seabass, Dicentrarchus labrax, innate immune response: An in vitro approach. Sci. Rep. 2017, 7, 18009. [Google Scholar] [CrossRef]
- Machado, M.; Azeredo, R.; Domingues, A.; Fernandez-Boo, S.; Dias, J.; Conceicao, L.E.C.; Costas, B. Dietary tryptophan deficiency and its supplementation compromises inflammatory mechanisms and disease resistance in a teleost fish. Sci. Rep. 2019, 9, 7689. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Azeredo, R.; Fontinha, F.; Fernandez-Boo, S.; Conceicao, L.E.C.; Dias, J.; Costas, B. Dietary methionine improves the European seabass (Dicentrarchus labrax) immune status, inflammatory response, and disease resistance. Front. Immunol. 2018, 9, 2672. [Google Scholar] [CrossRef]
- Ramos-Pinto, L.; Martos-Sitcha, J.A.; Reis, B.; Azeredo, R.; Fernandez-Boo, S.; Perez-Sanchez, J.; Calduch-Giner, J.A.; Engrola, S.; Conceicao, L.E.C.; Dias, J.; et al. Dietary tryptophan supplementation induces a transient immune enhancement of gilthead seabream (Sparus aurata) juveniles fed fishmeal-free diets. Fish Shellfish Immunol. 2019, 93, 240–250. [Google Scholar] [CrossRef]
- Cortes, J.; Alvarez, C.; Santana, P.; Torres, E.; Mercado, L. Indoleamine 2,3-dioxygenase: First evidence of expression in rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2016, 65, 73–78. [Google Scholar] [CrossRef]
- Cui, Z.W.; Zhang, X.Y.; Zhang, X.J.; Wu, N.; Lu, L.F.; Li, S.; Chen, D.D.; Zhang, Y.A. Molecular and functional characterization of the indoleamine 2,3-dioxygenase in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2019, 89, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Tsogka, K.; Sounidaki, M.; Liakopoulos, V.; Stefanidis, I. Indoleamine 2,3-dioxygenase downregulates T-cell receptor complex ζ-chain and c-Myc, and reduces proliferation, lactate dehydrogenase levels and mitochondrial glutaminase in human T-cells. Mol. Med. Rep. 2016, 13, 925–932. [Google Scholar] [CrossRef][Green Version]
- Yuasa, H.J.; Mizuno, K.; Ball, H.J. Low efficiency IDO2 enzymes are conserved in lower vertebrates, whereas higher efficiency IDO1 enzymes are dispensable. Febs J. 2015, 282, 2735–2745. [Google Scholar] [CrossRef]
- Grohmann, U.; Bronte, V. Control of immune response by amino acid metabolism. Immunol. Rev. 2010, 236, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, R.; Machado, M.; Martos-Sitcha, J.A.; Martinez-Rodriguez, G.; Moura, J.; Peres, H.; Oliva-Teles, A.; Afonso, A.; Mancera, J.M.; Costas, B. Dietary Tryptophan Induces Opposite Health-Related Responses in the Senegalese Sole (Solea senegalensis) Reared at Low or High Stocking Densities With Implications in Disease Resistance. Front. Physiol. 2019, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- Lepage, O.; Tottmar, O.; Winberg, S. Elevated dietary intake of L-tryptophan counteracts the stress-induced elevation of plasma cortisol in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2002, 205, 3679–3687. [Google Scholar] [CrossRef]
- Lim, J.E.; Porteus, C.S.; Bernier, N.J. Serotonin directly stimulates cortisol secretion from the interrenals in goldfish. Gen. Comp. Endocrinol. 2013, 192, 246–255. [Google Scholar] [CrossRef]
- Backstrom, T.; Winberg, S. Serotonin Coordinates Responses to Social Stress-What We Can Learn from Fish. Front. Neurosci. 2017, 11, 294719. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Caballero, M.J.; Mompel, D.; Montero, D.; Zamorano, M.J.; Robaina, L.; Rivero-Ramirez, F.; Karalazos, V.; Kaushik, S.; Izquierdo, M. Disease resistance and response against Vibrio anguillarum intestinal infection in European seabass (Dicentrarchus labrax) fed low fish meal and fish oil diets. Fish Shellfish Immunol. 2017, 67, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, M.J.; Ferraz, R.; Magnoni, L.J.; Pereira, R.; Goncalves, J.F.; Calduch-Giner, J.; Perez-Sanchez, J.; Ozorio, R.O.A. Protective effects of seaweed supplemented diet on antioxidant and immune responses in European seabass (Dicentrarchus labrax) subjected to bacterial infection. Sci. Rep. 2019, 9, 16134. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Machado, M.; Motac, C.S.C.; Abreu, H.; Silva, J.; Maia, M.R.G.; Kiron, V.; Costas, B.; Valente, L.M.P. Micro- and macroalgae blend modulates the mucosal and systemic immune responses of European seabass upon infection with Tenacibaculum maritimum. Aquaculture 2023, 566, 739222. [Google Scholar] [CrossRef]
- Machado, M.; Peixoto, D.; Santos, P.; Ricardo, A.; Duarte, I.; Carvalho, I.; Aragao, C.; Azeredo, R.; Costas, B. Tryptophan Modulatory Role in European Seabass (Dicentrarchus labrax) Immune Response to Acute Inflammation under Stressful Conditions. Int. J. Mol. Sci. 2022, 23, 12475. [Google Scholar] [CrossRef] [PubMed]
- Tafalla, C.; Bogwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.; Ellis, A.E.; Silva, M.T. The leucocyte population of the unstimulated peritoneal cavity of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 1997, 7, 335–348. [Google Scholar] [CrossRef]
- Machado, M.; Azeredo, R.; Diaz-Rosales, P.; Afonso, A.; Peres, H.; Oliva-Teles, A.; Costas, B. Dietary tryptophan and methionine as modulators of European seabass (Dicentrarchus labrax) immune status and inflammatory response. Fish Shellfish Immunol. 2015, 42, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.; Lousada, S.; Silva, J.; Ellis, A.E.; Silva, M.T. Neutrophil and macrophage responses to inflammation in the peritoneal cavity of rainbow trout Oncorhynchus mykiss. A light and electron microscopic cytochemical study. Dis. Aquat. Organ. 1998, 34, 27–37. [Google Scholar] [CrossRef]
- Costas, B.; Conceicao, L.E.C.; Dias, J.; Novoa, B.; Figueras, A.; Afonso, A. Dietary arginine and repeated handling increase disease resistance and modulate innate immune mechanisms of Senegalese sole (Solea senegalensis Kaup, 1858). Fish Shellfish Immunol. 2011, 31, 838–847. [Google Scholar] [CrossRef]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunop. 1997, 58, 239–248. [Google Scholar] [CrossRef]
- Graham, S.; Jeffries, A.H.; Secombes, C.J. A novel assay to detect macrophage bactericidal activity in fish: Factors influencing the killing of Aeromonas salmonicida. J. Fish. Dis. 1988, 11, 389–396. [Google Scholar] [CrossRef]
- Azeredo, R.; Machado, M.; Afonso, A.; Fierro-Castro, C.; Reyes-López, F.E.; Tort, L.; Gesto, M.; Conde-Sieira, M.; Míguez, J.M.; Soengas, J.L.; et al. Neuroendocrine and immune responses undertake different fates following tryptophan or methionine dietary treatment: Tales from a teleost model. Front. Immunol. 2017, 8, 1226. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, D.; Pinto, W.; Goncalves, A.T.; Machado, M.; Reis, B.; Silva, J.; Navalho, J.; Dias, J.; Conceicao, L.; Costas, B. Microalgal biomasses have potential as ingredients in microdiets for Senegalese sole (Solea senegalensis) post-larvae. J. Appl. Phycol. 2021, 33, 2241–2250. [Google Scholar] [CrossRef]
- Costas, B.; Couto, A.; Azeredo, R.; Machado, M.; Krogdahl, Å.; Oliva-Teles, A. Gilthead seabream (Sparus aurata) immune responses are modulated after feeding with purified antinutrients. Fish Shellfish Immunol. 2014, 41, 70–79. [Google Scholar] [CrossRef]
- Machado, M.; Arenas, F.; Svendsen, J.C.; Azeredo, R.; Pfeifer, L.J.; Wilson, J.M.; Costas, B. Effects of Water Acidification on Senegalese Sole Solea senegalensis Health Status and Metabolic Rate: Implications for Immune Responses and Energy Use. Front. Physiol. 2020, 11, 497078. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Oliveira, C.; Gravato, C.; Guilhermino, L. Linking behavioural alterations with biomarkers responses in the European seabass Dicentrarchus labrax L. exposed to the organophosphate pesticide fenitrothion. Ecotoxicology 2010, 19, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.; Moreira, S.M.; Rendon-Von Osten, J.; Soares, A.M.; Guilhermino, L. Biochemical responses of the marine mussel Mytilus galloprovincialis to petrochemical environmental contamination along the North-western coast of Portugal. Chemosphere 2007, 66, 1230–1242. [Google Scholar] [CrossRef]
- Hamre, K.; Penglase, S.J.; Rasinger, J.D.; Skjaerven, K.H.; Olsvik, P.A. Ontogeny of redox regulation in Atlantic cod (Gadus morhua) larvae. Free Radic. Biol. Med. 2014, 73, 337–348. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic Method for Quantitative Determination of Nanogram Amounts of Total and Oxidized Glutathione-Applications to Mammalian Blood and Other Tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase Activity. In CRC Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; p. 467. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M.; Gravato, C.; Quintaneiro, C.; Bordalo, M.D.; Barata, C.; Soares, A.M.; Pestana, J.L.T. Energetic costs and biochemical biomarkers associated with esfenvalerate exposure in Sericostoma vittatum. Chemosphere 2017, 189, 445–453. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Vela-Patiño, S.; Salazar, M.I.; Remba-Shapiro, I.; Peña-Martínez, E.; Silva-Roman, G.; Andoneui-Elguera, S.; Ordoñez-Garcia, J.D.; Taniguchi-Ponciano, K.; Bonifaz, L.; Aguilar-Flores, C.; et al. Neuroendocrine-immune Interface: Interactions of Two Complex Systems in Health and Disease. Arch. Med. Res. 2022, 53, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Verburg-van Kemenade, B.M.L.; Cohen, N.; Chadzinska, M. Neuroendocrine-immune interaction: Evolutionarily conserved mechanisms that maintain allostasis in an ever-changing environment. Dev. Comp. Immunol. 2017, 66, 2–23. [Google Scholar] [CrossRef]
- Bury, N.R.; Sturm, A. Evolution of the corticosteroid receptor signalling pathway in fish. Gen. Comp. Endocr. 2007, 153, 47–56. [Google Scholar] [CrossRef]
- Aluru, N.; Vijayan, M.M. Stress transcriptomics in fish: A role for genomic cortisol signaling. Gen. Comp. Endocr. 2009, 164, 142–150. [Google Scholar] [CrossRef]
- Sakai, M.; Hikima, J.-i.; Kono, T. Fish cytokines: Current research and applications. Fish. Sci. 2021, 87, 1–9. [Google Scholar] [CrossRef]
- Peixoto, D.; Machado, M.; Azeredo, R.; Costas, B. Chronic Inflammation Modulates Opioid Receptor Gene Expression and Triggers Respiratory Burst in a Teleost Model. Biology 2022, 11, 764. [Google Scholar] [CrossRef]
- Bayne, C.J.; Gerwick, L. The acute phase response and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 725–743. [Google Scholar] [CrossRef]
- Zhou, L.; Wei, J.-F.; Lin, K.-T.; Gan, L.; Wang, J.-J.; Sun, J.-J.; Xu, X.-P.; Liu, L.; Huang, X.-D. Intestinal microbial profiling of grass carp (Ctenopharyngodon idellus) challenged with Aeromonas hydrophila. Aquaculture 2020, 524, 735292. [Google Scholar] [CrossRef]
- Markandey, M.; Bajaj, A.; Ilott, N.E.; Kedia, S.; Travis, S.; Powrie, F.; Ahuja, V. Gut microbiota: Sculptors of the intestinal stem cell niche in health and inflammatory bowel disease. Gut Microbes 2021, 13, 1990827. [Google Scholar] [CrossRef] [PubMed]
- Maclean, J.A.; Schoenwaelder, S.M. Chapter 5—Serotonin in Platelets. In Serotonin; Pilowsky, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 91–119. [Google Scholar] [CrossRef]
- Caamano-Tubio, R.I.; Perez, J.; Ferreiro, S.; Aldegunde, M. Peripheral serotonin dynamics in the rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2007, 145, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Boulakirba, S.; Pfeifer, A.; Mhaidly, R.; Obba, S.; Goulard, M.; Schmitt, T.; Chaintreuil, P.; Calleja, A.; Furstoss, N.; Orange, F.; et al. IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci. Rep. 2018, 8, 256. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (%) | CTRL | TRP |
|---|---|---|
| Fish protein hydrolysate 1 | 5.00 | 5.00 |
| Fish gelatin 2 | 2.00 | 2.00 |
| Soy protein concentrate 3 | 25.00 | 25.00 |
| Pea protein concentrate 4 | 6.00 | 6.00 |
| Wheat gluten 5 | 10.00 | 10.00 |
| Corn gluten meal 6 | 15.00 | 15.00 |
| Wheat meal 7 | 15.80 | 15.50 |
| Vit and Min Premix 8 | 1.00 | 1.00 |
| Antioxidant 9 | 0.20 | 0.20 |
| Sodium propionate 10 | 0.10 | 0.10 |
| Monocalcium phosphate 11 | 3.00 | 3.00 |
| L-Lysine 99% 12 | 0.60 | 0.60 |
| L-Tryptophan 13 | 0.00 | 0.30 |
| DL-Methionine 14 | 0.20 | 0.20 |
| Soy lecithin 15 | 1.00 | 1.00 |
| Fish oil 16 | 15.10 | 15.10 |
| Total | 100.00 | 100.00 |
| Proximate analyses (% dry weight) | ||
| Crude protein (%) | 45.70 | 46.00 |
| Crude fat (%) | 18.00 | 18.00 |
| Fiber (%) | 1.70 | 1.70 |
| Starch (%) | 13.40 | 13.20 |
| Ash (%) | 6.80 | 6.80 |
| Energy (MJ kg−1) | 21.90 | 21.90 |
| Amino Acids (% Dry Weight) | CTRL | TRP |
|---|---|---|
| Arginine | 4.0 | 4.1 |
| Histidine | 1.2 | 1.2 |
| Lysine | 3.0 | 3.0 |
| Threonine | 1.8 | 1.9 |
| Isoleucine | 2.1 | 2.1 |
| Leucine | 4.2 | 4.3 |
| Valine | 2.3 | 2.3 |
| Tryptophan | 0.2 | 0.4 |
| Methionine | 1.1 | 1.2 |
| Phenylalanine | 3.0 | 3.2 |
| Cysteine | 0.4 | 0.4 |
| Tyrosine | 2.6 | 2.8 |
| Aspartic acid | 3.4 | 3.2 |
| Glutamic acid | 9.7 | 9.1 |
| Alanine | 2.4 | 2.5 |
| Glycine | 2.7 | 2.7 |
| Proline | 3.7 | 3.6 |
| Serine | 2.6 | 2.6 |
| Acronym | Gene | GenBank | Eff 2 | AT 3 | Product Length 4 | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|---|---|---|---|
| 40s | 40s Ribosomal protein | HE978789.1 | 105.7 | 60 | 79 | TGATTGTGACAGACCCTCGTG | CACAGAGCAATGGTGGGGAT |
| ef1α | Elongation factor 1 β | AJ866727.1 | 97.2 | 57 | 144 | AACTTCAACGCCCAGGTCAT | CTTCTTGCCAGAACGACGGT |
| gr1 | Glucocorticoid receptor 1 | AY619996.1 | 104.1 | 60 | 100 | AAATCTGCCTGGTGTGTTCC | TGCCCTTTCACTGCTCTCTT |
| mc2r | Melanocortin 2 receptor | DLAgn_00065140 1 | 101 | 60 | 118 | GAGGGCAAGGGGAGCATTTA | GACGGGCAGATGGCAGTTAT |
| tcrα | T-cell receptor α chain | DLAgn_00260540 1 | 105.3 | 60 | 72 | ACACTGGCTGAGAAACATCCT | GGCTGGGTCACTCTGTCTTC |
| ido2 | Indoleamine 2,3-dioxygenase | DLAgn_00014730 1 | 108.1 | 60 | 74 | TGAAGGTGTGAGCAATGAGC | CAAAGCACTGAATGGCTGAA |
| il1β | Interleukin 1 β | AJ269472.1 | 93.2 | 57 | 105 | AGCGACATGGTGCGATTTCT | CTCCTCTGCTGTGCTGATGT |
| mcsfr | Macrophage colony-stimulating factor receptor | DLAgn_00109630 1 | 83.0 | 62 | 200 | ATGTCCCAACCAGACTTTGC | GGCTCATCACACACTTCACC |
| cxcr4 | Chemokine CXC receptor 4 | FN687464.1 | 91.7 | 57 | 171 | ACCAGACCTTGTGTTTGCCA | ATGAAGCCCACCAGGATGTG |
| il34 | Interleukin 34 | DLAgn_00164750 1 | 105.3 | 60 | 129 | GGAAATACGCTTCAGGGATG | GGCACTCTGTCGGGTTCTT |
| tgfβ | Transforming growth factor β | AM421619.1 | 97.9 | 55 | 143 | ACCTACATCTGGAACGCTGA | ACCTACATCTGGAACGCTGA |
| il10 | Interleukin 10 | AM268529.1 | 112.75 | 55 | 164 | ACCCCGTTCGCTTGCCA | CATCTGGTGACATCACTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeredo, R.; Peixoto, D.; Santos, P.; Duarte, I.; Ricardo, A.; Aragão, C.; Machado, M.; Costas, B. Dietary Tryptophan Plays a Role as an Anti-Inflammatory Agent in European Seabass (Dicentrarchus labrax) Juveniles during Chronic Inflammation. Biology 2024, 13, 309. https://doi.org/10.3390/biology13050309
Azeredo R, Peixoto D, Santos P, Duarte I, Ricardo A, Aragão C, Machado M, Costas B. Dietary Tryptophan Plays a Role as an Anti-Inflammatory Agent in European Seabass (Dicentrarchus labrax) Juveniles during Chronic Inflammation. Biology. 2024; 13(5):309. https://doi.org/10.3390/biology13050309
Chicago/Turabian StyleAzeredo, Rita, Diogo Peixoto, Paulo Santos, Inês Duarte, Ana Ricardo, Cláudia Aragão, Marina Machado, and Benjamín Costas. 2024. "Dietary Tryptophan Plays a Role as an Anti-Inflammatory Agent in European Seabass (Dicentrarchus labrax) Juveniles during Chronic Inflammation" Biology 13, no. 5: 309. https://doi.org/10.3390/biology13050309
APA StyleAzeredo, R., Peixoto, D., Santos, P., Duarte, I., Ricardo, A., Aragão, C., Machado, M., & Costas, B. (2024). Dietary Tryptophan Plays a Role as an Anti-Inflammatory Agent in European Seabass (Dicentrarchus labrax) Juveniles during Chronic Inflammation. Biology, 13(5), 309. https://doi.org/10.3390/biology13050309









