Cloning and Functional Analysis of CsROP5 and CsROP10 Genes Involved in Cucumber Resistance to Corynespora cassiicola
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Pathogens and Inoculation
2.3. Sequence Analysis
2.4. Quantitative RT-qPCR
2.5. Virus-Induced Gene Silencing (VIGS)
2.6. Construction of the Overexpressing Vector
2.7. Histochemical Analysis
3. Results
3.1. Cloning and Sequence Analysis of CsROP5 and CsROP10
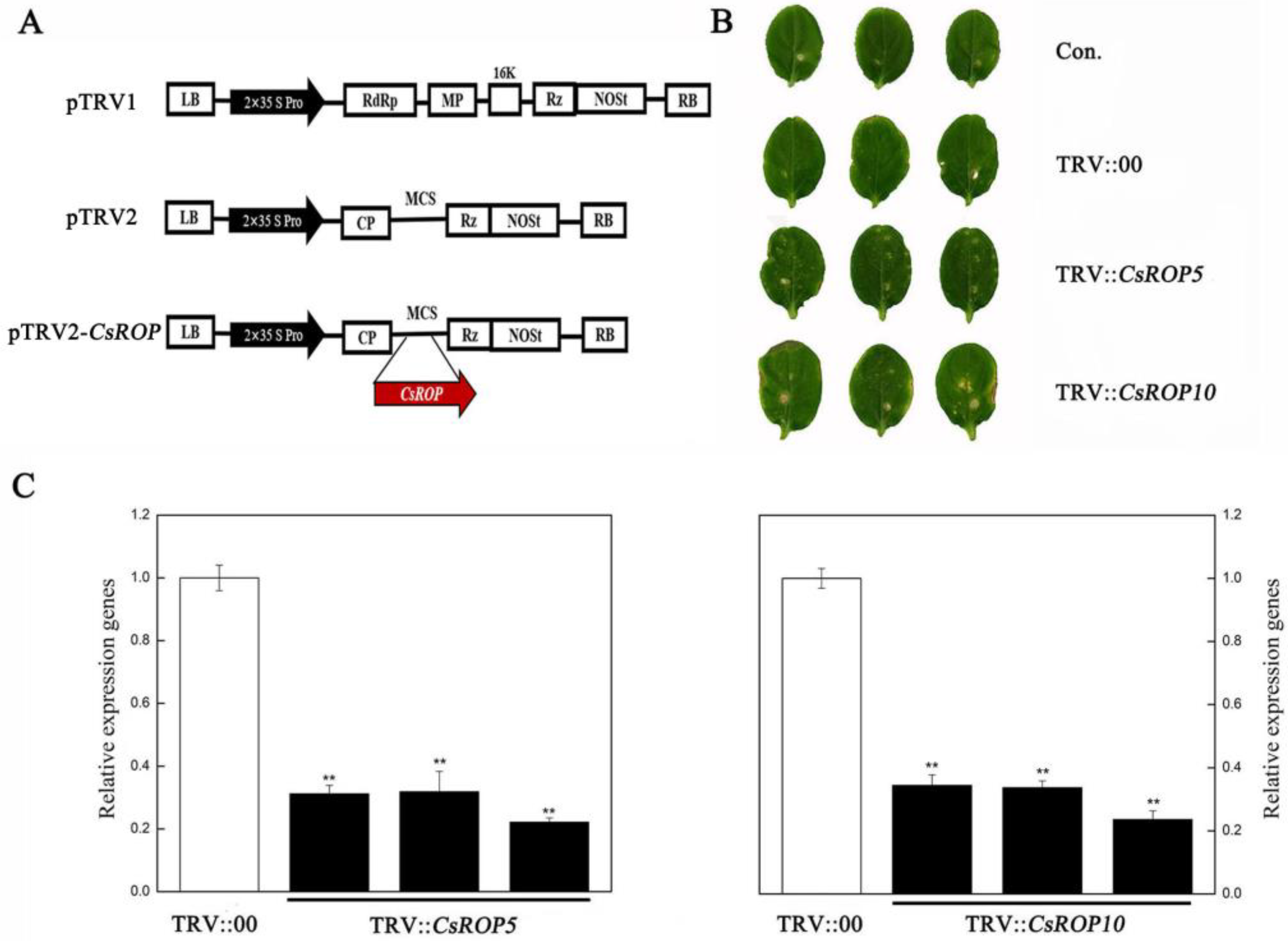
3.2. CsROP5 and CsROP10 Silencing in Cucumber Enhances Its Resistance to C. cassiicola
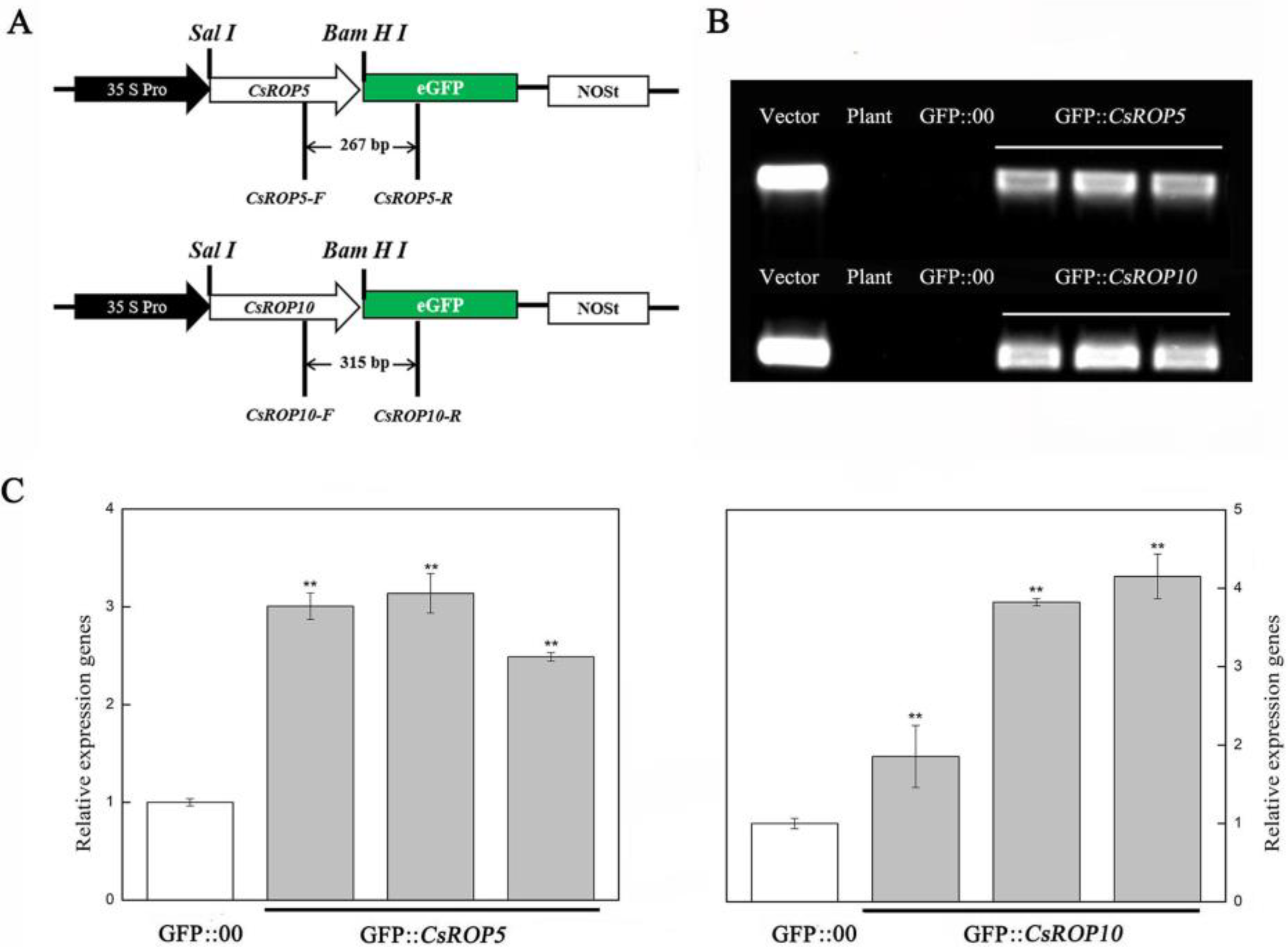
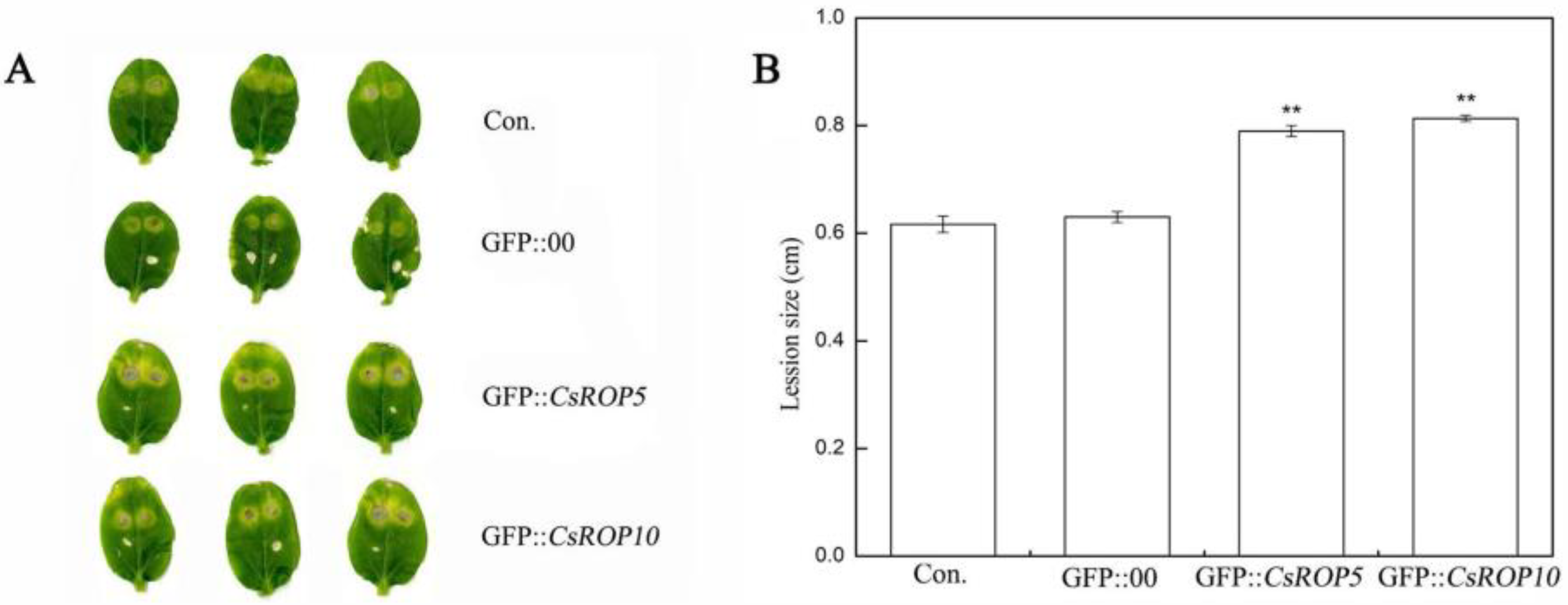
3.3. CsROP5 and CsROP10 Overexpression in Cucumber Cotyledons Impairs Resistance to C. cassiicola
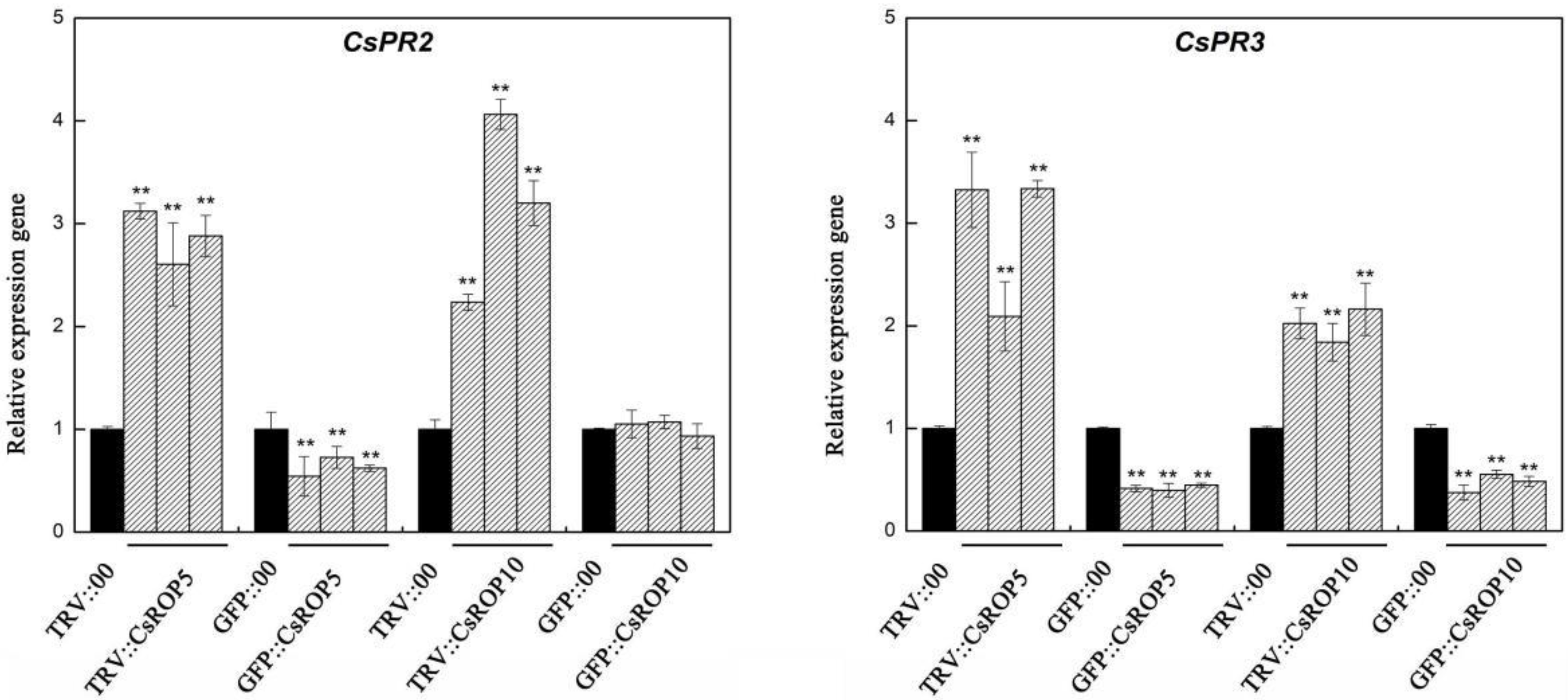
3.4. CsROP5 and CsROP10 Modulate the Expression Levels of Defense-Related Genes after C. cassiicola Challenge in Transgenic Plants
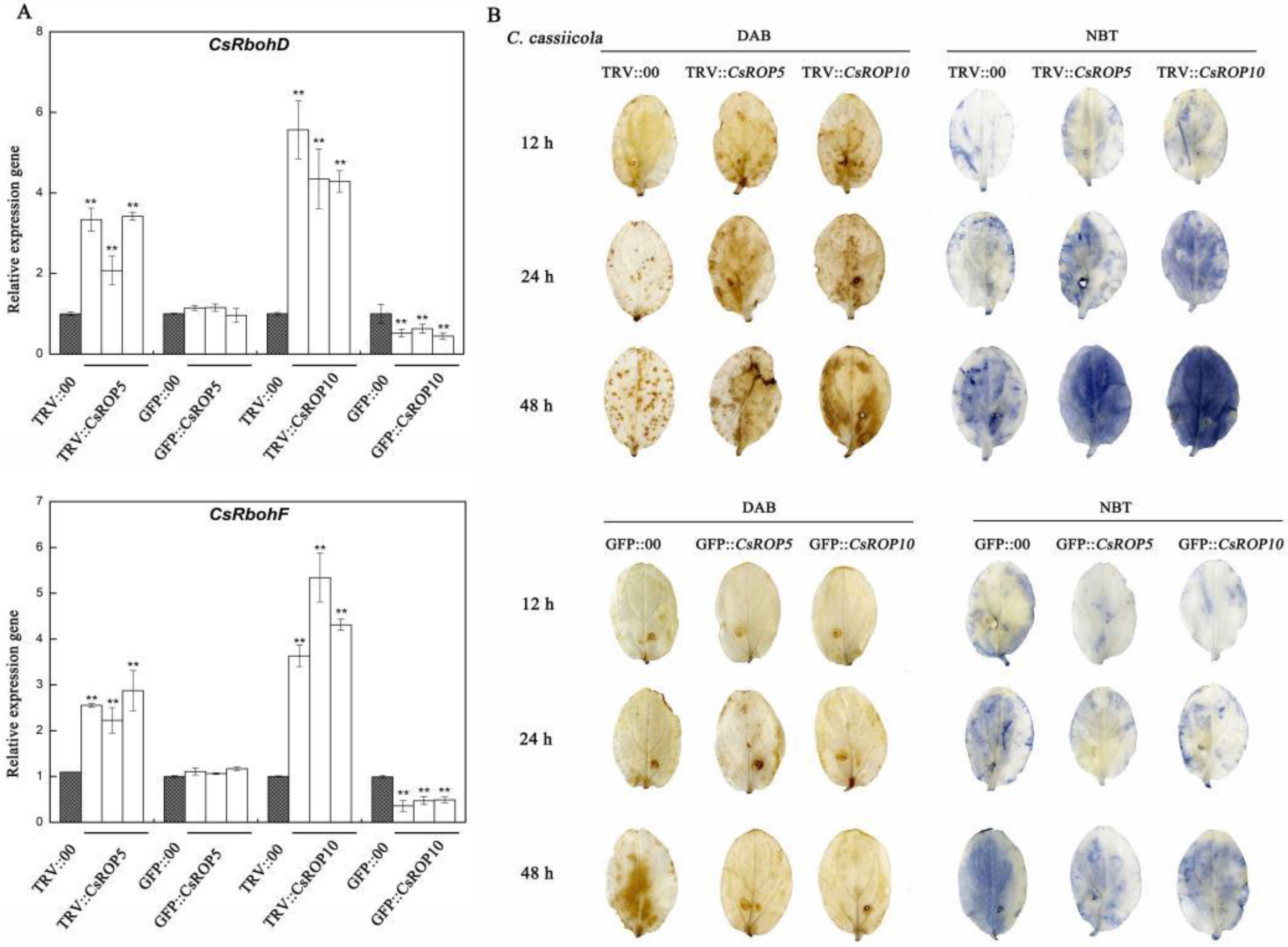
3.5. ROS Homoeostasis Is Crucial for CsROP5- and CsROP10-Mediated Resistance against C. cassiicola
3.6. CsROP5 and CsROP10 Modulate Abscisic Acid (ABA)-Signaling Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, C.; Mao, A.; Dong, C.; Liu, H.; Yu, S.; Guo, Y.D.; Weng, Y.; Xu, Y. Fine genetic mapping of target leaf spot resistance gene cca3 in cucumber, Cucumis sativus L. Theor. Appl. Genet. 2015, 128, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Déon, M.; Scomparin, A.; Tixier, A.; Mattos, C.R.R.; Leroy, T.; Seguin, M.; Roeckel-Drevet, P.; Pujade-Renaud, V. First characterization of endophytic Corynespora cassiicola isolates with variant cassicolin genes recovered from rubber trees in Brazil. Fungal Divers. 2012, 54, 87–99. [Google Scholar] [CrossRef]
- Déon, M.; Bourré, Y.; Gimenez, S.; Berger, A.; Bieysse, D.; De Lamotte, F.; Poncet, J.; Roussel, V.; Bonnot, F.; Oliver, G.; et al. Characterization of a cassicolin-encoding gene from Corynespora cassiicola, pathogen of rubbertree (Hevea brasiliensis). Plant Sci. 2012, 185, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Déon, M.; Fumanal, B.; Gimenez, S.; Bieysse, D.; Oliveira, R.R.; Shuib, S.S.; Breton, F.; Elumalai, S.; Vida, J.B.; Seguin, M.; et al. Diversity of the Cassicolin gene in Corynespora cassiicola and relation with the pathogenicity in Hevea brasiliensis. Fungal Biol. 2014, 118, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, X.W.; Shi, Y.; Chai, A.L.; Li, B.J. Analysis of pathogenic and genetic variability of Corynespora cassicola based on iPBSretrotransposons. Can. J. Plant Pathol. 2018, 41, 329–338. [Google Scholar]
- Ribeiro, S.; Label, P.; Garcia, D.; Montoro, P.; Pujade, R.V. Transcriptome profiling in susceptible and tolerant rubber tree clones in response to cassicolin Casl, a necrotrophic effector ffom Corynespora cassiicola. PLoS ONE 2021, 16, e0254541. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, J.; Liu, L.; Zhao, H.; Zhang, M.; Hong, J.; Meng, X.; Fan, H. Characterization of caffeoyl shikimate esterase gene family identifies CsCSE5 as a positive regulator of Podosphaera xanthii and Corynespora cassiicola pathogen resistance in cucumber. Plant Cell Rep. 2023, 42, 1937–1950. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, J.; Wang, X.; Ma, L.; Ma, Z.; Meng, X.; Fan, H. SnRK1 signaling regulates cucumber growth and resistance to Corynespora cassiicola. Plant Sci. 2023, 332, 111716. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Q.; Wang, X.; Meng, X.; Yu, Y.; Fan, H.; Cui, N. Mildew Resistance Locus O Genes CsMLO1 and CsMLO2 are negative modulators of the Cucumis sativus defense response to Corynespora cassiicola. Int. J. Mol. Sci. 2019, 20, 4793. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Yang, Z.B. The Rop GTPase switch turns on polar growth in pollen. Trends Plant Sci. 2000, 5, 298–303. [Google Scholar] [CrossRef]
- Hoefle, C.; Huesmann, C.; Schultheiss, H.; Börnke, F.; Hensel, G.; Kumlehn, J.; Hückelhoven, R. A barley ROP GTPase ACTIVATING PROTEIN associates with microtubules and regulates entry of the barley powdery mildew fungus into leaf epidermal cells. Plant Cell 2011, 23, 2422–2439. [Google Scholar] [CrossRef]
- Kawano, Y.; Akamatsu, A.; Hayashi, K.; Housen, Y.; Okuda, J.; Yao, A.; Nakashima, A.; Takahashi, H.; Yoshida, H.; Wong, H.L.; et al. Activation of a Rac GTPase by the NLR family disease resistance protein Pit plays a critical role in rice innate immunity. Cell Host Microbe 2010, 7, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Fu, Y.; Dowd, P.; Li, S.; Vernoud, V.; Gilroy, S.; Yang, Z. A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol. 2005, 169, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Henmi, K.; Ono, E.; Hatakeyama, S.; Iwano, M.; Satoh, H.; Shimamoto, K. The small GTP-binding protein Rac is a regulator of cell death in plants. Proc. Natl. Acad. Sci. USA 1999, 96, 10922–10926. [Google Scholar] [CrossRef]
- Ono, E.; Wong, H.L.; Kawasaki, T.; Hasegawa, M.; Kodama, O.; Shimamoto, K. Essential role of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. USA 2001, 98, 759–764. [Google Scholar] [CrossRef]
- Takai, Y.; Sasaki, T.; Matozaki, T. Small GTP-binding proteins. Physiol. Rev. 2001, 81, 153–208. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Yang, Z.B. The Rop GTPase: An emerging signaling switch in plants. Plant Mol. Biol. 2000, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.A.; Lee, A.Y.; An, K.J.; Sin-Ae Park, S.A. Horticultural therapy program for improving emotional well-being of elementary school students: An observational study. J. Integr. Med. Res. 2020, 9, 37–41. [Google Scholar] [CrossRef]
- Berken, A. ROPs in the spotlight of plant signal transduction. Cell Mol. Life Sci. 2006, 63, 2446–2459. [Google Scholar] [CrossRef]
- Yang, Z. Small GTPases: Versatile signaling switches in plants. Plant Cell 2002, 14 (Suppl. S1), S375–S388. [Google Scholar] [CrossRef]
- Bloch, D.; Lavy, M.; Efrat, Y.; Wasteneys, G.; Yang, Z. Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling. Mol. Biol. Cell 2005, 16, 1913–1927. [Google Scholar] [CrossRef]
- Fu, Y.; Gu, Y.; Zheng, Z.; Wasteneys, G.; Zhenbiao Yang, Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 2005, 120, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A.; Raymond, M.J.; Yang, Z.; Smirnoff, N. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J. Exp. Bot. 2007, 58, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Lavy, M.; Bloch, D.; Hazak, O.; Gutman, I.; Poraty, L.; Sorek, N.; Sternberg, H.; Yalovsky, S. A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr. Biol. 2007, 17, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.; Hensel, G.; Imani, J.; Broeders, S.; Sonnewald, U.; Kogel, K.H.; Kumlehn, J.; Hückelhoven, R. Ectopic expression of constitutively activated RACB in barley enhances susceptibility to powdery mildew and abiotic stress. Plant Physiol. 2005, 139, 353–362. [Google Scholar] [CrossRef]
- Xin, Z.; Zhao, Y.; Zheng, Z.L. Transcriptome analysis reveals specific modulation of abscisic acid signaling by ROP10 small GTPase in Arabidopsis. Plant Physiol. 2005, 139, 1350–1365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gao, E.; Wang, Y.; Li, Y. GhROP6 involved in cotton resistance to Verticillium wilt through regulating jasmonic acid synthesis and lignin metabolism. Cotton Sci. 2022, 34, 79–92. [Google Scholar]
- Zhou, Z.Z.; Pang, Z.Q.; Zhao, S.L.; Zhang, L.; Lv, Q.; Yin, D.; Li, D.; Liu, X.; Zhao, X.; Li, X.; et al. Importance of OsRac1 and RAI1 in signalling of nucleotide-binding site leucine-rich repeat protein-mediated resistance to rice blast disease. New Phytol. 2019, 223, 828–838. [Google Scholar] [CrossRef]
- Jung, Y.H.; Agrawal, G.K.; Rakwal, R.; Kim, J.A.; Lee, M.O.; Choi, P.G.; Kim, Y.J.; Kim, M.J.; Shibato, J.; Kim, S.H.; et al. Functional characterization of OsRacB GTPase–a potentially negative regulator of basal disease resistance in rice. Plant Physiol. Biochem. 2006, 44, 68–77. [Google Scholar] [CrossRef]
- Chen, L.; Shiotani, K.; Togashi, T.; Miki, D.; Aoyama, M.; Wong, H.L.; Kawasaki, T.; Shimamoto, K. Analysis of the Rac/Rop small GTPase family in rice: Expression: Subcellular localization and role in disease resistance. Plant Cell Physiol. 2010, 51, 585–595. [Google Scholar] [CrossRef]
- Opalski, K.S.; Schultheiss, H.; Kogel, K.H.; Hückelhoven, R. The receptor-like MLO protein and the RAC/ROP family G-protein RACB modulate actin reorganization in barley attacked by the biotrophic powdery mildew fungus Blumeria graminis f.sp. hordei. Plant J. 2005, 41, 291–303. [Google Scholar] [CrossRef]
- Moeder, W.; Yoshioka, K.; Klessig, D.F. Involvement of the small GTPase Rac in the defense responses of tobacco to pathogens. Mol. Plant-Microbe Interact. 2005, 18, 116–124. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Zhang, X.L.; Na, R.; Yang, S.; Tian, Z.; Zhao, Y.; Zhao, J. StRac1 plays an important role in potato resistance against Phytophthora infestans via regulating H2O2 production. J. Plant Physiol. 2020, 253, 153249. [Google Scholar] [CrossRef]
- Potikha, T.S.; Collins, C.C.; Johnson, D.I.; Delmer, D.P.; Levine, A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999, 119, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, H.J.; Lee, S.; Lee, T.; Yang, Z.; Lee, Y. Rac-related GTP-binding protein in elicitor-induced reactive oxygen generation by suspension-cultured soybean cells. Plant Physiol. 2000, 124, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Sakamoto, T.; Kawasaki, T.; Umemura, K.; Shimamoto, K. Down-regulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol. 2004, 135, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Pathuri, I.P.; Zellerhoff, N.; Schaffrath, U.; Hensel, G.; Kumlehn, J.; Kogel, K.H.; Eichmann, R.; Hückelhoven, R. Constitutively activated barley ROPs modulate epidermal cell size: Defense reactions and interactions with fungal leaf pathogens. Plant Cell Rep. 2008, 27, 1877–1887. [Google Scholar] [CrossRef]
- Lemichez, E.; Wu, Y.; Sanchez, J.P.; Mettouchi, A.; Mathur, J.; Chua, N.H. Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 2001, 15, 1808–1816. [Google Scholar] [CrossRef]
- Li, H.; Shen, J.J.; Zheng, Z.L.; Lin, Y.; Yang, Z. The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 2001, 126, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Feijo, J.A.; Sainhas, J.; Holdaway-Clarke, T.; Cordeiro, M.S.; Kunkel, J.G.; Hepler, P.K. Cellular oscillations and the regulation of growth: The pollen tube paradigm. Bioessays 2001, 23, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.L.; Nafisi, M.; Tam, A.; Li, H.; Crowell, D.N.; Chary, S.N.; Schroeder, J.I.; Shen, J.; Yang, Z. Plasma membrane-associated ROP10 small GTPase is a specific negative regulator of abscisic acid responses in Arabidopsis. Plant Cell 2002, 14, 2787–2797. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Duan, C.; Chen, P.; Li, Q.; Dai, S.; Sun, L.; Ji, K.; Sun, Y.; Xu, W.; et al. The expression profiling of the CsPYL, CsPP2C and CsSnRK2 gene families during fruit development and drought stress in cucumber. J. Plant Physiol. 2012, 169, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Molina, L.; Mongrand, S.; Mclachlin, D.T.; Chait, B.T.; Chua, N.H. ABI5 acts downstream of abi3 to execute an aba-dependent growth arrest during germination. Plant J. 2010, 32, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fan, Y.; Ren, N.; Zhang, X.; Yang, S.; Gao, J.; Fan, M.; Zhao, Y.; Zhao, J. AtROP1 negatively regulates potato resistance to Phytophthora infestans, via NADPH oxidase-mediated accumulation of H2O2. BMC Plant Biol. 2014, 14, 392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, H.; Yang, X.; Li, Q.; Ling, J.; Wang, H.; Gu, X.; Huang, S.; Jiang, W. CsWRKYW46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic-plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner. Plant Physiol. Biochem. 2016, 108, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Ma, Y.; Zhou, Y.; Zhang, H.; Duan, L.; Chen, H.; Zeng, J.; Zhou, Q.; Wang, S.; Gu, W.; et al. Biosynthesis, regulation, and domestication of bitterness in cucumber. Science 2014, 346, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Chen, F.; Cheng, Z.; Wang, Y.; Yang, P.; Zhang, Y.; Cha, Z. Manipulation of arginase expression modulates abiotic stress tolerance in Arabidopsis: Effect on arginine metabolism and ROS accumulation. J. Exp. Bot. 2013, 64, 1367–1379. [Google Scholar] [CrossRef]
- Mackay, J.G.; Hall, A. Rho GTPases. J. Biol. Chem. 1998, 273, 20685–20688. [Google Scholar] [CrossRef]
- Winge, P.; Brembu, T.; Kristensen, R.; Bones, A.M. Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics 2000, 156, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.M.; Vejlupkova, Z.; Sharma, Y.K.; Arthur, K.M.; Spatafora, J.W.; Albright, C.A.; Meeley, R.B.; Duvick, J.P.; Quatrano, R.S.; Fowler, J.E. Conserved subgroups and developmental regulation in the monocot rop gene family. Plant Physiol. 2003, 133, 1791–1808. [Google Scholar] [CrossRef]
- Ivanchenko, M.; Vejlupkova, Z.; Quatrano, R.S.; Fowler, J.E. Maize ROP7 GTPase contains a unique, CAAX box-independent plasma membrane targeting signal. Plant J. 2000, 24, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Lavy, M.; Bracha-Drori, K.; Sternberg, H.; Yalovsky, S. A cell-specific, prenylation-independent mechanism regulates targeting of type II RACs. Plant Cell 2002, 14, 2431–2450. [Google Scholar] [CrossRef]
- Schultheiss, H.; Dechert, C.; Kogel, K.H.; Hückelhoven, R. Functional analysis of barley RAC/Rop G-protein family members in susceptibility to the powdery mildew fungus. Plant J. 2003, 36, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Trutzenberg, A.; Huckelhoven, R. Regulation and Functions of ROP GTPasesin Plant-Microbe Interactions. Cells 2020, 9, 2016. [Google Scholar] [CrossRef]
- Schultheiss, H.; Dechert, C.; Kogel, K.H.; Hückelhoven, R. A Small GTP-Binding Host Protein Is Required for Entry of Powdery Mildew Fungus into Epidermal Cells of Barley. Plant Physiol. 2002, 128, 1447–1454. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Cui, N.; Yu, Y.; Yu, G.; Fan, H. Transcriptome and miRNA analyses of the response to Corynespora cassiicolain cucumber. Sci. Rep. 2018, 8, 7798. [Google Scholar] [CrossRef]
- Wong, H.L.; Pinontoan, R.; Hayashi, K.; Tabata, R.; Yaeno, T.; Hasegawa, K.; Kojima, C.; Yoshioka, H.; Iba, K.; Kawasaki, T.; et al. Regulation of Rice NADPH Oxidase byBinding of Rac GTPase to Its N-Terminal Extension. Plant Cell 2007, 19, 4022–4034. [Google Scholar] [CrossRef]
- Oda, T.; Hashimoto, H.; Kuwabara, N.; Akashi, S.; Hayashi, K.; Kojima, C.; Wong, H.L.; Kawasaki, T.; Shimamoto, K.; Sato, M.; et al. Structure of the N-terminal regulatory domain ofa plant NADPH oxidase and its functional implications. J. Biol. Chem. 2010, 285, 1435. [Google Scholar] [CrossRef]
- Niu, M.; Huang, Y.; Sun, S.; Sun, J.; Cao, H.; Shabala, S.; Bie, Z. Root respiratory burst oxidasehomologue-dependent H₂Oz production confers salt tolerance on a grafted cucumber by controlling Na+ exclusion and stomatal closure. J. Expl. Bot. 2018, 69, 3465–3476. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, H.M.; Murali, M.; Anup, C.P.M.; Melvin, P.; Sharada, M.S. Salicylic acid seed priming instigates defense mechanism by inducing PR-Proteins in Solanum melongena L. upon infection with Verticillium dahliae Kleb. Plant Physiol. Biochem. 2017, 117, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.H.; Elmohamedy, R.S.R. Induction of Defense-Related Physiological and Antioxidant Enzyme Response against Powdery Mildew Disease in Okra (Abelmoschus esculentus L.) Plant by Using Chitosan and Potassium Salts. Mycobiology 2017, 45, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yi, H. Enhanced Arabidopsis disease resistance against Botrytis cinerea induced by sulfur dioxide. Ecotoxicol. Environ. Saf. 2017, 147, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Koita, H.; Nakatsubo, T.; Hasegawa, K.; Wakabayashi, K.; Takahashi, H.; Umemura, K.; Umezawa, T.; Shimamoto, K. Cinnamoyl-CoA reductase, a key enzyme inlignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Oikawa, T.; Kyozuka, J.; Wong, H.L.; Umemura, K.; Kishi-Kaboshi, M.; Takahashi, A.; Kawano, Y.; Kawasaki, T.; Shimamoto, K. The bHLH Rac Immunity1 (RAI1)Is Activated byOsRacl via OsMAPK3 and OsMAPK6 in Rice Immunity. Plant Cell Physiol. 2012, 53, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Chen, L.; Nakashima, A.; Thao, N.P.; Chen, L.; Nakashima, A.; Hara, S.; Umemura, K.; Takahashi, A.; Shirasu, K.; et al. RARI and HSP90 form a complex with Rac/RopGTPase and function in innate-immune responses in rice. Plant Cell 2007, 19, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Poraty-Gavra, L.; Zimmermann, P.; Haigis, S.; Bednarek, P.; Hazak, O.; Stelmakh, O.R.; Sadot, E.; Schulze-Lefert, P.; Gruissem, W.; Yalovsky, S. The Arabidopsis Rho of plants GTPaseAtROP6 functions in developmental and pathogen response pathways. Plant Physiol. 2013, 161, 1172–1188. [Google Scholar] [CrossRef]
- Wasilewska, A.; Vlad, F.; Sirichandra, C.; Redko, Y.; Jammes, F.; Valon, C.; dit Frey, N.F.; Leung, J. An update on abscisic acid signaling in plants and more. Mol. Plant. 2008, 1, 198–217. [Google Scholar] [CrossRef]
- Asselbergh, B.; De, V.D.; Hofte, M. Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol. Plant–Microbe Interact. 2008, 21, 709–719. [Google Scholar] [CrossRef]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ulferts, S.; Delventhal, R.; Splivallo, R.; Karlovsky, P.; Schaffrath, U. Abscisic acid negatively interferes with basal defence of barley against Magnaporthe oryzae. BMC Plant Biol. 2015, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kang, J.; Sui, N.; Liu, D. ROP11 GTPase is a negative regulator of multiple ABA responses in Arabidopsis. J. Integr. Plant Biol. 2012, 54, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Qian, L.; Nibau, C.; Duan, Q.; Kita, D.; Levasseur, K.; Li, X.; Lu, C.; Li, H.; Hou, C.; et al. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc. Natl. Acad. Sci. USA 2012, 109, 14693–14698. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Raghavendra, A.S. Convergence and Divergence of Signaling Events in Guard Cellsduring Stomatal Closure by PlantHormones or Microbial Elicitors. Front. Plant Sci. 2016, 24, 1332. [Google Scholar]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Takeda, S.; Gapper, C.; Kaya, H.; Bell, E.; Kuchitsu, K.; Dolan, L. Local positive feedback regulation determines cell shape in root hair cells. Science 2008, 319, 1241–1244. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Jia, L.; Yu, N.; Feng, M.; Qu, Y. Cloning and Functional Analysis of CsROP5 and CsROP10 Genes Involved in Cucumber Resistance to Corynespora cassiicola. Biology 2024, 13, 308. https://doi.org/10.3390/biology13050308
Yu G, Jia L, Yu N, Feng M, Qu Y. Cloning and Functional Analysis of CsROP5 and CsROP10 Genes Involved in Cucumber Resistance to Corynespora cassiicola. Biology. 2024; 13(5):308. https://doi.org/10.3390/biology13050308
Chicago/Turabian StyleYu, Guangchao, Lian Jia, Ning Yu, Miao Feng, and Yue Qu. 2024. "Cloning and Functional Analysis of CsROP5 and CsROP10 Genes Involved in Cucumber Resistance to Corynespora cassiicola" Biology 13, no. 5: 308. https://doi.org/10.3390/biology13050308
APA StyleYu, G., Jia, L., Yu, N., Feng, M., & Qu, Y. (2024). Cloning and Functional Analysis of CsROP5 and CsROP10 Genes Involved in Cucumber Resistance to Corynespora cassiicola. Biology, 13(5), 308. https://doi.org/10.3390/biology13050308




