Ploidy’s Role in Daylily Plant Resilience to Drought Stress Challenges
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Location and Design
2.3. Data Collection
2.3.1. RWC
2.3.2. ROS
2.3.3. Morpho-Physiological Parameters
2.4. Drought Stress Response Index
2.5. Data Analysis
3. Results
3.1. Drought Stress Affects ROS Components and Leads to Yellow Leaves in Daylily Plants
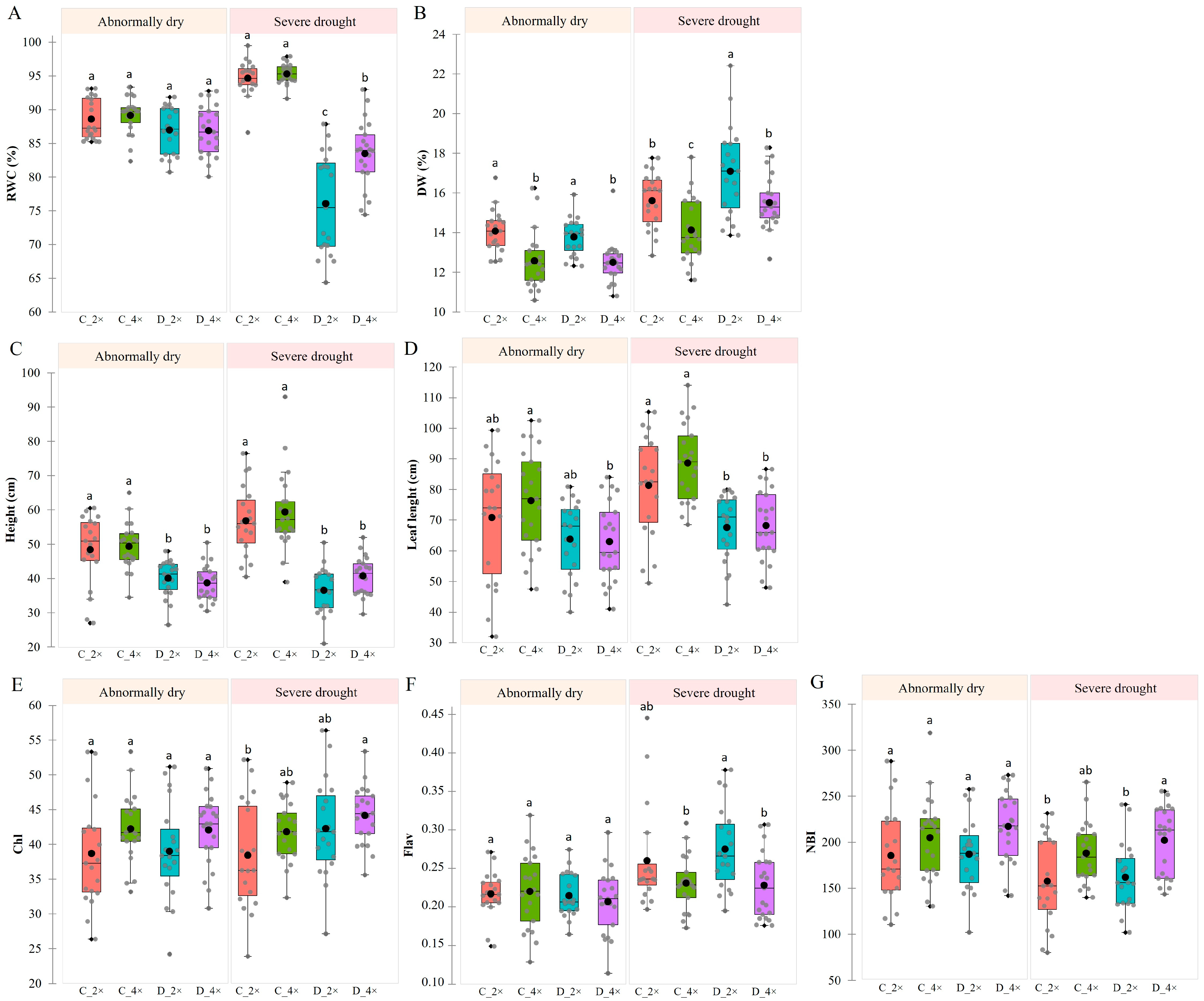
3.2. Effects of Drought Stress on Relative Water Content, Habit, and Leaf Pigment Indices of Daylilies
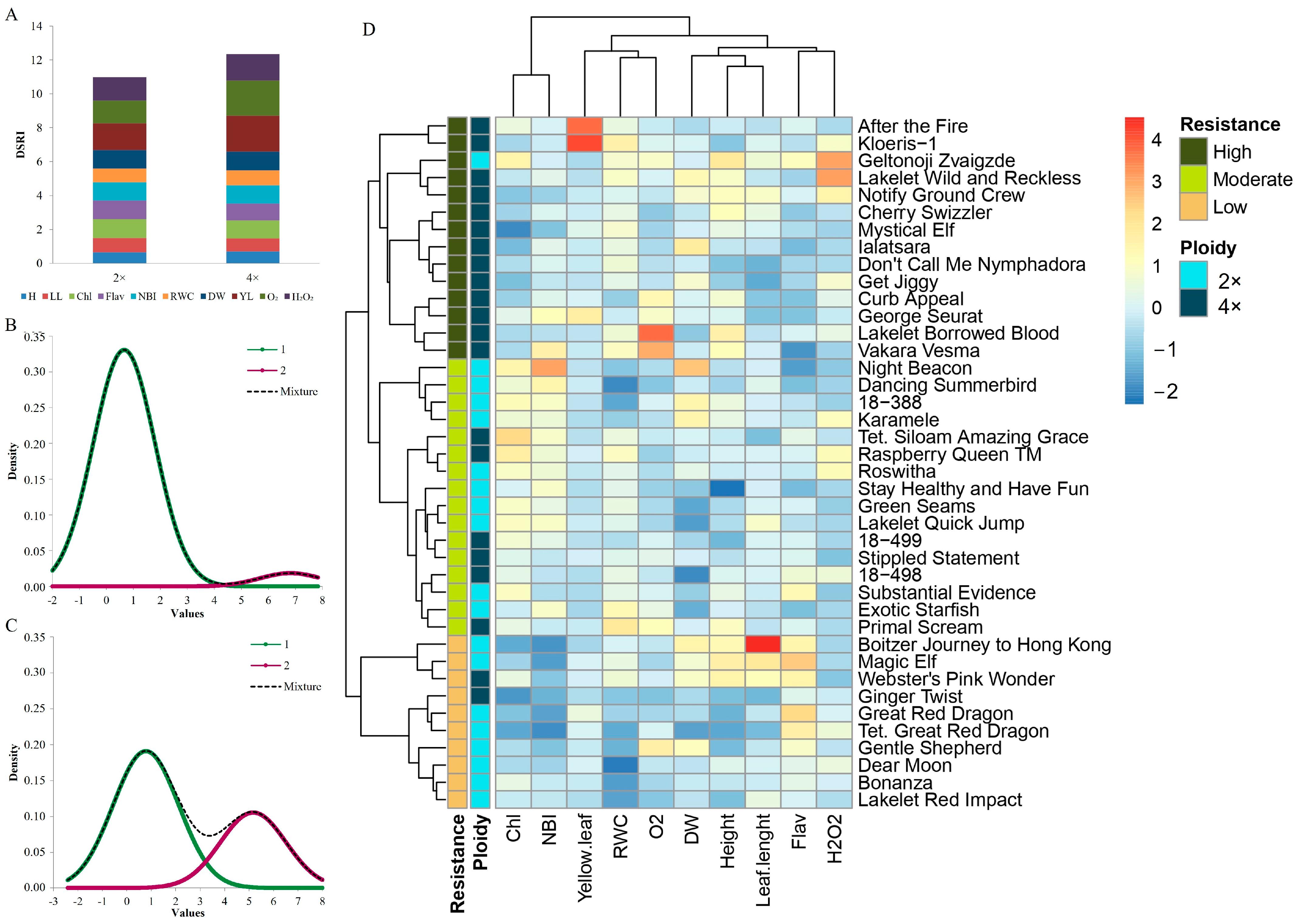
3.3. Drought Response Indexing in Daylilies
4. Discussion
4.1. Ploidy Level Affects Plant Morphological and Photosynthetic Pigments’ Response to Drought Stress
4.2. Tetraploid Plants Maintain Higher RWC under Drought Stress
4.3. Mechanisms for Tetraploid Plant Adaptation to Drought Tolerance
4.4. Drought Stress Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Tripathy, K.P.; Mukherjee, S.; Mishra, A.K.; Mann, M.E.; Williams, A.P. Climate change will accelerate the high-end risk of compound drought and heatwave events. Proc. Natl. Acad. Sci. USA 2023, 120, e2219825120. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Basu, P.S.; Kumar, M.; Ansari, J.; Shukla, A.; Thakur, S.; Singh, P.; Datta, S.; Chaturvedi, S.K.; Sheshshaeyee, M.S.; et al. Transgenic chickpea (Cicer arietinum L.) harbouring AtDREB1a are physiologically better adapted to water deficit. BMC Plant Biol. 2021, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.; Ahsan, M.; Sadaqat, H.A.; Awan, F. Screening of maize (Zea mays L.) inbred lines under water deficit conditions. Biol. Clin. Sci. Res. J. 2020, 2020, 7. [Google Scholar] [CrossRef]
- Maherali, H.; Walden, A.E.; Husband, B.C. Genome duplication and the evolution of physiological responses to water stress. New Phytol. 2009, 184, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.M.; Joshi, M.S.; Djidonou, D.; Joshi, V.; Nick, C.; Leskovar, D.I. Physiological and biochemical responses of tomato plants grafted onto Solanum pennellii and Solanum peruvianum under water-deficit conditions. Plants 2021, 10, 2236. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Wang, Y.; Xie, Z.; Guo, D.; Chen, C.; Fan, Q.; Deng, X.; Liu, J.H. Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata. Plant Biotechnol. J. 2019, 17, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.; Dias, M.C.; Castro, M.; Loureiro, J.; Castro, S. Impact of genome duplications in drought tolerance and distribution of the diploid-tetraploid Jasione maritima. Front. Plant Sci. 2023, 14, 1144678. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Mizunashi, K.; Tanaka, S.; Adachi, Y.; Nakano, M. Ploidy estimation in Hemerocallis species and cultivars by flow cytometry. Sci. Hortic. 2003, 97, 185–192. [Google Scholar] [CrossRef]
- Keene, S.A.; Johnson, T.S.; Sigler, C.L.; Kalk, T.N.; Genho, P.; Colquhoun, T.A. A survey of the floral volatile profiles of daylily species and hybrids. J. Am. Soc. Hortic. Sci. 2020, 145, 120–130. [Google Scholar] [CrossRef]
- American Daylily Society. AHS 2024. The American Daylily Society Online Daylily Database. Available online: https://daylilies.org/DaylilyDB/ (accessed on 25 March 2024).
- Cai, X.; Liu, J.; Zhao, F.; Wang, X. Transcriptome analysis of response strategy in Hemerocallis fulva under drought stress. Genes Genom. 2023, 45, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Misiukevičius, E.; Mažeikienė, I.; Gossard, J.; Starkus, A.; Stanys, V. Transcriptome Analysis of Diploid and Autotetraploid Hemerocallis Response to Drought Stress. Horticulturae 2023, 9, 1194. [Google Scholar] [CrossRef]
- Misiukevičius, E.; Frercks, B.; Šikšnianienė, J.B.; Kącki, Z.; Gębala, M.; Akulytė, P.; Trilikauskaitė, E.; Stanys, V. Assessing the genetic diversity of daylily germplasm using SSR markers: Implications for daylily breeding. Plants 2023, 12, 1752. [Google Scholar] [CrossRef]
- Andriūnaitė, E.; Rugienius, R.; Tamošiūnė, I.; Haimi, P.; Vinskienė, J.; Baniulis, D. Enhanced carbonylation of photosynthetic and glycolytic proteins in antibiotic timentin-treated tobacco in vitro shoot culture. Plants 2022, 11, 1572. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Iqbal, M.S.; Li, H.; Nazir, M.F.; Khalid, S.; Sarfraz, Z.; Hu, D.; Baojun, C.; Geng, X.; Tajo, S.M.; et al. Differential seedling growth and tolerance indices reflect drought tolerance in cotton. BMC Plant Biol. 2022, 22, 331. [Google Scholar] [CrossRef]
- Aversano, R.; Ercolano, M.R.; Caruso, I.; Fasano, C.; Rosellini, D.; Carputo, D. Molecular tools for exploring polyploid genomes in plants. Int. J. Mol. Sci. 2012, 13, 10316–10335. [Google Scholar] [CrossRef] [PubMed]
- Gulia, S.K.; Singh, B.P.; Carter, J.; Griesbach, R.J. Daylily: Botany, propagation, breeding. Hortic. Rev. 2009, 35, 193–220. [Google Scholar] [CrossRef]
- Zhang, X.X.; Liu, M.; Wang, M.Y.; Shi, C.Q.; Cheng, X.Y. Developmental and morphological study of the coleorhizae in Hemerocallis (Liliaceae). Pak. J. Bot. 2013, 45, 1673–1676. Available online: https://www.pakbs.org/pjbot/PDFs/47(1)/47.pdf (accessed on 26 March 2024).
- Podwyszyńska, M.; Gabryszewska, E.; Dyki, B.; Stępowska, A.A.; Kowalski, A.; Jasiński, A. Phenotypic and genome size changes (variation) in synthetic tetraploids of daylily (Hemerocallis) in relation to their diploid counterparts. Euphytica 2015, 203, 1–16. [Google Scholar] [CrossRef]
- Chen, Z.J. Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu. Rev. Plant Biol. 2007, 58, 377–406. [Google Scholar] [CrossRef]
- Semedo, J.N.; Rodrigues, A.L.; Lidon, F.C.; Pais, I.P.; Marques, I.; Gouveia, D.; Armengaud, J.; Silva, M.J.; Martins, S.; Semedo, M.C.; et al. Intrinsic non-stomatal resilience to drought of the photosynthetic apparatus in Coffea spp. is strengthened by elevated air [CO2]. Tree Physiol. 2021, 41, 708–727. [Google Scholar] [CrossRef]
- Elsalahy, H.H.; Reckling, M. Soybean resilience to drought is supported by partial recovery of photosynthetic traits. Front. Plant Sci. 2022, 13, 971893. [Google Scholar] [CrossRef]
- Walczyk, A.M.; Hersch-Green, E.I. Do water and soil nutrient scarcities differentially impact the performance of diploid and tetraploid Solidago gigantea (Giant Goldenrod, Asteraceae)? Plant Biol. 2022, 24, 1031–1042. [Google Scholar] [CrossRef]
- Thompson, K.A.; Husband, B.C.; Maherali, H. No influence of water limitation on the outcome of competition between diploid and tetraploid Chamerion angustifolium (Onagraceae). J. Ecol. 2015, 103, 733–741. [Google Scholar] [CrossRef]
- Abdolinejad, R.; Shekafandeh, A. Tetraploidy confers superior in vitro water-stress tolerance to the fig tree (Ficus carica) by reinforcing hormonal, physiological, and biochemical defensive systems. Front. Plant Sci. 2022, 12, 796215. [Google Scholar] [CrossRef]
- Bharati, R.; Gupta, A.; Novy, P.; Severová, L.; Šrédl, K.; Žiarovská, J.; Fernández-Cusimamani, E. Synthetic polyploid induction influences morphological, physiological, and photosynthetic characteristics in Melissa officinalis L. Front. Plant Sci. 2023, 14, 1332428. [Google Scholar] [CrossRef]
- Misiukevičius, E.; Stanys, V. Induction and analysis of polyploids in daylily (Hemerocallis L.) plants. Zemdirb.-Agric. 2022, 109, 373–382. [Google Scholar] [CrossRef]
- Hussain, S.; Sohail, H.; Noor, I.; Ahmad, S.; Ejaz, S.; Ali, M.A.; Haider, S.T.; Sohail, M.; Jaffer, H.; Ercisli, S.; et al. Physiological and biochemical determinants of drought tolerance in tetraploid vs diploid sour orange citrus rootstock. J. Hortic. Sci. Biotechnol. 2023, 98, 772–785. [Google Scholar] [CrossRef]
- Bortolin, G.S.; Galviz, Y.C.; Pedroso, C.E.; Souza, G.M.; Palta, J. Root/shoot responses to drought and flooding of bahiagrass at reproductive stage depends on genotype ploidy. Funct. Plant Biol. 2022, 49, 333–350. [Google Scholar] [CrossRef]
- Barceló-Anguiano, M.; Holbrook, N.M.; Hormaza, J.I.; Losada, J.M. Changes in ploidy affect vascular allometry and hydraulic function in Mangifera indica trees. Plant J. 2021, 108, 541–554. [Google Scholar] [CrossRef]
- Oraee, A.; Tehranifar, A. Evaluating the potential drought tolerance of pansy through its physiological and biochemical responses to drought and recovery periods. Sci. Hortic. 2020, 265, 109225. [Google Scholar] [CrossRef]
- Allario, T.; Brumós, J.; Colmenero-Flores, J.M.; Iglesias, D.J.; Pina, J.A.; Navarro, L.; Talon, M.; Ollitrault, P.; Morillón, R. Tetraploid Rangpur lime rootstock increases drought tolerance via enhanced constitutive root abscisic acid production. Plant Cell Environ. 2013, 36, 856–868. [Google Scholar] [CrossRef]
- Van Laere, K.; França, S.C.; Vansteenkiste, H.; Van Huylenbroeck, J.; Steppe, K.; Van Labeke, M.C. Influence of ploidy level on morphology, growth and drought susceptibility in Spathiphyllum wallisii. Acta Physiol. Plant. 2011, 33, 1149–1156. [Google Scholar] [CrossRef]
- Rao, S.; Tian, Y.; Xia, X.; Li, Y.; Chen, J. Chromosome doubling mediates superior drought tolerance in Lycium ruthenicum via abscisic acid signaling. Hortic. Res. 2020, 7, 40. [Google Scholar] [CrossRef]
- Zhang, F.; Xue, H.; Lu, X.; Zhang, B.; Wang, F.; Ma, Y.; Zhang, Z. Autotetraploidization enhances drought stress tolerance in two apple cultivars. Trees 2015, 29, 1773–1780. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2016, 67, 47–60. [Google Scholar] [CrossRef]
- Seleiman, M.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.; Battaglia, M. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Urano, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Drought stress responses and resistance in plants: From cellular responses to long-distance intercellular communication. Front. Plant Sci. 2020, 11, 556972. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.; He, T.; Gao, R.; Guo, G.; Lu, R.; Chen, Z.; Liu, C. Comparative analysis of morphology, photosynthetic physiology, and transcriptome between diploid and tetraploid barley derived from microspore culture. Front. Plant Sci. 2021, 12, 626916. [Google Scholar] [CrossRef]
- Liu, H.; Bao, L.; Han, S.; Hui, T.; Zhang, R.; Zhang, M.; Su, C.; Qian, Y.; Jiao, F. Secondary metabolism and hormone response reveal the molecular mechanism of triploid mulberry (Morus alba L.) trees against drought. Front. Plant Sci. 2021, 12, 720452. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Pozo, J.C.d. and Ramírez-Parra, E. Deciphering the molecular bases for drought tolerance in Arabidopsis autotetraploids. Plant Cell Environ. 2014, 37, 2722–2737. [Google Scholar] [CrossRef]
- Niu, S.; Wang, Y.; Zhao, Z.; Deng, M.; Cao, L.; Yang, L.; Fan, G. Transcriptome and degradome of microRNAs and their targets in response to drought stress in the plants of a diploid and its autotetraploid Paulownia australis. PLoS ONE 2016, 11, e0158750. [Google Scholar] [CrossRef]
- Correia, S.; Braga, A.; Martins, J.; Correia, B.; Pinto, G.; Canhoto, J. Effects of polyploidy on physiological performance of acclimatized Solanum betaceum Cav. plants under water deficit. Forests 2023, 14, 208. [Google Scholar] [CrossRef]
- Greer, B.T.; Still, C.; Cullinan, G.L.; Brooks, J.R.; Meinzer, F.C. Polyploidy influences plant–environment interactions in quaking aspen (Populus tremuloides Michx.). Tree Physiol. 2018, 38, 630–640. [Google Scholar] [CrossRef]
- Da Sois, L.; Mencuccini, M.; Castells, E.; Sanchez-Martinez, P.; Martínez-Vilalta, J. How are physiological responses to drought modulated by water relations and leaf economics’ traits in woody plants? Agric. Water Manag. 2024, 291, 108613. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misiukevičius, E.; Mažeikienė, I.; Stanys, V. Ploidy’s Role in Daylily Plant Resilience to Drought Stress Challenges. Biology 2024, 13, 289. https://doi.org/10.3390/biology13050289
Misiukevičius E, Mažeikienė I, Stanys V. Ploidy’s Role in Daylily Plant Resilience to Drought Stress Challenges. Biology. 2024; 13(5):289. https://doi.org/10.3390/biology13050289
Chicago/Turabian StyleMisiukevičius, Edvinas, Ingrida Mažeikienė, and Vidmantas Stanys. 2024. "Ploidy’s Role in Daylily Plant Resilience to Drought Stress Challenges" Biology 13, no. 5: 289. https://doi.org/10.3390/biology13050289
APA StyleMisiukevičius, E., Mažeikienė, I., & Stanys, V. (2024). Ploidy’s Role in Daylily Plant Resilience to Drought Stress Challenges. Biology, 13(5), 289. https://doi.org/10.3390/biology13050289






