1. The Heart and Psychostasis in Ancient Egypt and in Early Christianity
Cardiac neuromodulation represents the intricate physiological control exerted on the heart through a diverse array of endogenous and exogenous mechanisms, aimed at modulating nerve activity within the cardiovascular system. This field has a long history, dating back to ancient Egyptian psychostasis, which involved the use of a scale to weigh the heart after death to determine a person’s fate in the afterlife. The concept of psychostasis is based on the idea of balancing good and bad deeds, as represented by the weighing of the heart against a feather. In this review, we will explore the historical development of cardiac neuromodulation, drawing inspiration from the ancient Egyptian concept of psychostasis. We will describe how the balance of different hormonal, chemical, electrical, and contrasting nervous stimuli is akin to the weighing of the heart in psychostasis. By examining the historical evolution of cardiology knowledge from ancient Egyptian psychostasis to the contemporary understanding of cardiac neuromodulation, we aim to shed light on the potential benefits and challenges of these approaches for the treatment of cardiovascular diseases. We will begin by discussing the historical development of cardiac neuromodulation, tracing its roots from ancient Egyptian psychostasis to the discovery of cardiac nerves in the 17th century. We will then examine the mechanisms and therapies of cardiac neuromodulation, highlighting its potential applications in the treatment of various cardiovascular diseases. Finally, we will discuss the relevance of cardiac neuromodulation to psychostasis, drawing parallels between the balance of good and bad in psychostasis and the balance of different stimuli in cardiac neuromodulation.
The heart was revered in ancient Egypt as the seat of intelligence, religion, and spirituality, as well as the organic engine of the body. It was regarded as one of the eight essential bodily parts. Unlike other organs, the heart needed to be carefully conserved in the mummy to ensure its immortality. Early in the first dynasty, the heart was depicted as a hieroglyph connected to eight vessels [
1,
2].
30 centuries ago, Egyptian physicians established an original theory of cardiovascular physiology that has endured for 30 centuries [
3].
The Treatise on the Heart, the first cardiology book in human history, is one of the nine sections of the
Ebers Papyrus (1534 BC) [
4]. Unfortunately, the first translations of this papyrus were very inexact, which obscured awareness of the remarkable accomplishments of medicine, particularly of Pharaonic cardiology. Thanks to more precise translations, particularly that of Thierry Bardinet [
5], we can now recognize that the Egyptians were the real pioneers of medicine as well as cardiology.
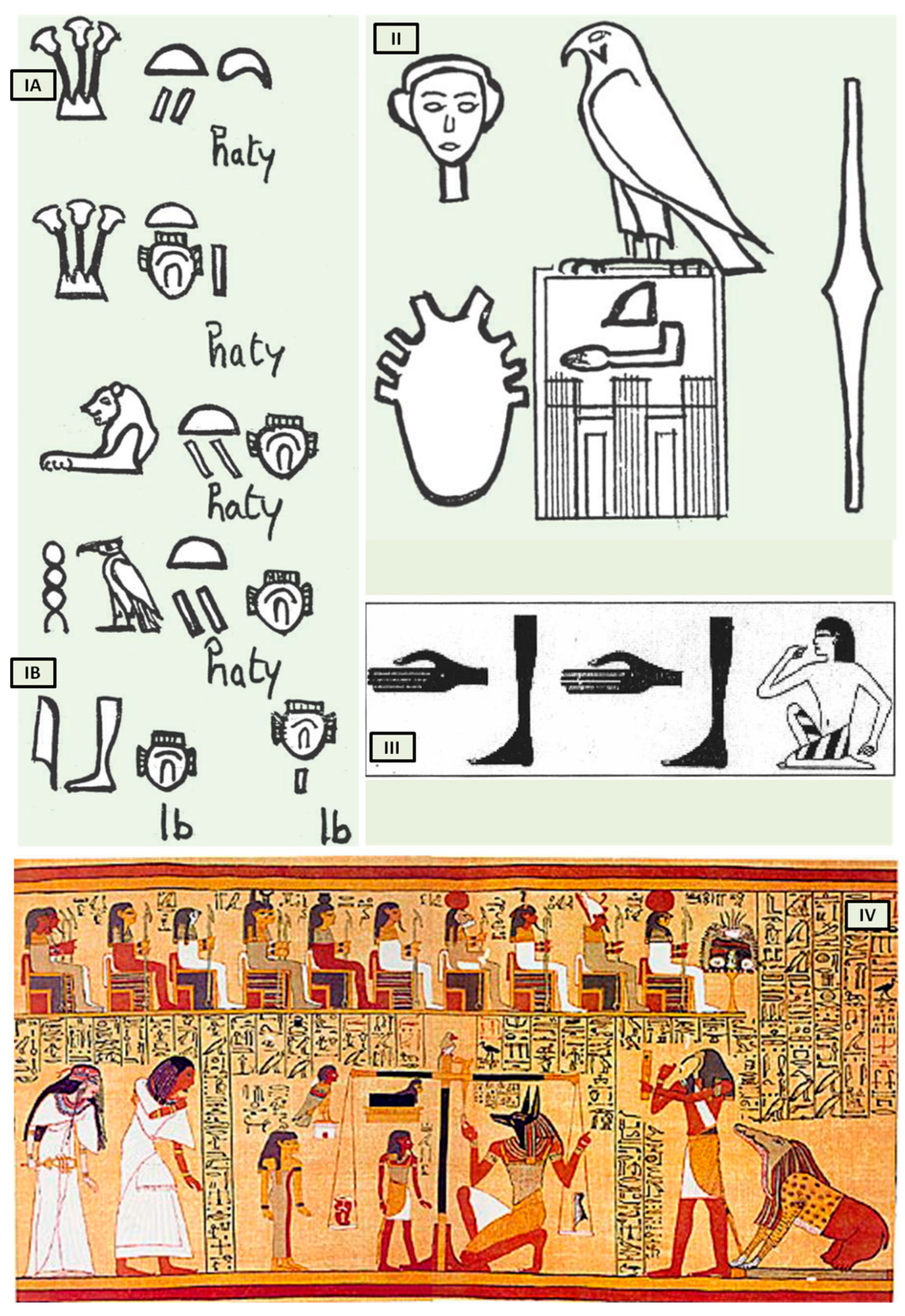
Bardinet elucidated the concept of the heart and its “components” in ancient Egypt based on graphic representations and the interpretation of hieroglyphs that represent the heart. The Egyptians, in fact, combined three distinct concepts under this term, both complementary and intricate (
Figure 1(IA)).
The first, termed heart-haty (
Figure 1(IA)), corresponded to the anatomical heart responsible for the circulation of fluids throughout the human body.
The second, defined as heart-ib or, according to Bardinet’s interpretation, interior-ib (
Figure 1(IB)), grouped the organism’s structures responsible for the whole vital function, with the exception of the heart-haty [
5].
The third, finally, symbolized the center of thought, intelligence, and memory, responsible for collecting all information from the senses, anticipating the concept of cardiac innervation.
However, the ancient Egyptians were unable to distinguish between arteries, veins, tendons, and nerves, which were all referred to as “metw”, i.e., network texture, supporting the cardiocentric doctrine (
Figure 1II). Their understanding of the heart’s structure was primarily influenced by their spiritual and religious convictions, but records demonstrate that they could link the pulse to cardiac contractions (
Figure 1III). The term heart-haty itself means “the one who stands in front, the one who commands” [
4,
6].
Despite the anatomical accuracy of the various hieroglyphs representing the heart, particularly the representation of eight vessels alluding to the pulmonary artery, aorta, superior and inferior vena cava including the four pulmonary veins (
Figure 1II), which dates back to the first dynasty (3000 BC), no other civilization contemporaneous to Pharaonic Egypt depicted the heart with such precision. This suggests that only a scholar with access to heart anatomy could have designed such hieroglyphs.
The third “constituent” of the heart, the “heart-soul”, embodies the concept of individual and social value, particularly in the context of the ancient Egyptian religion’s ceremony known as “psychostasis”. This ceremony, described in chapter 125 of the
Book of the Dead (
Papyrus of Ani, 1275 BC) [
7] (
Figure 1IV), involved the deceased being weighed against the feather of Maat, the goddess of order, truth, and righteousness. To the Egyptians, the heart, not the brain, was the source of human discernment, emotions, and memory. In the weighing ritual, the heart of the deceased was placed on a scale opposite the feather of Maat, and their fate was determined by whether the heart balanced the feather or weighed heavier (indicating a life of sin).
Although psychostasis was a fundamental and ubiquitous aspect of Egyptian thought, the earliest Egyptian literature on the afterlife, dating back to the Old Kingdom (2425–2300 BC), did not mention it. Instead, postmortem judgment was depicted using worldly courtroom symbolism and metaphors. This changed during the First Intermediate period (2200–2050 BC) with the introduction of the “Instruction for King Merikare”, which introduced the concept of measuring the deceased’s good and bad deeds in two separate piles. According to Samuel Brandon, this new element of court procedures was developed in response to Egyptians’ distrust of earthly justice [
8,
9]. By weighing good deeds against bad deeds, the afterlife judgment became more objective and impartial, ensuring that individuals’ destinies after death were based on their own actions, not on the biases of a divine judge. This concept of weighing was further developed in the Coffin Texts of the Middle Kingdom, and it was fully incorporated into Egyptian mythology during the New Kingdom (1580–1090 BC), with the Book of the Dead describing the weighing of the deceased’s heart against the feather of Maat on a set of scales.
The ancient Egyptians believed that the heart was a sentient entity that acted independently, sometimes even against its owner. Some Egyptians even personified the heart as a tiny deity residing within human beings. The heart was considered the repository of a person’s memories and intelligence, allowing them to retain both positive and negative recollections of their earthly life. Moreover, the heart represented the essence of an individual’s moral and ethical compass.
Figure 1.
The heart in ancient Egypt. (
I) The heart and the interior-ib in medical texts. (
A) Heart-haty. (
B) Heart-ib or interior-ib [adapted from B. Ziskind [
1]]; (
II) representation of the heart in the titling of the pharaoh. All the names brought by the king of Egypt constitute his title. Title of Horus Qâ, pharaoh of the 1st dynasty [adapted from B. Ziskind [
1]]; (
III) the “debdeb” hieroglyph; pulsate in the
Ebers Papyrus [adapted from B. Ziskind [
2]]; (
IV) Anubis weighing the heart of Ani. [adapted from
Papyrus of Ani, 1250 b.C, during the Nineteenth Dynasty of the New Kingdom of ancient Egypt. Egyptians dealing in illegal antiques in Luxor in 1888 were responsible for the discovery of the scroll. The purchase was made by E. A. Wallis Budge and, finally, by the British Museum [
7]].
Figure 1.
The heart in ancient Egypt. (
I) The heart and the interior-ib in medical texts. (
A) Heart-haty. (
B) Heart-ib or interior-ib [adapted from B. Ziskind [
1]]; (
II) representation of the heart in the titling of the pharaoh. All the names brought by the king of Egypt constitute his title. Title of Horus Qâ, pharaoh of the 1st dynasty [adapted from B. Ziskind [
1]]; (
III) the “debdeb” hieroglyph; pulsate in the
Ebers Papyrus [adapted from B. Ziskind [
2]]; (
IV) Anubis weighing the heart of Ani. [adapted from
Papyrus of Ani, 1250 b.C, during the Nineteenth Dynasty of the New Kingdom of ancient Egypt. Egyptians dealing in illegal antiques in Luxor in 1888 were responsible for the discovery of the scroll. The purchase was made by E. A. Wallis Budge and, finally, by the British Museum [
7]].
In Egyptian mythology, the goddess Maat, daughter of the sun god Ra, symbolized Egyptian ethics and cosmology. She embodied the Egyptian concept of cosmic and social order, while Ra himself embodied the ultimate cosmic order. Maat was regarded as the embodiment of “truth”, “justice”, “rectitude”, “equilibrium”, “cosmic law”, or “order” due to her association with social harmony. Essentially, Maat represented a standard or benchmark against which a person’s character and actions could be judged in this world [
10].
The concept of psychostasia originated in Egypt and spread to various cultures and religions [
10,
11]. In the practice of psychostasia, the ancient Greeks, Muslims, Hindus, and Buddhists, unlike the Egyptians, who weighed the heart, placed the entire body on the scales as a symbolic process to weigh the soul, fate, and actions of the individual. This symbolic act aimed to evaluate the soul based on one’s deeds and inner qualities rather than focusing on specific body parts.
The psychostasia of ancient Greece is depicted in the Iliad attributed to Homer, likely written in the late 8th or early 7th century BC. This poem uses the idea of “weighing of the souls” for judgment, with Zeus weighing the fates of Achilles and Hector (Il. 22. 209 ff., [
12]). The Greek adaptation of psychostasia differs from the Egyptian version by focusing on individual fates rather than moral worth, reflecting a pre-mortem judgment [
13,
14]. This adaptation illustrates how ancient literature tailored concepts to fit their beliefs. While specifics vary, the core purpose remains consistent: an impartial method for divine figures to determine judgment. This practice is common in many religions like Islam, Zoroastrianism, Hinduism, Buddhism, and medieval Christianity, describing post-mortem divine judgment using psychostasia imagery. In Islam, the Quran was revealed to Prophet Muhammad in the 7th century CE through the archangel Gabriel as guidance for humanity. While the Greeks weighed the fates or lives of warriors (Keres or psychai), the Quran [
15] focuses on weighing good deeds. Despite this difference, both traditions use scales for judgment, influencing one’s destiny based on what is weighed. Quranic verses like 7:8–9 and 23:102–103 mention psychostasia [
15], aligning with the ancient Greek concept of weighing souls.
In Christian depictions of the Last Judgment, Archangel Michael weighs souls on a scale, mirroring the Egyptian concept of Maat overseeing the weighing of hearts (
Figure 2).
While the Old Testament (3rd century BC) does not explicitly mention Michael weighing souls on a scale, it does metaphorically portray God assessing people’s attitudes and actions using the image of a scale of justice (Proverbs 16:11). The Testament of Abraham, a pseudepigraphic book of the Old Testament, narrates Abraham’s journey to heaven, guided by Michael. During this journey, they encounter an angel holding a scale for weighing souls. This text, likely written in the 2nd century AD, suggests that the concept of psychostasis had already permeated Jewish beliefs by this time.
Figure 2.
Archangel Michael weighs souls and defeats Satan [adapted from Guariento di Arpo (1310–1370 AD)—Padua, Museo Civico].
Figure 2.
Archangel Michael weighs souls and defeats Satan [adapted from Guariento di Arpo (1310–1370 AD)—Padua, Museo Civico].
In the New Testament (I century AD), the concept of psychostasis reappears in the original context of heart weighing:
34“Be careful, or your hearts will be weighed down with carousing, drunkenness, and the anxieties of life, and that day will close on you suddenly like a trap.” [
16].
According to Christianity, humanity has to live paying attention to its own heart, first of all, because it is the house of life and the door of God. The incarnation is not over; it happens continuously. In the Eucharist, we find the flesh of Jesus.
And again, in the New Testament, we read:
28“Come to me, all you who are weary and burdened, and I will give you rest.
29Take my yoke upon you and learn from me, for I am gentle and humble in heart, and you will find rest for your souls.
30For my yoke is easy and my burden is light.” [
17].
In Christianity, the weighing of the heart against the feather of Maat, Egyptian, which had a role of social punitive coercion (do not do evil because it damages society and you will be punished), is replaced with the weighing of one’s heart against the lightness of the incarnate sacramental bread of Christ. Jesus suffered in defense of the last and the oppressed; his heart is light because he offers mercy (from the Latin misericors and from misereor (I have pity) and cor (heart)). “Cor” of the Christian Divinity is an empathic heart. Nowadays, we could say that it is a “vagal” heart. That is, it has a calming effect on the heart itself, reduces sympathetic responsiveness, and promotes social engagement behaviors.
In this regard, all Catholic communities in the world have handed down the story of Eucharistic miracles [Blessed Carlo Acutis planned and organized The Eucharistic Miracles of the World, an international exhibition.
http://www.miracolieucaristici.org (accessed on 6 April 2022) [
18]].
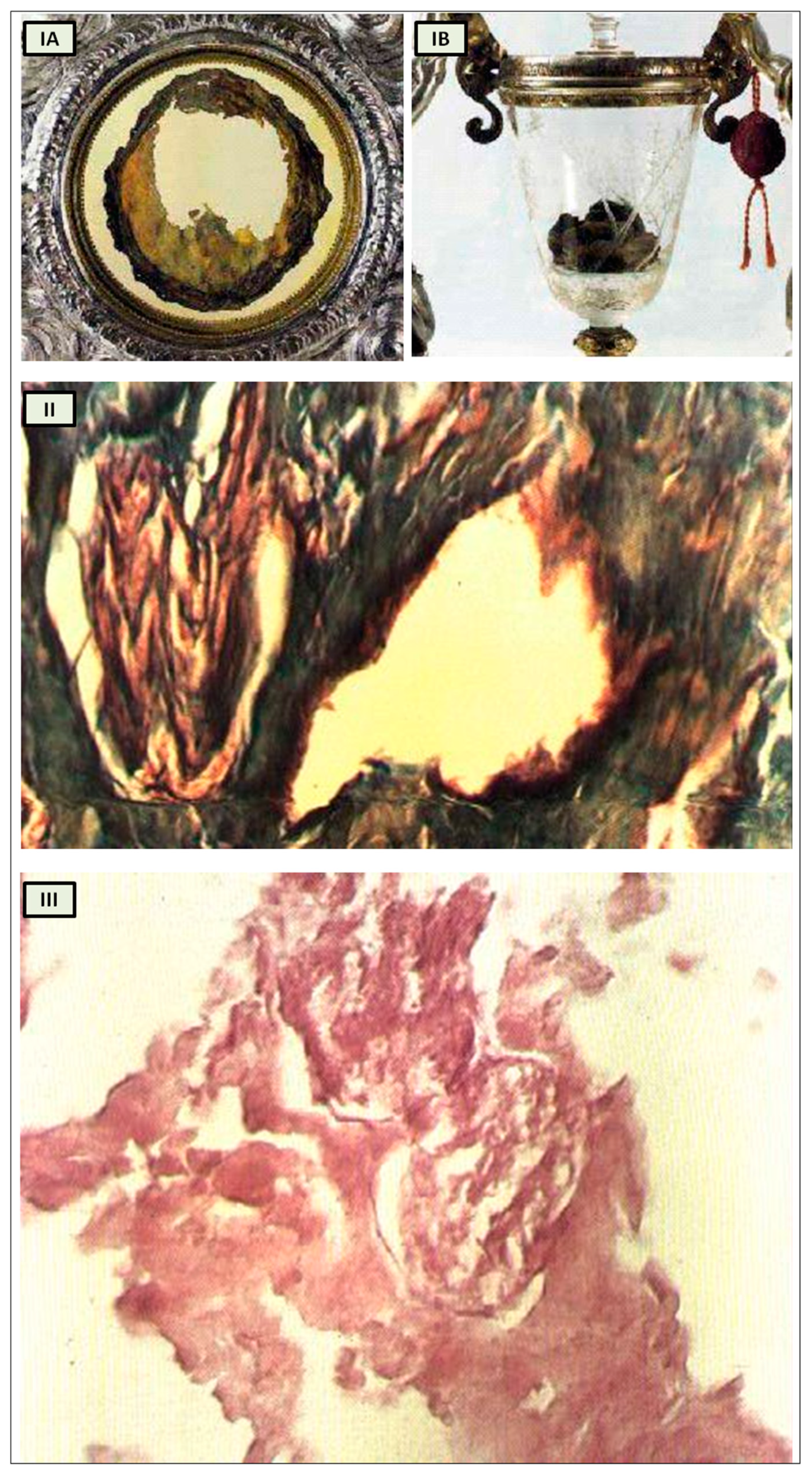
The Catholic faith does not hinge on Eucharistic miracles, and Christians are not obligated to believe in any private revelation, even those authorized by the Church. However, it is intriguing to revisit the Eucharistic miracle that occurred between 730 and 750 in Lanciano, Italy [
19], within the context of our analysis. According to historical tradition, a monk who harbored doubts about the genuine presence of Christ in the Eucharist witnessed the bread and wine transforming into flesh and blood during the Mass when he pronounced the words of consecration (
Figure 3I) [
19].
Numerous studies have attempted to analyze the relics, with varying outcomes influenced by scientific advancements over the centuries.
Professor Odoardo Linoli, a specialist in anatomy, histology, chemistry, and clinical microscopy and former head of the Pathology Laboratory at Arezzo’s “Spedali Riuniti” Hospital, conducted an examination of the specimens in 1971. He documented his findings in Quaderni Sclavo di Diagnostica Clinica e di Laboratori, which was indexed in PubMed [
20]. In 1981, Ruggero Bertelli, a retired professor of human anatomy at the University of Siena, corroborated Linoli’s analysis.
The reports of the investigations into the ancient flesh of Lanciano conducted in 1971 and 1981 indicate that it is striated muscle tissue that appears to be of cardiac origin owing to its extensive syncytial connections between the fibers, as well as arterial and venous vessels and two slender branches of the vagus nerve [
19].
While it undoubtedly does not confirm that the Lanciano host of the 8th century is the original one transformed into myocardium, this remaining an exclusively faith-based matter, it nonetheless offers us an anthropological and historical perspective from which to approach the study of cardiac innervation through the evolution of the psychostasis concept. Strikingly, the histological features of the Lanciano host’s flesh closely resemble, from a neuroanatomical standpoint as well, the previously proposed description of psychostasis in paleo-Christianity, particularly the “vagal heart” of Jesus (
Figure 3II,III) [
19].
Figure 3.
Miracle Heart in Lanciano, Italy. (
I) The relics of Lanciano’s Eucharistic miracle. When an 8th-century priest questioned Christ’s presence in the Eucharist, the host was turned into flesh (
A) and the wine into blood (
B); (
II) Miracle Heart in Lanciano. Mallory × 250. A branch of the vagal nerve and an artery; (
III) Miracle Heart in Lanciano. Eosine multiplied × 350. A vagal nerve branch. The perineurium is narrow, and the fascicular structure is well preserved. [adapted from N. Nasuti [
19]].
Figure 3.
Miracle Heart in Lanciano, Italy. (
I) The relics of Lanciano’s Eucharistic miracle. When an 8th-century priest questioned Christ’s presence in the Eucharist, the host was turned into flesh (
A) and the wine into blood (
B); (
II) Miracle Heart in Lanciano. Mallory × 250. A branch of the vagal nerve and an artery; (
III) Miracle Heart in Lanciano. Eosine multiplied × 350. A vagal nerve branch. The perineurium is narrow, and the fascicular structure is well preserved. [adapted from N. Nasuti [
19]].
In modern times, we can inquire into the scientific basis of anima-bios well-being as reflected in the concept of a “light heart”. Does it hold physiological significance to say that an individual approaches life with a “light heart”? Which autonomic nervous system (ANS) component could be activated in this sensation?
Conversely, what factors contribute to feelings of fatigue, a “heavy heart”, and depression, common symptoms associated with stress and pathologies prevalent in the industrial age?
Building on these considerations, let us examine the symptoms and sequelae of a novel respiratory infection that emerged in December 2019 in Wuhan, China.
This newly identified coronavirus, originating from animals (zoonotic), spread rapidly around the world. In response, the World Health Organization (WHO) designated it as 2019-nCoV, signifying a novel coronavirus from 2019. The disease caused by this virus was later named COVID-19, and on 11 March 2020, WHO declared it a global pandemic.
COVID-19 is responsible for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), manifesting as atypical pneumonia alongside flu-like respiratory symptoms. This infection poses a significant global health threat, leading to post-acute sequelae of COVID-19 (PASC) in affected individuals. Studies indicate that 30% of COVID-19 patients experience persistent symptoms for up to 9 months post-infection. Those recovering from COVID-19 may endure prolonged symptoms categorized as long COVID or post-COVID syndrome [
21,
22].
Long COVID presents various adverse outcomes, including cardiovascular, thrombotic, and cerebrovascular diseases, type 2 diabetes, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), postural orthostatic tachycardia syndrome, and other dysautonomic events. Effective treatments for these debilitating symptoms are yet to be validated, with some conditions expected to persist for years or even a lifetime. It remains unclear whether long COVID is a different disease entity with unclear pathophysiology or a spectrum of prolonged viral infection.
Respiratory distress observed in COVID-19 and long COVID patients is not solely attributed to atypical pneumonia. Research suggests that aberrant ventilatory responses may stem from direct brainstem involvement and para-infective mechanisms affecting the peripheral nervous system [
23,
24].
In particular, the study by Jareonsettasin et al. explores the intriguing phenomenon of impaired perception of dyspnea to tachypnea in COVID-19 patients, shedding light on the underlying mechanisms driving this inappropriate ventilatory response. The research uncovers the role of increased afferent feedback from chest wall mechanoreceptors and muscle stretch receptors, highlighting the abnormality where a significant portion of patients exhibit a reduced dyspneic response to tachypnoea. This anomaly is attributed to factors such as maintained neuromechanical coupling and potential interruptions in central processes when comparing the expected and actual consequences of breathing motor commands. Furthermore, the study delves into post-COVID syndrome, particularly focusing on the prevalence of breathing pattern disorder (BPD) post-discharge and the subsequent recovery trajectory for most patients. The findings suggest a complex interplay of factors influencing dyspneic perception and breathing control in COVID-19 patients, urging further research to unravel these intricate mechanisms for improved clinical management and long-term outcomes.
Literature data link autonomic dysfunction in long COVID with persistent feelings of fatigue, chest oppression, depression, postural orthostatic tachycardia syndrome (POTS), and cognitive impairment following SARS-CoV-2 infection [
25,
26].
The Greek myth of Sisyphus provides a powerful representation of the experience of angor, the dysautonomic chest discomfort that can linger in long COVID patients. Sisyphus was condemned by the gods to an eternal punishment: he had to roll a massive rock up a hill, only to watch it roll back down when it reached the top, forcing him to start over in an endless cycle. Paraphrasing Sisyphus, one could say that “Sisyphus’ pandemic boulder” primarily affects the heart as well as the mind, and the challenge of modern cardiological pathophysiology should be the attempt to recognize the psycho-vegetative mechanisms of cardiac oppression that characterize the cardiac alterations present in PASC and in the distress of modern life.
Just as with Albert Camus’ Sisyphus, scientific research endeavors to transform detrimental endogenous and exogenous stimuli impacting cardiac contractility into a regenerative process of plastic modulation that restores ‘cardiac lightness’ and a state of well-being. Camus’ Sisyphus manages to transmute daily toil into a sense of happiness and adequacy: ‘I leave Sisyphus at the foot of the mountain!’ One always finds one’s burden again. But Sisyphus teaches the higher fidelity that negates the gods and raises rocks. He, too, concludes that all is well. This universe henceforth without a master seems to him neither sterile nor futile. Each atom of that stone, each mineral flake of that night-filled mountain, in itself forms a world. The struggle itself toward the heights is enough to fill a man’s heart. One must imagine Sisyphus happy.’” [
27].
In the forthcoming sections, we will delve into the neural network governing cardiac activity by exploring the concept of cardiac neuromodulation. Our exploration will encompass the neurophysiological foundations of cardiac innervation, the principles underlying the modern ortho-parasympathetic integration concept, the emotional aspects of respiratory sinus arrhythmia (RSA), and the significance of identifying neurocardiac genes.
A comprehensive examination of both intrinsic and extrinsic cardiac innervation will undoubtedly enhance scientific research undertakings aimed at elucidating the neurovegetative mechanisms underlying dysautonomic disorders present in various diseases, including PASC syndromes.
4. The Cardiac Neuromodulation
The autonomic nervous system stands out as a distinct anatomical and functional division of the nervous system. This distinction traces back to Marie François Xavier Bichat (1771–1802).
In his seminal work “Physiological Researches upon Life and Death” (1800), Bichat defined life as the sum of those functions that resist death and observed that living organisms constantly strive to maintain themselves in a state of equilibrium despite the destructive forces that surround them. Bichat further divided life into two distinct domains: organic life (“vie organique”), also known as the vegetative system, which corresponded to the process of nourishment, and animal life, (“vie animale”), or the animal system, which corresponded to the process of interaction with the environment.
Bichat conceptualized organic life as the essential functions of the heart, intestines, and other internal organs, controlled by a system of ganglia (“ganglions”), clusters of independent neural centers (little indipendent brains) in the chest cavity. In contrast, animal life pertained to symmetrical organs (eyes, ears, and limbs), governed by habits and memories but primarily driven by intelligence and astuteness. This aligned with the brain’s characteristic attributes, yet it would not be feasible without the heart, the cornerstone of organic life. Bichat’s use of the term “animale” harkens back to the Latin noun “anima” or soul [
52]. In this manner, Bichat effectively shifts the traditional paleochristian conception of the soul’s abode from the “heart” to the “brain”, a hallmark of the Enlightenment era.
Building upon Bichat’s foundational work in delineating the autonomic nervous system, 19th-century researchers made significant strides in elucidating the specific neural mechanisms governing the heart’s function and cardiac autonomic control.
In 1846, the Weber brothers, Eduard Weber and Ernst Heinrich Weber, published their findings on the inhibitory effects of vagus nerve stimulation on the heart rate in their work “Muskelbewegung” [
53,
54,
55].
Concurrently, in the 1850s, researchers such as Carl Stelling [
56], Claude Bernard [
57], and Charles-Édouard Brown-Séquard [
58] identified the sympathetic nerves as pressor (blood pressure-raising) nerves, in contrast to the vagus nerve’s inhibitory effects, as documented in their works. Building on this, in 1866, Carl Ludwig [
59] and then Ewald Hering [
60,
61], described the vasomotor reflexes, which involve the autonomic regulation of blood vessel diameter and blood pressure.
In 1886, Langley employed nicotine and other medications to meticulously investigate the sympathetic and parasympathetic nervous systems, which he subsequently unified under the concept of the ‘autonomic nervous system’ [
62].
Finally, in 1946, Ulf Svante von Euler demonstrated that the sympathetic transmitter substance was noradrenaline (norepinephrine), a significant discovery in understanding autonomic neurotransmission in the cardiovascular system, as published in his paper “A Specific Sympathomimetic Ergone in Adrenergic Nerve Fibres (Sympathin) and its Relations to Adrenaline and Noradrenaline” [
63].
These pioneering researchers laid the foundation for the modern understanding of the autonomic nervous system’s influence on cardiac function and homeostasis.
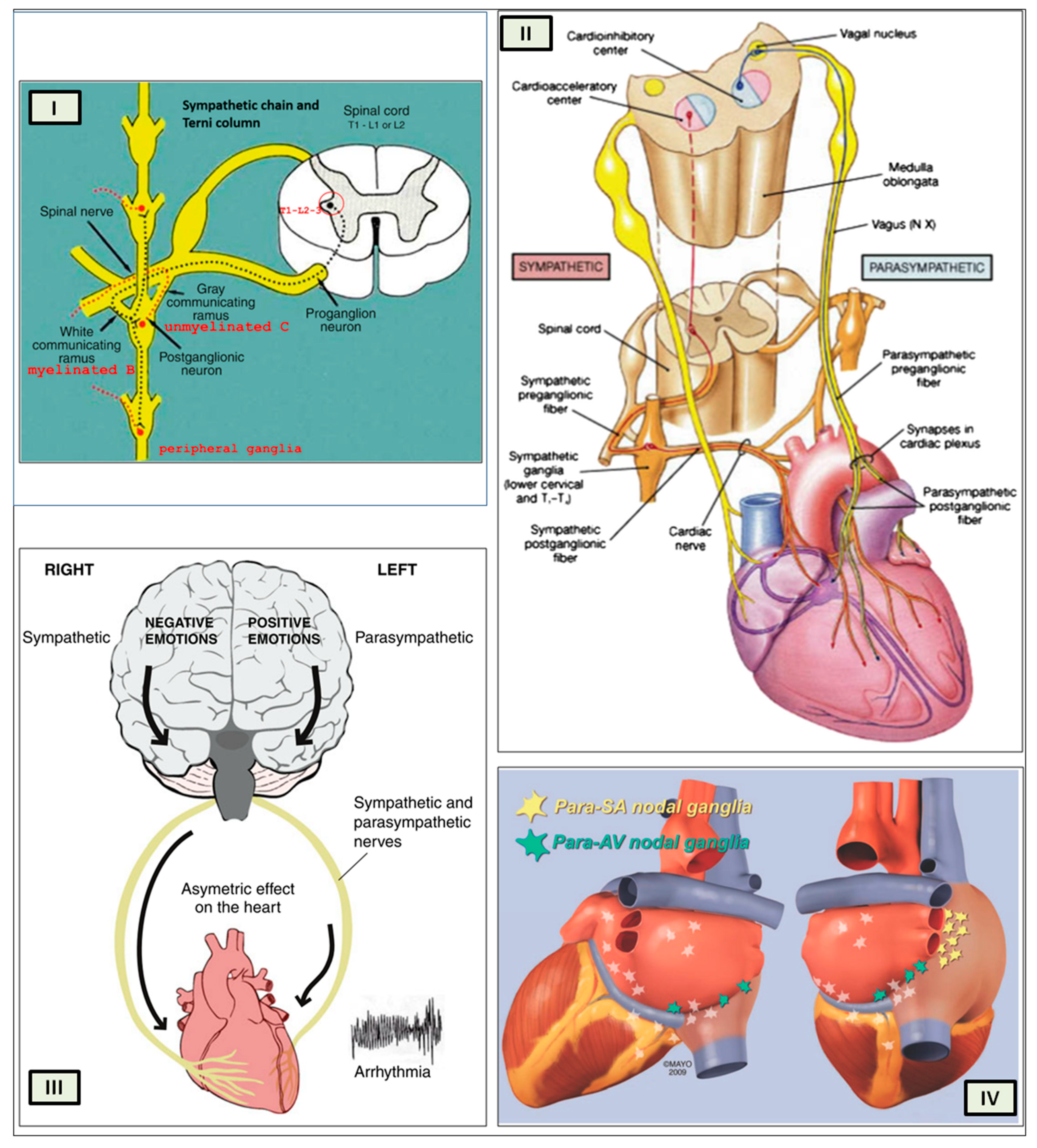
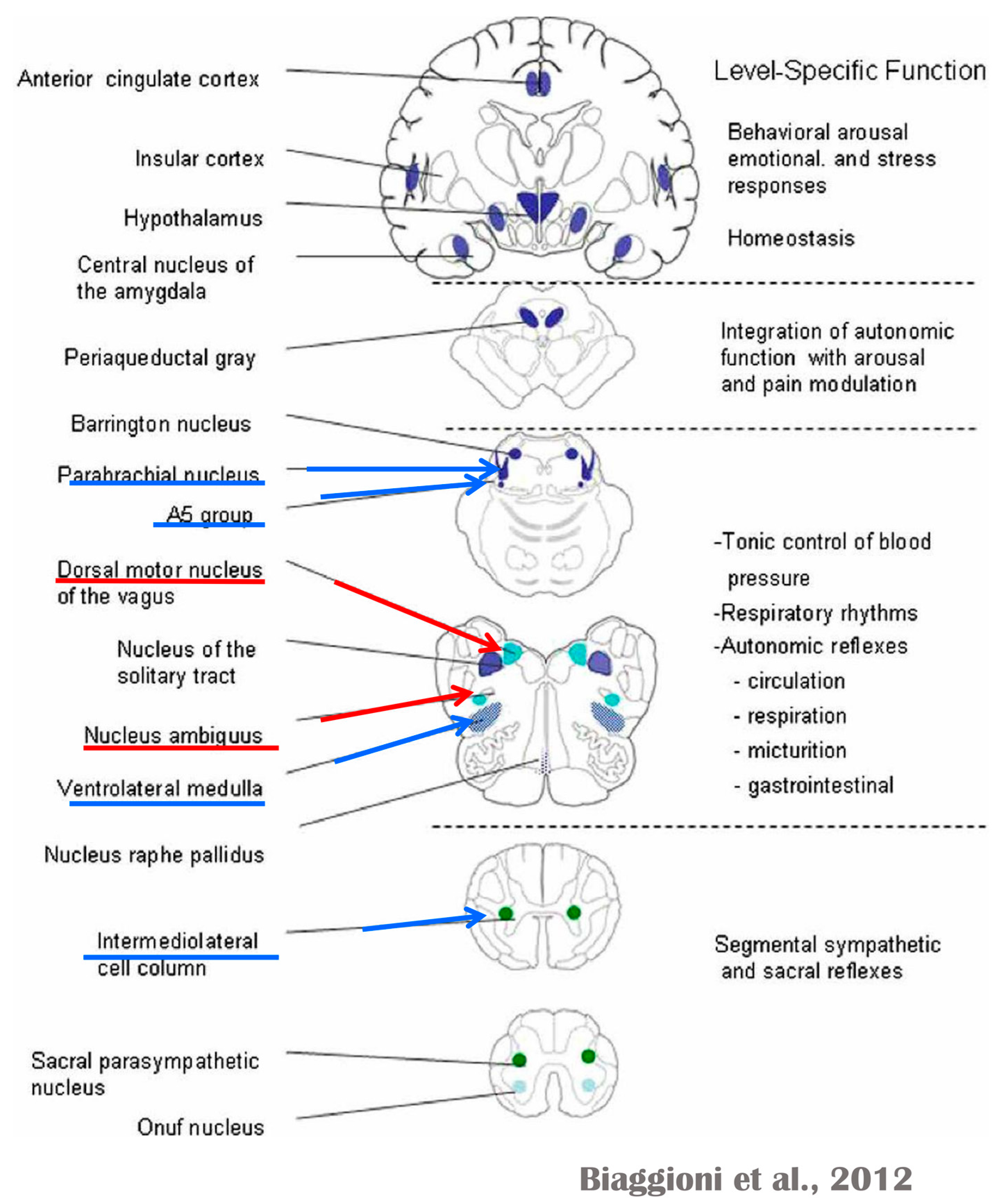
Several regions of the neuraxis orchestrate the central control of orthosympathetic and parasympathetic output. This intricate network of central autonomic connections plays a pivotal role in regulating visceral functions and maintaining homeostasis by adapting to internal and external stimuli. The central autonomic system is hierarchically organized into four distinct levels: spinal, bulbopontine, pontomesencephalic, and forebrain (
Figure 6 and
Figure 7) [
64,
65,
66,
67,
68,
69].
The cardiac preganglionic parasympathetic axons of the vagus nerve (
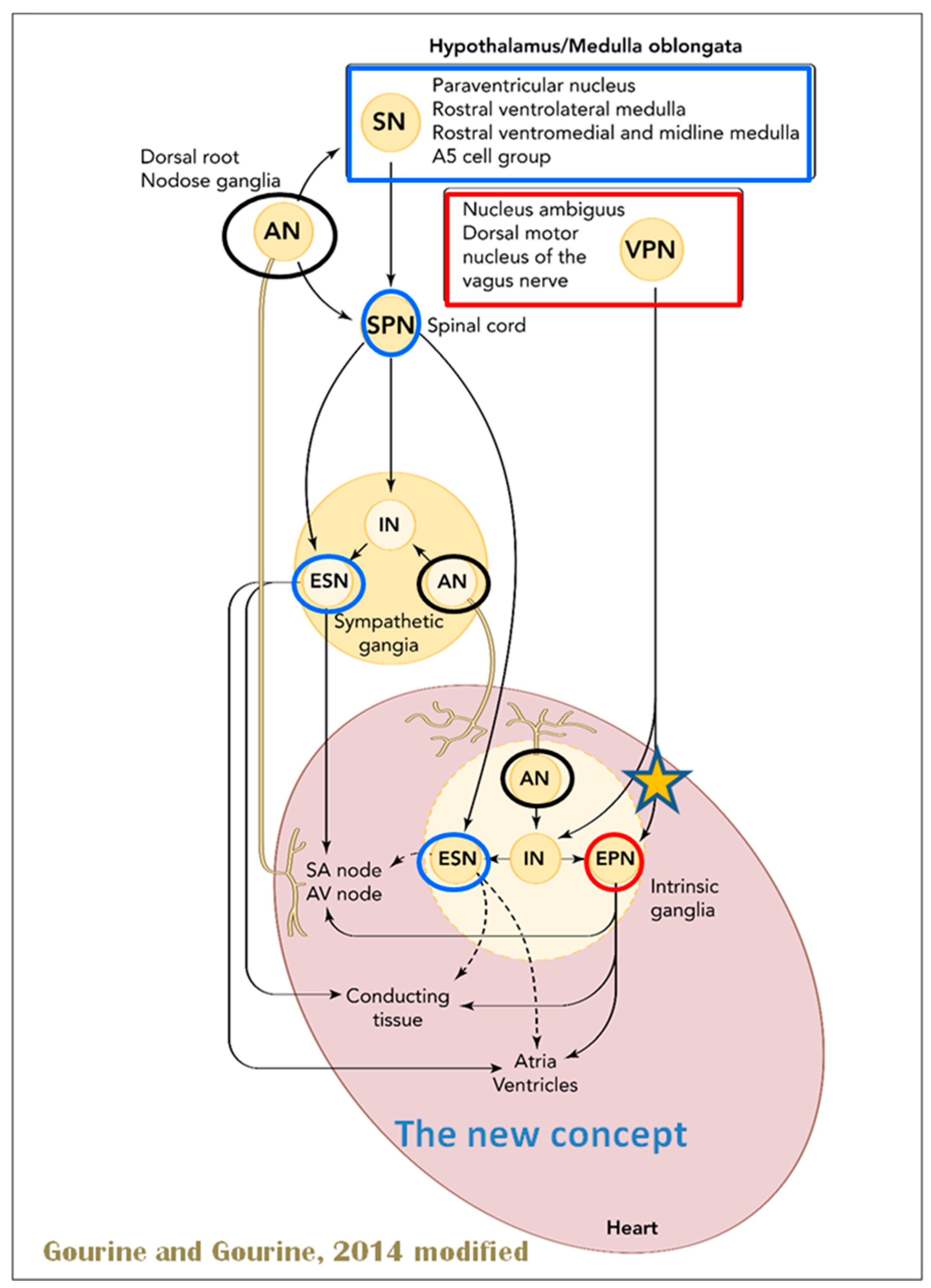
Figure 6 in red) primarily arise from medullar neurons placed in the dorsal motor nucleus of the vagus (DMV), expanding presumably wider into the ventrolateral portion of the nucleus ambiguus (NAmb). It has been suggested that preganglionic parasympathetic axons originating from neuronal somata adjacent to NAmb are B fibers slowing rapidly the heart rate, conduction, and force of myocardial contraction. A second group of cardiac parasympatethic preganglionic axons originates from the dorsal motor nucleus (DMV), and these are the C fibers propagating slower neural impulses. Selective stimulation of the DMV axons affects atrioventricular conduction and ventricular contraction and reduces the heart rate (HR); however, it starts off more slowly and exhibits a more varied pharmacology than what is observed after stimulating the vagus nerve’s B fibers. The two groups of neurons have somewhat different discharge patterns, apart from conduction speed. While the DMV neurons show a non-uniform, non-respiratory-dependent discharge and are unresponsive to baroreceptors and chemoreceptors, the cardiac neurons next to NAmb exhibit a respiratory rhythm and receive input from both sources. These features of medullar parasympathetic neurons have implied the theory that they presumably reflect the differential functions of the two groups. An experiment that targets distinct populations of intrinsic cardiac ganglionic cells by nerve terminals from the proper DMV and those near the NAmb, each anterogradely labeled with a different fluorescent tracer, supports this theory [
70]. It is also possible that DMV neurons innervate small, intensely fluorescent (SIF) cells in the ganglia. On the contrary, Jones [
71] has hypothesized that the tonic input transmitted by the more slowly moving C fibers may interconnect with the rhythmic respiratory input carried by the faster vagal B fibers on the same cardiac ganglia.
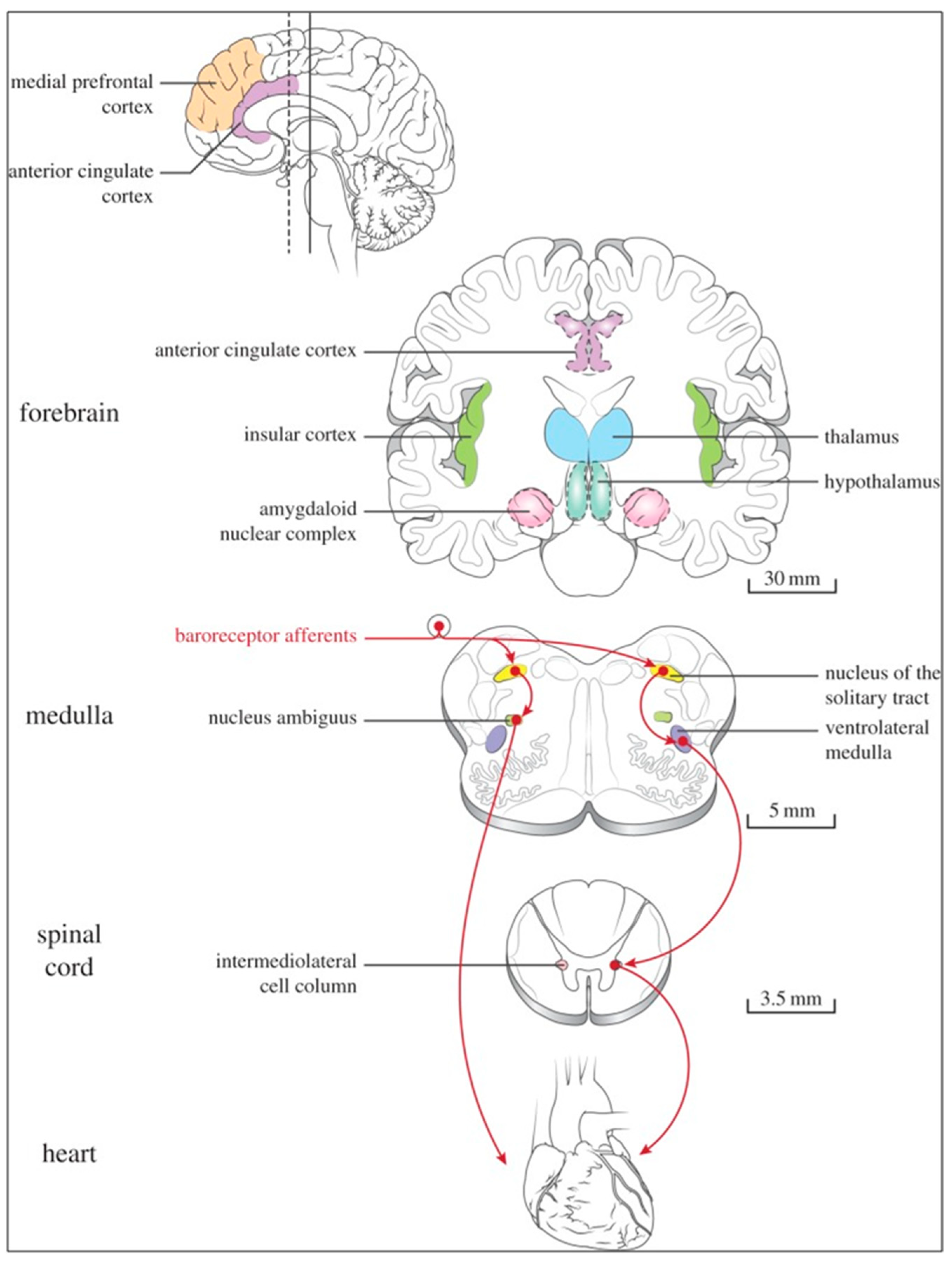
At the spinal cord level, segmental sympathetic or sacral parasympathetic reflexes mediate specific responses to stimuli, albeit under the influence of higher brain regions. These reflexes play a crucial role in maintaining cardiovascular, respiratory, gastrointestinal, and micturition functions. The lower brainstem, also known as the bulbopontine level, plays a pivotal role in reflex control of core physiological processes. It regulates circulation, respiration, gastrointestinal functions, and micturition through a network of interconnected neurons. The upper brainstem, referred to as the pontomesencephalic level, engages in sophisticated pain modulation and behavioral integration in response to stress. It orchestrates physiological and psychological adaptations to challenging situations. The forebrain level encompasses the hypothalamus, the master regulator of the autonomic nervous system. It interacts with the anterior limbic circuit, a network comprising the insula, anterior cingulate cortex, and amygdala, to modulate emotional and visceral responses.
The cortex of the insula functions as the primary interoceptive cortex and combines sensations of the viscera, pain, and temperature. The dorsal insula has an organization that is specialized for visceral processing and acquires inputs from gustative, visceral, muscle, and skin receptors through the thalamic region. Through their connections with limbic and neocortical association areas, the neurons of the dorsal portion of the insula extend to the right anterior insula, which connects these interoceptive signals with emotional and cognitive elaboration. As a result of this integration, one can consciously sense their body. Additionally, the insula acts as a visceromotor area and controls both sympathetic and parasympathetic outputs, primarily through a relay in the lateral hypothalamus.
The anterior cingulated cortex is connected to the anterior portion of the insula and is divided into ventral (emotional and default mode network), and dorsal (cognitive and frontoparietal awareness networks) regions. Subcallosal and precallosal regions make up the cortical ventral anterior part of the cingulate gyrus, which is connected to the brainstem, prefrontal cortex, amygdala, hypothalamus, and insula on an extensive level. Through their connections, the anterior cingulate cortex regulates autonomic systems.
The amygdala enriches the neuronal sensory afferents with an affective and/or emotional connotation through a downstream neuronal network with which it participates in the neuroendocrine and autonomic responses to stress. Through its extensive connections with the hypothalamus and brainstem, including the periaqueductal gray and the medullary reticular region, the central nucleus of the amygdala (CeA) plays a key role in the coordination of stress responses, particularly alarm responses. The hypothalamus is a center for visceromotor activity and starts selective patterns of autonomic and endocrine responses based on various stimuli such as variations in the temperature of the blood, hypoglycemia, osmolarity, or outside agents of stress. The preoptic-hypothalamic area is divided into three functional regions: periventricular, medial, and lateral. The periventricular zone comprises the circadian pacemaker, localized in the suprachiasmatic nucleus, and numerous regions implicated in neuroendocrine management through the pituitary gland. The medial zone includes the medial preoptic area, dorsomedial nucleus (DMH), and paraventricular nucleus (PVN), which are involved in osmoregulation, thermoregulation, and stress reactions. The PVN, DMH, and lateral hypothalamic areas are the primary autonomic efferences of the hypothalamus. The PVN contains several groups of neurons that are selectively activated during stress responses, including magnocellular neurons that release arginine-vasopressin (AVP) into the systemic circulation, neurons that release corticotropin-releasing hormone and stimulate the adrenocortical axis, and neurons that project to the brainstem and spinal cord autonomic nuclei. The PVN regulates stress reactions, food and sodium intake, glucose metabolism, and cardiovascular, renal, gastrointestinal, and respiratory functions through these efferences. The DMH is involved in stress responses, thermoregulation, and cardiovascular control. Arousal, eating, and reward-driven behaviors are all regulated by hypocretin/orexin neurons in the posterior lateral hypothalamus.
The periaqueductal gray matter of the midbrain (PAG), the parabrachial nucleus (PBN) of the pons, and various medullary regions, such as the nucleus of the solitary tract (NTS), the ventrolateral reticular formation of the medulla, and the medullary raphe (
Figure 6 and
Figure 7) are all involved in autonomic output [
65]. The PAG is composed of several longitudinal columns that coordinate the micturition reflex, participate in cardiovascular responses related to respiratory regulation, and modulate pain through their diverse spinal, brainstem, and cortical connections. The PAG integrates forebrain and lower brainstem activity in response to tasks including stress, pain control, and somatic adaptative changing. The PBN is an important location for relaying information to the hypothalamus, amygdala, and thalamus from the spinal cord’s converging visceral, thermoreceptive, and discomfort stimuli. The PBN in particular plays a significant role in the regulation of the digestive, cardiac, and breathing systems. The NTS has multiple subnuclei that are arranged “viscerotropically” (i.e., with a special affinity for various organs) and acts as the first relay station for flavor and visceral afferent information. Therefore, taste inputs are allowed in the rostral region of the NTS, gastrointestinal afferents are allowed in the intermediate part, and baroreceptor, cardiac, chemoreceptor, and pulmonary afferents are allowed in the caudal part. The NTS sends this neuronal input to the upper brainstem and forebrain areas, either directly or via the PBN. As a result, the NTS serves as the first central “switch” station for all medullary reflexes controlling the function of the heart (baroreflex and cardiac reflexes), the breathing process (carotid chemoreflex and pulmonary mechanoreflexes), and digestive tract peristalsis. The rostral ventrolateral medulla (RVLM), which contains the C1 group of adrenergic neurons holding epinephrine, is a crucial region for the control of arterial blood pressure. The sympathoexcitatory RVLM neurons collect and combine a wide range of inputs from the brainstem and forebrain, particularly the hypothalamus, including the PVN. The efferent neurotransmission of glutamate in the RVLM sends direct and rhythmic stimulation to sympathetic preganglionic neurons, which govern cardiac function and peripheral resistance in general. The cardiopulmonary reflexes, baroreflexes, and chemoreflexes belong to the reflexes under the control of the RVLM. These reflexes are mediated by inhibitory GABA neurons in the distal ventrolateral medulla, which receives inhibitory signals from the baroreceptor-sensitive neural pathway in the NTS.
GABAergic neural populations of the caudal ventrolateral medulla preserve a rhythmic, constantly slow inhibitory manage on the upper ventrolateral medulla and retransmit the inhibitory signals from the NTS, producing the inhibitory element of the sympathetic arterial baroreflex. Data from neural stimulations show that the caudal medulla holds pressure-dependent regions too. Furthermore, the A1 neuronal population in the distal ventrolateral medulla releases norepinephrine to the hypothalamus and is implicated in a reflex route that causes the production of vasopressin or arginine vasopressin (AVP) in reaction to hypovolemia or hypotension.
The upper portion of the ventromedial medulla, which includes the caudal raphe nuclei, is important for body temperature control, pain regulation, and autonomic respiratory control. Through input to preganglionic sympathetic neurons, which cause effective cutaneous vasoconstriction and thermogenesis without shivering in brown adipose tissue, one group of medullary raphe neurons initiates adrenergic responses to cold.
The maintenance of circulation, temperature control, and displacing of localized blood flow during stress and exercise depend heavily on the sympathetic efferences. Pregangliar neurons, which are mostly found in the intermediolateral cell column of the thoracolumbar spinal cord’s T1 to L2 segments, are the source of sympathetic efferences. The intermediolateral neurons are organized into segregated functional units that give the innervations to specific subgroups of sympathetic ganglion neurons and collect different separate portions of afferent inputs, triggering segmental somatic and viscerosympathetic reflexes. Premotor neurons in the brainstem and hypothalamus recruit different pregangliar sympathetic units in an integrated manner to start particular patterns of responses to particular internal or external events that cause stress, such as exposure to extreme temperature, low blood sugar, dehydration, changes of posture, physical activity, and/or stress. The principal sources of premotor sympathetic innervation include the RVLM, medullary raphe, A5 noradrenergic neuronal population of the pons, PVN, and lateral hypothalamic region.
Opposite to the sympathetic system, which affects multiple effectors, the parasympathetic system mediates organ-specific reflexes. Most vagal preganglionic parasympathetic neurons, which are arranged viscerotropically and innervate local ganglia in the liver, pancreas, enteric nervous system (ENS), and respiratory tract, are found in the DMV. The DMV generates all vago-vagal responses controlling digestive tract movement and secretion after receiving information from the NTS. The dorsal motor nucleus is the source of a substantial population of cardiac vagus nerves (
Figure 6).
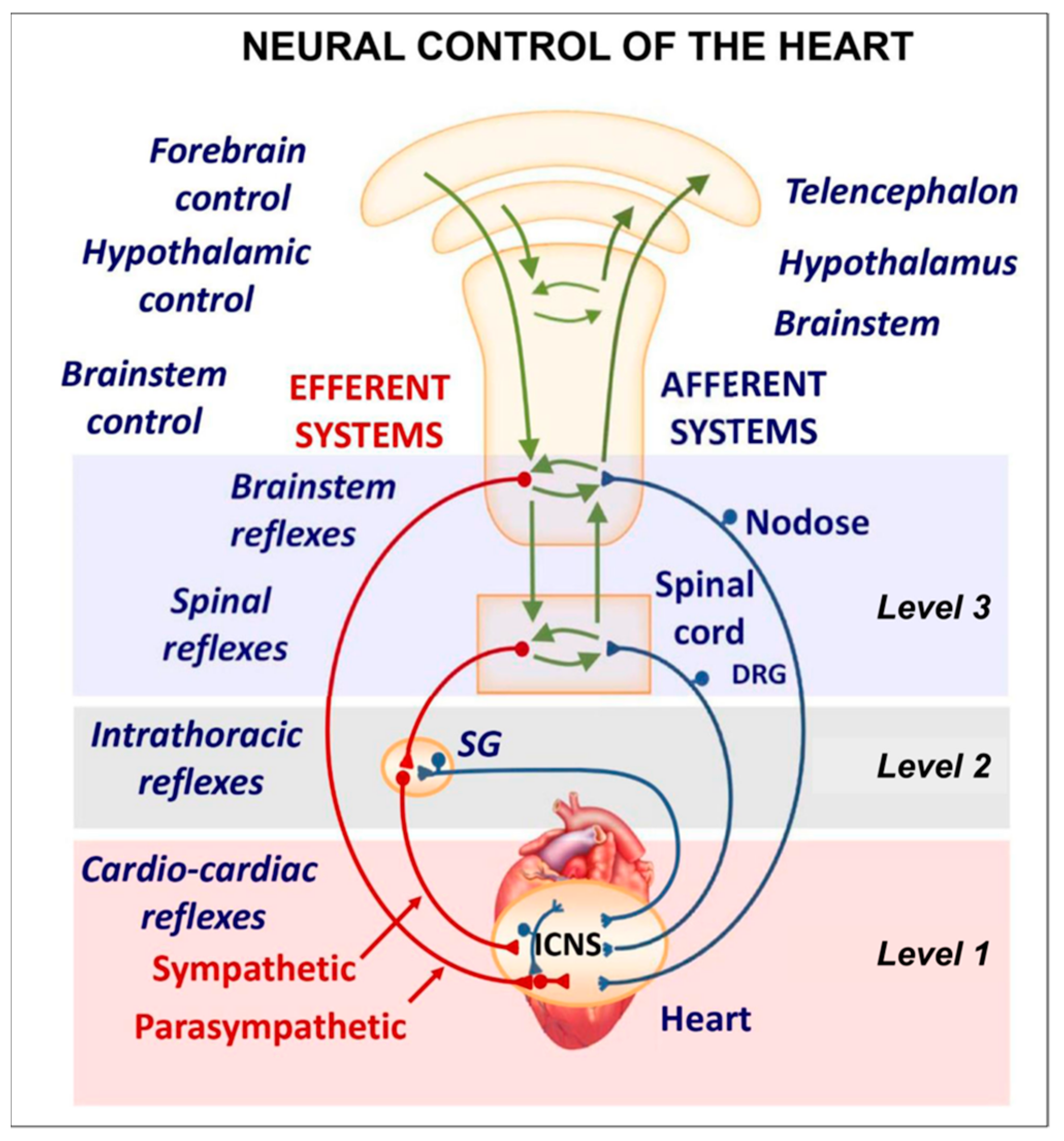
The NTS activates the cardiovagal neurons next to the NAmb during the baroreflex and inhibits them during inspiration, as will be covered in greater detail later. For life in a constantly changing environment and to maintain homeostasis, interactions between the heart and the brain are crucial (
Figure 8) [
72,
73]. Neurons located in the lateral gray matter at the S2–S4 segments of the sacral spinal cord are the source of the sacral preganglionic output. With synchronized interactions with both lumbar sympathetic neurons at the T12-L2 levels and somatic motor neurons of the Onuf nucleus at the S2–S4 levels, which innervate the external urinary sphincter and pelvic floor, these neurons play a crucial role in normal defecation, micturition, and sexual organ function (
Figure 6) [
64].
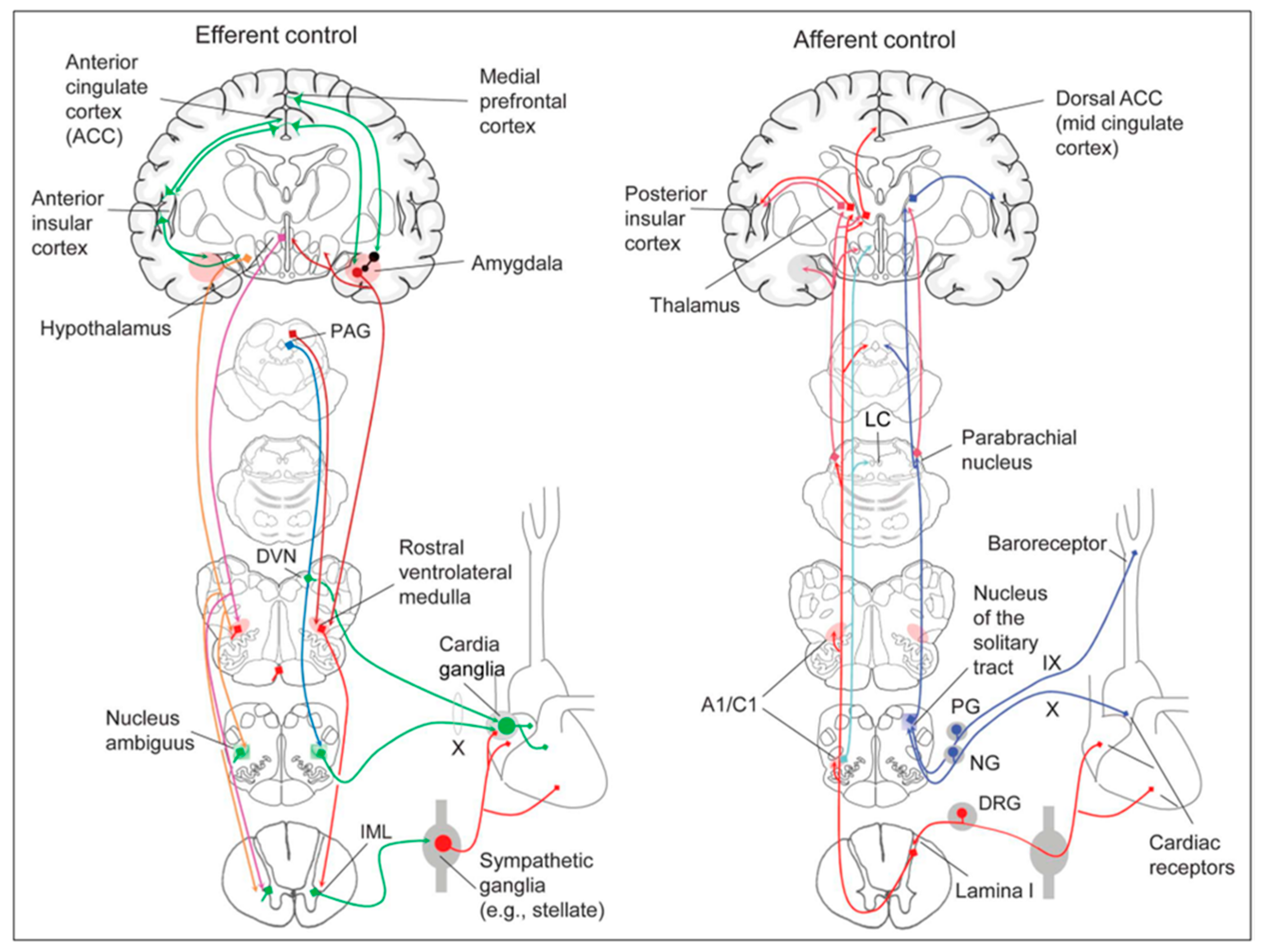
Cardiac function is controlled neurally by the anterior insular region, anterior part of the cingulate gyrus cortex (ACC), amygdala, hypothalamus, periaqueductal gray matter, parabrachial nucleus, and several medulla areas. Further, these regions are connected to homeostatic reflexes, responses to emotions, and stress reactions. By means of the sympathetic and parasympathetic neural systems, they govern and regulate heart rate (HR) and cardiac contractility (
Figure 9) [
75]. It is still unclear if this central control might be lateralized.
The heart’s intrinsic electrophysiological properties arise from pacemaker activity generated by specialized cardiomyocytes within the cardiac intrinsic conduction system, comprising the sinoatrial (SA) node, atrioventricular (AV) node, bundle of His, and Purkinje fiber network. The HR, excitability, and contractile function of these cardiomyocytes depend on the interplay between their intrinsic characteristics and regulation by the vagus and sympathetic nerves via the intrinsic cardiac ganglionated plexus, or intrinsic cardiac nervous system (ICNS) [
76].
The HR (chronotropism) is regulated by the SA node’s spontaneous depolarization (automatism), which is controlled by a “voltage regulator”. The voltage regulator is produced by the cyclic activation and deactivation of different membrane ion channels, and a “calcium regulator” that is triggered by the rhythmic release of Ca
2+ from the sarcoplasmic reticulum via the ryanodine receptor 2 (RYR2). The rhythmic increase in cytosolic Ca
2+ activates the Ca
2+-Na+ exchanger current, leading to depolarization [
77,
78,
79].
The cardiac cycle starts with depolarization spreading through connexin channels to neighboring cardiomyocytes, followed by the opening of voltage-gated Na+ (Nav1.5) channels. These channels are rapidly inactivated by depolarization, which activates both L-type calcium channels accountable for the action potential plateau and voltage-gated K+ channels responsible for repolarization. The synchronized activity of these channels produces the excitability of the His-Purkinje system (bathmotropism), the velocity of AV conduction, or dromotropism (PR interval), and the duration of the cardiac action potential (QT interval). Systolic contraction (inotropism) occurs through excitation-contraction coupling, when calcium released from the sarcoplasmic reticulum through RYR2 binds to the troponin complex and activates the contractile machinery. Cellular relaxation during diastole (lusitropism) occurs when cytosolic Ca2+ is removed by the sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) uptake pump, which is negatively regulated by the protein phospholamban (an inhibitor of Ca2+-ATPase).
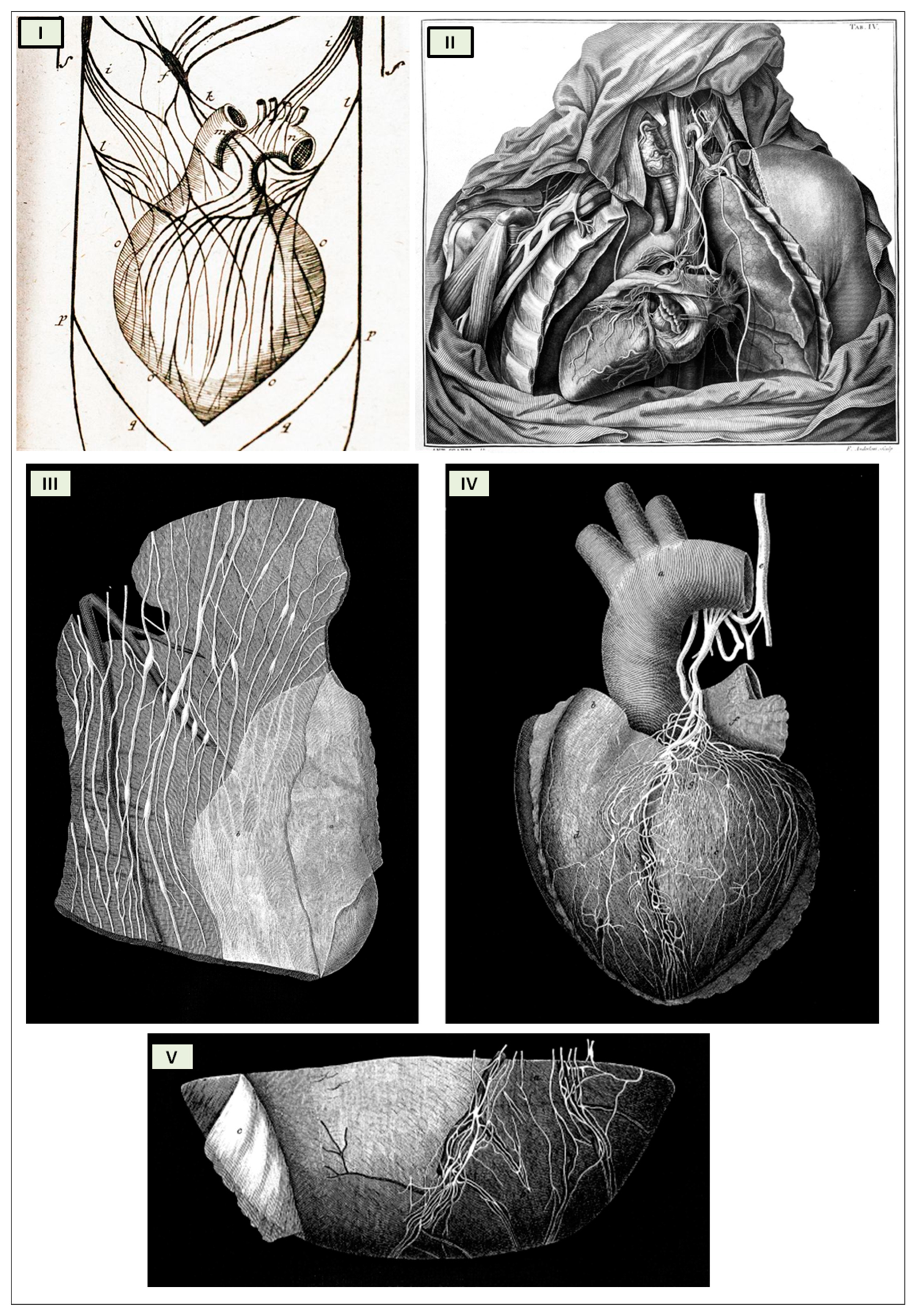
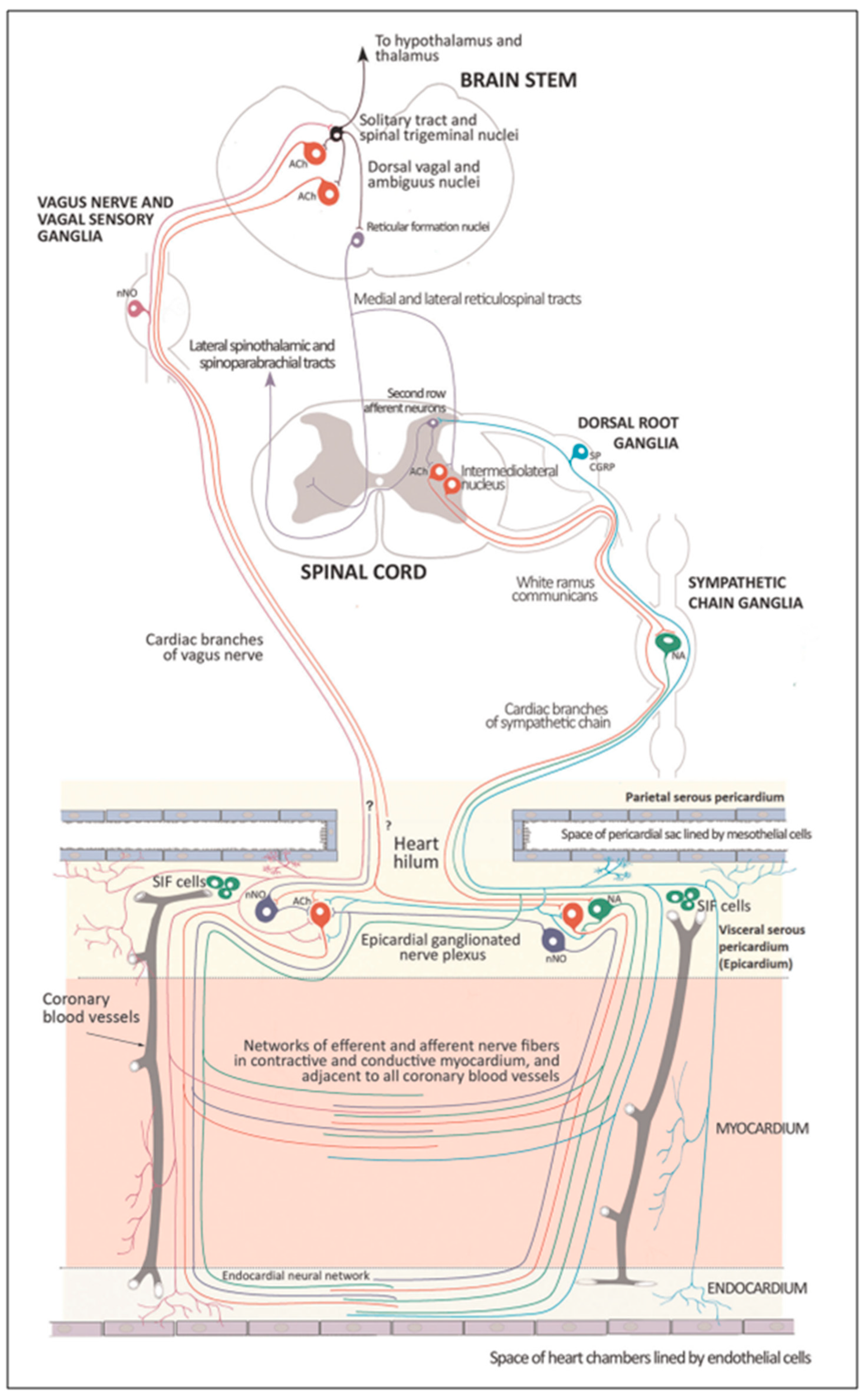
The cardiac nervous system is coupled to areas distributed throughout the neuraxis (
Figure 8 and
Figure 9) and includes intrinsic and extrinsic neural networks. Morphologically, the ICNS is an intrinsic neural plexus with epicardial ganglia, as described by Lee in 1849 (
Figure 4III–V). Its function is controlled by extrinsic influences mediated by the vagal and sympathetic nerves. A variety of different kinds of neurons are present in the intrinsic cardiac ganglia, most likely including the following subtypes: afferent neurons, motor (parasympathetic and sympathetic) neurons, and linking local circuit neurons (interneurons), which make up the first three types of neurons. Vagal or sympathetic inputs regulate their intrinsic reactivity. The modulation of cardio-cardiac reflexes is significantly influenced by this network of ganglionic cells (
Figure 10 and
Figure 11) [
80].
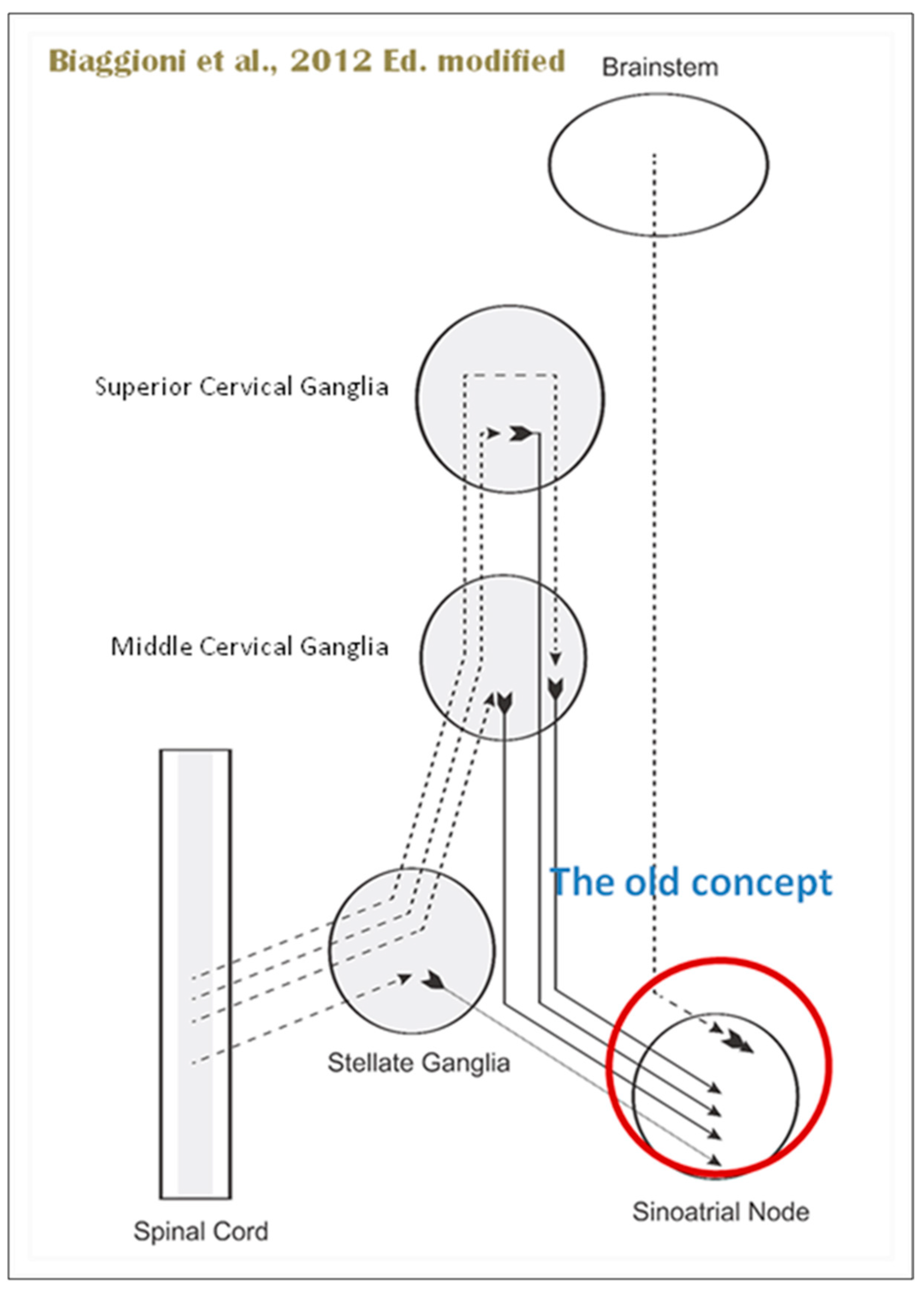
When the sympathetic efferent route is activated, the parasympathetic pathway is often inhibited, and vice versa, according to the obsolete theory of an autonomic balance. The dorsal motor nucleus of the brainstem and the spinal cord, respectively, both possess preganglionic efferents of the parasympathetic and sympathetic systems that innervate the heart. Sympathetic transmission occurs in the sympathetic ganglion chain near the spinal cord, from preganglionic (dashed) to postganglionic (solid). Preganglionic parasympathetic axons synapse with postganglionic parasympathetic components in the cardiac plexus, which is reached by postganglionic axons from the sympathetic trunk.
Furthermore, it is evident that certain physiological reactions can entail the simultaneous cardiac stimulation of both sympathetic and parasympathetic activation. In comparison to vagal regulation, the development of adrenergic innervation of the heart occurred relatively later in the course of evolution [it is absent in elasmobranch fish]; hence, the simultaneous activation of both autonomic pathways may be very important in expanding the heart’s functional capacity to fulfill the metabolic requirements of the body within constantly changing behavioral and environmental circumstances.
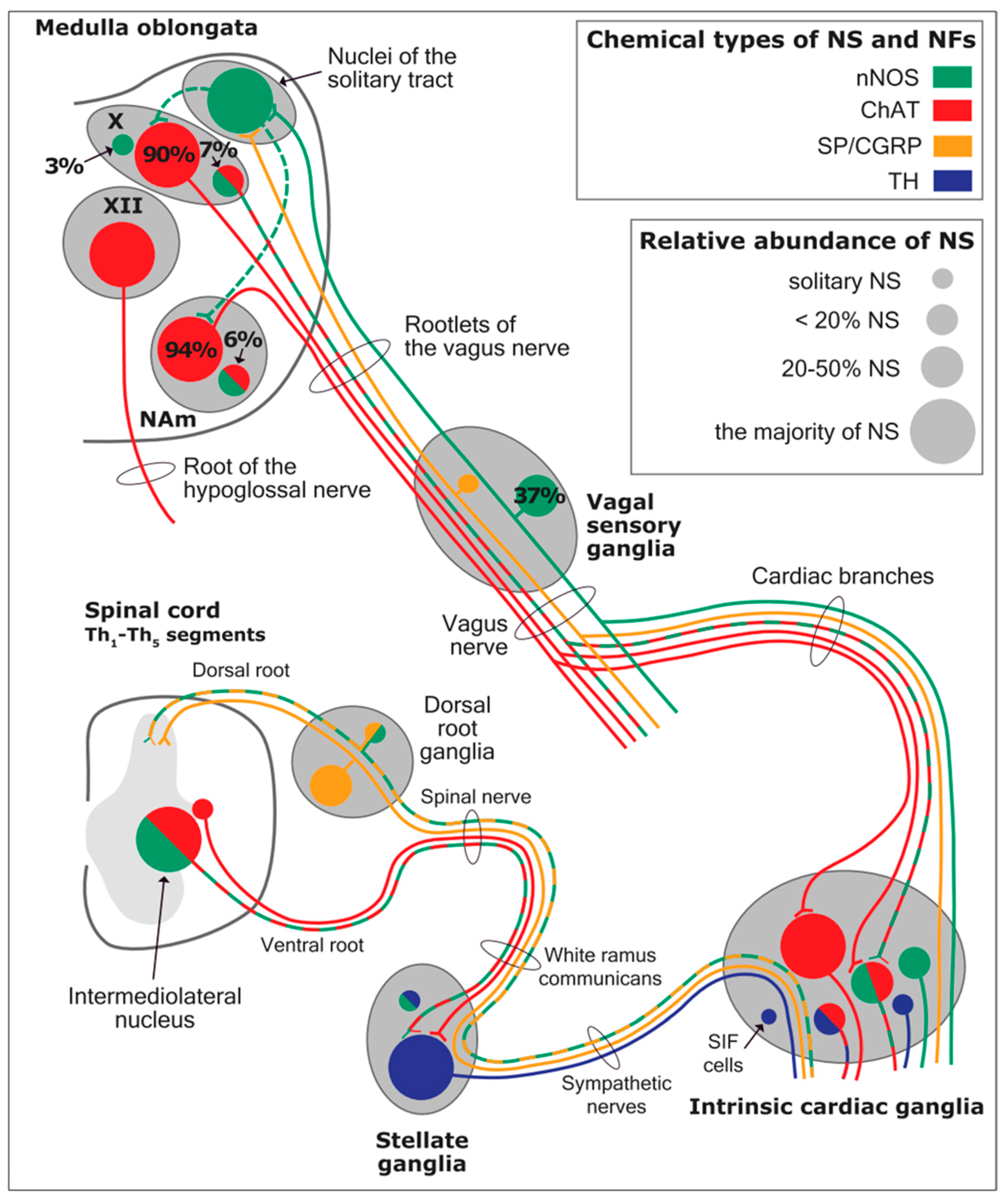
Using immunohistochemical methods, it has been evidentially proven that a range of neurochemical substances are present. Epicardial ganglia, as well as intracardiac ganglia situated within the boundaries of the heart hilum, exhibit immunoreactivity (IR) towards neurotransmitters and neuromodulators such as choline acetyltransferase (ChAT), the enzyme accountable for the synthesis of acetylcholine (ACh); tyrosine hydroxylase (TH), the enzyme which catalyze the production of the sympathetic neurotransmitter noradrenaline (NA); vasoactive intestinal peptide (VIP, recognized to co-release alongside ACh); neuropeptide Y (NPY, reputed to co-liberate together with NA); neuronal nitric oxide synthase (nNOS, involved in NO synthesis by parasympathetic, sympathetic, and non-adrenergic non-cholinergic (NANC) nerves) [
83] (
Figure 12); synaptophysin (a tracer for presynaptic fibers); substance P (sub P); and calcitonin gene-related peptide (CGRP).
Most ganglion cells utilize acetylcholine as their primary transmitter.
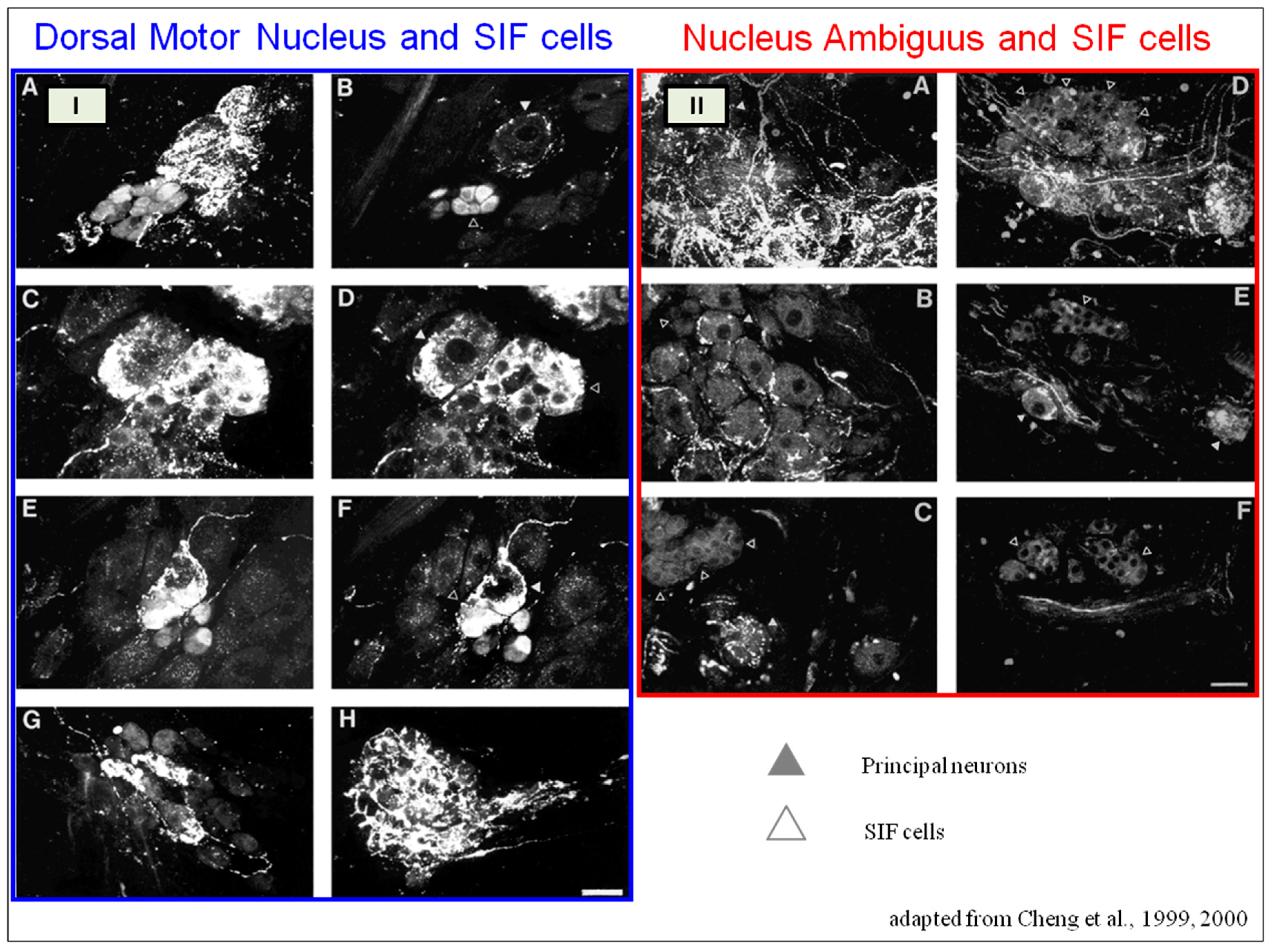
SIF cells are TH-positive and are often found inside larger ganglia, in tiny clusters, or scattered along the atrial and ventricular walls. The exact function of SIF cells in the heart is still unclear, since the majority of them are not adjacent to axons and presumably are not under neural control, according to numerous observations by Pauza and his co-workers [
87,
88,
89,
90]; Pauziene et al., [
86,
91].
An intriguing feature of the cardiac ganglia is the presence of biphenotypic neurons, i.e., neurons showing both ChAT-IR and TH-IR. These neurons account for 10 to 20% of all intrinsic cardiac neurons. Cholinergic cardiac neurons may also exhibit immunoreactivity for nitric oxide, i.e., such cholinergic neurons should be considered biphenotypic nitrergic [
92,
93] This feature of producing and perhaps releasing both neurotransmitters is most interesting and needs further investigation [
82,
94].
As mentioned above, with respect to the extrinsic sympathetic innervation, the cardiac preganglionic axons originate from neurons of the intermediolateral (IML) nucleus of the Terni’s column of the spinal cord (
Figure 5II). Rhythmic excitatory glutamatergic inputs from neurons of the rostral ventrolateral medulla (RVLM) are collected by IML neurons. The cardiac preganglionic sympathetic neurons are neurons that liberate acetylcholine and project to neurons with norepinephrine release of the superior and middle cervical, cervicothoracic (stellate), and first four thoracic ganglia, which give rise to axons that innervate the heart via the superior, middle, and inferior cardiac nerves [
49]. The right-to-left map of sympathetic nerves is asymmetrical [
51] and shows differences in expression between individuals; this may clarify their heterogeneous effect on cardiac electrophysiologic properties.
The cardiovagal innervation represents the extrinsic parasympathetic output. The preganglionic cardiovagal neurons are cholinergic, and their axons extend to the cardiac ganglia through superior cervical, inferior cervical, and thoracic rami, which merge with adrenergic nerves of the heart to form the extrinsic cardiac plexus in the mediastinum. Vagal nerve fibers distribute within the walls of the atria and ventricles, in the sinoatrial (SA) and atrioventricular (AV) nodes [
50].
Sympathetic activation elicits an increase in inotropism and dromotropism, faster conduction through the AV node, a boost in the stimulation of the His-Purkinje system, which determines a strengthening of contraction force during systole, and faster relaxation of the cardiac muscle cells during diastole.
The primary impact of the vagus, on the other hand, is suppression of the pacemaker activity of the SA node (reduction in heart rate, HR), decreased AV conduction, and diminished excitability of the His-Purkinje system by cholinergic neurons of the cardiac ganglia.
The tonic vagal control of the SA node automatism prevails over that of the sympathetic system during resting conditions. HR has a circadian pattern; it increases in the early morning because the sympathetic activity surges and then decreases during sleep, particularly during non-REM (rapid eye movement) sleep, for vagal predominance. However, phasic transient vagal interruption and sympathetic activation result in HR surges during REM sleep. Vagal activity rapidly decreases in response to orthostatic stress, hypovolemia, or exercise. In conditions with very low basal HR (e.g., athletes, during non-REM sleep, or patients with sinusal bradycardia), vagal activation could unexpectedly increase HR by reducing the interval between depolarizations of the atria. The vagal activation, particularly in the ventricles, is higher in the setting of prominent simultaneous adrenergic activation; this so-called “accentuated antagonism” depends on presynaptic inhibition of sympathetic transmission.
HR variability (HRV) results from interactions between the vagal and sympathetic influences on the SA node. HRV can be evaluated during deep breathing and during the Valsalva maneuver.
The cardiac preganglionic sympathetic IML neurons are tonically activated by premotor glutamatergic sympathoexcitatory neurons of RVLM, which act as a common effector of descending and reflex pathways controlling blood pressure (BP) and cardiac function; some of these neurons also synthesize epinephrine (C1 group). Psychological stress, pain, hypoxia, hypovolemia, and hypoglycemia activate RVLM neurons both directly and via descending inputs from the forebrain. RVLM is inhibited by the baroreflex via disynaptic inhibition from the NTS, mediated by GABA (gamma-aminobutyric acid) neurons of the caudal portion of the ventrolateral medulla (
Figure 8 and
Figure 5II). The NAmb contains the majority of cardioinhibitory vagal motoneurons that control SA automatism and AV node conduction. These neurons are activated by glutamatergic inputs from barosensitive neurons of the NTS and inhibited by local GABAergic neurons and by GABAergic neurons of the medullary ventral respiratory group that are active during inspiration. In this way, the vagal control of the HR is modulated on a beat-to-beat basis by respiration; cardiovagal activity is lower during inspiration and higher during expiration. This physiologic condition is known as respiratory sinus arrhythmia (RSA). RSA is a phenomenon that occurs during both regular breathing and enhanced breathing patterns like sighs [
95]. It is considered a healthy aspect of HRV and is thought to enhance the efficiency of gas exchange [
96] or help reduce cardiac workload while maintaining optimal blood gas levels [
97]. Sighs and the accompanying RSA typically occur at the beginning of arousal and are believed to play a role in reopening the airways after obstruction [
98]. In young, healthy individuals, RSA is often evaluated as a high-frequency HRV, reflecting the combined activity of the sympathetic and parasympathetic nervous systems. Even in full-term infants, cardiorespiratory coupling, as seen in RSA, is present from the early stages of life. This coupling strengthens with gestational age, indicating a shift towards parasympathetic dominance after birth. Conversely, premature infants may exhibit immature cardiorespiratory coupling, characterized by lower HRV in the high-frequency range, which can impair responses to stress and increase the risk of sudden death [
95]. Therefore, RSA is an important measure of cardiovagal output and health and declines linearly with age. In addition, the Hering–Breuer reflex generated by pulmonary mechanoreceptors via the NTS may contribute to the RSA. The caudal region of the solitary nucleus receives afferents mostly from vagal and glossopharyngeal afferents from baroreceptors, cardiac receptors, chemoreceptors, and pulmonary receptors. Respectively, the vagal afferents by aortic baroreceptor and chemoreceptor, cardiac and visceral sensory receptors in most organs of the thoracic and abdominal cavities with cell bodies in the nodose ganglion (NG), together with carotid baroreceptor and chemoreceptor of glossopharyngeal afferents with cell bodies in the petrosal ganglion (PG), provide inputs to the nucleus of the solitary tract. This nucleus begins a variety of cardiovascular reflexes and also carries cardiovascular receptor inputs to the thalamus and parabrachial nucleus. Therefore, it is the primary essential station for all reflexes of the medulla, inclusive of the baroreflex and cardiac reflexes controlling BP and HR.
The baroreceptor reflex (baroreflex) is a fundamental BP buffering tool and is activated by the mechanical deformation of vessel walls in the carotid sinus and aortic arch during systole. An increase in BP stimulates baroreceptor afferents of the glossopharyngeal and vagus nerves, which activate the NTS via a monosynaptic excitatory input. The barosensitive NTS neurons initiate sympathoinhibitory and cardioinhibitory responses with two different pathways. The sympathoinhibitory pathway controls total peripheral resistance via disynaptic inhibition of RVLM neurons mediated by gamma-aminobutyric acid neurons of the caudal ventrolateral medulla. Through the second pathway, the cardioinhibitory signal elicits a decrease in HR via direct excitatory inputs from the NTS to cardiovagal neurons of the NAmb. Numerous cardiovascular reflexes are triggered by afferents from the heart, coronary, and pulmonary arteries. Among these are cardiac unmyelinated afferents to the thoracic dorsal root ganglia, which run along the adrenergic nerves and provide input to the dorsal horn (primarily lamina I) and intermediate gray matter of the spinal cord, as well as myelinated and unmyelinated vagal afferents with cell bodies in the nodose ganglion, which provide input to the NTS. On the other side, myelinated vagal afferents are activated by atrial distension due to an increase in blood volume, which triggers the reflex of sympathetic input to the SA node, resulting in an increase in HR, as well as suppression of renal adrenergic activity and arginine vasopressin production, favoring salt and water excretion. Furthermore, unmyelinated spinal and vagal afferents innervating the ventricles are stimulated by strong mechanical or chemical stimuli, in particular products of ischemia or inflammation such as adenosine triphosphate, serotonin, and prostanoids.
Spinothalamic projections from lamina I neurons elicit the sensation of cardiac pain; these afferents can also trigger excitatory cardiac reflexes via local interneurons projecting to the IML (the so-called “cardio-cardiac reflex”). In response to the chemical stimulation of myocardial injury, unmyelinated vagal afferents in the ventricles may trigger a decrease in BP and HR (Bezold–Jarisch reflex). Stimulation of pulmonary arterial baroreceptors at physiologic pressure produces reflex vasoconstriction and respiratory stimulation. This could be implicated in cardiovascular control during exercise or in hypoxic conditions.
The cortex of the insula (IC), ACC, amygdala central nucleus (CeA), and numerous nuclei of the hypothalamus (
Figure 8) project to the medullary and spinal nuclei to control the activity of the heart. These projections are either direct or indirect via a relay in the periaqueductal gray. Afferent cardiovascular information carried by the dorsal horn (layer I) or NTS neurons reaches cortical areas through the thalamus. Visceral afferents are also conveyed to the parabrachial nucleus of the pons, which relays the information to the thalamus, hypothalamus, and amygdala, and to catecholaminergic neurons of the A1/C1 group of the ventrolateral medulla (
Figure 6). The role of these areas and the possible hemispheric lateralization of the control of cardiac function are yet poorly understood. The IC has been implicated in a wide range of functional activities as well as the underlying causes of several neurologic diseases. It is separated into two zones: dorsocaudal and rostroventral. The dorsocaudal zone contains many areas that receive thalamic-relay gustatory, viscerosensory, somatosensory, pain, and vestibular afferents. The rostroventral zone, which is connected to the ACC and the amygdala, is primarily engaged in affective neural elaboration. Electrical stimulation of the insula in patients undergoing surgical treatment for intractable epilepsy elicits a variety of visceromotor phenomena, including changes in BP and HR. Oversimplifying, stimulation of the left IC more frequently elicited a small decrease in HR and BP, whereas stimulation of the right IC elicited the opposite effect. These findings, further supported by fMRI studies, suggest that the left IC primarily regulates the parasympathetic and the right IC the sympathetic influence on the heart (
Figure 5III) [
50].
The “laterality hypothesis” has a “cardiotopic” counterpart of asymmetric autonomic nerve distribution around the heart. This complex rotation around the heart axis may be correlated to the remodeling/regression of the right aortic arch during embryonic development, whereas the left-sided arch persists and/or to the cardiac tube rotation during the formation of the four embryonic heart chambers. Typically, the right vagus regulates the activity of the cardiac atria, whereas the left vagus controls the activity of the ventricles. HR is therefore regulated by asymmetric sympathetic and parasympathetic innervation of the sinoatrial (SA) node. In factual terms, the right stellate cardiac nerve is the primary source of sympathetic input from the sinus atrial node, whereas the right vagus is the primary source of parasympathetic SA input. Contrarily, left side inputs are primarily responsible for controlling conduction time in atrioventricular fibers, which is enhanced by parasympathetic activity and reduced through sympathetic activity, again in both systems. However, left-side sympathetic stimulation is primarily responsible for increasing contraction of the myocardium. In conclusion, the right side primarily regulates cardiac frequency, whereas the left side mostly controls ventricular activity and pulse pressure. (
Figure 5IV). The evaluation of asymmetric anatomical and functional autonomic nerve distribution in humans is important to understand anomalies of the heart with left-right predominance and to identify new treatments [
50,
99,
100].
The ACC integrates autonomic responses with behavioral arousal via its wide associations with the IC, amygdala, prefrontal cortex, hypothalamus, and brainstem autonomic nuclei. Functional MRI (Magnetic Resonance Imaging) studies show that the ventral ACC is involved, as described above, in the “default mode network”, activated in the resting state in conditions of self-monitoring. Whereas the dorsal ACC, together with the anterior IC, is a central element of the so-called “salience network”; it is primarily recruited during tasks that demand cognitive control, such as conflict resolution, and is connected with an enhancement in sympathetic conduction, resulting in an HR increase. On the contrary, the subgenual ACC and adjacent ventromedial prefrontal cortex are inactivated at the same time. Pharmacological and neuroimaging studies show that subgenual ACC stimulation depends on HRV mediated by the vagus nerve, particularly in the right hemisphere.
The amygdala provides emotive meaning to inputs from sensory systems and is engaged in fear-conditioning processes. The amygdala includes several nuclei, among them the basolateral nuclear complex and the CeA. The medial aspect of the CeA projects to the hypothalamus and brainstem and triggers the autonomic, endocrine, and motor manifestations of fear responses. Sympathoexcitatory responses involve excitatory connections to the RVLM and inhibition of barosensitive neurons of the NTS. Functional neuroimaging studies have demonstrated a coactivation of the lateral and medial amygdala in relation to changes in HRV both at rest and during emotional tasks. The orbitofrontal and ventromedial prefrontal cortices offer an inhibition of the amygdala via the GABAergic neuronal population in the lateral CeA and in the intercalate nucleus between the basolateral amygdala and the CeA; these prefrontal modulations are involved in mechanisms of emotional regulation, including fear extinction. In fact, in addition to promoting vagal output, these prefrontal areas tonically inhibit sympathoexcitatory responses initiated in the amygdala.
Sympathoexcitatory responses during stress are mediated by projections from the hypothalamus. It tunes autonomic output to the heart via inputs that originate primarily from the PVN, DMH, and lateral hypothalamic area; these hypothalamic projections reach the periaqueductal gray parabrachial nucleus, RVLM, NAmb, DMV, NTS, and IML. THe hypothalamus modulates the baroreflex via NTS or NAmb connections. Experimental studies indicate that the inputs from the IC to the hypothalamus are mostly ipsilateral.
Lower HRV and baroreflex sensitivity (BRS) are important markers of cardiovascular risk, including an increased incidence of ventricular arrhythmias in individuals with primary cardiac disease, due to the fact that they reflect deficiencies in forebrain vagal or sympathetic drive, brainstem reflexes, or vagal or sympathetic output (as occurs in diabetic or amyloid neuropathy). Reduced HRV, mainly at the expense of decreased high-frequency HF (vagal) component and sometimes associated with indices of increased sympathetic activity, has been described in patients with ischemic stroke, epilepsy, multiple sclerosis, and Parkinson’s disease. An alteration in cardiac autonomic control, manifested by a reduction in HRV, has been correlated with sudden infant death syndrome (SIDS) and sudden unexpected death in epilepsy (SUDEP). Furthermore, new evidence suggests that a similar reduction in HRV is associated with long COVID [
101]. The observed decrease in HRV in patients experiencing persistent long COVID symptoms highlights dysautonomia. This underscores the critical need for in-depth research to investigate interventions, both pharmacological and non-pharmacological, aimed at addressing this dysautonomia [
102].
Central autonomic abnormalities can cause a wide range of cardiac arrhythmias, some of which are life-threatening. Vagal hyperactivity, for example, causes bradyarrhythmias such as AV block, whereas sympathetic overdrive causes both supraventricular and ventricular tachycardia, and both sympathetic and vagal hyperstimulation can cause atrial fibrillation (AF). Sympathetic activity in the ventricles is proarrhythmic, whereas vagal activation is antiarrhythmic.
Seizures and cardiac dysfunction are characterized by a number of shared features, including: cardiac arrhythmias as an ictal event that can localize the seizure focus, seizures and cardiac arrhythmias as co-occurring manifestations of channelopathies, and cardiovascular dysregulation and ictal arrhythmias as a potential mechanism underlying sudden unexpected death in epilepsy (SUDEP). These features suggest that seizures and cardiac dysfunction may be linked to common underlying mechanisms. Sinus tachycardia occurs in 80% to 100% of patients before, during, or after a temporal lobe seizure; paroxysmal AF, supraventricular or ventricular tachycardia, and ventricular fibrillation may also occur. Furthermore, temporal-lobe seizures may lead to ictal bradycardia and asystole. Seizures, more common in patients with left-sided seizures, can also produce alterations of cardiac repolarization as manifested by acute changes in the corrected QT (QTc) interval, which may be in part due to ictal hypoxemia. Mutations in genes encoding Na+ or K+ channels are associated with the coexistence of long QT syndrome (LQTS) or Brugada syndrome and epilepsy. Autonomic cardiovascular manifestations of seizures may have a role in the pathophysiology of sudden unexpected death in epilepsy, but this causal relationship is yet to be established.
Autonomic nervous system dysfunction is a widespread occurrence that relates significant heart diseases to neurologic problems. Many neurologic diseases, such as ischemic stroke, sudden cardiac death, and epilepsy, may have higher morbidity due to these severe autonomic effects on the heart.