Prognostic Electrocardiographic Signs in Arrhythmogenic Cardiomyopathy
Abstract
Simple Summary
Abstract
1. Introduction
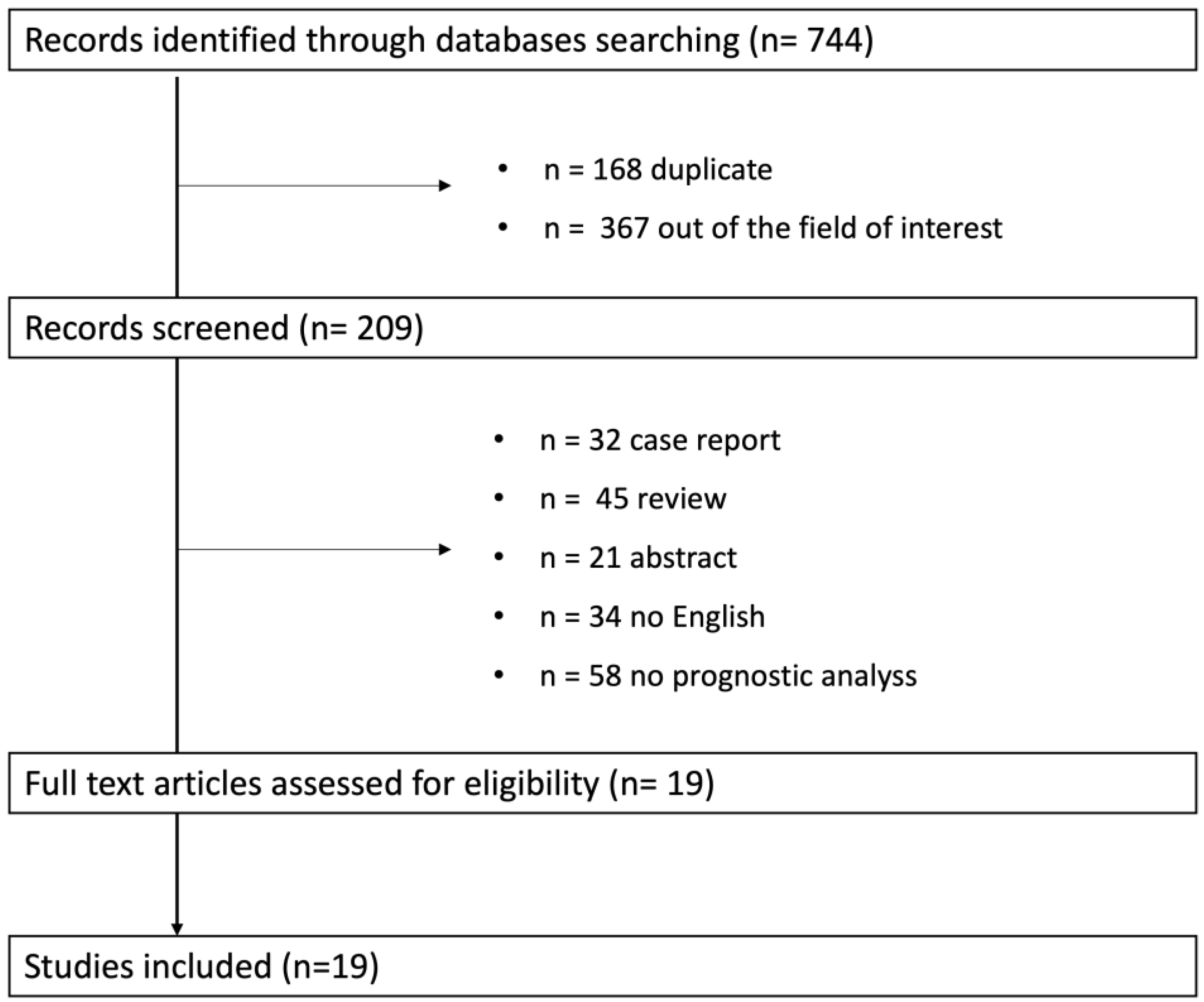
2. Methodological Considerations
3. Results
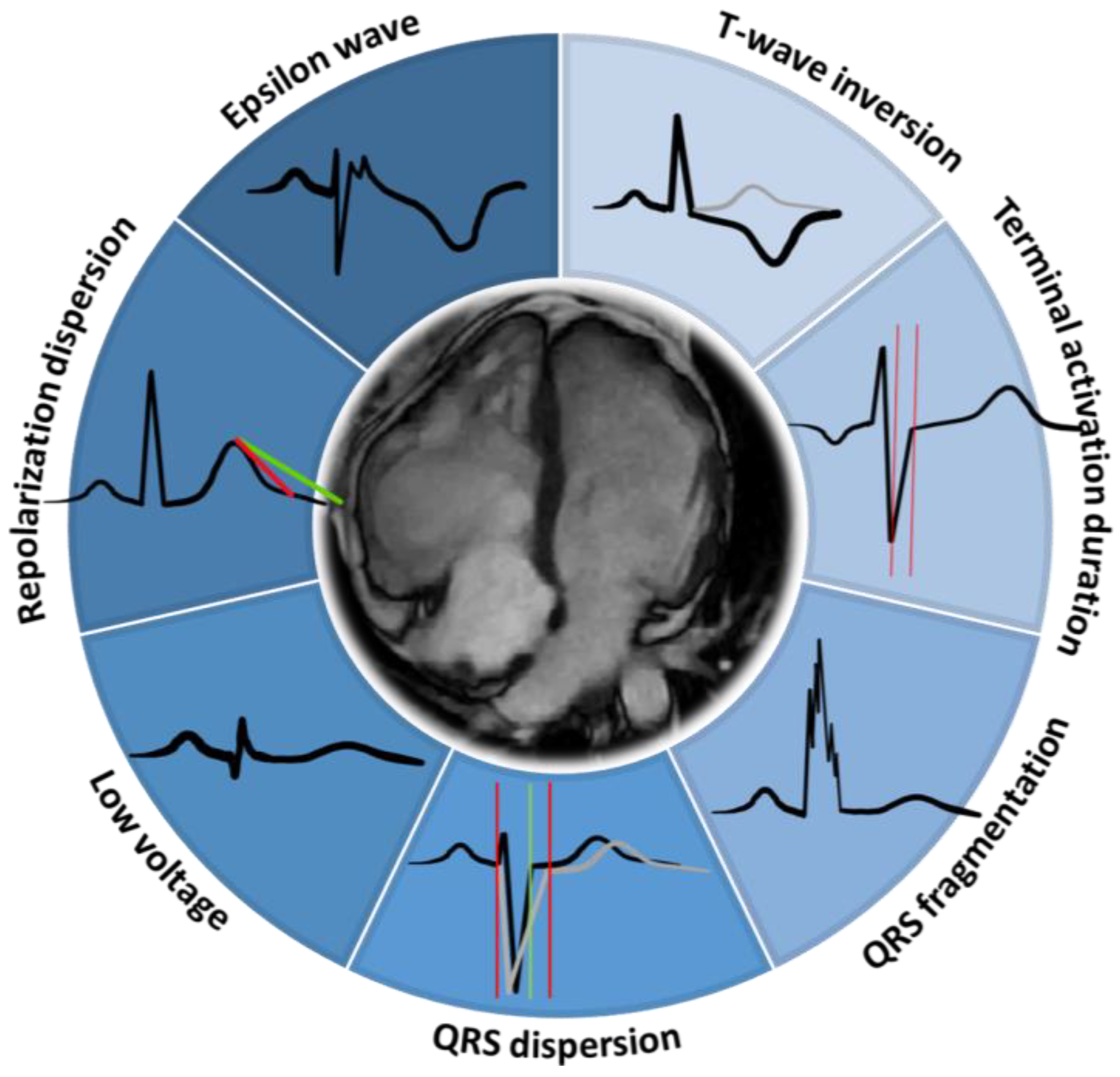
4. T-Wave Inversion
5. QRS Fragmentation
6. Low Voltage QRS
7. Epsilon Wave
8. QRS Dispersion
9. Repolarization Dispersion
10. Terminal Activation Duration
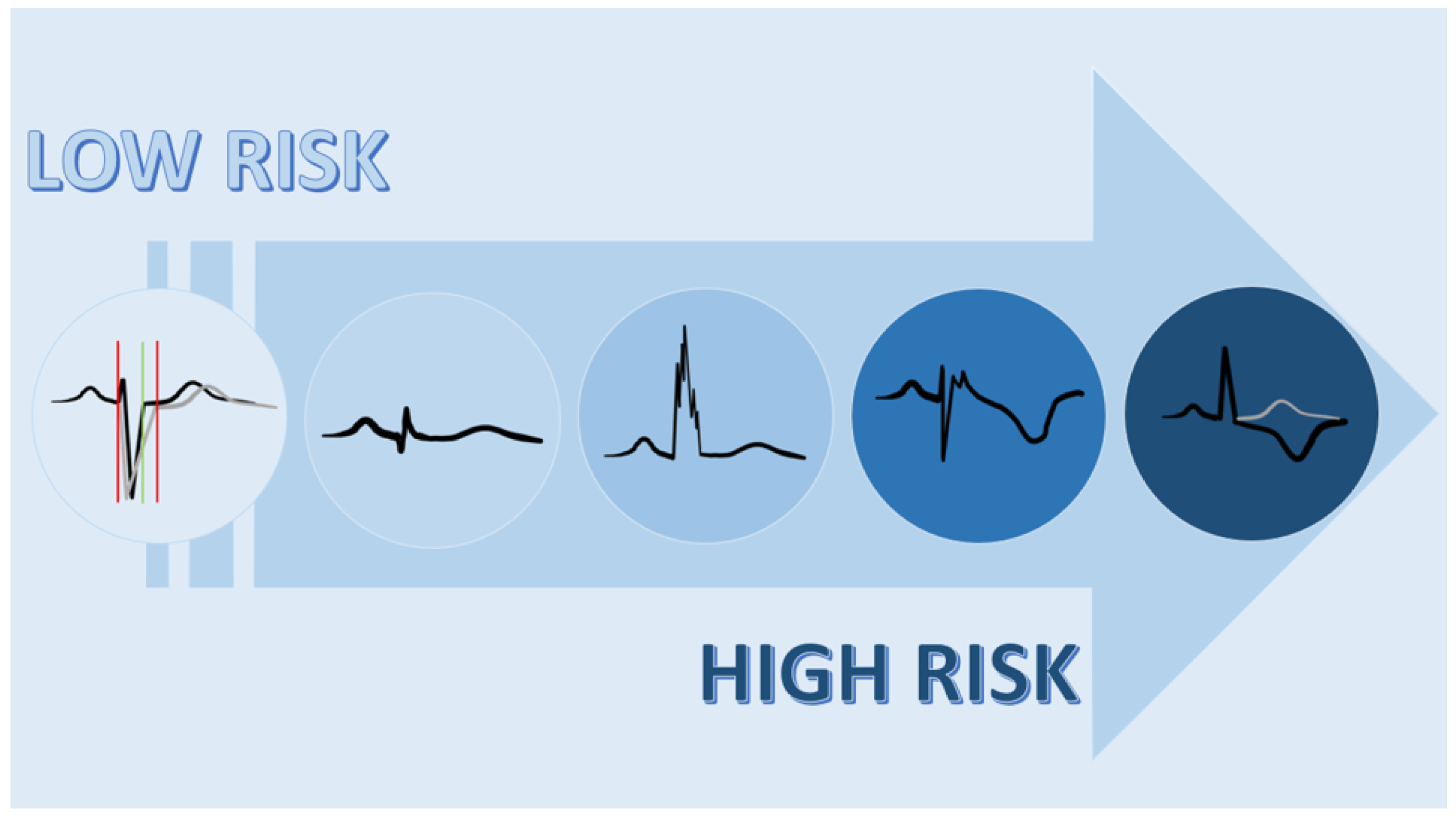
11. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the Task Force Criteria. Eur. Heart J. 2010, 31, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleeijnen, J.; Moher, D.; et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Migliore, F.; Elmaghawry, M.; Silvano, M.; Marra, M.P.; Niero, A.; Nguyen, K.; Rigato, I.; Bauce, B.; Basso, C.; et al. Electrocardiographic predictors of electroanatomic scar size in arrhythmogenic right ventricular cardiomyopathy: Implications for arrhythmic risk stratification. J. Cardiovasc Electrophysiol. 2013, 24, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Kalantarian, S.; Åström Aneq, M.; Svetlichnaya, J.; Sharma, S.; Vittinghoff, E.; Klein, L.; Scheinman, M. Long-Term Electrocardiographic and Echocardiographic Progression of Arrhythmogenic Right Ventricular Cardiomyopathy and Their Correlation With Ventricular Tachyarrhythmias. Circ. Heart Failure 2021, 14, e008121. [Google Scholar] [CrossRef] [PubMed]
- Bhonsale, A.; James, C.A.; Tichnell, C.; Murray, B.; Madhavan, S.; Philips, B.; Russell, S.D.; Abraham, T.; Tandri, H.; Judge, D.P.; et al. Risk stratification in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. Circ. Arrhythm. Electrophysiol. 2013, 6, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Saguner, A.M.; Ganahl, S.; Baldinger, S.H.; Kraus, A.; Medeiros-Domingo, A.; Nordbeck, S.; Saguner, A.R.; Mueller-Burri, A.S.; Haegeli, L.M.; Wolber, T.; et al. Usefulness of electrocardiographic parameters for risk prediction in arrhythmogenic right ventricular dysplasia. Am. J. Cardiol. 2014, 113, 1728–1734. [Google Scholar] [CrossRef]
- Link, M.S.; Laidlaw, D.; Polonsky, B.; Zareba, W.; McNitt, S.; Gear, K.; Marcus, F.; Estes, N.A., 3rd. Ventricular arrhythmias in the North American multidisciplinary study of ARVC: Predictors, characteristics, and treatment. J. Am. Coll Cardiol. 2014, 64, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Orgeron, G.M.; James, C.A.; Te Riele, A.; Tichnell, C.; Murray, B.; Bhonsale, A.; Kamel, I.R.; Zimmerman, S.L.; Judge, D.P.; Crosson, J.; et al. Implantable Cardioverter-Defibrillator Therapy in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy: Predictors of Appropriate Therapy, Outcomes, and Complications. J. Am. Heart Assoc. 2017, 6, e006242. [Google Scholar] [CrossRef]
- Leren, I.S.; Saberniak, J.; Haland, T.F.; Edvardsen, T.; Haugaa, K.H. Combination of ECG and Echocardiography for Identification of Arrhythmic Events in Early ARVC. JACC Cardiovasc. Imaging 2017, 10, 503–513. [Google Scholar] [CrossRef]
- Cadrin-Tourigny, J.; Bosman, L.P.; Wang, W.; Tadros, R.; Bhonsale, A.; Bourfiss, M.; Lie, Ø.H.; Saguner, A.M.; Svensson, A.; Andorin, A.; et al. Sudden cardiac death prediction in arrhythmogenic right ventricular cardiomyopathy: A multinational collaboration. Circ. Arrhythm. Electrophysiol. 2021, 14, e008509. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Truemmel, M.; Koehler, B. Prognostic value of QRS fragmentation in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. J. Cardiovasc. Med. (Hagerstown) 2012, 13, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Trümmel, M.; Koehler, B. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia-cardiomyopathy. Heart Rhythm. 2008, 5, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, N.Q.S.; Sacilotto, L.; Wulkan, F.; D’Arezzo Pessente, G.; Lombardi Peres de Carvalho, M.; Moleta, D.; Tessariol Hachul, D.; Veronese, P.; Hardy, C.; Pisani, C. Clinical Features, Genetic Findings, and Risk Stratification in Arrhythmogenic Right Ventricular Cardiomyopathy: Data From a Brazilian Cohort. Circ. Arrhythm Electrophysiol. 2023, 16, e011391. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.; Blandino, A.; Giustetto, C.; Anselmino, M.; Castagno, D.; Richiardi, E.; Gaita, F. Arrhythmogenic right ventricular cardiomyopathy: ECG progression over time and correlation with long-term follow-up. J. Cardiovasc. Med. (Hagerstown) 2016, 17, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Turrini, P.; Corrado, D.; Basso, C.; Nava, A.; Bauce, B.; Thiene, G. Dispersion of ventricular depolarization-repolarization: A noninvasive marker for risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation 2001, 103, 3075–3080. [Google Scholar] [CrossRef]
- Peters, S.; Peters, H.; Thierfelder, L. Risk stratification of sudden cardiac death and malignant ventricular arrhythmias in right ventricular dysplasia-cardiomyopathy. Int. J. Cardiol. 1999, 71, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.H.; Lin, C.Y.; Te, A.L.D.; Wu, C.I.; Chung, F.P.; Chang, Y.C.; Chang, S.L.; Lin, C.; Lo, L.W.; Hu, Y.F.; et al. A novel noninvasive surface ECG analysis using interlead QRS dispersion in arrhythmogenic right ventricular cardiomyopathy. PLoS ONE 2017, 12, e0182364. [Google Scholar] [CrossRef] [PubMed]
- Benn, M.; Hansen, P.S.; Pedersen, A.K. QT dispersion in patients with arrhythmogenic right ventricular dysplasia. Eur. Heart J. 1999, 20, 764–770. [Google Scholar] [CrossRef][Green Version]
- Cekirdekci, E.I.; Bugan, B. Can abnormal dispersion of ventricular repolarization be a predictor of mortality in arrhythmogenic right ventricular cardiomyopathy: The importance of Tp-e interval. Ann. Noninvasive. Electrocardiol. 2019, 24, e12619. [Google Scholar] [CrossRef]
- Jain, R.; Dalal, D.; Daly, A.; Tichnell, C.; James, C.; Evenson, A.; Jain, R.; Abraham, T.; Tan, B.Y.; Tandri, H.; et al. Electrocardiographic features of arrhythmogenic right ventricular dysplasia. Circulation 2009, 120, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Nava, A.; Canciani, B.; Buja, G.; Martini, B.; Daliento, L.; Scognamiglio, R.; Thiene, G. Electrovectorcardiographic study of negative T waves on precordial leads in arrhythmogenic right ventricular dysplasia: Relationship with right ventricular volumes. J. Electrocardiol. 1988, 21, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Suradi, H.; Maskoun, W.; Michael, M.A.; Shen, C.; Peng, J.; Dandamudi, G.; Mahenthiran, J. Fragmented wide QRS on a 12-lead ECG: A sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008, 1, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Roudijk, R.W.; Bosman, L.P.; van der Heijden, J.F.; de Bakker, J.M.T.; Hauer, R.N.W.; van Tintelen, J.P.; Asselbergs, F.W.; Te Riele, A.S.J.M.; Loh, P. Quantitative Approach to Fragmented QRS in Arrhythmogenic Cardiomyopathy: From Disease towards Asymptomatic Carriers of Pathogenic Variants. J. Clin. Med. 2020, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Bettella, N.; Tatangelo, M.; Del Monte, A.; Vessella, T.; Poscolieri, B.; Crescenzi, C.; Pegorin, D.; D’Ascenzi, F.; Pescatore, V.; et al. Prevalence and clinical significance of isolated low QRS voltages in young athletes. Europace 2022, 24, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Perazzolo Marra, M.; Zorzi, A.; Beffagna, G.; Cipriani, A.; Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; Rigato, I.; et al. Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int. J. Cardiol. 2020, 319, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Perrin, M.J.; Spears, D.; Gollob, M.H. Epsilon wave uncovered by exercise test in a patient with desmoplakin-positive arrhythmogenic right ventricular cardiomyopathy. Can. J. Cardiol. 2015, 31, 819.e1–819.e2. [Google Scholar] [CrossRef]
- Angaran, P.; Laksman, Z.; Zhang, H.; Porepa, L.F.; Rutberg, J.; James, C.; Krahn, A.D.; Judge, D.P.; Calkins, H.; Gollob, M.H. Exercise testing in asymptomatic gene carriers exposes a latent electrical substrate of arrhythmogenic right ventricular cardiomyopathy. J. Am. Coll. Cardiol. 2013, 62, 1772–1779. [Google Scholar] [CrossRef]
- Turrini, P.; Corrado, D.; Basso, C.; Nava, A.; Thiene, G. Noninvasive risk stratification in arrhythmogenic right ventricular cardiomyopathy. Ann. Noninvasive Electrocardiol. 2003, 8, 161–169. [Google Scholar] [CrossRef]
- Wu, S.; Wang, P.; Hou, Y.; Yang, P.; Xiao, Y.; Zhan, X. Epsilon wave in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Pacing Clin. Electrophysiol. 2009, 32, 59–63. [Google Scholar] [CrossRef]
- Lie, Ø.H.; Rootwelt-Norberg, C.; Dejgaard, L.A.; Leren, I.S.; Stokke, M.K.; Edvardsen, T.; Haugaa, K.H. Prediction of Life-Threatening Ventricular Arrhythmia in Patients With Arrhythmogenic Cardiomyopathy: A Primary Prevention Cohort Study. JACC Cardiovasc. Imaging 2018, 11, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Cheng, H.; Lu, M.; Jiang, S.; Yin, G.; Zhao, S. Cardiac magnetic resonance imaging in arrhythmogenic right ventricular cardiomyopathy: Correlation to the QRS dispersion. Magn. Reson. Imaging 2012, 30, 1454–1460. [Google Scholar] [CrossRef] [PubMed]

| Main Characteristics | Prognostic Value | ||
|---|---|---|---|
| T-wave inversion | Single-center retrospective study, 49 pts, year of publication 2013 [4] | Right ventricular electroanatomic scar-related arrhythmic risk | p = 0.03 |
| Single-center retrospective study, 405 ECG, year of publication 2021 [5] | Any ventricular tachycardia or adverse cardiac outcome | p = 0.04 | |
| Single-center retrospective study, 215 pts, year of publication 2013 [6] | Arrhythmic events including VT storms, appropriate ICD interventions and NSVT | p = 0.035 | |
| Single-center retrospective study, 111 pts, year of publication 2015 [7] | MACE | p = 0.020 | |
| Single-center prospective study, 137 pts, year of publication 2015 [8] | ICD treatment of VT | p < 0.001 | |
| Single-center prospective study, 312 pts, year of publication 2017 [9] | Appropriate ICD intervention | p = 0.018 | |
| Single-center retrospective study, 162 pts, year of publication 2017 [10] | Arrhythmic events even in early ACM | p < 0.001 | |
| Multicenter retrospective study, 864 pts, year of publication 2021 [11] | LTVA | p = 0.024 | |
| QRS fragmentation | Multicenter retrospective study, 335 pts, year of publication 2012 [12] | Arrhythmic events including VT, VF, SCD, and recurrent ICD discharges | p < 0.001 |
| Multicenter retrospective study, 360 pts, year of publication 2008 [13] | MACE | p < 0.029 | |
| LQRSV | Single-center prospective study, 111 pts, year of publication 2023 [14] | Heart failure death and heart transplantation | p = 0.010 |
| Epsilon wave | Single-center prospective study, 68 pts, year of publication 2016 [15] | Composite endpoint of SCD, heart failure-related mortality, and heart transplantation | p = 0.015 |
| QRS dispersion | Single-center retrospective study, 40 pts, year of publication 2001 [16] | SCD | p < 0.0001 |
| Single-center retrospective study, 121 pts, year of publication 1999 [17] | Recurrence of ventricular arrhythmias | p < 0.01 | |
| Single-center retrospective study, 71 pts, year of publication 2017 [18] | More advanced disease requiring epicardial ablation | p < 0.001 | |
| Single-center retrospective study, 25 pts, year of publication 1999 [19] | SCD, VT | p < 0.05 | |
| Repolarization dispersion | Single-center retrospective study, 40 pts, year of publication 2001 [16] | SCD | p < 0.0001 |
| Single-center prospective study, 105 pts, year of publication 2019 [20] | c Tp-e interval > all-cause mortality | p = 0.027 | |
| TAD | Single-center retrospective study, 57 pts, year of publication 2009 [21] | Delayed right ventricular activation, playing a role in ventricular tachycardia | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonet, E.; Vitali, F.; Amantea, V.; Azzolini, G.; Balla, C.; Micillo, M.; Lapolla, D.; Canovi, L.; Bertini, M. Prognostic Electrocardiographic Signs in Arrhythmogenic Cardiomyopathy. Biology 2024, 13, 265. https://doi.org/10.3390/biology13040265
Tonet E, Vitali F, Amantea V, Azzolini G, Balla C, Micillo M, Lapolla D, Canovi L, Bertini M. Prognostic Electrocardiographic Signs in Arrhythmogenic Cardiomyopathy. Biology. 2024; 13(4):265. https://doi.org/10.3390/biology13040265
Chicago/Turabian StyleTonet, Elisabetta, Francesco Vitali, Veronica Amantea, Giorgia Azzolini, Cristina Balla, Marco Micillo, Davide Lapolla, Luca Canovi, and Matteo Bertini. 2024. "Prognostic Electrocardiographic Signs in Arrhythmogenic Cardiomyopathy" Biology 13, no. 4: 265. https://doi.org/10.3390/biology13040265
APA StyleTonet, E., Vitali, F., Amantea, V., Azzolini, G., Balla, C., Micillo, M., Lapolla, D., Canovi, L., & Bertini, M. (2024). Prognostic Electrocardiographic Signs in Arrhythmogenic Cardiomyopathy. Biology, 13(4), 265. https://doi.org/10.3390/biology13040265








