Adult Feeding Experience Determines the Fecundity and Preference of the Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Plant
2.2. Insect Material
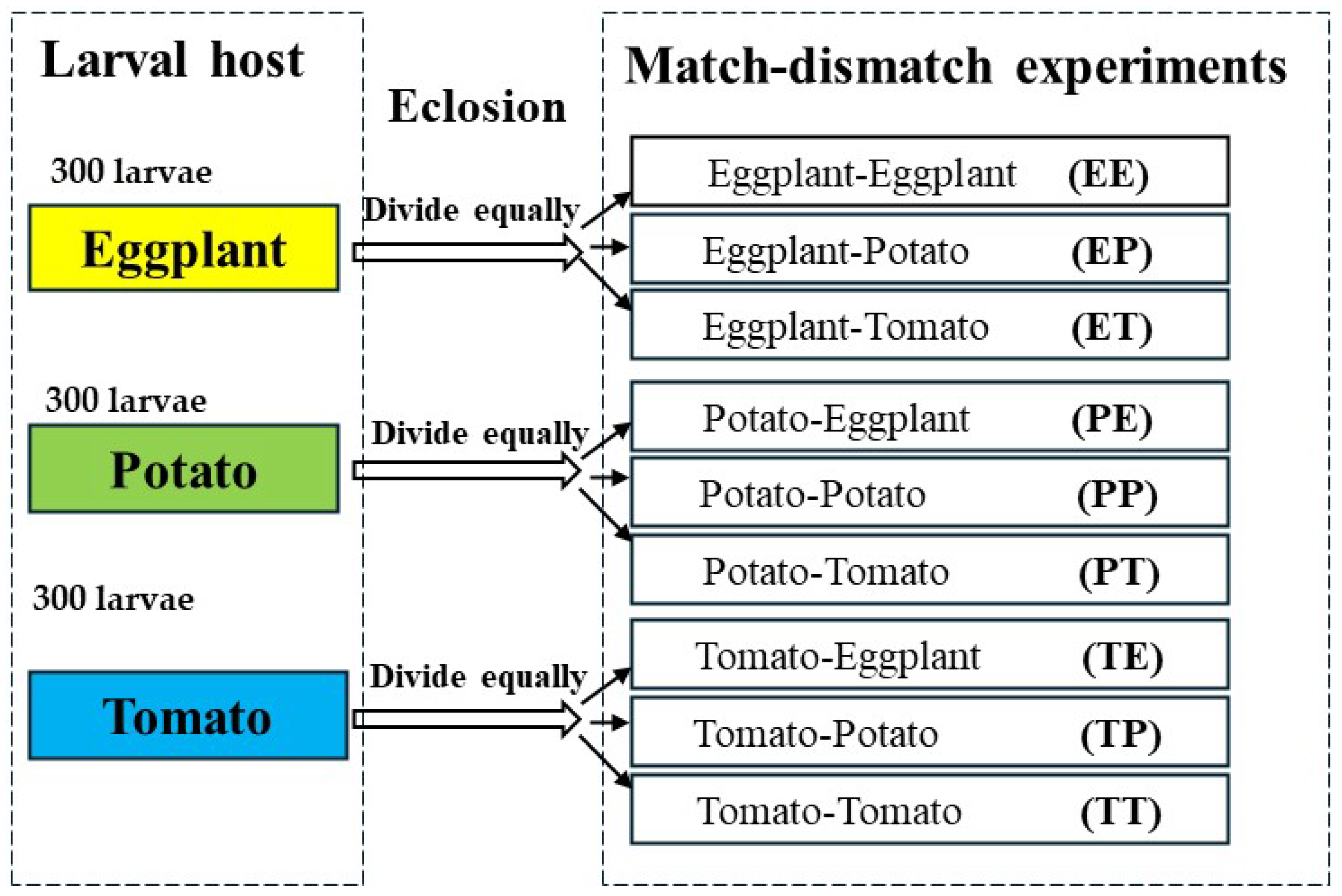
2.3. Match-Mismatch Experimental Design
2.4. Adult Feeding Preference Experience
2.5. Nutrient Components of Host Plant Leaves
2.6. Data Analyses
3. Results
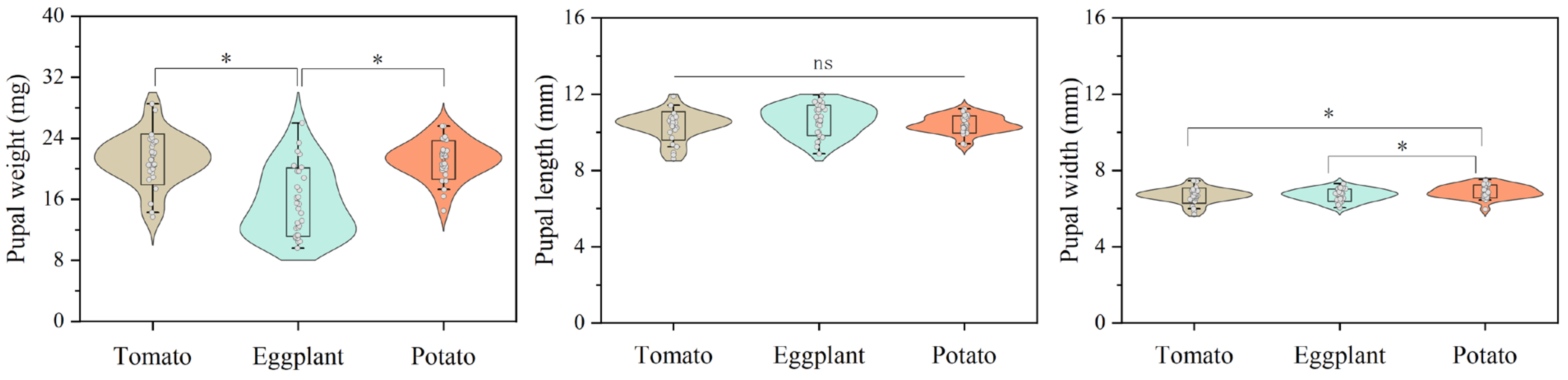
3.1. Effects of Host Plants on the Development of Larvae and Pupae of H. vigintioctopunctata
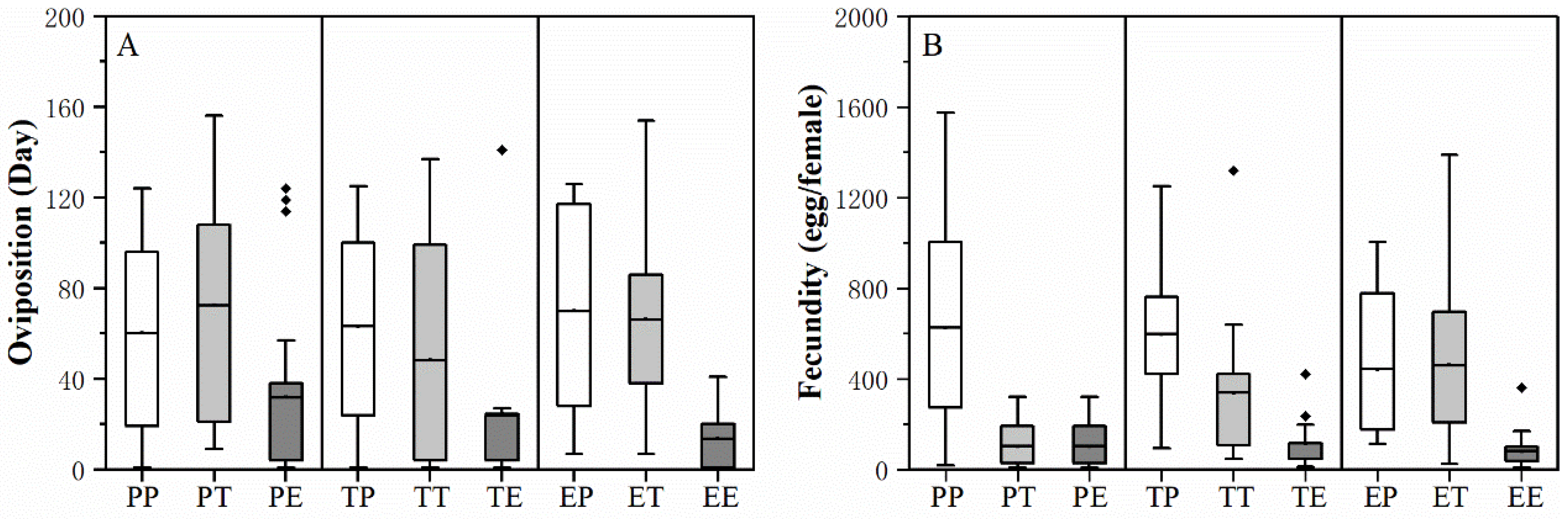
3.2. Effect of the Larval and Adult Host Plant Experience and Their Interaction on H. vigintioctopunctata Adult Performance
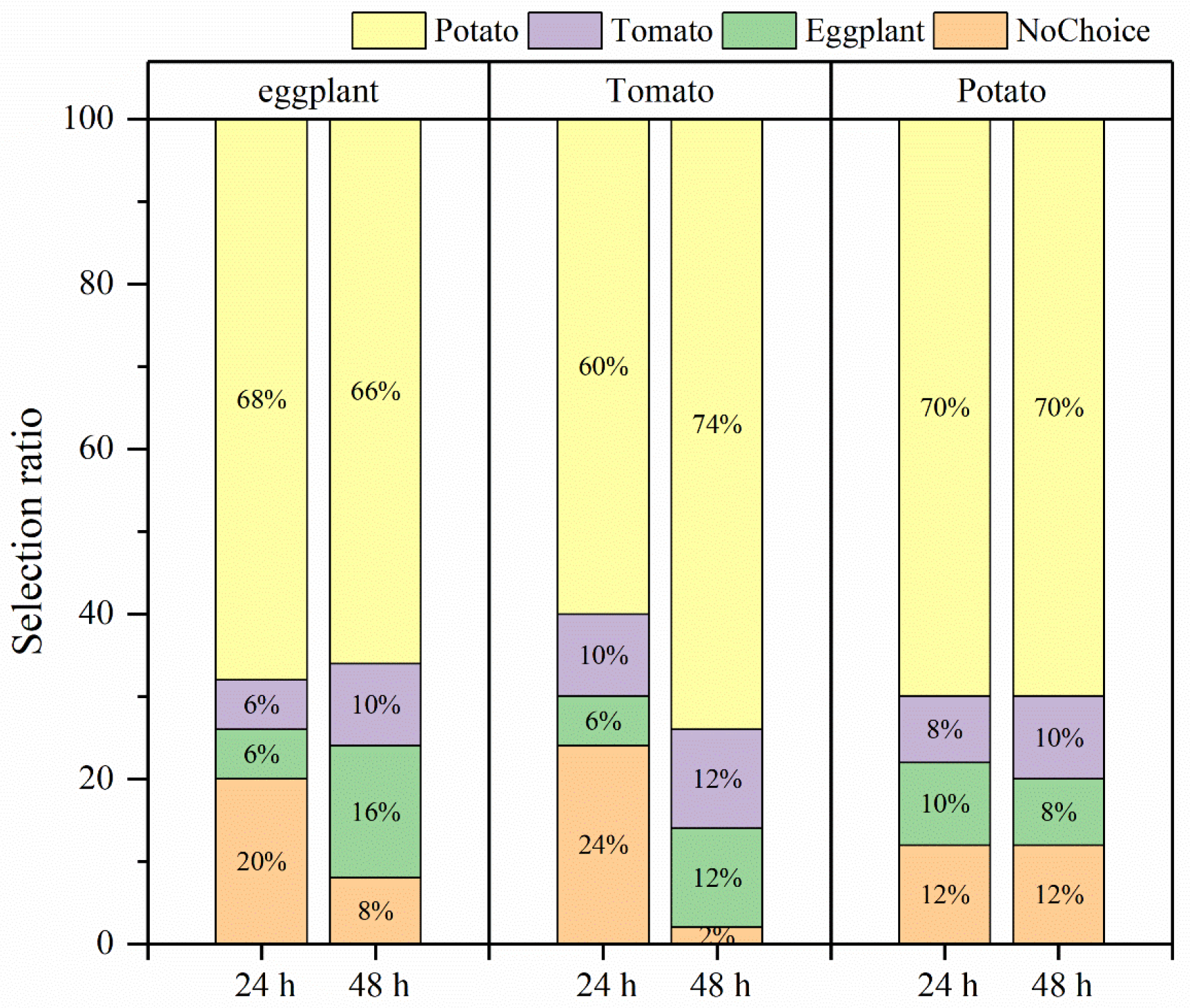
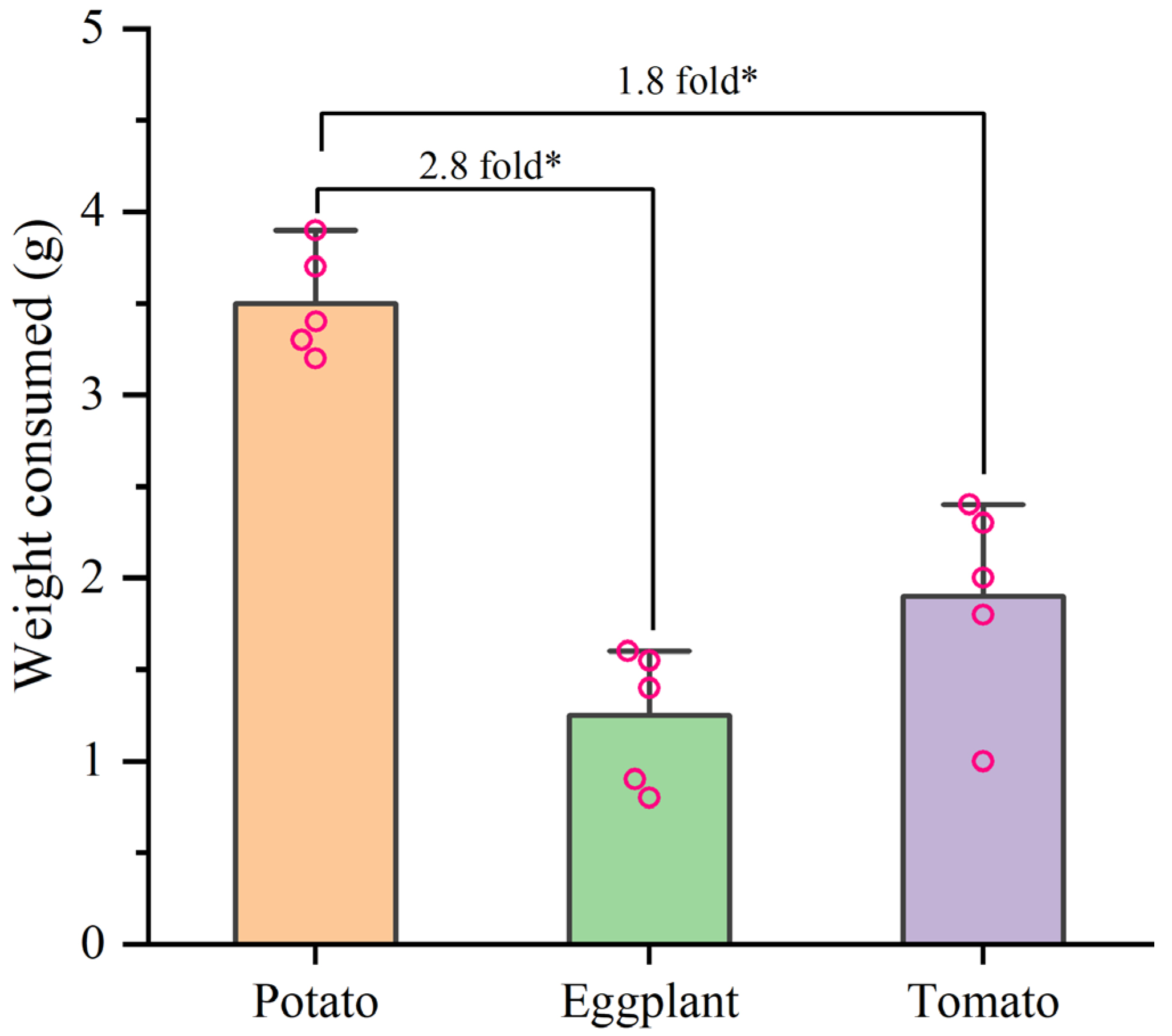
3.3. Effects of Larval Host Experience on Adult Preference and Feeding of H. vigintioctopunctata
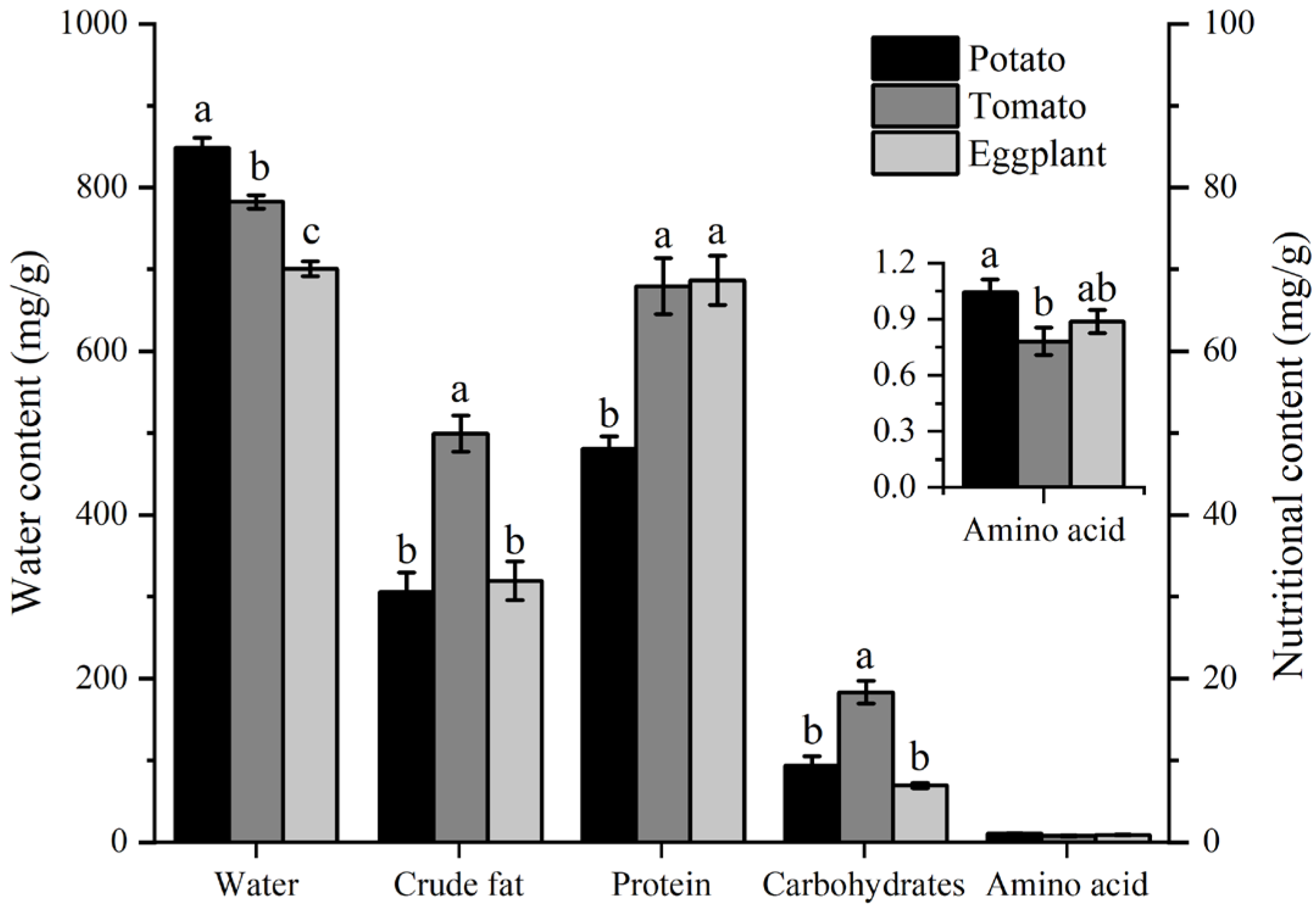
3.4. The Water and Nutritional Content of Host Plants
4. Discussion
4.1. Effects of Host Plant Nutritional Differences on the Performace H. vigintioctopunctata
4.2. Effect of Larval and Adult Feeding Experience on the Fecundity of H. vigintioctopunctata Adult
4.3. The Advantage of Host Switch between Larva and Adult for H. vigintioctopunctata
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wetzel, W.C.; Kharouba, H.M.; Robinson, M.; Holyoak, M.R. Variability in plant nutrients reduces insect herbivore performance. Nature 2016, 539, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Redak, R.; Wang, L.W. Host nutrition determines blood nutrient composition and mediates parasite developmental success: Manduca sexta L. parasitized by Cotesia congregata (Say). J. Exp. Biol. 2005, 208, 625–635. [Google Scholar] [CrossRef]
- Behmer, S.T.; Simpson, S.J.; Raubenheimer, D. Herbivore foraging in chemically heterogeneous environments: Nutrients and secondary metabolites. Ecology 2002, 83, 2489–2501. [Google Scholar] [CrossRef]
- Ponton, F.; Wilson, K.; Cotter, S.C.; Raubenheimer, D.; Simpson, S.J. Nutritional immunology: A multi-dimensional approach. PLoS Pathog. 2011, 7, e1002223. [Google Scholar] [CrossRef]
- Nemzer, B.; Al-Taher, F.; Abshiru, N. Phytochemical composition and nutritional value of different plant parts in two cultivated and wild purslane (Portulaca oleracea L.) genotypes. Food Chem. 2020, 320, 126621. [Google Scholar] [CrossRef] [PubMed]
- Gherlenda, A.N.; Haigh, A.M.; Moore, B.D.; Johnson, S.N.; Riegler, M. Climate change, nutrition and immunity: Effects of elevated CO2 and temperature on the immune function of an insect herbivore. J. Insect Physiol. 2016, 85, 57–64. [Google Scholar] [CrossRef]
- Hamby, K.A.; Bellamy, D.E.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; York, R.M.; Biondi, A. Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J. Pest Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef]
- Lee, M.A. A global comparison of the nutritive values of forage plants grown in contrasting environments. J. Plant Res. 2018, 131, 641–654. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Liu, C.H.; Shen, T.C. Effects of plant nutrient availability and host plant species on the performance of two Pierisbutterflies (Lepidoptera: Pieridae). Biochem. Syst. Ecol. 2008, 36, 505–513. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Salgado, A.L.; Saastamoinen, M. Developmental stage-dependent response and preference for host plant quality in an insect herbivore. Anim. Behav. 2019, 150, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Tilmon, K. Specialization, Speciation, and Radiation: The Evolutionary Biology of Herbivorous Insects; University of California Press: Berkeley, CA, USA, 2008. [Google Scholar]
- Singer, M.; Stireman Iii, J. Does anti-parasitoid defense explain host-plant selection by a polyphagous caterpillar? Oikos 2003, 100, 554–562. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Whalen, M.A.; Davenport, T.M.; Stone, J.P.; Duffy, J.E. Physiological effects of diet mixing on consumer fitness: Ameta-analysis. Ecology 2013, 94, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Müller, C. Adult beetles compensate for poor larval food conditions. J. Insect Physiol. 2016, 88, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, H.; Byrne, M.; Van den Berg, J. The effect of different host plants on the reproduction and longevity of Nysius natalensis. Entomol. Exp. Appl. 2012, 145, 209–214. [Google Scholar] [CrossRef]
- Karban, R.; Karban, C.; Huntzinger, M.; Pearse, I.; Crutsinger, G. Diet mixing enhances the performance of a generalist caterpillar, Platyprepia virginalis. Ecol. Entomol. 2010, 35, 92–99. [Google Scholar] [CrossRef]
- Rios, R.S.; Cárdenas, M.; González, K.; Cisternas, M.F.; Guerra, P.C.; Loayza, A.P.; Gianoli, E. Effects of host plant and maternal feeding experience on population vital rates of a specialized leaf beetle. Arthropod-Plant Inte. 2013, 7, 109–118. [Google Scholar] [CrossRef]
- Moran, N.A. Adaptation and constraint in the complex life cycles of animals. Annu. Rev. Ecol. Syst. 1994, 25, 573–600. [Google Scholar] [CrossRef]
- Renwick, J.A.A. Variable diets and changing taste in plant–insect relationships. J. Chem. Ecol. 2001, 27, 1063–1076. [Google Scholar] [CrossRef]
- Chow, J.K.; Akhtar, Y.; Isman, M.B. The effects of larval experience with a complex plant latex on subsequent feeding and oviposition by the cabbage looper moth: Trichoplusia ni (Lepidoptera: Noctuidae). Chemoecology 2005, 15, 129–133. [Google Scholar] [CrossRef]
- Santana, A.; Zucoloto, F.S. Influence of previous experience on the preference, food utilization and performance of Ascia monuste orseis wild larvae (Godart) (Lepidoptera: Pieridae) for three different hosts. Neotrop. Entomol. 2011, 40, 631–638. [Google Scholar] [PubMed]
- Bernays, E.A. Neural limitations in phytophagous insects: Implications for diet breadth and evolution of host affiliation. Annu. Rev. Entomol. 2001, 46, 703–727. [Google Scholar] [CrossRef]
- Soler, R.; Pineda, A.; Li, Y.; Ponzio, C.; van Loon, J.J.; Weldegergis, B.T.; Dicke, M. Neonates know better than their mothers when selecting a host plant. Oikos 2012, 121, 1923–1934. [Google Scholar] [CrossRef]
- Barron, A.B. The life and death of Hopkins’ host-selection principle. J. Insect Behav. 2001, 14, 725–737. [Google Scholar] [CrossRef]
- Silva, A.K.; Gonçalves, G.L.; Moreira, G.R.P. Larval feeding choices in heliconians: Induced preferences are not constrained by performance and host plant phylogeny. Anim. Behav. 2014, 89, 155–162. [Google Scholar] [CrossRef]
- Bernays, E.; Weiss, M. Induced food preferences in caterpillars: The need to identify mechanisms. Entomol. Exp. Appl. 1996, 78, 1–8. [Google Scholar] [CrossRef]
- del Campo, M.; Renwick, J.A.A. Induction of host specificity in larvae of Manduca sexta: Chemical dependence controlling host recognition and developmental rate. Chemoecology 2000, 10, 115–121. [Google Scholar] [CrossRef]
- Cai, X.M.; Sun, X.L.; Dong, W.X.; Wang, G.C.; Chen, Z.M. Herbivore species, infestation time, and herbivore density affect induced volatiles in tea plants. Chemoecology 2014, 24, 1–14. [Google Scholar] [CrossRef]
- Xiao, Y.; Qian, J.; Hou, X.; Zeng, L.; Xu, L.; Mei, G.; Liao, Y. Diurnal emission of herbivore-induced (Z)-3-hexenyl acetate and alloocimene activates sweet potato defense responses to sweet potato weevils. J. Integr. Agric. 2023, 22, 1782–1796. [Google Scholar] [CrossRef]
- Behmer, S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Cease, A.J. How Nutrients Mediate the Impacts of Global Change on Locust Outbreaks. Annu. Rev. Entomol. 2024, 69, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Clissold, F.J.; Lihoreau, M.; Ponton, F.; Wilder, S.M.; Raubenheimer, D. Recent advances in the integrative nutrition371of arthropods. Annu. Rev. Entomol. 2015, 60, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Groothuis, T.G.; Taborsky, B. Introducing biological realism into the study of developmental plasticity in behaviour. Front. Zool. 2015, 12, S6. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Sadek, M.; Larsson, M.; Hansson, B.; Thöming, G. Larval host plant experience modulates both mate finding and oviposition choice in a moth. Anim. Behav. 2013, 85, 1169–1175. [Google Scholar] [CrossRef]
- Desurmont, G.A.; Weston, P.A. Switched after birth: Performance of the viburnum leaf beetle [Pyrrhalta viburni (Paykull)] after transfer to a suboptimal host plant. Insects 2014, 5, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, C.; Yuan, J.; Li, S.; Wang, X.; Chi, H. Demographic comparison of Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae) reared on three cultivars of Solanum melongena L. and a wild hostplant Solanum nigrum L. J. Econ. Entomol. 2017, 110, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xie, B.; Wang, X.; Li, C.; Wang, W. Population dynamic of Henosepilachna vigintioctopunctata in different host plants in Jianghan plain. China J. North. Hort. 2015, 11, 103–105. [Google Scholar]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Özgökçe, M.; Güncan, A.; Gökçe, A.; Smith, C.; Benelli, G.; Guedes, R. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–735. [Google Scholar] [CrossRef]
- Bertram, D.F.; Strathmann, R.R. Effects of maternal and larval nutrition on growth and form of planktotrophic larvae. Ecology 1998, 79, 315–327. [Google Scholar] [CrossRef]
- Skoracka, K.; Ratajczak, A.E.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Female fertility and the nutritional approach: The most essential aspects. Adv. Nutr. 2021, 12, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Smykal, V.; Raikhel, A.S. Nutritional control of insect reproduction. Curr. Opin. Insect Sci. 2015, 11, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Li, C.; Xia, Z.; Li, S. Effect of dietary protein and carbohydrates on survival and growth in larvae of the Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae). J. Insect Sci. 2018, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Kawazu, K. Rearing the 28-spotted ladybird beetle, Henosepilachna vigintioctopunctata (Coleoptera: Coccinelidae), with a switchover from host plant leaves to artificial diet. Appl. Entomol. Zool. 2014, 49, 359–362. [Google Scholar] [CrossRef]
- Peschiutta, M.L.; Bucci, S.J.; Scholz, F.G.; Goldstein, G. Compensatory responses in plant-herbivore interactions: Impacts of insects on leaf water relations. Acta Oecol. 2016, 73, 71–79. [Google Scholar] [CrossRef]
- Content, P.N. Heavy Livestock Grazing Promotes Locust Outbreaks by Lowering. Science 2010, 329, 330. [Google Scholar]
- Behmer, S.T.; Joern, A. Coexisting generalist herbivores occupy unique nutritional feeding niches. Proc. Natl. Acad. Sci. USA 2008, 105, 1977–1982. [Google Scholar] [CrossRef]
- Behmer, S.T.; Cox, E.; Raubenheimer, D.; Simpson, S.J. Food distance and its effect on nutrient balancing in a mobile insect herbivore. Anim. Behav. 2003, 66, 665–675. [Google Scholar] [CrossRef]
- Lee, K.P.; Raubenheimer, D.; Behmer, S.T.; Simpson, S.J. A correlation between macronutrient balancing and insect host-plant range: Evidence from the specialist caterpillar Spodoptera exempta (Walker). J. Insect Physiol. 2003, 49, 1161–1171. [Google Scholar] [CrossRef]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef]
- Morimoto, J. Optimum ratio of dietary protein and carbohydrate that maximises lifespan is shared among related insect species. Aging Cell 2023, 23, e14067. [Google Scholar] [CrossRef] [PubMed]
- Fanson, B.G.; Weldon, C.W.; Pérez-Staples, D.; Simpson, S.J.; Taylor, P.W. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni). Aging Cell 2009, 8, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Effects of Macronutrient on the Development of Henisepilachna vigintioctopunctata Larvae. Master’s Thesis, Yangtze University, Jingzhou, China, 2019. [Google Scholar]
- Milner, S.E.; Brunton, N.P.; Jones, P.W.; O’Brien, N.M.; Collins, S.G.; Maguire, A.R. Bioactivities of glycoalkaloids and their aglycones from Solanum species. J. Agric. Food Chem. 2011, 59, 3454–3484. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J. Agric. Food Chem. 2015, 63, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Distribution of Eggplant Glycoalkaloid and Its Chemical Ecological Function. Ph.D. Thesis, Shenyang Agricultural University, Shenyang, China, 2009. [Google Scholar]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Ding, Y.; Liu, X. Content determination and distribution of α-solanine in eggplant. Bull. Bot. Res. 2009, 29, 380–384. [Google Scholar]
- Zbigniew, A.; Pawel, M.; Kazimierz, Z. Potato leaf extract and its component, α-solanine, exert similar impact on development and oxidative stress in Galleria mellonella L. Arch. Insect Biochem. 2014, 87, 26–39. [Google Scholar]
- Krishnan, N.; Sehnal, F. Compartmentalization of oxidative stress and antioxidant defense in the larval gut of Spodoptera littoralis. Arch. Insect Biochem. 2006, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Devanand, P.; Rani, P.U. Insect growth regulatory activity of the crude and purified fractions from Solanum melongena L., Lycopersicum esculentum Mill. and Capsicum annuum L. J. Biopestic. 2011, 4, 118. [Google Scholar]
- Gürbüz, N.; Gürbüz, S.; Frary, A.; Frary, A.; Gürbüz, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Zhang, J.; He, T.; Huang, J.; Zhang, Z.; Wang, Y.; Hafeez, M.; Zhou, S.; Ren, X. Comprehensive metabolome and volatilome analyses in eggplant and tomato reveal their differential responses to Tuta absoluta infestation. Front. Plant Sci. 2021, 12, 757230. [Google Scholar] [CrossRef]
- Monaghan, P. Early growth conditions, phenotypic development and environmental change. Philos. Trans. R. Soc. B 2008, 363, 1635–1645. [Google Scholar] [CrossRef]
- Dmitriew, C.; Rowe, L. The effects of larval nutrition on reproductive performance in a food-limited adult environment. PLoS ONE 2011, 6, e17399. [Google Scholar] [CrossRef] [PubMed]
- Day, T.; Rowe, L. Developmental thresholds and the evolution of reaction norms for age and size at life-history transitions. Am. Nat. 2002, 159, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, P.J. Herbivore host choice and optimal bad motherhood. Trends Ecol. Evol. 2001, 16, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M. Patterns of variation in the influence of natal experience on habitat choice. Q. Rev. Biol. 2008, 83, 363–380. [Google Scholar] [CrossRef]
- Boggs, C.L.; Ross, C.L. The effect of adult food limitation on life history traits in Speyeria mormonia (Lepidoptera: Nymphalidae). Ecology 1993, 74, 433–441. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.R.; Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef]
| Stage | Host Plant | ||
|---|---|---|---|
| Potato | Tomato | Eggplant | |
| L1 (d) | 3.47 ± 0.09 a | 3.65 ± 0.09 a | 3.73 ± 0.09 a |
| L2 (d) | 2.88 ± 0.09 b | 2.62 ± 0.06 c | 3.17 ± 0.10 a |
| L3 (d) | 2.85 ± 0.10 b | 2.82 ± 0.07 b | 3.75 ± 0.08 a |
| L4 (d) | 5.18 ± 0.13 c | 5.63 ± 0.06 b | 6.00 ± 0.09 a |
| Pupa (d) | 3.78 ± 0.06 a | 3.73 ± 0.05 a | 3.72 ± 0.05 a |
| Total larval stage (d) | 14.28 ± 0.19 a | 15.11 ± 0.09 b | 16.61 ± 0.19 a |
| Larval survival rate (%) | 68.0 ± 5.5 b | 91.0 ± 3.4 a | 74.9 ± 7.7 ab |
| Stage | Factor | F Value | p Value |
|---|---|---|---|
| Preoviposition period (d) | Larval host | 0.600 | 0.549 |
| Adult host | 1.073 | 0.344 | |
| Larval * adult host | 0.883 | 0.475 | |
| Oviposition days (d) | Larval host | 0.467 | 0.627 |
| Adult host | 15.839 | <0.0001 * | |
| Larval * adult host | 0.786 | 0.536 | |
| Female adult (d) | Larval host | 2.585 | 0.0780 |
| Adult host | 2.457 | 0.0883 | |
| Larval * adult host | 1.229 | 0.299 | |
| Male adult (d) | Larval host | 0.251 | 0.778 |
| Adult host | 0.575 | 0.564 | |
| Larval * adult host | 1.722 | 0.148 | |
| Fecundity (eggs) | Larval host | 1.410 | 0.322 |
| Adult host | 37.289 | <0.0001 * | |
| Larval * adult host | 5.557 | <0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Wang, X.; Zhang, T.; Li, C.; Wang, Z. Adult Feeding Experience Determines the Fecundity and Preference of the Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae). Biology 2024, 13, 250. https://doi.org/10.3390/biology13040250
Qi J, Wang X, Zhang T, Li C, Wang Z. Adult Feeding Experience Determines the Fecundity and Preference of the Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae). Biology. 2024; 13(4):250. https://doi.org/10.3390/biology13040250
Chicago/Turabian StyleQi, Jingwei, Xiangping Wang, Tingjia Zhang, Chuanren Li, and Zailing Wang. 2024. "Adult Feeding Experience Determines the Fecundity and Preference of the Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae)" Biology 13, no. 4: 250. https://doi.org/10.3390/biology13040250
APA StyleQi, J., Wang, X., Zhang, T., Li, C., & Wang, Z. (2024). Adult Feeding Experience Determines the Fecundity and Preference of the Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae). Biology, 13(4), 250. https://doi.org/10.3390/biology13040250






