Ultrastructural Analysis of Large Japanese Field Mouse (Apodemus speciosus) Testes Exposed to Low-Dose-Rate (LDR) Radiation after the Fukushima Nuclear Power Plant Accident
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Collection of Animals
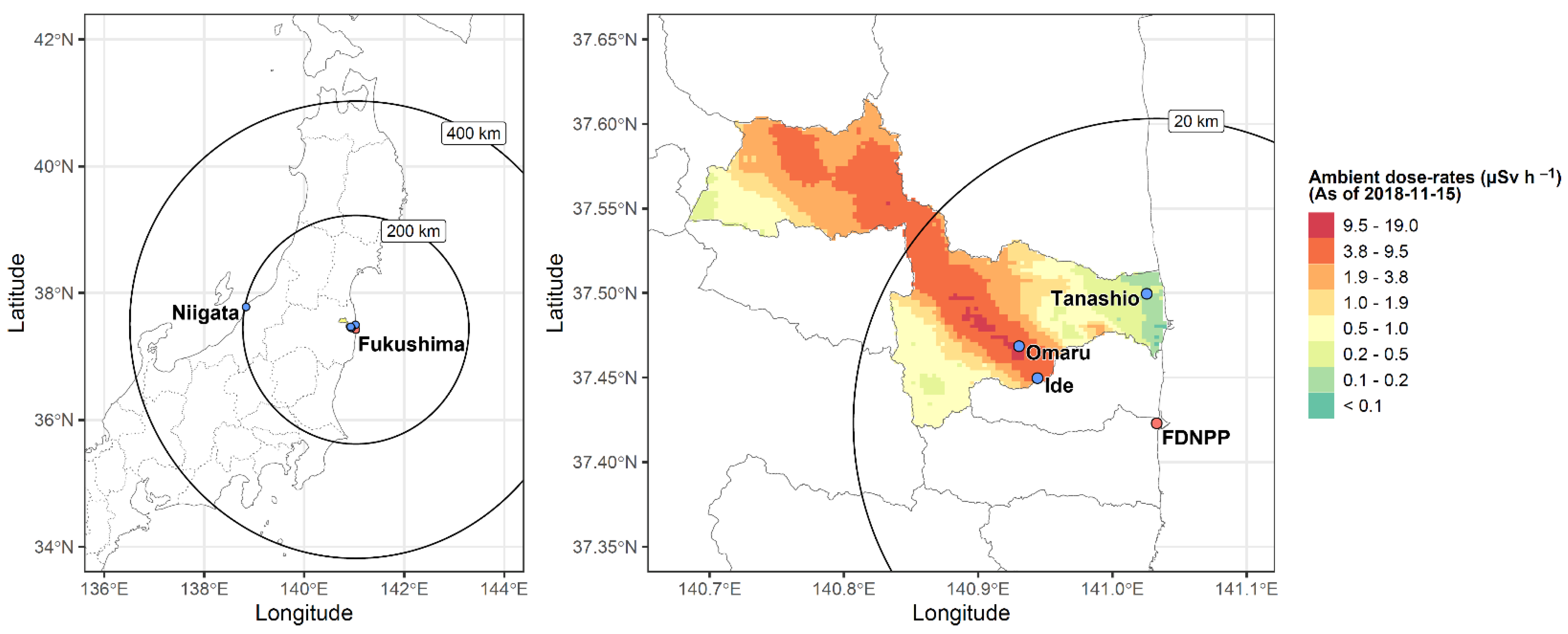
2.3. Ambient Radiation Dose rate Measurements at Sampling Sites
2.4. Internal Exposure to Radiation
2.5. Sample Preparation for Light Microscopy (LM) and Transmission Electron Microscopy (TEM)
2.6. Morphometric Analysis
2.7. Statistical Analysis
3. Results
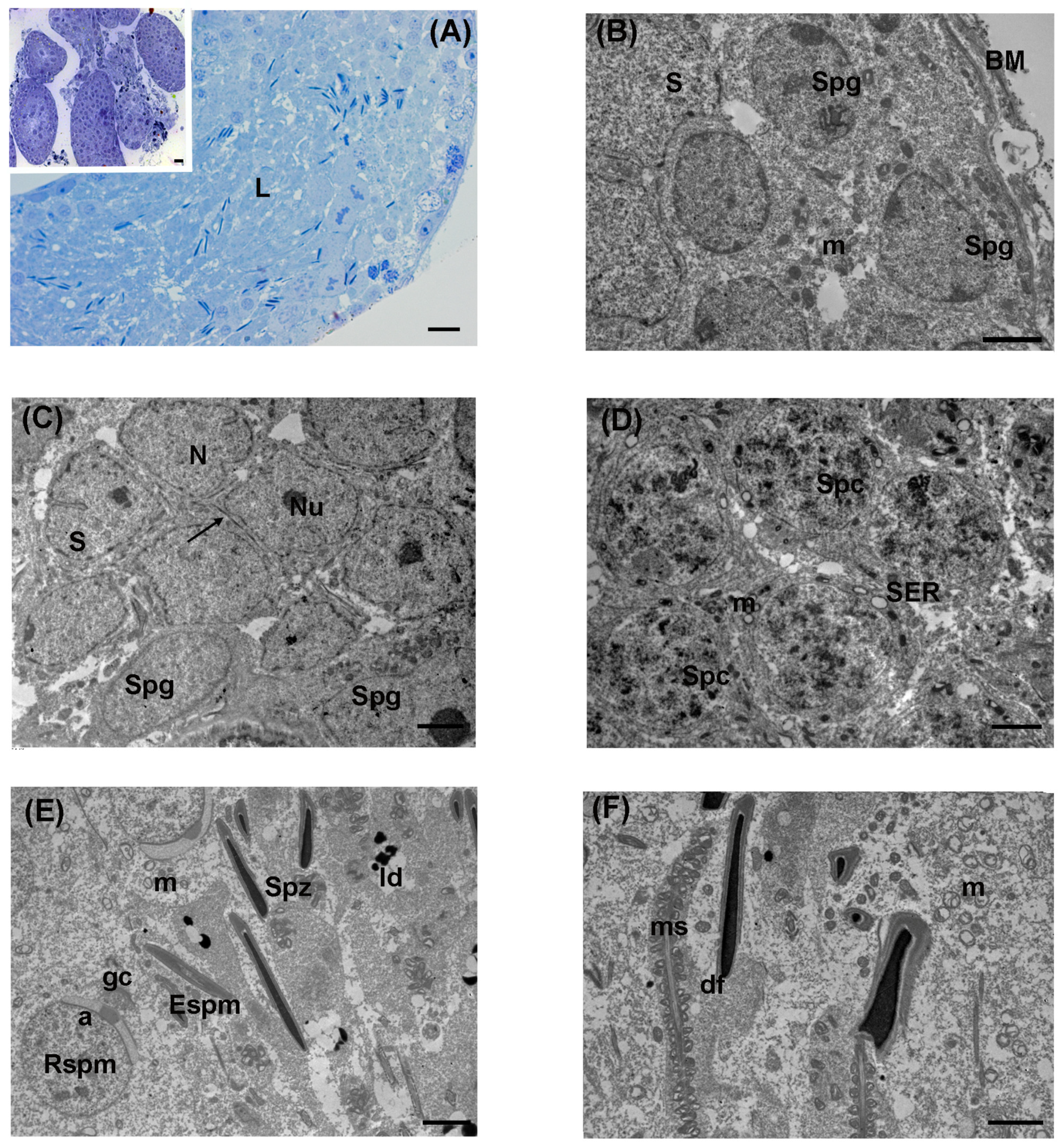
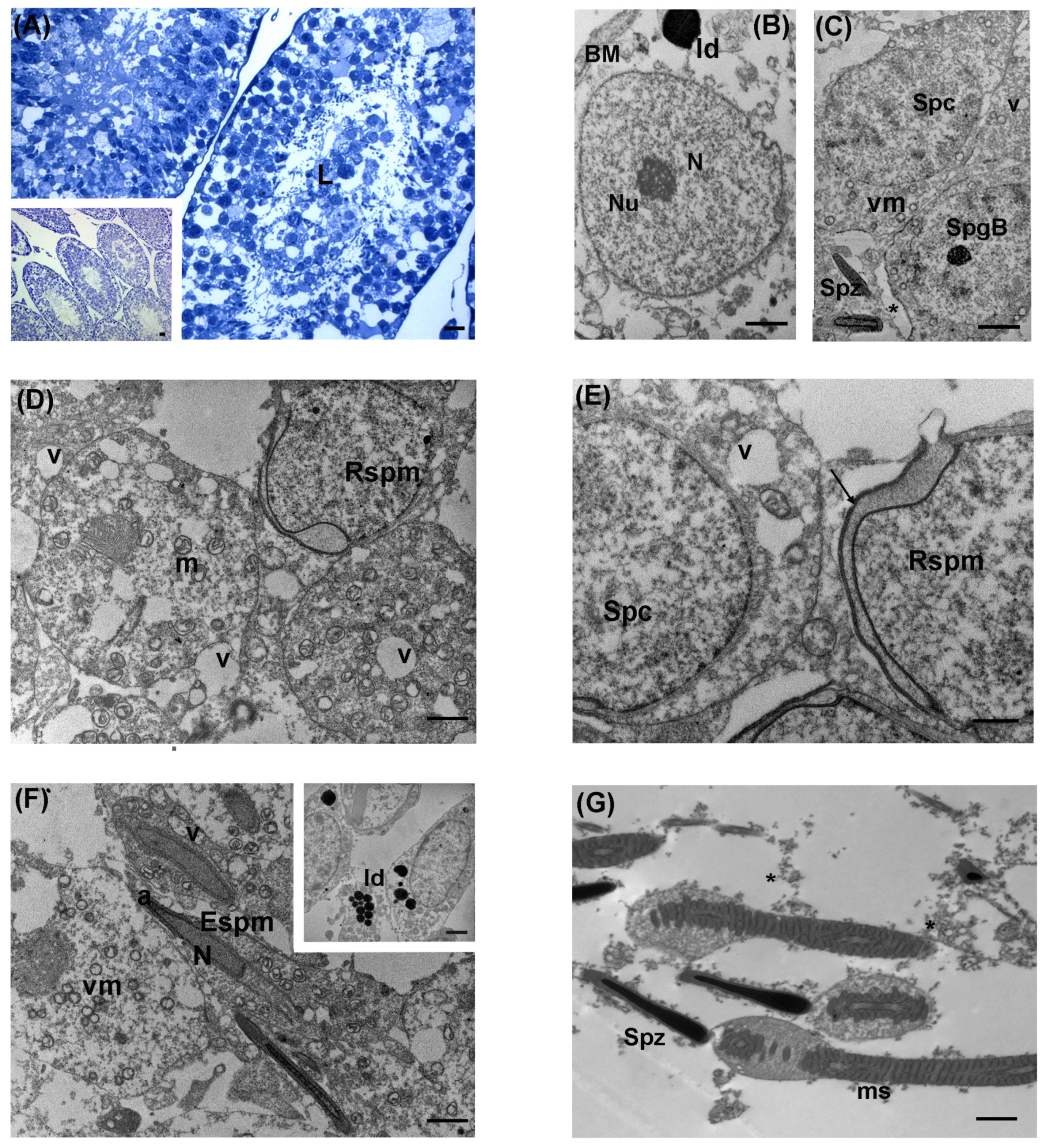
3.1. Control
3.2. Low Levels of LDR Radiation (Tanashio Site)
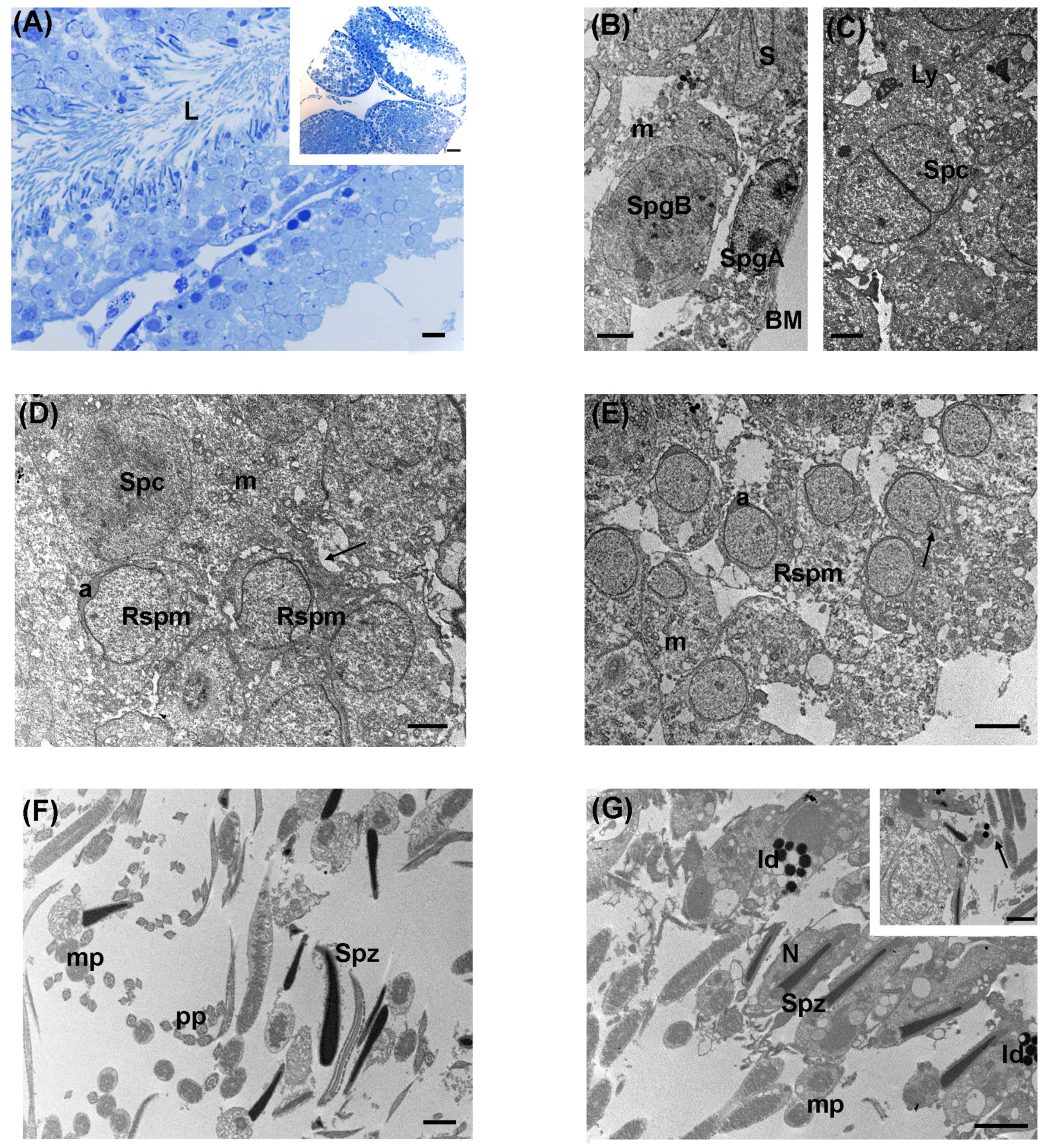
3.3. Intermediate Levels of LDR Radiation (Ide Site)
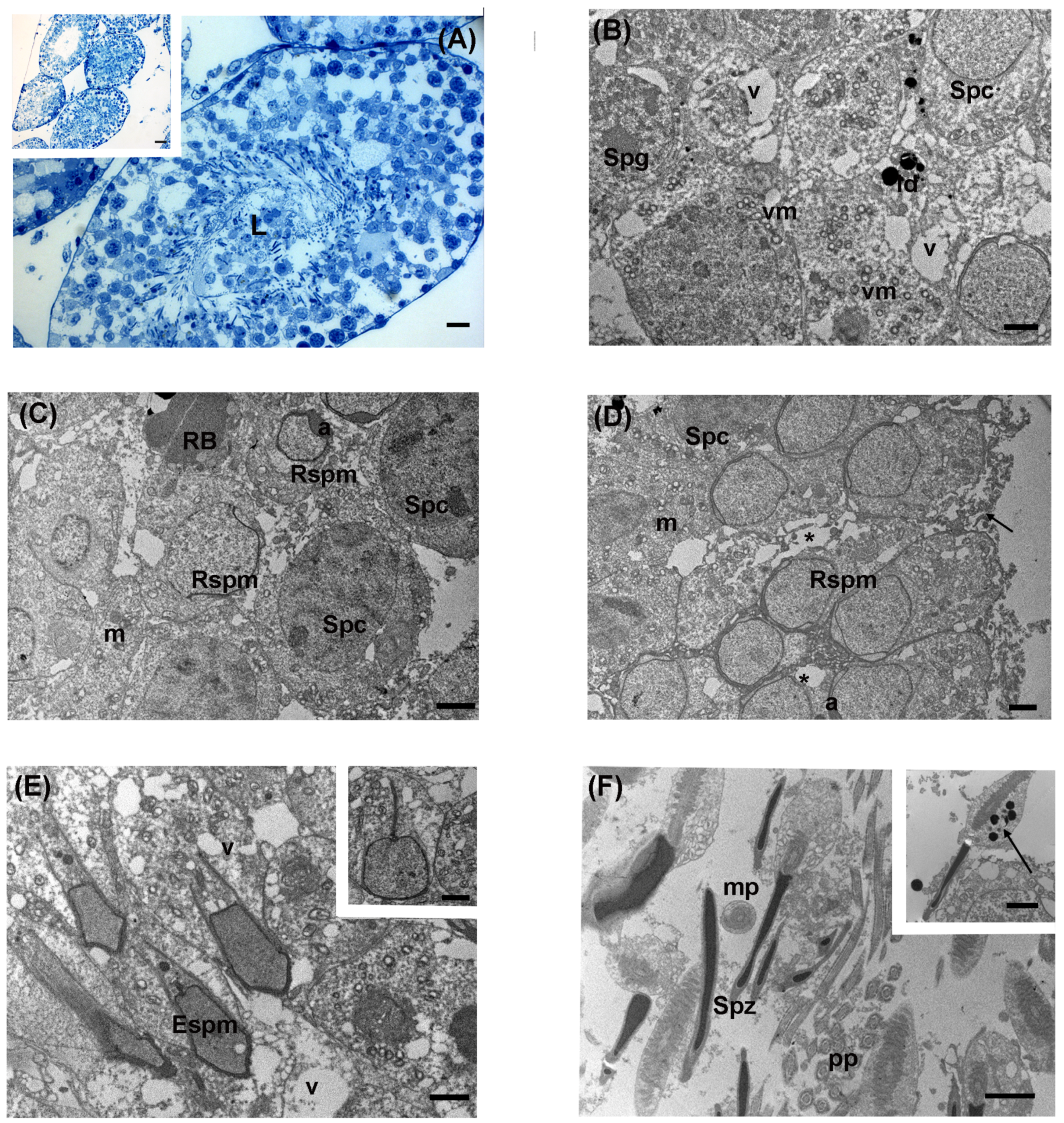
3.4. High Levels of LDR Radiation (Omaru Site)
3.5. Morphometric Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinoshita, N.; Sueki, K.; Sasa, K.; Kitagawa, J.; Ikarashi, S.; Nishimura, T.; Wong, Y.S.; Satou, Y.; Handa, K.; Takahashi, T.; et al. Assessment of individual radionuclide distributions from the Fukushima nuclear accident covering central-east Japan. Proc. Natl. Acad. Sci. USA 2011, 108, 19526–19529. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tagami, K.; Watanabe, Y.; Uchida, S.; Aono, T.; Ishii, N.; Yoshida, S.; Kubota, Y.; Fuma, S.; Ihara, S. Isotopic evidence of plutonium release into the environment from the Fukushima DNPP accident. Sci. Rep. 2012, 2, 304. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation, UNSCEAR 2008 Report to the General Assembly, with Scientific Annexes, Volume II: Scientific Annex E: Effects of Ionizing Radiation on Non-Human Biota. Available online: http://www.unscear.org/docs/reports/2008/11-80076_Report_2008_Annex_E.pdf (accessed on 7 April 2011).
- Hiyama, A.; Nohara, C.; Kinjo, S.; Taira, W.; Gima, S.; Tanahara, A.; Otaki, J.M. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012, 2, 570. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Hagiwara, A.; Matsui, S.; Kasahara, S.; Kawatsu, K.; Nishiumi, I.; Suzuki, H.; Ueda, K.; Mousseau, T.A. Abundance of birds in Fukushima as judged from Chernobyl. Environ. Pollut. 2012, 164, 36–39. [Google Scholar] [CrossRef]
- Murase, K.; Murase, J.; Horie, R.; Endo, K. Effects of the Fukushima Daiichi nuclear accident on goshawk reproduction. Sci. Rep. 2015, 5, 9405. [Google Scholar] [CrossRef]
- Sproull, M.; Hayes, J.; Ishiniwa, H.; Nanba, K.; Shankavaram, U.; Camphausen, K.; Johnson, T.E. Proteomic biomarker analysis of serum from Japanese Field mice (Apodemus Speciosus) collected within the Fukushima difficult-to-return zone. Health Phys. 2021, 121, 564–573. [Google Scholar] [CrossRef]
- Sato, I.; Sasaki, J.; Satoh, H.; Deguchi, Y.; Chida, H.; Natsuhori, M.; Otani, K.; Okada, K. Decreased blood cell counts were not observed in cattle living in the “difficult-to-return zone” of the Fukushima nuclear accident. Anim. Sci. J. 2019, 90, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sato, I.; Sasaki, J.; Satoh, H.; Natsuhori, M.; Murata, T.; Okada, K. Assessments of DNA damage and radiation exposure dose in cattle living in the contaminated area caused by the Fukushima nuclear accident. Bull. Environ. Contam. Toxicol. 2020, 105, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.; Hinton, T.G.; Luxton, J.J.; Bordman, A.; Okuda, K.; Taylor, L.E.; Hayes, J.; Gerke, H.C.; Chinn, S.M.; Anderson, D.; et al. Evaluation of DNA damage and stress in wildlife chronically exposed to low-dose, low-dose rate radiation from the Fukushima Dai-ichi nuclear power plant accident. Environ. Int. 2021, 155, 106675. [Google Scholar] [CrossRef]
- Shiomi, N.; Takahashi, H.; Watanabe, Y.; Fuma, S.; Aoki, M.; Kubota, M.; Furuhata, Y.; Morosawa, T.; Kubota, Y. Chromosomal aberrations in Large Japanese field mice (Apodemus speciosus) captured in various periods after Fukushima Daiichi nuclear power plant accident. Radiat. Res. 2022, 198, 347–356. [Google Scholar] [CrossRef]
- Okano, T.; Onuma, M.; Ishiniwa, H.; Azuma, N.; Tamaoki, M.; Nakajima, N.; Shindo, J.; Yokohata, Y. Classification of the spermatogenic cycle, seasonal changes of seminiferous tubule morphology and estimation of the breeding season of the large Japanese field mouse (Apodemus speciosus) in Toyama and Aomori prefectures, Japan. J. Vet. Med. Sci. 2015, 77, 799–807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawagoshi, T.; Shiomi, N.; Takahashi, H.; Watanabe, Y.; Fuma, S.; Doi, K.; Kawaguchi, I.; Aoki, M.; Kubota, M.; Furuhata, Y.; et al. Chromosomal aberrations in Large Japanese Field mice (Apodemus speciosus) captured near Fukushima Dai-ichi nuclear power plant. Environ. Sci. Technol. 2017, 51, 4632–4641. [Google Scholar] [CrossRef]
- Otala, M.; Suomalainen, L.; Pentikainen, M.O.; Kovanen, P.; Tenhunen, M.; Erkkila, K.; Toppari, J.; Dunkel, L. Protection from radiation-induced male germ cell loss by sphingosine-1-phosphate1. Biol. Reprod. 2004, 70, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gong, P.; Zhao, H.; Wang, Z.; Gong, S.; Cai, L. Effect of low level radiation on the death of male germ cells. Radiat. Res. 2006, 165, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Bazalytska, S.V.; Persidsky, Y.; Romanenko, A.M. Molecular mechanisms of initiation of carcinogenesis in the testis. Exp. Oncol. 2019, 41, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Li, J.; Le, W.; Zhang, J. Low-dose ionizing irradiation triggers apoptosis of undifferentiated spermatogonia in vivo and in vitro. Transl. Androl. Urol. 2019, 8, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.J.; Kang, M.K.; Kye, Y.U.; Baek, J.H.; Sim, Y.J.; Lee, H.J.; Kang, Y.R.; Jo, W.S.; Kim, J.S.; Lee, C.G. Differential effects of low and high radiation dose rates on mouse spermatogenesis. Int. J. Mol. Sci. 2021, 22, 12834. [Google Scholar] [CrossRef]
- Takino, S.; Yamashiro, H.; Sugano, Y.; Fujishima, Y.; Nakata, A.; Kasai, K.; Hayashi, G.; Urushihara, Y.; Suzuki, M.; Shinoda, H.; et al. Analysis of the effect of chronic and low-dose radiation exposure on spermatogenic cells of male large Japanese field mice (Apodemus speciosus) after the Fukushima Daiichi nuclear power plant accident. Radiat. Res. 2017, 187, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Onuma, M.; Endo, D.; Ishinawa, H.; Tamaoki, M. Estimation of dose rate for the large Japanese field mouse (Apodemus speciosus) distributed in the “difficult-to-return zone” in Fukushima prefecture. In Low-Dose Radiation Effects on Animals and Ecosystems, Long-Term Study on the Fukushima Nuclear Accident; Fukumoto, M., Ed.; Springer: Singapore, 2020; Volume I, Chapter 2; pp. 17–30. [Google Scholar]
- Ito, J.; Meguro, K.; Komatsu, K.; Ohdaira, T.; Shoji, R.; Yamada, T.; Sugimura, S.; Fujishima, Y.; Nakata, A.; Fukumoto, M.; et al. Seasonal changes in the spermatogenesis of the large Japanese field mice (Apodemus speciosus) controlled by proliferation and apoptosis of germ cells. Anim. Reprod. Sci. 2020, 214, 106288. [Google Scholar] [CrossRef]
- Braga-Tanaka, I.; Tanaka, S.; Kohda, A.; Takai, D.; Nakamura, S.; Ono, T.; Tanaka, K.; Komura, J.I. Experimental studies on the biological effects of chronic low dose-rate radiation exposure in mice: Overview of the studies at the Institute for Environmental Sciences. Int. J. Radiat. Biol. 2018, 94, 423–433. [Google Scholar] [CrossRef]
- Kojima, Y.; Yokoya, S.; Kurita, N.; Idaka, T.; Ishikawa, T.; Tanaka, H.; Ezawa, Y.; Ohto, H. Cryptorchidism after the Fukushima Daiichi Nuclear Power Plant accident:causation or coincidence? Fukushima J. Med. Sci. 2019, 65, 76–98. [Google Scholar] [CrossRef]
- Hacker-Klom, U. Long term effects of ionizing radiation on mouse spermatogenesis. Acta Radiol. Oncol. 1985, 24, 363–367. [Google Scholar] [CrossRef]
- Gong, E.J.; Shin, I.S.; Son, T.G.; Yang, K.; Heo, K.; Kim, J.S. Low-dose-rate radiation exposure leads to testicular damage with decreases in DNMT1 and HDAC1 in the murine testis. J. Radiat. Res. 2014, 55, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Iwasaki, T.; Murata, K.; Yamashiro, H.; Goh, V.S.T.; Nakayama, R.; Fujishima, Y.; Ono, T.; Kino, Y.; Simizu, Y.; et al. Morphological reproductive characteristics of testes and fertilization capacity of cryopreserved sperm after the Fukushima accident in raccoon (Procyon lotor). Reprod. Domest. Anim. 2020, 56, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Nihei, K.; Tokita, S.; Yamashiro, H.; Ting, V.G.S.; Nakayama, R.; Fujishima, Y.; Kino, Y.; Shimizu, Y.; Shinoda, H.; Ariyoshi, K.; et al. Evaluation of sperm fertilization capacity of large Japanese field mice (Apodemus speciosus) exposed to chronic low dose-rate radiation after the Fukushima accident. J. Radiat. Res. Appl. Sci. 2022, 15, 186–190. [Google Scholar] [CrossRef]
- Higley, K.; Real, A.; Chambers, D. ICRP Publication 148: Radiation weighting for reference animals and plants. Ann. ICRP 2021, 50, 9–133. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Tsuji, H.; Kawagoshi, T.; Shiomi, N.; Takahashi, H.; Watanabe, Y.; Fuma, S.; Doi, K.; Kawaguchi, I.; Aoki, M.; et al. Chromosomal aberrations in wild mice captured in areas differentially contaminated by the Fukushima Dai–ichi nuclear power plant accident. Environ. Sci. Technol. 2015, 49, 10074–10083. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Chiba, M.; Tanahara, A.; Aida, J.; Shimizu, Y.; Suzuki, T.; Murakami, S.; Koarai, K.; Ono, T.; Oka, T.; et al. Radioactivity and radionuclides in deciduous teeth formed before the Fukushima-Daiichi Nuclear Power Plant accident. Sci. Rep. 2021, 11, 10335. [Google Scholar] [CrossRef]
- Palmerini, M.G.; Zhurabekova, G.; Balmagambetova, A.; Nottola, S.A.; Miglietta, S.; Belli, M.; Bianchi, S.; Cecconi, S.; Di Nisio, V.; Familiari, G.; et al. The pesticide Lindane induces dose-dependent damage to granulosa cells in an in vitro culture. Reprod. Biol. 2017, 17, 349–356. [Google Scholar] [CrossRef]
- Belli, M.; Rinaudo, P.; Palmerini, M.G.; Ruggeri, E.; Antonouli, S.; Nottola, S.A.; Macchiarelli, G. Pre-implantation mouse embryos cultured in vitro under different oxygen concentrations show altered ultrastructures. Int. J. Environ. Res. Public Health 2020, 17, 3384. [Google Scholar] [CrossRef]
- Palmerini, M.G.; Belli, M.; Nottola, S.A.; Miglietta, S.; Bianchi, S.; Bernardi, S.; Antonouli, S.; Cecconi, S.; Familiari, G.; Macchiarelli, G. Mancozeb impairs the ultrastructure of mouse granulosa cells in a dose-dependent manner. J. Reprod. Dev. 2018, 64, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Zhang, L.; Liu, X.; Donjacour, A.; Ruggeri, E.; Palmerini, M.G.; Nottola, S.A.; Macchiarelli, G.; Rinaudo, P. Oxygen concentration alters mitochondrial structure and function in in vitro fertilized preimplantation mouse embryos. Hum. Reprod. 2019, 34, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Belli, M.; De Rubeis, M.; Khalili, M.A.; Familiari, G.; Nottola, S.A.; Macchiarelli, G.; Hajderi, E.; Palmerini, M.G. Ultrastructural evaluation of mouse oocytes exposed in vitro to different concentrations of the fungicide Mancozeb. Biology 2023, 12, 698. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.T.; De Souza, D.B.; Costa, W.S.; Pereira-Sampaio, M.A.; Sampaio, F.J. Effects of testicular transfixation on seminiferous tubule morphology and sperm parameters of prepubertal, pubertal, and adult rats. Theriogenology 2015, 84, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Wang, B.; Doi, K.; Ishibashi, K.; Ichikawa, S.; Furuhata, Y.; Kubota, M.; Watanabe, Y. Radiation effects on wild medaka around Fukushima Dai-ichi Nuclear Power Plant assessed by micronucleus assay. J. Radiat. Res. 2021, 62, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, H.; Butterworth, K.T.; Yokoya, A.; Ogawa, T.; Prise, K.M. Low-dose radiation-induced risk in spermatogenesis. Int. J. Radiat. Biol. 2017, 93, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, A.; Skrzypek, M.; Stec, M.; Panasiuk, L. Effect of ionizing radiation on the male reproductive system. Ann. Agric. Environ. Med. 2019, 26, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L.; Hunter, N.R.; Suzuki, N.; Trostle, P.K.; Withers, H.R. Gradual regeneration of mouse testicular stem cells after exposure to ionizing radiation. Radiat. Res. 1978, 74, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Intano, G.W.; McCarrey, J.R.; Walter, R.B.; McMahan, C.A.; Walter, C.A. Recovery of a low mutant frequency after ionizing radiation-induced mutagenesis during spermatogenesis. Mutat. Res. 2008, 654, 150–157. [Google Scholar] [CrossRef]
- Elbakary, R.H.; Tawfik, S.M.; Amer, R.M. Evaluation of the possible protective effect of alpha lipoic acid on testicular toxicity induced by polychlorinated biphenyl in adult albino rats: A histological study. J. Microsc. Ultrastruct. 2020, 8, 42–50. [Google Scholar]
- Bizarro, P.; Acevedo, S.; Niño-Cabrera, G.; Mussali-Galante, P.; Pasos, F.; Avila-Costa, M.R.; Fortoul, T.I. Ultrastructural modifications in the mitochondrion of mouse Sertoli cells after inhalation of lead, cadmium or lead-cadmium mixture. Reprod. Toxicol. 2003, 17, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Abuelhija, M.; Weng, C.C.; Shetty, G.; Meistrich, M.L. Differences in radiation sensitivity of recovery of spermatogenesis between rat strains. Toxicol. Sci. 2012, 126, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Krishna, H.; Changil, A.; Srinivas, M.; Roy, T.S.; Jacob, T.G. Ultrastructural study of rat testis following conventional phototherapy during neonatal period. J. Microsc. Ultrastruct. 2018, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.; Saber, A.; Omar, A.; Soliman, A. Effect of cadmium on the testes of adult albino rats and the ameliorating effect of zinc and Vitamin E. Br. J. Sci. 2014, 11, 72–95. [Google Scholar]
- Hussein, M.R.; Abu-Dief, E.E.; Abou El-Ghait, A.T.; Adly, M.A.; Abdelraheem, M.H. Morphological evaluation of the radioprotective effects of melatonin against X-ray-induced early and acute testis damage in Albino rats: An animal model. Int. J. Exp. Pathol. 2006, 87, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, B.M.; Jimenez, G.C.; de Morais, R.N.; Peixoto, C.A.; de Albuquerque Nogueira, R.; da Silva, V.A., Jr. Evaluation of testicular degeneration induced by low-frequency electromagnetic fields. J. Appl. Toxicol. 2012, 32, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A. Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinology 1995, 136, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Furuchi, T.; Masuko, K.; Nishimune, Y.; Obinata, M.; Matsui, Y. Inhibition of testicular germ cell apoptosis and differentiation in mice misexpressing Bcl-2 in spermatogonia. Development 1996, 122, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Miao, J.; Wang, Y. Mitochondrial regulation in spermatogenesis. Reproduction 2022, 163, R55–R69. [Google Scholar] [CrossRef]
- Vertika, S.; Singh, K.K.; Rajender, S. Mitochondria, spermatogenesis, and male infertility—An update. Mitochondrion 2020, 54, 26–40. [Google Scholar] [CrossRef]
- Shi, L.; Xun, W.; Zhou, H.; Hou, G.; Yue, W.; Zhang, C.; Ren, Y.; Yang, R. Ultrastructure of germ cells, Sertoli cells and mitochondria during spermatogenesis in mature testis of the Chinese Taihang black goats (Capra hircus). Micron 2013, 50, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Tirinato, L.; Marafioti, M.G.; Pagliari, F.; Jansen, J.; Aversa, I.; Hanley, R.; Nisticò, C.; Garcia-Calderón, D.; Genard, G.; Guerreiro, J.F.; et al. Lipid droplets and ferritin heavy chain: A devilish liaison in human cancer cell radioresistance. eLife 2021, 10, e72943. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.H.; Liu, T.; Wang, H.; Xue, L.X.; Wang, J.J. Fatty acids metabolism: The bridge between ferroptosis and ionizing radiation. Front. Cell Dev. Biol. 2021, 9, 675617. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, C.; Aramaki, S.; Xu, L.; Tsuge, S.; Sakamoto, T.; Mamun, M.A.; Islam, A.; Hayakawa, T.; Takanashi, Y.; et al. Lipid polyunsaturated fatty acid chains in mouse kidneys were increased within 5 min of a single high dose whole body irradiation. Int. J. Mol. Sci. 2023, 24, 12439. [Google Scholar] [CrossRef]
- Meistrich, M.L.; Kangasniemi, M. Hormone treatment after irradiation stimulates recovery of rat spermatogenesis from surviving spermatogonia. J. Androl. 1997, 18, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zeng, Y.; Zhao, J.; Zhu, C.J.; Hou, W.G.; Zhang, S. Upregulation and nuclear translocation of testicular ghrelin protects differentiating spermatogonia from ionizing radiation injury. Cell Death Dis. 2014, 5, e1248. [Google Scholar] [CrossRef]
- Eugeni, E.; Arato, I.; Del Sordo, R.; Sidoni, A.; Garolla, A.; Ferlin, A.; Calafiore, R.; Brancorsini, S.; Mancuso, F.; Luca, G. Fertility preservation and restoration options for pre-pubertal male cancer patients: Current approaches. Front. Endocrinol. 2022, 13, 877537. [Google Scholar] [CrossRef]
- Shalet, S.M.; Tsatsoulis, A.; Whitehead, E.; Read, G. Vulnerability of the human leydig cell to radiation damage is dependent upon age. J. Endocrinol. 1989, 120, 161–165. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Wu, Z.B.; Zhao, J.; Zhang, S.; Li, W. Protection of murine spermatogenesis against ionizing radiation-induced testicular injury by a green tea polyphenol. Biol. Reprod. 2015, 92, 6. [Google Scholar] [CrossRef]
- Albuquerque, A.V.; Almeida, F.R.; Weng, C.C.; Shetty, G.; Meistrich, M.L.; Chiarini-Garcia, H. Spermatogonial behavior in rats during radiation-induced arrest and recovery after hormone suppression. Reproduction 2013, 146, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Takahashi, H.; Watanabe, Y.; Fuma, S.; Kawaguchi, I.; Aoki, M.; Kubota, M.; Furuhata, Y.; Shigemura, Y.; Yamada, F.; et al. Estimation of absorbed radiation dose rates in wild rodents inhabiting a site severely contaminated by the Fukushima Dai-ichi nuclear power plant accident. J. Environ. Radioact. 2015, 142, 124–131. [Google Scholar] [CrossRef] [PubMed]
| Group | Number of Animals | ID | Site | Breeding Season | Sampling Date | Body Weight (g) | Ambient Dose Rate (µSv/h) | Average Diameter of Seminiferous Tubule (µm) |
|---|---|---|---|---|---|---|---|---|
| Niigata (Control) | 4 | Ni_36 | Kakuta | TP | 02/06/2021 | 61.6 | 0.048 * | 200.3 |
| Ni_38 | Kakuta | TP | 02/06/2021 | 52.1 | 0.049 * | 144.8 | ||
| Ni_41 | Kakuta | TP | 02/07/2021 | 62.6 | 0.049 * | 129.4 | ||
| Ni_44 | Kakuta | TP | 02/07/2021 | 45.7 | 0.048 * | 138.7 | ||
| Fukushima | 4 | Fu_730 | Tanashio | RS | 17/04/2018 | 39.3 | 0.29 | 244.7 |
| Fu_733 | Omaru | RS | 18/04/2018 | 39.8 | 11.80 | 245.5 | ||
| Fu_735 | Ide | RS | 18/04/2018 | 17.0 | 5.11 | 236.4 | ||
| Fu_737 | Omaru | RS | 19/04/2018 | 28.8 | 11.80 | 243.7 |
| Controls (Niigata Site) | Low Levels of LDR Radiation (Tanashio Site) | Intermediate Levels of LDR Radiation (Ide Site) | High Levels of LDR Radiation (Omaru Site) | |
|---|---|---|---|---|
| Seminiferous tubules Diameter (µm) | 172.5 ± 50.3 a | 244.7 ± 75.8 a | 236.4 ± 63.9 a | 245.8 ± 67.4 a |
| Germinal epithelium Height (µm) | 56.0 ± 15.5 a | 47.8 ± 10.1 a | 47.6 ± 11.8 a | 52.8 ± 16.4 a |
| Controls (Niigata Site) | Low Levels of LDR Radiation (Tanashio Site) | Intermediate Levels of LDR Radiation (Ide Site) | High Levels of LDR Radiation (Omaru Site) | |
|---|---|---|---|---|
| Intercellular space (µm) | 0.88 ± 0.27 a | 1.10 ± 0.30 a,b | 1.17 ± 0.32 a,b | 1.7 ± 0.40 c |
| Cytoplasmatic vacuolization (500 µm2) | 5.6 ± 2.3 a | 11.6 ± 1.5 b | 14.8 ± 2.0 b | 14.2 ± 3.5 b |
| Vacuolated mitochondria (500 µm2) | 5.4 ± 1.6 a | 9.2 ± 2.5 a,b,c | 11.6 ± 3.4 b,c | 13.2 ± 2.5 b,c |
| Lipid droplets (500 µm2) | 5 ± 2.5 a | 6.8 ± 5.3 a,b | 7.2 ± 2.3 a,b | 12 ± 4.2 b |
| Acrosomal vesicles (500 µm2) | 4.6 ± 2 a | 2.6 ± 1.3 a,b | 2 ± 1 b | 2.4 ± 1.1 a,b |
| Electron-dense mitochondria (with no visible cristae) (500 µm2) | 5.2 ± 0.8 a | 6.8 ± 1.9 a,b | 8.8 ± 2.4 b | 9.2 ± 1.9 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, M.; Belli, M.; De Rubeis, M.; Tokita, S.; Ikema, H.; Yamashiro, H.; Fujishima, Y.; Anderson, D.; Goh, V.S.T.; Shinoda, H.; et al. Ultrastructural Analysis of Large Japanese Field Mouse (Apodemus speciosus) Testes Exposed to Low-Dose-Rate (LDR) Radiation after the Fukushima Nuclear Power Plant Accident. Biology 2024, 13, 239. https://doi.org/10.3390/biology13040239
Gatti M, Belli M, De Rubeis M, Tokita S, Ikema H, Yamashiro H, Fujishima Y, Anderson D, Goh VST, Shinoda H, et al. Ultrastructural Analysis of Large Japanese Field Mouse (Apodemus speciosus) Testes Exposed to Low-Dose-Rate (LDR) Radiation after the Fukushima Nuclear Power Plant Accident. Biology. 2024; 13(4):239. https://doi.org/10.3390/biology13040239
Chicago/Turabian StyleGatti, Marta, Manuel Belli, Mariacarla De Rubeis, Syun Tokita, Hikari Ikema, Hideaki Yamashiro, Yohei Fujishima, Donovan Anderson, Valerie Swee Ting Goh, Hisashi Shinoda, and et al. 2024. "Ultrastructural Analysis of Large Japanese Field Mouse (Apodemus speciosus) Testes Exposed to Low-Dose-Rate (LDR) Radiation after the Fukushima Nuclear Power Plant Accident" Biology 13, no. 4: 239. https://doi.org/10.3390/biology13040239
APA StyleGatti, M., Belli, M., De Rubeis, M., Tokita, S., Ikema, H., Yamashiro, H., Fujishima, Y., Anderson, D., Goh, V. S. T., Shinoda, H., Nakata, A., Fukumoto, M., Miura, T., Nottola, S. A., Macchiarelli, G., & Palmerini, M. G. (2024). Ultrastructural Analysis of Large Japanese Field Mouse (Apodemus speciosus) Testes Exposed to Low-Dose-Rate (LDR) Radiation after the Fukushima Nuclear Power Plant Accident. Biology, 13(4), 239. https://doi.org/10.3390/biology13040239







