Simple Summary
Cancer cells often show high levels of use of glucose, leading to acidic conditions around tumors. This acidity can make cancer more aggressive, spreading to other parts of the body and worsening patients’ outcomes. The imbalance in acidity can be both a cause and a result of changes in some cell functions. This review looks into why cancer cells become acidic and how they adapt to survive in this harsh environment. It also discusses the challenges of measuring acidity inside cells, which is crucial to understanding how cells regulate acidity. Finally, it shows how acidity affects tumor growth and whether it helps or hinders cancer treatments.
Abstract
Cancer cells are associated with high glycolytic activity, which results in acidification of the tumor microenvironment. The occurrence of this stressful condition fosters tumor aggressiveness, with the outcome of invasiveness and metastasis that are linked to a poor clinical prognosis. Acidosis can be both the cause or consequence of alterations in the functions and expressions of transporters involved in intracellular acidity regulation. This review aims to explore the origin of acidity in cancer cells and the various mechanisms existing in tumors to resist, survive, or thrive in the acidic environment. It highlights the difficulties in measuring the intracellular pH evolution that impedes our understanding of the many regulatory and feedback mechanisms. It finally presents the consequences of acidity on tumor development as well as the friend or foe role of acidity in therapy.
1. Origin of Tumor Acidity
A striking but common feature of the tumor microenvironment is a high level of acidity [1,2,3]. Tumor acidity primarily originates from the increased glycolytic activity of cancer cells. This heightened metabolic process leads to the accumulation of lactic acid in the tumor microenvironment, resulting in acidification, but is glycolysis—or, more exactly, anaerobic glycolysis—the only driver of the increased acidity?
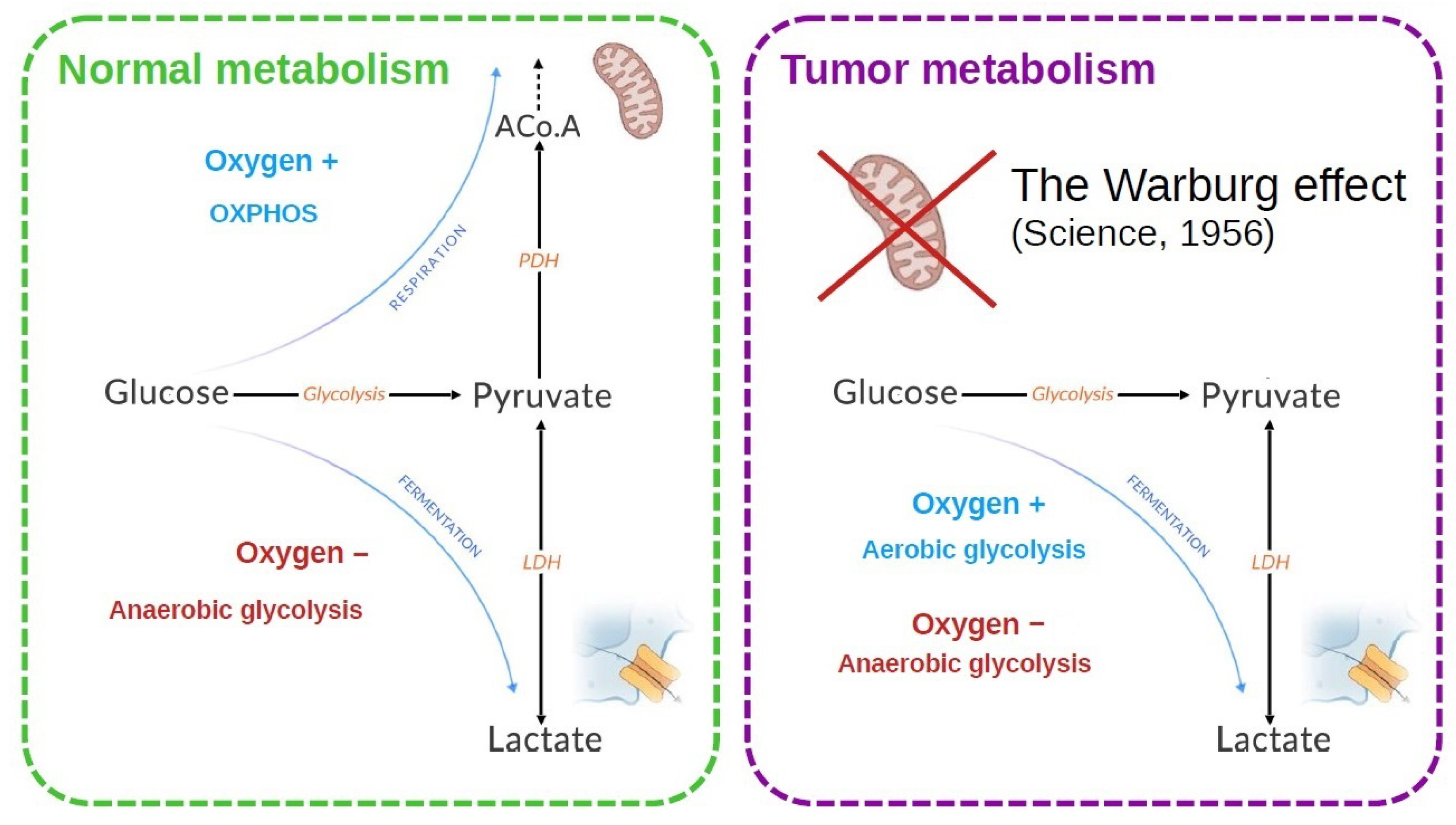
Glycolysis is indeed the common starting point for the two main pathways of energy metabolism: mitochondrial respiration and fermentation, which depend on the presence and absence of oxygen, respectively (Figure 1). In the presence of oxygen, most differentiated cells metabolize glucose to pyruvate by glycolysis, then completely oxidize most of this pyruvate to carbon dioxide during the Krebs cycle in the mitochondrial compartment. It is the mitochondrial respiration or oxidative phosphorylation (OXPHOS), which produces the largest amount of ATP by metabolizing glucose. Conversely, anaerobic glycolysis, or fermentation, is the reaction that converts glucose to lactic acid in the absence of oxygen, producing large amounts of lactate and little ATP (Figure 1). Although glycolysis produces less ATP per mole of glucose than OXPHOS, it allows faster generation of ATP [4].
Cells in the proliferative state need energy and organic resources in order to reproduce the components of the cell (DNA, membranes, and proteins). Glucose is essential because, apart from ATP, it also provides the metabolic intermediates that take part in anabolic reactions. In the case of tumor cells, the majority develops a more glycolytic than oxidative metabolism, often accompanying a greater aggressiveness of these tumors. This led Warburg to suspect a deficit in mitochondrial function responsible for this metabolic orientation [5,6]. It now appears that mitochondria function normally in tumor cells and that the blocking of OXPHOS constitutes an adaptive event (Figure 1).
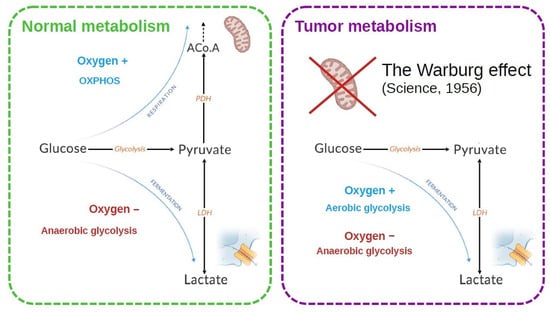
Figure 1.
Energy metabolism is classically described for a normal and a tumor cell. In the normal cell, the two metabolic modes depend on the presence of oxygen. In tumor cells, an increased lactate production is observed. Warburg [6] made the hypothesis that the mitochondria were defective to explain this observation. It was later shown that this is not necessarily the case.
Figure 1.
Energy metabolism is classically described for a normal and a tumor cell. In the normal cell, the two metabolic modes depend on the presence of oxygen. In tumor cells, an increased lactate production is observed. Warburg [6] made the hypothesis that the mitochondria were defective to explain this observation. It was later shown that this is not necessarily the case.
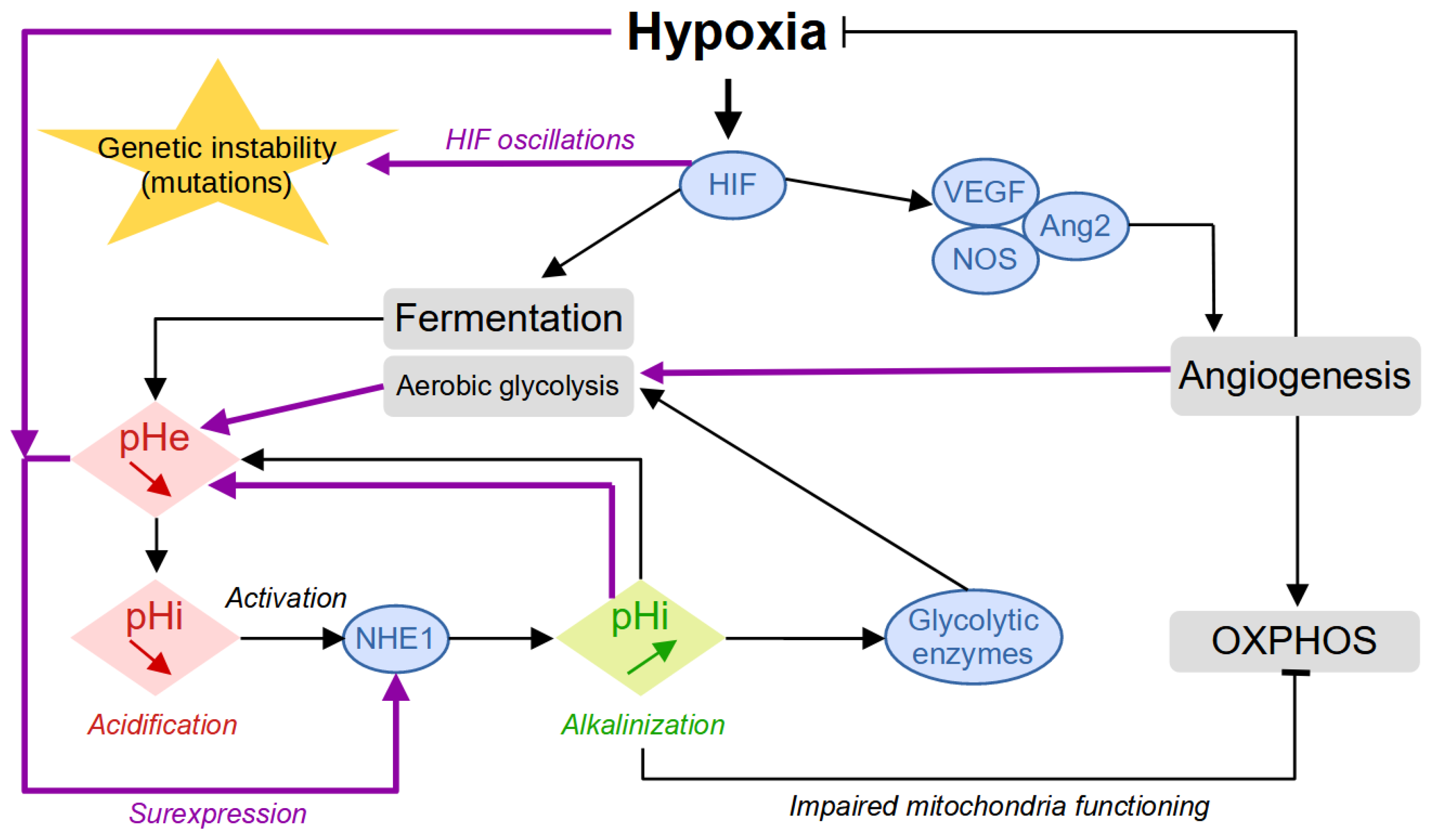
In addition to the role of glucose, oxygen is an essential molecule for cell survival since it is used as a final electron acceptor during the production of energy by mitochondrial respiration. Oxygen can diffuse up to a distance of about 100 μm from a blood vessel, and this is why the presence of many vessels is necessary to supply all the cells of a tissue [7,8]. As it develops, the tumor destroys the microvascular network which ensures the physiological and homogeneous oxygenation of the tissue. Tumor cells that divide very quickly are therefore large consumers of oxygen and nutrients and will then quickly run out of resources. They then enter a quiescent state, where they drastically reduce their metabolism and no longer divide. The activation of the Hypoxia-Inducible Factor (HIF) family of transcription factors represents one of the main oxygen-responsive signaling pathways that allow the adaptation of tumor cells to this hypoxic environment [7] (Figure 2). Therefore, hypoxic tumor cells activate the HIF pathway that leads to the production of VEGF (vascular endothelial growth factor), which stimulates the growth of new vessels. The tumor is then vascularized by the process of angiogenesis or neovascularization. Some cells return to the proliferative state where they can divide, and the tumor can thus continue to grow. While hypoxia is harmful to both healthy and tumor cells—where healthy cells can cope with hypoxia through anaerobic glycolysis for a limited time—tumor cells have the ability to adapt to it in several ways and even seem to take advantage of the tumor microenvironment to promote their growth and dissemination via various interactions [9].
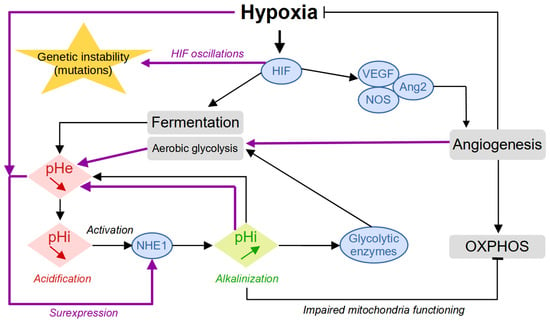
Figure 2.
Building up acidity in the tumor microenvironment. The first reaction to hypoxia is represented with the black arrows. The secondary response, promoted by the worsening of the conditions with increased environmental acidity and intermittent oxygen levels induced by angiogenesis, is represented by the purple arrows.
Moreover, when cells experience low oxygen levels (hypoxia), their signaling pathways, particularly HIF signaling, shift energy production from oxygen-dependent respiration to fermentation. This allows cells to keep generating ATP despite the lack of oxygen. In hypoxic tumors, this metabolic shift results in increased glycolytic activity, leading to the secretion of large amounts of lactate and acidic hydrogen ions (H+). This secretion alters the intracellular pH (pHi) of cancer cells. To cope with the excess lactate and H+ ions, tumor cells activate various proteins involved in regulating the pH, such as CAIX, NHE1, MCT4, and V-ATPase. These proteins help export lactate and H+ ions out of the cell, maintaining the pHi within normal levels. However, once outside the cell, lactate and H+ ions contribute to a decrease in extracellular pH (pHe, resulting in acidification of the tumor microenvironment.
The combination of hypoxia and acidity creates a chronic hostile microenvironment that directly participates in the adaptation of the tumor cells through phenotypic plasticity [10] and acquired genetic instability. Whereas the reaction to hypoxia proceeds similarly to most cell types whether normal or tumoral, the building up of acidity is enhanced by self-reinforcing mechanisms that kill normal cells and select for and promote tumor aggressiveness. For example, the acidity-induced activation of the NHE1 protein leads to the alkalinization of the intracellular pH, which will participate in the activation of glycolytic enzymes, inhibition of OXPHOS through impaired mitochondria functioning, and cell proliferation [11] (Figure 2). Collectively, these events will increase aerobic glycolysis and environmental acidity, which, in turn, will promote NHE1 overexpression and sustain the cycle of events thus worsening the conditions. The onset of angiogenesis temporarily reduces hypoxia, which leads to HIF oscillations associated with an increase in genetic instability [12].
2. Regulation of Intracellular Acidity
It is often reported in the literature that tumor cells are characterized by an inverse pH gradient compared to normal cells, but what does this really mean? The tumor microenvironment is characterized by a high level of acidity. As a consequence, the tumor cells, to survive, activate some regulation mechanisms to maintain an intracellular pH close to the physiological value of about 7.4 in order to keep functioning [13,14,15]. Typically alkaline pHi values from 7.1 to 7.6 are measured for acidic pHe values from 6.2 to 6.9 [16]. On the other hand, normal cells experiment with an extracellular physiological pHe, and their pHi is usually found to be lower, around 7.0–7.2 [17,18]. Therefore, the reverse pH gradient is not a property of the tumor cells, as we might be led to think, but rather it is merely an observation. Normal cells are rarely observed in acidic conditions, but, if artificially submitted to acidic conditions, they also activate the same regulatory mechanisms. Reciprocally, tumor cells immersed in alkaline pH are also very efficient in restoring an intracellular physiological pH [19].
The reverse pH for tumor cells is, therefore, a misleading concept, since any cell—tumoral or normal—in acidic conditions will try to maintain an intracellular physiological pH, with a pHi higher than the pHe. Reciprocally, in alkaline conditions (above 7.0), the cell will also try to keep its internal physiological pH, this time with a reverse situation in which the pHi tends to be lower than the pHe. So, the reverse pH gradient is not a property of the tumor cell per se but is entirely contextual.
As a consequence, another question is: do tumor cells regulate intracellular acidity in a different way than normal cells? Moreover, do they regulate it more efficiently? To answer these questions, we will first recall the mechanisms involved in this regulation and then discuss the tumor versus normal cells’ differences.
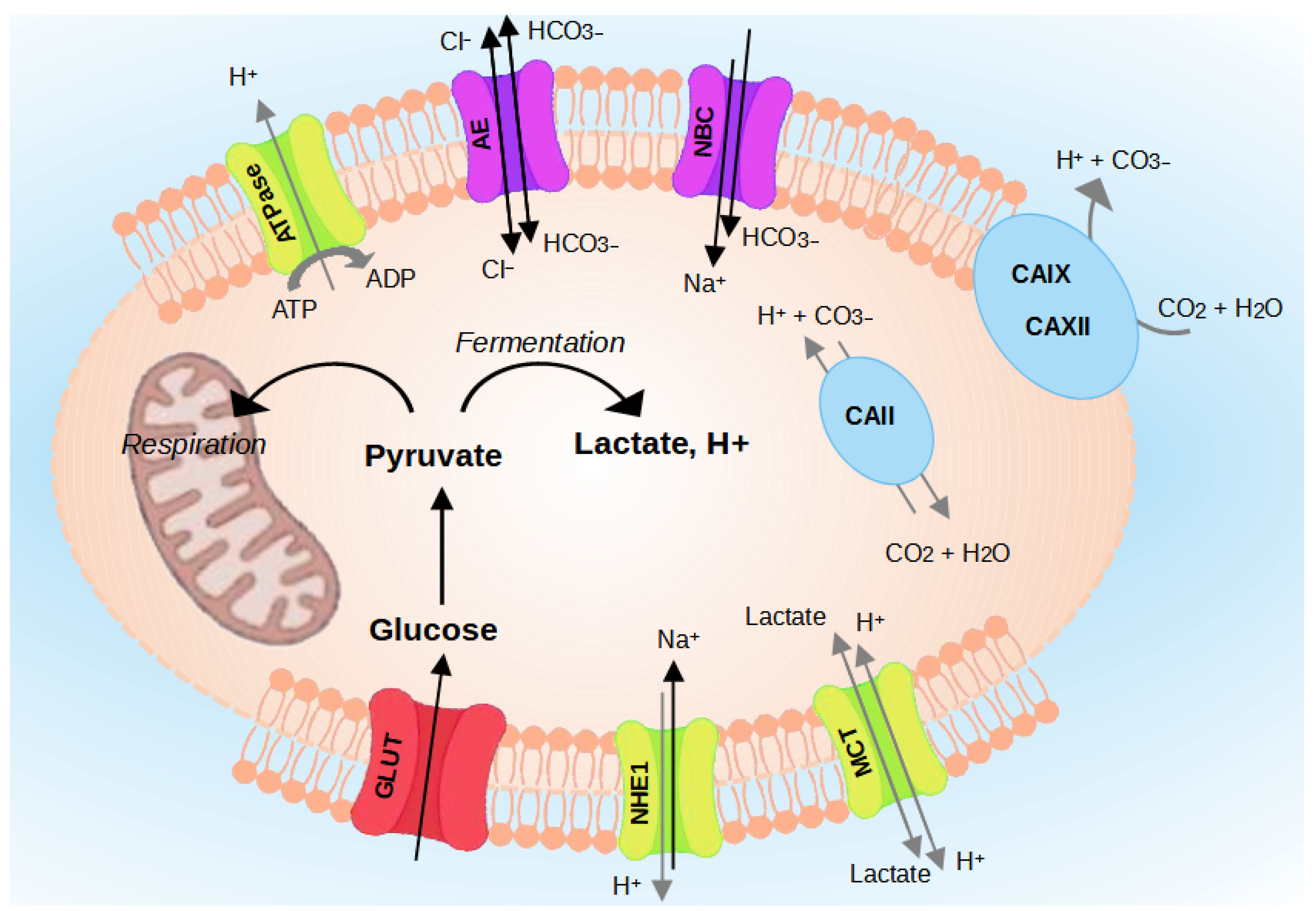
The pH regulating proteins comprise Na+/H+ exchangers (NHEs) [20], V-ATPase [21], the monocarboxylate transporters (MCTs) [22], Carbonic Anhydrases (CA) [20,23], and the transporters [24] (Figure 3). The role of each regulator is detailed in the following paragraphs.
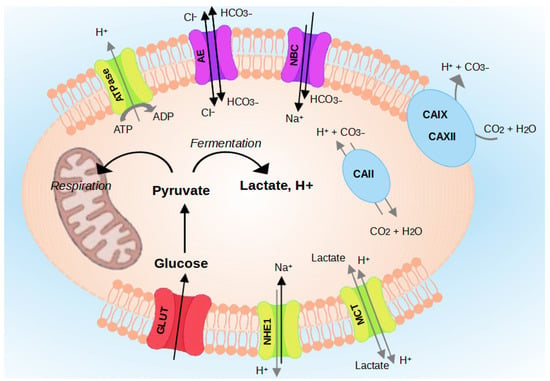
Figure 3.
Regulation of intracellular pH (pHi) in cancer cells. Cancer cells survive in an acidic environment by coordinating the function of pH-regulating proteins, such as NHE1, CAs, MCTs, V-ATPase, and transporters.
2.1. The Family of Na+/H+ Exchangers (NHEs)
Sodium-proton exchangers play a major role in regulation of the pHi. These Na+/H+ exchangers allow the electroneutral exchange of a Na+ for an H+. A 2:2 stoichiometry has also been proposed [25]. This family includes nine isoforms (NHE1 to NHE9) with varied subcellular and tissue distributions. Only three have been identified in the regulation of pHi: NHE1, NHE2, and NHE3. NHE2 and NHE3 have been localized only in the intestine and kidney, while NHE-1 is abundantly expressed throughout the body. NHE1 is localized at the level of the plasma membrane of the cell, and its role is to expel an H+ ion in exchange for the entry of a sodium Na+ ion, thereby increasing the pHi. NHE1 has a great potential to maintain a normal pHi. Chiche et al. (2010) [3] reported the following experiment: the injection of a large number of H+ ions into hamster cells expressing NHE1, NHE2, and NHE3 gives rapid recovery to a normal pHi, suitable for their survival. Contrariwise, mutated cells in which a lack of NHE1 is observed did not recover their pHi after the injection of H+ and died in less than an hour due to the persistent acidification. The ubiquitous NHE1 isoform is the only one whose activity has been associated with several properties of cancer cells [26,27,28].
2.2. The Enzyme V-ATPase
The vacuolar-type proton pumping ATPase (V-ATPase) is a multiprotein complex using the energy of ATP hydrolysis to electrogenically transport protons [29,30]. It is an enzyme catalyzing the following reaction:
ATP + H2O + H +intracellular → ADP + phosphate + H +extracellular
V-ATPase is expressed at the plasma membrane of cancer cells where it has a role in regulating the pHi [31,32] and, consequently, contributes to modification of the pHe. Its expression at the plasma membrane level and its activity are found in highly invasive cancer cells, and it is proposed that its activity is correlated with the aggressiveness of cancer cells [33,34].
2.3. Proton-Lactate Transporters (MCTs)
Monocarboxylate transporters (MCTs) allow the transport of lactate, ketone bodies, or pyruvate. This family includes 14 isoforms, of which only the first four play a role in pHi regulation. The MCT1, MCT2, MCT3, and MCT4 isoforms are electroneutral membrane cotransporters of protons and lactate with a stoichiometry of 1:1. In cancer cells, significant expression of MCT1 and MCT4 has been demonstrated. MCT4 allows the export of lactate produced by glycolytic (hypoxic) cancer cells, which will then be captured by MCT1 expressed in oxidative cancer cells located at the periphery of the tumor [35]. MCT1 allows the export of lactate outside the cell and also the import inside it. In contrast, MCT4 allows the export of lactate outside the cell but is unsuitable for importing it. MCT1 and MCT4 have a role in the invasiveness of lung cancer cells [36], and MCT4 is involved in the migration of breast cancer cells [37]. MCT1 expression has been shown to be correlated with glioma progression [38].
2.4. Carbonic Anhydrases (CA)
The regulation of cell pH involves, in addition to protons, bicarbonate ions. The carbon dioxide/bicarbonate buffer system helps regulate pH and is the most important physiological buffer system. Carbonic anhydrases are able to catalyze the reaction of CO2 with H2O to produce bicarbonates and protons: CO2 + H2O ⇌ H+ + . These bicarbonates can be transported into cells to neutralize protons and increase intracellular pH. The transport of bicarbonate ions can be carried out by various co-transporters or exchangers using different associated ions.
Carbonic anhydrases form a superfamily comprising 16 isoforms that catalyze the reversible hydration of CO2 into and H+ and are mainly involved in gas exchange. They can be anchored in the plasma, cytoplasmic, or mitochondrial membranes, or they can even be secreted [39,40]. Among the active enzymes, five carbonic anhydrases are cytosolic (CAI, CAII, CAIII, CAVII, and CAXIII), five are membrane-associated and oriented toward the extracellular environment (CAIV, CAIX, CAXII, CAXIV, and CAXV), two are mitochondrial (CAVA and CAVB), and CAVI is secreted. Three are devoid of catalytic activity (CAVIII, CAX, and CAXI). CAs are involved in many physiological phenomena including respiration and transport of CO2 and to the tissues and the lungs, pH and CO2 homeostasis, certain biosynthetic reactions, and bone formation or resorption.
High expression of CAIX and CAXII has been found in breast cancer cells [41,42] and in many other types of cancers [3]. CAIX and CAXII are membrane-bound, and their catalytic site is oriented toward the outside of the cell. Thus, CO2, produced by cellular respiration and which diffuses freely outside the cell, is hydrated into protons and bicarbonate ions (). This action participates in the acidification of the extracellular environment [43] but has also been shown to regulate pHi [44,45,46,47]. The expression of CAIX and CAXII is induced by hypoxia via the HIF-1 transcription factor [44]. Their expression can also be increased even in normoxia in the case of mutations making HIF-1α constitutively active [48,49]. In addition, the activity of CAIX is increased by an acid medium such as that found in tumors [50]. In hypoxia, induction of CAIX expression contributes to acidifying the extracellular environment of cancer cells and therefore participates in pH regulation [44,51].
An intracellular carbonic anhydrase, CAII, also seems to be found in certain cancers such as brain cancers [52] and to be associated with a poor prognosis in medulloblastomas [53], while its expression is associated with better survival in gastrointestinal stromal tumors [54]. There are also compensation relationships since the reduction in CAIX expression induces an increase in CAII expression and vice versa [55]. Particular attention should be given to CAIX since this isoform is found very infrequently in healthy tissues (in certain parts of the gastrointestinal tract) but is expressed in many tumor tissues, probably due to its hypoxic induction as well as the selective advantage it confers on tumor cells that express it [56].
2.5. Transporters
These proteins fall into three main categories: electroneutral / exchangers belonging to the SLC4 family, Na+/ co-transporters from the NBC family, and anion transporters within the SLC26 family. These transporters allow the importation of bicarbonates which are the main buffer species for H+ hydrogen ions, thus contributing to alkalizing the cytoplasm of the cell.
Little is known about the involvement of bicarbonate transporters in cancer cells. These transporters have been proposed to be involved in the pH regulation of cancer cells in different reviews [24,57,58]. Their involvement was initially studied by the use of a pharmacological inhibitor, DIDS, which is not very specific for a single transporter and also inhibits channels permeable to ions. This inhibitor decreases the pHi of cancer cells in vitro [59] and decreases tumor growth in vivo but has a toxicity limiting its potential therapeutic use [60].
Different cellular pathways can be established in cells exhibiting varying degrees of invasiveness or metastatic abilities [34,61]. Moreover, research has shown variations in the subcellular distribution of pH regulators within brain tumor models [62].
From this brief survey on the pH-regulating proteins, it is evident that they consistently exhibit elevated expression levels in tumor cells compared to normal cells. This heightened expression can be attributed to a variety of interrelated factors, including the hostile hypoxic and acidic tumor microenvironment, heightened metabolic demands, dysregulated signaling pathways, and increased genetic alterations.
3. Consequences of Acidity
Acidic conditions within the tumor microenvironment promote tumor progression by enhancing cell proliferation, migration, and many other events that contribute to facilitating the selection of more aggressive and invasive cancer cell phenotypes.
3.1. Acidity and Tumor Invasion
It has been established that extracellular acidity plays an important role in the process of tumor invasion. Acidity promotes tumor invasion by two main mechanisms. On the one hand, acidity induces toxicity on normal cells, whereas tumor cells are resistant to acid stress; as normal cells die, tumor cells continue to proliferate and invade the open space. On the other hand, the acidity induces the degradation of the extracellular matrix via the release and activation of proteases, such as cathepsin B [63,64], and of matrix metalloproteases (MMPs), such as MMP-1, 2, and 9 [65,66], which are generally believed to be involved in local invasion and tissue remodeling. In a xenograft model with breast and colon cancer cells, Estrella et al. (2013) [67] observed a correlation between acidity and tumor invasion. In tumors, areas with the highest invasion corresponded to areas with the lowest pHs. Interestingly, systematic treatment with sodium bicarbonate decreased the proton gradient in the tumor and prevented tumor invasion [68].
3.2. Acidity and Cellular Plasticity
Although the role of extracellular acidity in regulating the stem state of cancer cells has been less characterized, there are a few studies on the regulation of cellular plasticity by acidity. The acidic condition was shown to stimulate the development of a cancer stem cell phenotype [69]. Exposure of glioma cells to an acidic culture medium (pH 6.5) promotes the expression of stem cell markers, such as Olig2, Oct4, and Nanog, and increases the secretion of angiogenic factors, such as VEGF. This happens, in particular, through increasing the expression of HIF-2α, which is involved in the phenotype of stem cells and the growth of tumor cells. Additionally, lactate has been shown to attract human mesenchymal stem cells to tumor cells and heighten stem cell migration [70].
3.3. Acidity and Immunosuppression
During their development, malignant tumors are able to evade the response of the immune system [71]. Recent studies have shown that the anti-tumor immune response is modulated by the tumor microenvironment, including acidity. Lactic acid produced by tumor cells inhibits the production of cytokines from cytotoxic T lymphocytes in vitro [72]. Activated T cells themselves use glycolysis and rely on the efficient secretion of lactic acid. Lactic acid blocks the efflux of lactate produced in the tumor environment and thus disrupts T cell metabolism. In another study, treatment with the proton pump inhibitor esomeprazole, by reducing tumor acidity, restored the functioning of T lymphocytes [73].
3.4. Acidity and Warburg Effect
The Warburg effect is the observation made by Otto Warburg back in the 1920s of an increased glucose uptake and conversion to lactate in cancer cells [5]. He hypothesized that an irreversible alteration of mitochondrial respiration is at the origin of the development of cancer [6]. This hypothesis was ignored for more than fifty years by a large part of the scientific community, so much so that metabolism was not part of the “Hallmarks of cancer” listed in the famous review by Hanahan and Weinberg published in 2000 [74]. It was not until these were updated in 2011 that abnormal metabolism made its appearance with the mention of “deregulating cellular energetics” [71].
In honor of its discoverer, the activation of glycolysis under aerobic conditions has been dubbed the “Warburg effect”. At the same time that this process was admitted, it became a paradox to be resolved: why malignant cells, a priori presumed to have an increased need for ATP to proliferate, not use the OXPHOS pathway, despite its significantly higher energy yield?
Today, with the progress of knowledge on the origin and mechanisms of malignant transformation, Warburg’s hypothesis on the origin of uncontrolled cell proliferation is no longer accepted for two reasons. The first is that aerobic glycolysis is not directly related to malignancy. Many mammalian cell lines (lymphocytes and fibroblasts) develop aerobic glycolysis under proliferating conditions [75]. In cultured lymphocytes, after stimulation of proliferation, it has been shown that these cells convert a significant amount of consumed glucose into lactate [76,77,78]. The second reason is that the development of aerobic glycolysis may be independent of whether the mitochondria are functional or not. Indeed, several works have proven that mitochondria function normally in cancer cells and that blocking oxidative phosphorylation constitutes an adaptive event [79,80,81].
In addition, a study has investigated the impact of environmental acidity on tumor mitochondrial function [82]. The authors of this review reported that cancer cells reverted to OXPHOS when they were exposed to lactic acidosis, a common factor in tumor microenvironment. To draw this conclusion, they quantitatively determined the percentage of ATP production from glycolysis and OXPHOS with and without lactic acidosis. They found that in the absence of lactic acidosis, cancer cells exhibited excessive glycolysis and produced a large amount of lactate; while, in the presence of lactic acidosis, cancer cells showed a low level of glycolysis and produced a negligible amount of lactate. These quantitative data supported therefore their notion that cancer cells reverted from the Warburg effect to the OXPHOS phenotype under acidic conditions.
A reduced model of the cell energy metabolism was recently proposed to test the role of pyruvate in the distribution of fluxes toward respiration or fermentation, to specify the importance of lactate and to include the glycolytic inhibition by acidity [83]. The model is based on experimental data highlighting the role of acidity in the regulation of metabolism [84]. The results obtained from this model showed, with regards to the production of ATP, that keeping a constant level of pyruvate was necessary to direct the glycolytic fluxes toward fermentation when this exceeded the maximum activity of the pyruvate dehydrogenase (PDH) complex. Conversely, when the glycolytic flow is reduced by acidity, it is necessary to consume lactate. Thus, the oxidative activity is maintained as long as the oxygen level allows it and gradually passes to fermentation as long as the acidity is not too high, a transition dictated by the maintenance of the pyruvate concentration. This model tends to show that the Warburg effect is not necessarily an inherent characteristic of the tumor cell but a spontaneous and transient adaptation mechanism to a disturbed environment [85].
Beyond its influence on the Warburg effect, acidity in the tumor microenvironment has many effects on the evolution of cancer and its progression to malignancy. It has also been proposed that exposure to chronic acidosis induces genome instability through chromosome breakages and translocations driving somatic evolution [86,87]. On the other hand, acidity can be cytotoxic, inhibit cancer cell proliferation, and promote stress response and cell apoptosis [88,89,90,91]. Collectively, acidosis has been described as a defining hallmark of the tumor microenvironment. However, the mechanisms by which cancer cells, immune cells, and blood vessels sense acidosis and respond to it are yet to be completely established but may be advantageous for a more comprehensive understanding of tumor biology. This depends on the ability to make measurements, which we will now discuss.
4. Evolution of the Methods for Intracellular pH Measurements
In this section, we present some methods that have been developed to measure the intracellular pH of cells for a range of biological applications. The focus is here to illustrate the techniques and the contexts in which they have been developed. We note that most of the methods have been developed in contexts unrelated to cancer. However, all of the techniques are transposable to measure the intracellular pH in some other cell types, including cancer cells.
Measurement of the intracellular pH is indeed very challenging. Especially if we intend to make measurements at the single cell level in a non-disruptive and less intrusive way so as to be able to monitor acute pH changes and pH evolutions on a longer time scale (several hours to days). Although changes in the pHi had been observed much earlier, the oldest method of what might loosely be described as pHi measurement was employed in 1912 by Michaelis and Davidoff with red blood cells (for a review, see [92]). Using platinum/hydrogen electrodes, they noted that red cell lysis caused a change in bulk pH. Although cell lysis was the most appropriate method for estimating pHi, the potentially serious problems with this technique were recognized early on. For example, the metabolism continues after cell lysis and leads to a drop in pH due to the production of lactic acid and carbon dioxide. Therefore, various studies in the field of intracellular pH have been characterized by better developments in measurement techniques, by increasing knowledge of the mechanism of control of pHi, and by progress in establishing relationships between pHi and cellular metabolism.
4.1. pH Microelectrodes
One of the methods developed to measure pHi is the pH-sensitive microelectrodes method [93]. Although this method is technically very exacting, it has provided very important information. It is particularly suitable for providing direct, continuous monitoring of local pH and has been used extensively to measure cytosolic pH.
Due to the existence of electrical potential differences across the cell membrane, two electrodes must enter the cell to measure the pHi: a reference microelectrode filled with potassium chloride (KCl) to measure the membrane potential and a pH-sensitive microelectrode to measure a combination of pH and membrane potential. By subtracting the potential recorded by the two electrodes, one obtains an electric potential proportional to the local pH.
Using this method, intracellular pH measurement was performed in papillary muscle cells of streptozotocin-induced diabetic rats [94]. The aim of this study was to investigate the in vivo regulation of pHi following an intracellular acid or alkaline loading in diabetic rats. Accordingly, diabetes was induced in male Wistar rats by the injection of streptozotocin into the femoral vein. The results show differences between diabetic and normal muscles in the regulation of pHi in response to induced acidity. This suggests that diabetes is associated with a change in the activity of the Na+/H+ exchange [95], which is a major pathway for the export of H+ ions from cells during acidity [25].
Further, pH-sensitive microelectrodes have been used to measure the pHi of mammalian cardiac cells. The first measurement in vivo was performed by Ellis and Thomas, (1976) [96] who studied the effect of CO2 on the pHi of Purkinje fiber cells cut from sheep ventricles. The results showed that the pHi was strongly influenced by CO2 in the equilibration gas. The mean pHi in the presence of CO2 is close to 7.02 and is significantly lower than when CO2 is absent.
In fact, when the dimensions of the cells are too small, such as epithelial cells, it is difficult and often impossible to accommodate two separate electrodes in the same cell. In this case, two different cells can be penetrated and a distinct membrane electrical potential can be noted by the reference microelectrode and the pH-sensitive microelectrode. The measurement of pHi is therefore carried out by calculating the average values of the electrical potentials of several penetrations [97]. In order to circumvent most of these problems, a double-barreled pH microelectrode was introduced [98]. It allows the penetration of both electrodes into the same cell, thus measuring the same membrane potential.
A repetitive pHi measurement of Purkinje fiber cells was obtained using the double-barreled pH microelectrode [98]. The results show that the pHi varies between 7.1 and 7.2, which is, however, significantly greater than that reported in [96]. It has been reported that this difference is probably due to the different concentration in the control solution, compared to that used in [96].
Hagberg et al. (1983) [99] measured in vivo the pHi of rabbit skeletal muscle fibers using the double-barreled pH microelectrode. The pHi of 7.00 ± 0.09 found in this study was lower than that found in mouse soleus fibers in vitro using pH-sensitive microelectrodes (7.07) [100] or in rat soleus using double-barreled electrodes (7.1–7.2) [98]. They assumed that the higher bath pH and the lower level of CO2 in those studies could explain this small discrepancy. In addition, the pHi could be different in the soleus (composed mainly of red fibers) than in the predominantly white gastrocnemius muscle.
Despite the widespread use of pH-sensitive microelectrodes for pHi measurements, their use has been rather limited because they can cause cell membrane damage and cytosolic leakage that alters pHi measurements. In addition, the use of pH-sensitive microelectrodes is impossible on mobile cells such as amoebae [101].
4.2. 31P NMR Spectroscopy
Following the pioneering work of Moon and Richards [102], who, in 1973, used the principle of the variation in the chemical shift of mainly cytosolic inorganic phosphate (Pi) with pH, phosphorus nuclear magnetic resonance (31P NMR) spectroscopy was used as a non-invasive pHi measurement technique. In this case, the cells are incubated in the presence of probes, such as phosphate or methylphosphonate comprising 31P, which penetrate into the cells.
Several cardiac pH measurements were performed using 31P NMR spectroscopy [103,104,105,106]. Since cardiac cellular acidosis has been introduced as one of the central mechanisms to explain irreversible cell damage during ischemia [107], cardiac cellular pH is therefore an important parameter to measure in various types of ischemic states. For example, a 31P NMR measurement of cardiac pH was performed in an isolated rat heart during ischemia and reperfusion [105]. The results show that in the pre-ischemic rat heart, the pHi was approximately 7.15. After 4 min of ischemia, the pHi decreased to approximately 6.16. On reperfusion, the pHi returned to pre-ischemic levels within 2.5 min. In addition, 31P NMR spectroscopy has also been used to monitor renal intracellular pH [108,109,110]. It has been suggested that intracellular acidification could be a primary signal that stimulates renal ammoniogenesis [111]. Therefore, knowing renal intracellular pH may be a key issue in understanding the regulation of ammoniogenesis. In this context, the intrarenal pH was measured in vivo in perfused rat kidneys where an acidification, from pH 7.4 to pH 6.9, was induced by the addition of hydrochloric acid (HCl) to the perfusion medium [109]. The results showed that the kidney pH fell from 7.20 to 6.75 and reached a plateau of pH 6.85, 30 min after addition of HCl. In parallel, it was found that the rate of ammonia (NH3) production at 7.4 was doubled when the perfusate was acidified to pH 6.9. This is therefore consistent with the hypothesis that intracellular acidification may participate in the stimulation of ammoniogenesis.
In addition to measuring intracellular pH, 31P NMR spectroscopy has also been used to visualize and quantify the phosphorylated metabolites, which are directly involved in energy metabolism. We will cite, as examples, ATP which constitutes the main source of energy of the organism; phosphocreatine, which is an energy buffer (It makes it possible to maintain the concentration of available ATP constant.); and the inorganic phosphate Pi, which is the substrate for the ATP synthesis reaction. In addition to quantification, 31P spectra provide unique access to dynamic parameters, such as the speed of ATP synthesis [112,113]. Although this method can provide very important information, it remains little used. This is due to relatively high equipment costs and the need to work with suspensions of high cell concentrations. In addition, it has the disadvantage of giving relatively long analysis times (spectrum acquisition), which do not make it possible to see rapid variations in the pHi [114,115,116].
4.3. Weak Acids and Bases
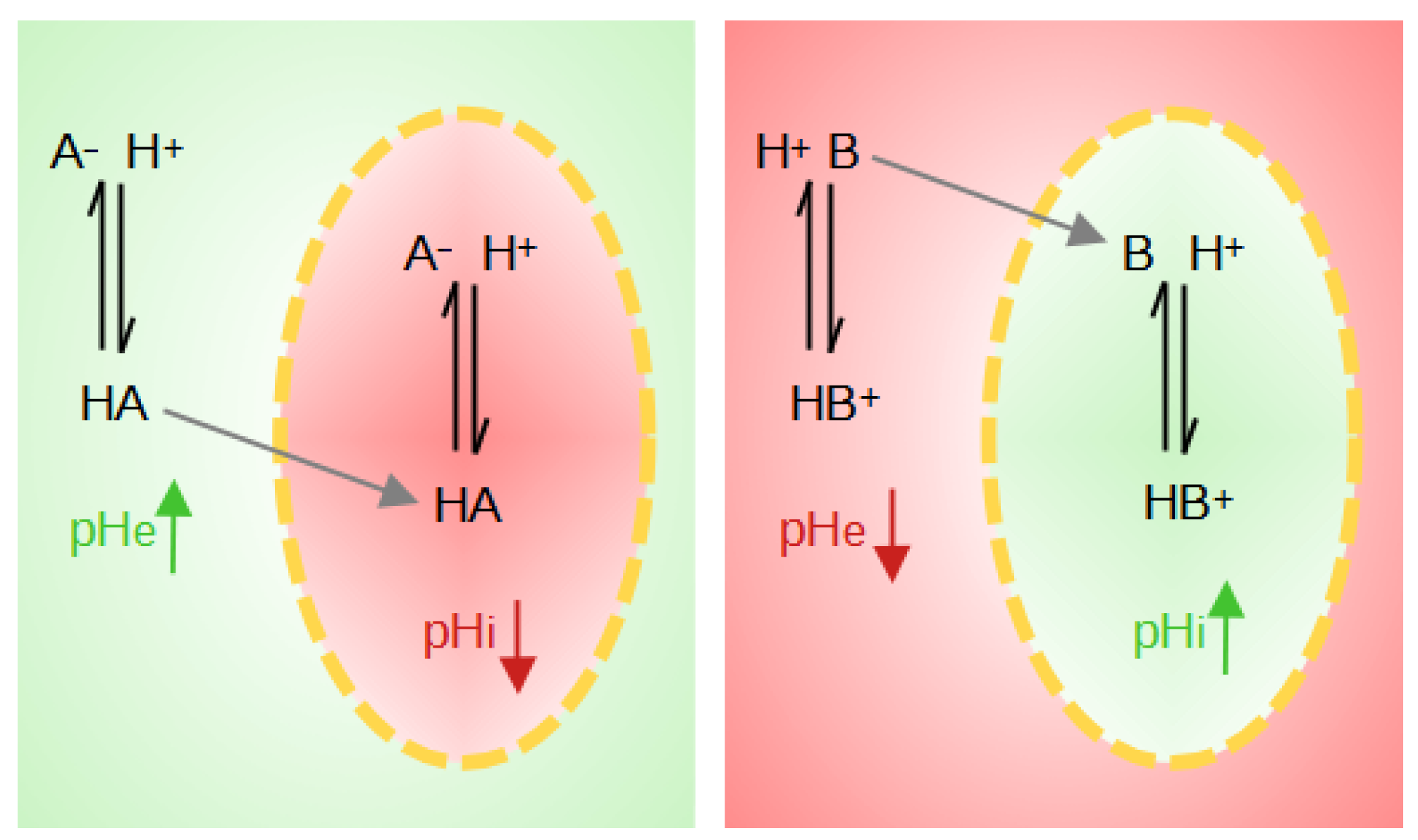
The weak acids and bases distribution method has also been introduced to measure intracellular pH [117]. The basis of this method is the use of weak acids HA when pHi > pHe or of weak bases B when pHi < pHe. The neutral species of these probes diffuses freely through the membrane while the ion is impermeable. The weak protonated acid, HA, transports its proton across the membrane and can then dissociate inside the cell, lowering the intracellular pH and increasing the extracellular pH (pHe). The weak deprotonated base B will penetrate the cell, leaving a proton behind, and will tend to pick up a proton inside the cell, raising the pHi and lowering the pHe (Figure 4).
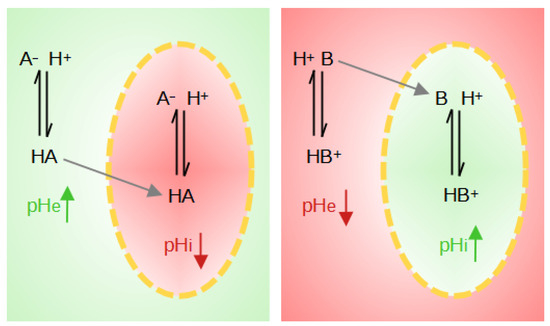
Figure 4.
Illustration of the effects on local pH when weak acids (HA) or weak bases (B) are present. The yellow dotted line represents the cell membrane. The red zones correspond to a pH drop, whereas green zones correspond to a pH rise.
In the case of a weak acid, []i = []e at equilibrium. Moreover, since the acid dissociates on both sides, it is therefore assumed that the dissociation constant is the same in the extracellular and intracellular media. From this equilibrium, the following equation results:
Under this condition, and if pHe > pKa + 1, most of the acid is ionized on both sides of the membrane, and the measurement of the acid distribution ratio allows calculation of the intracellular pH at equilibrium. Since the extracellular pH can be easily measured, the calculation of the pHi then becomes possible. The quantities of ionized acid are assayed by titrimetry or radiometry in the case of labeled probes, such as acetate and methylamine [118].
Waddell and Butler (1959) [119] measured the pHi of the skeletal muscle of the dog using the weak acid DMO (5,5-Dimethyl-2,4-oxazolidinedione). The effects of various treatments altering the pH or the pCO2 (plasma CO2) of plasma were studied. In 11 dogs, the calculated pHi values of normal, resting muscle were all in the range from 6.96 to 7.10 with an average of 7.04. The greatest changes in muscle pHi are those produced by increasing blood carbon dioxide tension. The pHi of muscle is lowered by a high tension of carbon dioxide whether or not the blood pH is lowered. Lowering blood pH by intravenous infusion of hydrochloric acid (HCl) has less effect on lowering the muscle pHi than the high tension of carbon dioxide. While raising blood pH, sodium bicarbonate (NaHCO3) injection raises the muscle pHi only to a small extent.
Although this technique is easy, accurate and applicable to small cells, its use is limited by the fact that it requires the destruction of the tissue and therefore cannot easily be used to measure pHi changes over time. In addition, the presence of acidic or basic molecules can disrupt the establishment of the electrochemical gradient [116].
4.4. Fluorescence Microscopy
Another method developed over the past thirty years to deal with these drawbacks is fluorescence microscopy. By the protonation of a chemical function, it is possible to modify the spectroscopic properties of the molecule and to obtain a probe sensitive to the variation in the proton concentration.
Currently, pH-sensitive probes are often in the esterified form of the probe (AM). The presence of these ester groups facilitates the diffusion of fluorophores through the cytoplasmic membrane. Inside the cells and under the action of intracellular esterases, the ester groups are cleaved and the more or less negatively charged probes are retained intracellularly and are then potentially fluorescent.
Gores et al. (1989) [120] used the BCECF-AM probe to measure the pHi in cultured rat hepatocytes after ATP depletion by metabolic inhibition with KCN (potassium cyanide) and iodoacetate acid (IAA)(chemical hypoxia). KCN and IAA block cellular ATP formation through inhibition of oxidative phosphorylation and glycolysis, respectively. During chemical hypoxia at a pHe of 7.4, the pHi dropped from 7.36 to 6.33 within 10 min and remained at 6.1–6.5 for 30–40 min (plateau phase). Subsequently, the pHi began to increase, and cells ensued within minutes, as evidenced by nuclear staining with propidium iodide. Exposure of hepatocytes to an acidic pHe simultaneously with KCN and IAA produced a slightly greater drop in the pHi, prolonged the plateau phase of intracellular acidosis, and delayed the onset of cell death. In addition, inhibition of the Na+/H+ exchange with amiloride also produced an increase in cell survival and a prolongation of the plateau phase of intracellular acidosis. The results suggest that intracellular acidosis after ATP depletion protects against hepatocellular death from ATP depletion, a phenomenon that may represent a protective adaptation against hypoxia-induced stress [120].
It has been suggested that such an alteration in the pHi of brain cells could contribute to neurological dysfunctions in patients with AIDS. Therefore, Makutonina et al. (1996) [121] used the BCECF-AM probe to quantitate the pHi in HIV-1-infected T cells (human immunodeficiency virus type 1). The results obtained show that an infection of RH9 cells, an established line of T-lymphoblastoid cells, with HIV-1, resulted in a significant decrease in the pHi from 7.2 in mock-infected cells to approximately 6.6 by day 4 after the infection when cells were undergoing acute cytopathic effects. Fluorescence concentration analysis using BCECF indicated that the pHi in persistently infected cells that survived the acute cytopathic effects of HIV-1 was from approximately 6.8 to 7.0, values which are modestly lower than those in mock-infected cells. The mechanism by which HIV reduces the pHi in T-lymphoblastoid cells is not established in this study. However, prior studies indicated that HIV infection induces intracellular acidification in lymphocytes due to dysfunction of the plasma membrane ion transport system Na+/H+ [122,123]. Exposure to gp120, the HIV-1 surface glycoprotein (SU), activates the Na+/H+ exchange system, resulting in an increase in the active transport of protons out of the cell and intracellular alkalinization. The results of these studies suggest that alterations in the pHi may mediate certain cytopathic effects of HIV-1, thereby contributing to depletion of the T lymphocytes in patients with AIDS.
Salvi et al. (2002) [124] evaluated the performance of three pH-sensitive probes, SNARF-1-AM, BCECF-AM, and Cell-Tracker Green CMFDA, in Chinese hamster ovary (CHO), human embryonic kidney (HEK293), and two human colon carcinoma (Caco-2) cells to measure pHi changes in a multi-well plate format. The stability of the pHi measurements was strongly affected by the rate of the probe efflux, which was found to depend on the pH-sensitive probe, temperature, pHe, cell type, and the use of transport inhibitors to prevent probe export. The results show that the performance of the Cell-Tracker Green CMFDA was significantly better with respect to the cellular probe efflux. Therefore, this probe was selected for use in further studies with CHO, HEK293, and Caco-2 cells. To investigate whether the effect of hypertonic stress on the pHi can be detected, the medium was made hypertonic by the addition of sucrose. The exposure of HEK293 and CHO cells to the hypertonic medium induced pHi increases of 0.08 pH unit and 0.16 pH unit, respectively, whereas no significant pHi rise was observed in the Caco-2 cells. To examine whether the activation of G-protein coupled receptors was connected to a pHi change, acetylcholine was injected into HEK293-M1 and CHO-M1 cells. In both cell lines expressing a high level of muscarinic receptors, activation by acetylcholine did not cause any significant pHi response compared with wild-type cells. Similarly, stimulation of endogenously expressed β-adrenergic receptors by isoproterenol had no effect. The effects of human dipeptide transport activity on the pHi were also investigated in this study. A pHi decrease of 0.15 pH unit was observed in CHO cells expressing the human H+/peptide transporter PEPT1 upon addition of dipeptide substrates. The authors of this study succeeded in developing a rapid fluorimetric assay using the multi-well plate format to measure pHi changes in living cells and examine pHi changes induced by various effectors.
A large number of fluorescent probes, or fluorophores, have been developed for pHi sensing; these probes have the advantage of low toxicity to cells and are not metabolized [116]. In addition, the specificity of localization can allow the exclusive study of certain cellular compartments, such as lysosomes in macrophages [125]. Since its introduction by Tsien in the early 1980s, 2′,7′ bis(carboxyethyl)-5(and 6)-carboxyfluorescein (BCECF) has been by far the most widely used pH-sensitive fluorescent probe [126].
In a recent study, we developed a methodology that allowed us to improve the precision of pH measurements made with the BCECF probe and to extend the pH range that can be measured with this probe. We validated this methodology by measuring the pH drift observed in the cell culture medium [127]. We then applied it to monitor simultaneously the intra and extracellular pH in two glioma cell lines (murine F98 and human U87 gliomas) that were submitted to a range of acute pH changes from 5.0 to 8.4 [19]. We showed that the two cell lines reacted differently to acidity. The F98 cells exhibited a weak pHi regulation capability, whereas the U87 cells were more efficient at raising their pHi under acidic environmental conditions. On the other hand, the F98 cells exhibited a higher survival rate compared to that of the U87 cells under those conditions. We hypothesized that the F98 cells were activating the V-ATPase pump to encapsulate the protons in the lysosomes to neutralize the excess acidity and keep functioning. Although this mechanism is well known, it remains to be verified in this specific case.
5. Acidity and Therapy
Until very recently, the emphasis in anti-cancer drug research and development solely targeted tumor cells. Conventional chemotherapy primarily aimed at hindering cancer cell proliferation, while more recent targeted agents focused on specific tumor proteins or neovascularization. However, with minimal prognosis improvement and a growing understanding of acidity’s role in tumor progression and treatment response, significant attention has shifted toward strategies addressing tumor acidity. These strategies, which directly target acidity or leverage its characteristics, are now under development and show promising initial results. For example, some new strategies focus on exploiting acidity to improve drug uptake by the tumor cells, to activate the drugs, or to enhance their toxic effects. Another approach involves inhibition of the proteins responsible for maintaining an intracellular pH, vital for tumor survival in acidic conditions. This is achieved through the use of small molecule inhibitors or antibodies targeting these proteins.
5.1. Extracellular Acidity and Chemotherapy
Acidosis of the tumor microenvironment leads to a selection of cancer cells with a stem cell phenotype, with greater invasive and metastatic potentials, and failure with treatment strategies involving chemotherapy [67,128,129,130]. The association between extracellular acidity and increased chemotherapeutic resistance has been reported in previous in vitro and in vivo studies on human melanoma [131], osteosarcoma [132], prostate carcinoma [133], colon [134], breast [135], and human oral squamous cell carcinoma [136]. Therefore, the following paragraph is devoted to a literature review in order to elucidate the biological mechanisms underlying the effects of acidosis on chemotherapies (Table 1).
The passage of chemotherapeutic agents through the cell membrane is often compromised by the nature of the molecule. Uncharged and small molecules can easily diffuse whereas the passage of charged or bigger molecules can be much more difficult. ATP-Binding Cassette (ABC) transporters, also known as primary active transporters, are a broad family of proteins involved in the transport of hydrophobic compounds against their concentration gradients, from a wide variety of substrates, including peptides, lipids, and chemotherapeutic agents [137,138]. ABC transporters use the energy provided by the hydrolysis of the ATP at the level of NBDs (nucleotide-binding domains) to function. Genes encoding ABC transporters are grouped into subfamilies according to their domain organization and amino acid homology. In humans, seven subfamilies have been identified (from ABCA to ABCG).
Cheng et al. (2012) [134] assessed in vitro the regulation of the ABCG2 transporter in human colon carcinoma, HCT-116, and S1 and its resistant S1M1-80 cell lines overexpressing ABCG2 in response to an acidic pH. The data revealed increased regulation of ABCG2 gene transcription, with the exception of the S1 cell line, under extracellular acidity, and increased resistance to the drugs Cisplatin (CIS) and mitoxantrone [134].
Federici et al. (2014) [139] have investigated the role of both extracellular acidosis and exosome release in resistance of different malignant cell lines (human breast cancer, human metastatic melanoma, and human colon carcinoma) to Cisplatin. They demonstrated that tumor cell lines exhibited different sensitivity to the Cisplatin and that the acid culture condition reduced sensitivity to Cisplatin in all tumor cell lines tested compared to the same cells cultured at a neutral pH. In addition, the results showed that an acidic pH increased exosome release by tumor cells with a higher accumulation of Cisplatin, which may be one of the mechanisms by which cells protect themselves by expelling the drug. Melanoma cells’ pre-treatment with lansoprazole, a proton pump inhibitor (PPI), induced a 50% reduction in the amount of Cisplatin present in the exosomes purified from the cell culture supernatant, as compared to the exosomes purified from the supernatant of the cell cultures that were not treated with lansoprazole. This supported the hypothesis that pretreatment with a proton pump inhibitor may lead both to exosome release inhibition and an increased drug retention by tumor cells [139].
The most important human ABC transporter involved in drug disposition is ABCB1, also known as multidrug resistance protein 1 (MDR1) or P-glycoprotein (Pgp) [140]. P-glycoprotein prevents cellular uptake of a large number of structurally and functionally diverse compounds by acting as an efflux pump and transporting the chemotherapeutics out of the cell and, in this way, causes multidrug resistance (MDR) [141,142].
A group of researchers have been working on the impact of extracellular acidity on the activity of Pgp and the cytotoxicity of chemotherapeutic drugs [133]. They found that exposing prostate carcinoma cells (AT1) to an acidic extracellular environment (pH 6.6) for a period from 3 to 6 h doubled the activity of Pgp and reduced the cytotoxic efficacy of two chemotherapeutic drugs, Daunorubicin (DNR) and Cisplatin. In order to assess the cytotoxic activity of the chemotherapeutic drugs, the relative caspase 3 (a proapoptotic protein) activity normalized with respect to untreated control cells was measured. With Cisplatin, caspase 3 activity was significantly reduced by the acidic environment compared to the normal pH. However, even though Cisplatin-induced caspase 3 activation was markedly reduced in the acidic microenvironment, overall cell death was not significantly different in cells incubated with Cisplatin at different pH levels. These results indicate that the cytotoxicity of Cisplatin was only marginally affected by the acidic microenvironment. In contrast, DNR-induced cell death was markedly reduced by the acidic microenvironment. In order to investigate the underlying mechanisms by which the extracellular pH may influence pGP activity, changes in the intracellular levels of calcium ions () and protein kinase C (PKC) were measured. It was found that the increased Pgp activity under acidic conditions was related to the decreased intracellular calcium levels and inhibition of PKC. Based on the results of these experiments, the authors concluded that the chemoresistance to chemotherapeutic agents generated by extracellular acidosis may be the result of an increase in Pgp activity, which, in turn, is potentially caused by a decreased intracellular calcium concentration and PKC activity (The decreased intracellular calcium concentration can also be induced by reducing the extracellular one which leads to the same effect as acidosis) [133].
Thews et al. (2014) [143] studied the cytotoxicity of three chemotherapeutic drugs, DNR, CIS, and docetaxel (DOC), and, their dependence on Pgp activity during acidosis was analyzed. In vitro and in vivo experiments were performed using the same treatments and the same cell lines as in their previous study. Under acidic (pH 6.6) conditions, they observed a decrease in the cytotoxicity of DNR or DOC, whereas Cisplatin-induced cell death was almost pH-independent. The Pgp inhibitor (verapamil) reversed the acidosis-induced chemoresistance against DNR and DOC. The cytotoxicity in vivo was assessed by forcing glycolytic metabolism “acidosis treatment”) in the tumor tissue. In vivo results confirmed that induction of acidity in animals reduced the cytotoxicity of DNR and DOC, whereas the cytotoxicity of CIS remained almost independent from the tumor pH. Thus, it was suggested that when there are clinical indications for DAU and DOC treatment, the Pgp-mediated chemoresistance can be counteracted by inhibition of the drug transporter [143].

Table 1.
Literature review on the effects of pH on chemotherapies.
Table 1.
Literature review on the effects of pH on chemotherapies.
| Reference | Cancer Type | Cell Model | Tested Drug | pH Assessed | Mechanisms Evaluated | Main Results |
|---|---|---|---|---|---|---|
| Mellor and Callaghan (2011) [144] | Colorectal adenocarcinoma (DLD-1), colon adenocarcinoma (HT29), and ovarian adenocarcinoma (NCIADR) | in vitro | Doxorubicin (DOX) | 6.9–7.0 and 7.2–7.3 | Growth of tumor spheroids in normoxia and hypoxia, intracellular accumulation of DOX, and inhibition of Pgp by Tariquidar (XR9576) | The distribution and accumulation of DOX were heterogeneous in all cell lines evaluated. The acidity generated by hypoxia decreased the accumulation of DOX in tumor spheroid. The inibition of Pgp by Tariquidar (XR9576) increased the accumulation of DOX in tumor spheroids. |
| Avnet et al. (2016) [132] | Osteosarcoma | in vivo | Doxorubicin (DOX) | 6.5 and 7.4 | Combined treatment of DOX and omeprazole (a proton pump inhibitor targeting lysosomal acidity) | The combined treatment of DOX with omeprazole showed a higher necrotic areas, smaller tumor volumes, and less body weight loss. |
| Fan et al. (2012) [135] | Breast cancer (MCF-7/ADR) | in vitro and in vivo | Doxorubicin (DOX), Cisplatin (CIS), and 5-fluorouracil (5-FU) | Acidic pH (unknown value) | Induction of LASS2 expression | The overexpression of LASS2 in MCF-7/ADR breast cancer cells increased the effect of several chemotherapeutic agents. LASS2 inhibited the function of V-ATPase. More DOX entered the cells and stayed in the nuclei of cells, inducing increased rates of apoptosis. |
| Visioli et al. 2014 [136] | Endothelial cells from human oral squamous cell carcinomas (OSCC) | in vitro | Sunitinib | 6.0–6.4 | Activation of UPR: quantification of Grp78 in endothelial cells and cytotoxicity using SRB | Extracellular acidity increased expression of the UPR marker (Grp78) and its inhibition reversed the drug sensitivity to Sunitinib. |
| Cheng and To (2012) [134] | Human colon carcinoma HCT-116 and S1 and its ABCG2-overexpressing resistant S1M1-80 cell lines | in vitro | Cisplatin (CIS) and mitoxantrone | 5.00 | Regulation of ABCG2 under adverse conditions within the tumor microenvironment (hypoxia, glucose deprivation, and acidosis) | Glucose depletion, decreased extracellular pH, and hypoxia can all upregulate ABCG2 transcript level leading to multidrug resistance. Acidic pH did not significantly alter the level of ABCG2 in S1 cells. |
| Federici et al. 2014 [139] | MCF7 (human breast cancer), Me30966 and Me501 (human metastatic melanoma), and SW480 (human colon carcinoma) cell lines | both in vitro and in vivo experiments | Cisplatin (CIS) | acidic (5.0–6.0), buffered (7.4), and unbuffered media | Cisplatin cellular resistance and effects of proton pump inhibitor (lansoprazole) on Cisplatin tumor uptake | Treatment with lansoprazole increased the intracellular absorption of Cisplatin and reduced the amount of Cisplatin present in the exosomes. |
| Thews et al. (2006) [133] | Rat prostate cancer (AT1) | in vitro | Daunorubicin (DNR) and Cisplatin (CIS) | 6.6 and 7.4 | Caspase 3 activity and cell toxicity assay | Exposure to acidic extracellular environment doubled Pgp activity and reduced cytotoxic efficacy of CIS and DNR. |
| Thews et al. (2014) [143] | Rat prostate cancer (AT1) | in vitro and in vivo | Daunorubicin (DNR), Cisplatin (CIS), and docetaxel (DOC) | 6.6 and 7.4 | Measurement of apoptosis induction (caspase 3) and cell survival | Acidity reduced the cytotoxicity of DAU, CIS, and DOC. The Pgp inhibitor (verapamil) reversed the acidosis-induced chemoresistance against DNR and DOC. |
In an acidic microenvironment, combined chemotherapy and proton pump inhibitors have been shown to increase the sensitivity of cancer cells to treatment. Avnet et al. (2016) [132] injected osteosarcoma cells into immunodeficient mice (NOD/SCID animals) and treated them with the chemotherapeutic drug DOX and omeprazole, a proton pump inhibitor targeting lysosomal acidity. Exposure to an acidic pHe significantly increased the number and acidity of lysosomes, and the combination of DOX with omeprazole significantly enhanced DOX cytotoxicity. In addition, the results of this study demonstrated that mice treated with the combination of DOX and omeprazole showed a higher necrotic index, smaller tumor volumes, and less body weight loss compared to the groups treated with DOX alone. Therefore, it can be inferred that proton pump inhibitor drugs may offer novel solutions to overcoming drug resistance.
The role of V-ATPase in the efficacy of chemotherapy treatment has been evaluated in breast cancer. Fan et al. (2012) [135] observed that LASS2 (Homo sapiens longevity assurance homolog 2 of yeast LAG1) is able to enhance breast cancer chemosensitivity by counteracting the acidic tumor microenvironment by inhibiting proton pump V-ATPase activity. Low expression of LASS2 was associated with poor prognosis in breast tumors. The results showed that the overexpression of LASS2 in MCF-7/ADR breast cancer cells increased the effect of several chemotherapeutic agents (DOX, CIS, and 5-fluorouracil (5-FU)). In these cells, they detected that LASS2 inhibited the function of V-ATPase by binding to its C-subunit. This effect was mediated by a significant increase in pHe and lysosomal pH, and DOX that was previously sequestered in the cytosol was released in the nuclei of cells, inducing increased rates of apoptosis. These results suggest that LASS2 is involved in chemotherapeutic outcomes and that a low expression of LASS2 may predict drug resistance.
Visioli et al. (2014) [136] investigated the effect of acidic pH stress on tumor-associated endothelial cells (TECs) and found that the mechanisms underlying cell adaptation and resistance to antiangiogenic chemotherapeutic agents may depend on the activation of the unfolded protein response (UPR). Primary human dermal microvascular endothelial cells (HDMECs) cultured in an acidic-conditioned medium showed increased expression of the UPR marker (Grp78, glucose-regulated protein 78) and concomitantly greater resistance to cytotoxicity induced by the drug Sunitinib. Inhibition of GRP78 resensitizes HDMECs to drug treatment. Therefore, this work suggested that UPR induction in endothelial cells under acidic pH conditions may results in therapeutic failures in response to chemotherapy. Targeting Grp78, the key component of the UPR pathway, may provide a promising alternative for cancer treatment.
Tumor spheroids have also found application in assessing tumor resistance since they reproduce the tumor microenvironment conditions in a more accurate way and provide more physiologically relevant information and more predictive data for in vivo tests. Mellor and Callaghan, (2011) [144] used such a model to evaluate chemoresistance in colorectal adenocarcinoma cells (DLD-1), colon adenocarcinoma cells (HT29), and ovarian adenocarcinoma cells (NCIADR). The accumulation and distribution of doxorubicin (DOX), a chemotherapeutic drug, were measured in tumor spheroids. DOX is fluorescent and thus allows detection by confocal microscopy. Tumor spheroids demonstrated a high level of DOX in cells on the tissue surface. An inner ring, only in the DLD-1 and HT29 cell lines, consistent with the hypoxic zone, was almost completely devoid of DOX staining, reflecting poor intracellular accumulation in this zone. In addition, the authors of this study observed that the pH was acidic (6.9–7.0) in all the cell lines exposed to hypoxia. The influence of Pgp on doxorubicin accumulation was examined under hypoxic conditions in TS comprising all three cell types. The inhibition of Pgp by the modulator Tariquidar (XR9576) increased the accumulation of DOX in all three cell lines evaluated. The study concluded that exposure of 3D tumor spheroids to hypoxia generates an acidic microenvironment; this leads to increased expression of drug transporters and, therefore, a decrease in DOX-induced cytotoxicity [144].
5.2. The Case of Temozolomide
Temozolomide (TMZ) is a DNA alkylating agent discovered in the early 1980s that is now a reference in the treatment of glioblastomas. Temozolomide has the ability to cross the blood–brain barrier and has excellent biodistribution in the central nervous system as well as high stability at acidic pH, allowing its oral administration. The activation of this molecule takes place via the spontaneous conversion of temozolomide into 3-methyl-(triazen-1-yl) imidazole-4-carboxamide (MTIC), which is subsequently degraded into a methyldiazonium cation, the DNA-methylating species. The methyldiazonium cation activates the DNA repair system MMR (mismatch repair), which will lead to DNA breaks and trigger cell death via the p53 protein [145,146,147].
The degradation rates of both TMZ and MTIC exhibit strong opposite pH dependencies, with an exponential increase and decrease observed as pH values vary, respectively. Consequently, only a narrow pH range facilitates the hydrolysis of TMZ into MTIC and, subsequently, into the DNA alkylating group [145]. Stéphanou and Ballesta (2019) [148] have designed a theoretical framework to study the antitumor efficacy of TMZ, integrating the effect of the pHe, the spatial configuration of the tumor, and the microenvironment in order to identify this range. Based on the relationship between the pHe and pHi for a given cell type, it provides quantitative predictions regarding the differential impact of TMZ on cancer cells and optimal pH values leading to a greater amount of DNA damage. Therefore, by experimentally characterizing the cell’s ability to regulate its pHi as a function of the pHe, the model highlights potential ways to improve the efficacy of TMZ on tumor cells. We subsequently characterized the pH regulation ability of two glioma cell lines and were indeed able to show that different optimal pHs for TMZ efficacy were identified [19].
Despite the treatment’s promise, the use of TMZ as an oral chemotherapy agent has certain limitations. Unfortunately, more than 50% of GBM patients treated with TMZ do not respond to therapy [149,150]. In addition, some patients subsequently suffer from recurrences or their tumor continues to grow. This represents treatment failure that is commonly associated with the phenomenon known as drug resistance, which is a major problem with TMZ treatment. This calls for urgent means to circumvent those limitations.
5.3. Targeting the Membrane Transporters
The activity of NHE-1 participates in the phenomena of resistance to anti-cancer drugs. Cells resistant to chemotherapy agents generally have a more alkaline cytoplasmic pH and more acidic intracellular vesicles than cells sensitive to these agents [151]. It has been shown that the expression and activity of NHE-1 were increased in cells resistant to doxorubicin and that inhibition of NHE-1 by a pharmacological inhibitor restores sensitivity to this anti-cancer agent [152]. Inhibition of NHE-1 is also involved in the action of certain anti-cancer agents that induce cancer cell apoptosis such as paclitaxel [11] and Cisplatin [153].
Targeting V-ATPase activity has been proposed as a new cancer treatment strategy [154]. A V-ATPase inhibitor molecule, esomeprazole used as an acidic pH-activated prodrug, decreases tumor growth and tumor pH gradient in vivo [155]. V-ATPase inhibitors could also be used in the context of resistance to chemotherapy. Indeed, the expression of several V-ATPase subunits is increased by a chemotherapy agent, Cisplatin [156,157]. This increase in expression is involved in chemoresistance phenomena; therefore, it has been proposed that inhibition of V-ATPase would increase the cytotoxicity of Cisplatin and could be a new strategy for avoiding resistance to this compound.
The use of an MCT1 inhibitor, α-cyano-4-hydroxycinnamate (CHC), has been proposed in order to inhibit the entry of lactate into oxidative cells, which will then have to use glucose as an energy substrate and which will not be, therefore, more available to the glycolytic cells in the center of the tumor [35]. The use of CHC shows a decrease in tumor growth and radiosensitization in mouse tumor models [35]. Similar effects are found when the expression of MCT1 is inhibited by an siRNA [158]. Other inhibitors have been identified [159,160]. No specific MCT4 molecule is available, and a targeting problem may arise since MCT4 is located in glycolytic cells far from blood vessels.
CAIX represents a particularly interesting therapeutic target and is the subject of intense research around the development of specific inhibitors [161].
6. Conclusions
Normal and tumor cells share a fundamental similarity in their regulation of intracellular pH, both striving to maintain it near the physiological levels necessary for cellular function. However, chronic adverse conditions within the tumor microenvironment favor the selection of resistant, invasive, and aggressive cell populations. These cells develop enhanced abilities to withstand, manage, or regulate acidity, in contrast to normal cells that may succumb to such conditions and perish.
While many experiments have investigated cells within their native environments, understanding their intrinsic functioning often requires exposure to controlled conditions outside the norm, for instance, exploring the dynamic capacity of cells to regulate the intracellular pH necessitates subjecting them to a broad range of pH levels, from highly acidic to basic, in a systematic manner. Our research with two glioma cell lines demonstrated the potential to optimize temozolomide efficacy through this approach [19], although it has faced criticism for exposing cells to extreme conditions not typically encountered in vivo.
A significant challenge in such experiments lies in simultaneously measuring the extracellular and intracellular pH levels in individual cells over extended periods, spanning from hours to days. Fluorescence microscopy employing pH-sensitive probes offers a viable alternative, though protocols must be carefully adjusted to prevent cell overexposure to light excitation or signal extinction within tumor masses.
This review, spanning the past century, highlights a persistent interest in acidity regulation and measurement. Today, there is a renewed interest in understanding the role of acidity in driving cellular progression toward malignancy and increased aggressiveness, as well as its potential implications for therapy.
The exploration of acidity regulation and measurement underscores the critical role of the acidic tumor microenvironment in driving cancer progression and therapy resistance. Understanding how cells respond to varying pH conditions provides valuable insights into the development of targeted cancer therapies. By leveraging this knowledge, innovative approaches can be designed to disrupt tumor survival pathways and enhance treatment efficacy. Strategies such as pH-modulating agents and proton pump inhibitors offer promising avenues for combating acidity-related challenges in cancer treatment. By targeting acidity as a therapeutic vulnerability, personalized medicine approaches can be tailored to individual tumor characteristics, maximizing treatment effectiveness. Thus, the renewed interest in acidity as a therapeutic target aligns with the ongoing efforts to advance cancer treatment strategies and improve patient outcomes.
Author Contributions
Writing—original draft preparation, A.T.; Writing—review and editing, A.S.; Supervision, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project received financial support from the CNRS through the MITI interdisciplinary programs (MetaMod Project 2021–2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Kallinowski, F.; Vaupel, P. pH distributions in spontaneous and isotransplanted rat tumours. British J. Cancer 1988, 58, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Chiche, J.; Brahimi-Horn, M.C.; Pouysségur, J. Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J. Cell. Mol. Med. 2010, 14, 771–794. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. Ueber den Stoffwechsel der Tumoren: Arbeiten aus dem Kaiser Wilhelm-Institut für Biologie, Berlin-Dahlem. JAMA 1926, 87, 1671. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Kerbel, R.; Folkman, J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer 2002, 2, 727–739. [Google Scholar] [CrossRef]
- Serres, S.; O’Brien, E.R.; Sibson, N.R. Imaging Angiogenesis, Inflammation, and Metastasis in the Tumor Microenvironment with Magnetic Resonance Imaging. In Tumor Microenvironment and Cellular Stress. Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Gupta, P.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell 2019, 24, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Reshkin, S.; Bellizzi, A.; Cardone, R.; Tommasino, M.; Casavola, V.; Paradiso, A. Paclitaxel induces apoptosis via protein kinase A- and p38 mitogen-activated protein-dependent inhibition of the Na+/H+ exchanger (NHE) NHE isoform 1 in human breast cancer cells. Clin. Cancer Res. 2003, 9, 2366–2373. [Google Scholar]
- Bedessem, B.; Stéphanou, A. Role of Compartmentalization on HiF-1a Degradation Dynamics during Changing Oxygen Conditions: A Computational Approach. PLoS ONE 2014, 9, e110495. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.; Boron, W.F. Intracellular pH. Physiol. Rev. 1981, 61, 296–434. [Google Scholar] [CrossRef] [PubMed]
- Madshus, I.H. Regulation of intracellular pH in eukaryotic cells. Biochem. J. 1988, 250, 1–8. [Google Scholar] [CrossRef]
- Zhuang, Y.X.; Cragoe, E.J.; Shaikewitz, T.; Glaser, L.; Cassel, D. Characterization of potent sodium/proton exchange inhibitors from the amiloride series in A431 cells. Biochemistry 1984, 23, 4481–4488. [Google Scholar] [CrossRef] [PubMed]
- Druzhkova, I.; Shirmanova, M.; Lukina, M.; Dudenkova, V.; Sergeeva, T.; Belousov, V.; Lukyanov, S.; Zagaynova, E. Registration of intracellular pH in cancer cells with genetically encoded ratiometric sensor. In Proceedings of the European Conference on Biomedical Optics 2015, Munich, Germany, 21–25 June 2015. [Google Scholar] [CrossRef]
- Shirmanova, M.V.; Druzhkova, I.N.; Lukina, M.M.; Matlashov, M.E.; Belousov, V.V.; Snopova, L.B.; Prodanetz, N.N.; Dudenkova, V.V.; Lukyanov, S.A.; Zagaynova, E.V. Intracellular pH imaging in cancer cells in vitro and tumors in vivo using the new genetically encoded sensor SypHer2. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 1905–1911. [Google Scholar] [CrossRef]
- Gillies, R.J.; Raghunand, N.; Karczmar, G.S.; Bhujwalla, Z.M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging 2002, 16, 430–450. [Google Scholar] [CrossRef] [PubMed]
- Tafech, A.; Jacquet, P.; Beaujean, C.; Fertin, A.; Usson, Y.; Stéphanou, A. Characterization of the Intracellular Acidity Regulation of Brain Tumor Cells and Consequences for Therapeutic Optimization of Temozolomide. Biology 2023, 12, 1221. [Google Scholar] [CrossRef] [PubMed]
- Barathova, M.; Takacova, M.; Holotnakova, T.; Gibadulinova, A.; Ohradanova, A.; Zatovicova, M.; Hulikova, A.; Kopacek, J.; Parkkila, S.; Supuran, C.T.; et al. Alternative splicing variant of the hypoxia marker carbonic anhydrase IX expressed independently of hypoxia and tumour phenotype. Br. J. Cancer 2007, 98, 129–136. [Google Scholar] [CrossRef]
- Hinton, A.; Sennoune, S.R.; Bond, S.; Fang, M.; Reuveni, M.; Sahagian, G.G.; Jay, D.; Martinez-Zaguilan, R.; Forgac, M. Function of a Subunit Isoforms of the V-ATPase in pH Homeostasis and in Vitro Invasion of MDA-MB231 Human Breast Cancer Cells. J. Biol. Chem. 2009, 284, 16400–16408. [Google Scholar] [CrossRef]
- Enerson, B.E.; Drewes, L.R. Molecular Features, Regulation, and Function of Monocarboxylate Transporters: Implications for Drug Delivery. J. Pharm. Sci. 2003, 92, 1531–1544. [Google Scholar] [CrossRef]
- Supuran, C.T.; Fiore, A.D.; Alterio, V.; Montib, S.M.; Simone, G.D. Recent Advances in Structural Studies of the Carbonic Anhydrase Family: The Crystal Structure of Human CA IX and CA XIII. Curr. Pharm. Des. 2010, 16, 3246–3254. [Google Scholar] [CrossRef] [PubMed]
- Pouysségur, J.; Dayan, F.; Mazure, N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006, 441, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Fuster, D.; Moe, O.W.; Hilgemann, D.W. Lipid- and mechanosensitivities of sodium/hydrogen exchangers analyzed by electrical methods. Proc. Natl. Acad. Sci. USA 2004, 101, 10482–10487. [Google Scholar] [CrossRef] [PubMed]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Harguindey, S.; Orive, G.; Pedraz, J.L.; Paradiso, A.; Reshkin, S.J. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin—One single nature. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2005, 1756, 1–24. [Google Scholar] [CrossRef]
- Stock, C.; Schwab, A. Protons make tumor cells move like clockwork. Pflügers Archiv.-Eur. J. Physiol. 2009, 458, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Gluck, S.; Hartwig, J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J. Cell Biol. 1987, 105, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Forgac, M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol. Rev. 1989, 69, 765–796. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zaguilan, R.; Gillies, R.J. A Plasma Membrane V-type H+-ATPase May Contribute to Elevated Intracellular pH (pHin) in Some Human Tumor Cells. Ann. N. Y. Acad. Sci. 1992, 671, 478–480. [Google Scholar] [CrossRef]
- Martinez-Zaguilan, R.; Lynch, R.M.; Martinez, G.M.; Gillies, R.J. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Cell Physiol. 1993, 265, C1015–C1029. [Google Scholar] [CrossRef]
- Martínez-Zaguilán, R.; Martinez, G.M.; Gomez, A.; Hendrix, M.J.C.; Gillies, R.J. Distinct regulation of pHin and [Ca2+]in in human melanoma cells with different metastatic potential. J. Cell. Physiol. 1998, 176, 196–205. [Google Scholar] [CrossRef]
- Sennoune, S.R.; Bakunts, K.; Martínez, G.M.; Chua-Tuan, J.L.; Kebir, Y.; Attaya, M.N.; Martínez-Zaguilán, R. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: Distribution and functional activity. Am. J. Physiol.-Cell Physiol. 2004, 286, C1443–C1452. [Google Scholar] [CrossRef] [PubMed]
- Sonveaux, P.; Végran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; Saedeleer, C.J.D.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Takahashi, M.; Uramoto, H.; Nakayama, Y.; Oyama, T.; Wang, K.Y.; Sasaguri, Y.; Nishizawa, S.; Kohno, K. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci. 2011, 102, 1007–1013. [Google Scholar] [CrossRef]
- Gallagher, S.M.; Castorino, J.J.; Wang, D.; Philp, N.J. Monocarboxylate Transporter 4 Regulates Maturation and Trafficking of CD147 to the Plasma Membrane in the Metastatic Breast Cancer Cell Line MDA-MB-231. Cancer Res. 2007, 67, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Froberg, M.K.; Gerhart, D.Z.; Enerson, B.E.; Manivel, C.; Guzman-Paz, M.; Seacotte, N.; Drewes, L.R. Expression of monocarboxylate transporter MCT1 in normal and neoplastic human CNS tissues. Neuroreport 2001, 12, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, K. Perspectives on carbonic anhydrase. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Parkkila, S.; Pastorek, J.; Supuran, C.T. Review Article. J. Enzym. Inhib. Med. Chem. 2004, 19, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Bartošová, M.; Parkkila, S.; Pohlodek, K.; Karttunen, T.J.; Galbavý, Š.; Mucha, V.; Harris, A.L.; Pastorek, J.; Pastoreková, S. Expression of carbonic anhydrase IX in breast is associated with malignant tissues and is related to overexpression of c-erbB2. J. Pathol. 2002, 197, 314–321. [Google Scholar] [CrossRef]
- Ivanov, S.; Liao, S.Y.; Ivanova, A.; Danilkovitch-Miagkova, A.; Tarasova, N.; Weirich, G.; Merrill, M.J.; Proescholdt, M.A.; Oldfield, E.H.; Lee, J.; et al. Expression of Hypoxia-Inducible Cell-Surface Transmembrane Carbonic Anhydrases in Human Cancer. Am. J. Pathol. 2001, 158, 905–919. [Google Scholar] [CrossRef]
- Švastová, E.; Hulıková, A.; Rafajová, M.; Zat’ovičová, M.; Gibadulinová, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.; et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004, 577, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Chiche, J.; Ilc, K.; Laferrière, J.; Trottier, E.; Dayan, F.; Mazure, N.M.; Brahimi-Horn, M.C.; Pouysségur, J. Hypoxia-Inducible Carbonic Anhydrase IX and XII Promote Tumor Cell Growth by Counteracting Acidosis through the Regulation of the Intracellular pH. Cancer Res. 2008, 69, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.K.; Chiche, J.; Pouyssegur, J. pH control mechanisms of tumor survival and growth. J. Cell. Physiol. 2010, 226, 299–308. [Google Scholar] [CrossRef]
- Parks, S.K.; Mazure, N.M.; Counillon, L.; Pouysségur, J. Hypoxia promotes tumor cell survival in acidic conditions by preserving ATP levels. J. Cell. Physiol. 2013, 228, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The Role of Carbonic Anhydrase 9 in Regulating Extracellular and Intracellular pH in Three-dimensional Tumor Cell Growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef] [PubMed]
- Grabmaier, K.; de Weijert, M.C.; Verhaegh, G.W.; Schalken, J.A.; Oosterwijk, E. Strict regulation of CAIXG250/MN by HIF-1α in clear cell renal cell carcinoma. Oncogene 2004, 23, 5624–5631. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.V.; Kuzmin, I.; Wei, M.H.; Pack, S.; Geil, L.; Johnson, B.E.; Stanbridge, E.J.; Lerman, M.I. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc. Natl. Acad. Sci. USA 1998, 95, 12596–12601. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; Hilvo, M.; Fiore, A.D.; Supuran, C.T.; Pan, P.; Parkkila, S.; Scaloni, A.; Pastorek, J.; Pastorekova, S.; Pedone, C.; et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc. Natl. Acad. Sci. USA 2009, 106, 16233–16238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Oosterwijk, E.; Tu, C.; Shiverick, K.T.; Silverman, D.N.; Frost, S.C. Expression and Activity of Carbonic Anhydrase IX Is Associated With Metabolic Dysfunction in MDA-MB-231 Breast Cancer Cells. Cancer Investig. 2009, 27, 613–623. [Google Scholar] [CrossRef]
- Parkkila, A.K.; Herva, R.; Parkkila, S.; Rajaniemi, H. Immunohistochemical demonstration of human carbonic anhydrase isoenzyme II in brain tumours. Histochem. J. 1995, 27, 974–982. [Google Scholar] [CrossRef]
- Nordfors, K.; Haapasalo, J.; Korja, M.; Niemelä, A.; Laine, J.; Parkkila, A.K.; Pastorekova, S.; Pastorek, J.; Waheed, A.; Sly, W.S.; et al. The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: An association of CA IX with poor prognosis. BMC Cancer 2010, 10, 148. [Google Scholar] [CrossRef]
- Parkkila, S.; Lasota, J.; Fletcher, J.A.; bin Ou, W.; Kivelä, A.J.; Nuorva, K.; Parkkila, A.K.; Ollikainen, J.; Sly, W.S.; Waheed, A.; et al. Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Mod. Pathol. 2010, 23, 743–750. [Google Scholar] [CrossRef]
- Pan, P.; Leppilampi, M.; Pastorekova, S.; Pastorek, J.; Waheed, A.; Sly, W.S.; Parkkila, S. Carbonic anhydrase gene expression in CA II-deficient (Car2−/−) and CA IX-deficient (Car9−/−) mice. J. Physiol. 2006, 571, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.K.; Chiche, J.; Pouysségur, J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 2013, 13, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Harguindey, S.; Arranz, J.L.; Wahl, M.L.; Orive, G.; Reshkin, S.J. Proton Transport Inhibitors as Potentially Selective Anticancer Drugs. Anticancer Res. 2009, 29, 2127–2136. [Google Scholar] [PubMed]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007, 26, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Rotin, D.; Wan, P.; Grinstein, S.; Tannock, I.F. Cytotoxicity of compounds that interfere with the regulation of intracellular pH: A potential new class of anticancer drugs. Cancer Res. 1987, 47, 1497–1504. [Google Scholar] [PubMed]
- Yamagata, M.; Tannock, I. The chronic administration of drugs that inhibit the regulation of intracellular pH: In vitro and anti-tumour effects. Br. J. Cancer 1996, 73, 1328–1334. [Google Scholar] [CrossRef]
- Montcourrier, P.; Silver, I.; Farnoud, R.; Bird, I.; Rochefort, H. Breast cancer cells have a high capacity to acidify extracellular milieu by a dual mechanism. Clin. Exp. Metastasis 1997, 15, 382–392. [Google Scholar] [CrossRef]
- Grillon, E.; Farion, R.; Fablet, K.; Waard, M.D.; Tse, C.M.; Donowitz, M.; Rémy, C.; Coles, J.A. The Spatial Organization of Proton and Lactate Transport in a Rat Brain Tumor. PLoS ONE 2011, 6, e17416. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Singleton, P.A.; Diedrich, F.; Stern, R.; Gilad, E. CD44 Interaction with Na+-H+ Exchanger (NHE1) Creates Acidic Microenvironments Leading to Hyaluronidase-2 and Cathepsin B Activation and Breast Tumor Cell Invasion. J. Biol. Chem. 2004, 279, 26991–27007. [Google Scholar] [CrossRef] [PubMed]
- Rozhin, J.; Sameni, M.; Ziegler, G.; Sloane, B.F. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994, 54, 6517–6525. [Google Scholar] [PubMed]
- Yang, X.; Wang, D.; Dong, W.; Song, Z.; Dou, K. Inhibition of Na+/H+ exchanger 1 by 5-(N-ethyl-N-isopropyl) amiloride reduces hypoxia-induced hepatocellular carcinoma invasion and motility. Cancer Lett. 2010, 295, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Lambert, C.A.; Colige, A.C.; Mineur, P.; Noël, A.; Frankenne, F.; Foidart, J.M.; Baba, M.; Hata, R.I.; Miyazaki, K.; et al. Acidic Extracellular pH Induces Matrix Metalloproteinase-9 Expression in Mouse Metastatic Melanoma Cells through the Phospholipase D-Mitogen-activated Protein Kinase Signaling. J. Biol. Chem. 2005, 280, 10938–10944. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Robey, I.F.; Nesbit, L.A. Investigating Mechanisms of Alkalinization for Reducing Primary Breast Tumor Invasion. BioMed Res. Int. 2013, 2013, 485196. [Google Scholar] [CrossRef]
- Hjelmeland, A.B.; Wu, Q.; Heddleston, J.M.; Choudhary, G.S.; MacSwords, J.; Lathia, J.D.; McLendon, R.; Lindner, D.; Sloan, A.; Rich, J.N. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2010, 18, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, Y.I.; Patel, B.B.; Ackerstaff, E.; Sukenick, G.; Koutcher, J.A.; Glod, J.W.; Banerjee, D. Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment. Exp. Cell Res. 2012, 318, 326–335. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Calcinotto, A.; Filipazzi, P.; Grioni, M.; Iero, M.; Milito, A.D.; Ricupito, A.; Cova, A.; Canese, R.; Jachetti, E.; Rossetti, M.; et al. Modulation of Microenvironment Acidity Reverses Anergy in Human and Murine Tumor-Infiltrating T Lymphocytes. Cancer Res. 2012, 72, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Lunt, S.Y.; Heiden, M.G.V. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Roos, D.; Loos, J. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. Exp. Cell Res. 1973, 77, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Marquardt, C.; Foker, J. Aerobic glycolysis during lymphocyte proliferation. Nature 1976, 261, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Hedeskov, C.J. Early effects of phytohaemagglutinin on glucose metabolism of normal human lymphocytes. Biochem. J. 1968, 110, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Razungles, J.; Cavaillès, V.; Jalaguier, S.; Teyssier, C. L’effet Warburg. Med. Sci. (Paris) 2013, 29, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Thompson, C.B. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Gentric, G.; Mieulet, V.; Mechta-Grigoriou, F. Heterogeneity in Cancer Metabolism: New Concepts in an Old Field. Antioxidants Redox Signal. 2017, 26, 462–485. [Google Scholar] [CrossRef]
- Wu, H.; Ying, M.; Hu, X. Lactic acidosis switches cancer cells from aerobic glycolysis back to dominant oxidative phosphorylation. Oncotarget 2016, 7, 40621–40629. [Google Scholar] [CrossRef]
- Jacquet, P.; Stéphanou, A. A reduced model of cell metabolism to revisit the glycolysis-OXPHOS relationship in the deregulated tumor microenvironment. J. Theor. Biol. 2023, 562, 111434. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, H.; Dai, C.; Pan, Q.; Ding, Z.; Hu, D.; Ji, B.; Luo, Y.; Hu, X. Beyond Warburg effect—Dual metabolic nature of cancer cells. Sci. Rep. 2014, 4, 4927. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Stéphanou, A. Searching for the Metabolic Signature of Cancer: A Review from Warburg’s Time to Now. Biomolecules 2022, 12, 1412. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Nagaki, T.; Fukuda, I.; Okumura, K. Clastogenicity of low pH to various cultured mammalian cells. Fundam. Mol. Mech. Mutagen. 1992, 268, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, T.K.; Yang, J.M.; Liu, L.F. Acidic pH induces topoisomerase II-mediated DNA damage. Proc. Natl. Acad. Sci. USA 2003, 100, 5205–5210. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, T.; Wang, X.; Takahashi, A.; Ohnishi, K.; Saito, H.; Song, C.W.; Ohnishi, T. p53-dependent induction of WAF1 by a low-pH culture condition in human glioblastoma cells. Cancer Res. 1997, 57, 3910–3913. [Google Scholar]
- Williams, A.C.; Collard, T.J.; Paraskeva, C. An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: Implications for clonal selection during colorectal carcinogenesis. Oncogene 1999, 18, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Putney, L.K.; Barber, D.L. Na-H Exchange-dependent Increase in Intracellular pH Times G2/M Entry and Transition. J. Biol. Chem. 2003, 278, 44645–44649. [Google Scholar] [CrossRef] [PubMed]
- Smallbone, K.; Maini, P.K.; Gatenby, R.A. Episodic, transient systemic acidosis delays evolution of the malignant phenotype: Possible mechanism for cancer prevention by increased physical activity. BMC Biol. Direct 2010, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, P.C. Intracellular pH. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1956; pp. 229–277. [Google Scholar] [CrossRef]
- Thomas, R.C. Intracellular pH of snail neurones measured with a new pH-sensitive glass micro-electrode. J. Physiol. 1974, 238, 159–180. [Google Scholar] [CrossRef]
- Lagadic-Gossmann, D.; Chesnais, J.M.; Feuvray, D. Intracellular pH regulation in papillary muscle cells from streptozotocin diabetic rats: An ion-sensitive microelectrode study. Pflügers Archiv.-Eur. J. Physiol. 1988, 412, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.D. Effects of insulin upon ion transport. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1983, 737, 1–49. [Google Scholar] [CrossRef]
- Ellis, D.; Thomas, R.C. Microelectrode measurement of the intracellular pH of mammalian heart cells. Nature 1976, 262, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Zeuthen, T. Chapter 3 How to Make and Use Double-Barreled Ion-Selective Microelectrodes. In Current Topics in Membranes and Transport; Elsevier: Amsterdam, The Netherlands, 1980; pp. 31–47. [Google Scholar] [CrossRef]
- de Hemptinne, A. Intracellular pH and surface pH in skeletal and cardiac muscle measured with a double-barrelled pH microelectrode. Pflügers Archiv.-Eur. J. Physiol. 1980, 386, 121–126. [Google Scholar] [CrossRef]
- Hagberg, H.; Larsson, S.; Haljamae, H. A new design of double-barrelled microelectrodes for intracellular pH-measurement in vivo. Acta Physiol. Scand. 1983, 118, 149–153. [Google Scholar] [CrossRef]
- Claire, C.; Thomas, R.C. Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J. Physiol. 1977, 267, 791–810. [Google Scholar] [CrossRef]
- Heiple, J.M.; Taylor, D.L. Intracellular pH in single motile cells. J. Cell Biol. 1980, 86, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.B.; Richards, J.H. Determination of Intracellular pH by 31P Magnetic Resonance. J. Biol. Chem. 1973, 248, 7276–7278. [Google Scholar] [CrossRef]
- Salhany, J. 31P nuclear magnetic resonance measurement of cardiac pH in perfused guinea-pig hearts. J. Mol. Cell. Cardiol. 1979, 11, 601–610. [Google Scholar] [CrossRef]
- Brindle, K.M.; Rajagopalan, B.; Williams, D.S.; Detre, J.A.; Simplaceanu, E.; Ho, C.; Radda, G.K. 31P NMR measurements of myocardial pH invivo. Biochem. Biophys. Res. Commun. 1988, 151, 70–77. [Google Scholar] [CrossRef]
- Clarke, K.; Stewart, L.C.; Neubauer, S.; Balschi, J.A.; Smith, T.W.; Ingwall, J.S.; Nédélec, J.F.; Humphrey, S.M.; Kléber, A.G.; Springer, C.S. Extracellular volume and transsarcolemmal proton movement during ischemia and reperfusion: A31P NMR spectroscopic study of the isovolumic rat heart. NMR Biomed. 1993, 6, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.A.; Swietach, P.; Atherton, H.J.; Gallagher, F.A.; Lee, P.; Radda, G.K.; Clarke, K.; Tyler, D.J. Measuring intracellular pH in the heart using hyperpolarized carbon dioxide and bicarbonate: A 13C and 31P magnetic resonance spectroscopy study. Cardiovasc. Res. 2009, 86, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.R.; Schaffer, S.W.; Ford, C.; Safer, B. Contribution of tissue acidosis to ischemic injury in the perfused rat heart. Circulation 1976, 53 (Suppl. S3), I3-14. [Google Scholar] [PubMed]
- Ackerman, J.J.; Lowry, M.; Radda, G.K.; Ross, B.D.; Wong, G.G. The role of intrarenal pH in regulation of ammoniagenesis: [31P]NMR studies of the isolated perfused rat kidney. J. Physiol. 1981, 319, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Adam, W.R.; Koretsky, A.P.; Weiner, M.W. Measurement of Renal Intracellular pH by 31P NMR. In Contributions to Nephrology; S.Karger AG: Basel, Switzerland, 1985; pp. 15–21. [Google Scholar] [CrossRef]
- Adam, W.R.; Koretsky, A.P.; Weiner, M.W. 31P-NMR in vivo measurement of renal intracellular pH: Effects of acidosis and K+ depletion in rats. Am. J. Physiol.-Ren. Physiol. 1986, 251, F904–F910. [Google Scholar] [CrossRef] [PubMed]
- Tannen, R.L. Ammonia metabolism. Am. J. Physiol.-Ren. Physiol. 1978, 235, F265–F277. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Ugurbil, K.; Chen, W. Measurement of unidirectional Pi to ATP flux in human visual cortex at 7 T by using 31 P magnetic resonance spectroscopy. Proc. Natl. Acad. Sci. USA 2003, 100, 14409–14414. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhu, X.H.; Zhang, X.L.; Ugurbil, K.; Chen, W. In vivo31P magnetic resonance spectroscopy of human brain at 7 T: An initial experience. Magn. Reson. Med. 2003, 49, 199–205. [Google Scholar] [CrossRef]
- Leyval, D.; Debay, F.; Engasser, J.M.; Goergen, J.L. Flow cytometry for the intracellular pH measurement of glutamate producing Corynebacterium glutamicum. J. Microbiol. Methods 1997, 29, 121–127. [Google Scholar] [CrossRef]
- Imai, T.; Ohno, T. Measurement of yeast intracellular pH by image processing and the change it undergoes during growth phase. J. Biotechnol. 1995, 38, 165–172. [Google Scholar] [CrossRef]
- Molenaar, D.; Abee, T.; Konings, W.N. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1991, 1115, 75–83. [Google Scholar] [CrossRef]
- Brown, D.A.; Garthwaite, J. Intracellular pH and the distribution of weak acids and bases in isolated rat superior cervical ganglia. J. Physiol. 1979, 297, 597–620. [Google Scholar] [CrossRef] [PubMed]
- Padan, E.; Zilberstein, D.; Schuldiner, S. pH homesstasis in bacteria. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1981, 650, 151–166. [Google Scholar] [CrossRef]
- Waddell, W.J.; Butler, T.C. Calculation of intracellular pH from the distribution of 5,5-Dimethyl-2,4-Oxazolidinedione (DMO). Application to skeletal muscle of the dog. J. Clin. Investig. 1959, 38, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Gores, G.J.; Nieminen, A.L.; Wray, B.E.; Herman, B.; Lemasters, J.J. Intracellular pH during “chemical hypoxia” in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J. Clin. Investig. 1989, 83, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Makutonina, A.; Voss, T.G.; Plymale, D.R.; Fermin, C.D.; Norris, C.H.; Vigh, S.; Garry, R.F. Human immunodeficiency virus infection of T-lymphoblastoid cells reduces intracellular pH. J. Virol. 1996, 70, 7049–7055. [Google Scholar] [CrossRef]
- Garry, R.F.; Gottlieb, A.A.; Zuckerman, K.P.; Pace, J.R.; Frank, T.W.; Bostick, D.A. Cell surface effects of human immunodeficiency virus. Biosci. Rep. 1988, 8, 35–48. [Google Scholar] [CrossRef]
- Voss, T.G.; Fermin, C.D.; Levy, J.A.; Vigh, S.; Choi, B.; Garry, R.F. Alteration of intracellular potassium and sodium concentrations correlates with induction of cytopathic effects by human immunodeficiency virus. J. Virol. 1996, 70, 5447–5454. [Google Scholar] [CrossRef] [PubMed]
- Salvi, A.; Quillan, J.M.; Sadée, W. Monitoring intracellular pH changes in response to osmotic stress and membrane transport activity using 5-chloromethylfluorescein. AAPS PharmSci 2002, 4, 21–28. [Google Scholar] [CrossRef]
- Ohkuma, S.; Poole, B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA 1978, 75, 3327–3331. [Google Scholar] [CrossRef]
- Tsien, R.Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature 1981, 290, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Tafech, A.; Beaujean, C.; Usson, Y.; Stéphanou, A. Generalization of the Ratiometric Method to Extend pH Range Measurements of the BCECF Probe. Biomolecules 2023, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Moellering, R.E.; Black, K.C.; Krishnamurty, C.; Baggett, B.K.; Stafford, P.; Rain, M.; Gatenby, R.A.; Gillies, R.J. Acid treatment of melanoma cells selects for invasive phenotypes. Clin. Exp. Metastasis 2008, 25, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.S.; Gillies, R.D.; Gatenby, R.A. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin. Cancer Biol. 2008, 18, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.R.; Damaghi, M.; Marunaka, Y.; Spugnini, E.P.; Fais, S.; Gillies, R.J. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019, 38, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.M. An electrochemical cell for reduction of biochemicals: Its application to the study of the effect of pH and redox potential on the activity of hydrogenases. Anal. Biochem. 1983, 130, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Avnet, S.; Lemma, S.; Cortini, M.; Pellegrini, P.; Perut, F.; Zini, N.; Kusuzaki, K.; Chano, T.; Grisendi, G.; Dominici, M.; et al. Altered pH gradient at the plasma membrane of osteosarcoma cells is a key mechanism of drug resistance. Oncotarget 2016, 7, 63408–63423. [Google Scholar] [CrossRef]
- Thews, O.; Gassner, B.; Kelleher, D.K.; Schwerd, G.; Gekle, M. Impact of Extracellular Acidity on the Activity of P-glycoprotein and the Cytotoxicity of Chemotherapeutic Drugs. Neoplasia 2006, 8, 143–152. [Google Scholar] [CrossRef]
- Cheng, G.M.Y.; To, K.K.W. Adverse Cell Culture Conditions Mimicking the Tumor Microenvironment Upregulate ABCG2 to Mediate Multidrug Resistance and a More Malignant Phenotype. ISRN Oncol. 2012, 2012, 746025. [Google Scholar] [CrossRef]
- Fan, S.; Niu, Y.; Tan, N.; Wu, Z.; Wang, Y.; You, H.; Ke, R.; Song, J.; Shen, Q.; Wang, W.; et al. LASS2 enhances chemosensitivity of breast cancer by counteracting acidic tumor microenvironment through inhibiting activity of V-ATPase proton pump. Oncogene 2012, 32, 1682–1690. [Google Scholar] [CrossRef]
- Visioli, F.; Wang, Y.; Alam, G.N.; Ning, Y.; Rados, P.V.; Nör, J.E.; Polverini, P.J. Glucose-Regulated Protein 78 (Grp78) Confers Chemoresistance to Tumor Endothelial Cells under Acidic Stress. PLoS ONE 2014, 9, e101053. [Google Scholar] [CrossRef]
- Tarling, E.J.; de Aguiar Vallim, T.Q.; Edwards, P.A. Role of ABC transporters in lipid transport and human disease. Trends Endocrinol. Metab. 2013, 24, 342–350. [Google Scholar] [CrossRef]
- Gillet, J.; Remacle, J. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2007, 1775, 237–262. [Google Scholar] [CrossRef] [PubMed]
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T.; et al. Exosome Release and Low pH Belong to a Framework of Resistance of Human Melanoma Cells to Cisplatin. PLoS ONE 2014, 9, e88193. [Google Scholar] [CrossRef] [PubMed]
- Genovese, I.; Ilari, A.; Assaraf, Y.G.; Fazi, F.; Colotti, G. Not only P-glycoprotein: Amplification of the ABCB1- containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resist. Updat. 2017, 32, 23–46. [Google Scholar] [CrossRef]
- Lam, F.C.; Liu, R.; Lu, P.; Shapiro, A.B.; Renoir, J.M.; Sharom, F.J.; Reiner, P.B. β-Amyloid efflux mediated by p-glycoprotein. J. Neurochem. 2001, 76, 1121–1128. [Google Scholar] [CrossRef]
- Ponte-Sucre, A. Availability and applications of ATP-binding cassette (ABC) transporter blockers. Appl. Microbiol. Biotechnol. 2007, 76, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Thews, O.; Riemann, A.; Nowak, M.; Gekle, M. Impact of Hypoxia-Related Tumor Acidosis on Cytotoxicity of Different Chemotherapeutic Drugs In Vitro and In Vivo. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; pp. 51–58. [Google Scholar] [CrossRef]
- Mellor, H.R.; Callaghan, R. Accumulation and distribution of doxorubicin in tumour spheroids: The influence of acidity and expression of P-glycoprotein. Cancer Chemother. Pharmacol. 2011, 68, 1179–1190. [Google Scholar] [CrossRef]
- Denny, B.J.; Wheelhouse, R.T.; Stevens, M.F.G.; Tsang, L.L.H.; Slack, J.A. NMR and Molecular Modeling Investigation of the Mechanism of Activation of the Antitumor Drug Temozolomide and Its Interaction with DNA. Biochemistry 1994, 33, 9045–9051. [Google Scholar] [CrossRef]
- Ballesta, A.; Zhou, Q.; Zhang, X.; Lv, H.; Gallo, J. Multiscale Design of Cell-Type-Specific Pharmacokinetic/Pharmacodynamic Models for Personalized Medicine: Application to Temozolomide in Brain Tumors. CPT Pharmacometrics Syst. Pharmacol. 2014, 3, 112. [Google Scholar] [CrossRef]
- Thomas, A.; Tanaka, M.; Trepel, J.; Reinhold, W.C.; Rajapakse, V.N.; Pommier, Y. Temozolomide in the Era of Precision Medicine. Cancer Res. 2017, 77, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Stéphanou, A.; Ballesta, A. pH as a potential therapeutic target to improve temozolomide antitumor efficacy: A mechanistic modeling study. Pharmacol. Res. Perspect. 2019, 7, e00454. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—A comprehensive review. Cancer Drug Resist. 2020, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.K.; Escargueil, A.E.; Skladanowski, A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol. Ther. 2000, 85, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, E.; Viarisio, D.; Riganti, C.; Costamagna, C.; Ghigo, D.; Bosia, A. Na+/H+ exchanger activity is increased in doxorubicin-resistant human colon cancer cells and its modulation modifies the sensitivity of the cells to doxorubicin. Int. J. Cancer 2005, 115, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Rebillard, A.; Tekpli, X.; Meurette, O.; Sergent, O.; LeMoigne-Muller, G.; Vernhet, L.; Gorria, M.; Chevanne, M.; Christmann, M.; Kaina, B.; et al. Cisplatin-Induced Apoptosis Involves Membrane Fluidification via Inhibition of NHE1 in Human Colon Cancer Cells. Cancer Res. 2007, 67, 7865–7874. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Citro, G.; Fais, S. Proton pump inhibitors as anti vacuolar-ATPases drugs: A novel anticancer strategy. J. Exp. Clin. Cancer Res. 2010, 29, 44. [Google Scholar] [CrossRef]
- Milito, A.D.; Canese, R.; Marino, M.L.; Borghi, M.; Iero, M.; Villa, A.; Venturi, G.; Lozupone, F.; Iessi, E.; Logozzi, M.; et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int. J. Cancer 2009, 127, 207–219. [Google Scholar] [CrossRef]
- Murakami, T.; Shibuya, I.; Ise, T.; Chen, Z.S.; ichi Akiyama, S.; Nakagawa, M.; Izumi, H.; Nakamura, T.; ichi Matsuo, K.; Yamada, Y.; et al. Elevated expression of vacuolar proton pump genes and cellular ph in cisplatin resistance. Int. J. Cancer 2001, 93, 869–874. [Google Scholar] [CrossRef]
- Torigoe, T.; Izumi, H.; Ishiguchi, H.; Uramoto, H.; Murakami, T.; Ise, T.; Yoshida, Y.; Tanabe, M.; Nomoto, M.; Itoh, H.; et al. Enhanced Expression of the Human Vacuolar H+-ATPase c subunit Gene (ATP6L) in Response to Anticancer Agents. J. Biol. Chem. 2002, 277, 36534–36543. [Google Scholar] [CrossRef] [PubMed]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate Influx through the Endothelial Cell Monocarboxylate Transporter MCT1 Supports an NF-κB/IL-8 Pathway that Drives Tumor Angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Bueno, V.; Binet, I.; Steger, U.; Bundick, R.; Ferguson, D.; Murray, C.; Donald, D.; Wood, K. The Specific Monocarboxylate Transporter (MCT1) Inhibitor, AR-C117977, a Novel Immunosuppressant, Prolongs Allograft Survival in the Mouse. Transplantation 2007, 84, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, H.; Qi, Z.; Pahlman, C.; Veress, B.; Bundick, R.V.; Craggs, R.I.; Holness, E.; Edwards, S.; Murray, C.M.; Ferguson, D.; et al. The Specific Monocarboxylate Transporter-1 (MCT-1) Inhibitor, AR-C117977, Induces Donor-Specific Suppression, Reducing Acute and Chronic Allograft Rejection in the Rat. Transplantation 2007, 84, 1191–1199. [Google Scholar] [CrossRef]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).