Distribution Model Reveals Rapid Decline in Habitat Extent for Endangered Hispid Hare: Implications for Wildlife Management and Conservation Planning in Future Climate Change Scenarios
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
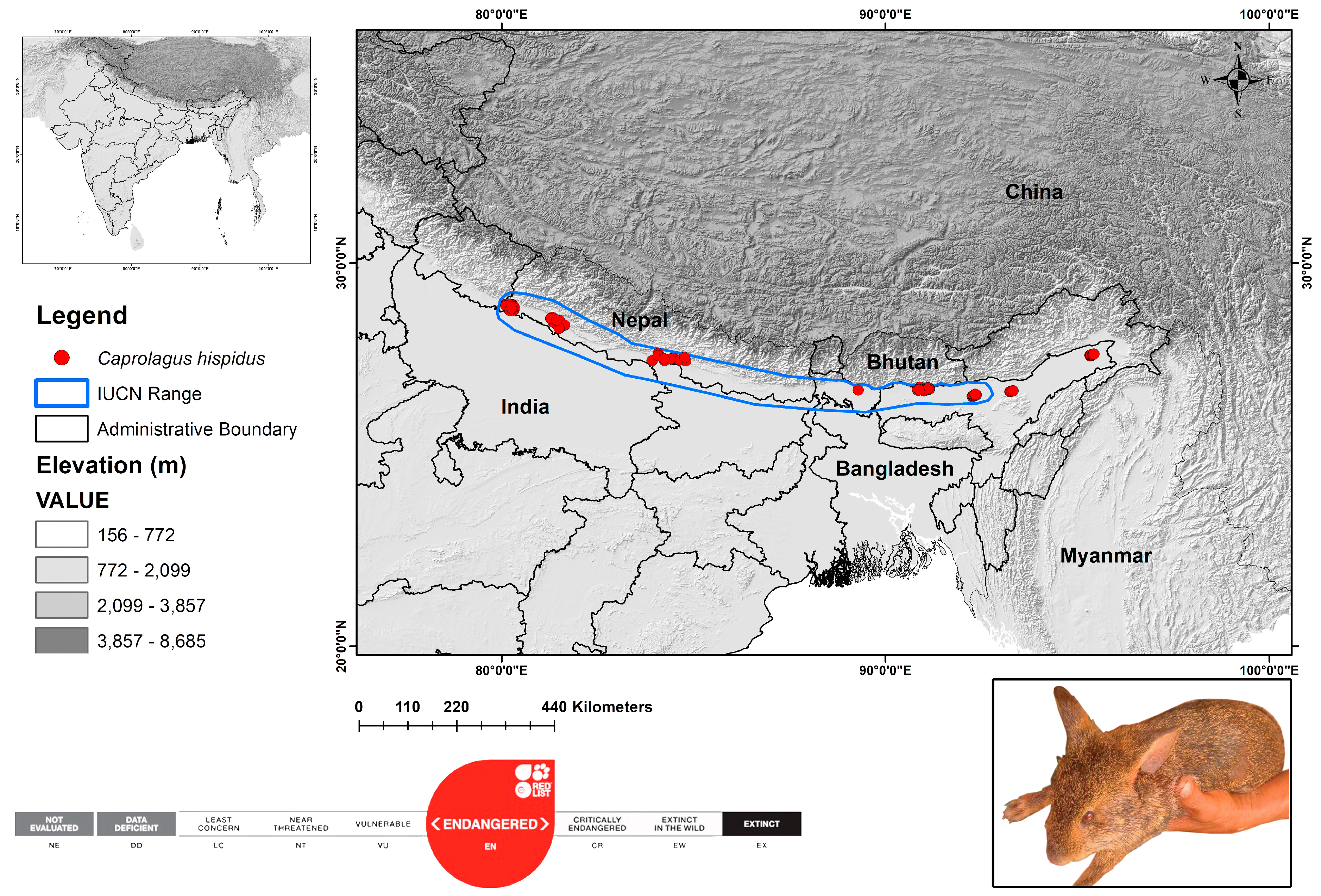
2.1. Study Area and Species Occurrence Records (SORs)
2.2. Model Covariates
2.3. Model Development
2.4. Habitat Quality Assessment
3. Results
3.1. Species Distribution Model
3.2. Habitat Quality, Geometry, and Complexity
3.3. Representativeness of the Protected Area for Conservation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, R.S.; Smith, A.T. Order Lagomorpha. In Mammal Species of the World; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 185–211. [Google Scholar]
- Leach, K.; Kelly, R.; Cameron, A.; Montgomery, W.I.; Reid, N. Expertly Validated Models and Phylogenetically-Controlled Analysis Suggests Responses to Climate Change Are Related to Species Traits in the Order Lagomorpha. PLoS ONE 2015, 10, e0122267. [Google Scholar] [CrossRef]
- Chapman, J.A.; Flux, J.E.C. Introduction to the Lagomorpha. In Lagomorph Biology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–9. [Google Scholar]
- Ge, D.; Wen, Z.; Xia, L.; Zhang, Z.; Erbajeva, M.; Huang, C.; Yang, Q. Evolutionary History of Lagomorphs in Response to Global Environmental Change. PLoS ONE 2013, 8, e59668. [Google Scholar] [CrossRef]
- Dhami, B.; Neupane, B.; Nishan, K.C.; Maraseni, T.; Basyal, C.R.; Joshi, L.R.; Adhikari, H. Ecological Factors Associated with Hispid Hare (Caprolagus hispidus) Habitat Use and Conservation Threats in the Terai Arc Landscape of Nepal. Glob. Ecol. Conserv. 2023, 43, e02437. [Google Scholar] [CrossRef]
- Molur, S.; Srinivasulu, C.; Srinivasulu, B.; Walker, S.; Nameer, P.O.; Ravikumar, L. Status of South Asian Non-Volant Small Mammals: Conservation Assessment and Management Plan (C.A.M.P.) Workshop Report; Zoo Outreach Organisation/CBSG-South Asia: Coimbatore, India, 2005; 618p. [Google Scholar]
- Maheswaran, G. Ecology and conservation of the endangered hispid hare Caprolagus hispidus in Jaldapra Wildlife Sanctuary, West Bengal, India. J. Bombay Nat. Hist. Soc. 2006, 103, 191. [Google Scholar]
- Nath, N.K. Rapid Survey of Endangered Hispid Hare, Caprolagus hispidus in North Bank Landscape, North-East India. Indian For. 2015, 141, 1029–1033. [Google Scholar]
- Tandan, P.; Dhakal, B. Population Status, Habitat Utilization, Distribution and Conservation Threats of Hispid Hare (Caprolagus hispidus, Pearson, 1839) in Bardia National Park of Western Nepal. Environ. Sci. Biol. 2013, 40, 7–13. [Google Scholar]
- Sadadev, B.M.; Silwal, T.; Dhami, B.; Thapa, N.; Neupane, B.; Rana, A.; Singh, H.B. Do Grassland Burning Practices Affect the Distribution of the Hispid Hare, Caprolagus hispidus (Pearson, 1839)? A Study at the Shuklaphanta National Park, Nepal. J. Anim. Divers. 2021, 3, 86–92. [Google Scholar] [CrossRef]
- Khadka, B.B.; Yadav, B.P.; Aryal, N.; Aryal, A. Rediscovery of the Hispid Hare (Caprolagus hispidus) in Chitwan National Park, Nepal after Three Decades. Conserv. Sci. 2017, 5, 10–12. [Google Scholar] [CrossRef]
- Mallinson, J.C. A Note on the Hispid Hare Caprolagus hispidus (Pearson, 1839); Jersey Wildlife Preservation Trust Annual Report 8: 70; Jersey Wildlife Preservation Trust: Jersey, UK, 1971. [Google Scholar]
- Tessier-Yandell, J. The Hispid Hare Caprolagus hispidus (Pearson, 1839). Cheetal 1972, 15, 34–36. [Google Scholar]
- Nath, N.K.; Machary, K. An Ecological Assessment of Hispid Hare Caprolagus hispidus (Mammalia: Lagomorpha: Leporidae) in Manas National Park, Assam, India. J. Threat. Taxa 2015, 7, 8195. [Google Scholar] [CrossRef]
- Bell, D.J.; Oliver, W.L.R.; Ghose, R.K. The Hispid Hare Caprolagus hispidus. In Rabbits, Hares and Pikas: Status Survey and Conservation Action Plan; Chapman, J.A., Flux, J.E.C., Eds.; IUCN: Gland, Switzerland, 1990; pp. 128–136. [Google Scholar]
- Aryal, A.; Yadav, B. Caprolagus hispidus. In The IUCN Red List of Threatened Species 2019: E. T3833A45176688; IUCN: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Aryal, A.; Brunton, D.; Ji, W.; Yadav, H.K.; Adhikari, B.; Raubenheimer, D. Diet and Habitat Use of Hispid Hare Caprolagus Hispidus in Shuklaphanta Wildlife Reserve, Nepal. Mamm. Stud. 2012, 37, 147–154. [Google Scholar] [CrossRef]
- Rastogi, S.; Raj, R.K.; Chauhan, B.K. A Rare Camera Trap Record of the Hispid Hare Caprolagus hispidus from Dudhwa Tiger Reserve, Terai Arc Landscape, India. J. Threat. Taxa 2020, 12, 17024–17027. [Google Scholar] [CrossRef]
- Oliver, W.L.R. The Pygmy Hog: The Biology and Conservation of the Pygmy Hog, Sus Salvanius and the Hispid Hare, Caprolagus hispidus; Special Scientific Report No. 1; Jersey Wildlife Preservation Trust: Jersey, UK, 1980; 120p. [Google Scholar]
- White, R.P.; Siobhan, M.; Mark, R. Pilot Analysis of Global Ecosystems. Grassland Ecosystems; World Resources Institute: Washington, DC, USA, 2000. [Google Scholar]
- Yadav, B.P.; Sathyakumar, S.; Koirala, R.K.; Pokharel, C. Status, distribution and habitat use of Hispid Hare (Caprolagus hispidus) in Royal Suklaphanta Wildlife Reserve, Nepal. Tiger Pap. 2008, 35, 8–14. [Google Scholar]
- Jnawali, S.R.; Baral, H.S.; Lee, S.; Acharya, K.P.; Upadhyay, G.P.; Pandey, M.; Shrestha, R.; Joshi, D.; Lamichhane, B.R.; Griffiths, J. The Status of Nepal’s Mammals: The National Red List Series-IUCN; The National Trust for Nature Conservation: Kathmandu, Nepal, 2011. [Google Scholar]
- Chand, D.B.; Khanal, L.; Chalise, M.K. Distribution and Habitat Preference of Hispid Hare (Caprolagus hispidus) In Shuklaphanta National Park, Nepal. Tribhuvan Univ. J. 2017, 31, 1–16. [Google Scholar] [CrossRef]
- Mukherjee, T.; Sharma, L.K.; Thakur, M.; Saha, G.K.; Chandra, K. Changing Landscape Configuration Demands Ecological Planning: Retrospect and Prospect for Megaherbivores of North Bengal. PLoS ONE 2019, 14, e0225398. [Google Scholar] [CrossRef] [PubMed]
- Nidup, B.T. Endangered Hispid Hare (Caprolagus Hispidus-Pearson 1839) in the Royal Manas National Park. J. Bhutan Ecol. Society. 2018, 3, 56–64. [Google Scholar]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond Predictions: Biodiversity Conservation in a Changing Climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef]
- Warren, R.; Vanderwal, J.; Price, J.; Welbergen, J.A.; Atkinson, I.; Ramirez-Villegas, J.; Osborn, T.J.; Jarvis, A.; Shoo, L.P.; Williams, S.E.; et al. Quantifying the Benefit of Early Climate Change Mitigation in Avoiding Biodiversity Loss. Nat. Clim. Chang. 2013, 3, 678–682. [Google Scholar] [CrossRef]
- Holt, R.D. The Microevolutionary Consequences of Climate Change. Trends Ecol. Evol. 1990, 5, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.D. Bringing the Hutchinsonian Niche into the 21st Century: Ecological and Evolutionary Perspectives. Proc. Natl. Acad. Sci. USA 2009, 106, 19659–19665. [Google Scholar] [CrossRef]
- Maheswaran, G.; Sharma, L.K.; Mondal, H.S.; Mukherjee, T. White-Bellied Heron a Species on the Verge of Extinction: Ensemble Model Reveals Loss of Habitats and Resultant Prolonged Isolation Driving the Species to Extinction. Ecol. Inform. 2021, 64, 101383. [Google Scholar] [CrossRef]
- Ortega-Huerta, M.A.; Peterson, A.T. Modelling Spatial Patterns of Biodiversity for Conservation Prioritization in North-Eastern Mexico. Divers. Distrib. 2004, 10, 39–54. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive Habitat Distribution Models in Ecology. Ecol. Modell. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Pearson, R.G. Species’ Distribution Modeling for Conservation Educators and Practitioners. Netw. Conserv. Educ. Pract. Cent. Biodivers. Conserv. Am. Mus. Nat. Hist. 2010, 3, 54–89. [Google Scholar]
- Kujala, H.; Moilanen, A.; Araújo, M.B.; Cabeza, M. Conservation Planning with Uncertain Climate Change Projections. PLoS ONE 2013, 8, e53315. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Sharma, L.K.; Kumar, V.; Sharief, A.; Dutta, R.; Kumar, M.; Joshi, B.D.; Thakur, M.; Venkatraman, C.; Chandra, K. Adaptive Spatial Planning of Protected Area Network for Conserving the Himalayan Brown Bear. Sci. Total Environ. 2021, 754, 142416. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Mukherjee, T.; Dutta, R.; Sharief, A.; Kumar, V.; Joshi, B.D.; Chandra, K.; Thakur, M.; Sharma, L.K. Future Simulated Landscape Predicts Habitat Loss for the Golden Langur (Trachypithecus geei): A Range Level Analysis for an Endangered Primate. Sci. Total Environ. 2022, 826, 154081. [Google Scholar] [CrossRef] [PubMed]
- Ranjan Deka, J.; Hazarika, A.; Boruah, A.; Prasad Das, J.; Tanti, R.; Ainul Hussain, S. The Impact of Climate Change and Potential Distribution of the Endangered White Winged Wood Duck (Asarcornis scutulata, 1882) in Indian Eastern Himalaya. J. Nat. Conserv. 2022, 70, 126279. [Google Scholar] [CrossRef]
- Kundu, S.; Mukherjee, T.; Kamalakannan, M.; Barhadiya, G.; Ghosh, C.; Kim, H.-W. Matrilineal Phylogeny and Habitat Suitability of the Endangered Spotted Pond Turtle (Geoclemys hamiltonii; Testudines: Geoemydidae): A Two-Dimensional Approach to Forecasting Future Conservation Consequences. PeerJ 2023, 11, e15975. [Google Scholar] [CrossRef] [PubMed]
- Bachman, S.; Moat, J.; Hill, A.W.; de laTorre, J.; Scott, B. Supporting Red List Threat Assessments with GeoCAT: Geospatial Conservation Assessment Tool. Zookeys 2011, 150, 117–126. [Google Scholar] [CrossRef]
- GBIF. GBIF Occurrence Download. 2024. Available online: https://doi.org/10.15468/dl.ey6nbf (accessed on 15 February 2024).
- Maheswaran, G. Ecology and Conservation of Endangered Hispid Hare Caprolagus hispidus in India. In Rare Animals of India; Bentham Science Publishers Ltd.: Potomac, MD, USA, 2013; pp. 179–203. ISBN 9781608056293. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Buchhorn, M.; Bertels, L.; Smets, B.; De Roo, B.; Lesiv, M.; Tsendbazar, N.E.; Masiliunas, D.; Li, L. Copernicus Global Land Service: Land Cover 100 m: Version 3 Globe 2015–2019: Algorithm Theoretical Basis Document; Laboratory of Geoinformation Science and Remote Sensing: Gelderland, The Netherlands, 2020. [Google Scholar]
- Mukherjee, T.; Chongder, I.; Ghosh, S.; Dutta, A.; Singh, A.; Dutta, R.; Joshi, B.D.; Thakur, M.; Sharma, L.K.; Venkatraman, C.; et al. Indian Grey Wolf and Striped Hyaena Sharing from the Same Bowl: High Niche Overlap between Top Predators in a Human-Dominated Landscape. Glob. Ecol. Conserv. 2021, 28, e01682. [Google Scholar] [CrossRef]
- Pekel, J.F.; Cottam, A.; Gorelick, N.; Belward, A.S. High-Resolution Mapping of Global Surface Water and Its Long-Term Changes. Nature 2016, 540, 418–422. [Google Scholar] [CrossRef]
- Cong, M.; Li, Y.; Yang, W. Potential Distribution of Bryophyte, Entodon challengeri (Entodontaceae), under Climate Warming in China. Diversity 2023, 15, 871. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Norgate, M.; Tiwari, P.R.; Das, S.; Kumar, D. On the Heat Waves over India and Their Future Projections under Different SSP Scenarios from CMIP6 Models. Int. J. Climatol. 2024. ahead of print. [Google Scholar] [CrossRef]
- Desmet, Q.; Ngo-Duc, T. A Novel Method for Ranking CMIP6 Global Climate Models over the Southeast Asian Region. Int. J. Climatol. 2022, 42, 97–117. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Erinjery, J.J.; Singh, M.; Kent, R. Diet-Dependent Habitat Shifts at Different Life Stages of Two Sympatric Primate Species. J. Biosci. 2019, 44, 43. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Halvorsen, R.; Mazzoni, S.; Dirksen, J.W.; Næsset, E.; Gobakken, T.; Ohlson, M. How Important Are Choice of Model Selection Method and Spatial Autocorrelation of Presence Data for Distribution Modelling by MaxEnt? Ecol. Modell. 2016, 328, 108–118. [Google Scholar] [CrossRef]
- Johnson, S.E.; Delmore, K.E.; Brown, K.A.; Wyman, T.M.; Louis, E.E. Niche Divergence in a Brown Lemur (Eulemur spp.) Hybrid Zone: Using Ecological Niche Models to Test Models of Stability. Int. J. Primatol. 2016, 37, 69–88. [Google Scholar] [CrossRef]
- Kamilar, J.M.; Tecot, S.R. Anthropogenic and Climatic Effects on the Distribution of Eulemur Species: An Ecological Niche Modeling Approach. Int. J. Primatol. 2016, 37, 47–68. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, W.H. Application of True Skill Statistics as a Practical Method for Quantitatively Assessing CLIMEX Performance. Ecol. Indic. 2023, 146, 109830. [Google Scholar] [CrossRef]
- McGarigal, K. FRAGSTATS Help; University of Massachusetts: Amherst, MA, USA, 2015. [Google Scholar]
- Sertel, E.; Topaloğlu, R.H.; Şallı, B.; Yay Algan, I.; Aksu, G.A. Comparison of Landscape Metrics for Three Different Level Land Cover/Land Use Maps. ISPRS Int. J. Geo-Inf. 2018, 7, 408. [Google Scholar] [CrossRef]
- Mukherjee, T.; Sharma, V.; Sharma, L.K.; Thakur, M.; Joshi, B.D.; Sharief, A.; Thapa, A.; Dutta, R.; Dolker, S.; Tripathy, B.; et al. Landscape-Level Habitat Management Plan through Geometric Reserve Design for Critically Endangered Hangul (Cervus hanglu hanglu). Sci. Total Environ. 2021, 777, 146031. [Google Scholar] [CrossRef]
- Aryal, A.; Yadav, H.K. First cameras trap sighting of critically endangered hispid hare (Caprolagus hispidus) in Shuklaphanta wildlife reserve-Nepal. World Appl. Sci. J. 2010, 9, 367–371. [Google Scholar]
- Tandan, P.; Dhakal, B.; Karki, K.; Aryal, A. Tropical Grasslands Supporting the Endangered Hispid Hare (Caprolagus hispidus) Population in the Bardia National Park, Nepal. Curr. Sci. 2013, 105, 691–694. [Google Scholar]
- Maheshwaran, G.; Kumar, A. Trapping Success and Inventory of Small Mammals in Jaldapara Wildlife Sanctuary, India. Tiger Pap. 2008, 35, 22–28. [Google Scholar]
- Subedi, N.; Lamichhane, B.R.; Amin, R.; Jnawali, S.R.; Jhala, Y.V. Demography and Viability of the Largest Population of Greater One-Horned Rhinoceros in Nepal. Glob. Ecol. Conserv. 2017, 12, 241–252. [Google Scholar] [CrossRef]
- Pant, G.; Maraseni, T.; Apan, A.; Allen, B.L. Predicted Declines in Suitable Habitat for Greater One-Horned Rhinoceros (Rhinoceros unicornis) under Future Climate and Land Use Change Scenarios. Ecol. Evol. 2021, 11, 18288–18304. [Google Scholar] [CrossRef]




| Scenarios | NP | PD | LPI | TE | ED | LSI | AI |
|---|---|---|---|---|---|---|---|
| Present | 415 | 2,014,490 | 0.1116 | 67.808 | 3291.53 | 19.8178 | 82.1292 |
| SSP 126 (2041–2060) | 340 | 1,650,425 | 0.1115 | 55.408 | 2689.61 | 16.8309 | 84.4197 |
| SSP 126 (2061–2080) | 321 | 1,558,196 | 0.0732 | 55.848 | 2710.97 | 17.53 | 83.2506 |
| SSP 245 (2041–2060) | 401 | 1,946,531 | 0.1055 | 69.184 | 3358.32 | 18.029 | 85.6869 |
| SSP 245 (2061–2080) | 229 | 1,111,610 | 0.0615 | 32.176 | 1561.89 | 13.6959 | 82.5469 |
| SSP 585 (2041–2060) | 293 | 1,422,278 | 0.0902 | 46.656 | 2264.77 | 16.1209 | 83.1186 |
| SSP 585 (2061–2080) | 184 | 893,171 | 0.042 | 26.384 | 1280.73 | 12.6061 | 81.8698 |
| Sl. No. | Country | State/Province | Protected Area | Mean Suitability (Present) | SSP126 (2041–2060) | SSP126 (2061–2080) | SSP245 (2041–2060) | SSP245 (2061–2080) | SSP585 (2041–2060) | SSP585 (2061–2080) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nepal | Mahakali Province | ShNP | 0.837 | 0.827 | 0.816 | 0.898 | 0.888 | 0.749 | 0.587 |
| 2 | India | Assam | DSNP | 0.631 | 0.485 | 0.388 | 0.427 | 0.340 | 0.378 | 0.216 |
| 3 | India | Assam | ONP | 0.572 | 0.491 | 0.425 | 0.427 | 0.239 | 0.231 | 0.213 |
| 4 | India | Uttarakhand | CNP | 0.530 | 0.759 | 0.681 | 0.772 | 0.601 | 0.587 | 0.479 |
| 5 | India | Arunachal Pradesh | DMWLS | 0.477 | 0.389 | 0.235 | 0.340 | 0.272 | 0.282 | 0.163 |
| 6 | India | Uttar Pradesh | DNP | 0.464 | 0.478 | 0.496 | 0.634 | 0.252 | 0.344 | 0.209 |
| 7 | India | Assam | KNP | 0.463 | 0.495 | 0.199 | 0.343 | 0.266 | 0.280 | 0.175 |
| 8 | Nepal | Bagmati Province | ChNP | 0.446 | 0.297 | 0.353 | 0.526 | 0.168 | 0.404 | 0.301 |
| 9 | India | Assam | BWLS | 0.437 | 0.407 | 0.351 | 0.349 | 0.190 | 0.191 | 0.170 |
| 10 | India | Uttarakhand | SWLS | 0.423 | 0.726 | 0.593 | 0.646 | 0.565 | 0.603 | 0.399 |
| 11 | Nepal | Lumbini Province | BNP | 0.384 | 0.330 | 0.387 | 0.485 | 0.175 | 0.352 | 0.213 |
| 12 | India | Assam | NNP | 0.376 | 0.336 | 0.171 | 0.201 | 0.077 | 0.123 | 0.091 |
| 13 | India | Assam | LWLS | 0.329 | 0.311 | 0.237 | 0.276 | 0.143 | 0.156 | 0.126 |
| 14 | India | Assam | PDWLS | 0.318 | 0.333 | 0.164 | 0.276 | 0.227 | 0.245 | 0.151 |
| 15 | India | Assam | SRWLS | 0.266 | 0.172 | 0.103 | 0.115 | 0.046 | 0.044 | 0.047 |
| 16 | India | Assam | MTR | 0.245 | 0.133 | 0.077 | 0.096 | 0.064 | 0.079 | 0.029 |
| 17 | India | Bihar | VNP | 0.216 | 0.067 | 0.120 | 0.357 | 0.088 | 0.208 | 0.120 |
| 18 | India | Uttar Pradesh | KgWLS | 0.192 | 0.211 | 0.209 | 0.270 | 0.095 | 0.101 | 0.096 |
| 19 | India | Uttar Pradesh | KWLS | 0.108 | 0.716 | 0.539 | 0.407 | 0.513 | 0.228 | 0.138 |
| 20 | India | Assam | BoRWLS | 0.103 | 0.105 | 0.106 | 0.102 | 0.103 | 0.102 | 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedin, I.; Mukherjee, T.; Kim, A.R.; Kim, H.-W.; Kang, H.-E.; Kundu, S. Distribution Model Reveals Rapid Decline in Habitat Extent for Endangered Hispid Hare: Implications for Wildlife Management and Conservation Planning in Future Climate Change Scenarios. Biology 2024, 13, 198. https://doi.org/10.3390/biology13030198
Abedin I, Mukherjee T, Kim AR, Kim H-W, Kang H-E, Kundu S. Distribution Model Reveals Rapid Decline in Habitat Extent for Endangered Hispid Hare: Implications for Wildlife Management and Conservation Planning in Future Climate Change Scenarios. Biology. 2024; 13(3):198. https://doi.org/10.3390/biology13030198
Chicago/Turabian StyleAbedin, Imon, Tanoy Mukherjee, Ah Ran Kim, Hyun-Woo Kim, Hye-Eun Kang, and Shantanu Kundu. 2024. "Distribution Model Reveals Rapid Decline in Habitat Extent for Endangered Hispid Hare: Implications for Wildlife Management and Conservation Planning in Future Climate Change Scenarios" Biology 13, no. 3: 198. https://doi.org/10.3390/biology13030198
APA StyleAbedin, I., Mukherjee, T., Kim, A. R., Kim, H.-W., Kang, H.-E., & Kundu, S. (2024). Distribution Model Reveals Rapid Decline in Habitat Extent for Endangered Hispid Hare: Implications for Wildlife Management and Conservation Planning in Future Climate Change Scenarios. Biology, 13(3), 198. https://doi.org/10.3390/biology13030198









