Putative Molecular Mechanisms Underpinning the Inverse Roles of Mitochondrial Respiration and Heme Function in Lung Cancer and Alzheimer’s Disease
Simple Summary
Abstract
1. Introduction
2. The Role of Heme and Heme Oxygenase in Cancer and AD
2.1. Ferroptosis in Cancer and AD
2.2. P53, Ferroptosis, and Heme
2.3. Heme Oxygenase in Cancer and AD
3. Common Risk Factors in Cancer and AD
3.1. Aging, Cancer, and AD: Unraveling the Connection
3.2. The Role of Obesity in Cancer and AD
3.2.1. Leptin in Cancer
3.2.2. Leptin in AD
3.2.3. Adiponectin in Cancer
3.2.4. Adiponectin in AD
3.3. Understanding the Link between Diabetes, Cancer, and AD
3.3.1. Diabetes in Lung Cancer
3.3.2. Diabetes in AD
3.4. The Link between Tobacco and the Onset of Cancer and AD
3.4.1. Tobacco in Lung Cancer
3.4.2. Tobacco in AD
4. Common Signaling Pathways in Both Cancer and AD: P53, Wnt, Pin1
4.1. P53 in Lung Cancer
4.2. P53 in AD
4.3. Wnt in Cancer and AD
4.3.1. Wnt in Lung Cancer
4.3.2. Wnt in AD
4.4. Pin1 in Cancer and AD
5. Mitochondria in Cancer and AD
5.1. Mitochondrial Changes in Lung Cancer
5.2. Mitochondrial Dysfunction in AD
5.3. Wnt, P53, Pin1, and Mitochondria
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zabłocka, A.; Kazana, W.; Sochocka, M.; Stańczykiewicz, B.; Janusz, M.; Leszek, J.; Orzechowska, B. Inverse correlation between Alzheimer’s disease and cancer: Short overview. Mol. Neurobiol. 2021, 58, 6335–6349. [Google Scholar] [CrossRef]
- Mogavero, M.P.; Silvani, A.; DelRosso, L.M.; Salemi, M.; Ferri, R. Focus on the complex interconnection between cancer, narcolepsy, and other neurodegenerative diseases: A possible case of orexin-dependent inverse comorbidity. Cancers 2021, 13, 2612. [Google Scholar] [CrossRef]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Seromata, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sanchez-Valle, J.; Tejero, H.; Ibañez, K.; Portero, J.L.; Krallinger, M.; Al-Shahrour, F.; Valencia, A. A molecular hypothesis to explain direct and inverse co-morbidities between Alzheimer’s disease, Glioblastoma and Lung cancer. Sci. Rep. 2017, 7, 4474. [Google Scholar] [CrossRef]
- Ospina-Romero, M.; Abdiwahab, E.; Kobayashi, L.; Filshtein, T.; Brenowitz, W.D.; Mayeda, E.R.; Glymour, M.M. Rate of memory change before and after cancer diagnosis. JAMA Netw. Open 2019, 2, e196160. [Google Scholar] [CrossRef]
- Frain, L.; Swanson, D.; Cho, K.; Gagnon, D.; Lu, K.P.; Betensky, R.A.; Driver, J. Association of cancer and Alzheimer’s disease risk in a national cohort of veterans. Alzheimer’s Dement 2017, 13, 1364–1370. [Google Scholar] [CrossRef]
- Bowles, E.J.A.; Walker, R.L.; Anderson, M.L.; Dublin, S.; Crane, P.K.; Larson, E.B. Risk of Alzheimer’s disease or dementia following a cancer diagnosis. PLoS ONE 2017, 12, e0179857. [Google Scholar] [CrossRef]
- Ganguli, M. Cancer and dementia: It’s complicated. Alzheimer Dis. Assoc. Disord. 2015, 29, 177. [Google Scholar] [CrossRef]
- Seo, J.; Park, M. Molecular crosstalk between cancer and neurodegenerative diseases. Cell Mol. Life Sci. 2020, 77, 2659–2680. [Google Scholar] [CrossRef]
- Ng, L.F.; Kaur, P.; Bunnag, N.; Suresh, J.; Sung, I.C.H.; Tan, Q.H.; Gruber, J.; Tolwinski, N.S. WNT signaling in disease. Cell J. 2019, 8, 826. [Google Scholar] [CrossRef]
- Lamb, R.; Ablett, M.P.; Spence, K.; Landberg, G.; Sims, A.H.; Clarke, R.B. Wnt pathway activity in breast cancer sub-types and stem-like cells. PLoS ONE 2013, 8, e67811. [Google Scholar] [CrossRef]
- Hong, C.-F.; Chen, W.-Y.; Wu, C.-W. Upregulation of Wnt signaling under hypoxia promotes lung cancer progression. Oncol. Rep. 2017, 38, 1706–1714. [Google Scholar] [CrossRef]
- Manandhar, S.; Kabekkodu, S.P.; Pai, K.S.R. Aberrant canonical Wnt signaling: Phytochemical based modulation. Phytomedicine 2020, 76, 153243. [Google Scholar] [CrossRef]
- Driver, J.A. Understanding the link between cancer and neurodegeneration. J. Geriatr. Oncol. 2012, 3, 58–67. [Google Scholar] [CrossRef]
- Schwartz, L.; Peres, S.; Jolicoeur, M.; da Veiga Moreira, J. Cancer and Alzheimer’s disease: Intracellular pH scales the metabolic disorders. Biogerontology 2020, 21, 683–694. [Google Scholar] [CrossRef]
- Valentine, D.; Teerlink, C.C.; Farnham, J.M.; Rowe, K.; Kaddas, H.; Tschanz, J.; Kauwe, J.S.K.; Cannon-Albright, L.A. Comorbidity and Cancer Disease Rates among Those at High-Risk for Alzheimer’s disease: A Population Database Analysis. Int. J. Environ. Res. 2022, 19, 16419. [Google Scholar] [CrossRef]
- Lanni, C.; Masi, M.; Racchi, M.; Govoni, S. Cancer and Alzheimer’s disease inverse relationship: An age-associated diverging derailment of shared pathways. Mol. Psychiatry 2021, 26, 280–295. [Google Scholar] [CrossRef]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The role of immunosenescence in the development of age-related diseases. Rev. Investig. Clin. J. 2016, 68, 84–91. [Google Scholar]
- Edwards, G.A., III; Gamez, N.; Escobedo, G., Jr.; Calderon, O.; Moreno-Gonzalez, I. Modifiable risk factors for Alzheimer’s disease. Front. Aging Neurosci. 2019, 11, 146. [Google Scholar] [CrossRef]
- Harding, J.L.; Andes, L.J.; Gregg, E.W.; Cheng, Y.J.; Weir, H.K.; Bullard, K.M.; Burrows, N.R.; Imperatore, G. Trends in cancer mortality among people with vs without diabetes in the USA, 1988–2015. Diabetologia 2020, 63, 75–84. [Google Scholar] [CrossRef]
- Kimura, N. Diabetes mellitus induces Alzheimer’s disease pathology: Histopathological evidence from animal models. Int. J. Mol. Sci. 2016, 17, 503. [Google Scholar] [CrossRef]
- Potenza, M.A.; Sgarra, L.; Desantis, V.; Nacci, C.; Montagnani, M. Diabetes and Alzheimer’s disease: Might mitochondrial dysfunction help deciphering the common path? Antioxid. Act. 2021, 10, 1257. [Google Scholar] [CrossRef]
- Dikalov, S.; Itani, H.; Richmond, B.; Arslanbaeva, L.; Vergeade, A.; Rahman, S.M.J.; Boutaud, O.; Blackwell, T.; Massion, P.P.; Harrison, D.G.; et al. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H639–H646. [Google Scholar] [CrossRef]
- Kussainova, A.; Bulgakova, O.; Aripova, A.; Khalid, Z.; Bersimbaev, R.; Izzotti, A. The role of mitochondrial miRNAs in the development of radon-induced lung cancer. Biomedicines 2022, 10, 428. [Google Scholar] [CrossRef]
- Afsar, A.; Castro, M.d.C.C.; Soladogun, A.S.; Zhang, L. Recent Development in the Understanding of Molecular and Cellular Mechanisms Underlying the Etiopathogenesis of Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 7258. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Luque-Contreras, D.; Carvajal, K.; Toral-Rios, D.; Franco-Bocanegra, D.; Campos-Peña, V. Oxidative stress and metabolic syndrome: Cause or consequence of Alzheimer’s disease? Oxid. Med. Cell Longev. 2014, 2014, 497802. [Google Scholar] [CrossRef]
- Harris, R.A.; Tindale, L.; Cumming, R.C. Cumming. Age-dependent metabolic dysregulation in cancer and Alzheimer’s disease. Biogerontology 2014, 15, 559–577. [Google Scholar] [CrossRef]
- Sohoni, S.; Ghosh, P.; Wang, T.; Kalainayakan, S.P.; Vidal, C.; Dey, S.; Konduri, P.C.; Zhang, L. Elevated Heme Synthesis and Uptake Underpin Intensified Oxidative Metabolism and Tumorigenic Functions in Non–Small Cell Lung Cancer Cells. Cancer Res. 2019, 79, 2511–2525. [Google Scholar] [CrossRef]
- Ghosh, P.; Vidal, C.; Dey, S.; Zhang, L. Mitochondria targeting as an effective strategy for cancer therapy. Int. J. Mol. Sci. 2020, 21, 3363. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, V.; Chiabrando, D.; Petrillo, S.; Bertino, F.; Tolosano, E. The multifaceted role of heme in cancer. Front. Oncol. 2020, 9, 1540. [Google Scholar] [CrossRef]
- Hooda, J.; Maksudul Alam, M.; Zhang, L. Evaluating the association of heme and heme metabolites with lung cancer bioenergetics and progression. J. Metab. 2015, 5, 1000150. [Google Scholar]
- Hooda, J.; Cadinu, D.; Alam, M.; Shah, A.; Cao, T.M.; Sullivan, L.A.; Brekken, R.; Zhang, L. Enhanced heme function and mitochondrial respiration promote the progression of lung cancer cells. PLoS ONE 2013, 8, e63402. [Google Scholar] [CrossRef]
- Chua, J.J.E. HEBP1-An early trigger for neuronal cell death and circuit dysfunction in Alzheimer’s disease. Semin. Cell Dev. Biol. 2023, 139, 102–110. [Google Scholar] [CrossRef]
- Atamna, H.; Frey, W.H. A role for heme in Alzheimer’s disease: Heme binds amyloid β and has altered metabolism. Proc. Natl. Acad. Sci. USA 2004, 101, 11153–11158. [Google Scholar] [CrossRef]
- Darius, L., Jr.; Ayton, S.; Bush, A.I. Iron and Alzheimer’s disease: An update on emerging mechanisms. J. Alzheimer’s Dis. 2018, 64, S379–S395. [Google Scholar]
- Smith, M.A.; Harris, P.L.R.; Sayre, L.M.; Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Kim, Y.-M. Beneficial and detrimental roles of heme oxygenase-1 in the neurovascular system. Int. J. Mol. Sci. 2022, 23, 7041. [Google Scholar] [CrossRef] [PubMed]
- Xiaodong, W.; Kang, S. Ferroptosis in myocardial infarction: Not a marker but a maker. Open Biol. 2021, 11, 200367. [Google Scholar]
- Ponka, P. Cell biology of heme. Am. J. Med. Sci. 1999, 318, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Joe, Y.; Chen, Y.; Park, G.H.; Kim, U.H.; Chung, H.T. Carbon monoxide attenuates amyloidogenesis via down-regulation of NF-κB-mediated BACE1 gene expression. Aging Cell. 2019, 18, e12864. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Vergallo, A. The β-secretase BACE1 in Alzheimer’s disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Singh, N.; Yao, A.Y.; Zhou, J.; He, W.; Hu, X.; Yan, R. BACE1 controls synaptic function through modulating release of synaptic vesicles. Mol. Psychiatry 2021, 26, 6394–6410. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P. Hemachandra. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp. Neurol. 2009, 218, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H. Heme binding to Amyloid-β peptide: Mechanistic role in Alzheimer’s disease. J. Alzheimer’s Dis. 2006, 10, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Frey, W.H. Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion 2007, 7, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Boyle, K. Amyloid-β peptide binds with heme to form a peroxidase: Relationship to the cytopathologies of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 3381–3386. [Google Scholar] [CrossRef]
- Vidal, C.; Daescu, K.; Fitzgerald, K.E.; Starokadomska, A.; Bezprozvanny, I.; Zhang, L. Amyloid β perturbs elevated heme flux induced with neuronal development. Alzheimers Dement TRCI 2019, 5, 27–37. [Google Scholar] [CrossRef]
- Atamna, H.; Killilea, D.W.; Killilea, A.N.; Ames, B.N. Heme deficiency may be a factor in the mitochondrial and neuronal decay of aging. Proc. Natl. Acad. Sci. USA 2002, 99, 14807–14812. [Google Scholar] [CrossRef]
- Atamna, H.; Liu, J.; Ames, B.N. Heme deficiency selectively interrupts assembly of mitochondrial complex IV in human fibroblasts: Relevance to aging. J. Biol. Chem. 2001, 276, 48410–48416. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell J. 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- dos Santos, A.F.; Fazeli, G.; da Silva, T.N.X.; Angeli, J.P.F. Ferroptosis: Mechanisms and implications for cancer development and therapy response. Trends Cell Biol. 2023, 33, 1062–1076. [Google Scholar] [CrossRef]
- Dixon, S.J.; Pratt, D.A. Ferroptosis: A flexible constellation of related biochemical mechanisms. Mol. Cell 2023, 83, 1030–1042. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell J. 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Deng, W.; Deng, Y.; Liu, Y.; Xiao, S.; Luo, Y.; Xiang, W.; He, Q. Mechanisms of ferroptosis regulating oxidative stress and energy metabolism in myocardial ischemia-reperfusion injury and a novel perspective of natural plant active ingredients for its treatment. Biomed. Pharmacother. 2023, 165, 114706. [Google Scholar] [CrossRef] [PubMed]
- Yagoda, N.; von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Stockwell, B.R. RAS–RAF–MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007, 447, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of mitochondria in ferroptosis. Mol. Cell 2019, 73, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell J. 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Chen, L.; Hambright, W.S.; Na, R.; Ran, Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J. Biol. Chem. 2015, 290, 28097–28106. [Google Scholar] [CrossRef] [PubMed]
- Gascon, S.; Murenu, E.; Masserdotti, G.; Ortega, F.; Russo, G.L.; Petrik, D.; Deshpande, A.; Heinrich, C.; Karow, M.; Robertson, S.P.; et al. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem. Cell 2016, 18, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, X.; Lan, X.; Gao, Y.; Wan, J.; Durham, F.; Cheng, T.; Yang, J.; Wang, Z.; Jiang, C.; et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2017, 2, e90777. [Google Scholar] [CrossRef] [PubMed]
- Vavra, J.; Sergunin, A.; Pompach, P.; Savchenko, D.; Hraniček, J.; Šloufova, I.; Shimizu, T.; Martinkova, M. Characterization of the interaction between the tumour suppressor p53 and heme and its role in the protein conformational dynamics studied by various spectroscopic techniques and hydrogen/deuterium exchange coupled with mass spectrometry. J. Inorg. Biochem. 2023, 243, 112180. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Kon, N.; Chen, D.; Li, T.; Liu, T.; Jiang, L.; Song, S.; Tavana, O.; Gu, W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 2019, 21, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Wang, S.-J.; Li, D.; Ou, Y.; Jiang, L.; Chen, Y.; Zhao, Y.; Gu, W. Acetylation is crucial for p53-mediated ferroptosis and tumor suppression. Cell Rep. 2016, 17, 366–373. [Google Scholar] [CrossRef]
- Shen, J.; Sheng, X.; Chang, Z.; Wu, Q.; Wang, S.; Xuan, Z.; Li, D.; Wu, Y.; Shang, Y.; Kong, X.; et al. Iron metabolism regulates p53 signaling through direct heme-p53 interaction and modulation of p53 localization, stability, and function. Cell Rep. 2014, 7, 180–193. [Google Scholar] [CrossRef]
- Gamage, S.M.K.; Cheng, T.; Lee, K.T.-W.; Dissabandara, L.; Lam, A.K.-Y.; Gopalan, V. Hemin, a major heme molecule, induced cellular and genetic alterations in normal colonic and colon cancer cells. Pathol. Res. Pract. 2021, 224, 153530. [Google Scholar] [CrossRef]
- Shen, J.; Sheng, X.; Chang, Z.; Wu, Q.; Xie, D.; Wang, F.; Hu, R. The heme–p53 interaction: Linking iron metabolism to p53 signaling and tumorigenesis. Mol. Cell Oncol. 2016, 3, e965642. [Google Scholar] [CrossRef][Green Version]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Andrysik, Z.; Sullivan, K.D.; Kieft, J.S.; Espinosa, J.M. PPM1D suppresses p53-dependent transactivation and cell death by inhibiting the Integrated Stress Response. Nat. Commun. 2022, 13, 7400. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fierro, A.; Funes, S.C.; Rios, M.; Covian, C.; Gonzalez, J.; Kalergis, A.M. Immune modulation by inhibitors of the HO system. Int. J. Mol. Sci. 2020, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lü, Y.; Sun, J.Y.; Tang, Y.; Yu, L. Mechanisms of Natural Food Dyes Curcumin on Regulation of HO-1/HO-2 and Inhibition of Aβ-Heme Compound in Alzheimer’s disease. Adv. Mat. Res. 2013, 781, 1148–1151. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Kochert, B.A.; Fleischhacker, A.S.; Wales, T.E.; Becker, D.F.; Engen, J.R.; Ragsdale, S.W. Dynamic and structural differences between heme oxygenase-1 and-2 are due to differences in their C-terminal regions. J. Biol. Chem. 2019, 294, 8259–8272. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Salerno, L.; Ciaffaglione, V.; Leonardi, C.; Fallica, A.N.; Carota, G.; Amata, E.; Marrazzo, A.; Pittala, V.; Romeo, G. Heme Oxygenase-2 (HO-2) as a therapeutic target: Activators and inhibitors. Eur. J. Med. Chem. 2019, 183, 111703. [Google Scholar] [CrossRef]
- Lu, J.-J.; Abudukeyoumu, A.; Zhang, X.; Liu, L.-B.; Li, M.-Q.; Xie, F. Heme oxygenase 1: A novel oncogene in multiple gynecological cancers. Int. J. Biol. Sci. 2021, 17, 2252. [Google Scholar] [CrossRef]
- Dey, S.; Sayers, C.M.; Verginadis, I.I.; Lehman, S.L.; Cheng, Y.; Cerniglia, G.J.; Tuttle, S.W.; Feldman, M.D.; Zhang, P.J.; Fuchs, S.Y.; et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J. Clin. Investig. 2015, 125, 2592–2608. [Google Scholar] [CrossRef]
- Nitti, M.; Piras, S.; Marinari, U.M.; Moretta, L.; Pronzato, M.A.; Furfaro, A.L. HO-1 induction in cancer progression: A matter of cell adaptation. Antioxidants 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Andres, N.C.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Ferro, A.; Donna, L.G.; Curino, A.C.; Facchinetti, M.M. Heme oxygenase-1 has antitumoral effects in colorectal cancer: Involvement of p53. Exp. Mol. Pathol. 2014, 97, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Mascaro, M.; Alonso, E.N.; Alonso, E.G.; Lacunza, E.; Curino, A.C.; Facchinetti, M.M. Nuclear localization of heme oxygenase-1 in pathophysiological conditions: Does it explain the dual role in cancer? Antioxidants 2021, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Toro, A.; Anselmino, N.; Solari, C.; Francia, M.; Oses, C.; Sanchis, P.; Bizzotto, J.; Echegaray, C.V.; Petrone, M.V.; Levi, V.; et al. Novel Interplay between p53 and HO-1 in embryonic stem cells. Cell J. 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Degese, M.S.; Mendizabal, J.E.; Gandini, N.A.; Gutkind, J.S.; Molinolo, A.; Hewitt, S.M.; Curino, A.C.; Coso, O.A.; Facchinetti, M.M. Expression of heme oxygenase-1 in non-small cell lung cancer (NSCLC) and its correlation with clinical data. Lung Cancer 2012, 77, 168–175. [Google Scholar] [CrossRef]
- Hsu, F.-F.; Yeh, C.-T.; Sun, Y.-J.; Chiang, M.-T.; Lan, W.-M.; Li, F.-A.; Lee, W.-H.; Chau, L.-Y. Signal peptide peptidasemediated nuclear localization of heme oxygenase-1 promotes cancer cell proliferation and invasion independent of its enzymatic activity. Oncogene 2015, 34, 2360–2370. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.-F.; Chou, Y.-T.; Chiang, M.-T.; Li, F.-A.; Yeh, C.-T.; Lee, W.-H.; Chau, L.-Y. Signal peptide peptidase promotes tumor progression via facilitating FKBP8 degradation. Oncogene 2019, 38, 1688–1701. [Google Scholar] [CrossRef]
- Hsu, F.-F.; Chiang, M.-T.; Li, F.-A.; Yeh, C.-T.; Lee, W.-H.; Chau, L.-Y. Acetylation is essential for nuclear heme oxygenase-1-enhanced tumor growth and invasiveness. Oncogene 2017, 36, 6805–6814. [Google Scholar] [CrossRef]
- Nemeth, Z.; Csizmadia, E.; Vikstrom, L.; Li, M.; Bisht, K.; Feizi, A.; Otterbein, S.; Zuckerbraun, B.; Costa, D.B.; Pandolfi, P.P.; et al. Alterations of tumor microenvironment by carbon monoxide impedes lung cancer growth. Oncotarget 2016, 7, 23919–23932. [Google Scholar] [CrossRef]
- Chau, L.-Y. Heme oxygenase-1: Emerging target of cancer therapy. J. Biomed. Sci. 2015, 22, 22. [Google Scholar] [CrossRef]
- Shao, L.; Gu, Y.-Y.; Jiang, C.-H.; Liu, C.-Y.; Lv, L.-P.; Liu, J.-N.; Zou, Y. Carbon monoxide releasing molecule-2 suppresses proliferation, migration, invasion, and promotes apoptosis in non-small cell lung cancer Calu-3 cells. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1948–1957. [Google Scholar] [PubMed]
- Krukowska, K.; Magierowski, M. Carbon monoxide (CO)/heme oxygenase (HO)-1 in gastrointestinal tumors pathophysiology and pharmacology-possible anti-and pro-cancer activities. Biochem. Pharmacol. 2022, 201, 115058. [Google Scholar] [CrossRef]
- Spampinato, M.; Sferrazzo, G.; Pittala, V.; Di Rosa, M.; Vanella, L.; Salerno, L.; Sorrenti, V.; Carota, G.; Parrinello, N.; Raffaele, M.; et al. N Non-competitive heme oxygenase-1 activity inhibitor reduces non-small cell lung cancer glutathione content and regulates cell proliferation. Mol. Biol. Rep. 2020, 47, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Tertil, M.; Golda, S.; Ciesla, M.; Weglarczyk, K.; Collet, G.; Guichard, A.; Kozakowska, M.; Boczkowski, J.; Was, H.; et al. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid. Redox Signal 2013, 19, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Podkalicka, P.; Mucha, O.; Jozkowicz, A.; Dulak, J.; Łoboda, A. Heme oxygenase inhibition in cancers: Possible tools and targets. Contemp. Oncol. Wspolczesna Onkol. 2018, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-K.; Chen, S.-E.; Chang, L.-C. The role of HO-1 and its crosstalk with oxidative stress in cancer cell survival. Cell J. 2021, 10, 2401. [Google Scholar] [CrossRef] [PubMed]
- Tertil, M.; Golda, S.; Skrzypek, K.; Florczyk, U.; Weglarczyk, K.; Kotlinowski, J.; Maleszewska, M.; Czauderna, S.; Pichon, C.; Kieda, C.; et al. Nrf2-heme oxygenase-1 axis in mucoepidermoid carcinoma of the lung: Antitumoral effects associated with down-regulation of matrix metalloproteinases. Free Radic. Biol. Med. 2015, 89, 147–157. [Google Scholar] [CrossRef]
- Fahrer, J.; Wittmann, S.; Wolf, A.-C.; Kostka, T. Heme Oxygenase-1 and Its Role in Colorectal Cancer. Antioxidants 2023, 12, 1989. [Google Scholar] [CrossRef]
- Kim, H.; Yin, K.; Falcon, D.M.; Xue, X. The interaction of Hemin and Sestrin2 modulates oxidative stress and colon tumor growth. Toxicol. Appl. Pharmacol. 2019, 374, 77–85. [Google Scholar] [CrossRef]
- Chen, K.-B.; Xuan, Y.; Shi, W.-J.; Chi, F.; Xing, R.; Zeng, Y.-C. Sestrin2 expression is a favorable prognostic factor in patients with non-small cell lung cancer. Am. J. Transl. Res. 2016, 8, 1903. [Google Scholar]
- Chae, H.S.; Gil, M.; Saha, S.K.; Kwak, H.J.; Park, H.-W.; Vellingiri, B.; Cho, S.-G. Sestrin2 expression has regulatory properties and prognostic value in lung cancer. J. Pers. Med. 2020, 10, 109. [Google Scholar] [CrossRef]
- Nitti, M.; Piras, S.; Brondolo, L.; Marinari, U.M.; Pronzato, M.A.; Furfaro, A.L. Heme oxygenase 1 in the nervous system: Does it favor neuronal cell survival or induce neurodegeneration? Int. J. Mol. Sci. 2018, 19, 2260. [Google Scholar] [CrossRef]
- Si, Z.; Wang, X. The neuroprotective and neurodegeneration effects of heme oxygenase-1 in Alzheimer’s disease. J. Alzheimer’s Dis. 2020, 78, 1259–1272. [Google Scholar] [CrossRef]
- Wang, D.; Hui, Y.; Peng, Y.; Tang, L.; Jin, J.; He, R.; Li, Y.; Zhang, S.; Li, L.; Zhou, Y.; et al. Overexpression of heme oxygenase 1 causes cognitive decline and affects pathways for tauopathy in mice. J. Alzheimer’s Dis. 2015, 43, 519–534. [Google Scholar] [CrossRef]
- Hui, Y.; Wang, D.; Li, W.; Zhang, L.; Jin, J.; Ma, N.; Gao, X. Long-term overexpression of heme oxygenase 1 promotes tau aggregation in mouse brain by inducing tauphosphorylation. J. Alzheimer’s Dis. 2011, 26, 299–313. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Hui, Y.; Zhang, S.; Zhou, Y.; Li, D.; Li, J.; Si, Z.; Li, J.; Wang, D.; et al. Overexpression of heme oxygenase 1 impairs cognitive ability and changes the plasticity of the synapse. J. Alzheimer’s Dis. 2015, 47, 595–608. [Google Scholar] [CrossRef]
- Afsar, A.; Chen, M.; Xuan, Z.; Zhang, L. A glance through the effects of CD4+ T cells, CD8+ T cells, and cytokines on Alzheimer’s disease. Comput. Struct. Biotechnol. J. 2023, 21, 5662–5675. [Google Scholar] [CrossRef]
- Jiao, W.; Wang, Y.; Kong, L.; Ou-Yang, T.; Meng, Q.; Fu, Q.; Hu, Z. CART peptide activates the Nrf2/HO-1 antioxidant pathway and protects hippocampal neurons in a rat model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2018, 501, 1016–1022. [Google Scholar] [CrossRef]
- Hettiarachchi, N.; Dallas, M.; Al-Owais, M.; Griffiths, H.; Hooper, N.; Scragg, J.; Boyle, J.; Peers, C. Heme oxygenase-1 protects against Alzheimer’s amyloid-β1-42-induced toxicity via carbon monoxide production. Cell Death Dis. 2014, 5, e1569. [Google Scholar] [CrossRef]
- Hettiarachchi, N.T.; Boyle, J.P.; Dallas, M.L.; Al-Owais, M.M.; Scragg, J.L.; Peers, C. Heme oxygenase-1 derived carbon monoxide suppresses Aβ1–42 toxicity in astrocytes. Cell Death Dis. 2017, 8, e2884. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Pretorius, E.; Bester, J.; Jacobsen, W.K.; Boyle, P.K.; Reinhard, J.P. Carbon monoxide and iron modulate plasmatic coagulation in Alzheimer’s disease. Curr. Neurovasc. Res. 2015, 12, 31–39. [Google Scholar] [CrossRef][Green Version]
- Schipper, H.M.; Song, W.; Tavitian, A.; Cressatti, M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog. Neurobiol. 2019, 172, 40–70. [Google Scholar] [CrossRef]
- Liu, L.; Dumbrepatil, A.B.; Fleischhacker, A.S.; Marsh, E.N.G.; Ragsdale, S.W. Heme oxygenase-2 is post-translationally regulated by heme occupancy in the catalytic site. J. Biol. Chem. 2020, 295, 17227–17240. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; Chen, Q.; Zhang, T.; Zhu, Y.; Xian, X.; Han, X. Heme oxygenase-2 suppresses acute inflammation and improves the survival of skin allografts. Int. Immunopharmacol. 2018, 63, 191–197. [Google Scholar] [CrossRef]
- Jayaraman, A.; Pike, C.J. Alzheimer’s disease and type 2 diabetes: Multiple mechanisms contribute to interactions. Curr. Diab. Rep. 2014, 14, 476. [Google Scholar] [CrossRef]
- Durham, A.; Adcock, I. The relationship between COPD and lung cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef]
- de Jesus, B.B.; Blasco, M.A. Telomerase at the intersection of cancer and aging. Trends Genet. 2013, 29, 513–520. [Google Scholar] [CrossRef]
- Aunan, J.R.; Cho, W.C.; Søreide, K. The biology of aging and cancer: A brief overview of shared and divergent molecular hallmarks. Aging Dis. 2017, 8, 628–642. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Shay, J.W. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef]
- Lex, K.; Gil, M.M.; Lopes-Bastos, B.; Figueira, M.; Marzullo, M.; Giannetti, K.; Carvalho, T.; Ferreira, M.G. Telomere shortening produces an inflammatory environment that increases tumor incidence in zebrafish. Proc. Natl. Acad. Sci. USA 2020, 117, 15066–15074. [Google Scholar] [CrossRef]
- Begus-Nahrmann, Y.; Hartmann, D.; Kraus, J.; Eshraghi, P.; Scheffold, A.; Grieb, M.; Rasche, V.; Schirmacher, P.; Lee, H.-W.; Kestler, H.A.; et al. Transient telomere dysfunction induces chromosomal instability and promotes carcinogenesis. J. Clin. Investig. 2012, 122, 2283–2288. [Google Scholar] [CrossRef]
- Teng, Y.; Huang, D.Q.; Li, R.X.; Yi, C.; Zhan, Y.Q. Association Between Telomere Length and Risk of Lung Cancer in an Asian Population: A Mendelian Randomization Study. World J. Oncol. 2023, 14, 277–284. [Google Scholar] [CrossRef]
- Asaithamby, A.; Shay, J.W.; Minna, J.D. Cellular senescence and lung cancer prognosis. Transl. Lung Cancer Res. 2022, 11, 1982–1987. [Google Scholar] [CrossRef]
- Zhu, Y.I.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Prata, L.G.L.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018, 40, 101275. [Google Scholar] [CrossRef]
- Song, P.; An, J.; Zou, M.-H. Immune clearance of senescent cells to combat ageing and chronic diseases. Cell J. 2020, 9, 671. [Google Scholar] [CrossRef]
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.-Y.; Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef]
- Prasanna, P.G.; E Citrin, D.; Hildesheim, J.; Ahmed, M.M.; Venkatachalam, S.; Riscuta, G.; Xi, D.; Zheng, G.; van Deursen, J.; Goronzy, J.; et al. Therapy-induced senescence: Opportunities to improve anticancer therapy. J. Natl. Cancer Inst. 2021, 113, 1285–1298. [Google Scholar] [CrossRef]
- Beck, J.; Turnquist, C.; Horikawa, I.; Harris, C.C. Targeting cellular senescence in cancer and aging: Roles of p53 and its isoforms. Carcinogenesis 2020, 41, 1017–1029. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Grimm, A.; Friedland, K.; Eckert, A. Mitochondrial dysfunction: The missing link between aging and sporadic Alzheimer’s disease. Biogerontology 2016, 17, 281–296. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction, and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Kullmann, S.; Heni, M.; Hallschmid, M.; Fritsche, A.; Preissl, H.; Häring, H.-U. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol. Rev. 2016, 96, 1169–1209. [Google Scholar] [CrossRef]
- Frölich, L.; Blum-Degen, D.; Bernstein, H.-G.; Engelsberger, S.; Humrich, J.; Laufer, S.; Muschner, D.; Thalheimer, A.; Türk, A.; Hoyer, S.; et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J. Neural Transm. 1998, 105, 423–438. [Google Scholar] [CrossRef]
- Akintola, A.A.; Berg, A.v.D.; Altmann-Schneider, I.; Jansen, S.W.; van Buchem, M.A.; Slagboom, P.E.; Westendorp, R.G.; van Heemst, D.; van der Grond, J. Parameters of glucose metabolism and the aging brain: A magnetization transfer imaging study of brain macro-and micro-structure in older adults without diabetes. Age 2015, 37, 9802. [Google Scholar] [CrossRef]
- Akintola, A.A.; van Opstal, A.M.; Westendorp, R.G.; Postmus, I.; van der Grond, J.; van Heemst, D. Effect of intranasally administered insulin on cerebral blood flow and perfusion; a randomized experiment in young and older adults. Aging 2017, 9, 790–802. [Google Scholar] [CrossRef]
- Akintola, A.A.; Berg, A.v.D.; van Buchem, M.A.; Jansen, S.W.; Slagboom, E.P.; Westendorp, R.G.; van der Grond, J.; van Heemst, D. Associations between insulin action and integrity of brain microstructure differ with familial longevity and with age. Front. Aging Neurosci. 2015, 7, 92. [Google Scholar] [CrossRef]
- Nixon, D.W. The inverse relationship between cancer and Alzheimer’s disease: A possible mechanism. Curr. Alzheimer Res. 2017, 14, 883–893. [Google Scholar] [CrossRef]
- Bonda, D.J.; Stone, J.G.; Torres, S.L.; Siedlak, S.L.; Perry, G.; Kryscio, R.; Jicha, G.; Casadesus, G.; Smith, M.A.; Zhu, X.; et al. Dysregulation of leptin signaling in Alzheimer disease: Evidence for neuronal leptin resistance. J. Neurochem. 2014, 128, 162–172. [Google Scholar] [CrossRef]
- Fan, X.; Yuan, W.; Huang, W.; Lin, Z. Recent progress in leptin signaling from a structural perspective and its implications for diseases. Biochimie 2023, 212, 60–75. [Google Scholar] [CrossRef]
- Pan, H.; Deng, L.L.; Cui, J.Q.; Shi, L.; Yang, Y.C.; Luo, J.H.; Wang, L. Association between serum leptin levels and breast cancer risk: An updated systematic review and meta-analysis. Medicine 2018, 97, e11345. [Google Scholar] [CrossRef]
- Jutant, E.-M.; Tu, L.; Humbert, M.; Guignabert, C.; Huertas, A. The thousand faces of leptin in the lung. Chest 2021, 159, 239–248. [Google Scholar] [CrossRef]
- Yuan, Q.-H.; Zhang, L.-L.; Xu, Y.; Chen, X.; Zhang, B.; Li, L.-X.; Li, S.; Shang, D. Circulating leptin and adiponectin levels in patients with pancreatic cancer. Chin. Med. J. 2021, 134, 2134–2136. [Google Scholar] [CrossRef]
- Song, C.-H.; Liao, J.; Deng, Z.-H.; Zhang, J.-Y.; Xue, H.; Li, Y.-M.; Liang, C.; Han, M.; Zhang, K.; Yan, G.-T. Is leptin a predictive factor in patients with lung cancer? Clin. Biochem. 2014, 47, 230–232. [Google Scholar] [CrossRef]
- Karatas, F.; Yalcin, B.; Sahin, S.; Akbulut, H.; Utkan, G.; Demirkazik, A.; Icli, F. The significance of serum leptin level in patients with early stage nonsmall cell lung cancer. J. Canc. Res. Ther. 2017, 13, 204–207. [Google Scholar] [CrossRef]
- Terzidis, A.; Sergentanis, T.N.; Antonopoulos, G.; Syrigos, C.; Efremidis, A.; Polyzos, A.; Dessypris, N.; Petridou, E.T. Petridou. Elevated serum leptin levels: A risk factor for non-small-cell lung cancer? Oncology 2008, 76, 19–25. [Google Scholar] [CrossRef]
- Du, J.; Han, J.; Zhang, Y.; Qi, G.; Li, H. Relationship between serum leptin levels and non-small cell lung carcinoma: A meta-analysis. Genet. Mol. Res. 2015, 14, 13699–13708. [Google Scholar] [CrossRef]
- Kerenidi, T.; Lada, M.; Tsaroucha, A.; Georgoulias, P.; Mystridou, P.; Gourgoulianis, K.I. Gourgoulianis. Clinical significance of serum adipokines levels in lung cancer. Med. Oncol. 2013, 30, 507. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Zhou, Q.; He, J.; Peng, B.; Liu, S.; Yan, Z.; Yang, X.; Fan, H. Serum and tissue leptin in lung cancer: A meta-analysis. Oncotarget 2017, 8, 19699. [Google Scholar] [CrossRef]
- Gulen, S.T.; Karadag, F.; Karul, A.B.; Kilicarslan, N.; Ceylan, E.; Kuman, N.K.; Cildag, O. Adipokines and systemic inflammation in weight-losing lung cancer patients. Lung 2012, 190, 327–332. [Google Scholar] [CrossRef]
- Jimenez-Cortegana, C.; Lopez-Saavedra, A.; Sanchez-Jimenez, F.; Perez-Perez, A.; Castiñeiras, J.; Virizuela-Echaburu, J.A.; de la de la Cruz-Merino, L.; Sanchez-Margalet, V. Leptin, both bad and good actor in cancer. Biomolecules 2021, 11, 913. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernandez, C.; Mera, A.; Gonzalez-Gay, M.A.; Gomez, R.; Gualillo, O. Obesity, fat mass, and immune system: Role for leptin. Front Physiol. 2018, 9, 640. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Figueiredo, I.P.; Afsar, A.; Burroughs, N.J.; Pinto, A.A.; Oliveira, B.M. The effect of a linear tuning between the antigenic stimulations of CD4+ T cells and CD4+ Tregs. Mathematics 2020, 8, 293. [Google Scholar] [CrossRef]
- Afsar, A.; Martins, F.; Oliveira, B.M.P.M.; Pinto, A.A. Immune response model fitting to CD4+ T Cell data in lymphocytic choriomeningitis virus LCMV infection. In Modeling, Dynamics, Optimization and Bioeconomics, 4th ed.; Springer Proceedings in Mathematics & Statistics: Berlin/Heidelberg, Germany, 2017; Volume 365, pp. 1–10. [Google Scholar]
- Afsar, A.; Martins, F.; Oliveira, B.M.P.M.; Pinto, A.A. A fit of CD4+ T cell immune response to an infection by lymphocytic choriomeningitis virus. Math. Biosci. Eng. 2019, 16, 7009–7021. [Google Scholar] [CrossRef]
- Afsar, A. Applications of Game Theory and Dynamical Systems to Biology and Economy. Ph.D. Thesis, University of Porto, Porto, Portugal, 2022. [Google Scholar]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martin-Romero, C.; Santos-Alvarez, J.; Goberna, R.; Sanchez-Margalet, V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell. Immunol. 2000, 199, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.Y.; Yeh, H.; Chu, C.S.; Schlienger, K.; Carroll, R.G.; Riley, J.L.; Kaiser, L.R.; June, C.H. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J. Immunol. 2002, 168, 4272–4276. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, G.; Huang, D.; Sui, M.; Xu, Y. Cancer immunotherapy based on natural killer cells: Current progress and new opportunities. Front. Immunol. 2019, 10, 1205. [Google Scholar] [CrossRef]
- Forny-Germano, L.; De Felice, F.G.; Vieira, M.N.D.N. The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer’s disease. Front. Neurosci. 2019, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Bik, A.; Bik, W.; Styczynska, M.; Chodakowska-Zebrowska, M.; Barcikowska, M.; Wolinska-Witort, E.; Kalisz, M.; Martynska, L.; Baranowska, B. Plasma leptin levels and free leptin index in women with Alzheimer’s disease. Neuropeptides 2015, 52, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Khemka, V.K.; Bagchi, D.; Bandyopadhyay, K.; Bir, A.; Chattopadhyay, M.; Biswas, A.; Basu, D.; Chakrabarti, S. Altered serum levels of adipokines and insulin in probable Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 41, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Tian, S.; Huang, R.; Cai, R.; Guo, D.; Lin, H.; Wang, J.; Wang, S. Low plasma leptin and high soluble leptin receptor levels are associated with mild cognitive impairment in type 2 diabetic patients. Front. Aging Neurosci. 2018, 10, 132. [Google Scholar] [CrossRef]
- Maioli, S.; Lodeiro, M.; Merino-Serrais, P.; Falahati, F.; Khan, W.; Puerta, E.; Codita, A.; Rimondini, R.; Ramirez, M.J.; Simmons, A.; et al. Alterations in brain leptin signalling in spite of unchanged CSF leptin levels in Alzheimer’s disease. Aging Cell 2015, 14, 122–129. [Google Scholar] [CrossRef]
- McGuire, M.J.; Makoto, I. Leptin dysfunction and Alzheimer’s disease: Evidence from cellular, animal, and human studies. Cell. Mol. Neurobiol. 2016, 36, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Cecon, E.; Lhomme, T.; Maurice, T.; Luka, M.; Chen, M.; Silva, A.; Wauman, J.; Zabeau, L.; Tavernier, J.; Prevot, V.; et al. Amyloid beta peptide is an endogenous negative allosteric modulator of leptin receptor. Neuroendocrinology 2021, 111, 370–387. [Google Scholar] [CrossRef] [PubMed]
- Ulker, M.; Kenangil, G. The relation of circulating levels of leptin with cognition in patients with Alzheimer’s disease. Noro Psikiyatr Ars. 2018, 55, 211–214. [Google Scholar] [CrossRef]
- Flores-Cordero, J.A.; Perez-Perez, A.; Jimenez-Cortegana, C.; Alba, G.; Flores-Barragan, A.; Sanchez-Margalet, V. Obesity as a risk factor for dementia and Alzheimer’s disease: The role of leptin. Int. J. Mol. Sci. 2022, 23, 5202. [Google Scholar] [CrossRef]
- Irving, A.J.; Harvey, J. Leptin regulation of hippocampal synaptic function in health and disease. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130155. [Google Scholar] [CrossRef]
- Mejido, D.C.; Peny, J.A.; Vieira, M.N.; Ferreira, S.T.; De Felice, F.G. Insulin and leptin as potential cognitive enhancers in metabolic disorders and Alzheimer’s disease. Neuropharmacology 2020, 171, 108115. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.J.; Hamzelou, A.; Johnston, J.M.; Smith, M.A.; Ashford, J.W.; Tezapsidis, N. Leptin boosts cellular metabolism by activating AMPK and the sirtuins to reduce tau phosphorylation and β-amyloid in neurons. Biochem. Biophys. Res. Commun. 2011, 414, 170–174. [Google Scholar] [CrossRef]
- Shah, S.A.; Yoon, G.H.; Chung, S.S.; Abid, M.N.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer’s disease neuropathological deficits. Mol. Psychiatry 2017, 22, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Oomura, Y.; Hori, N.; Shiraishi, T.; Fukunaga, K.; Takeda, H.; Tsuji, M.; Matsumiya, T.; Ishibashi, M.; Aou, S.; Li, X.; et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides 2006, 27, 2738–2749. [Google Scholar] [CrossRef]
- Gilbert, T.; Roche, S.; Blond, E.; Bar, J.-Y.; Drai, J.; Cuerq, C.; Haution-Bitker, M.; Ecochard, R.; Bonnefoy, M. Association between peripheral leptin and adiponectin levels and cognitive decline in patients with neurocognitive disorders ≥ 65 years. J. Alzheimer’s Dis. 2018, 66, 1255–1264. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ha, J.; Kim, M.; Cho, S.Y.; Kim, H.; Kim, E. Plasma adiponectin levels predict cognitive decline and cortical thinning in mild cognitive impairment with beta-amyloid pathology. Alzheimer’s Res. Ther. 2022, 14, 165. [Google Scholar] [CrossRef]
- Nigro, E.; Stiuso, P.; Matera, M.; Monaco, M.; Caraglia, M.; Maniscalco, M.; Perrotta, F.; Mazzarella, G.; Daniele, A.; Bianco, A. The anti-proliferative effects of adiponectin on human lung adenocarcinoma A549 cells and oxidative stress involvement. Pulm. Pharmacol. Ther. 2019, 55, 25–30. [Google Scholar] [CrossRef]
- Tsai, J.-R.; Liu, P.-L.; Chen, Y.-H.; Chou, S.-H.; Cheng, Y.-J.; Hwang, J.-J.; Chong, I.-W. Curcumin inhibits non-small cell lung cancer cells metastasis through the adiponectin/NF-κb/MMPs signaling pathway. PLoS ONE 2015, 10, e0144462. [Google Scholar] [CrossRef] [PubMed]
- Barb, D.; Pazaitou-Panayiotou, K.; Mantzoros, C.S. Adiponectin: A link between obesity and cancer. Expert. Opin. Investig. Drugs 2006, 15, 917–931. [Google Scholar] [CrossRef]
- Katira, A.; Peng, H.T. Evolving role of adiponectin in cancer-controversies and update. Cancer Biol. Med. 2016, 13, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, E.; Polito, R.; Bartollino, S.; Nigro, E.; Porcile, C.; Bianco, A.; Daniele, A.; Moncharmont, B. Adiponectin as link factor between adipose tissue and cancer. Int. J. Mol. Sci. 2019, 20, 839. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.T.; Mitsiades, N.; Gialamas, S.; Angelopoulos, M.; Skalkidou, A.; Dessypris, N.; Hsi, A.; Lazaris, N.; Polyzos, A.; Syrigos, C.; et al. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: Two case-control studies. Oncology 2008, 73, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Perrotta, F.; Monaco, M.L.; Polito, R.; Pafundi, P.C.; Matera, M.G.; Daniele, A.; Bianco, A. Implications of the adiponectin system in non-Small cell lung cancer patients: A case-control study. Biomolecules 2020, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Xu, Y.; Niu, W. Causal relevance of circulating adiponectin with cancer: A meta-analysis implementing Mendelian randomization. Tumour. Biol. 2015, 36, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Guo, H.; Shen, M.; Yu, H.; Gu, D.; Mao, W.; Wang, X. Adiponectin inhibits migration and invasion by reversing epithelial-mesenchymal transition in non-small cell lung carcinoma. Oncol. Rep. 2018, 40, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Veyssiere, J.; Gandin, C.; Zsürger, N.; Pietri, M.; Heurteaux, C.; Glaichenhaus, N.; Petit-Paitel, A.; Chabry, J. Neurogenesis-independent antidepressant-like effects of enriched environment is dependent on adiponectin. Psychoneuroendocrinology 2015, 57, 72–83. [Google Scholar] [CrossRef]
- Nicolas, S.; Cazareth, J.; Zarif, H.; Guyon, A.; Heurteaux, C.; Chabry, J.; Petit-Paitel, A. Globular adiponectin limits microglia pro-inflammatory phenotype through an AdipoR1/NF-κB signaling pathway. Front. Cell Neurosc. 2017, 11, 352. [Google Scholar] [CrossRef]
- Waragai, M.; Adame, A.; Trinh, I.; Sekiyama, K.; Takamatsu, Y.; Une, K.; Hashimoto, M. Possible involvement of adiponectin, the anti-diabetes molecule, in the pathogenesis of Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 52, 1453–1459. [Google Scholar] [CrossRef]
- Letra, L.; Matafome, P.; Rodrigues, T.; Duro, D.; Lemos, R.; Baldeiras, I.; Patricio, M.; Castelo-Branco, M.; Caetano, G.; Seiça, R.; et al. Association between adipokines and biomarkers of Alzheimer’s disease: A cross-sectional study. J. Alzheimer’s Dis. 2019, 67, 725–735. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Diniz, B.S.; Campos, A.C.; Miranda, A.S.; Rocha, N.P.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Decreased levels of circulating adiponectin in mild cognitive impairment and Alzheimer’s disease. Neuromol. Med. 2013, 15, 115–121. [Google Scholar] [CrossRef]
- Kitagawa, K.; Miwa, K.; Okazaki, S.; Sakaguchi, M.; Mochizuki, H. Serum high-molecular-weight adiponectin level and incident dementia in patients with vascular risk factors. Eur. J. Neurol. 2016, 23, 641–647. [Google Scholar] [CrossRef]
- van Andel, M.; van Schoor, N.M.; Korten, N.C.; Comijs, H.C.; Heijboer, A.C.; Drent, M.L. The association between high-molecular-weight adiponectin, ghrelin and leptin and age-related cognitive decline: Results from longitudinal aging study Amsterdam. J. Gerontol 2021, 76, 131–140. [Google Scholar] [CrossRef]
- Chabry, J.; Nicolas, S.; Cazareth, J.; Murris, E.; Guyon, A.; Glaichenhaus, N.; Heurteaux, C.; Petit-Paitel, A. Enriched environment decreases microglia and brain macrophages inflammatory phenotypes through adiponectin-dependent mechanisms: Relevance to depressive-like behavior. Brain Behav. Immun. 2015, 50, 275–287. [Google Scholar] [CrossRef]
- Ng, R.C.-L.; Cheng, O.-Y.; Jian, M.; Kwan, J.S.-C.; Ho, P.W.-L.; Cheng, K.K.-Y.; Yeung, P.K.K.; Zhou, L.L.; Hoo, R.L.-C.; Chung, S.K.; et al. Chronic adiponectin deficiency leads to Alzheimer’s disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Mol. Neurodegener. 2016, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.-L.; Chan, K.-H. Potential neuroprotective effects of adiponectin in Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 592. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Barua, S.; Jeong, Y.J.; Lee, J.E. Adiponectin: The potential regulator and therapeutic target of obesity and Alzheimer’s disease. Int. J. Mol. Sci. 2021, 21, 6419. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; bin Abid, N.; Jo, M.H.; Jo, M.G.; Yoon, G.H.; Kim, M.O. Suppression of adiponectin receptor 1 promotes memory dysfunction and Alzheimer’s disease-like pathologies. Sci. Rep. 2017, 7, 12435. [Google Scholar] [CrossRef] [PubMed]
- Samant, N.P.; Gupta, G.L. Adiponectin: A potential target for obesity-associated Alzheimer’s disease. Metab. Brain Dis. 2021, 36, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hu, Y.; Zhao, Y.; Xie, S.; Wang, C. Impact of Type 2 Diabetes Mellitus on the Prognosis of Non-Small Cell Lung Cancer. J. Clin. Med. 2022, 12, 321. [Google Scholar] [CrossRef]
- Ding, J.; Li, X.; Ge, J.; Gong, Y.; Zhou, Y.; Xiao, J.; Yang, Q.; Chen, J.; Mao, M. Survival Risk Analysis of Small Cell Lung Cancer Patients with Pre-Existing Type 2 Diabetes Mellitus: A Single-Center Retrospective Cohort Study. Cancer Manag. Res. 2022, 14, 1313–1322. [Google Scholar] [CrossRef]
- Kurishima, K.; Watanabe, H.; Ishikawa, H.; Satoh, H.; Hizawa, N. Survival of patients with lung cancer and diabetes mellitus. Mol. Clin. Oncol. 2017, 6, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.C.; Georgescu, D.; Mireștean, C.C.; Badulescu, F. Cancer and Diabetes: Predictive Factors in Patients with Metabolic Syndrome. Diagnostics 2013, 13, 2647. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hendryx, M.; Qi, L.; Ho, G.Y.; Margolis, K.L. Margolis. Pre-existing diabetes and lung cancer prognosis. Br. J. Cancer 2016, 115, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Luther, Y.; Xiong, G.; Ni, Y.; Yun, F.; Chen, J.; Yang, Z.; Zhang, Q.; Kuang, Y.; Zhu, Y. Association between diabetes mellitus and lung cancer: Meta-analysis. Eur. J. Clin. Investig. 2020, 50, e13332. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Chen, X.; Cai, X.; Chen, Y.; Wang, H.; Fan, L.; Bai, L.; Qiu, H.; Zhang, B. Inflammation-based markers can predict the prognosis of geriatric patients with metastatic colorectal cancer receiving first-line chemotherapy. Transl. Cancer Res. 2019, 8, 1137. [Google Scholar] [CrossRef] [PubMed]
- van de Poll-Franse, L.V.; Houterman, S.; Janssen-Heijnen, M.L.; Dercksen, M.W.; Coebergh, J.W.W.; Haak, H.R. Less aggressive treatment and worse overall survival in cancer patients with diabetes: A large population based analysis. Int. J. Cancer 2007, 120, 1986–1992. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D.; Chen, T.-S.; Liou, S.-Y.; Kuo, C.-H.; Pan, L.-F.; Yeh, Y.-L.; Liou, J.; Padma, V.V.; Yao, C.-H.; et al. Obesity and diabetes: The increased risk of cancer and cancer-related mortality. Physiol. Rev. 2015, 95, 727–748. [Google Scholar] [CrossRef]
- Leiter, A.; Charokopos, A.; Bailey, S.; Gallagher, E.J.; Hirsch, F.R.; LeRoith, D.; Wisnivesky, J.P. Assessing the association of diabetes with lung cancer risk. Transl. Lung Cancer Res. 2021, 10, 4200. [Google Scholar] [CrossRef]
- Liao, Y.F.; Yin, S.; Chen, Z.Q.; Li, F.; Zhao, B. High glucose promotes tumor cell proliferation and migration in lung adenocarcinoma via the RAGE-NOXs pathway Corrigendum in/10.3892/mmr. 2018.9555. Mol. Med. Rep. 2018, 17, 8536–8541. [Google Scholar]
- Xiao, K.; Liu, F.; Liu, J.; Xu, J.; Wu, Q.; Li, X. The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: A meta-analysis. J. Clin. Pharm. Ther. 2020, 45, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jansåker, F.; Sundquist, J.; Crump, C.; Hamano, T.; Sundquist, K. Neighborhood deprivation in relation to lung cancer in individuals with type 2 diabetes—A nationwide cohort study (2005–2018). PLoS ONE 2023, 18, e0288959. [Google Scholar] [CrossRef] [PubMed]
- Daugan, M.; Wojcicki, A.D.; D’hayer, B.; Boudy, V. Metformin: An anti-diabetic drug to fight cancer. Pharmacol. Res. 2016, 113, 675–685. [Google Scholar] [CrossRef]
- Kim, T.H.; Suh, D.H.; Kim, M.-K.; Song, Y.S. Metformin against cancer stem cells through the modulation of energy metabolism: Special considerations on ovarian cancer. Biomed. Res. Int. 2014, 2014, 132702. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Prisco, M.; Ertel, A.; Tsirigos, A.; Lin, Z.; Pavlides, S.; Lisanti, M.P. Ketones and lactate increase cancer cell “stemness”, driving recurrence, metastasis and poor clinical outcome in breast cancer: Achieving personalized medicine via Metabolo-Genomics. Cell Cycle 2011, 10, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Pasto, A.; Bellio, C.; Pilotto, G.; Ciminale, V.; Silic-Benussi, M.; Guzzo, G.; Rasola, A.; Frasson, C.; Nardo, G.; Zulato, E.; et al. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget 2014, 5, 4305. [Google Scholar] [CrossRef]
- Mayer, M.J.; Klotz, L.H.; Venkateswaran, V. Metformin and prostate cancer stem cells: A novel therapeutic target. Prostate Cancer Prostatic Dis. 2015, 18, 303–309. [Google Scholar] [CrossRef]
- Bedi, M.; Ray, M.; Ghosh, A. Active mitochondrial respiration in cancer: A target for the drug. Mol. Cell. Biochem. 2022, 477, 345–361. [Google Scholar] [CrossRef]
- Zendehdel, K.; Nyren, O.; Östenson, C.-G.; Adami, H.-O.; Ekbom, A.; Ye, W. Cancer incidence in patients with type 1 diabetes mellitus: A population-based cohort study in Sweden. J. Natl. Cancer Inst. 2003, 95, 1797–1800. [Google Scholar] [CrossRef]
- Szablewski, L. Diabetes mellitus: Influences on cancer risk. Diabetes Metab. Res. Rev. 2014, 30, 543–553. [Google Scholar] [CrossRef]
- Nelson, A.R.; Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim. Biophys. Acta 2016, 1862, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Frittitta, L. Type 2 diabetes mellitus and Alzheimer’s disease: Role of insulin signalling and therapeutic implications. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 19, 3306. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Peters, S.A.E.; Woodward, M.; Arango, S.M.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.N.; et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Hamze, R.; Delangre, E.; Tolu, S.; Moreau, M.; Janel, N.; Bailbe, D.; Movassat, J. Type 2 diabetes mellitus and Alzheimer’s disease: Shared molecular mechanisms and potential common therapeutic targets. Int. J. Mol. Sci. 2022, 23, 15287. [Google Scholar] [CrossRef]
- Stanley, M.; Macauley, S.L.; Holtzman, D.M. Changes in insulin and insulin signaling in Alzheimer’s disease: Cause or consequence? J. Exp. Med. 2016, 213, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, A.; Bik, W.; Kalisz, M.; Baranowska-Bik, A. Inflammasome NLRP3 potentially links obesity-associated low-grade systemic inflammation and insulin resistance with Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 5603. [Google Scholar] [CrossRef]
- Gray, S.M.; Aylor, K.W.; Barrett, E.J. Unravelling the regulation of insulin transport across the brain endothelial cell. Diabetologia 2017, 60, 1512–1521. [Google Scholar] [CrossRef]
- Heni, M.; Schöpfer, P.; Peter, A.; Sartorius, T.; Fritsche, A.; Synofzik, M.; Häring, H.-U.; Maetzler, W.; Hennige, A.M. Evidence for altered transport of insulin across the blood–brain barrier in insulin-resistant humans. Acta Diabetol. 2014, 51, 679–681. [Google Scholar] [CrossRef]
- Sartorius, T.; Peter, A.; Heni, M.; Maetzler, W.; Fritsche, A.; Häring, H.-U.; Hennige, A.M. The brain response to peripheral insulin declines with age: A contribution of the blood-brain barrier? PLoS ONE 2015, 10, e0126804. [Google Scholar] [CrossRef]
- Suzanne, M.d.l.M. Therapeutic targets of brain insulin resistance in sporadic Alzheimer’s disease. Front. Biosci. 2012, 4, 1582–1605. [Google Scholar]
- Kellar, D.; Craft, S. Brain insulin resistance in Alzheimer’s disease and related disorders: Mechanisms and therapeutic approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and insulin resistance in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef]
- Claxton, A.; Baker, L.D.; Hanson, A.; Trittschuh, E.H.; Cholerton, B.; Morgan, A.; Callaghan, M.; Arbuckle, M.; Behl, C.; Craft, S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J. Alzheimer’s Dis. 2015, 44, 897–906. [Google Scholar] [CrossRef]
- Reger, M.A.; Watson, G.S.; Green, P.S.; Wilkinson, C.W.; Baker, L.D.; Cholerton, B.; Fishel, M.A.; Plymate, S.R.; Breitner, J.; DeGroodt, W.; et al. Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology 2008, 70, 440–448. [Google Scholar] [CrossRef]
- Rahman, A.; Effat, J. Molecules associated with the development of lung cancer. Cell Adh Migr. 2023, 4, 130–145. [Google Scholar]
- Astori, E.; Garavaglia, M.L.; Colombo, G.; Landoni, L.; Portinaro, N.M.; Milzani, A.; Dalle-Donne, I. Antioxidants in smokers. Nutr. Res. Rev. 2022, 35, 70–97. [Google Scholar] [CrossRef]
- Kim, A.-S.; Ko, H.-J.; Kwon, J.-H.; Lee, J.-M. Exposure to secondhand smoke and risk of cancer in never smokers: A meta-analysis of epidemiologic studies. Int. J. Environ. Res. Public Health 2018, 15, 1981. [Google Scholar] [CrossRef]
- Scheffler, S.; Dieken, H.; Krischenowski, O.; Förster, C.; Branscheid, D.; Aufderheide, M. Evaluation of E-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int. J. Environ. Res. Public Health 2015, 12, 3915–3925. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.W.; Kang, H.S.; Park, C.K.; Kim, S.K.; Kim, J.S.; Kim, J.W.; Kim, S.J.; Lee, S.H.; Yeo, C.D. Regional emphysema score is associated with tumor location and poor prognosis in completely resected NSCLC patients. BMC Pulm. Med. 2020, 20, 242. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health. 2009, 6, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Halvorsen, A.R.; Bengtson, M.-B.; Tasken, K.A.; Mælandsmo, G.M.; Yndestad, A.; Halvorsen, B.; Brustugun, O.T.; Aukrust, P.; Ueland, T.; et al. Levels and prognostic impact of circulating markers of inflammation, endothelial activation, and extracellular matrix remodelling in patients with lung cancer and chronic obstructive pulmonary disease. BMC Cancer 2018, 18, 739. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Salvato, I.; Ricciardi, L.; Nucera, F.; Nigro, A.; Col, J.D.; Monaco, F.; Caramori, G.; Stellato, C. RNA-Binding Proteins as a Molecular Link between COPD and Lung Cancer. COPD J. Chronic Obstr. Pulm. Dis. 2023, 20, 18–30. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory endotypes in COPD. Allergy 2019, 74, 1249–1256. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Birch, J.; Anderson, R.K.; Correia-Melo, C.; Jurk, D.; Hewitt, G.; Marques, F.M.; Green, N.J.; Moisey, E.; Birrell, M.A.; Belvisi, M.G.; et al. DNA damage response at telomeres contributes to lung aging and chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1124–L1137. [Google Scholar] [CrossRef]
- Kaluza, J.; Larsson, S.C.; Orsini, N.; Linden, A.; Wolk, A. Fruit and vegetable consumption and risk of COPD: A prospective cohort study of men. Thorax 2017, 72, 500–509. [Google Scholar] [CrossRef]
- Middha, P.; Weinstein, S.J.; Männistö, S.; Albanes, D.; Mondul, A.M. β-carotene supplementation and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: The role of tar and nicotine. Nicotine Tob. Res. J. 2019, 21, 1045–1050. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Skavicus, S.; Card, J.; Stadler, A.; Levin, E.D.; Seidler, F.J. Developmental neurotoxicity of tobacco smoke directed toward cholinergic and serotonergic systems: More than just nicotine. Toxicol. Sci. 2015, 147, 178–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waisman Campos, M.; Serebrisky, D.; Mauricio Castaldelli-Maia, J. Smoking and cognition. Curr. Drug Abuse. Rev. 2016, 9, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Wang, J.; Xue, Q.; Yang, X.; Kang, Y.; Li, M.; Xu, J.; Li, G.; Li, C.; et al. Association of cigarette smoking with cerebrospinal fluid biomarkers of neurodegeneration, neuroinflammation, and oxidation. JAMA Netw. Open 2020, 3, e2018777. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.C.; Mattsson, N.; Weiner, M.W. Alzheimer’s disease Neuroimaging Initiative. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimer’s Dement 2014, 10, S122–S145. [Google Scholar] [CrossRef]
- Cho, H.; Kim, C.; Kim, H.J.; Ye, B.S.; Kim, Y.J.; Jung, N.; Son, T.O.; Cho, E.B.; Jang, H.; Kang, M.; et al. Impact of smoking on neurodegeneration and cerebrovascular disease markers in cognitively normal men. Eur. J. Neurol. 2016, 23, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Luo, Y.; Roberts, A.R. Secondhand smoke and women’s cognitive function in China. Am. J. Epidemiol. 2018, 187, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Chen, R. Association of environmental tobacco smoke with dementia and Alzheimer’s disease among never smokers. Alzheimer’s Dement 2012, 8, 590–595. [Google Scholar] [CrossRef]
- Chen, M.; Hu, C.; Dong, H.; Yan, H.; Wu, P.; Alzheimer’s disease Neuroimaging Initiative. A history of cigarette smoking is associated with faster functional decline and reduction of entorhinal cortex volume in mild cognitive impairment. Aging 2021, 13, 6205–6213. [Google Scholar] [CrossRef]
- Morris, J.C.; Storandt, M.; Miller, J.P.; McKeel, D.W.; Price, J.L.; Rubin, E.H.; Berg, L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 2001, 58, 397–405. [Google Scholar] [CrossRef]
- Wallin, C.; Sholts, S.B.; Österlund, N.; Luo, J.; Jarvet, J.; Roos, P.M.; Wärmländer, S.K. Alzheimer’s disease and cigarette smoke components: Effects of nicotine, PAHs, and Cd (II), Cr (III), Pb (II), Pb (IV) ions on amyloid-β peptide aggregation. Sci. Rep. 2017, 7, 14423. [Google Scholar] [CrossRef]
- de Oliveira, A.S.A.; Santiago, F.E.; Balioni, L.F.; Ferrari, M.d.F.R.; Almeida, M.C.; Carrettiero, D.C. BAG2 expression dictates a functional intracellular switch between the p38-dependent effects of nicotine on tau phosphorylation levels via the α7 nicotinic receptor. Exp. Neurol. 2016, 275, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q.; Chen, Q.; Liu, N.-Q.; Li, F.-L.; Lu, Z.-B.; Qin, C.; Zhu, H.; Huang, Y.-Y.; He, W.; et al. Nicotine attenuates the β-amyloid neurotoxicity through regulating metal homeostasis. FASEB J. 2006, 20, 1212–1214. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mu, Q.; Kang, Y.; Yang, X.; Shan, L.; Wang, M.; Li, C.; Liu, Y.; Wang, F. Association of cigarette smoking with male cognitive impairment and metal ions in cerebrospinal fluid. Front. Psychiatry 2021, 12, 738358. [Google Scholar] [CrossRef] [PubMed]
- Monacelli, F.; Cea, M.; Borghi, R.; Odetti, P.; Nencioni, A. Do cancer drugs counteract neurodegeneration? Repurposing for Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 55, 1295–1306. [Google Scholar] [CrossRef]
- Shi, H.-B.; Tang, B.; Liu, Y.-W.; Wang, X.-F.; Chen, G.-J. Alzheimer disease and cancer risk: A meta-analysis. J. Cancer Res. Clin. Oncol. 2015, 141, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Donehower, L.A.; Harvey, M.; Slagle, B.L.; McArthur, M.J.; Montgomery, C.A., Jr.; Butel, J.S.; Bradley, A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992, 356, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Chan, C.S.; Dudgeon, C.; Puzio-Kuter, A.; Hainaut, P. The evolution of tumors in mice and humans with germline p53 mutations. Cold Spring Harb Symp. Quant. Biol. 2015, 80, 139–145. [Google Scholar] [CrossRef][Green Version]
- Levine, A.J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 2020, 20, 471–480. [Google Scholar] [CrossRef]
- Mehta, S.; Tsai, P.; Lasham, A.; Campbell, H.; Reddel, R.; Braithwaite, A.; Print, C. A study of TP53 RNA splicing illustrates pitfalls of RNA-seq methodology. Cancer Res. 2016, 76, 7151–7159. [Google Scholar] [CrossRef]
- Kazantseva, M.; Eiholzer, R.A.; Mehta, S.; Taha, A.; Bowie, S.; Roth, I.; Braithwaite, A.W. Elevation of the TP53 isoform Δ133p53β in glioblastomas: An alternative to mutant p53 in promoting tumor development. J. Pathol. 2018, 246, 77–88. [Google Scholar] [CrossRef]
- Horikawa, I.; Park, K.-Y.; Isogaya, K.; Hiyoshi, Y.; Li, H.; Anami, K.; I Robles, A.; Mondal, A.M.; Fujita, K.; Serrano, M.; et al. Δ133p53 represses p53-inducible senescence genes and enhances the generation of human induced pluripotent stem cells. Cell Death Differ. 2017, 24, 1017–1028. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, F.; Li, F.; Wang, B.; Hu, Y.; Li, X. Autocrined leptin promotes proliferation of non-small cell lung cancer (NSCLC) via PI3K/AKT and p53 pathways. Ann. Transl. Med. 2021, 9, 568. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Deng, S.; Ai, Y.; Mo, Y.; Li, W.; Peng, Q.; Huang, L.; Zhang, L. MicroRNA-125b alleviates hydrogen-peroxide-induced abnormal mitochondrial dynamics in HT22 cells by inhibiting p53. Metab. Brain Dis. 2021, 36, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.-Q.; Luo, T.-T.; Luo, S.-C.; Wang, J.-Q.; Wang, S.-M.; Bai, Y.-H.; Yang, Y.-L.; Wang, Y.-Y. p53 and mitochondrial dysfunction: Novel insight of neurodegenerative diseases. J. Bioenerg. Biomembr. 2016, 48, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Ohyagi, Y.; Asahara, H.; Chui, D.-H.; Tsuruta, Y.; Sakae, N.; Miyoshi, K.; Yamada, T.; Kikuchi, H.; Taniwaki, T.; Murai, H.; et al. I Intracellular Aβ42 activates p53 promoter: A pathway to neurodegeneration in Alzheimer’s disease. FASEB J. 2005, 19, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.M.; Ghag, G.; Puangmalai, N.; Montalbano, M.; Bhatt, N.; Kayed, R. P53 aggregation, interactions with tau, and impaired DNA damage response in Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 132. [Google Scholar] [CrossRef]
- Das, M.M.; Svendsen, C.N. Astrocytes show reduced support of motor neurons with aging that is accelerated in a rodent model of ALS. Neurobiol. Aging 2015, 36, 1130–1139. [Google Scholar] [CrossRef]
- Turnquist, C.; Horikawa, I.; Foran, E.; Major, E.O.; Vojtesek, B.; Lane, D.P.; Lu, X.; Harris, B.T.; Harris, C.C. p53 isoforms regulate astrocyte-mediated neuroprotection and neurodegeneration. Cell Death Differ. 2016, 23, 1515–1528. [Google Scholar] [CrossRef]
- Cohen, J.; Torres, C. Astrocyte senescence: Evidence and significance. Aging Cell 2019, 18, e12937. [Google Scholar] [CrossRef]
- Fujita, K. P53 isoforms in cellular senescence-and ageing-associated biological and physiological functions. Int. J. Mol. Sci. 2019, 20, 6023. [Google Scholar] [CrossRef]
- Turnquist, C.; Beck, J.A.; Horikawa, I.; Obiorah, I.E.; Von Muhlinen, N.; Vojtesek, B.; Lane, D.P.; Grunseich, C.; Chahine, J.J.; Ames, H.M.; et al. Radiation-induced astrocyte senescence is rescued by Δ133p53. Neuro. Oncol. 2019, 21, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Siddharth, S.; Sharma, D. Adiponectin, obesity, and cancer: Clash of the bigwigs in health and disease. Int. J. Mol. Sci. 2019, 20, 2519. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-B.; Kim, J.-W.; Baek, K.-H. Regulation of Wnt signaling through ubiquitination and deubiquitination in cancers. Int. J. Mol. Sci. 2020, 21, 3904. [Google Scholar] [CrossRef] [PubMed]
- El-Sahli, S.; Xie, Y.; Wang, L.; Liu, S. Wnt signaling in cancer metabolism and immunity. Cancers 2019, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Pate, K.T.; Stringari, C.; Sprowl-Tanio, S.; Wang, K.; TeSlaa, T.; Hoverter, N.P.; McQuade, M.M.; Garner, C.; A Digman, M.; A Teitell, M.; et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014, 33, 1454–1473. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Hua, F.; Hu, Z.-W. The regulation of β-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.N.; Chen, J.; Li, Z.; Yan, H.; Yin, Y.; Wo, D.; Zhu, W. LRP5/6 directly bind to Frizzled and prevent Frizzled-regulated tumour metastasis. Nat. Commun. 2015, 6, 6906. [Google Scholar] [CrossRef]
- Roslan, Z.; Muhamad, M.; Selvaratnam, L.; Ab-Rahim, S. The roles of low-density lipoprotein receptor-related proteins 5, 6, and 8 in cancer: A Review. J Oncol. 2019, 2019, 4536302. [Google Scholar] [CrossRef]
- Joiner, D.M.; Ke, J.; Zhong, Z.; Xu, H.E.; Williams, B.O. LRP5 and LRP6 in development and disease. Trends Endocrinol. Metab. 2013, 24, 31–39. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Bao, W.; Kong, X. Low-density lipoprotein receptor-related protein 6 (LRP6) rs10845498 polymorphism is associated with a decreased risk of non-small cell lung cancer. Int. J. Med. Sci. 2014, 11, 685–690. [Google Scholar] [CrossRef] [PubMed]
- DiMeo, T.A.; Anderson, K.; Phadke, P.; Feng, C.; Perou, C.M.; Naber, S.; Kuperwasser, C. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009, 69, 5364–5373. [Google Scholar] [CrossRef]
- Tacchelly-Benites, O.; Wang, Z.; Yang, E.; Benchabane, H.; Tian, A.; Randall, M.P.; Ahmed, Y. Axin phosphorylation in both Wnt-off and Wnt-on states requires the tumor suppressor APC. PLoS Genet. 2018, 14, e1007178. [Google Scholar] [CrossRef]
- Yang, L.-H.; Han, Y.; Li, G.; Xu, H.-T.; Jiang, G.-Y.; Miao, Y.; Zhang, X.-P.; Zhao, H.-Y.; Xu, Z.-F.; Stoecker, M.; et al. Axin gene methylation status correlates with radiosensitivity of lung cancer cells. BMC Cancer 2013, 13, 368. [Google Scholar] [CrossRef]
- Salahshor, S.; Woodgett, J.R. The links between axin and carcinogenesis. J. Clin. Pathol. 2005, 58, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Liu, X.; Hua, S.; Liu, X. The roles of AXIN2 in tumorigenesis and epigenetic regulation. Fam. Cancer 2015, 14, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Tseng, R.C.; Lin, R.K.; Wen, C.K.; Tseng, C.; Hsu, H.S.; Hsu, W.H.; Wang, Y.C. Epigenetic silencing of AXIN2/betaTrCP and deregulation of p53-mediated control lead to wild-type β-catenin nuclear accumulation in lung tumorigenesis. Oncogene 2008, 27, 4488–4496. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Zhu, L.; Zhang, W.; Mi, Y.; Sun, H.; Zhang, L.; Yue, C.; Wu, X.; Zuo, L.; Bai, Y. The association between three AXIN2 variants and cancer risk. J. Cell Biochem. 2019, 120, 15561–15571. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Gao, H.; Xue, J.; Lin, W.; Zheng, L. The association between AXIN2 gene polymorphisms and the risk of breast cancer in Chinese women. Genet. Test Mol. Biomark. 2019, 23, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Q.; Brabletz, T.; Fearon, E.; Willis, A.L.; Hu, C.Y.; Li, X.Y.; Weiss, S.J. Canonical Wnt suppressor, Axin2, promotes colon carcinoma oncogenic activity. Proc. Natl. Acad. Sci. USA 2012, 109, 11312–11317. [Google Scholar] [CrossRef]
- Folke, J.; Pakkenberg, B.; Brudek, T. Impaired Wnt signaling in the prefrontal cortex of Alzheimer’s disease. Mol. Neurobiol. 2019, 56, 873–891. [Google Scholar] [CrossRef]
- Wan, W.; Xia, S.; Kalionis, B.; Liu, L.; Li, Y. The role of Wnt signaling in the development of Alzheimer’s disease: A potential therapeutic target? Biomed. Res. Int. 2014, 2014, 301575. [Google Scholar] [CrossRef]
- Palomer, E.; Buechler, J.; Salinas, P.C. Wnt signaling deregulation in the aging and Alzheimer’s brain. Front. Cell Neurosci. 2019, 13, 227. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Tapia-Rojas, C.; Lindsay, C.B.; Zolezzi, J.M. Wnt signaling pathway dysregulation in the aging brain: Lessons from the Octodon degus. Front Cell Dev. Biol. 2020, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Marzo, A.; Galli, S.; Lopes, D.; McLeod, F.; Podpolny, M.; Segovia-Roldan, M.; Ciani, L.; Purro, S.; Cacucci, F.; Gibb, A.; et al. Reversal of synapse degeneration by restoring Wnt signaling in the adult hippocampus. Curr. Biol. 2016, 26, 2551–2561. [Google Scholar] [CrossRef]
- Behrens, M.I.; Ponce, D.P.; Roe, C.M.; Salech, F. A common biological mechanism in cancer and Alzheimer’s disease? Curr. Alzheimer Res. 2009, 6, 196–204. [Google Scholar] [CrossRef]
- De Ferrari, G.V.; A Chacon, M.; I Barria, M.; Garrido, J.L.; A Godoy, J.; Olivares, G.; E Reyes, A.; Alvarez, A.; Bronfman, M.; Inestrosa, N.C. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by β-amyloid fibrils. Mol. Psychiatry 2003, 8, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Caricasole, A.; Copani, A.; Caraci, F.; Aronica, E.; Rozemuller, A.J.; Caruso, A.; Nicoletti, F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J. Neurosci. 2004, 24, 6021–6027. [Google Scholar] [CrossRef]
- Garner, B.; Lezanne, O. Wnt is here! Could Wnt signalling be promoted to protect against Alzheimer disease? An Editorial for Wnt signaling loss accelerates the appearance of neuropathological hallmarks of Alzheimer’s disease in J20-APP transgenic and wild-type mice. J. Neurochem. 2018, 144, 356–359. [Google Scholar] [CrossRef]
- Zhang, L.; Bahety, P.; Ee, P.L.R. Wnt co-receptor LRP5/6 overexpression confers protection against hydrogen peroxide-induced neurotoxicity and reduces tau phosphorylation in SH-SY5Y cells. Neurochem. Int. 2015, 87, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Jia, L.; Liu, C.-C.; Rong, Z.; Zhong, L.; Yang, L.; Chen, X.-F.; Fryer, J.D.; Wang, X.; Zhang, Y.-W.; et al. TREM2 promotes microglial survival by activating Wnt/β-catenin pathway. J. Neurosci. 2017, 37, 1772–1784. [Google Scholar] [CrossRef]
- Zheng, F.; Li, Y.; Zhang, F.; Sun, Y.; Zheng, C.; Luo, Z.; Wang, Y.-L.; Aschner, M.; Zheng, H.; Lin, L.; et al. Cobalt induces neurodegenerative damages through Pin1 inactivation in mice and human neuroglioma cells. J. Hazard Mater. 2021, 419, 126378. [Google Scholar] [CrossRef]
- Sherzai, A.Z.; Parasram, M.; Haider, J.M.; Sherzai, D. Alzheimer disease and cancer: A national inpatient sample analysis. Alzheimer Dis. Assoc. Disord. 2020, 34, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-W.; Tse, E. PIN1 in cell cycle control and cancer. Front. Pharmacol. 2018, 9, 1367. [Google Scholar] [CrossRef]
- Yu, J.H.; Im, C.Y.; Min, S.-H. Function of PIN1 in cancer development and its inhibitors as cancer therapeutics. Front. Cell Dev. Biol. 2020, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-H.; Zhen, Y.-Y.; Tsai, Y.-C.; Chuang, C.-H.; Huang, M.-S.; Hsiao, M.; Yang, C.-J. Targeting Pin1 for modulation of cell motility and cancer therapy. Biomedicines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Z.; Lu, K.P. The isomerase PIN1 controls numerous cancer-driving pathways and is a unique drug target. Nat. Rev. Cancer 2016, 16, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.-R.; Yang, H.-Y.; Li, X.-Z.; Jie, M.-M.; Hu, C.-J.; Wu, Y.-Y.; Yang, S.-M.; Yang, Y.-B. Prolyl isomerase Pin1: A promoter of cancer and a target for therapy. Cell Death Dis. 2018, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- van der Mijn, J.C.; Panka, D.J.; Geissler, A.K.; Verheul, H.M.; Mier, J.W. Novel drugs that target the metabolic reprogramming in renal cell cancer. Cancer Metab. 2016, 4, 14. [Google Scholar] [CrossRef]
- Driver, J.A.; Zhou, X.Z.; Lu, K.P. Pin1 dysregulation helps to explain the inverse association between cancer and Alzheimer’s disease. Biochim. Biophys. Acta 2015, 1850, 2069–2076. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Abdul, H.M.; Opii, W.; Newman, S.F.; Joshi, G.; Ansari, M.A.; Sultana, R. Pin1 in Alzheimer’s disease. J. Neurochem. 2006, 98, 1697–1706. [Google Scholar] [CrossRef]
- Xu, L.; Ren, Z.; Chow, F.E.; Tsai, R.; Liu, T.; Rizzolio, F.; Boffo, S.; Xu, Y.; Huang, S.; Lippa, C.F.; et al. Pathological role of peptidyl-prolyl isomerase Pin1 in the disruption of synaptic plasticity in Alzheimer’s disease. Neural Plast. 2017, 2017, 3270725. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-J.; Wulf, G.; Zhou, X.Z.; Davies, P.; Lu, K.P. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 1999, 399, 784–788. [Google Scholar] [CrossRef]
- Xiong, K.; Wang, S.-C.; Hu, X.-M. The regulatory role of Pin1 in neuronal death. Neural Regen. Res. 2023, 18, 74. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Chen, D.; Lee, T.H. Peptidyl-prolyl cis/trans isomerase Pin1 and Alzheimer’s disease. Front. Cell Dev. Biol. 2020, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Albayram, O.; Kondo, A.; Wang, B.; Kim, N.; Arai, K.; Tsai, C.-Y.; Bassal, M.A.; Herbert, M.K.; Washida, K.; et al. Cis P-tau underlies vascular contribution to cognitive impairment and dementia and can be effectively targeted by immunotherapy in mice. Sci. Transl. Med. 2021, 13, eaaz7615. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Zhou, X.Z.; Lu, K.P. Cis phosphorylated tau as the earliest detectable pathogenic conformation in Alzheimer disease, offering novel diagnostic and therapeutic strategies. Prion 2017, 7, 117–120. [Google Scholar] [CrossRef]
- Katsumoto, A.; Takeuchi, H.; Tanaka, F. Tau pathology in chronic traumatic encephalopathy and Alzheimer’s disease: Similarities and differences. Front. Neurol. 2019, 10, 980. [Google Scholar] [CrossRef]
- Kondo, A.; Shahpasand, K.; Mannix, R.; Qiu, J.; Moncaster, J.; Chen, C.-H.; Yao, Y.; Lin, Y.-M.; Driver, J.A.; Sun, Y.; et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 2015, 523, 431–436. [Google Scholar] [CrossRef]
- Ittner, L.M.; Götz, J. Amyloid-β and tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 67–72. [Google Scholar] [CrossRef]
- Shentu, Y.P.; Huo, Y.; Feng, X.L.; Gilbert, J.; Zhang, Q.; Liuyang, Z.Y.; Liu, R. CIP2A causes Tau/APP phosphorylation, synaptopathy, and memory deficits in Alzheimer’s disease. Cell Rep. 2018, 24, 713–723. [Google Scholar] [CrossRef]
- Pastorino, L.; Sun, A.; Lu, P.-J.; Zhou, X.Z.; Balastik, M.; Finn, G.; Wulf, G.; Lim, J.; Li, S.-H.; Li, X.; et al. The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-β production. Nature 2006, 440, 528–534. [Google Scholar] [CrossRef]
- Nowotny, P.; Bertelsen, S.; Hinrichs, A.L.; Kauwe, J.S.; Mayo, K.; Jacquart, S.; Morris, J.C.; Goate, A. Association studies between common variants in prolyl isomerase Pin1 and the risk for late-onset Alzheimer’s disease. Neurosci. Lett. 2007, 419, 15–17. [Google Scholar] [CrossRef][Green Version]
- Kwong, F.N.K.; Nicholson, A.G.; Harrison, C.L.; Hansbro, P.M.; Adcock, I.M.; Chung, K.F. Is mitochondrial dysfunction a driving mechanism linking COPD to nonsmall cell lung carcinoma? Eur. Respir. Rev. 2017, 26, 170040. [Google Scholar] [CrossRef]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Veeramachaneni, N. Targeting interleukin-1β and inflammation in lung cancer. Biomark. Res. 2022, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Limonta, P. The multifaceted roles of mitochondria at the crossroads of cell life and death in cancer. Free Radic. Biol. Med. 2021, 176, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Audano, M.; Pedretti, S.; Ligorio, S.; Crestani, M.; Caruso, D.; De Fabiani, E.; Mitro, N. The Loss of Golden Touch: Mitochondria-Organelle Interactions, Metabolism, and Cancer. Cells 2020, 9, 2519. [Google Scholar] [CrossRef] [PubMed]
- Peruzzo, R.; Costa, R.; Bachmann, M.; Leanza, L.; Szabo, I. Mitochondrial metabolism, contact sites, and cellular calcium signaling: Implications for tumorigenesis. Cancers 2020, 12, 2574. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.; Sandoval-Borquez, A.; Bravo-Sagua, R.; Quest, A.F.G.; Lavandero, S. Perspectives on organelle interaction, protein dysregulation, and cancer disease. Front. Cell Dev. Biol. 2021, 9, 613336. [Google Scholar] [CrossRef]
- Lynes, E.M.; Bui, M.; Yap, M.C.; Benson, M.D.; Schneider, B.; Ellgaard, L.; Berthiaume, L.G.; Simmen, T. Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. EMBO J. 2012, 31, 457–470. [Google Scholar] [CrossRef]
- Luu Hoang, K.N.; Anstee, J.E.; Arnold, J.N. The diverse roles of heme oxygenase-1 in tumor progression. Front. Immunol. 2021, 12, 658315. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Audano, M.; Beretta, G.; Procacci, P.; Sartori, P.; Mitro, N.; Limonta, P. Mitochondrial functional and structural impairment is involved in the antitumor activity of δ-tocotrienol in prostate cancer cells. Free Radic. Biol. Med. 2020, 160, 376–390. [Google Scholar] [CrossRef]
- Mignard, V.; Dubois, N.; Lanoe, D.; Joalland, M.-P.; Oliver, L.; Pecqueur, C.; Heymann, D.; Paris, F.; Vallette, F.M.; Lalier, L. Sphingolipid distribution at mitochondria-associated membranes (MAMs) upon induction of apoptosis. J. Lipid Res. 2020, 61, 1025–1037. [Google Scholar] [CrossRef]
- Marchi, S.; Giorgi, C.; Oparka, M.; Duszynski, J.; Wieckowski, M.R.; Pinton, P. Oncogenic and oncosuppressive signal transduction at mitochondria-associated endoplasmic reticulum membranes. Mol. Cell Oncol. 2014, 1, e956469. [Google Scholar] [CrossRef]
- Giorgi, C.; Bonora, M.; Sorrentino, G.; Missiroli, S.; Poletti, F.; Suski, J.M.; Ramirez, F.G.; Rizzuto, R.; Di Virgilio, F.; Zito, E.; et al. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc. Natl. Acad. Sci. USA 2015, 112, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Raturi, A.; Gutierrez, T.; Ortiz-Sandoval, C.; Ruangkittisakul, A.; Herrera-Cruz, M.S.; Rockley, J.P.; Gesson, K.; Ourdev, D.; Lou, P.-H.; Lucchinetti, E.; et al. TMX1 determines cancer cell metabolism as a thiol-based modulator of ER–mitochondria Ca2+ flux. J. Cell Biol. 2016, 214, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Kalainayakan, S.P.; Fitzgerald, K.E.; Konduri, P.C.; Vidal, C.; Zhang, L. Essential roles of mitochondrial and heme function in lung cancer bioenergetics and tumorigenesis. Cell Biosci. 2018, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Kumari, S.; Kalainayakan, S.P.; Campbell, J., 3rd; Ghosh, P.; Zhou, H.; FitzGerald, K.E.; Li, M.; Mason, R.P.; Zhang, L.; et al. The vascular disrupting agent combretastatin A-4 phosphate causes prolonged elevation of proteins involved in heme flux and function in resistant tumor cells. Oncotarget 2018, 9, 4090. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huang, C.; Gunda, V.; Sun, J.; Chellappan, S.P.; Li, Z.; Izumi, V.; Fang, B.; Koomen, J.; Singh, P.K.; et al. Fascin controls metastatic colonization and mitochondrial oxidative phosphorylation by remodeling mitochondrial actin filaments. Cell Rep. 2019, 28, 2824–2836. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizio, A.; Limonta, P. The emerging role of paraptosis in tumor cell biology: Perspectives for cancer prevention and therapy with natural compounds. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188338. [Google Scholar] [CrossRef] [PubMed]
- Nedungadi, D.; Binoy, A.; Pandurangan, N.; Pal, S.; Nair, B.G.; Mishra, N. 6-Shogaol induces caspase-independent paraptosis in cancer cells via proteasomal inhibition. Exp. Cell Res. 2018, 364, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Binoy, A.; Nedungadi, D.; Katiyar, N.; Bose, C.; Shankarappa, S.A.; Nair, B.G.; Mishra, N. Plumbagin induces paraptosis in cancer cells by disrupting the sulfhydryl homeostasis and proteasomal function. Chem. Biol. Interact. 2019, 310, 108733. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.-G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Bulati, M.; Buffa, S.; Pellicano, M.; Caruso, C.; Candore, G.; Colonna-Romano, G. Immunosenescence, inflammation, and Alzheimer’s disease. Longev. Healthspan. 2012, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Koutsouraki, E.; Gąsiorowski, K.; Leszek, J. Vascular oxidative stress and mitochondrial failure in the pathobiology of Alzheimer’s disease: A new approach to therapy. CNS Neurol. Disord Drug Targets 2013, 12, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, D.; Suski, J.M.; Oules, B.; Debayle, D.; Gay, A.S.; Lacas-Gervais, S.; Bussiere, R.; Bauer, C.; Pinton, P.; Paterlini-Brechot, P.; et al. Localization and processing of the amyloid-β protein precursor in mitochondria-associated membranes. J. Alzheimer’s Dis. 2017, 55, 1549–1570. [Google Scholar] [CrossRef]
- Pera, M.; Larrea, D.; Guardia-Laguarta, C.; Montesinos, J.; Velasco, K.R.; Agrawal, R.R.; Area-Gomez, E. Increased localization of APP-C99 in mitochondria-associated ER membranes causes mitochondrial dysfunction in Alzheimer disease. EMBO J. 2017, 3356–3371. [Google Scholar] [CrossRef] [PubMed]
- Völgyi, K.; Badics, K.; Sialana, F.J.; Gulyassy, P.; Udvari, E.B.; Kis, V.; Drahos, L.; Lubec, G.; Kekesi, K.A.; Juhasz, G. Early presymptomatic changes in the proteome of mitochondria-associated membrane in the APP/PS1 mouse model of Alzheimer’s disease. Mol. Neurobiol. 2018, 36, 7839–7857. [Google Scholar] [CrossRef]
- Schreiner, B.; Hedskog, L.; Wiehager, B.; Ankarcrona, M. Amyloid-β peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J. Alzheimer’s Dis. 2015, 43, 369–374. [Google Scholar] [CrossRef]
- Area-Gomez, E.; de Groof, A.; Bonilla, E.; Montesinos, J.; Tanji, K.; Boldogh, I.; Pon, L.; Schon, E.A. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis. 2018, 9, 335. [Google Scholar] [CrossRef]
- Sarasija, S.; Laboy, J.T.; Ashkavand, Z.; Bonner, J.; Tang, Y.; Norman, K.R. Presenilin mutations deregulate mitochondrial Ca2+ homeostasis and metabolic activity causing neurodegeneration in Caenorhabditis elegans. eLife 2018, 7, e33052. [Google Scholar] [CrossRef]
- Sarasija, S.; Norman, K.R. Norman. A γ-secretase independent role for presenilin in calcium homeostasis impacts mitochondrial function and morphology in Caenorhabditis elegans. Genetics 2015, 201, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, C.; Filadi, R.; Rossi, A.; Mammucari, C.; Pizzo, P. PSEN2 (presenilin 2) mutants linked to familial Alzheimer disease impair autophagy by altering Ca2+ homeostasis. Autophagy 2019, 15, 2044–2062. [Google Scholar] [CrossRef] [PubMed]
- Kipanyula, M.J.; Contreras, L.; Zampese, E.; Lazzari, C.; Wong, A.K.C.; Pizzo, P.; Fasolato, C.; Pozzan, T. Ca2+ dysregulation in neurons from transgenic mice expressing mutant presenilin 2. Aging Cell 2012, 11, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, M.; Petersen, A.J.; Naumenko, N.; Puttonen, K.; Lehtonen, Š.; Olive, M.G.; Koistinaho, J. PSEN1 mutant iPSC-derived model reveals severe astrocyte pathology in Alzheimer’s disease. Stem. Cell Rep. 2017, 9, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Tambini, M.D.; Pera, M.; Kanter, E.; Yang, H.; Guardia-Laguarta, C.; Holtzman, D.; Sulzer, D.; Area-Gomez, E.; Schon, E.A. ApoE4 upregulates the activity of mitochondria-associated ER membranes. EMBO Rep. 2016, 17, 27–36. [Google Scholar] [CrossRef]
- Kerr, J.S.; Adriaanse, B.A.; Greig, N.H.; Mattson, M.P.; Cader, M.Z.; Bohr, V.A.; Fang, E.F. Mitophagy and Alzheimer’s disease: Cellular and molecular mechanisms. Trends Neurosci. 2017, 40, 151–166. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Khan, S.M. A mitochondrial cascade hypothesis for sporadic Alzheimer’s disease. Med. Hypotheses 2004, 63, 8–20. [Google Scholar] [CrossRef]
- Fong, S.; Teo, E.; Ng, L.F.; Chen, C.-B.; Lakshmanan, L.N.; Tsoi, S.Y.; Moore, P.K.; Inoue, T.; Halliwell, B.; Gruber, J. Energy crisis precedes global metabolic failure in a novel Caenorhabditis elegans Alzheimer Disease model. Sci. Rep. 2016, 6, 33781. [Google Scholar] [CrossRef]
- Dosanjh, L.E.; Brown, M.K.; Rao, G.; Link, C.D.; Luo, Y. Behavioral phenotyping of a transgenic Caenorhabditis elegans expressing neuronal amyloid-β. J. Alzheimer’s Dis. 2010, 19, 681–690. [Google Scholar] [CrossRef]
- Andra, A.; Tanigawa, S.; Bito, T.; Ishihara, A.; Watanabe, F.; Yabuta, Y. Effects of vitamin B12 deficiency on amyloid-β toxicity in Caenorhabditis elegans. Antioxidants 2021, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A convenient in vivo model for assessing the impact of food bioactive compounds on obesity, aging, and Alzheimer’s disease. Annu. Rev. Food Sci. Technol. 2018, 9, 012709. [Google Scholar] [CrossRef] [PubMed]
- McColl, G.; Roberts, B.R.; Pukala, T.L.; Kenche, V.B.; Roberts, C.M.; Link, C.D.; Cherny, R.A. Utility of an improved model of amyloid-beta (Aβ1-42) toxicity in Caenorhabditis elegansfor drug screening for Alzheimer’s disease. Mol. Neurodegener. 2012, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.; Gergou, A.; Wacker, I.; Fath, T.; Hutter, H. A Caenorhabditis elegans model of tau hyperphosphorylation: Induction of developmental defects by transgenic overexpression of Alzheimer’s disease-like modified tau. Neurobiol. Aging 2009, 30, 22–33. [Google Scholar] [CrossRef]
- Sorrentino, V.; Romani, M.; Mouchiroud, L.; Beck, J.S.; Zhang, H.; D’Amico, D.; Moullan, N.; Potenza, F.; Schmid, A.W.; Rietsch, S.; et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 2017, 552, 187–193. [Google Scholar] [CrossRef]
- Delgado-Deida, Y.; Alula, K.M.; Theiss, A.L. The influence of mitochondrial-directed regulation of Wnt signaling on tumorigenesis. Gastroenterol. Rep. 2020, 8, 215–223. [Google Scholar] [CrossRef]
- Wen, Y.-A.; Xiong, X.; Scott, T.; Li, A.T.; Wang, C.; Weiss, H.L.; Tan, L.; Bradford, E.; Fan, T.W.M.; Chandel, N.S.; et al. The mitochondrial retrograde signaling regulates Wnt signaling to promote tumorigenesis in colon cancer. Cell Death Differ. 2019, 26, 1955–1969. [Google Scholar] [CrossRef]
- Costa, R.; Peruzzo, R.; Bachmann, M.; Monta, G.D.; Vicario, M.; Santinon, G.; Mattarei, A.; Moro, E.; Quintana-Cabrera, R.; Scorrano, L.; et al. Impaired mitochondrial ATP production downregulates Wnt signaling via ER stress induction. Cell Rep. 2019, 28, 1949–1960. [Google Scholar] [CrossRef]
- Martin, L.J.; Semenkow, S.; Hanaford, A.; Wong, M. Wnt signaling prevents the Aβ oligomer-induced mitochondrial permeability transition pore opening preserving mitochondrial structure in hippocampal neurons. PLoS ONE 2017, 12, e0168840. [Google Scholar]
- Godoy, J.A.; Arrazola, M.S.; Ordenes, D.; Silva-Alvarez, C.; Braidy, N.; Inestrosa, N.C. Wnt-5a ligand modulates mitochondrial fission-fusion in rat hippocampal neurons. J. Biol. Chem. 2014, 289, 36179–36193. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Tseng, L.-M.; Lee, H.-C. Role of mitochondrial dysfunction in cancer progression. Exp. Biol. Med. 2016, 241, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Achanta, G.; Sasaki, R.; Feng, L.; Carew, J.S.; Lu, W.; Pelicano, H.; Keating, M.J.; Huang, P. Novel role of p53 in maintaining mitochondrial genetic stability through interaction with DNA Pol γ. EMBO J. 2005, 24, 3482–3492. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, Y.; Meisenhelder, J.; Yang, W.; Hawke, D.H.; Zheng, Y.; Xia, Y.; Aldape, K.; He, J.; Hunter, T.; et al. Mitochondriatranslocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol. Cell 2016, 61, 705–719. [Google Scholar] [CrossRef]
- Sorrentino, G.; Mioni, M.; Giorgi, C.; Ruggeri, N.; Pinton, P.; Moll, U.; Mantovani, F.; Del Sal, G. The prolyl-isomerase Pin1 activates the mitochondrial death program of p53. Cell Death Differ. 2013, 20, 198–208. [Google Scholar] [CrossRef] [PubMed]
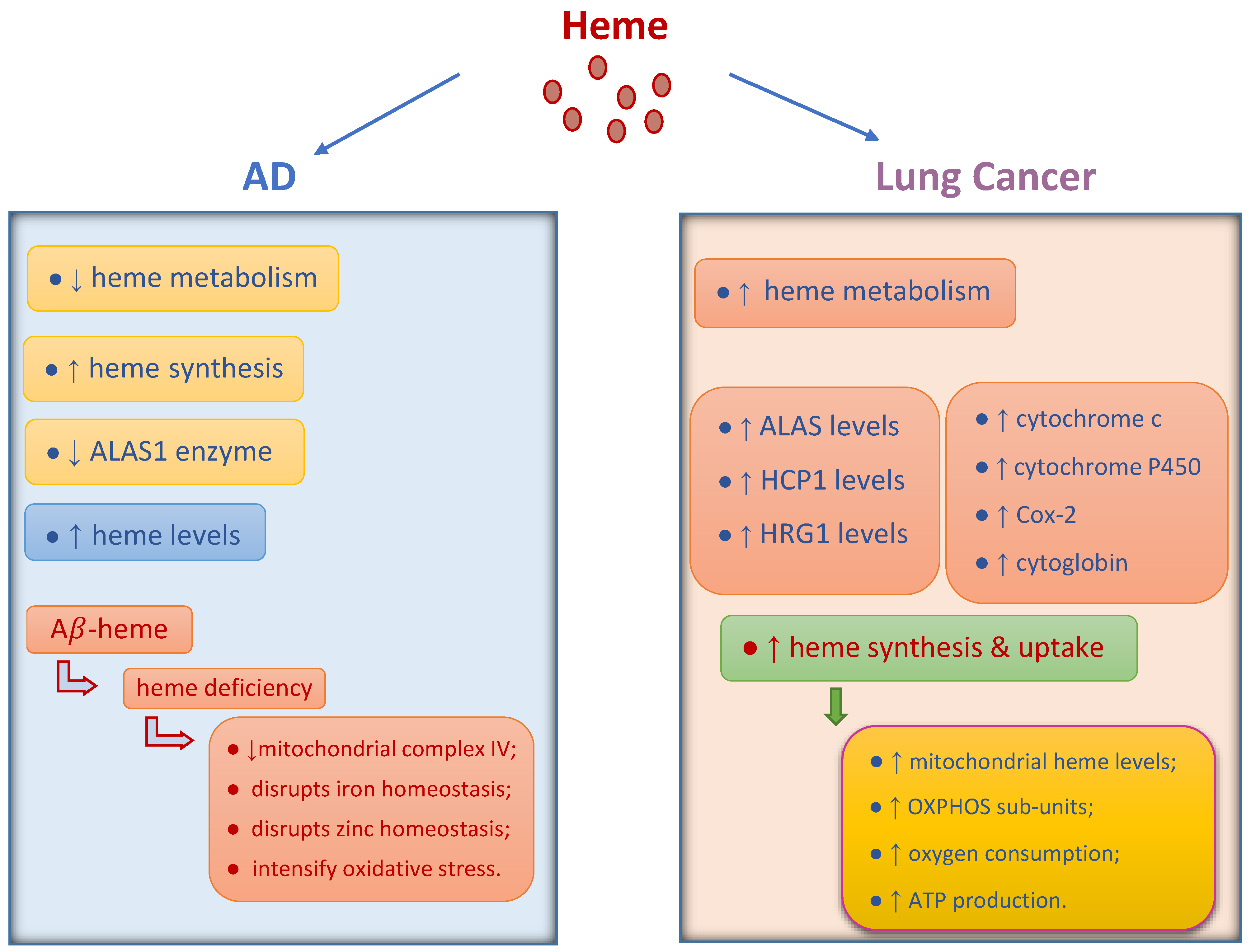
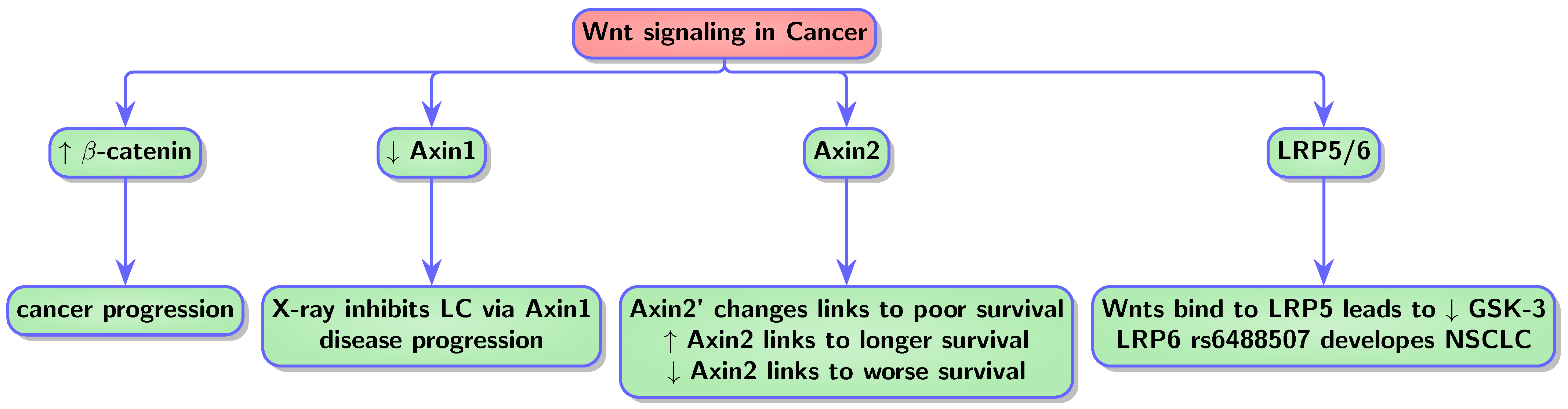
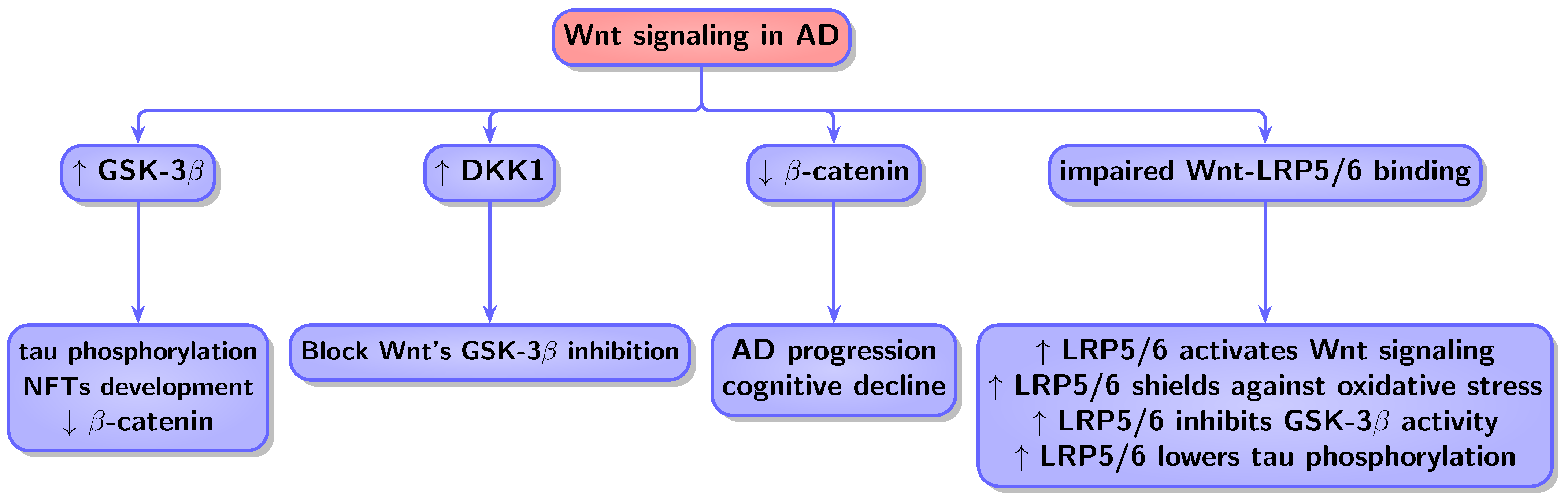
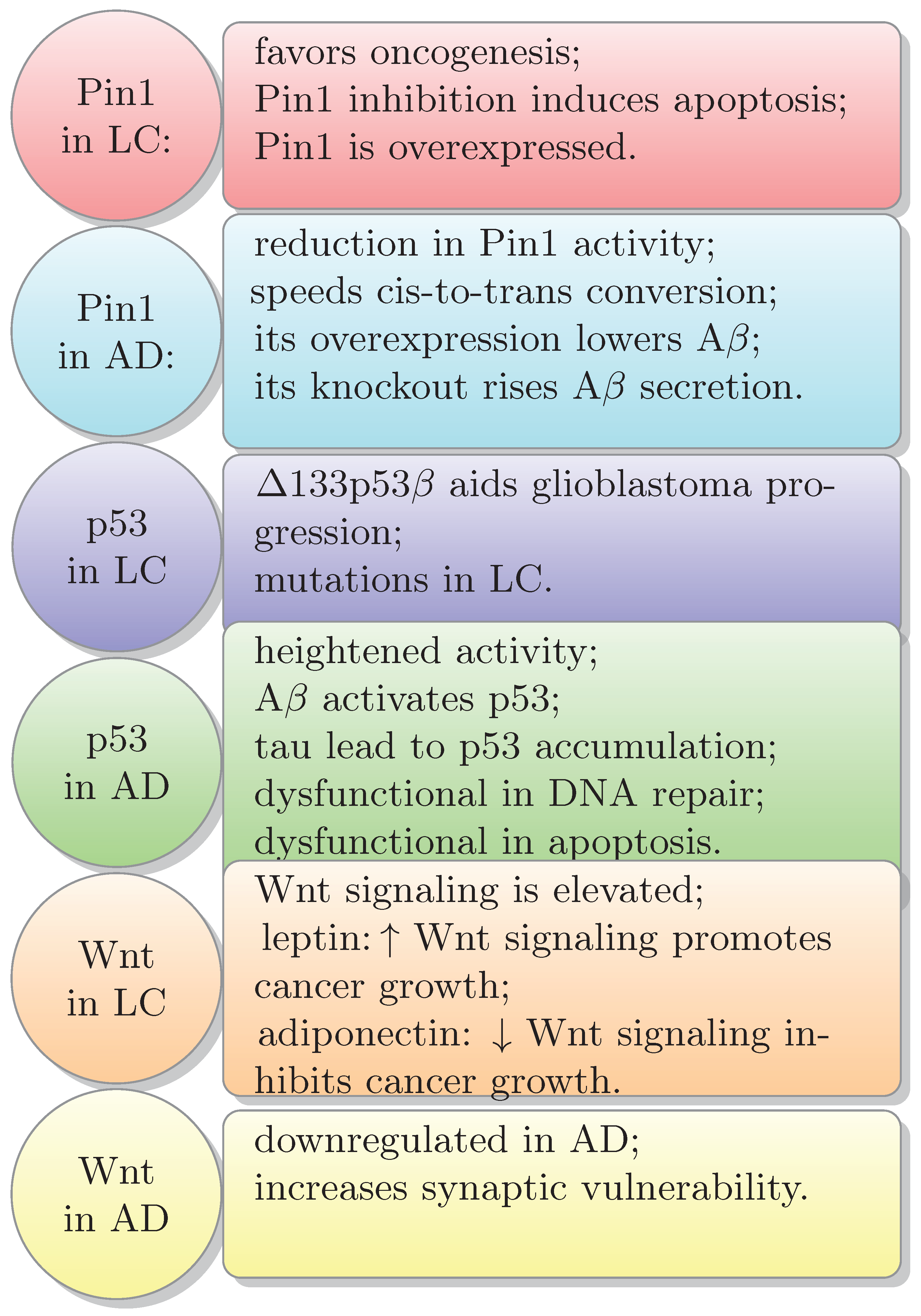
| Effects | References | |
|---|---|---|
| Cancer |
| Degese et al., 2012, [86] Hsu et al., 2015, [87] Hsu et al., 2017, [89] Skrzypek et al., 2013, [95] Sohoni et al., 2019, [30] |
| AD |
| Intagliata et al., 2019, [79] Chen et al., 2018, [115] Liu et al., 2020, [114] Hettiarachchi et al., 2014, [110] Hettiarachchi et al., 2017, [111] Choi et al., 2022, [39] Wang et al., 2015, [105] Hui et al., 2011, [106] Li et al., 2015, [107] Schipper et al., 2019, [113] Choi et al., 2022, [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afsar, A.; Zhang, L. Putative Molecular Mechanisms Underpinning the Inverse Roles of Mitochondrial Respiration and Heme Function in Lung Cancer and Alzheimer’s Disease. Biology 2024, 13, 185. https://doi.org/10.3390/biology13030185
Afsar A, Zhang L. Putative Molecular Mechanisms Underpinning the Inverse Roles of Mitochondrial Respiration and Heme Function in Lung Cancer and Alzheimer’s Disease. Biology. 2024; 13(3):185. https://doi.org/10.3390/biology13030185
Chicago/Turabian StyleAfsar, Atefeh, and Li Zhang. 2024. "Putative Molecular Mechanisms Underpinning the Inverse Roles of Mitochondrial Respiration and Heme Function in Lung Cancer and Alzheimer’s Disease" Biology 13, no. 3: 185. https://doi.org/10.3390/biology13030185
APA StyleAfsar, A., & Zhang, L. (2024). Putative Molecular Mechanisms Underpinning the Inverse Roles of Mitochondrial Respiration and Heme Function in Lung Cancer and Alzheimer’s Disease. Biology, 13(3), 185. https://doi.org/10.3390/biology13030185







