The Cardiometabolic Impact of Rebaudioside A Exposure during the Reproductive Stage
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
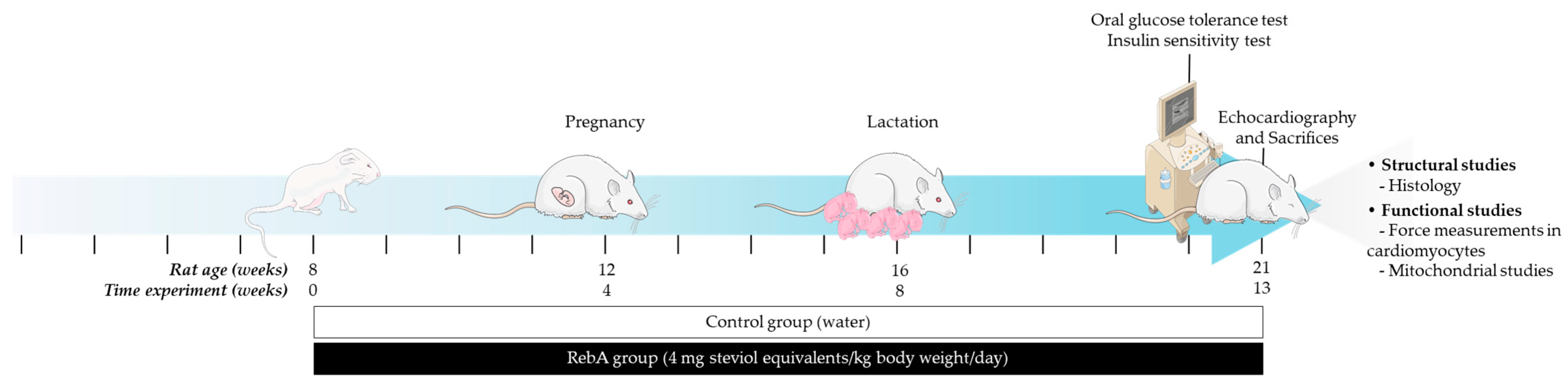
2.1. Animals and Treatments
2.2. Oral Glucose Tolerance Assessment and Insulin Sensitivity Assessment
2.3. Plasma Biochemical Markers Determination
2.4. Echocardiographic Evaluation
2.5. Mitochondrial Respiration Evaluation Using Permeabilized Cardiac Fibers
2.6. Force Measurements in Isolated Permeabilized Cardiomyocytes
2.7. Histology
2.8. Biomarker of Myocardial Fibrotic Assessment
2.9. Statistical Analysis
3. Results
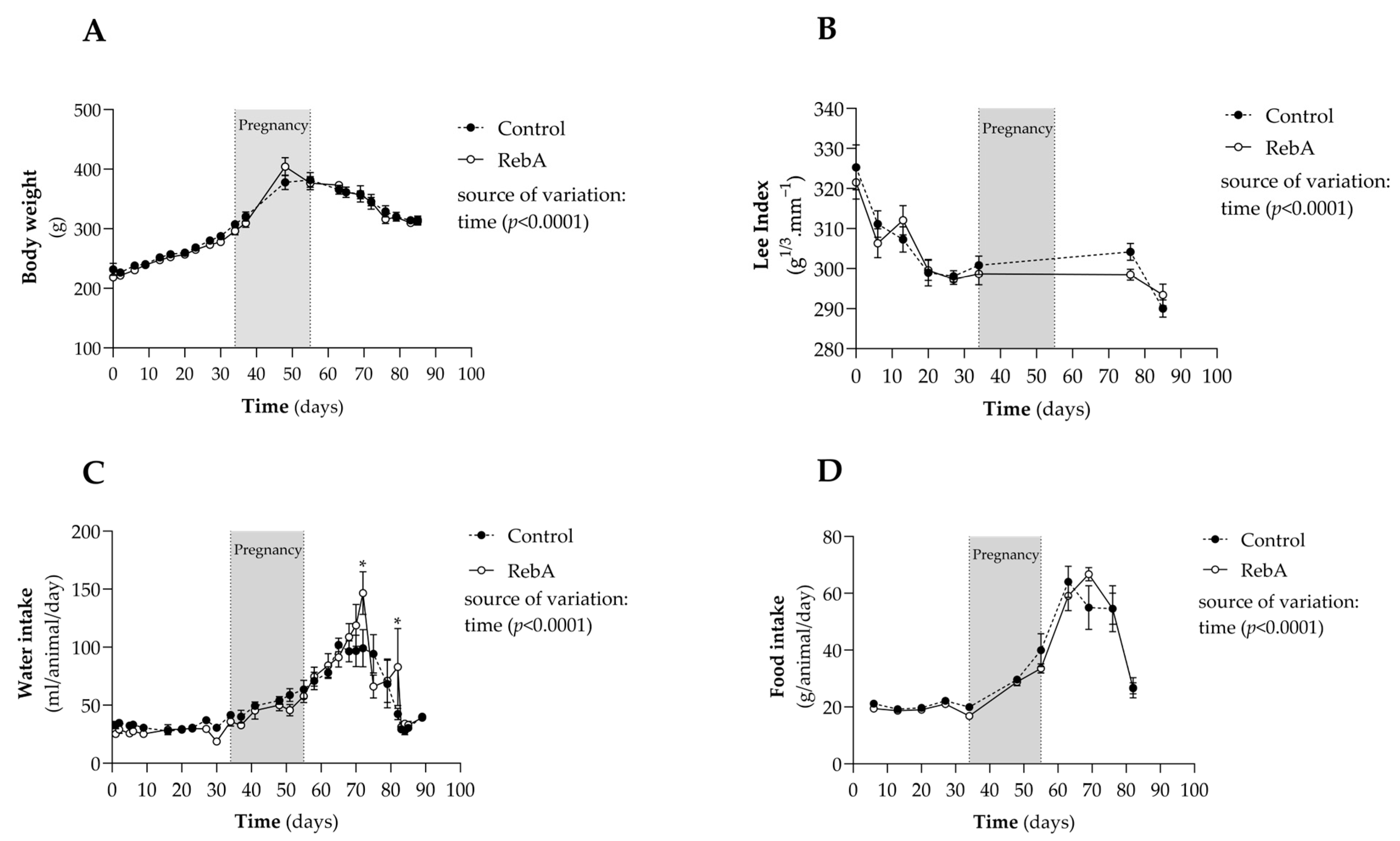
3.1. Gestational and Morphometric Data
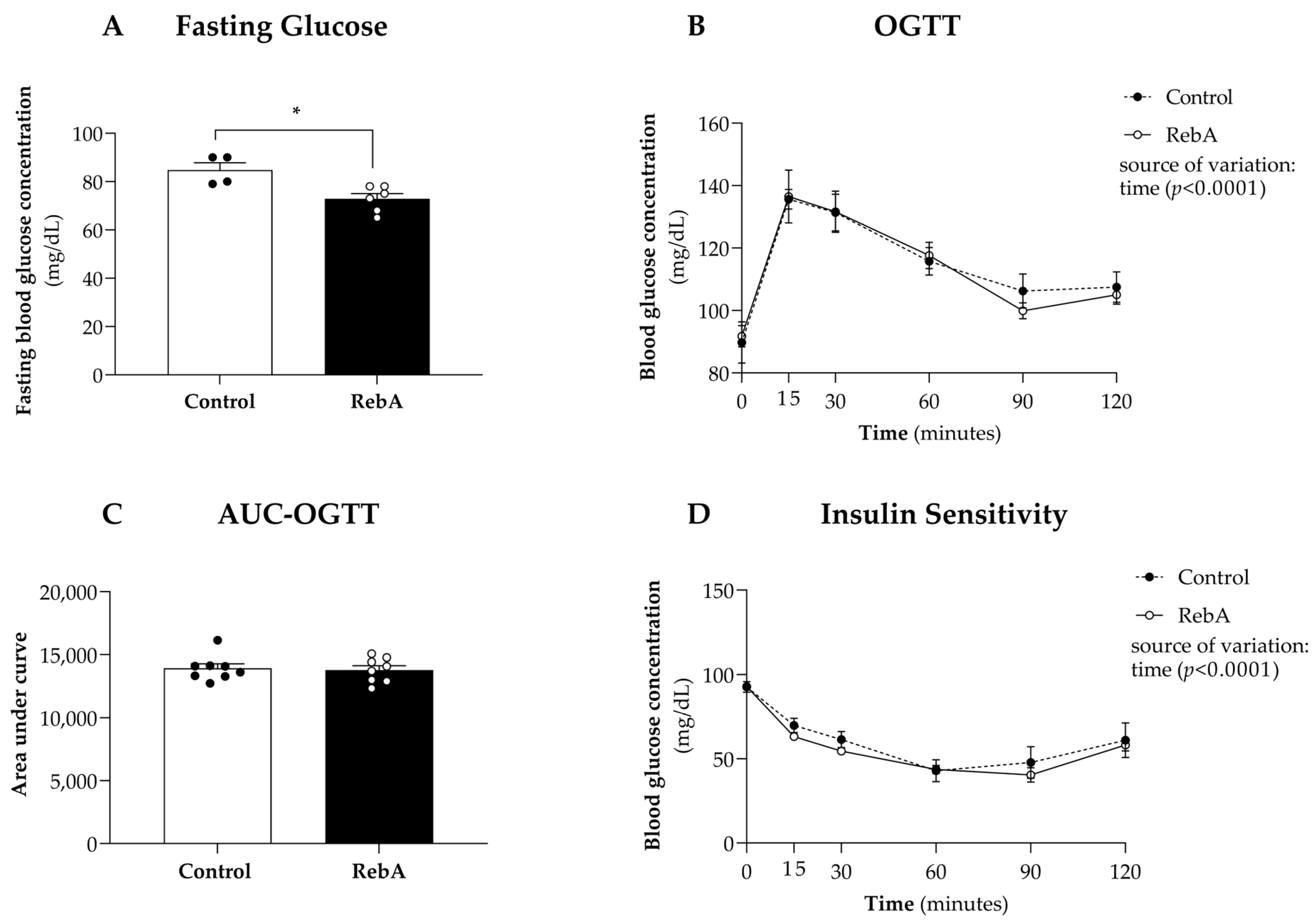
3.2. Glycemic Control
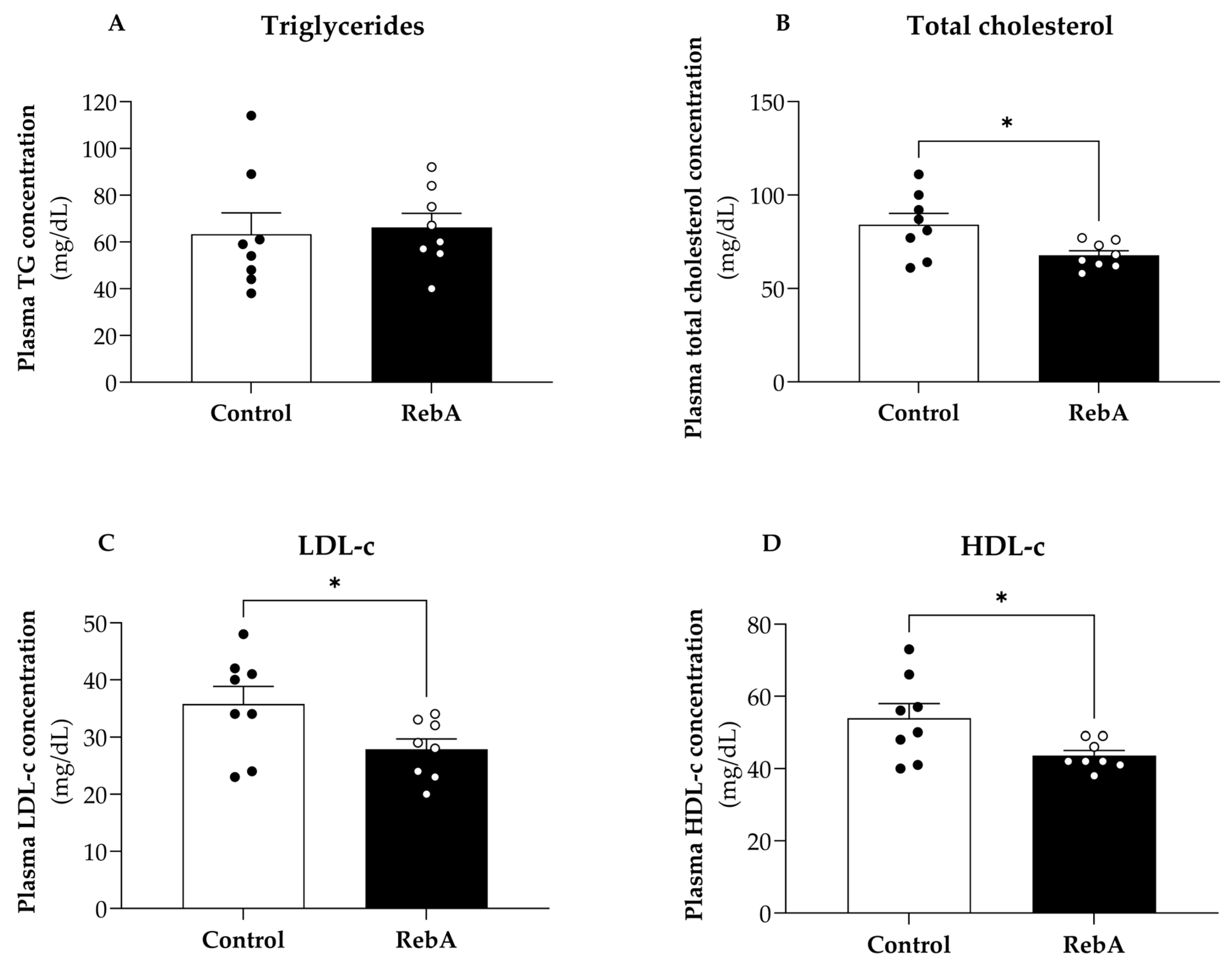
3.3. Plasma Biochemical Markers
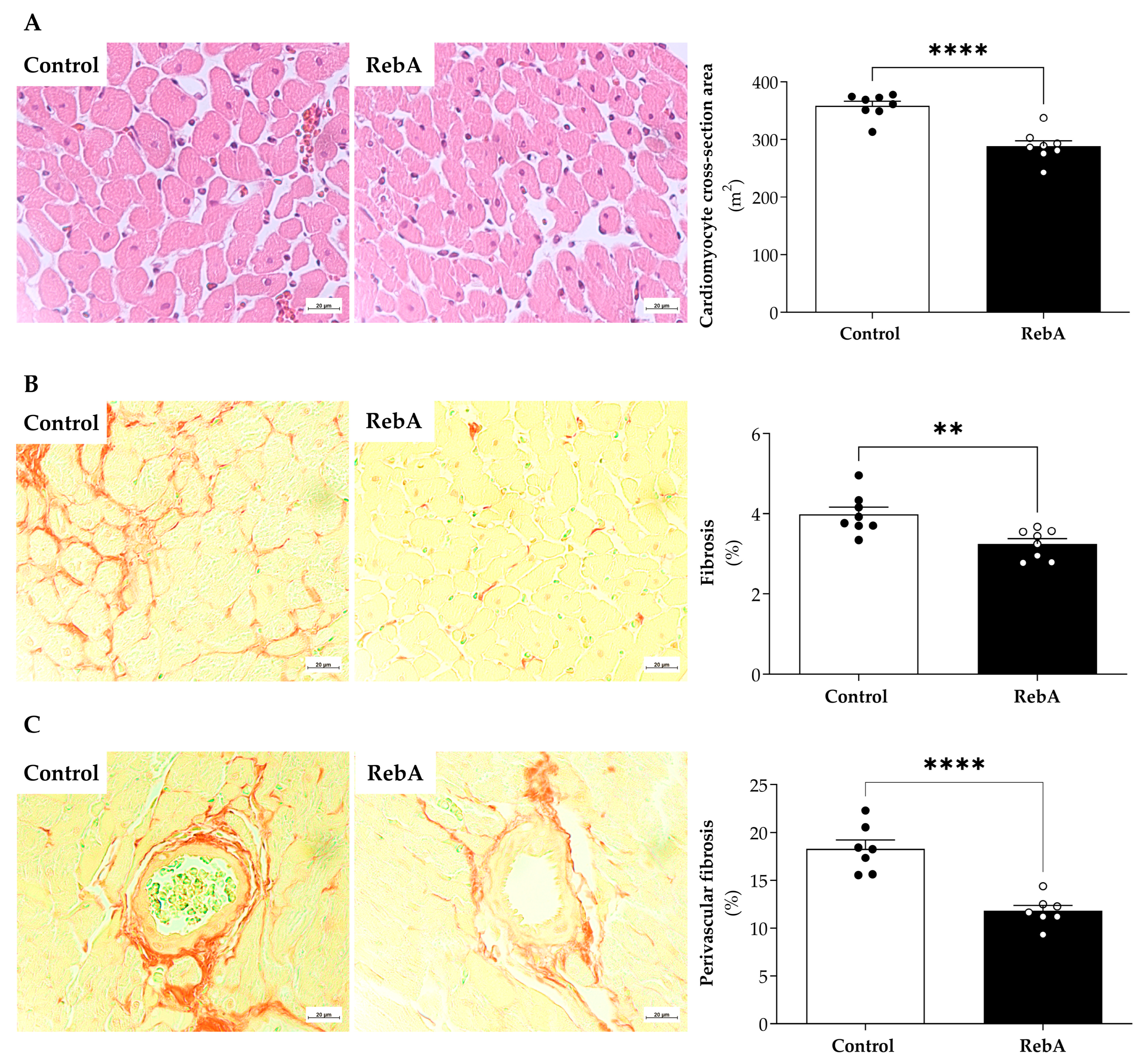
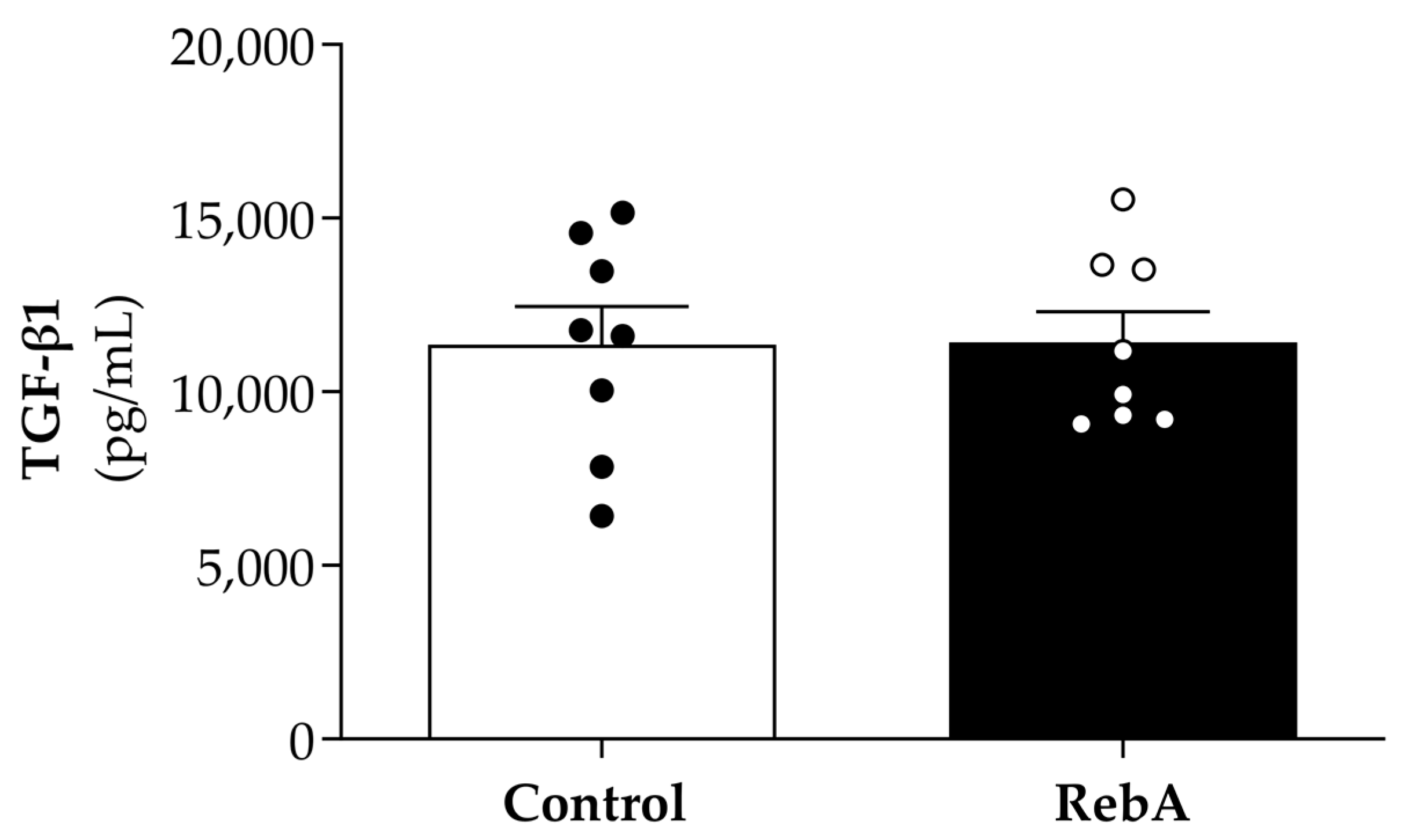
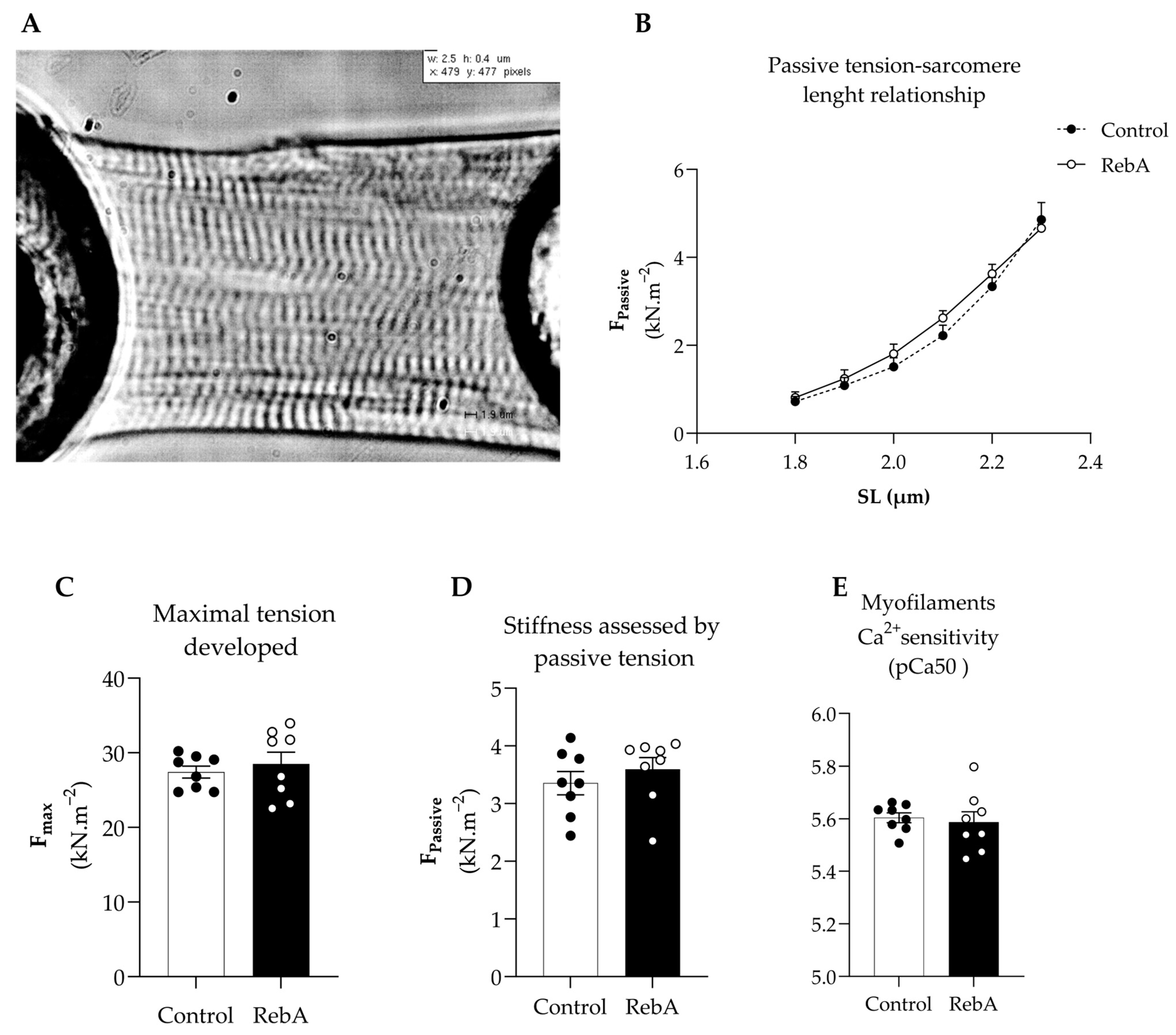
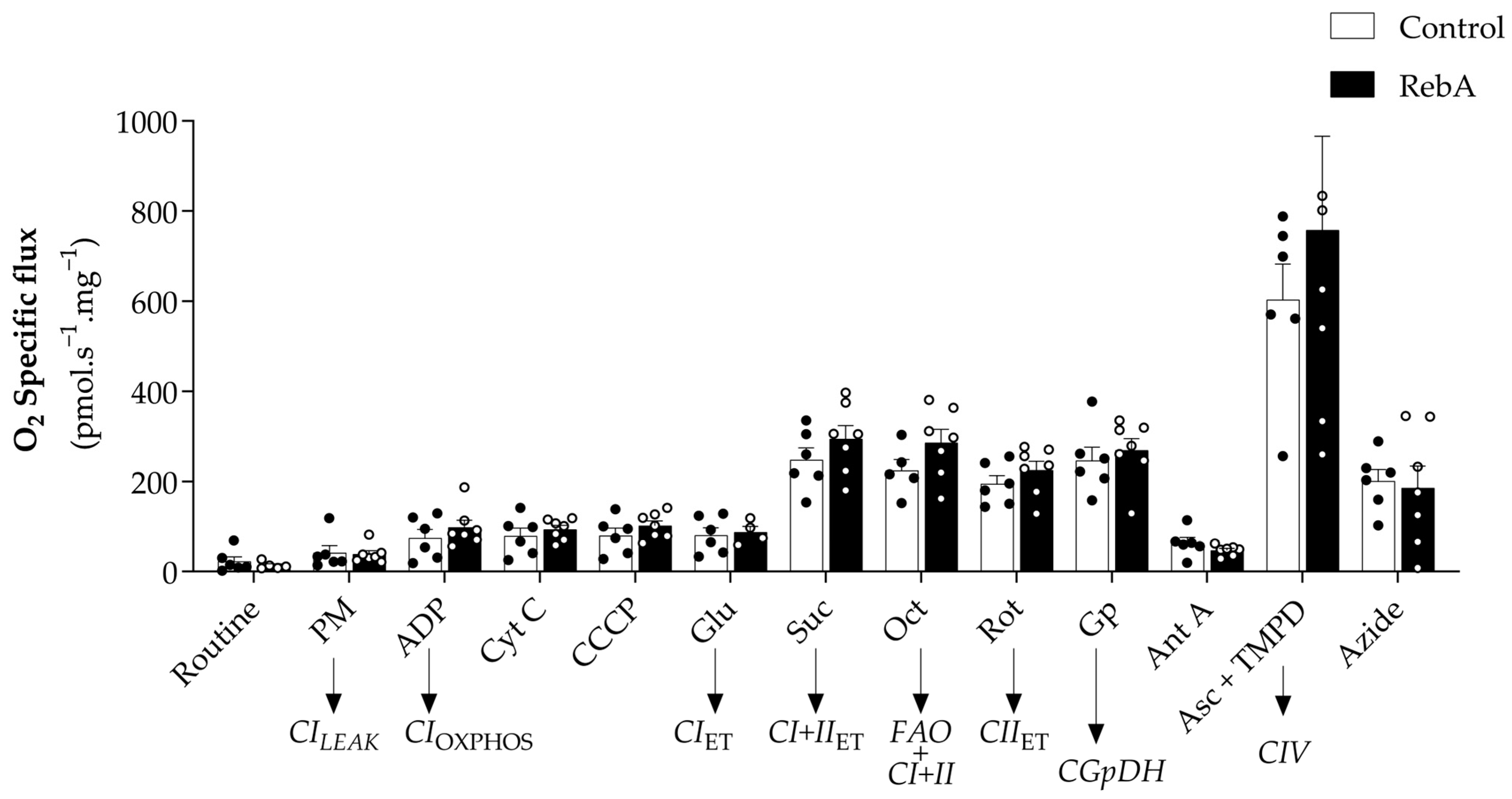
3.4. Left Ventricle Cardiac Structure and Functional Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022.
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary Sugars Intake and Cardiovascular Health. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Figueroa, J.; Rother, K.I.; Goran, M.I.; Welsh, J.A. Trends in Low-Calorie Sweetener Consumption Among Pregnant Women in the United States. Curr. Dev. Nutr. 2019, 3, nzz004. [Google Scholar] [CrossRef] [PubMed]
- Ashwell, M. Stevia, Nature’s Zero-Calorie Sustainable Sweetener: A New Player in the Fight Against Obesity. Nutr. Today 2015, 50, 129–134. [Google Scholar] [CrossRef]
- Fowler, S.P.G. Low-calorie sweetener use and energy balance: Results from experimental studies in animals, and large-scale prospective studies in humans. Physiol. Behav. 2016, 164, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. Can. Med. Assoc. J. 2017, 189, E929–E939. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Engel, K.H.; Fowler, P.J.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; et al. Safety evaluation of glucosylated steviol glycosides as a food additive in different food categories. EFSA J. 2022, 20, e07066. [Google Scholar] [CrossRef]
- Use of Non-Sugar Sweeteners: WHO Guideline; World Health Organization: Geneva, Switzerland, 2023.
- EFSA. Scientific Opinion on the safety of steviol glycosides for the proposed uses as a food additive. EFSA J. 2010, 8, 1537. [Google Scholar] [CrossRef]
- Hamdani, N.; Franssen, C.; Lourenco, A.; Falcao-Pires, I.; Fontoura, D.; Leite, S.; Plettig, L.; Lopez, B.; Ottenheijm, C.A.; Becher, P.M.; et al. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ. Heart Fail. 2013, 6, 1239–1249. [Google Scholar] [CrossRef]
- Pesta, D.; Gnaiger, E. Preparation of permeabilized muscle fibers for diagnosis of mitochondrial respiratory function. Mitochondr. Physiol. Netw. 2015, 14, 1–5. [Google Scholar]
- Gonçalves-Rodrigues, P.; Almeida-Coelho, J.; Gonçalves, A.; Amorim, F.; Leite-Moreira, A.F.; Stienen, G.J.M.; Falcão-Pires, I. In Vitro Assessment of Cardiac Function Using Skinned Cardiomyocytes. J. Vis. Exp. 2020, e60427. [Google Scholar] [CrossRef]
- Kirilmaz, O.B.; Salegaonkar, A.R.; Shiau, J.; Uzun, G.; Ko, H.S.; Lee, H.F.; Park, S.; Kwon, G. Study of blood glucose and insulin infusion rate in real-time in diabetic rats using an artificial pancreas system. PLoS ONE 2021, 16, e0254718. [Google Scholar] [CrossRef] [PubMed]
- Suanarunsawat, T.; Klongpanichapak, S.; Rungseesantivanon, S.; Chaiyabutr, N. Glycemic effect of stevioside and Stevia rebaudiana in streptozotocin-induced diabetic rats. East. J. Med. 2004, 9, 51–56. [Google Scholar]
- Maki, K.C.; Curry, L.L.; Reeves, M.S.; Toth, P.D.; McKenney, J.M.; Farmer, M.V.; Schwartz, S.L.; Lubin, B.C.; Boileau, A.C.; Dicklin, M.R.; et al. Chronic consumption of rebaudioside A, a steviol glycoside, in men and women with type 2 diabetes mellitus. Food Chem. Toxicol. 2008, 46 (Suppl. S7), S47–S53. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lee, J.H.; Kang, M.S.; Kim, T.H.; Jeong, S.J.; Kim, C.H.; Kim, S.S.; Kim, I.J. Glycemic Effects of Rebaudioside A and Erythritol in People with Glucose Intolerance. Diabetes Metab. J. 2016, 40, 283–289. [Google Scholar] [CrossRef]
- Jan, S.A.; Habib, N.; Shinwari, Z.K.; Ali, M.; Ali, N. The anti-diabetic activities of natural sweetener plant Stevia: An updated review. SN Appl. Sci. 2021, 3, 517. [Google Scholar] [CrossRef]
- Suanarunsawat, T.; Chaiyabutr, N. The effect of stevioside on glucose metabolism in rat. Can. J. Physiol. Pharmacol. 1997, 75, 976–982. [Google Scholar] [CrossRef]
- Prata, C.; Zambonin, L.; Rizzo, B.; Maraldi, T.; Angeloni, C.; Vieceli Dalla Sega, F.; Fiorentini, D.; Hrelia, S. Glycosides from Stevia rebaudiana Bertoni Possess Insulin-Mimetic and Antioxidant Activities in Rat Cardiac Fibroblasts. Oxid. Med. Cell Longev. 2017, 2017, 3724545. [Google Scholar] [CrossRef]
- Casas-Grajales, S.; Reyes-Gordillo, K.; Cerda-García-Rojas, C.M.; Tsutsumi, V.; Lakshman, M.R.; Muriel, P. Rebaudioside A administration prevents experimental liver fibrosis: An in vivo and in vitro study of the mechanisms of action involved. J. Appl. Toxicol. 2019, 39, 1118–1131. [Google Scholar] [CrossRef]
- Xi, D.; Bhattacharjee, J.; Salazar-Gonzalez, R.-M.; Park, S.; Jang, A.; Warren, M.; Merritt, R.; Michail, S.; Bouret, S.; Kohli, R. Rebaudioside affords hepatoprotection ameliorating sugar sweetened beverage- induced nonalcoholic steatohepatitis. Sci. Rep. 2020, 10, 6689. [Google Scholar] [CrossRef]
- Bartlett, A.Q.; Vesco, K.K.; Purnell, J.Q.; Francisco, M.; Goddard, E.; Guan, X.; DeBarber, A.; Leo, M.C.; Baetscher, E.; Rooney, W.; et al. Pregnancy and weaning regulate human maternal liver size and function. Proc. Natl. Acad. Sci. USA 2021, 118, e2107269118. [Google Scholar] [CrossRef]
- Saravanan, R.; Ramachandran, V. Modulating efficacy of Rebaudioside A, a diterpenoid on antioxidant and circulatory lipids in experimental diabetic rats. Environ. Toxicol. Pharmacol. 2013, 36, 472–483. [Google Scholar] [CrossRef]
- Ilias, A.N.; Ismail, I.S.; Hamzah, H.; Mohd Mohidin, T.B.; Idris, M.F.; Ajat, M. Rebaudioside A Enhances LDL Cholesterol Uptake in HepG2 Cells via Suppression of HMGCR Expression. Rep. Biochem. Mol. Biol. 2021, 10, 477–487. [Google Scholar] [CrossRef]
- Wang, J.; Shen, W.; Zhang, J.-Y.; Jia, C.-H.; Xie, M.-L. Stevioside attenuates isoproterenol-induced mouse myocardial fibrosis through inhibition of the myocardial NF-κB/TGF-β1/Smad signaling pathway. Food Funct. 2019, 10, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Osorio, J.C.; Manlhiot, C.; Gruber, D.; Homma, S.; Mital, S. Hypertrophy signaling during peripartum cardiac remodeling. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3008–H3013. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, J.; Qin, L.; Zhang, X.; Mei, Y. Stevioside improved hyperglycemia-induced cardiac dysfunction by attenuating the development of fibrosis and promoting the degradation of established fibrosis. J. Funct. Foods 2020, 68, 103895. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Kang, M.J. Mitochondrial dysfunction and damage associated molecular patterns (DAMPs) in chronic inflammatory diseases. Mitochondrion 2018, 41, 37–44. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef]
- Wallace, D.C.; Fan, W.; Procaccio, V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 2010, 5, 297–348. [Google Scholar] [CrossRef] [PubMed]
- Satapati, S.; He, T.; Inagaki, T.; Potthoff, M.; Merritt, M.E.; Esser, V.; Mangelsdorf, D.J.; Kliewer, S.A.; Browning, J.D.; Burgess, S.C. Partial resistance to peroxisome proliferator-activated receptor-alpha agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes 2008, 57, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Z.; Lam, S.M.; Shui, G. Rebaudioside A Enhances Resistance to Oxidative Stress and Extends Lifespan and Healthspan in Caenorhabditis elegans. Antioxidants 2021, 10, 262. [Google Scholar] [CrossRef]
- Franco-Obregón, A.; Gilbert, J.A. The Microbiome-Mitochondrion Connection: Common Ancestries, Common Mechanisms, Common Goals. mSystems 2017, 2, e00018-17. [Google Scholar] [CrossRef]
- Gesper, M.; Nonnast, A.B.H.; Kumowski, N.; Stoehr, R.; Schuett, K.; Marx, N.; Kappel, B.A. Gut-Derived Metabolite Indole-3-Propionic Acid Modulates Mitochondrial Function in Cardiomyocytes and Alters Cardiac Function. Front. Med. 2021, 8, 648259. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.E.; Cho, N.A.; Klancic, T.; Nicolucci, A.C.; Shearer, J.; Borgland, S.L.; Johnston, L.A.; Ramay, H.R.; Noye Tuplin, E.; Chleilat, F.; et al. Maternal low-dose aspartame and stevia consumption with an obesogenic diet alters metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring. Gut 2020, 69, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.E.; Klancic, T.; Schick, A.; Choo, A.C.; Shearer, J.; Borgland, S.L.; Chleilat, F.; Mayengbam, S.; Reimer, R.A. Low-Dose Stevia (Rebaudioside A) Consumption Perturbs Gut Microbiota and the Mesolimbic Dopamine Reward System. Nutrients 2019, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- El-Hattab, A.W.; Scaglia, F. Mitochondrial Cardiomyopathies. Front. Cardiovasc. Med. 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Pulakat, L.; Whaley-Connell, A.; Sowers, J.R. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J. Mol. Med. 2010, 88, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Sengupta, S.; Bandyopadhyay, T.K.; Bhattacharyya, A. Stevioside induced ROS-mediated apoptosis through mitochondrial pathway in human breast cancer cell line MCF-7. Nutr. Cancer 2012, 64, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Lima, Y.C.; Kurauti, M.A.; da Fonseca Alves, G.; Ferezini, J.; Piovan, S.; Malta, A.; de Almeida, F.L.A.; Gomes, R.M.; de Freitas Mathias, P.C.; Milani, P.G.; et al. Whey protein sweetened with Stevia rebaudiana Bertoni (Bert.) increases mitochondrial biogenesis markers in the skeletal muscle of resistance-trained rats. Nutr. Metab. 2019, 16, 65. [Google Scholar] [CrossRef]
- Han, J.-Y.; Park, M.; Lee, H.-J. Stevia (Stevia rebaudiana) extract ameliorates insulin resistance by regulating mitochondrial function and oxidative stress in the skeletal muscle of db/db mice. BMC Complement. Med. Ther. 2023, 23, 264. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Heneghan, C.J. Effect of the natural sweetener, steviol glycoside, on cardiovascular risk factors: A systematic review and meta-analysis of randomised clinical trials. Eur. J. Prev. Cardiol. 2015, 22, 1575–1587. [Google Scholar] [CrossRef]
- Redruello-Requejo, M.; González-Rodríguez, M.; Samaniego-Vaesken, M.D.L.; Montero-Bravo, A.; Partearroyo, T.; Varela-Moreiras, G. Low- and No-Calorie Sweetener (LNCS) Consumption Patterns Amongst the Spanish Adult Population. Nutrients 2021, 13, 1845. [Google Scholar] [CrossRef] [PubMed]
- Halasa, B.C.; Sylvetsky, A.C.; Conway, E.M.; Shouppe, E.L.; Walter, M.F.; Walter, P.J.; Cai, H.; Hui, L.; Rother, K.I. Non-Nutritive Sweeteners in Human Amniotic Fluid and Cord Blood: Evidence of Transplacental Fetal Exposure. Am. J. Perinatol. 2023, 40, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
| Control | RebA | p-Value b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SEM | (n) | Mean | ± | SEM | (n) | ||
| Mating efficiency (n mating attempts) a | 1.9 | ± | 0.4 | (8) | 2.5 | ± | 0.5 | (8) | 0.300 |
| Gestational age at birth (days) | 21.5 | ± | 0.3 | (8) | 21.9 | ± | 0.1 | (8) | 0.224 |
| Litter size (pups) | 12.5 | ± | 1.1 | (8) | 14.3 | ± | 0.5 | (8) | 0.526 |
| Female-to-male ratio | 1.1 | ± | 0.2 | (6) | 0.8 | ± | 0.1 | (8) | 0.138 |
| Body weight at euthanasia (g) | 323.4 | ± | 4.8 | (8) | 314.5 | ± | 5.8 | (8) | 0.257 |
| Organ Weight | Control | RebA | p-Value b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SEM | (n) | Mean | ± | SEM | (n) | ||
| Liver/BSA (mg/cm2) | 23.3 | ± | 0.38 | (8) | 21.6 | ± | 0.31 | (8) | 0.005 |
| Pancreas/BSA (mg/cm2) | 1.94 | ± | 0.22 | (7) | 2.42 | ± | 0.21 | (8) | 0.188 |
| Heart/BSA (mg/cm2) | 26.8 | ± | 0.05 | (8) | 25.0 | ± | 0.04 | (8) | 0.031 |
| Skeletal muscle/BSA (mg/cm2) | 4.73 | ± | 0.17 | (8) | 4.87 | ± | 0.06 | (7) | 0.505 |
| Parameter | Control Mean ± SEM | (n) | RebA Mean ± SEM | (n) | p-Value |
|---|---|---|---|---|---|
| Heart rate (bpm) | 287.5 ± 6.9 | (8) | 269.2 ± 9.4 | (8) | 0.165 |
| LV end-diastolic volume/BSA (cm3.cm−2) | 1.260 ± 0.065 | (8) | 1.20 ± 0.054 | (8) | 0.604 |
| LV end-systolic volume/BSA (cm3.cm−2) | 0.049 ± 0.042 | (8) | 0.050 ± 0.040 | (8) | 0.969 |
| Interventricular septum (mm) | 1.48 ± 0.13 | (8) | 1.39 ± 0.13 | (8) | 0.171 |
| Posterior LV wall (mm) | 0.004 ± 85 × 10−5 | (8) | 0.003 ± 12 × 10−5 | (8) | 0.047 |
| LV mass/BSA (g·cm−2) | 1.614 ± 0.081 | (8) | 1.508 ± 0.060 | (8) | 0.329 |
| Relative wall thickness | 0.890 ± 0.025 | (8) | 0.780 ± 0.029 | (8) | 0.025 |
| Ejection fraction (%) | 75.38 ± 2.30 | (8) | 71.28 ± 1.43 | (8) | 0.176 |
| Cardiac index mL·min·cm−2 | 0.113 ± 0.005 | (8) | 0.102 ± 0.005 | (8) | 0.152 |
| E/A | 1.88 ± 0.54 | (8) | 2.15 ± 0.69 | (8) | 0.398 |
| Tei index | 0.44 ± 0.06 | (8) | 0.46 ± 0.10 | (8) | 0.748 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bracchi, I.; Morais, J.; Coelho, J.A.; Ferreira, A.F.; Alves, I.; Mendes, C.; Correia, B.; Gonçalves, A.; Guimarães, J.T.; Falcão-Pires, I.; et al. The Cardiometabolic Impact of Rebaudioside A Exposure during the Reproductive Stage. Biology 2024, 13, 163. https://doi.org/10.3390/biology13030163
Bracchi I, Morais J, Coelho JA, Ferreira AF, Alves I, Mendes C, Correia B, Gonçalves A, Guimarães JT, Falcão-Pires I, et al. The Cardiometabolic Impact of Rebaudioside A Exposure during the Reproductive Stage. Biology. 2024; 13(3):163. https://doi.org/10.3390/biology13030163
Chicago/Turabian StyleBracchi, Isabella, Juliana Morais, João Almeida Coelho, Ana Filipa Ferreira, Inês Alves, Cláudia Mendes, Beatriz Correia, Alexandre Gonçalves, João Tiago Guimarães, Inês Falcão-Pires, and et al. 2024. "The Cardiometabolic Impact of Rebaudioside A Exposure during the Reproductive Stage" Biology 13, no. 3: 163. https://doi.org/10.3390/biology13030163
APA StyleBracchi, I., Morais, J., Coelho, J. A., Ferreira, A. F., Alves, I., Mendes, C., Correia, B., Gonçalves, A., Guimarães, J. T., Falcão-Pires, I., Keating, E., & Negrão, R. (2024). The Cardiometabolic Impact of Rebaudioside A Exposure during the Reproductive Stage. Biology, 13(3), 163. https://doi.org/10.3390/biology13030163








