Influence of Brainstem’s Area A5 on Sympathetic Outflow and Cardiorespiratory Dynamics
Abstract
Simple Summary
Abstract
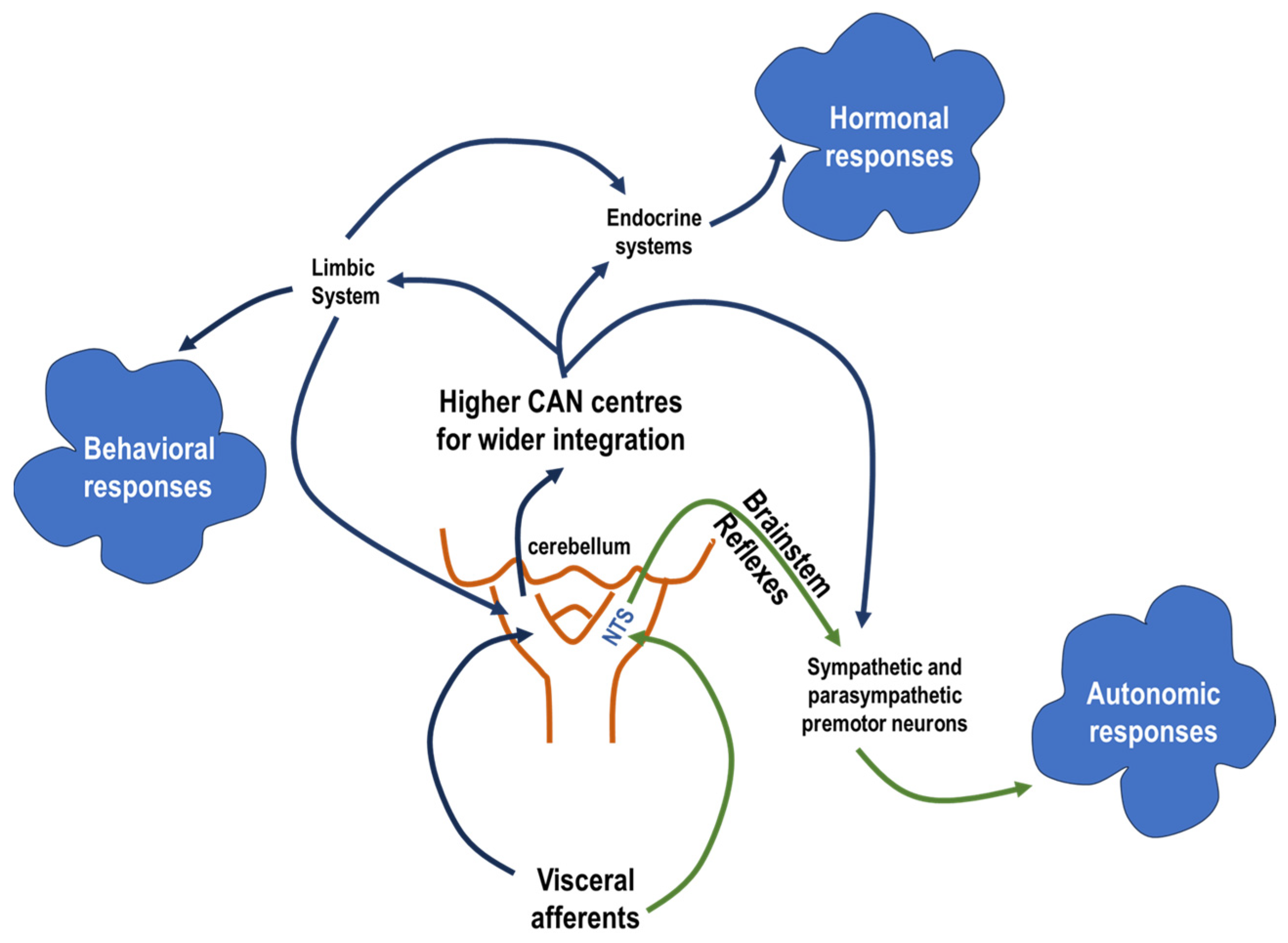
1. Introduction
2. Neuroanatomical Overview of Area A5
3. Neurofunctional Characteristics of A5 Neurones
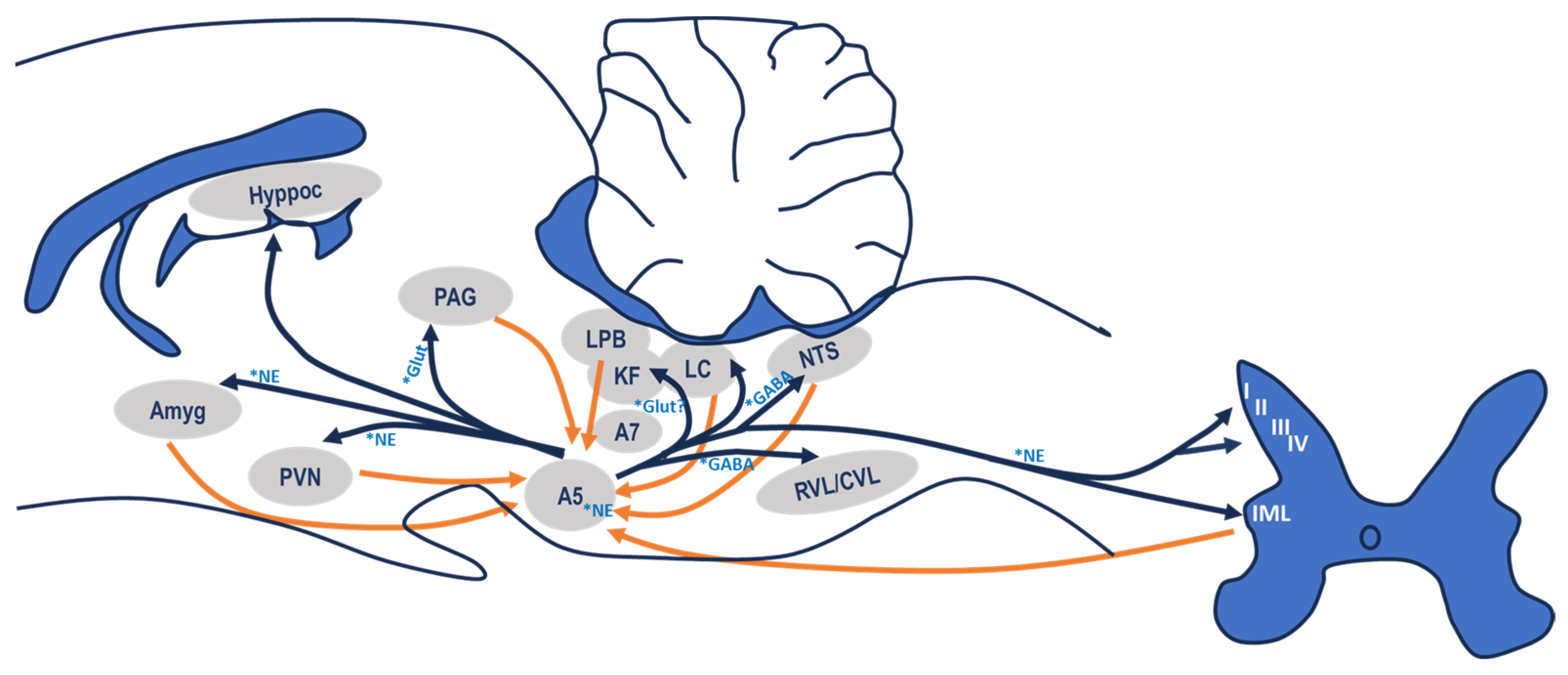
3.1. Afferent Connections
3.2. Efferent Connections
4. Area A5 and Cardiovascular Regulation
5. The Role of Area A5 in the Control of Breathing
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laranjo, S.; Geraldes, V.; Oliveira, M.; Rocha, I. Insights into the background of autonomic medicine. Rev. Port. Cardiol. 2007, 36, 757–771. [Google Scholar] [CrossRef]
- Jänig, W. The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Coote, J.H.; Spyer, K.M. Central control of autonomic function. Brain Neurosci. Adv. 2018, 2, 2398212818812012. [Google Scholar] [CrossRef]
- Braun, J.; Patel, M.; Kameneva, T.; Keatch, C.; Lambert, G.; Lambert, E. Central stress path-ways in the development of cardiovascular disease. Clin. Auton. Res. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Goldstein, D.S. Stress and the “extended” autonomic system. Auton. Neurosci. 2021, 236, 102889. [Google Scholar] [CrossRef]
- Saha, S. Role of the central nucleus of the amygdala in the control of blood pressure: Descending pathways to medullary cardiovascular nuclei. Clin. Exp. Pharmacol. Physiol. 2005, 32, 450–456. [Google Scholar] [CrossRef]
- Díaz-Casares, A.; López-González, M.V.; Peinado-Aragonés, C.A.; González-Barón, S.; Dawid-Milner, M.S. Parabrachial complex glutamate receptors modulate the cardiorespir-atory response evoked from hypothalamic defense area. Auton. Neurosci. 2012, 169, 124–134. [Google Scholar] [CrossRef]
- López-González, M.V.; González-García, M.; Peinado-Aragonés, C.A.; Barbancho, M.A.; Díaz-Casares, A.; Dawid-Milner, M.S. Pontine A5 region modulation of the cardiorespiratory response evoked from the midbrain dorsolateral periaqueductal grey. J. Physiol. Biochem. 2020, 76, 561–572. [Google Scholar] [CrossRef]
- Benarroch, E.E. Physiology and pathophysiology of the autonomic nervous system. CONTINUUM Lifelong Learn. Neurol. 2020, 26, 12–24. [Google Scholar] [CrossRef]
- Byrum, C.E.; Guyenet, P.G. Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J. Comp. Neurol. 1987, 261, 529–542. [Google Scholar] [CrossRef]
- Jodkowski, J.S.; Coles, S.K.; Dick, T.E. Prolongation in expiration evoked from ven-trolateral pons of adult rats. J. Appl. Physiol. 1997, 82, 377–381. [Google Scholar] [CrossRef]
- Goodchild, A.K.; Phillips, J.K.; Lipski, J.; Pilowsky, P.M. Differential expression of cate-cholamine synthetic enzymes in the caudal ventral pons. J. Comp. Neurol. 2001, 438, 457–467. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K. Localization of monoamines in the lower brain stem. Experientia 1964, 20, 398–399. [Google Scholar] [CrossRef]
- Byrum, C.E.; Stornetta, R.; Guyenet, P.G. Electrophysiological properties of spinal-ly-projecting A5 noradrenergic neurons. Brain Res. 1984, 303, 15–29. [Google Scholar] [CrossRef]
- Blessing, W.W.; Goodchild, A.K.; Dampney, R.A.; Chalmers, J.P. Cell groups in the lower brain stem of the rabbit projecting to the spinal cord, with special reference to catechola-mine-containing neurons. Brain Res. 1981, 221, 35–55. [Google Scholar] [CrossRef]
- Lackner, K.J. Mapping of monoamine neurones and fibres in the cat lower brainstem and spinal cord. Anat. Embryol. 1980, 161, 169–195. [Google Scholar] [CrossRef]
- Viemari, J.C. Noradrenergic modulation of the respiratory neural network. Respir. Physiol. Neurobiol. 2008, 164, 123–130. [Google Scholar] [CrossRef]
- Galgani, A.; Bartolini, E.; D’Amora, M.; Faraguna, U.; Giorgi, F.S. The Ce-tral Noradrenergic System in Neurodevelopmental Disorders: Merging Experimental and Clin-ical Evidence. Int. J. Mol. Sci. 2023, 24, 5805. [Google Scholar] [CrossRef]
- Galland, B.C.; Elder, D.E. Sudden unexpected death in infancy: Biological mechanisms. Paediatr. Respir. Rev. 2014, 15, 287–292. [Google Scholar] [CrossRef]
- Marien, M.R.; Colpaert, F.C.; Rosenquist, A.C. Noradrenergic mechanisms in neurodegenerative diseases: A theory. Brain Res. Rev. 2004, 45, 38–78. [Google Scholar] [CrossRef]
- Minghetti, L.; Carnevale, D.; Simone, R.D. Microglia-neuron interaction in inflammatory and degenerative diseases: Role of cholinergic and noradrenergic systems. CNS Neurol. Dis.-Drug Targets 2007, 6, 388–397. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic modulation of cognition in health and disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef]
- Krohn, F.; Lancini, E.; Ludwig, M.; Leiman, M.; Guruprasath, G.; Haag, L.; Panczyszyn, J.; Düzel, E.; Hämmerer, D.; Betts, M. Noradrenergic neuromodulation in ageing and disease. Neurosci. Biobehav. Rev. 2023, 152, 105311. [Google Scholar] [CrossRef]
- Llewellyn-Smith, I.J. Sympathetic preganglionic neurons. In Central Regulation of Autonomic Functions, 2nd ed.; Llewellyn-Smith, I.J., Verberne, A.J., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 98–119. [Google Scholar]
- Huangfu, D.; Hwang, T.; Riley, T.A.; Guyenet, P.G. Splanchnic nerve response to A5-area stimulation in rats. Am. J. Physiol. 1992, 263, R437–R446. [Google Scholar] [CrossRef]
- Woodruff, M.L.; Baisden, R.H.; Whittington, D.L.; Kelly, J.E. Inputs to the pontine A5 noradrenergic cell group: A horseradish peroxidase study. Exp. Neurol. 1986, 94, 782–787. [Google Scholar] [CrossRef]
- Díaz-Casares, A.; López-González, M.V.; Peinado-Aragonés, C.A.; Lara, J.P.; González-Barón, S.; Dawid-Milner, M.S. Role of the parabrachial complex in the cardiorespir-atory response evoked from hypothalamic defense area stimulation in the anesthetized rat. Brain Res. 2009, 1279, 58–70. [Google Scholar] [CrossRef]
- López-González, M.V.; Díaz-Casares, A.; Peinado-Aragonés, C.A.; Lara, J.P.; Barbancho, M.A.; Dawid-Milner, M.S. Neurons of the A5 region are required for the tach-ycardia evoked by electrical stimulation of the hypothalamic defence area in anaesthetized rats. Exp. Physiol. 2013, 98, 1279–1294. [Google Scholar] [CrossRef]
- Song, G.; Xu, H.; Wang, H.; Macdonald, S.M.; Poon, C.S. Hypoxia-excited neurons in NTS send axonal projections to Kölliker-Fuse/parabrachial complex in dorsolateral pons. Neuroscience 2011, 175, 145–153. [Google Scholar] [CrossRef]
- Kanbar, R.; Depuy, S.D.; West, G.H.; Stornetta, R.L.; Guyenet, P.G. Regulation of visceral sympathetic tone by A5 noradrenergic neurons in rodents. J. Physiol. 2011, 589, 903–917. [Google Scholar] [CrossRef]
- Bajic, D.; Van Bockstaele, E.J.; Proudfit, H.K. Ultrastructural analysis of rat ventrolateral periaqueductal gray projections to the A5 cell group. Neuroscience 2012, 224, 145–159. [Google Scholar] [CrossRef]
- González-García, M.; Carrillo-Franco, L.; Peinado-Aragonés, C.A.; Díaz-Casares, A.; Gago, B.; López-González, M.V.; Dawid-Milner, M.S. Impact of the glutamatergic neurotransmis-sion within the A5 region on the cardiorespiratory response evoked from the midbrain dlPAG. Pflugers Arch. 2023, 75, 505–516. [Google Scholar] [CrossRef]
- Dampney, R.A. Central neural control of the cardiovascular system: Current perspec-tives. Adv. Physiol. Educ. 2016, 40, 283–296. [Google Scholar] [CrossRef]
- Rosin, D.L.; Chang, D.A.; Guyenet, P.G. Afferent and efferent connections of the rat retrotrapezoid nucleus. J. Comp. Neurol. 2006, 499, 64–89. [Google Scholar] [CrossRef]
- Bruinstroop, E.; Cano, G.; Vanderhorst, V.G.; Cavalcante, J.C.; Wirth, J.; Sena-Esteves, M.; Saper, C.B. Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. J. Comp. Neurol. 2012, 520, 1985–2001. [Google Scholar] [CrossRef]
- Benarroch, E.E. Descending monoaminergic pain modulation: Bidirectional control and clinical relevance. Neurology 2008, 71, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, A. The noradrenergic pain regulation system: A potential target for pain therapy. Eur. J. Pharmacol. 2013, 716, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Delbono, O.; Wang, Z.M.; Messi, M.L. Brainstem noradrenergic neurons: Identifying a hub at the intersection of cognition, motility, and skeletal muscle regulation. Acta Physiol. 2022, 236, e13887. [Google Scholar] [CrossRef] [PubMed]
- Huckstepp, R.T.; Cardoza, K.P.; Henderson, L.E.; Feldman, J.L. Distinct parafacial regions in control of breathing in adult rats. PLoS ONE 2018, 13, e0201485. [Google Scholar] [CrossRef] [PubMed]
- Herbert, H.; Moga, M.M.; Saper, C.B. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol. 1990, 93, 540–580. [Google Scholar] [CrossRef] [PubMed]
- Rukhadze, I.; Rubin, L. Mesopontine cholinergic projections to the hypo-glossal motor nucleus. Neurosci. Lett. 2007, 413, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Specht, L.A.; Pickel, V.M.; Joh, T.H.; Reis, D.J. Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain. I. Early ontogeny. J. Comp. Neurol. 1981, 199, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Specht, L.A.; Pickel, V.M.; Joh, T.H.; Reis, D.J. Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain. II. Late ontogeny. J. Comp. Neurol. 1981, 199, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, D.H.; Koshiya, N.; Guyenet, P.G. A5 noradrenergic unit activity and sympathetic nerve discharge in rats. Am. J. Physiol. 1991, 261, R393–R402. [Google Scholar] [CrossRef] [PubMed]
- Neil, J.J.; Loewy, A.D. Decreases in blood pressure in response to Lglutamate mi-croinjections into the A5 catecholamine cell group. Brain Res. 1982, 241, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.; Aghajanian, G.K. Single cell activity in the noradrenergic A-5 region: Responses to drugs and peripheral manipulations of blood pressure. Brain Res. 1982, 242, 125–135. [Google Scholar] [CrossRef]
- Guyenet, P.G. Baroreceptor-mediated inhibition of A5 noradrenergic neurons. Brain Res. 1984, 303, 31–40. [Google Scholar] [CrossRef]
- Jeske, I.; Reis, D.J.; Milner, T.A. Neurons in the barosensory area of the caudal ventrolateral medulla project monosynaptically on to sympathoexcitatory bulbospinal neurons in the rostral ventrolateral medulla. Neuroscience 1995, 65, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Kwiat, G.C.; Liu, H.; Williamson, A.M.; Basbaum, A.I. GABAergic regulation of noradrenergic spinal projection neurons of the A5 cell group in the rat: An electron microscopic analysis. J. Comp. Neurol. 1993, 330, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G.; Byrum, C.E. Comparative effects of sciatic nerve stimulation, blood pressure, and morphine on the activity of A5 and A6 pontine noradrenergic neurons. Brain Res. 1985, 327, 191–201. [Google Scholar] [CrossRef]
- Li, Y.W.; Dampney, R.A.L. Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience 1994, 61, 613–634. [Google Scholar] [CrossRef]
- Polson, J.W.; Mrljak, S.; Potts, P.D.; Dampney, R.A. Fos expression in spinally projecting neurons after hypotension in the conscious rabbit. Auton. Neurosci. 2002, 100, 10–20. [Google Scholar] [CrossRef]
- Chan, R.K.W.; Sawchenko, P.E. Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholamilriergic cell groups induced by cardiovascular challenges in the rat. J. Comp. Neurol. 1994, 348, 433–460. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.S. Papel da área A5 na resposta simpato-excitatória do quimiorreflexo em ratos não-anestesiados. Ph.D. Thesis, Departamento de Fisiologia e Biofísica, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2002. [Google Scholar]
- Coote, J.H.; Macleod, V.H. Evidence for the involvement in the baroreceptor reflex of a descending inhibitory pathway. J. Physiol. 1974, 241, 477–496. [Google Scholar] [CrossRef]
- Westlund, K.N.; Bowker, R.M.; Ziegler, M.G.; Coulter, J.D. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983, 263, 15–31. [Google Scholar] [CrossRef]
- Taxini, C.L.; Takakura, A.C.; Gargaglioni, L.H.; Moreira, T.S. Control of the central chemoreflex by A5 noradrenergic neurons in rats. Neuroscience 2011, 199, 177–186. [Google Scholar] [CrossRef]
- Pilowsky, P.M.; Goodchild, A.K. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J. Hypertens. 2002, 20, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Dampney, R.A.; Horiuchi, J.; Tagawa, T.; Fontes, M.A.; Potts, P.D.; Polson, J.W. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol. Scand. 2003, 177, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Taxini, C.L.; Marques, D.A.; Bícego, K.C.; Gargaglioni, L.H. A5 noradren-ergic neurons and breathing control in neonate rats. Pflügers Arch.-Eur. J. Physiol. 2021, 473, 859–872. [Google Scholar] [CrossRef]
- Taxini, C.L.; Moreira, T.S.; Takakura, A.C.; Bícego, K.C.; Gargaglioni, L.H.; Zoccal, D.B. Role of A5 noradrenergic neurons in the chemoreflex control of respiratory and sympathetic activities in unanesthetized conditions. Neuroscience 2017, 354, 146–157. [Google Scholar] [CrossRef]
- López-González, M.V.; Díaz-Casares, A.; González-García, M.; Peinado-Aragonés, C.A.; Barbancho, M.A.; Carrillo de Albornoz, M.; Dawid-Milner, M.S. Glutamate receptors of the A5 region modulate cardiovascular responses evoked from the dorsomedial hypothalamic nucleus and perifornical area. J. Physiol. Biochem. 2018, 74, 325–334. [Google Scholar] [CrossRef]
- Souza, G.M.; Stornetta, D.S.; Vitali, A.J.; Wildner, H.; Zeilhofer, H.U.; Campbell, J.N.; Abbott, S.B. Chemogenetic activation of noradrenergic A5 neurons increases blood pressure and visceral sympathetic activity in adult rats. Am. J. Physiol.-Reg. Int. Comp. Physiol. 2022, 323, R512–R531. [Google Scholar] [CrossRef]
- Del Negro, C.A.; Funk, G.D.; Feldman, J.L. Breathing matters. Nat. Rev. Neurosci. 2008, 19, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.; Moreira, T.S.; Kuo, F.S.; Mulkey, D.K.; Takakura, A.C. α1- and α2-adrenergic receptors in the retrotrapezoid nucleus differentially regulate breathing in anesthetized adult rats. J. Neurophysiol. 2016, 116, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, T. The regulation of respiration: Part I. J. Physiol. 1923, 58, 81–91. [Google Scholar] [CrossRef]
- Dutschmann, M.; Dick, T.E. Pontine mechanisms of respiratory control. Compr. Physiol. 2012, 2, 2443–2469. [Google Scholar]
- Navarrete-Opazo, A.A.; Cook-Snyder, D.R.; Miller, J.R.; Callison, J.J.; McCarthy, N.; Palkovic, B.; Stuth, E.A.E.; Zuperku, E.J.; Stucke, A.G. Endogenous glutamatergic inputs to the Para-brachial Nucleus/Kölliker-Fuse Complex determine respiratory rate. Respir. Physiol. Neurobiol. 2020, 277, 103401. [Google Scholar] [CrossRef]
- Monteau, R.; Gauthier, P.; Rega, P.; Hilaire, G. Effects of N-methyl-D-aspartate (NMDA) antagonist MK-801 on breathing pattern in rats. Neurosci. Lett. 1990, 109, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Dawid-Milner, M.S.; Lara, J.P.; González-Barón, S.; Spyer, K.M. Respiratory effects of stimulation of cell bodies of the A5 region in the anaesthetised rat. Eur. J. Physiol. 2001, 441, 434–443. [Google Scholar] [CrossRef]
- Loewy, A.D.; Gregorie, E.M.; McKellar, S.; Baker, R.P. Electrophysiological evidence that the A5 catecholamine cell group is a vasomotor center. Brain Res. 1979, 178, 196–200. [Google Scholar] [CrossRef]
- Hilaire, G.; Viemari, J.C.; Coulon, P.; Simonneau, M.; Bévengut, M. Modulation of the res-piratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir. Physiol. Neurobiol. 2004, 143, 187–197. [Google Scholar] [CrossRef]
- Viemari, J.C.; Bévengut, M.; Coulon, P.; Hilaire, G. Nasal trigeminal inputs release the A5 inhibition received by the respiratory rhythm generator of the mouse neonate. J. Neurophysiol. 2004, 91, 746–758. [Google Scholar] [CrossRef][Green Version]
- Ito, Y.; Oyamada, Y.; Hakuno, H.; Yamaguchi, K. Morphological analysis of developmental changes in pontine noradrenergic neuronal groups in the neonatal rat. Brain Res. 2002, 925, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.K.; Dick, T.E. Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. J. Physiol. 1996, 497, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, E.H.; Prestbo, A. Elimination of the post-hypoxic frequency decline in conscious rats lesioned in pontine A5 region. Respir. Physiol. Neurobiol. 2003, 138, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.K.; Ernsberger, P.; Dick, T.E. Post-hypoxic frequency decline does not depend on α2-adrenergic receptors in the adult rat. Brain Res. 1998, 794, 267–273. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, I.; González-García, M.; Carrillo-Franco, L.; Dawid-Milner, M.S.; López-González, M.V. Influence of Brainstem’s Area A5 on Sympathetic Outflow and Cardiorespiratory Dynamics. Biology 2024, 13, 161. https://doi.org/10.3390/biology13030161
Rocha I, González-García M, Carrillo-Franco L, Dawid-Milner MS, López-González MV. Influence of Brainstem’s Area A5 on Sympathetic Outflow and Cardiorespiratory Dynamics. Biology. 2024; 13(3):161. https://doi.org/10.3390/biology13030161
Chicago/Turabian StyleRocha, Isabel, Marta González-García, Laura Carrillo-Franco, Marc Stefan Dawid-Milner, and Manuel Victor López-González. 2024. "Influence of Brainstem’s Area A5 on Sympathetic Outflow and Cardiorespiratory Dynamics" Biology 13, no. 3: 161. https://doi.org/10.3390/biology13030161
APA StyleRocha, I., González-García, M., Carrillo-Franco, L., Dawid-Milner, M. S., & López-González, M. V. (2024). Influence of Brainstem’s Area A5 on Sympathetic Outflow and Cardiorespiratory Dynamics. Biology, 13(3), 161. https://doi.org/10.3390/biology13030161





