Simple Summary
Natural killer cells and cytotoxic CD8+ T cells play a complementary role in controlling cytomegalovirus, a ubiquitous herpesvirus that establishes a lifelong latent infection in the host. Due to its ability to modulate the immune response, cytomegalovirus can impact the course of multiple sclerosis, an immune-mediated disease of the central nervous system. However, whether this herpesvirus may play a beneficial or a detrimental role in multiple sclerosis is currently uncertain. The cytomegalovirus seropositivity status increases both the expansion of highly differentiated CD8+ T cells and the number of natural killer cells expressing the NKG2C receptor. Given that NKG2C is an activating receptor that enhances natural killer cell-mediated cytotoxicity and recruitment of inflammatory cells, it is reasonable to investigate the involvement of this receptor in immune-mediated diseases. In the present study, we explored the magnitude of cytomegalovirus-induced immune changes in multiple sclerosis in order to increase the knowledge of the relationship between viruses and immune-mediated diseases.
Abstract
Multiple sclerosis (MS) is a debilitating neurological disease that has been classified as an immune-mediated attack on myelin, the protective sheath of nerves. Some aspects of its pathogenesis are still unclear; nevertheless, it is generally established that viral infections influence the course of the disease. Cytomegalovirus (CMV) is a major pathogen involved in alterations of the immune system, including the expansion of highly differentiated cytotoxic CD8+ T cells and the accumulation of adaptive natural killer (NK) cells expressing high levels of the NKG2C receptor. In this study, we evaluated the impact of latent CMV infection on MS patients through the characterization of peripheral NK cells, CD8+ T cells, and NKT-like cells using flow cytometry. We evaluated the associations between immune cell profiles and clinical features such as MS duration and MS progression, evaluated using the Expanded Disability Status Scale (EDSS). We showed that NK cells, CD8+ T cells, and NKT-like cells had an altered phenotype in CMV-infected MS patients and displayed high levels of the NKG2C receptor. Moreover, in MS patients, increased NKG2C expression levels were found to be associated with higher EDSS scores. Overall, these results support the hypothesis that CMV infection imprints the immune system by modifying the phenotype and receptor repertoire of NK and CD8+ T cells, suggesting a detrimental role of CMV on MS progression.
Keywords:
cytomegalovirus; multiple sclerosis; NK cells; CD8+ T cells; NKT-like cells; NKG2C; flow cytometry 1. Introduction
Multiple sclerosis (MS) is a multifactorial demyelinating and neurodegenerative disorder of the central nervous system (CNS); the deregulation of the immune response plays a crucial role in its pathophysiology [1,2]. Genetic and environmental factors such as viral infections are strongly linked to MS susceptibility and progression [3]. Viruses can cause damage directly by infecting the CNS or indirectly through modifications of the immune response, and several authors have focused on herpesvirus involvement for its ability to induce a persistent latent infection in the hosts [4,5]. The Epstein–Barr virus (EBV) appears to play a major role in MS; however, many studies have also focused on the putative involvement of cytomegalovirus (CMV) [6,7]. There is controversy regarding the role of CMV in MS pathogenesis. Some studies reported that CMV is associated with a lower MS susceptibility [8], while others suggested that CMV may contribute to the exacerbation of MS [9,10]. CMV is a ubiquitous beta herpesvirus that infects 40–80% of adults in industrialized countries and the entire population in developing countries [11,12]. Usually, the primary infection is asymptomatic, but some individuals develop a mononucleosis-like disease. Once infected, the virus persists in the host for life as a latent infection in undifferentiated cells of the myeloid lineage, such as CD34+ hematopoietic progenitor cells [13]. Natural killer cells (NK) and CD8+ T cells are the main effector cells that play a crucial role in the defense against CMV [14,15].
NK cells are innate lymphoid cells that recognize and eliminate CMV-infected cells. CMV has a complex relationship with NK cells: it plays a crucial role in shaping the NK cell receptor repertoire and drives the expansion of the population of NK cells with memory-like features. In humans, memory-like NK cells are commonly associated with the expression of NKG2C [16]. NKG2C is an activating NK cell receptor of the C-type lectin superfamily that binds the human non-classical MHC class I molecule HLA-E on the cell surface [17,18]. NKG2C-positive NK cells display higher cytotoxic capacity, elevated sensitivity to cytokines, such as interleukin (IL)-12 and IL-18, and increased ability for interferon (IFN)-γ production compared to their NKG2C-negative counterparts [19,20]. The mechanisms by which CMV drives the expression of NKG2C on NK subsets are unknown; however, it is well established that NKG2C+ cells play a role in controlling CMV infection, both in vitro and in vivo [21]. Both human studies and animal models of MS support the role of NK cells in the pathogenesis of this disease [22,23]. However, whether the interaction between NKG2C and HLA-E plays a role in the pathogenesis of MS has been evaluated only in one in vitro study [24].
CD8+ T cells play a critical role in the immune response to viral infections. Persistent CMV infection represents a major contributor to the senescence of CD8+ T cells [25]. Specifically, CMV promotes the accumulation of late-differentiated CD8+ T cells characterized by replicative senescence, the inability to proliferate in response to different triggers, the loss of the CD28 co-stimulatory marker, and the acquisition of the CD57 receptor [26,27]. Quantitative changes in the late-differentiated CD8+ T cell population are observed in MS and other immune-mediated diseases [28].
Considering the profound impact that CMV exerts on the immune system, a putative influence in MS immunopathology is conceivable. However, it is currently unclear whether CMV through these immune changes may have a beneficial or a detrimental effect on MS. In this research, we explored the impact of CMV on MS through a characterization of peripheral NK cells and T cells to provide a better understanding of CMV-associated immune cell alterations and to investigate CMV putative involvement in MS.
2. Materials and Methods
2.1. Study Population
At the Neuroinfectious Unit of Policlinico Umberto I Hospital, Sapienza University of Rome, we enrolled MS patients. For each patient, blood samples were collected before starting disease-modifying therapies (DMTs). The following parameters were evaluated: sex, age, disease duration, and disease progression, the latter through the EDSS (Expanded Disability Status Scale), which scores disease progression from 0 to 10 in 0.5-unit increments, with higher values representing higher levels of disability [29]. We also enrolled an age- and gender-matched control population that included healthy donors (HDs). From each subject, a total of two samples were collected: one sample for plasma and cells, and the other for serum. The study was approved by the Ethics Committee of Policlinico Umberto I, Sapienza University of Rome (protocol numbers 130/13 and 353/20), and all subjects signed an informed consent form before blood collection.
2.2. CMV Serology
A standard serological diagnostic analysis was performed to evaluate specific CMV-IgG and CMV-IgM antibodies. The serostatus was determined by the Liaison® CMV IgG, IgM assay (DiaSorin, Saluggia, Italy), and the results are expressed as index value.
2.3. Detection of CMV DNA by Real-Time PCR
Real-time PCR was performed to reveal CMV DNA in the plasma samples. Viral DNA was extracted from 200 μL of plasma by the use of High Pure Viral Nucleic Acid Kit (Roche Biochemicals, Mannheim, Germany). The purified DNA was eluted and stored at −20 °C until use. For real-time PCR, the following primers and probes (TIB MOLBIOL, Berlin, Germany) were mixed with 2 μL of template DNA, 4 mM MgCl2, and 4 μL of LightCycler-FastStart DNA (Master Hybridization Probes kit; Roche Biochemicals, Mannheim, Germany): 0.4 μM forward primer, 0.4 μM reverse primer, 0.2 μM fluorescein hybridization probe, and 0.2 μM LC-Red 640 probe. The sequences of the used specific primers for the glycoprotein B gene (254 bp) of CMV were previously reported (GenBank accession no. A13758). The hybridization probe sequences (5′-3′) comprised the donor fluorophore probe (CGTTTCGTCGTAGCTACGCRTACAT-fluorescein) and the acceptor fluorophore probe (LC-Red 640-ACACCACTTATCTYCTGGGCAGC-phosphate). The primers and probes were produced by TIB MOLBIOL (Berlin, Germany). The real-time PCR assays were run in the LightCycler® 2.0 instrument (Roche Applied Science, Penzberg, Germany) using the following protocol: 10 min at 95 °C, followed by 45 cycles of 10 s denaturation at 95 °C, 10 s annealing at 58 °C, and 12 s extension at 72 °C. The melting temperature for the probe set was 59.2 °C.
2.4. Isolation of Peripheral Blood Mononuclear Cells
As previously described [30], peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples collected in ethylenediaminetetraacetic acid (EDTA) tubes by density gradient centrifugation (Histopaque® 1077-1, Sigma-Aldrich, Saint Louis, MO, USA). The isolated PBMCs (1 × 106) were resuspended in 90% heat-inactivated fetal bovine serum (FBS) (Sigma-Aldrich, Saint Louis, MO, USA) and 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Saint Louis, MO, USA) and stored in liquid nitrogen.
2.5. Flow Cytometry Antibody Staining
We performed multiparameter flow cytometry to characterize the phenotype of NK cells, T cells, and NKT-like cells. As previously described [31], after thawing, PBMCs were incubated with specific mAbs in the dark for 30 min at 4 °C. To exclude dead cells from the analysis, a fixable viability dye (Zombie Aqua, BioLegend, San Diego, CA, USA) was included in the staining mixture. The following mAbs were used in the study: anti-CD3, pacific blue (PB)-conjugated (clone HIT3a), anti-CD16, phycoerythrin-cyanin7 (PE-cy7)-conjugated (clone 3G8), anti-CD8, allophycocyanin (APC)-conjugated (clone SK1), anti-CD57, PE-conjugated (clone HNK-1), anti-CD56, APC-cy7-conjugated (clone 5.1H11), anti-CD28, fluorescein isothiocyanate (FITC)-conjugated (clone CD28.2), anti-NKG2C, (CD159c), and peridinin–chlorophyll protein complex–cyanine5.5 (PerCP-Cy 5.5)-conjugated (clone 134591). All the mAbs were purchased from BioLegend (San Diego, CA, USA), except for anti-NKG2C, which was from BD Biosciences (Franklin Lakes, NJ, USA).
2.6. Flow Cytometry Data Analysis
Flow cytometry was performed using the MACSQuant® Analyzer 10 (Miltenyi Biotec, Bergisch Gladbach, Germany). By combining mAbs, we identified the following cell populations: NK cells (CD3− CD56+), CD56bright NK cells (CD3− CD56++ CD16+/−), CD56dim NK cells (CD3− CD56+ CD16+), CD57+ NK cells (CD3− CD56+ CD16+ CD57+), T cells (CD3+ CD56−), NKT-like cells (CD3+ CD56+), CD8+ T cells (CD3+ CD56− CD8+), early-differentiated CD8+ T cells (CD3+ CD56− CD8+ CD28+ CD57−), and late-differentiated CD8+ T cells (CD3+ CD56− CD8+ CD28− CD57+). The expression of the NKG2C receptor was evaluated on CD57+ NK cells by median fluorescence intensity (MFI). The analysis was performed using FlowJo (version 10.8.1) software. The gating strategy is shown in Supplementary Materials Figure S1.
2.7. Statistical Analysis
Statistical analysis was performed with GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA). The Mann–Whitney U test was used to compare the medians of the two groups. Spearman correlation was used to evaluate the relationships between two continuous variables. The data from the experiments are expressed as median (range interquartile, IQR). Statistical significance was set at p < 0.05.
3. Results
3.1. Study Population
A total of 74 MS patients (39 males and 35 females) with a median age (IQR) of 51 (43–58) years were included in the study. At the time of enrollment, the median EDSS score (IQR) was 5.0 (3.0–6.0), while the median MS duration (IQR) was 11 (5–19) years. In addition, 18 HDs (7 males and 11 females) with a median age (IQR) of 52 (38–59) years were enrolled. Table 1 summarizes the demographic, clinical, and serological data of the 74 MS patients and 18 HDs included in the analyses.

Table 1.
Demographics and clinical characteristics of the study population.
3.2. Detection of Anti-CMV IgG Antibodies and CMV DNA in MS Patients
As reported in Table 1, the prevalence of anti-CMV IgG antibodies among the MS patients was 69%, specifically, 51% in males and 49% in females. No anti-CMV IgM antibodies, suggestive of recent primary infection, were detected. We found that the CMV serostatus in the MS patients was significantly associated with older age (p = 0.02). In contrast, the CMV serostatus was not associated with clinical variables such as MS duration and EDSS (Table 2). Real-time PCR was used to detect CMV DNA in plasma samples using specific primers and probe for a portion of the CMV glycoprotein B gene. Our results showed that CMV DNA was detected in the plasma samples of 16% of the MS patients. The patients who had detectable CMV DNA were CMV-seropositive. CMV DNAemia was not associated with aging or clinical variables (Table 2).

Table 2.
Distribution of anti-CMV IgG and presence of CMV DNA among MS patients according to their demographic and clinical characteristics.
3.3. Increased NKG2C Expression Levels in MS Patients Compared to Healthy Donors
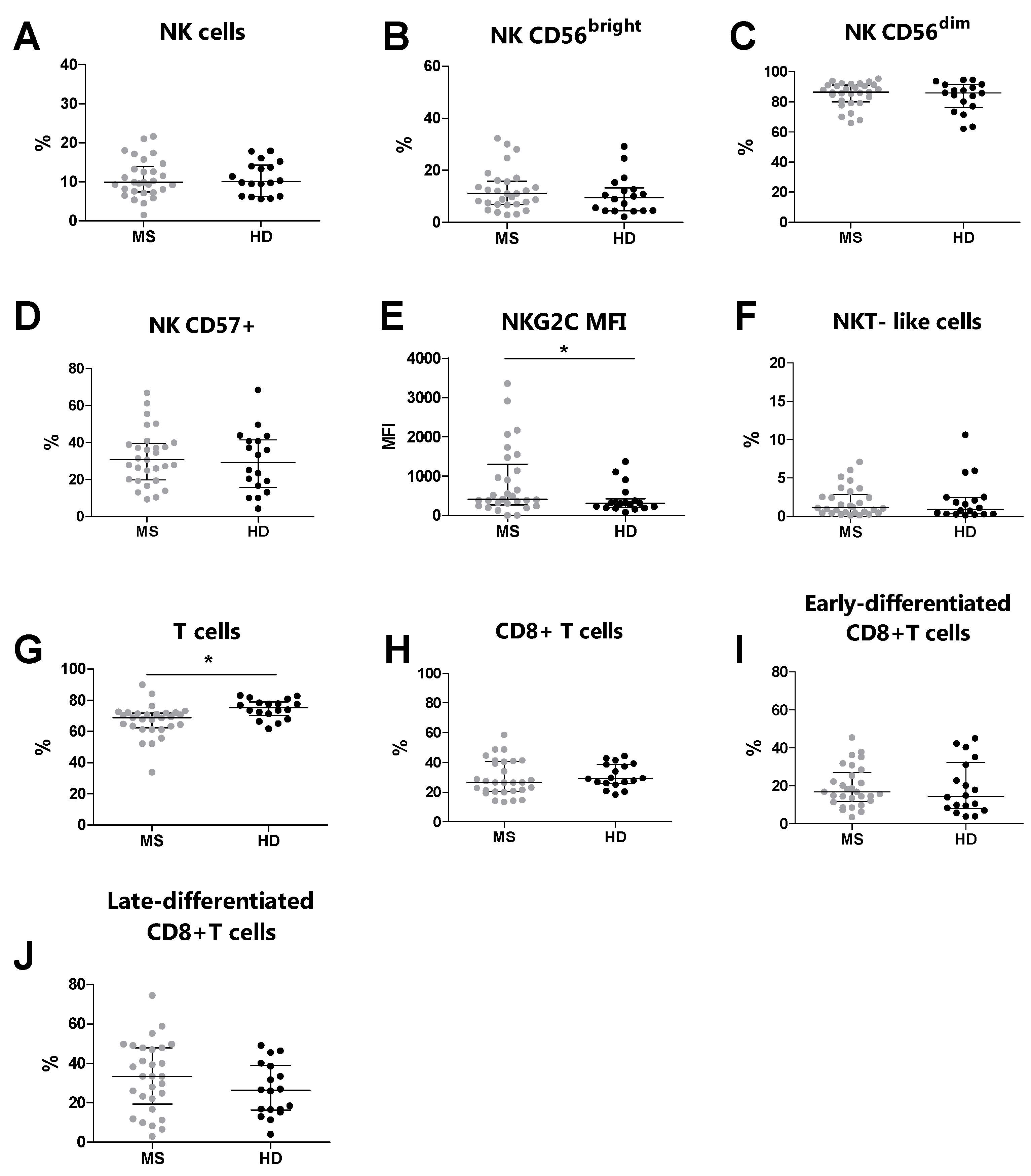
Overall, by flow cytometry, we analyzed 29 MS patients and 18 HDs. We compared the phenotype of NK cells T cells, NKT-like cells, and their subsets between MS patients and HDs (Table 3). Similar percentages of NK cells and NKT-like cells were observed in MS patients and HDs (Figure 1A and Figure 1F, respectively). Moreover, no differences were identified in the percentages of CD56bright, CD56dim, and CD57+ NK cells (Figure 1B, Figure 1C and Figure 1D, respectively). Conversely, the MS patients presented increased expression levels of NKG2C (p = 0.041) on the CD57+ NK cell subset compared to the HDs (Figure 1E). Moreover, no differences were observed in the percentages of CD8+ T cells, early-differentiated CD8+ T cells, and late-differentiated CD8+ T cells (Figure 1H, Figure 1I and Figure 1J, respectively). Conversely, the MS patients showed an increased percentage of total T cells (p = 0.001) compared to the HDs (Figure 1G).

Table 3.
Comparison between peripheral blood immune cell profiles in MS patients and HDs.
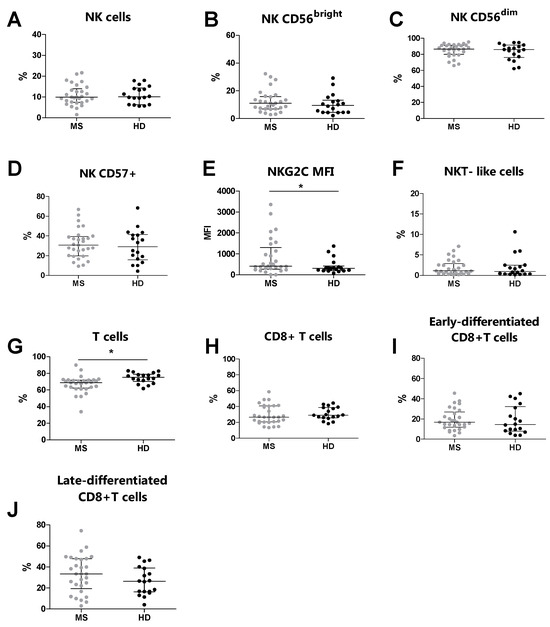
Figure 1.
Immunophenotyping analysis was performed in MS patients (grey dots) and HDs (black dots). (A) The total % of NK cells, (B) % of CD56bright NK cells, (C) % of CD56dim NK cells, (D) % of CD57+ NK cells, (E) MFI of NKG2C on CD57+, (F) % of NKT-like cells, (G) total % of T cells, (H) % of CD8+ T cells, (I) % of early-differentiated CD8+ T cells, (J) % of late-differentiated CD8+ T cells. p < 0.05 was considered significant (*: p < 0.05). The data are shown as the median and IQR. MS: multiple sclerosis; HDs: healthy donors; MFI: median fluorescence intensity.
3.4. The CMV Serostatus Influences the Peripheral Blood Phenotype in MS Patients
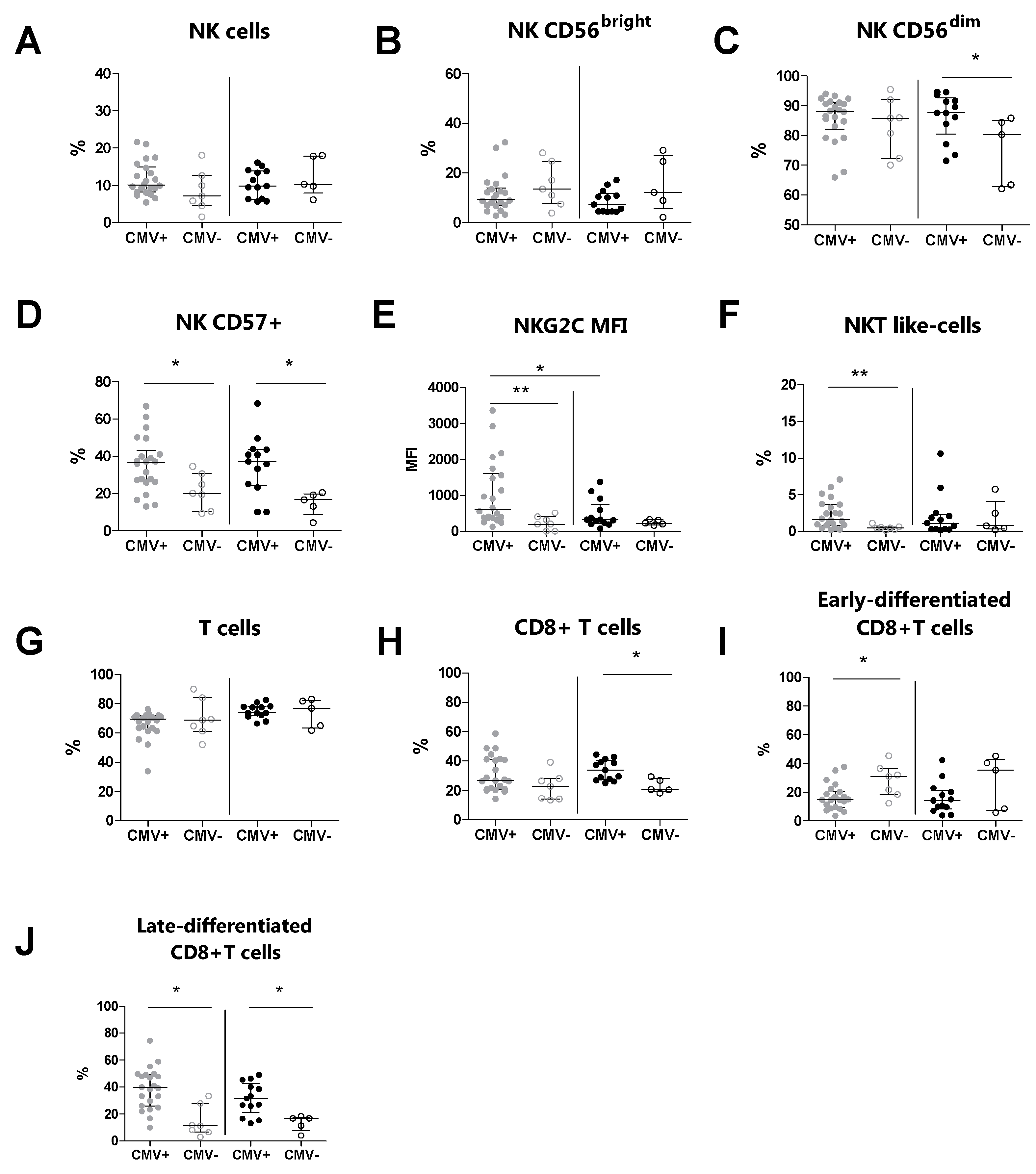
We subsequently evaluated the immunophenotype of peripheral NK, T, and NKT-like cells and their relative subsets in MS patients and HDs, according to their CMV serostatus (Table 4). We observed an increased percentage of CD57+ NK cells (p = 0.020) and NKT-like cells (p = 0.008) in CMV-seropositive MS patients compared to CMV-seronegative MS patients (Figure 2D and Figure 2F, respectively). Moreover, the expression level (MFI) of NKG2C on CD57+ NK cells (p = 0.008) was increased in CMV-seropositive MS patients compared to CMV-seronegative MS patients (Figure 2E). Conversely, the percentages of total NK cells and of the CD56bright and CD56dim NK cell subsets were comparable between CMV-seropositive MS patients and CMV-seronegative MS patients (Figure 2B and Figure 2C, respectively). Additionally, the expression level (MFI) of NKG2C (p = 0.022) was increased in CMV-seropositive MS patients compared to CMV-seropositive HDs (Figure 2E).

Table 4.
The peripheral blood immune cell profiles. MS patients and HDs were stratified according to their CMV serostatus and classified as either CMV-seropositive or CMV-seronegative.
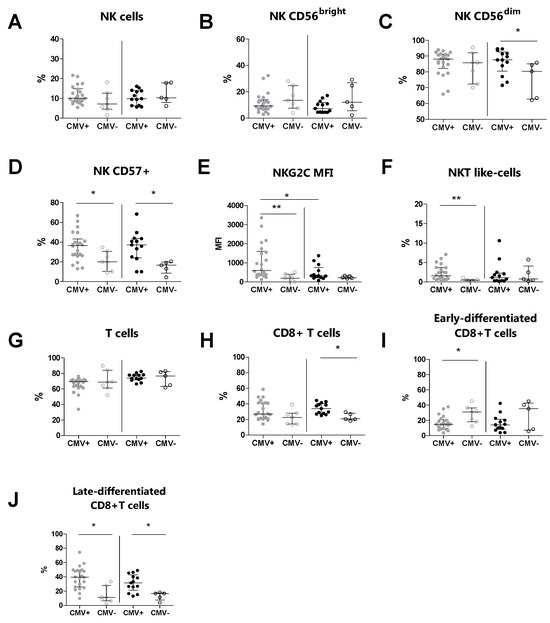
Figure 2.
Immunophenotyping analysis was performed in MS patients (grey dots) and HD subjects (black dots) stratified for their CMV serostatus. (A) The total % of NK cells, (B) % of CD56bright NK cells, (C) % of CD56dim NK cells, (D) % of CD57+ NK cells, (E) MFI of NKG2C on CD57+, (F) % of NKT-like cells, (G) total % of T cells, (H) % of CD8+ T cells, (I) % of early-differentiated CD8+ T cells, (J) % of late-differentiated CD8+ T cells. p < 0.05 was considered significant; *: p < 0.05, **: 0.01 < p < 0.001. The data are shown as median and IQR. CMV+: CMV-seropositive; CMV−: CMV-seronegative; MS: multiple sclerosis; HDs: healthy donors; MFI: median fluorescence intensity.
T cell immunophenotyping showed that the percentages of total T cells were comparable between CMV-seropositive MS patients and CMV-seronegative MS patients (Figure 2G). Also, for the CD8+ T cell subset, no statistically significant difference was found (Figure 2H). In contrast, we found a decreased percentage of the early-differentiated CD8+ subset (p = 0.023) and an increased percentage of the late-differentiated CD8+ subset (p = 0.002) in CMV-seropositive MS patients compared to CMV-seronegative MS patients (Figure 2I and Figure 2J, respectively).
3.5. Correlations
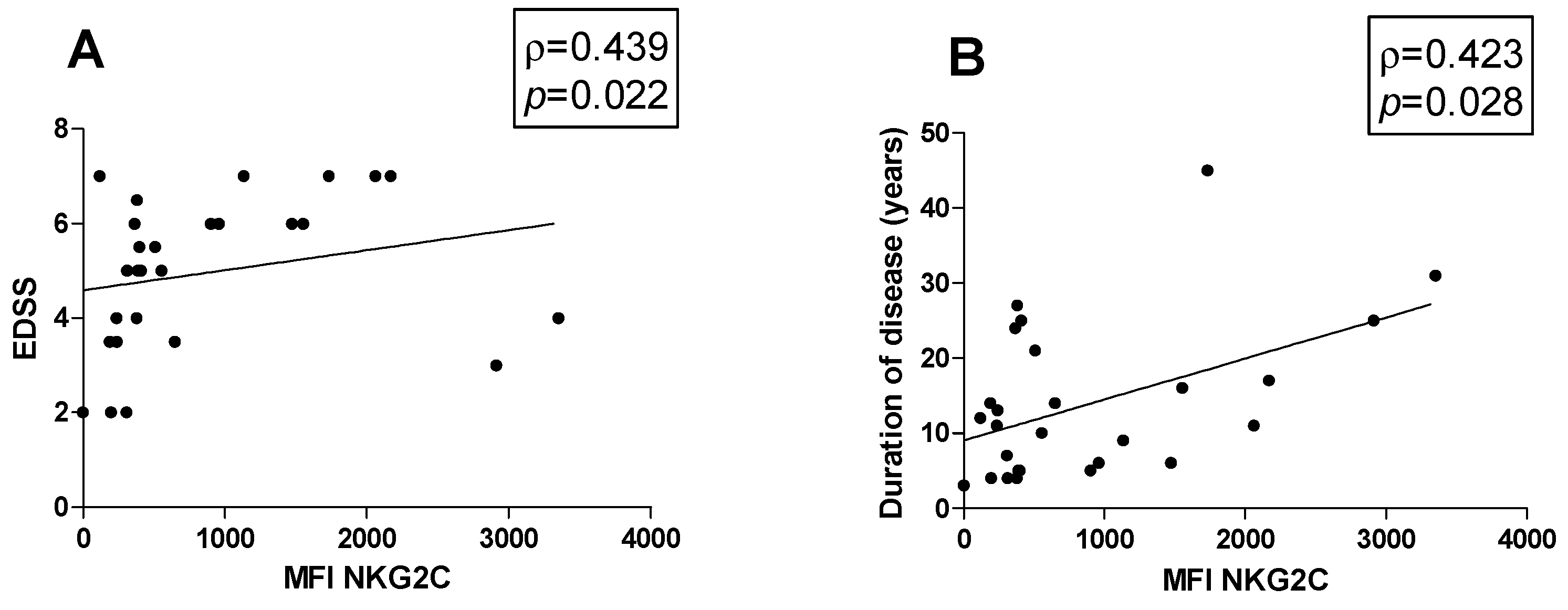
Possible associations between the percentage of cell subsets and clinical parameters were investigated in MS patients. Figures show all the statistically significant correlations detected by the Spearman’s rank correlation coefficient (p < 0.05). A positive correlation between the MFI of NKG2C on CD57+ NK cells with both EDSS (ρ = 0.439, p = 0.022) and disease duration was observed (ρ = 0.423, p = 0.028) (Figure 3A and Figure 3B, respectively).
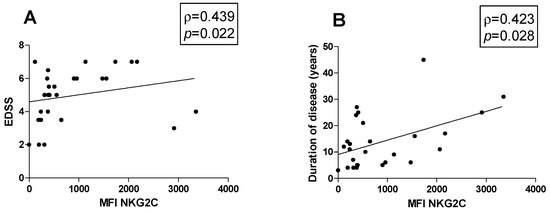
Figure 3.
Correlations. Statistically significant correlations (p < 0.05) in MS patients between (A) EDSS and MFI value of NKG2C and between disease duration in years and (B) MFI of NKG2C. Data are shown as regression lines with the number of patients. The Spearman’s correlation coefficient ρ (Rho) and the p values are shown in the graphs.
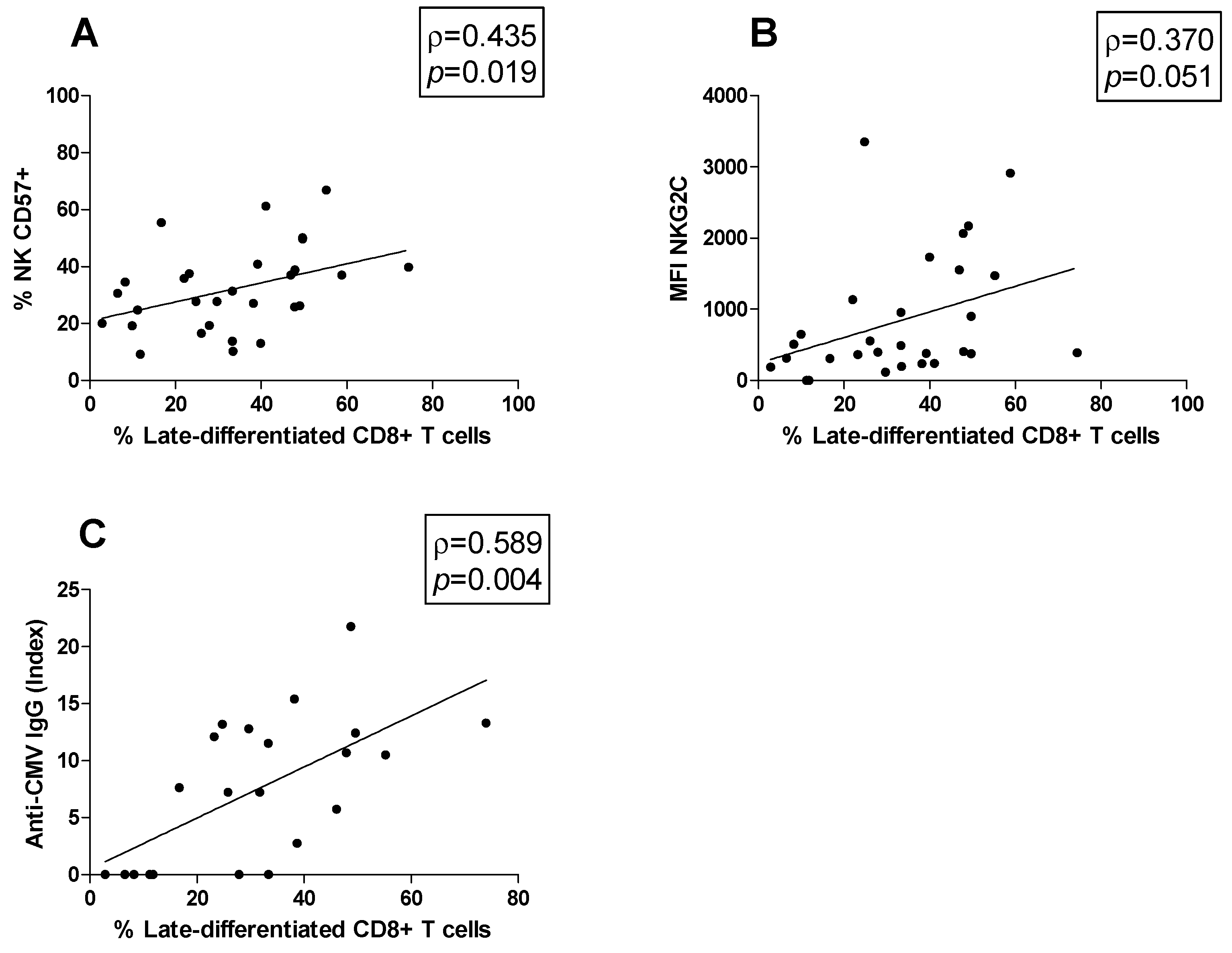
Moreover, possible correlations between cell subsets were studied. The late-differentiated CD8+ T cells percentage was positively correlated both with the CD57+ NK cell percentage (ρ = 0.435, p = 0.019; Figure 4A) and the MFI of NKG2C on CD57+ NK cells (ρ = 0.370, p = 0.051; Figure 4B). Moreover, the association between the anti-CMV IgG titer and the peripheral blood immune cell profile was investigated. We found that the anti-CMV IgG titer was positively correlated with the late-differentiated CD8+ T cell subset percentage (ρ = 0.589, p = 0.004) (Figure 4C).
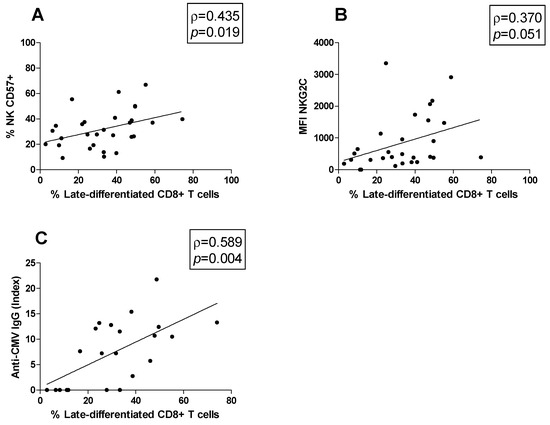
Figure 4.
Correlations. Statistically significant correlations (p < 0.05) in MS patients between (A) the percentage of late-differentiated CD8+ T cells and that of CD57+ NK cells, (B) the percentage of late-differentiated CD8+ T cells and the MFI of NKG2C, (C) the percentage of late-differentiated CD8+ T cells and the anti-CMV IgG titer. Data are shown as regression lines with the number of patients. The Spearman’s correlation coefficient ρ (Rho) and the p values are shown in the graphs.
4. Discussion
MS is a complex immune-mediate disorder characterized by the abnormal activation of immune cells which attack myelin in the CNS. Previous studies reported that CMV may have a detrimental or a beneficial role in MS, in both clinical and experimental settings, and can contribute to MS directly, through different mechanisms such as molecular mimicry and epitope spreading, or indirectly, via the activation and the expansion of specific immune cells [7,32]. Despite a few decades of investigation, the association between CMV and MS remains inconclusive. Together with other studies, we speculated on the detrimental role of CMV and its involvement in MS etiology and progression [33,34].
In line with other studies, we found no differences in the phenotype of total NK cells, CD56bright cells, and CD56dim cells in untreated MS patients compared to HDs [35]. Concerning T cells, MS patients did not differ from HDs in terms of percentages of CD8+ T cells and their subsets, including early-differentiated CD8+ T cells and late-differentiated CD8+ T cells. Otherwise, in line with previous reports, the MS patients presented lower levels of total T cells compared to the HDs [36].
Here, we found a significant increase in NKG2C expression levels on CD56dimCD57+ cells in the MS patients compared to the HDs, independently of their CMV serostatus. Importantly, higher NKG2C levels were positively associated with EDSS, suggesting that MS patients expressing higher levels of the NKG2C receptor are characterized by disease progression and the accumulation of disability over time. We observed a significant increase in NKG2C levels in CMV-seropositive MS patients compared to CMV-seronegative MS patients. In contrast, we did not find significant differences between CMV-seropositive and CMV-seronegative HDs, suggesting that the CMV-related increase in NKG2C was more pronounced in the MS patients. Moreover, our results showed that the NKG2C levels were increased in CMV-seropositive MS patients compared to CMV-seropositive HDs. These findings may identify some differences that could prove useful in MS monitoring. Nevertheless, they need to be confirmed in a larger group of patients to increase the power of the study.
Until the last decade, NK cells have largely been ignored in the MS field, differently from T and B cells, which have been recognized for their involvement in MS pathogenesis [37]. However, the involvement of NK cells remains controversial. Previous studies supported both deleterious and beneficial roles of NK cells in experimental autoimmune encephalomyelitis (EAE) [38]. The activation of NK cells is regulated by a balance between inhibitory and activating signals derived from membrane receptors, which interact with several ligands on their target cells [39]. Once activated, NK cells can induce apoptosis or release cytotoxic granules containing perforin and granzymes. The involvement of NK cells in MS pathogenesis is attributed to their ability to induce glial damage [40]. NKG2C is an activating receptor that plays an important role in promoting NK activation. This receptor interacts with non-classical HLA-E molecules of major histocompatibility (MHC) class I proteins expressed on the cell surface for triggering the NK cell cytotoxicity [41,42,43]. HLA-E molecules may be highly expressed in oligodendrocytes and microglia, as observed in active demyelinating lesions and, so, can be targeted by NK cells through NKG2C interaction [44,45]. Zaguia et al. performed immunohistochemical analyses on MS brain samples and found that HLA-E and NKG2C receptor are expressed in MS brain tissues [24].
The mechanisms by which CMV drives the expression of NKG2C on NK subsets are unknown; however, it is well established that NKG2C+ cells play a role in controlling CMV infection, both in vitro and in vivo [16]. Our findings suggest that CMV could act as a trigger in promoting an increase in the NKG2C expression levels, which may be deleterious in MS patients. We speculate that the NKG2C receptor might contribute to a self-directed attack on myelin resulting in CNS injury.
NK cells are critical for the control of viral infection and reactivation, and it is well known that CMV has a broad impact on their phenotype and function [46]. During CMV infection, the levels of the most differentiated and cytotoxic NK cells, CD56dimCD57+ (NK CD57+) cells, which exert a crucial role in CMV-infected cells, are increased [47]. These cells express high levels of CD57 receptor, a useful marker of NK maturation. In our study, the percentages of the most differentiated cell subset, i.e., NK CD57+ cells, were increased in CMV-seropositive MS patients compared to CMV-seronegative patients. We found no differences in terms of percentages for the most immature CD56bright cells and the intermediate CD56dim cells in CMV-seropositive MS patients compared to CMV-seronegative MS patients [48].
CD8+ T cells, together with NK cells, are essential for the control of viral infections. CMV exerts a persistent stimulation on CD8+ T cells, resulting in the development of a highly differentiated subset that is incapable of cell division. The advanced differentiation state is characterized by the acquisition of the CD57 marker and the lack of expression of the costimulatory receptor CD28, which is inversely expressed in early-differentiated CD8+ T cells [49]. The impact of late-differentiated CD8+ T cells on MS is not yet clear. Mikulkova et al. reported quantitative changes in the late-differentiated CD8+ T cell population in MS patients, considering these cells as immunosuppressive, although the expression of immunosuppressive markers was not evaluated in their study [28]. Conversely, in our study, the late-differentiated CD8+ T cell percentages did not differ between the MS patients and the HDs, suggesting that this population is not implicated in MS. Moreover, stratifying the population based on the serostatus, we found that CMV infection was associated with a significant increase in late-differentiated CD8+ T cells both in the MS patients and in the HDs [50]. These data confirmed that CMV may induce the accumulation of late-differentiated CD8+ T cells, both in health and in disease states. Moreover, these findings showed that CMV is a major viral driver of immunosenescence, and the expansion of this subset is a result of a constant stimulation by persistent antigens. Here, we also found that in MS patients, higher percentages of late-differentiated CD8+ T cells were associated with higher levels of anti-CMV antibodies. Immunosenescence may be associated with increased levels of anti-CMV antibodies and may be indicative of a poor control of the infection due to the worsening immunological status [51]. In contrast, we did not observe the same correlation in HDs. We hypothesize that MS patients may suffer from a high number of CMV reactivations and consequently exhibit increased interactions between the virus and CD8+ T cells, which become anergic and late-differentiated following the repeated antigenic stimulation. In our study, the MS patients with higher percentages of late-differentiated CD8+ T cells presented higher percentages of NK CD57+ cells, suggesting that CD57 expression is coordinately regulated as the immune system matures due to the persistent antigenic stimulation induced by CMV infection.
In addition to NK cells and CD8+ T cells, CD3+CD56+ natural killer T (NKT)-like cells represent a heterogeneous subset that shares some morphological and functional characteristics with both NK and T cells and are particularly interesting due to their dual role in the immune system [52]. NKT-like cells represent the first line of defense against several viral pathogens such as the influenza A and chikungunya viruses [53,54,55]. Van Dommelen et al. [56] demonstrated in the murine CMV model (MCMV) that NKT-like cells induce an antiviral state through the secretion of important cytokines, enhancing the immune response against MCMV. In line with McKay et al. [57], we did not find any differences in the percentages of NKT-like cells between MS patients and HDs. However, we observed a significantly higher percentage of NKT-like cells in CMV-seropositive MS patients compared to those who were CMV-seronegative. To the best of our knowledge, this is the first study investigating NKT-like cell expansion in CMV-seropositive MS patients. In conclusion, as we previously observed for other cell subsets, the increased percentage of NKT-like cells in MS patients may be explained by their protective role against CMV in preventing viral reactivation.
5. Conclusions
Cytomegalovirus, a ubiquitous herpesvirus, has long been studied for its putative association with MS, in both disease progression and pathogenesis. Due to its prolonged interaction with the immune system, it seems reasonable that this virus may impact MS at various levels. However, there are controversial reports on the association between CMV infection and MS. Our findings showed that CMV infection shapes the receptor repertoire of the immune system, both in healthy subjects and in MS patients. However, we identified some differences that could prove useful in MS monitoring. In this work, we hypothesized that CMV may contribute to immune-mediated processes, increasing the risk of disability worsening in patients showing a CMV-driven NKG2C receptor increase. This receptor could represent a pathogenic contribution, exacerbating the inflammatory response and consequently influencing the worsening of MS. However, we can only speculate a causal relationship between CMV infection and the risk of disability progression. Our results provide a starting point to identify specific biomarkers of disease progression, because our research was based on a limited number of samples, and our findings must be validated in larger studies. Further work is needed to understand the involvement of CMV in the multifaceted pathological picture of MS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology13030154/s1, Figure S1. Gating strategy. PBMC were gated based on forward (FSC) and side scatter (SSC), and then gated for singlets. After the exclusion of dead cells with Zombie Aqua dye, NK, T and NKT-like cells were identified based on CD3 and CD56 expression. NK cells were identified as CD3− CD56+. Based on CD16 and CD56 expression, CD56dim and CD56bright NK cells were identified. CD57+ NK cells were identified in CD56dim gate. The NKG2C median fluorescence intensity (MFI) was evaluated on CD57+ NK cells subset. NKT-like cells were identified as CD3+ CD56+. T cells were identified as CD3+ CD56−. In T-cell gate, we identified CD8+ based on CD3 and CD8 expression. In CD8 gate, we further identified (A) late-differentiated CD8 T cells and (B) early-differentiated CD8 T cells based on CD28/CD57 expression.
Author Contributions
Conceptualization, V.P. and M.A.Z.; data curation, V.P., M.A.Z. and P.P.; formal analysis, V.P. and M.A.Z.; investigation, P.P., F.C. and M.T.; methodology, V.P. and M.A.Z.; resources, M.T., V.B., L.M., G.F. and M.R.C.; supervision, C.M.M., A.C. and M.R.C.; visualization, V.P., M.A.Z. and P.P.; writing—original draft, V.P.; writing—review and editing, V.P., M.A.Z., P.P. and M.R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Ethics Committee of Policlinico Umberto I, Sapienza University of Rome (protocol numbers 130/13 and 353/20).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was supported by the Department of Public Health and Infectious Diseases, Sapienza University of Rome.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Compston, A.; Coles, A. Multiple Sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple Sclerosis. N. Engl. J. Med. 2000, 343, 938–952. [Google Scholar] [CrossRef]
- Cavallo, S. Immune-Mediated Genesis of Multiple Sclerosis. J. Transl. Autoimmun. 2020, 3, 100039. [Google Scholar] [CrossRef] [PubMed]
- Tarlinton, R.E.; Martynova, E.; Rizvanov, A.A.; Khaiboullina, S.; Verma, S. Role of Viruses in the Pathogenesis of Multiple Sclerosis. Viruses 2020, 12, 643. [Google Scholar] [CrossRef]
- Mechelli, R.; Romano, C.; Reniè, R.; Manfrè, G.; Buscarinu, M.C.; Romano, S.; Marrone, A.; Bigi, R.; Bellucci, G.; Ballerini, C.; et al. Viruses and Neuroinflammation in Multiple Sclerosis. Neuroimmunol. Neuroinflamm. 2021, 8, 269. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal Analysis Reveals High Prevalence of Epstein-Barr Virus Associated with Multiple Sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Vanheusden, M.; Stinissen, P.; ’t Hart, B.A.; Hellings, N. Cytomegalovirus: A Culprit or Protector in Multiple Sclerosis? Trends Mol. Med. 2015, 21, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Alari-Pahissa, E.; Moreira, A.; Zabalza, A.; Alvarez-Lafuente, R.; Munteis, E.; Vera, A.; Arroyo, R.; Alvarez-Cermeño, J.C.; Villar, L.M.; López-Botet, M.; et al. Low Cytomegalovirus Seroprevalence in Early Multiple Sclerosis: A Case for the “Hygiene Hypothesis”? Eur. J. Neurol. 2018, 25, 925–933. [Google Scholar] [CrossRef]
- Vanheusden, M.; Broux, B.; Welten, S.P.M.; Peeters, L.M.; Panagioti, E.; Van Wijmeersch, B.; Somers, V.; Stinissen, P.; Arens, R.; Hellings, N. Cytomegalovirus Infection Exacerbates Autoimmune Mediated Neuroinflammation. Sci. Rep. 2017, 7, 663. [Google Scholar] [CrossRef]
- Sanadgol, N.; Ramroodi, N.; Ahmadi, G.A.; Komijani, M.; Moghtaderi, A.; Bouzari, M.; Rezaei, M.; Kardi, M.T.; Dabiri, S.; Moradi, M.; et al. Prevalence of Cytomegalovirus Infection and Its Role in Total Immunoglobulin Pattern in Iranian Patients with Different Subtypes of Multiple Sclerosis. New Microbiol. 2011, 34, 263–274. [Google Scholar]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of Cytomegalovirus Seroprevalence and Demographic Characteristics Associated with Infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Kothari, A.; Ramachandran, V.G.; Gupta, P.; Singh, B.; Talwar, V. Seroprevalence of Cytomegalovirus among Voluntary Blood Donors in Delhi, India. J. Health Popul. Nutr. 2002, 20, 348–351. [Google Scholar] [PubMed]
- Mendelson, M.; Monard, S.; Sissons, P.; Sinclair, J. Detection of Endogenous Human Cytomegalovirus in CD34+ Bone Marrow Progenitors. J. Gen. Virol. 1996, 77 Pt 12, 3099–3102. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sejas, N.; Campos, C.; Hassouneh, F.; Sanchez-Correa, B.; Tarazona, R.; Pera, A.; Solana, R. Effect of CMV and Aging on the Differential Expression of CD300a, CD161, T-Bet, and Eomes on NK Cell Subsets. Front. Immunol. 2016, 7, 476. [Google Scholar] [CrossRef]
- Sester, M.; Sester, U.; Gärtner, B.C.; Girndt, M.; Meyerhans, A.; Köhler, H. Dominance of Virus-Specific CD8 T Cells in Human Primary Cytomegalovirus Infection. J. Am. Soc. Nephrol. 2002, 13, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Gumá, M.; Budt, M.; Sáez, A.; Brckalo, T.; Hengel, H.; Angulo, A.; López-Botet, M. Expansion of CD94/NKG2C+ NK Cells in Response to Human Cytomegalovirus-Infected Fibroblasts. Blood 2006, 107, 3624–3631. [Google Scholar] [CrossRef] [PubMed]
- Wikby, A.; Johansson, B.; Olsson, J.; Löfgren, S.; Nilsson, B.O.; Ferguson, F. Expansions of Peripheral Blood CD8 T-Lymphocyte Subpopulations and an Association with Cytomegalovirus Seropositivity in the Elderly: The Swedish NONA Immune Study. Exp. Gerontol. 2002, 37, 445–453. [Google Scholar] [CrossRef]
- Solana, R.; Tarazona, R.; Aiello, A.E.; Akbar, A.N.; Appay, V.; Beswick, M.; Bosch, J.A.; Campos, C.; Cantisán, S.; Cicin-Sain, L.; et al. CMV and Immunosenescence: From Basics to Clinics. Immun. Ageing 2012, 9, 23. [Google Scholar] [CrossRef]
- Luetke-Eversloh, M.; Hammer, Q.; Durek, P.; Nordström, K.; Gasparoni, G.; Pink, M.; Hamann, A.; Walter, J.; Chang, H.-D.; Dong, J.; et al. Human Cytomegalovirus Drives Epigenetic Imprinting of the IFNG Locus in NKG2Chi Natural Killer Cells. PLoS Pathog. 2014, 10, e1004441. [Google Scholar] [CrossRef]
- Muntasell, A.; Pupuleku, A.; Cisneros, E.; Vera, A.; Moraru, M.; Vilches, C.; López-Botet, M. Relationship of NKG2C Copy Number with the Distribution of Distinct Cytomegalovirus-Induced Adaptive NK Cell Subsets. J. Immunol. 2016, 196, 3818–3827. [Google Scholar] [CrossRef]
- Gumá, M.; Angulo, A.; Vilches, C.; Gómez-Lozano, N.; Malats, N.; López-Botet, M. Imprint of Human Cytomegalovirus Infection on the NK Cell Receptor Repertoire. Blood 2004, 104, 3664–3671. [Google Scholar] [CrossRef] [PubMed]
- Kastrukoff, L.F.; Lau, A.; Wee, R.; Zecchini, D.; White, R.; Paty, D.W. Clinical Relapses of Multiple Sclerosis Are Associated with “novel” Valleys in Natural Killer Cell Functional Activity. J. Neuroimmunol. 2003, 145, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Infante-Duarte, C.; Weber, A.; Krätzschmar, J.; Prozorovski, T.; Pikol, S.; Hamann, I.; Bellmann-Strobl, J.; Aktas, O.; Dörr, J.; Wuerfel, J.; et al. Frequency of Blood CX3CR1-Positive Natural Killer Cells Correlates with Disease Activity in Multiple Sclerosis Patients. FASEB J. 2005, 19, 1902–1904. [Google Scholar] [CrossRef] [PubMed]
- Zaguia, F.; Saikali, P.; Ludwin, S.; Newcombe, J.; Beauseigle, D.; McCrea, E.; Duquette, P.; Prat, A.; Antel, J.P.; Arbour, N. Cytotoxic NKG2C+ CD4 T Cells Target Oligodendrocytes in Multiple Sclerosis. J. Immunol. 2013, 190, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, S.P.H.; Pardieck, I.N.; Lanfermeijer, J.; Sauce, D.; Klenerman, P.; van Baarle, D.; Arens, R. The Hallmarks of CMV-Specific CD8 T-Cell Differentiation. Med. Microbiol. Immunol. 2019, 208, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Parish, S.T.; Wu, J.E.; Effros, R.B. Sustained CD28 Expression Delays Multiple Features of Replicative Senescence in Human CD8 T Lymphocytes. J. Clin. Immunol. 2010, 30, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Weng, N.-P.; Akbar, A.N.; Goronzy, J. CD28− T Cells: Their Role in the Age-Associated Decline of Immune Function. Trends Immunol. 2009, 30, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Mikulkova, Z.; Praksova, P.; Stourac, P.; Bednarik, J.; Strajtova, L.; Pacasova, R.; Belobradkova, J.; Dite, P.; Michalek, J. Numerical Defects in CD8+CD28− T-Suppressor Lymphocyte Population in Patients with Type 1 Diabetes Mellitus and Multiple Sclerosis. Cell Immunol. 2010, 262, 75–79. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Zingaropoli, M.A.; Iannetta, M.; Pontecorvo, S.; Anzivino, E.; Prezioso, C.; Rodio, D.M.; Morreale, M.; D’Abramo, A.; Oliva, A.; Lichtner, M.; et al. JC Virus-DNA Detection Is Associated with CD8 Effector Accumulation in Peripheral Blood of Patients with Multiple Sclerosis under Natalizumab Treatment, Independently from JC Virus Serostatus. BioMed Res. Int. 2018, 2018, 5297980. [Google Scholar] [CrossRef]
- Zingaropoli, M.A.; Parente, A.; Kertusha, B.; Campagna, R.; Tieghi, T.; Garattini, S.; Marocco, R.; Carraro, A.; Tortellini, E.; Guardiani, M.; et al. Longitudinal Virological and Immunological Profile in a Case of Human Monkeypox Infection. Open Forum Infect. Dis. 2022, 9, ofac569. [Google Scholar] [CrossRef]
- Olival, G.S.D.; Lima, B.M.; Sumita, L.M.; Serafim, V.; Fink, M.C.; Nali, L.H.; Romano, C.M.; Thomaz, R.B.; Cavenaghi, V.B.; Tilbery, C.P.; et al. Multiple Sclerosis and Herpesvirus Interaction. Arq. Neuropsiquiatr. 2013, 71, 727–730. [Google Scholar] [CrossRef]
- Horakova, D.; Zivadinov, R.; Weinstock-Guttman, B.; Havrdova, E.; Qu, J.; Tamaño-Blanco, M.; Badgett, D.; Tyblova, M.; Bergsland, N.; Hussein, S.; et al. Environmental Factors Associated with Disease Progression after the First Demyelinating Event: Results from the Multi-Center SET Study. PLoS ONE 2013, 8, e53996. [Google Scholar] [CrossRef] [PubMed]
- Smyk, D.S.; Alexander, A.K.; Walker, M.; Walker, M. Acute Disseminated Encephalomyelitis Progressing to Multiple Sclerosis: Are Infectious Triggers Involved? Immunol. Res. 2014, 60, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Laroni, A.; Armentani, E.; Kerlero de Rosbo, N.; Ivaldi, F.; Marcenaro, E.; Sivori, S.; Gandhi, R.; Weiner, H.L.; Moretta, A.; Mancardi, G.L.; et al. Dysregulation of Regulatory CD56bright NK Cells/T Cells Interactions in Multiple Sclerosis. J. Autoimmun. 2016, 72, 8–18. [Google Scholar] [CrossRef]
- Canto-Gomes, J.; Da Silva-Ferreira, S.; Silva, C.S.; Boleixa, D.; Martins da Silva, A.; González-Suárez, I.; Cerqueira, J.J.; Correia-Neves, M.; Nobrega, C. People with Primary Progressive Multiple Sclerosis Have a Lower Number of Central Memory T Cells and HLA-DR+ Tregs. Cells 2023, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- van Langelaar, J.; Rijvers, L.; Smolders, J.; van Luijn, M.M. B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Filaci, G.; Bacchetta, R.; Zanetti, M. Is There a Role for NK Cells in the Pathogenesis of Multiple Sclerosis? A Case Study. Hum. Immunol. 1999, 60, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling NK Cell Responses: Integration of Signals for Activation and Inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Laroni, A.; Weiner, H.L. Role of the Innate Immune System in the Pathogenesis of Multiple Sclerosis. J. Neuroimmunol. 2010, 221, 7–14. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Petersen, J.; Rossjohn, J.; Fugger, L. HLA Variation and Disease. Nat. Rev. Immunol. 2018, 18, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Trowsdale, J.; Fugger, L. Natural Killer Cells and Their Receptors in Multiple Sclerosis. Brain 2013, 136, 2657–2676. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Evolutionary Struggles between NK Cells and Viruses. Nat. Rev. Immunol. 2008, 8, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Durrenberger, P.F.; Webb, L.V.; Sim, M.J.W.; Nicholas, R.S.; Altmann, D.M.; Boyton, R.J. Increased HLA-E Expression in White Matter Lesions in Multiple Sclerosis. Immunology 2012, 137, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Höftberger, R.; Aboul-Enein, F.; Brueck, W.; Lucchinetti, C.; Rodriguez, M.; Schmidbauer, M.; Jellinger, K.; Lassmann, H. Expression of Major Histocompatibility Complex Class I Molecules on the Different Cell Types in Multiple Sclerosis Lesions. Brain Pathol. 2004, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.M.; White, M.J.; Goodier, M.R.; Riley, E.M. Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Front. Immunol. 2013, 4, 422. [Google Scholar] [CrossRef] [PubMed]
- Foley, B.; Cooley, S.; Verneris, M.R.; Pitt, M.; Curtsinger, J.; Luo, X.; Lopez-Vergès, S.; Lanier, L.L.; Weisdorf, D.; Miller, J.S. Cytomegalovirus Reactivation after Allogeneic Transplantation Promotes a Lasting Increase in Educated NKG2C+ Natural Killer Cells with Potent Function. Blood 2012, 119, 2665–2674. [Google Scholar] [CrossRef]
- Strioga, M.; Pasukoniene, V.; Characiejus, D. CD8+ CD28- and CD8+ CD57+ T Cells and Their Role in Health and Disease. Immunology 2011, 134, 17–32. [Google Scholar] [CrossRef]
- Akbar, A.N.; Henson, S.M.; Lanna, A. Senescence of T Lymphocytes: Implications for Enhancing Human Immunity. Trends Immunol. 2016, 37, 866–876. [Google Scholar] [CrossRef]
- Iglesias-Escudero, M.; Moro-García, M.A.; Marcos-Fernández, R.; García-Torre, A.; Álvarez-Argüelles, M.E.; Suárez-Fernández, M.L.; Martínez-Camblor, P.; Rodríguez, M.; Alonso-Arias, R. Levels of Anti-CMV Antibodies Are Modulated by the Frequency and Intensity of Virus Reactivations in Kidney Transplant Patients. PLoS ONE 2018, 13, e0194789. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, D.; Hokland, M.; Kuppen, P.J.K. The Role of Natural Killer T Cells in Cancer-A Phenotypical and Functional Approach. Front. Immunol. 2018, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.A.; Evans, B.L.; Durafourt, B.A.; Blain, M.; Lapierre, Y.; Bar-Or, A.; Antel, J.P. Reduction of the Peripheral Blood CD56bright NK Lymphocyte Subset in FTY720-Treated Multiple Sclerosis Patients. J. Immunol. 2011, 187, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Thanapati, S.; Das, R.; Tripathy, A.S. Phenotypic and Functional Analyses of NK and NKT-like Populations during the Early Stages of Chikungunya Infection. Front. Microbiol. 2015, 6, 895. [Google Scholar] [CrossRef]
- Ho, L.-P.; Denney, L.; Luhn, K.; Teoh, D.; Clelland, C.; McMichael, A.J. Activation of Invariant NKT Cells Enhances the Innate Immune Response and Improves the Disease Course in Influenza A Virus Infection. Eur. J. Immunol. 2008, 38, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- van Dommelen, S.L.H.; Tabarias, H.A.; Smyth, M.J.; Degli-Esposti, M.A. Activation of Natural Killer (NK) T Cells during Murine Cytomegalovirus Infection Enhances the Antiviral Response Mediated by NK Cells. J. Virol. 2003, 77, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- McKay, F.C.; Swain, L.I.; Schibeci, S.D.; Rubio, J.P.; Kilpatrick, T.J.; Heard, R.N.; Stewart, G.J.; Booth, D.R. CD127 Immunophenotyping Suggests Altered CD4+ T Cell Regulation in Primary Progressive Multiple Sclerosis. J. Autoimmun. 2008, 31, 52–58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).