Distribution of Acetogenic Naphthoquinones in Droseraceae and Their Chemotaxonomic Utility
Simple Summary
Abstract
1. Introduction
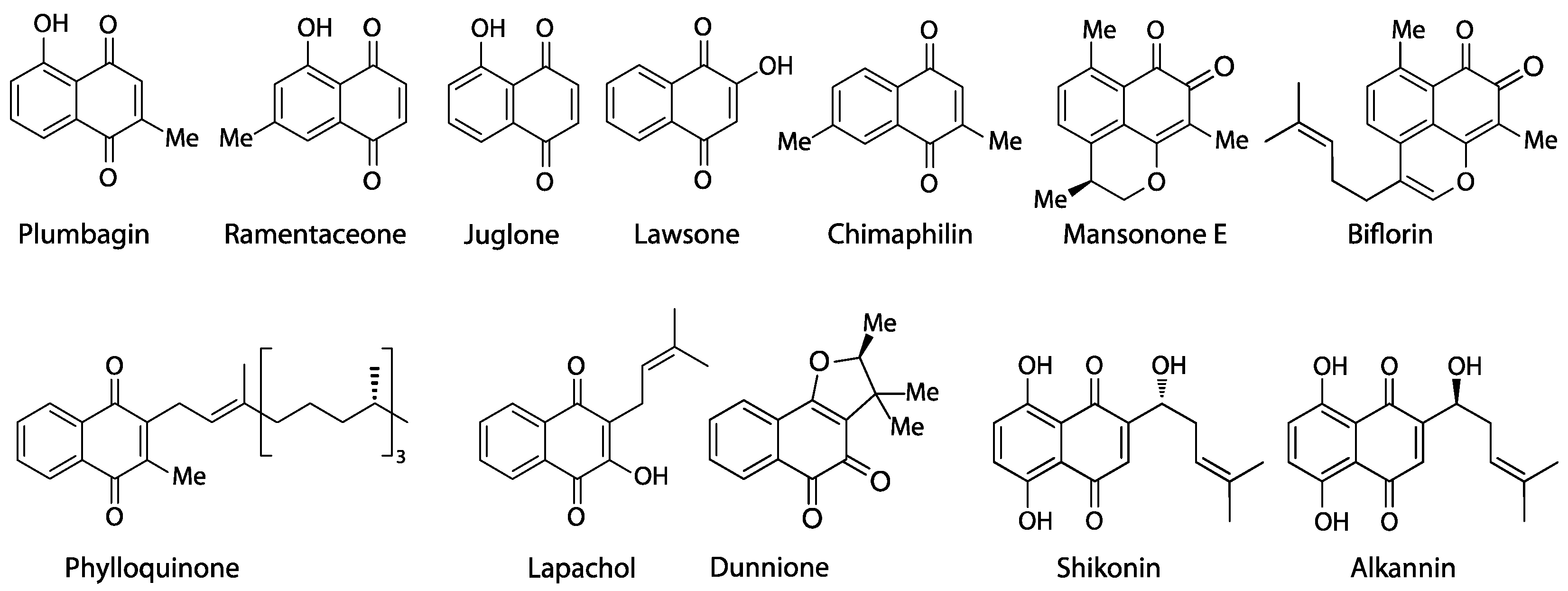
- The acetate-polymalonate or polyketide pathway (plumbagin, ramentaceone), a versatile and widespread route commonly used to produce aromatic compounds characterized by oxygen substitution at every second atom of the carbon backbone that is even retained in metabolites of mixed biosynthetic origin (like, e.g., flavonoids and stilbenoids);
- The o-succinylbenzoate pathway (juglone, lawsone, lapachol, phylloquinone, dunnione), a route that is almost ubiquitous in plants, as it leads to the essential phylloquinone (vitamin K group);
- The homogentisate/mevalonate pathway (chimaphilin) so far known only from Ericaceae-Pyroloideae to produce chimaphilin and its immediate derivatives;
- The 4-hydroxybenzoate/geranyl-pyrophosphate pathway (shikonin, alkannin), known with certainty only from Boraginaceae;
- The farnesyl-pyrophosphate pathway (mansonones) common to the huge and widespread class of sesquiterpenoids, of which only a comparatively limited number become quinones by oxidation.
2. Review of Naphthoquinone Data from Droseraceae
3. Discussion
3.1. Screening Approaches to Naphthoquinones in Droseraceae
3.2. Role of Naphthoquinones in Carnivory
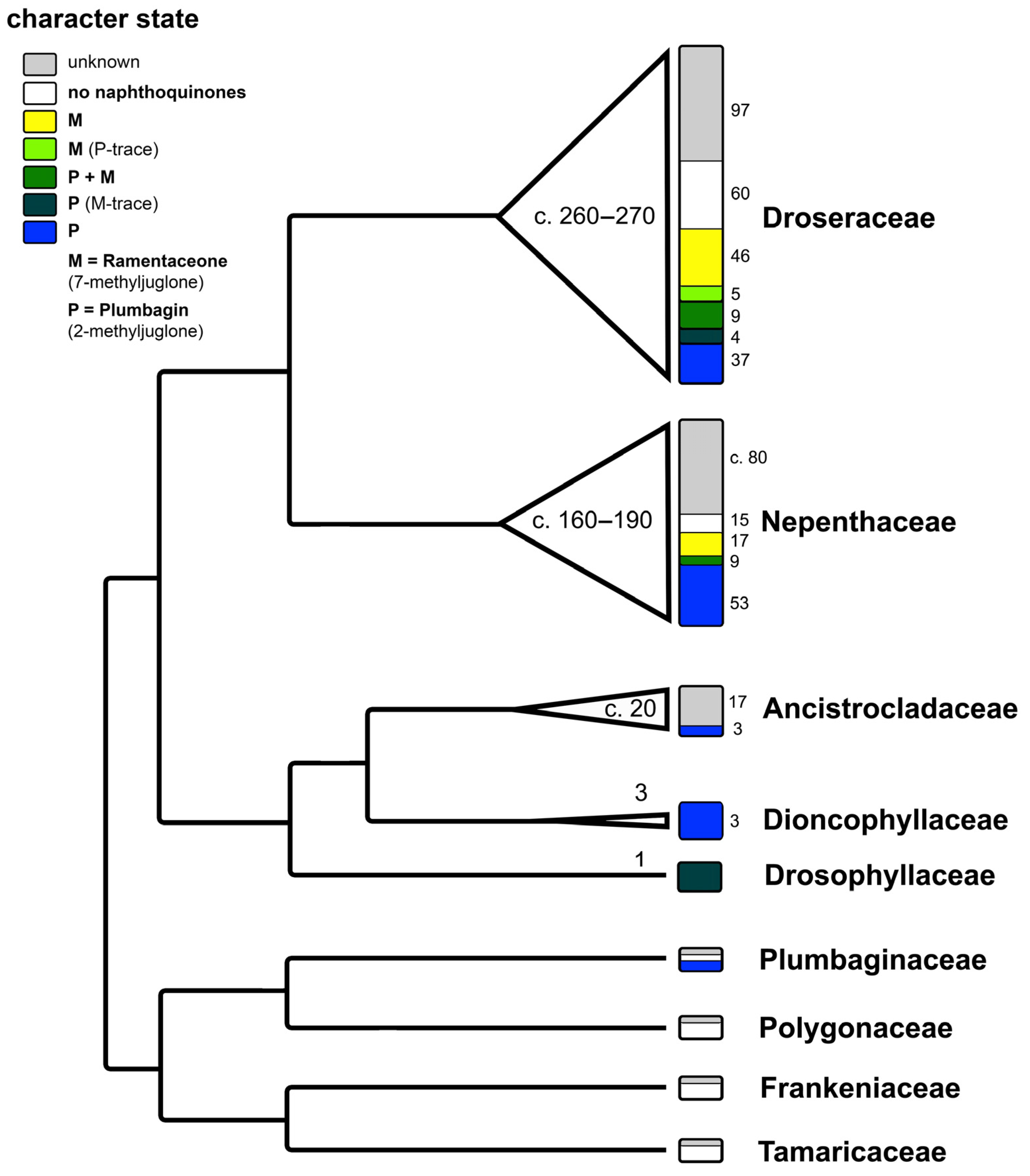
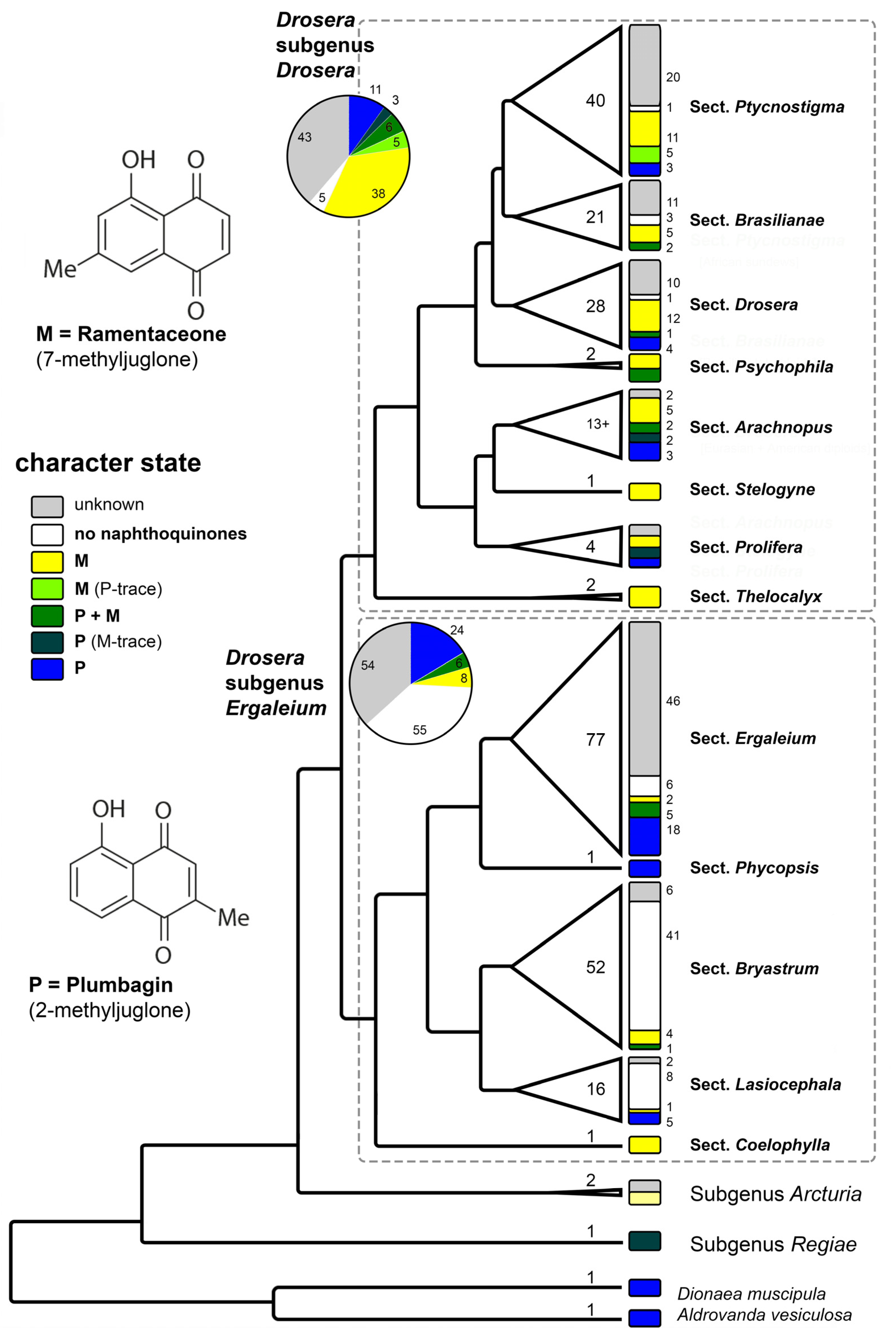
3.3. Taxonomic Implications
3.3.1. Quinone Relationships of Droseraceae and Nepenthales
3.3.2. Quinone Relationships within Drosera
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hook, I.; Mills, C.; Sheridan, H. Bioactive naphthoquinones from higher plants. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 41, pp. 119–160. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Rhodes, D. Biosynthesis and molecular actions of specialized 1,4-naphthoquinone natural products produced by horticultural plants. Hortic. Res. 2016, 3, 16046. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.W.; Bahamon Naranjo, M.A.; Widhalm, J.R. Convergent evolution of plant specialized 1,4-naphthoquinones: Metabolism, trafficking, and resistance to their allelopathic effects. J. Exp. Bot. 2021, 72, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Gerschler, S.; Neumann, N.; Schultze, N.; Guenther, S.; Schulze, C. Quality parameters for the medicinal plant Drosera rotundifolia L.: A new approach with established techniques. Arch. Pharm. 2023, 357, e2300436. [Google Scholar] [CrossRef] [PubMed]
- Izhaki, I. Emodin—A secondary metabolite with multiple ecological functions in higher plants. New Phytol. 2002, 155, 205–217. [Google Scholar] [CrossRef]
- Timoneda, A.; Feng, T.; Sheehan, H.; Walker-Hale, N.; Pucker, B.; Lopez-Nieves, S.; Guo, R.; Brockington, S. The evolution of betalain biosynthesis in Caryophyllales. New Phytol. 2019, 224, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Henarejos-Escudero, P.; Guadarrama-Flores, B.; García-Carmona, F.; Gandía-Herrero, F. Digestive glands extraction and precise pigment analysis support the exclusion of the carnivorous plant Dionaea muscipula Ellis from the Caryophyllales order. Plant Sci. 2018, 274, 342–348. [Google Scholar] [CrossRef]
- Dávila-Lara, A.; Reichelt, M.; Wang, D.; Vogel, H.; Mithöfer, A. Proof of anthocyanins in the carnivorous plant genus Nepenthes. FEBS Open Bio 2021, 11, 2576–2585. [Google Scholar] [CrossRef]
- Fleischmann, A.; Schlauer, J.; Smith, S.A.; Givnish, T.J. Evolution of carnivory in angiosperms. In Carnivorous Plants: Physiology, Ecology, and Evolution; Adamec, L., Ellison, A., Eds.; Oxford University Press: London, UK, 2017; pp. 22–42. [Google Scholar] [CrossRef]
- Vasav, A.P.; Meshram, B.G.; Pable, A.A.; Vitthal, T.; Barvkar, V.T. Artificial microRNA mediated silencing of cyclase and aldo–keto reductase genes reveal their involvement in the plumbagin biosynthetic pathway. J. Plant Res. 2023, 136, 47–62. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I.; Seppänen-Laakso, T.; Rischer, H. Contrasting dihydronaphthoquinone patterns in closely related Drosera (sundew) species enable taxonomic distinction and identification. Plants 2021, 10, 1601. [Google Scholar] [CrossRef]
- Brown, A.G.; Thomson, R.H. Ebenaceae Extractives. Part II Naphthaldehydes from Diospyros ebenum Koen. J. Chem. Soc. 1965, 4292–4295. [Google Scholar] [CrossRef]
- Matsushita, Y.; Jang, I.; Imai, T.; Fukushima, K.; Lee, S. Naphthalene derivatives from Diospyros kaki. J. Wood Sci. 2010, 56, 418–421. [Google Scholar] [CrossRef]
- Schlauer, J.; Fleischmann, A. Chemical evidence for hybridity in Drosera (Droseraceae). Biochem. Syst. Ecol. 2016, 66, 33–36. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I. Unexpected discovery of Ramentaceone (7-Methyljuglone) in several Australian sundews. Carniv. Pl. Newslett. 2017, 46, 20–22. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I.; Hennern, H.; Hennern, A. Sundew chemistry and emergence updates. Carniv. Plant Newsl. 2018, 47, 10–17. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I.; Hennern, H.; Hennern, A. New sundew quinone and emergence data. Carniv. Plant Newsl. 2019, 48, 6–12. [Google Scholar] [CrossRef]
- Schlauer, J.; Fleischmann, A.; Carow, T. Quinones from “Gondwanan” Drosera taxa. Carniv. Plant Newsl. 2019, 48, 13–17. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I. Chemistry and surface micromorphology of the Queensland sundews. Carniv. Plant Newsl. 2019, 48, 111–116. [Google Scholar] [CrossRef]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I. Quinone Patterns and Identification of Japanese Spider Leg Sundews. Carniv. Plant Newsl. 2019, 48, 161–163. [Google Scholar] [CrossRef]
- Schlauer, J.; Fleischmann, A. Naphthoquinones in Pygmy Sundews (Drosera sect. Bryastrum). Carniv. Plant Newsl. 2021, 50, 111–117. [Google Scholar] [CrossRef]
- Schlauer, J.; Fleischmann, A. Refined Taxon Sampling Discloses New Quinone Patterns and Relationships among Sundews (Drosera, Droseraceae). Carniv. Plant Newsl. 2022, 51, 70–73. [Google Scholar] [CrossRef]
- Devi, S.P.; Kumaria, S.; Rao, S.R.; Tandon, P. Carnivorous Plants as a Source of Potent Bioactive Compound: Naphthoquinones. Trop. Plant Biol. 2016, 9, 267–279. [Google Scholar] [CrossRef]
- Badwaik, H.R.; Kumari, L.; Nakhate, K.; Verma, V.S.; Sakure, K. Phytoconstituent plumbagin: Chemical, biotechnological and pharmaceutical aspects. Stud. Nat. Prod. Chem. 2019, 63, 415–460. [Google Scholar] [CrossRef]
- Wójciak, M.; Feldo, M.; Stolarczyk, P.; Płachno, B.J. Carnivorous Plants from Nepenthaceae and Droseraceae as a Source of Secondary Metabolites. Molecules 2023, 28, 2155. [Google Scholar] [CrossRef] [PubMed]
- Zenk, M.H.; Fürbringer, M.; Steglich, W. Occurrence and distribution of ramentaceone and plumbagin in the Droseraceae. Phytochemistry 1969, 8, 2199–2200. [Google Scholar] [CrossRef]
- Culham, A.; Gornall, R.J. The taxonomic significance of naphthoquinones in the Droseraceae. Biochem. Syst. Ecol. 1994, 22, 507–515. [Google Scholar] [CrossRef]
- Schlauer, J. Literature Reviews. Carniv. Plant Newsl. 2012, 41, 121. Available online: https://cpn.carnivorousplants.org/articles/CPNv41n3p121.pdf (accessed on 30 January 2024).
- Länger, R.; Pein, I.; Kopp, B. Glandular hairs in the genus Drosera (Droseraceae). Plant Syst. Evol. 1995, 194, 163–172. [Google Scholar] [CrossRef]
- Durand, R.; Zenk, M.H. Homogentisate ring-cleavage pathway in the biosynthesis of acetate-derived naphthoquinones of the Droseraceae. Phytochemistry 1974, 13, 1483–1492. [Google Scholar] [CrossRef]
- Nahrstedt, A. Absence of cyanogenesis from Droseraceae. Phytochemistry 1980, 19, 2757–2758. [Google Scholar] [CrossRef]
- Kovacik, J.; Repcak, M. Naphthoquinones Content of Some Sundews (Drosera L.). Carniv. Plant Newsl. 2006, 35, 49–51. [Google Scholar] [CrossRef]
- Egan, P.A.; van der Kooy, F. Phytochemistry of the Carnivorous Sundew Genus Drosera (Droseraceae)—Future Perspectives and Ethnopharmacological Relevance. Chem. Biodivers. 2013, 10, 1774–1790. [Google Scholar] [CrossRef] [PubMed]
- Budzianowski, J. Naphthoquinone Glucosides of Drosera gigantea from in vitro Cultures. Planta Med. 2000, 66, 667–669. [Google Scholar] [CrossRef]
- Le Clercq, J.; Angenot, L. À propos du Drosera peltata et de la standardisation de la teinture de Drosera. J. Pharm. Belg. 1984, 39, 269–274. Available online: https://hdl.handle.net/2268/41270 (accessed on 30 January 2024).
- Krenn, L.; Länger, R.; Kopp, B. Qualitätsprüfung von Sonnentaukraut. 2. Botanische Identitätsprüfung sowie qualitative und quantitative Naphthochinonbestimmung an Handelsmustern. Dtsch. Apoth. Ztg. 1995, 135, 867–870. [Google Scholar]
- Putalun, W.; Udomsin, O.; Yusakul, G.; Juengwatanatrakul, T.; Sakamoto, S.; Tanaka, H. Enhanced plumbagin production from in vitro cultures of Drosera burmanii using elicitation. Biotechnol. Lett. 2010, 32, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Trevisan Ferreira, D.; Andrei, C.C.; Ostrensky Saridakis, H.; Faria, T.J.; Vinhato, E.; Carvalho, K.E.; Feijó Souza, D.J.; Machado, S.L.; Panayotis Saridakis, D.; Braz-Filho, R. Antimicrobial Activity and Chemical Investigation of Brazilian Drosera. Mem. Inst. Oswaldo Cruz Rio Jan. 2004, 99, 753–755. [Google Scholar] [CrossRef]
- Sauerwein, M.; Schmidt, S.; Reichling, J.; Wink, M. Naphthoquinone production in in vitro cultures of Drosera communis. St. Hil. BIOforum Extra Prague 1994, 94, 26–27. [Google Scholar]
- Bonnet, M.; Coumans, M.; Hofinger, M.; Ramaut, J.L.; Gaspar, T. High-performance gas chromatography of 1,4-naphthoquinomes from Droseraceae. Chromatographia 1984, 18, 621–622. [Google Scholar] [CrossRef]
- Crouch, I.J.; Finnie, J.F.; van Staden, J. Studies on the isolation of plumbagin from in vitro and in vivo grown Drosera species. Plant Cell Tissue Organ Cult. 1990, 21, 78–82. [Google Scholar] [CrossRef]
- Egan, P.A.; van der Kooy, F. Coproduction and Ecological Significance of Naphthoquinones in Carnivorous Sundews (Drosera). Chem. Biodivers. 2012, 9, 1033–1044. [Google Scholar] [CrossRef]
- Fleischmann, A.; Cross, A.T.; Gibson, R.; Gonella, P.M.; Dixon, K.W. Systematics and Evolution of Droseraceae. In Carnivorous Plants: Physiology, Ecology, and Evolution; Adamec, L., Ellison, A., Eds.; Oxford University Press: London, UK, 2017; pp. 45–57. [Google Scholar] [CrossRef]
- Eilenberg, H.; Pnini-Cohen, S.; Rahamim, Y.; Sionov, E.; Segal, E.; Carmeli, S.; Zilberstein, A. Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. J. Exp. Bot. 2010, 61, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Rischer, H.; Hamm, A.; Bringmann, G. Nepenthes insignis uses a C2-portion of the carbon skeleton of L-alanine acquired via its carnivorous organs, to build up the allelochemical plumbagin. Phytochemistry 2002, 59, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, C.R.; Ryves, D.B.; Millett, J. The Function of Secondary Metabolites in Plant Carnivory. Ann. Bot. 2020, 125, 399–411. [Google Scholar] [CrossRef]
- Schlauer, J.; Wistuba, A.; Hartmeyer, S.R.H.; Hartmeyer, I. The taxonomic relevance of naphthoquinones in tropical pitcher plants (Nepenthes L., Nepenthaceae). Carniv. Plant Newsl. 2022, 51, 185–193. [Google Scholar] [CrossRef]
- Hotti, H.; Gopalacharyulu, P.; Seppänen-Laakso, T.; Rischer, H. Metabolite profiling of the carnivorous pitcher plants Darlingtonia and Sarracenia. PLoS ONE 2017, 12, e0171078. [Google Scholar] [CrossRef]
- Hanson, S.W.; Crawford, M.; Thanasingh, D.P.J. (+)-Isoshinanolone and 2-methylbenzofuran-4-carbaldehyde from the Fish-Stunning Plant Habropetalum dawei. Phytochemistry 1981, 20, 1162–1164. [Google Scholar] [CrossRef]
- Bringmann, G.; Wohlfarth, M.; Rischer, H.; Grüne, M.; Schlauer, J. A New Biosynthetic Pathway to Alkaloids in Plants: Acetogenic Isoquinolines. Angew. Chem. Int. Ed. 2000, 39, 1464–1466. Available online: http://10.1002/(SICI)1521-3773(20000417)39:8<1464::AID-ANIE1464>3.0.CO;2-%23 (accessed on 30 January 2024). [CrossRef]
- Funayama, S.; Cordell, G.A. Alkaloids—A Treasury of Poisons and Medicines; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 263–273. [Google Scholar] [CrossRef]
- Ogihara, K.; Zhao, J.; Higa, M.; Yogi, S. Studies on the Constituents of Aristolochia liukiuensis 2. Bull. Coll. Science. Univ. Ryukyus 1992, 54, 17–28. Available online: https://u-ryukyu.repo.nii.ac.jp/record/2005300/files/No54p017.pdf (accessed on 30 January 2024).
- Connolly, J.D.; Hill, R.A. Dictionary of Terpenoids; Chapman & Hall: London, UK, 1991. [Google Scholar]
- Forster, P.G.; Ghisalberti, E.L.; Jefferies, P.R.; Poletti, V.M.; Whiteside, N.J. Serrulatane Diterpenes from Eremophila spp. Phytochemistry 1986, 25, 1377–1383. [Google Scholar] [CrossRef]
- van der Vijver, L.M. Distribution of Plumbagin in the Plumbaginaceae. Phytochemistry 1972, 11, 3247–3248. [Google Scholar] [CrossRef]
- Shcherbanovskii, L.R. Plumbagin from Plumbagella micrantha. Chem. Nat. Compd. 1974, 10, 518. [Google Scholar] [CrossRef]
- Budzianowski, J.; Budzianowska, A.; Kromer, K. Naphthalene Glucoside and Other Phenolics from the Shoot and Callus Cultures of Drosophyllum lusitanicum. Phytochemistry 2002, 61, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Poppinga, S.; Hartmeyer, S.R.H.; Seidel, R.; Masselter, T.; Hartmeyer, I.; Speck, T. Catapulting Tentacles in a Sticky Carnivorous Plant. PLoS ONE 2012, 7, e45735. [Google Scholar] [CrossRef]
- Feineis, D.; Bringmann, G. Ancistrocladus Naphthylisoquinoline Alkaloids. Progress. Chem. Org. Nat. Prod. 2023, 119, 1–334. [Google Scholar] [CrossRef]
| Classification 1 | Provenance 2 | Taxon | Quinones 3 | References | Comments |
|---|---|---|---|---|---|
| Dionaea | SE USA | D. muscipula | P | [26,27] | |
| Aldrovanda | Old World | A. vesiculosa | P | [26,27] | |
| Drosera subg. Regiae | ZA | D. regia | P, (M-tr) | [18,27] | |
| Drosera subg. Arcturia | Tasmania | D. arcturi | (M-tr) | [18] | 0 in [27] |
| Drosera subg. Ergaleium | |||||
| Drosera sect. Coelophylla | SW AU | D. glanduligera | M | [15] | |
| Drosera sect. Lasiocephala | N AU, N.Guin. | D. banksii | (P-tr) | [22] | |
| NT | D. brevicornis | 0 | [17,22] | ||
| NT | D. broomensis | P | [22] | ||
| N AU | D. caduca | P | [22] | ||
| Kimberley | D. darwinensis | 0 | [22] | ||
| Kimberley | D. derbyensis | P | [22] | ||
| Kimberley, NT | D. dilatatopetiolaris | 0 | [22] | ||
| NT | D. falconeri | 0 | [22] | ||
| NT | D. fulva | M | [22] | ||
| Kimberley, NT | D. kenneallyi | P | [22] | ||
| Qld. | D. lanata | 0 | [22] | ||
| NT | D. aff. lanata | 0 | [22] | ||
| NT | D. aff. paradoxa (“swamp form”) | 0 | [17,22] | ||
| NT | D. aff. paradoxa (“NT form”) | 0 | [22] | as D. paradoxa “type form” | |
| NT | D. aff. paradoxa (orange form) | 0 | [22] | ||
| NT | D. cf. petiolaris | 0 | [22] | ||
| Qld. | D. petiolaris | 0 | [22] | ||
| N AU, N.Guin. | “D. petiolaris” | 0 | [27] | ||
| Drosera sect. Bryastrum | SW AU | D. androsacea | 0 | [21] | |
| SW AU | D. australis | 0 | [21] | ||
| SW AU | D. barbigera | 0 | [27] | ||
| SW AU | D. bindoon | 0 | [21] | ||
| SW AU | D. callistos | 0 | [21] | ||
| SW AU | D. citrina | 0 | [21] | ||
| SW AU | D. closterostigma | 0 | [21] | ||
| SW AU | D. coomallo | 0 | [21] | ||
| SW AU | D. depauperata | 0 | [21] | ||
| SW AU | D. echinoblastus | 0 | [21] | ||
| SW AU | D. enneabba | 0 | [21] | ||
| SW AU | D. gibsonii | 0 | [21] | ||
| SW AU | D. grievei | 0 | [21] | ||
| SW AU | D. helodes | 0 | [21] | ||
| SW AU | D. hyperostigma | 0 | [21] | ||
| SW AU | D. lasiantha | 0 | [21] | ||
| SW AU | D. leucoblasta | 0 | [27] | ||
| SW AU | D. leucostigma | 0 | [21] | ||
| SW AU | D. mannii | 0 | [21] | ||
| BR, Venezuela | D. meristocaulis | 0 | [28] | ||
| SW AU | D. micrantha | 0 | [21] | ||
| SW AU | D. microscapa | 0 | [21] | ||
| SW AU | D. miniata | 0 | [21] | ||
| SW AU | D. minutiflora | M | [21] | ||
| SW AU | D. nitidula | 0 | [27] | ||
| SW AU | D. nivea | 0 | [21] | ||
| SW AU | D. occidentalis | 0 | [27] | ||
| SW AU | D. omissa | M | [21] | 0 in [27] | |
| SW AU | D. oreopodion | 0 | [21] | ||
| SW AU | D. paleacea | 0 | [21,27] | ||
| SW AU | D. parvula | 0 | [27] | ||
| SW AU | D. patens | 0 | [21] | ||
| SW AU | D. pedicellaris | M | [21] | ||
| SW AU | D. platystigma | 0 | [27] | ||
| SW AU | D. pulchella | P + M | [21] | 0 in [27] | |
| SW AU | D. pulchella × nitidula | 0 | [27] | ||
| SW AU | D. pulchella × occidentalis | 0 | [27] | ||
| SW AU | D. pycnoblasta | 0 | [27] | ||
| E AU | D. pygmaea | P | [29] | most probably in err. | |
| E AU | D. pygmaea | 0 | [27] | ||
| SW AU | D. roseana | 0 | [21] | ||
| SW AU | D. sargentii | M | [21] | ||
| SW AU | D. scorpioides | 0 | [27] | ||
| SW AU | D. sewelliae | 0 | [21] | ||
| SW AU | D. silvicola | 0 | [21] | ||
| SW AU | D. stelliflora | 0 | [21] | ||
| SW AU | D. trichocaulis | 0 | [21] | ||
| SW AU | D. verrucata | 0 | [21,27] | (D. dichrosepala auct. in err.) | |
| SW AU | D. walyunga | 0 | [21] | ||
| Drosera sect. Phycopsis | E AU | D. binata | P | [26,27,30,31,32,33] | |
| E AU | D. binata (var. dichotoma) | P | [26,30,33] | ||
| Drosera sect. Ergaleium—“Ergaleium” | SW AU | D. andersoniana | P | [27] | |
| E AU | D. auriculata | P, (M-tr) | [26,27,30,33] | ||
| SE AU | D. auriculata | P | [22] | ||
| SW AU | D. gigantea | P | [33,34] | ||
| N.Guin. | D. gracilis | P, (M-tr) | [22] | ||
| E AU | D. gunniana | P, (M-tr) | [22] | ||
| SE AU | D. hookeri | P, (M-tr) | [22] | ||
| Thailand | D. lunata | P + M | [22] | ||
| E Asia | D. lunata | P | [26,27,30,33,35,36] | ||
| SW AU | D. macrantha s. str. | P | [22] | 0 in [27] | |
| SW AU | D. marchantii | 0 | [27] | ||
| SW AU | D. menziesii | P | [17] | 0 in [27] | |
| SW AU | D. microphylla | P | [27,30] | ||
| SW AU | D. modesta | P | [17,27] | ||
| SW AU | D. moorei | (P-tr) | [22] | ||
| SW AU | D. myriantha | 0 | [27] | ||
| SW AU | D. neesii | 0 | [27] | ||
| SW AU | D. planchonii | P | [17] | ||
| SW AU | D. radicans | 0 | [27] | ||
| SW AU | D. subhirtella | 0 | [27] | ||
| SW AU | D. zigzagia | P | [22] | ||
| Drosera sect. Ergaleium—“Erythrorhiza” | SW AU | D. aberrans | P | [17] | |
| SW AU | D. aberrans | P + M | [22] | ||
| SW AU | D. bulbosa | P | [27] | ||
| SW AU | D. erythrorhiza | P | [27,30] | ||
| SW AU | D. lowriei | P | [17] | ||
| SW AU | D. macrophylla | P | [27] | ||
| SW AU | D. major | 0 | [17] | ||
| SW AU | D. tubaestylis | P | [17] | ||
| SW AU | D. whittakeri | P | [26,27,30,31,33] | ||
| Drosera sect. Ergaleium—“Stoloniferae” | SW AU | D. platypoda | P | [27] | |
| SW AU | D. rupicola | M | [17] | P in [27] | |
| SW AU | D. stolonifera | M | [27,30] | 0 in [22] | |
| Drosera subg. Drosera | |||||
| Drosera sect. Thelocalyx | NT, India | D. burmannii | M | [16,27] | P in [33,37], probably in err. (method does not identify isomer) |
| BR | D. sessilifolia | M | [16] | ||
| Drosera sect. Prolifera | Qld. | D. adelae | (P-tr), M | [19,27,32,33] | |
| Qld. | D. prolifera | P | [19,27,32,33] | ||
| cult. (Qld.) | D. prolifera × schizandra | P + M | [19] | ||
| Qld. | D. schizandra | M | [19] | 0 in [27] | |
| Drosera sect. Arachnopus | NT | D. aquatica | M | [15] | |
| Kimberley | D. aurantiaca | M | [16] | ||
| Kimberley | D. barrettiorum | M | [22] | ||
| Kimberley | D. cucullata | P | [15,16] | ||
| NT | D. finlaysoniana | P, (M-tr) | [15,16] | ||
| NT | D. fragrans | P | [15] | ||
| Kimberley | D. hartmeyerorum | M | [15,16] | ||
| ?AU | “D. indica” | P | [26] | “D. indica” included all spp. of D. sect. Arachnopus at time of study | |
| Vietnam, Ivory Coast | D. indica | P + M | [16,27] | ||
| Japan | D. makinoi | P + M | [20] | ||
| Kimberley | D. margaritacea | P, (M-tr) | [22] | ||
| NT | D. nana | M | [17] | ||
| NT, Qld., Japan | D. serpens | P | [15,16,20] | ||
| Drosera sect. Stelogyne | SW AU | D. hamiltonii | M | [18,26,27,32,33] | |
| Drosera sect. Psychophila | New Zealand | D. stenopetala | P + M | [18] | |
| Chile | D. uniflora | M | [17] | ||
| Drosera sect. Drosera | BR | D. amazonica | M | [22] | |
| Colombia | D. amazonica × biflora | P, (M-tr) | [22] | ||
| Germany | D. anglica | M | [27,29,33] | (syn. D. longifolia) | |
| Germany | D. anglica × rotundifolia | M | [27] | (syn. D. ×obovata) | |
| Venezuela | D. arenicola | M | [18] | ||
| Venezuela | D. biflora | P | [22] | ||
| Venezuela | D. biflora × esmeraldae | P + M | [22] | ||
| BR | D. brevifolia | P, (M-tr) | [22] | ||
| USA | D. brevifolia | P? | [38] | ||
| ? | D. capillaris | P | [30,31] | ||
| BR, USA | D. capillaris | M | [17] | ||
| ? | “D. communis” | M | [32,33] | probably in err. (misidentified D. spatulata from cult.?) | |
| BR | D. communis | P | [18,38,39] | ||
| Venezuela | D. esmeraldae | M | [22] | ||
| Venezuela | D. felix | M | [17] | ||
| USA | D. filiformis | M | [14,27] | ||
| USA | D. filiformis subsp. tracyi | M | [26,30,33] | ||
| USA | D. filiformis var. floridana | M | [17] | ||
| Venezuela | D. intermedia | P | [22] | ||
| Germany | D. intermedia | P, (M-tr) | [14,26,27,30,31,33,40] | (D. longifolia auct. in err.) | |
| USA | D. intermedia × filiformis | P + M | [14] | (syn. D. ×hybrida) | |
| USA (introd. in Europe) | D. intermedia × rotundifolia | P + M | [14] | (syn. D. ×eloisiana, D. ×belezeana auct. in err.) | |
| Venezuela | D. kaieteurensis | 0 | [18] | ||
| USA | D. linearis | M | [17] | ||
| NC | D. neocaledonica | M | [17] | ||
| China | D. oblanceolata | M | [18] | ||
| Germany | D. rotundifolia | (P-tr), M | [14,27,29,30,31,33,40] | ||
| E Asia | D. spatulata | M | [18,26,27,30,31,32,33] | ||
| Palawan | D. ultramafica | P + M | [17] | ||
| Drosera sect. Ptycnostigma | ZA | D. admirabilis | M | [18,32] | |
| NE Namibia | D. affinis | P | [17] | ||
| ZA | D. aliciae | (P-tr), M | [26,27,30,31,33] | ||
| cult. (ZA) | D. aliciae × capensis | M | [27] | ||
| ZA | D. burkeana | M | [18,26,27,30,31,32,33] | ||
| ZA | D. capensis | (P-tr), M | [26,27,30,31,32,33,41] | ||
| ZA | D. cistiflora | (P-tr), M | [17,26,27,33] | ||
| ZA | D. collinsiae | M | [18,32,33] | 0 in [27] | |
| ZA | D. cuneifolia | (P-tr), M | [18,26,27,33] | ||
| ZA | D. dielsiana | M | [29] | ||
| Zambia | D. flexicaulis | P | [22] | ||
| ZA | D. glabripes | (P-tr), (M-tr) | [42] | possibly in err. | |
| ZA | D. hilaris | M | [27] | ||
| Madagascar | D. madagascariensis | M | [22] | ||
| Madagascar | D. madagascariensis | (P-tr), M | [18,26,27,33,36] | (D. ramentacea auct. in err.) | |
| Zambia | D. madagascariensis | M | [22] | ||
| ZA | D. natalensis | (P-tr), M? | [41] | ||
| ZA | D. nidiformis | M | [18] | ||
| Zambia | D. pilosa | M | [22] | ||
| ZA | D. ramentacea | M | [18] | ||
| ZA | D. rubrifolia | 0 | [18] | ||
| ZA | D. slackii | P | [18,27] | ||
| ZA | D. trinervia | M | [26,27,33] | ||
| ZA | D. venusta | M | [32,33] | P in [27], probably in err. | |
| Drosera sect. Brasilianae | BR | D. camporupestris | 0 | [18] | |
| BR | D. chrysolepis | 0 | [18] | ||
| BR | D. grantsaui × tomentosa | M | [18] | (syn. D. ×fontinalis) | |
| BR | D. graminifolia | M | [22] | ||
| BR | D. grantsaui | M | [18] | ||
| BR | D. graomogolensis | 0 | [18] | ||
| BR | D. latifolia | P + M | [18] | ||
| BR | D. magnifica | M | [22] | ||
| BR | D. montana | P? | [38] | possibly in err. | |
| BR | D. spiralis | M | [18] | ||
| BR | D. tentaculata | M | [22] | ||
| BR | D. villosa | P + M | [18,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlauer, J.; Fleischmann, A.; Hartmeyer, S.R.H.; Hartmeyer, I.; Rischer, H. Distribution of Acetogenic Naphthoquinones in Droseraceae and Their Chemotaxonomic Utility. Biology 2024, 13, 97. https://doi.org/10.3390/biology13020097
Schlauer J, Fleischmann A, Hartmeyer SRH, Hartmeyer I, Rischer H. Distribution of Acetogenic Naphthoquinones in Droseraceae and Their Chemotaxonomic Utility. Biology. 2024; 13(2):97. https://doi.org/10.3390/biology13020097
Chicago/Turabian StyleSchlauer, Jan, Andreas Fleischmann, Siegfried R. H. Hartmeyer, Irmgard Hartmeyer, and Heiko Rischer. 2024. "Distribution of Acetogenic Naphthoquinones in Droseraceae and Their Chemotaxonomic Utility" Biology 13, no. 2: 97. https://doi.org/10.3390/biology13020097
APA StyleSchlauer, J., Fleischmann, A., Hartmeyer, S. R. H., Hartmeyer, I., & Rischer, H. (2024). Distribution of Acetogenic Naphthoquinones in Droseraceae and Their Chemotaxonomic Utility. Biology, 13(2), 97. https://doi.org/10.3390/biology13020097








